Abstract
Plant cell growth is controlled by the balance between turgor pressure and the extensibility of the cell wall. Several distinct classes of wall polysaccharides and their interactions contribute to the architecture and the emergent features of the wall. As a result, remarkable tensile strength is achieved without relinquishing extensibility. The control of growth and development does not only require a precisely regulated biosynthesis of cell wall components, but also constant remodeling and modification after deposition of the polymers. This is especially evident given the fact that wall deposition and cell expansion are largely uncoupled. Pectins form a functionally and structurally diverse class of galacturonic acid-rich polysaccharides which can undergo abundant modification with a concomitant change in physicochemical properties. This review focuses on homogalacturonan demethylesterification catalyzed by the ubiquitous enzyme pectin methylesterase (PME) as a growth control module. Special attention is drawn to the recently discovered role of this process in primordial development in the shoot apical meristem.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
While turgor pressure is the driving force behind plant growth, the parameter solely responsible for the control of cell expansion is the extensibility of the cell wall (Geitmann and Ortega 2009; Boudaoud 2010; Baskin 2005). The plant cell wall is a highly complex and dynamic structure composed of polysaccharides, structural proteins and phenolic compounds (Cosgrove 2005; Somerville et al. 2004). It can be regarded as a fiber-enforced composite material, in which tensile strength is conferred by cellulose microfibrils which are embedded and cross-linked in a matrix composed of hemicelluloses and pectins (Dick-Perez et al. 2011; Kerstens et al. 2001), with a contribution from structural proteins (Lamport et al. 2011; Cannon et al. 2008). Pectins are characterized as a heterogenous group of galacturonic acid-rich polysaccharides which form up to 35% of dicot and nongrass monocot primary walls (Mohnen 2008). Both structurally and functionally, pectins are the most complex wall components and have a major impact on the physicochemical characteristics of the cell wall. One way in which pectin seems to exert an effect on wall properties is through its role during cellulose deposition (Shea et al. 1989; Yoneda et al. 2010). In vitro reconstruction of cellulose/pectin composite networks using cellulose-producing bacteria grown in the presence of pectin suggested that pectin strongly increased the extensibility of the composite compared to cellulose alone, an effect which prevailed even after removal of pectin from the network (Chanliaud and Gidley 1999). In addition, cross-linking of pectin subdomains by the essential plant micronutrient boron is required for normal growth and development (O'Neill et al. 2001; Noguchi et al. 1997; Bonin et al. 1997). This review focuses on the dynamic modification of homogalacturonan (HG), a pectin subclass, which has recently emerged as a vital regulator of plant growth.
Homogalacturonan and plant development
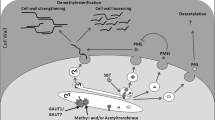
HG, the most abundant pectin (23% of the dry weight of total leaf cell wall in Arabidopsis (Caffall and Mohnen 2009)) can undergo major modification after deposition in the cell wall. It is polymerized in the Golgi apparatus by glycosyl transferases, substituted with methyl groups at the C6 position and secreted to the cell wall in a highly methyl-esterified state (Zhang and Staehelin 1992; Li et al. 1997; Sterling et al. 2006). In the wall, and potentially en route, pectin methylesterase (PME) can remove the methyl groups, dramatically altering the physical properties of the polymer (Wolf et al. 2009). As a result of PME activity, free carboxylic acid groups are created, and methanol and protons are released (Fig. 1a). Given the fact that HG is the most abundant pectin and that it becomes largely demethylesterified during the course of development, substantial amounts of methanol are produced. The fate of this methanol is still not entirely clear. A part of it will certainly evaporate and escape via stomata as it is a volatile (boiling point 65°C) compound (Fall and Benson 1996). Feeding studies with [13C]methanol indicate that plant cells can slowly metabolize CH3OH to serine and methionine via formaldehyde dehydrogenase. However, this seems not to be the major cause of methanol loss, as higher plants do not possess methanol oxidase, the key enzyme for methanol fixation (Gout et al. 2000). In contrast, methylobacteria do not only possess this enzyme but can also survive with volatile C1 compounds as the sole carbon source. These bacteria are frequently found on leaf surfaces, potentially relying on the emitted methanol. In addition, a model of mutual symbiosis was proposed, as some methanol-consuming bacteria are able to produce and secrete cytokinins and auxin (Kutschera 2007).
Schematic representation of homogalacturonic acid demethyl esterification. a The reaction catalyzed by pectin methylesterase (PME) leads to a free carboxylic acid group and the release of methanol and a proton, respectively. PMEI can block the reaction through interaction with PME. b Possible consequences of PME activity. 1 The decrease in pH caused by the reaction outlined in a might alter cell wall properties indirectly through changing the activity of cell wall remodeling enzymes. 2 Continuous demethyl esterification of more than nine galacturonic acid residues can lead to the Ca2+ cross-linking of two adjacent HG molecules, which in vitro, leads to gelation and stiffening of the pectin. 3 Demethyl esterification can promote hydration, which in turn leads to a reduction in wall stiffness. 4 PME activity is a prerequisite for HG degradation by polygalacturonase (PG). In addition to the wall loosening effect of pectin removal, PG activity can result in the production of oligogalacturonides (OGAs), which can act as signaling molecules during pathogen attack and normal development
Depending on regulatory factors such as pH, ion availability, methyl esterification state of the substrate and potentially intrinsic differences in mode of action of the respective isoforms, PME activity leads to a multitude of different methyl esterification patterns (Cameron et al. 2008; Cameron et al. 2011; Catoire et al. 1998; Denes et al. 2000; Willats et al. 2001). These patterns, or epitopes, differ both in terms of mechanical properties and degradability, which makes it difficult to transfer in vitro findings to the in vivo situation. Various pectin methyl esterification epitopes are recognized, in many cases somewhat promiscuously (Clausen et al. 2003), by an array of pectin-specific antibodies, which have greatly promoted the research in the field. Naturally, labeling with antibody probes directed against pectins provides only a snapshot of pectin dynamics, and it is difficult, if not impossible, to deduce with confidence a prediction for the mechanical properties of the cell wall, since HG is only one part of its composite structure. Moreover, since demethyl esterification precedes degradation (Francis et al. 2006; Wakabayashi et al. 2000; Wakabayashi et al. 2003), a seemingly rigid wall epitope could, in fact, represent an early stage of degradation. The use of techniques which allow the direct measurement of mechanical properties at high resolution such as microindentation and Atomic Force Microscopy (AFM) would allow, at least, the comparison of cell wall properties as a whole with the abundance and localization of certain pectic methyl esterification epitopes in the same respective tissue.
Despite the above-mentioned caveats, it can be safely stated that a low level of pectin methyl esterification is often associated with reduced wall extensibility and the cessation of growth, e.g., in pollen tubes and hypocotyls (Derbyshire et al. 2007; Parre and Geitmann 2005; Bosch et al. 2005; Pelletier et al. 2010). It is conceivable that Ca2+-mediated cross-linking of demethylesterified HG chains represents a means to abolish cell wall creep, prevent embedded cellulose microfibrils from sliding and, hence, render the (potentially elastic) initial extension of the cell wall permanent (plastic). However, this wall consolidation is only one of the possible consequences of PME activity (Fig. 1). As mentioned above, pectin demethyl esterification might also lead to wall loosening through enabling the action of HG degrading polygalacturonase, which has important consequences for cell adhesion. For example, mutation of the PME QUARTET1 (QRT1), prevents the degradation of primary cell wall material connecting the microspores in a pollen tetrade by QRT3, a polygalacturonase (Francis et al. 2006). In addition, breakdown of demethylesterified HG can liberate oligogalacturonides (OGA), which function as signaling molecules during defense and normal plant development (Branca et al. 1988; Bellincampi et al. 1996; Ayers et al. 1976; Hahn et al. 1981; Davis et al. 1986). Lastly, demethyl esterification promotes wall hydration and decreases the pH of the wall which, in turn, has several potential direct and indirect consequences for wall properties and enzyme activities (Pelloux et al. 2007).
Wall consolidation and beyond
PME-mediated demethyl esterification of HG as a wall consolidation mechanism apparently evolved before plants developed multicellularity, based on the presence of HG methyl esterification epitopes in present-day taxa of the charophycean green algae (Domozych et al. 2007; Eder and Lutz-Meindl 2008). This mechanism seems to be still operational in higher plants, perhaps most prominently in the pollen tube with its reduced wall complexity. Here, a good correlation between antibody labeling, deduced mechanical properties and actual measurements of cell wall stiffness by microindentation has been observed (Fayant et al. 2010; Zerzour et al. 2009; Parre and Geitmann 2005; Bosch et al. 2005). The pollen tube grows by massive incorporation of secretory vesicles into the plasma membrane exclusively at the tip (Bosch and Hepler 2005; McKenna et al. 2009). Notably, only highly methyl-esterified pectin is present in the growing apex, whereas demethylesterified pectin is found along the shank of the tube in accordance with a role of the latter type in wall consolidation (Parre and Geitmann 2005; Bosch et al. 2005). Based on protein localization studies, a possible mechanism for the maintenance of this distribution was proposed (Rockel et al. 2008). A PME inhibitor protein (PMEI) is exclusively localized at the pollen tube tip wall, where it could interact with the ubiquitous PME, thus preventing PME activity here. PMEI undergoes selective endocytosis, presumably near the transition region between tip and shank, thereby liberating PME for its wall solidifying role in the lateral cell wall (Rockel et al. 2008).
In accordance with the role of pollen PMEs in wall consolidation, mutation of VGD1, a highly expressed Arabidopsis pollen PME isoform, results in unstable tubes which lack the mechanical integrity to successfully penetrate the female tissue (Jiang et al. 2005). Similar effects, albeit to a weaker extent, were observed after genetic interference with other pollen PMEs in tobacco and Arabidopsis (Tian et al. 2006; Bosch and Hepler 2006). Conversely, ubiquitous overexpression of VGD1, leading to ectopic labeling of demethylesterified pectin, results in dwarf plants, a phenotype which could be explained by reduced cell wall extensibility (Fig. 2a). Interestingly, labeling with an antibody directed against HG with a low degree of methyl esterification showed a high abundance of this epitope in the outer epidermal wall of stem sections, whereas it is apparently undetectable in these walls in the wildtype (Fig. 2b). This is noteworthy with respect to the discussion on growth control exerted by the epidermis over the inner tissues (Savaldi-Goldstein et al. 2007).
Effects of ectopic overexpression of VGD1, a pollen-specific PME. a Morphological phenotype of 35S:VGD1 plants compared with the Col-0 wildtype. b Immunolabeling of Col-0 and VGD1 stem sections with antibodies raised against pectin with a low degree of methyl esterification (DM, upper panel) and high DM (lower panel). Note the ectopic appearance of demethylesterified pectin in the outer epidermal wall of 35S:VGD1 plants and the absence of a strong label for highly methylesterified pectin in the parenchyma
However, genetic interference with PME expression and activity does not always result in effects as clear as observed with VGD1. The overwhelming majority of PME mutants analyzed do not seem to show a morphological growth phenotype. While this can certainly, at least in part, be attributed to genetic redundancy (there are 66 PME isoforms in Arabidopsis), evidence for feedback regulation has been observed (unpublished data). With this respect, it is interesting to note that an increase in pectin abundance and a decrease in the degree of methyl esterification is a hallmark of the response to interference with cellulose biosynthesis (Burton et al. 2000; Manfield et al. 2004; His et al. 2001), indicating that the state of the cell wall is under surveillance of cell wall integrity signaling pathways.
HG methyl esterification and the mechanical forces shaping the plant
Recently, phyllotactic patterning at the shoot apical meristem (SAM) has been identified as a novel function of regulated pectin demethyl esterification. Labeling of SAM sections with antibodies specific for demethylesterified pectin showed that in the meristem dome, the degree of methyl esterification appears to be relatively high, whereas primordial outgrowths and incipient primordia are strongly labeled, indicating strong PME activity at these sites (Peaucelle et al. 2008). Furthermore, using SAM-expressed PME and PMEI isoforms, the authors demonstrated that elevated PME levels resulted in an increased number of primordia and disturbed phyllotactic patterning, whereas overexpression of a PMEI completely blocked lateral organ formation and led to a naked meristem phenotype. Most intriguingly, deposition of a sepharose bead loaded with PME resulted in a new primordium which developed into a normal floral meristem. In contrast to similar and now classic experiments with expansin-loaded beads (Fleming et al. 1997), application of PME beads to the meristem strongly disturbed both phyllotactic patterning and plastochrone (timing of primordium formation). In summary, these results strongly suggest that PME-mediated pectin demethyl esterification is necessary and sufficient for triggering primordium formation. The relation between pectin methyl esterification and other known SAM regulators such as auxin (Reinhardt et al. 2003) and microtubules (Hamant et al. 2008) remains to be established.
In a follow-up study, Peaucelle et al. analyzed the elastic properties of meristem cell walls by recording the apparent Young's modulus over the length and width of plasmolyzed SAMs through the use of AFM (Peaucelle et al. 2011a). Marked differences between the meristem dome, the primordia and the incipient primordia were observed. Both primordia and incipient primordia showed a lower apparent Young's modulus (or reduced stiffness) compared to the meristem center (summarized in Fig. 3). Interestingly, this suggests that a very low degree of methyl esterification does not necessarily lead to wall stiffening. As mentioned above, pectin turnover could possibly explain apparent discrepancies between expected and measured mechanical properties. In addition, the availability of calcium ions might influence pectin mechanics. Clearly, more work is needed to understand the differing properties of demethylesterified pectin in the SAM and the pollen tube (Fig. 3).
Schematic representation of mechanical properties and degree of pectin methyl esterification in shoot apical meristems and pollen tubes, respectively. In the SAM (upper left panel), pectin demethyl esterification is initiated in the L2 layer of incipient primordia and afterwards spreads to the L1. This pectin modification is accompanied by a reduction of stiffness (apparent Young's modulus EA) as measured by AFM (Peaucelle et al. 2011a; Peaucelle et al. 2008). Conversely, in pollen tubes (lower left panel), reduced methyl esterification in the shank of the pollen tube is associated with an increase in stiffness (Zerzour et al. 2009). In the case of elevated PME activity (middle panels), EA is decreased and ectopic primordia are formed in the SAM, whereas pollen tube growth is reduced or arrested, and thickening/stiffening of the apical wall occurs (Peaucelle et al. 2011a; Rockel et al. 2008; Parre and Geitmann 2005; Bosch et al. 2005; Peaucelle et al. 2008). In response to reduced PME activity through PMEI overexpression (right panels), primordium formation is blocked in the SAM with a concomitant rise in stiffness (Peaucelle et al. 2011a; Peaucelle et al. 2008). In pollen tubes, overexpression of PMEI results in increased elongation (Rockel et al. 2008). Pectin distribution and mechanical properties have not been analyzed in tubes overexpressing PMEI. Pectin methyl esterification in SAMs was analyzed with 2F4 antibody, whereas JIM7 and JIM5 were used with pollen tubes. Cell walls with high degree of HG methyl esterification are represented by gray color; low degree of HG methyl esterification is represented by red color. M meristem dome, P primordium, IP incipient primordium, EA apparent Young's modulus
Extending their analysis to transgenic plants, Peaucelle et al. established that meristems with elevated PME activity had an overall increased elasticity similar to what is observed in WT primordia, whereas overexpression of PMEI resulted in lower elasticity similar to what was observed in the WT meristem dome. Furthermore, by using two different sizes of beads connected to the AFM's cantilever, Peaucelle et al. suggest that the pectin-inflicted changes in mechanical properties are initiated in the L2 rather than the epidermal L1 layer (Peaucelle et al. 2011a).
Further, strengthening the link between pectin methyl esterification and phyllotaxis, a recent study showed that in bellringer-6 (blr-6), a transcription factor mutant with disturbed phyllotactic patterning, a PME (PME5, the same isoform used in the above-mentioned studies) is deregulated in the SAM. This leads to an increase of meristematic PME activity and reduced methyl esterification, as determined with antibody labeling and FTIR microspectroscopy (Peaucelle et al. 2011b). Introduction of a PME5 knockout allele into the blr-6 background was sufficient to revert phyllotactic patterning to near WT phenotype, supporting the notion that ectopic and elevated PME5 activity is the main determinant of the phyllotactic defect in blr-6 (Peaucelle et al. 2011b). These studies demonstrate spectacularly that demethyl esterification can have profound impact on plant development and that great caution has to be taken when inferring mechanical properties based on data concerning degree and pattern of methyl esterification.
References
Ayers AR, Valent B, Ebel J, Albersheim P (1976) Host–pathogen interactions: XI. Composition and structure of wall-released elicitor fractions. Plant Physiol 57(5):766–774
Baskin TI (2005) Anisotropic expansion of the plant cell wall. Annu Rev Cell Dev Biol 21:203–222. doi:10.1146/annurev.cellbio.20.082503.103053
Bellincampi D, Cardarelli M, Zaghi D, Serino G, Salvi G, Gatz C, Cervone F, Altamura MM, Costantino P, Lorenzo GD (1996) Oligogalacturonides prevent rhizogenesis in rolB-transformed tobacco explants by inhibiting auxin-induced expression of the rolB gene. Plant Cell 8(3):477–487
Bonin CP, Potter I, Vanzin GF, Reiter WD (1997) The MUR1 gene of Arabidopsis thaliana encodes an isoform of GDP-D-mannose-4,6-dehydratase, catalyzing the first step in the de novo synthesis of GDP-L-fucose. Proc Natl Acad Sci USA 94(5):2085–2090
Bosch M, Hepler PK (2005) Pectin methylesterases and pectin dynamics in pollen tubes. Plant Cell 17(12):3219–3226. doi:10.1105/tpc.105.037473
Bosch M, Hepler PK (2006) Silencing of the tobacco pollen pectin methylesterase NtPPME1 results in retarded in vivo pollen tube growth. Planta 223(4):736–745
Bosch M, Cheung AY, Hepler PK (2005) Pectin methylesterase, a regulator of pollen tube growth. Plant Physiol 138(3):1334–1346
Boudaoud A (2010) An introduction to the mechanics of morphogenesis for plant biologists. Trends Plant Sci 15(6):353–360. doi:10.1016/j.tplants.2010.04.002
Branca C, Lorenzo GD, Cervone F (1988) Competitive inhibition of the auxin-induced elongation by α-D-oligogalacturonides in pea stem segments. Physiol Plant 72(3):499–504
Burton RA, Gibeaut DM, Bacic A, Findlay K, Roberts K, Hamilton A, Baulcombe DC, Fincher GB (2000) Virus-induced silencing of a plant cellulose synthase gene. Plant Cell 12(5):691–706
Caffall KH, Mohnen D (2009) The structure, function, and biosynthesis of plant cell wall pectic polysaccharides. Carbohydr Res 344(14):1879–1900
Cameron RG, Luzio GA, Goodner K, Williams MAK (2008) Demethylation of a model homogalacturonan with a salt-independent pectin methylesterase from citrus: I. Effect of pH on demethylated block size, block number and enzyme mode of action. Carbohydr Polym 71(2):287–299
Cameron RG, Luzio GA, Vasu P, Savary BJ, Williams MA (2011) Enzymatic modification of a model homogalacturonan with the thermally tolerant pectin methylesterase from Citrus: 1. Nanostructural characterization, enzyme mode of action, and effect of pH. J Agric Food Chem 59(6):2717–2724
Cannon MC, Terneus K, Hall Q, Tan L, Wang Y, Wegenhart BL, Chen L, Lamport DT, Chen Y, Kieliszewski MJ (2008) Self-assembly of the plant cell wall requires an extensin scaffold. Proc Natl Acad Sci USA 105(6):2226–2231
Catoire L, Pierron M, Morvan C, du Penhoat CH, Goldberg R (1998) Investigation of the action patterns of pectinmethylesterase isoforms through kinetic analyses and NMR spectroscopy. Implications In cell wall expansion. J Biol Chem 273(50):33150–33156
Chanliaud E, Gidley MJ (1999) In vitro synthesis and properties of pectin/Acetobacter xylinus cellulose composites. Plant J 20(1):25–35
Clausen MH, Willats WG, Knox JP (2003) Synthetic methyl hexagalacturonate hapten inhibitors of anti-homogalacturonan monoclonal antibodies LM7, JIM5 and JIM7. Carbohydr Res 338(17):1797–1800
Cosgrove DJ (2005) Growth of the plant cell wall. Nat Rev Mol Cell Biol 6(11):850–861
Davis KR, Darvill AG, Albersheim P, Dell A (1986) Host–pathogen interactions: XXIX. Oligogalacturonides released from sodium polypectate by endopolygalacturonic acid lyase are elicitors of phytoalexins in soybean. Plant Physiol 80(2):568–577
Denes JM, Baron A, Renard CM, Pean C, Drilleau JF (2000) Different action patterns for apple pectin methylesterase at pH 7.0 and 4.5. Carbohydr Res 327(4):385–393
Derbyshire P, McCann MC, Roberts K (2007) Restricted cell elongation in Arabidopsis hypocotyls is associated with a reduced average pectin esterification level. BMC Plant Biol 7:31
Dick-Perez M, Zhang Y, Hayes J, Salazar A, Zabotina OA, Hong M (2011) Structure and interactions of plant cell-wall polysaccharides by two- and three-dimensional magic-angle-spinning solid-state NMR. Biochemistry 50(6):989–1000
Domozych DS, Serfis A, Kiemle SN, Gretz MR (2007) The structure and biochemistry of charophycean cell walls: I. Pectins of Penium margaritaceum. Protoplasma 230(1–2):99–115
Eder M, Lutz-Meindl U (2008) Pectin-like carbohydrates in the green alga Micrasterias characterized by cytochemical analysis and energy filtering TEM. J Microsc 231(2):201–214
Fall R, Benson AA (1996) Leaf methanol—the simplest natural product from plants. Trends Plant Sci 1(9):296–301. doi:10.1016/s1360-1385(96)88175-0
Fayant P, Girlanda O, Chebli Y, Aubin CE, Villemure I, Geitmann A (2010) Finite element model of polar growth in pollen tubes. Plant Cell 22(8):2579–2593
Fleming AJ, McQueen-Mason S, Mandel T, Kuhlemeier C (1997) Induction of leaf primordia by the cell wall protein expansin. Science 276(5317):1415–1418. doi:10.1126/science.276.5317.1415
Francis KE, Lam SY, Copenhaver GP (2006) Separation of Arabidopsis pollen tetrads is regulated by QUARTET1, a pectin methylesterase gene. Plant Physiol 142(3):1004–1013
Geitmann A, Ortega JK (2009) Mechanics and modeling of plant cell growth. Trends Plant Sci 14(9):467–478. doi:10.1016/j.tplants.2009.07.006
Gout E, Aubert S, Bligny R, Rebeille F, Nonomura AR, Benson AA, Douce R (2000) Metabolism of methanol in plant cells. Carbon-13 nuclear magnetic resonance studies. Plant Physiol 123(1):287–296
Hahn MG, Darvill AG, Albersheim P (1981) Host–pathogen interactions: XIX. The endogenous elicitor, a fragment of a plant cell wall polysaccharide that elicits phytoalexin accumulation in soybeans. Plant Physiol 68(5):1161–1169
Hamant O, Heisler MG, Jonsson H, Krupinski P, Uyttewaal M, Bokov P, Corson F, Sahlin P, Boudaoud A, Meyerowitz EM, Couder Y, Traas J (2008) Developmental patterning by mechanical signals in Arabidopsis. Science 322(5908):1650–1655. doi:10.1126/science.1165594
His I, Driouich A, Nicol F, Jauneau A, Hofte H (2001) Altered pectin composition in primary cell walls of korrigan, a dwarf mutant of Arabidopsis deficient in a membrane-bound endo-1,4-beta-glucanase. Planta 212(3):348–358
Jiang L, Yang SL, Xie LF, Puah CS, Zhang XQ, Yang WC, Sundaresan V, Ye D (2005) VANGUARD1 encodes a pectin methylesterase that enhances pollen tube growth in the Arabidopsis style and transmitting tract. Plant Cell 17(2):584–596
Kerstens S, Decraemer WF, Verbelen JP (2001) Cell walls at the plant surface behave mechanically like fiber-reinforced composite materials. Plant Physiol 127(2):381–385
Kutschera U (2007) Plant-associated methylobacteria as co-evolved phytosymbionts: a hypothesis. Plant Signal Behav 2(2):74–78
Lamport DT, Kieliszewski MJ, Chen Y, Cannon MC (2011) Role of the extensin superfamily in primary cell wall architecture. Plant Physiol 156(1):11–19
Li YQ, Moscatelli A, Cai G, Cresti M (1997) Functional interactions among cytoskeleton, membranes, and cell wall in the pollen tube of flowering plants. Int Rev Cytol 176:133–199
Manfield IW, Orfila C, McCartney L, Harholt J, Bernal AJ, Scheller HV, Gilmartin PM, Mikkelsen JD, Paul Knox J, Willats WG (2004) Novel cell wall architecture of isoxaben-habituated Arabidopsis suspension-cultured cells: global transcript profiling and cellular analysis. Plant J 40(2):260–275
McKenna ST, Kunkel JG, Bosch M, Rounds CM, Vidali L, Winship LJ, Hepler PK (2009) Exocytosis precedes and predicts the increase in growth in oscillating pollen tubes. Plant Cell 21(10):3026–3040
Mohnen D (2008) Pectin structure and biosynthesis. Curr Opin Plant Biol 11(3):266–277
Noguchi K, Yasumori M, Imai T, Naito S, Matsunaga T, Oda H, Hayashi H, Chino M, Fujiwara T (1997) bor1-1, an Arabidopsis thaliana mutant that requires a high level of boron. Plant Physiol 115(3):901–906
O'Neill MA, Eberhard S, Albersheim P, Darvill AG (2001) Requirement of borate cross-linking of cell wall rhamnogalacturonan II for Arabidopsis growth. Science 294(5543):846–849. doi:10.1126/science.1062319
Parre E, Geitmann A (2005) Pectin and the role of the physical properties of the cell wall in pollen tube growth of Solanum chacoense. Planta 220(4):582–592
Peaucelle A, Louvet R, Johansen JN, Hofte H, Laufs P, Pelloux J, Mouille G (2008) Arabidopsis phyllotaxis is controlled by the methyl-esterification status of cell-wall pectins. Curr Biol 18(24):1943–1948
Peaucelle A, Braybrook SA, Le Guillou L, Bron E, Kuhlemeier C, Hofte H (2011a) Pectin-induced changes in cell wall mechanics underlie organ initiation in Arabidopsis. Curr Biol 21(20):1720–1726
Peaucelle A, Louvet R, Johansen JN, Salsac F, Morin H, Fournet F, Belcram K, Gillet F, Hofte H, Laufs P, Mouille G, Pelloux J (2011b) The transcription factor BELLRINGER modulates phyllotaxis by regulating the expression of a pectin methylesterase in Arabidopsis. Development 138(21):4733–4741
Pelletier S, Van Orden J, Wolf S, Vissenberg K, Delacourt J, Ndong YA, Pelloux J, Bischoff V, Urbain A, Mouille G, Lemonnier G, Renou JP, Hofte H (2010) A role for pectin de-methylesterification in a developmentally regulated growth acceleration in dark-grown Arabidopsis hypocotyls. New Phytol 188(3):726–739
Pelloux J, Rusterucci C, Mellerowicz EJ (2007) New insights into pectin methylesterase structure and function. Trends Plant Sci 12(6):267–277. doi:10.1016/j.tplants.2007.04.001
Reinhardt D, Pesce ER, Stieger P, Mandel T, Baltensperger K, Bennett M, Traas J, Friml J, Kuhlemeier C (2003) Regulation of phyllotaxis by polar auxin transport. Nature 426(6964):255–260. doi:10.1038/nature02081
Rockel N, Wolf S, Kost B, Rausch T, Greiner S (2008) Elaborate spatial patterning of cell-wall PME and PMEI at the pollen tube tip involves PMEI endocytosis, and reflects the distribution of esterified and de-esterified pectins. Plant J 53(1):133–143
Savaldi-Goldstein S, Peto C, Chory J (2007) The epidermis both drives and restricts plant shoot growth. Nature 446(7132):199–202. doi:10.1038/nature05618
Shea EM, Gibeaut DM, Carpita NC (1989) Structural analysis of the cell walls regenerated by carrot protoplasts. Planta 179(3):293–308. doi:10.1007/bf00391074
Somerville C, Bauer S, Brininstool G, Facette M, Hamann T, Milne J, Osborne E, Paredez A, Persson S, Raab T, Vorwerk S, Youngs H (2004) Toward a systems approach to understanding plant cell walls. Science 306(5705):2206–2211
Sterling JD, Atmodjo MA, Inwood SE, Kumar Kolli VS, Quigley HF, Hahn MG, Mohnen D (2006) Functional identification of an Arabidopsis pectin biosynthetic homogalacturonan galacturonosyltransferase. Proc Natl Acad Sci USA 103(13):5236–5241
Tian GW, Chen MH, Zaltsman A, Citovsky V (2006) Pollen-specific pectin methylesterase involved in pollen tube growth. Dev Biol 294(1):83–91. doi:10.1016/j.ydbio.2006.02.026
Wakabayashi K, Chun J-P, Huber DJ (2000) Extensive solubilization and depolymerization of cell wall polysaccharides during avocado (Persea americana) ripening involves concerted action of polygalacturonase and pectinmethylesterase. Physiol Plant 108(4):345–352
Wakabayashi K, Hoson T, Huber DJ (2003) Methyl de-esterification as a major factor regulating the extent of pectin depolymerization during fruit ripening: a comparison of the action of avocado (Persea americana) and tomato (Lycopersicon esculentum) polygalacturonases. J Plant Physiol 160(6):667–673
Willats WG, McCartney L, Mackie W, Knox JP (2001) Pectin: cell biology and prospects for functional analysis. Plant Mol Biol 47(1–2):9–27
Wolf S, Mouille G, Pelloux J (2009) Homogalacturonan methyl-esterification and plant development. Mol Plant 2(5):851–860
Yoneda A, Ito T, Higaki T, Kutsuna N, Saito T, Ishimizu T, Osada H, Hasezawa S, Matsui M, Demura T (2010) Cobtorin target analysis reveals that pectin functions in the deposition of cellulose microfibrils in parallel with cortical microtubules. Plant J 64(4):657–667
Zerzour R, Kroeger J, Geitmann A (2009) Polar growth in pollen tubes is associated with spatially confined dynamic changes in cell mechanical properties. Dev Biol 334(2):437–446
Zhang GF, Staehelin LA (1992) Functional compartmentation of the Golgi apparatus of plant cells: immunocytochemical analysis of high-pressure frozen- and freeze-substituted sycamore maple suspension culture cells. Plant Physiol 99(3):1070–1083
Acknowledgments
We apologize to those colleagues whose work could not be cited due to space constraints. SW is recipient of a research fellowship from the German Research Foundation (Deutsche Forschungsgemeinschaft, DFG).
Conflict of interest
The authors hereby declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Peter Nick
Rights and permissions
About this article
Cite this article
Wolf, S., Greiner, S. Growth control by cell wall pectins. Protoplasma 249 (Suppl 2), 169–175 (2012). https://doi.org/10.1007/s00709-011-0371-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00709-011-0371-5