Abstract
Uptake and translocation of chromium (Cr) by two willow species was investigated. Intact pre-rooted weeping willows (Salix babylonica L.) and hankow willows (Salix matsudana Koidz) were grown hydroponically and spiked with hexavalent chromium [Cr (VI)] or trivalent chromium [Cr (III)] at 25.0 ± 0.5°C for 120 h. Removal of leaves was also performed as a treatment to quantify the effect of transpiration on uptake and translocation of either of the Cr species. Although the two willow species were able to eliminate Cr (VI) and Cr (III) from the hydroponic solution, significant differences in the removal rate for both chemical species were observed between the two willows (p < 0.05): faster removal rate for Cr (III) than Cr (VI) was detected in both willow species; hankow willows showed higher removal potential for both chemical species than weeping willows. Remarkable decreases in the removal rates for both Cr species were detected in the willows with leaves removed (p < 0.05). The results from the treatments spiked with Cr (VI) also revealed that Cr was more mobile in plant materials of hankow willows than that in weeping willows (p < 0.01), while higher translocation efficiency of Cr was observed in weeping willows than hankow willows for the Cr (III) treated (p < 0.01). However, a convincing decrease in the translocation efficiency due to the removal of leaves was only observed in the treatments spiked with Cr (VI) (p < 0.05). Substantial differences existed in the distribution of Cr species in plant materials after exposure of either of the chemical forms: roots and lower stems were the major sites for accumulation in weeping willows exposed to Cr (VI) and Cr (III), respectively; in contrast roots were the only sink in hankow willows exposed to both chemical species. The capacity of willows to assimilate both Cr species was also evaluated using detached leaves and roots of both willow species in sealed glass vessels in vivo. The results indicated that detached roots showed a more remarkable capacity to remove Cr (III) from the hydroponic solution than Cr (VI) (p < 0.01). Although detached leaves of both willow species were able to efficiently eliminate Cr (III), neither of them reduced the concentration of Cr (VI) in the solution. The results suggests that different mechanisms for uptake, assimilation and translocation of Cr (VI) and Cr (III) exist in different willow species and phytoremediation of Cr should consider this factor for the proposed target effectively.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Chromium is a metal of serious environmental concern. It can exist in a number of states in natural environment. Among them, hexavalent chromium [Cr (VI)] draws serious public health and legislative concerns because of its extremely high toxicity, mutagenicity and carcinogenicity. Due to the strong corrosion resistance, Cr (VI) has been widely applied in a wide range of industries, including electroplating, wood preservation, leather-tanning and alloy production (Kimbrough et al. 1999; Khan 2001; Dixit et al. 2002). Furthermore, the high solubility of Cr (VI) enhances its mobility and bioavailability, posing extensive hazards to humans and ecosystems at contaminated sites due to contamination of groundwater (Katz and Salem 1994). Conventional physicochemical treatments have been tested and proposed (Xu et al. 2005a, b), but they are prohibitively expensive for large-scale in situ cleaning up of heterogenous media, e.g., soils. Alternatively, bioremediation through sorption, accumulation and transformation have drawn increasing interests (McIntyre 2003; Kuffner et al. 2008). Selective microorganisms have been found to be capable of reducing Cr (VI) to non-toxic insoluble Cr (III) [commonly as Cr(OH)3] under either sulfate reducing or aerobic conditions (Cheung and Gu 2003, 2005; Ryan et al. 2002). Biological reduction of Cr (VI) can be achieved indirectly with metabolites, such as ascorbic acid (Xu et al. 2005a, b) and H2S (Cheung and Gu 2005), or through direct enzymatic reactions (Cheung et al. 2006). However, microbial remediation suffers from inability in removal of the metal species from the environment, especially when dealing with sediment contamination.
Phytoremediation of metals have shown promising results because plants are capable of extracting metal from the environment and mobilize them into different parts of the biomass, including the above-ground parts (Licina et al. 2007; Overesch et al. 2007; Yu and Gu 2007a; Yu et al. 2007). Among the plant species tested, willow has shown ability in assimilation of iron cyanide complexes and methyl tert-butyl ether (MTBE) (Yu and Gu 2006; Yu et al. 2006), and Cr (VI) and Cr (III) (Quaggiotti et al. 2007; Yu and Gu 2007a; Yu et al. 2007), as well as selenate and selenite (Yu and Gu 2007b). Accumulation of Cr (VI) in hydroponically grown hybrid willow (Salix matsudana Koidz × alba L.) results in accumulation mostly in roots reaching an approximately 50% and little in shoots (Yu et al. 2007). Similar trend has also been observed for Cr (III) with removal of more than 90% from the solution for an exposure concentration of < 7.5 mg/l (Yu and Gu 2007a). Additionally, effects of synthetic chelating agent EDTA and external nitrogen on the uptake and accumulation of Cr (VI) and Cr (III) in willows have been studied (Yu and Gu 2008a, b). A very important information in understanding the uptake of Cr by plants is the initial process of transport from solution into the roots. Because of the structural similarity between chromate ( \( {\text{CrO}}^{{2 - }}_{4} \)), a predominant Cr (VI) oxyanion, to sulfate (\( {\text{SO}}^{{2 - }}_{4} \)), Cr may penetrate through sulfate-transport system in membrane of root systems. Current information available suggests that the two Cr species may use different mechanisms for their entry into plant roots (Yu and Gu 2007a; Yu et al. 2007). The objectives of this study were to evaluate assimilation of both Cr species by two distinctively different willow species and compare their fractionation of metals into various parts of plant materials.
Materials and methods
Uptake experiments with trees
Weeping willows (Salix babylonica L.) and hankow willows (Salix matsudana Koidz) were selected in the current experiments. These willows were sampled from the campus of Hunan Agricultural University, P.R. China. Tree cuttings (40 cm in length) were removed from a mature tree and all cuttings used in this study were obtained from a single tree of respective species. They were placed in buckets of tap water at room temperature of 15–18°C under natural sunlight until new roots and leaves appeared. After a 2-month period of growth, each young rooted cutting was transferred to a 250 ml Erlenmeyer flask filled with approximately 200 ml modified ISO 8692 standard nutrient solution as described by Yu et al. (2007). The flasks were all sealed with cork stoppers and silicon sealant (Dow Chemical Co, Midland, MI) to prevent escape of water, and wrapped with aluminum foil to inhibit potential growth of algae. For each treatment, five replicates were prepared. All flasks were housed in a climate control chamber maintained at a constant temperature of 25.0 ± 0.5°C under natural sunlight (light: dark cycle 14:10 h). The plants were conditioned for 48 h first to adapt to the new environmental conditions. Then, the weight of the plant-flask system was measured and recorded individually. The flasks including the tree cuttings were weighed again after 24 h. By doing this way, the transpiration rate of each flask was determined. Trees with a similar transpiration rate were selected for the tests and grouped in the same treatment as replicates. The nutrient solution in each flask was replaced by spiked solution. Potassium chromate (K2CrO4) and chromium chloride (CrCl3) of analytical grade with ≥ 95% purity was used.
Four different treatments were prepared for the Cr uptake experiments for each plant species: (1) nutrient solution with Cr (VI) and intact willows; (2) nutrient solution with Cr (VI) and willows with removal of leaves; (3) nutrient solution with Cr (III) and intact willows; (4) nutrient solution with Cr (III) and willows with removal of leaves. In addition, one control in five replicates was also made. The control was with intact trees in the nutrient solution without addition of Cr to quantify the transpiration rate of trees without any exposure of Cr.
The concentration of Cr in the hydroponic solution was measured before the tree cuttings were transferred in and then at an interval of 24 h for an exposure period of 120 h.
Uptake experiments with detached leaves or roots
To further clarify the uptake mechanism of Cr, additional experiments were performed. Sealed glass vessels containing Cr (VI) or Cr (III) and plant materials were used. Plant leaves or roots were cut into small pieces, precisely weighted (1.0 g fresh weight) and placed in 100 ml Erlenmeyer flasks. Then 100 ml of spiked aqueous solution (deionized oxygen-saturated water) were added. The flasks were closed with glass stoppers and all placed at an incubator with a constant temperature of 25°C for 24 h. The initial concentrations of Cr (VI) and Cr (III) spiked solution were 1.60 and 1.51 mg Cr/l, respectively, at which three separate measurements were conducted for each plant materials. The concentrations of Cr in solutions and plant materials were measured after 24 h of exposure. Preparation and extraction of samples for total Cr analysis were identical to those described by Banks et al. (2006).
Chemical analysis
The concentration of Cr in the aqueous solution was analyzed by flame atomic absorption spectrophotometry. Preparation and extraction of root, stem and leaf samples for total Cr were conducted according to the method described by Banks et al. (2006). Plant materials from the treated plants were harvested after the exposure period. The lower stem was the part of plant materials in the Erlenmeyer flask, while the rest was the higher stem. The plants were washed with tap and distilled water followed by thorough rinsing, and then oven dried at 90°C for 48 h. Dried plant samples were ground in an electrical blender, except for the roots due to the small quantity of the total harvested material. The ground materials were sieved to pass 2 mm sieve and then placed in individual glass bottle and dried for 24 h at 65°C to remove any moisture absorbed during the processing step. The bottles were sealed and placed in a desiccator.
Root, stem and leaf samples were extracted for total Cr using a nitric/perchloric acid digestion method. Exactly 0.25 g of oven dried and ground plant materials was placed in a 50 ml digestion tube, mixed with 10 ml of HNO3/HClO4, (1:1, v/v) and allowed to stand overnight. The samples were then placed in a digestion block and heated for 2 h at 200°C until the digested liquid was clear. The contents in the digestion tube were diluted to 25 ml with deionized water and filtered (Whatman #1 filter paper, Fisher Scientific, Pittsburgh, PA) into 120 ml Erlenmeyer flasks. The filtrates were analyzed by flame atomic absorption spectrophotometry. The detection limits, determined as three times the standard deviation of 10 replicates of blank, were 0.001 mg Cr/l for water samples and 0.005 mg Cr/kg DW for plant materials. The sample preparation methods used were also checked against the spiked sample which is the certified solution standards; mean recovery was 96.49%. The precision of Cr determination, based on variations of replicate analyses (n = 2) for the same sample, was <15%.
Determination of the removal rates of Cr
In the absence of volatilization and negligible background Cr in controls with plants, all loss from the system can be contributed to removal by plants. The removal velocity v (μg Cr/g·d) was calculated from final and initial mass using the formula
where m (0) is the total mass (μg) of Cr in the solution at the beginning, and m (t) is the total mass (μg) of Cr in solution at time t; Δt is the time period (d), and M is the biomass of the plant (g).
Determination of transpiration rate
Inhibition of transpiration is a rapid and easy measure for the toxic effect of a chemical to the growing trees (Trapp et al. 2000). The effect of Cr was quantified by measuring the transpiration rate of the pre-rooted trees in the flasks. The weight loss of the plant-flask system over time was expressed as the transpiration rate for further data analysis.
Determination of translocation efficiency
The translocation efficiency (τ) as the fraction that, after root uptake, is successfully translocated to the upper parts of plants as defined by Meers et al. (2004)
where C (root), C (stem) and C (leaf) are the total Cr concentration in different plant materials, and DW (root), DW (stem) and DW (leaf) are the dry weight of plant materials.
Statistical analyses
Analysis of variance (ANOVA) and Tukey’s multiple range test was used to determine the statistical significance at 0.01 or 0.05 level between plant performances.
Results
Uptake of Cr (VI) from hydroponic solution by willows
The change of the total Cr concentrations in hydroponic solution spiked with Cr (VI) over time of incubation is shown in Fig. 1. Due to water loss by plant transpiration, Cr concentration in hydroponic solution with intact weeping willows increased slightly from 1.81 mg Cr/l initially to 1.96 mg Cr/l (±0.07), but a decrease of 15.16 (±3.72)% was observed for the total Cr over a 120 h period of exposure. Similarly, Cr concentrations also increased from 1.79 mg Cr/l initially to 1.87 mg Cr/l (±0.03) at the end of exposure in the hydroponic solution in the presence of weeping willows with removal of all leaves, but a decrease of 7.81 (±1.31)% was detected for the total Cr. Intact weeping willows showed a significantly higher uptake rate (%) of Cr (VI) than that without leaves (p < 0.01). For hankow willows with treatments, 34.87 (±2.85)% and 24.62 (±1.49)% of the applied Cr (VI) was removed from the hydroponic solution by intact willows and willows without leaves, respectively. The difference in removal of the applied Cr (VI) in hydroponic solution between the two willow species was significant (p < 0.01).
Mass balance for willows exposed to Cr (VI)
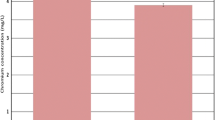
The concentrations of Cr in plant materials of willows exposed to Cr (VI) are shown in Fig. 2. Cr was detected in all parts of plants from all treatments, except for leaves (Cr concentration was below the detection limit), confirming uptake and translocation of Cr (VI) into different parts of plant materials from the hydroponic solution. However, substantial differences existed in the distribution of Cr (VI) in plant materials between the two willow species.
Measured Cr concentration (mg Cr/kg DW) in roots, lower stems, higher stems and leaves of both willows exposed to Cr (VI). The exposure period was 120 h. The values are the mean of five replicates for samples. Vertical lines represent standard deviation. DW: dried weight. Asterisk refers to the respective trees without leaves; Cr concentration in roots: ×102 mg Cr/kg DW
For intact weeping willows exposed to Cr (VI), the highest concentration of Cr in plant materials was found in roots (558.93 ± 24.77 mg Cr/kg DW). Similar levels of Cr concentrations were detected in the lower stems and higher stems of intact weeping willows, with values of 2.61 ± 0.14 mg Cr/kg DW and 2.54 ± 0.27 mg Cr/kg DW), respectively. For the weeping willows without leaves, the highest was also found in roots (503.78 ± 57.91 mg Cr/kg DW). Cr concentrations in lower stems and higher stems were significantly lower than those of intact weeping willows (p < 0.01). Significantly higher Cr concentrations in plant materials of hankow willows than weeping willows were found (p < 0.01). The highest Cr concentration was detected in roots of hankow willows (745.87 ± 45.79 mg Cr/kg DW). Similar levels of Cr concentrations were detected in the lower stems and higher stems of the intact hankow willows, with values of 10.51 ± 2.09 mg Cr/kg DW and 10.00 ± 1.77 mg Cr/kg DW, respectively. Cr concentration in roots of hankow willows with leaves removed (904.30 ± 80.32 mg Cr/kg DW) was significantly higher than that of intact hankow willows (p < 0.01). Slightly lower Cr concentrations were detected in lower stems and higher stems of hankow willows with leaves removed than those of the intact willows (p > 0.05).
The mass balance of Cr (VI) was made from total Cr in plant materials and removed from the hydroponic solution (Table 1). Majority of the applied Cr (VI) was associated with the roots of intact weeping willows in which the total Cr remaining in roots accounted for 54.98 ± 7.87%, but more Cr (69.85 ± 2.88%) was found in roots of weeping willows without leaves (p < 0.05). Approximately, 23.15 ± 3.98% and 21.87 ± 5.16% of the total Cr from the solution was found in the lower and higher stem of intact willows, respectively. Less Cr was detected in the weeping willows with leaves removed comparing with intact willows (p < 0.05). About 40.47 ± 1.21% and 48.15 ± 5.73% of the total Cr from solution was recovered in the roots of intact hankow willows and the hankow willows without leaves, followed by lower stems with values of 31.17 ± 4.24% and 28.98 ± 3.94%, respectively. A high recovery of Cr was obtained for all treatments within the cumulative ranges of measurement errors of solution and biomass. The removal rate for Cr (VI) was determined to be 0.41 and 0.79 μg Cr/g FW·d for intact weeping willows and intact hankow willows, respectively, whereas the uptake rates for Cr (VI) were 0.33 and 0.56 μg Cr/g FW·d for trees with removal of leaves, respectively. The difference in the removal rate for Cr (VI) between the intact trees and the trees without leaves of both willow species was significant (p < 0.01).
Uptake of Cr (III) from hydroponic solution by willows
Compared to the treatments spiked with Cr (VI), more Cr (III) was eliminated from the hydroponic solution by plants (p < 0.01) and the Cr concentrations in hydroponic solution spiked with Cr (III) over time are presented in Fig. 3. Cr concentration in hydroponic solution with intact weeping willows fell from 1.86 mg Cr/l initially to 0.35 mg Cr/l (±0.03) at the end of the exposure period, with a decrease of 84.77 (±2.22)% of the applied Cr (III) over an exposure period of 120 h, whereas 79.99 (±2.71)% decrease was detected in the treatment with the weeping willows with leaves removed. The difference in the uptake rate (%) of Cr (III) between the intact plants and the willows without leaves was significant (p < 0.05). A decrease of 87.97 (±2.85)% and 83.35 (±2.86)% was found for the initial Cr (III) in the hydroponic solution by the end of experimental period with intact hankow willows and the hankow willows without leaves, respectively.
Mass balance for willows exposed to Cr (III)
Figure 4 presents the concentrations of total Cr in plant materials of willows exposed to Cr (III) after 120 h of exposure. The background Cr concentration in non-treated control trees was 0.05 mg Cr/kg DW for roots (n = 2) and no Cr concentration above the detection limit was found for other plant tissues. Cr concentrations in different parts of the exposed willows were all significantly elevated comparing with the background. However, apparent difference existed in the distribution of Cr in different parts of plant materials between the two willows species.
Measured Cr concentration (mg Cr/kg DW) in roots, lower stems, higher stems and leaves of both willows exposed to Cr (III). The exposure period was 120 h. The values are the mean of five replicates for samples. Vertical lines represent standard deviation. DW: dried weight. Asterisk refers to the respective trees without leaves; Cr concentration in roots: ×102 mg Cr/kg DW
The highest concentration was detected in the roots of intact weeping willows (2110.55 ± 183.12 mg Cr/kg DW), followed by the lower stems with a value of 31.30 ± 2.81 mg Cr/kg DW. The lowest (9.59 ± 1.77 mg Cr/kg DW) was associated with the leaves of the intact weeping willows. For the weeping willows with leaves removed, significantly higher concentrations were found in the roots and higher stems with values of 2811.66 ± 171.72 mg Cr/kg DW and 24.60 ± 2.87 mg Cr/kg DW, respectively comparing with the intact willows (p < 0.01). In contrast, a slightly lower concentration was detected in the lower stems (28.78 ± 3.64 mg Cr/kg DW) (p > 0.05). A similar distribution pattern was also found in the treatments with intact hankow willows in which the highest concentration was in the roots (2623.65 ± 245.79 mg Cr/kg DW), followed by the lower stems (34.31 ± 2.47 mg Cr/kg DW). The lowest concentration was found in the leaves with a value of 12.44 ± 2.67 mg Cr/kg DW. A significantly higher concentration was detected in the roots of hankow willows without leaves (3535.84 ± 170.77 mg Cr/kg DW) comparing with the intact willows (p < 0.01). Similar levels of Cr concentrations were found in the low stems and higher stems (p > 0.05).
The mass balance of Cr (III) is presented in Table 2. Lower stems of weeping willows were the major site for Cr accumulation in plant materials in which the lower stems accounted for 40.80 ± 5.32% and 36.57 ± 2.59% of the total Cr accumulated by intact weeping willows and weeping willows without leaves, respectively. More Cr (30.51 ± 1.30%) was found in the higher stems of weeping willows without leaves than that of intact weeping willows (22.82 ± 1.13%) (p < 0.01). For the treatments with hankow willows, majority of the applied Cr (III) was associated with the roots of intact willows and willows without leaves with values of 48.65 ± 3.89% and 52.98 ± 3.39% of the total Cr accumulated, respectively. Similar amounts of Cr were detected in the lower stems and higher stems of intact willows and willows without leaves (p > 0.05). The removal rate for Cr (III) was determined to be 2.35 and 2.65 μg Cr/g FW·d for intact weeping willows and hankow willows, respectively, whereas the uptake rates for Cr (III) were 2.18 and 2.16 μg Cr/g FW·d for trees with removal of leaves, respectively. The difference in the removal rate of Cr (III) between the intact trees and the trees without leaves of both willow species was significant (p < 0.01).
Uptake of Cr by detached leaves and roots of willows
The potential of hankow willows and weeping willows to remove Cr was also tested (Tables 3 and 4). Concentration of both Cr species in the hydroponic solution without plant materials did not change after a 24 h of exposure (data not shown). Negligible change of the Cr concentrations in hydroponic solution spiked with Cr (VI) was observed for the flasks with detached leaves of both willows species over a 24 h period of incubation. Approximately, 18.13 (±2.65)% and 23.75 (±2.65)% of the applied Cr (VI) was removed by roots of weeping willows and hankow willows, respectively, from the incubation solution. The difference between the two treatments was significant (p < 0.05). More than 90% of the Cr removed from the hydroponic solution was recovered in roots.
In the flasks with detached leaves supplied with Cr (III), significant amounts of the applied Cr (III) in sealed vessels were removed by leaves of both willows species. Concentrations of Cr (III) in the hydroponic solution with leaves of weeping willows declined from 1.51 mg Cr/l initially to 0.86 mg Cr/l (±0.03) at the end of experiment, a 43.05 (±1.87)% decrease of the total Cr over a 24 h period of incubation. A slightly lower reduction by 42.05 (±3.28)% was found in the vessel with leaves of hankow willows (p > 0.05). For the treatments with roots, 45.70 (±1.87)% and 47.02 (±1.87)% of the applied Cr (III) was removed by weeping willows and hankow willows, respectively and the difference between the two species was not significant (p > 0.05).
Response of plant transpiration to Cr exposure
Table 5 shows the absolute transpiration of intact willows and willows with removal of leaves. Compared to the intact trees, remarkable decreases by 83.71 (±1.70)% and 79.62 (±1.87)% in transpiration rates were observed for the weeping willows with removal of leaves exposed to Cr (VI) and Cr (III), respectively. In contrast, a lower decrease by 67.70 (±2.04)% and 66.94 (±0.90)% in transpiration rates was detected for the hankow willows with removal of leaves exposed to (VI) and Cr (III), respectively. It is of interest to note that significant difference in the absolute transpiration rate between the two willows in the removal of leaves (p < 0.05), probably due to the different species used. There was no significant difference in the plant growth between treated and non-treated plants (data not shown) for both plant species over the period of incubation. Symptoms of chlorosis of leaves were not observed in any plant, implying that the trees cuttings could maintain their physiological functioning over the entire test period without any measurable and observed toxic effects.
Discussion
It has been reported that Cr (VI) is more water-soluble than Cr (III) and both chemical species are easily taken up by plants (Vajpayee et al. 2000). In our observation, more than 80% of the applied Cr (III) can be eliminated from the hydroponic solution by intact willows after a 120 h of exposure, whereas less than 35% of the initial (VI) can be removed by willows. The difference in the removal rates between the two Cr species was significant (p < 0.01), indicating that plants take up the Cr by dissimilar mechanisms and the conversion of Cr (VI) to Cr (III) in the hydroponic solution prior to uptake is unlikely to take place. It is of interest to note that intact hankow willows showed a higher uptake potential for both Cr chemical forms than intact weeping willows (p < 0.05), implying that uptake of Cr is highly dependent upon the genus and species diversity. This is in complete agreement with the conclusion drawn by Shahandeh and Hossner (2001). Significant difference in the uptake of both chemical species between the intact willows and the willows with leaves removed (p < 0.05) implies that plant transpiration also affects uptake of these Cr species.
A convincing decrease in the translocation efficiency due to the removal of leaves was only observed in the treatments spiked with (VI) (p < 0.05), implying that plant transpiration has a minor impact on the translocation of Cr (III) within plant materials. In our observation, (VI) is more mobile in plant materials of hankow willows than that in weeping willows (p < 0.01), while weeping willows show higher translocation efficiency for Cr (III) than hankow willows (p < 0.01). Therefore, we have a good reason to assume that the conversion of (VI) to Cr (III) within plant materials during transport is unlikely, and both Cr species in different parts of plant tissues are probably still in the same original chemical forms as they were in solution. Our data also showed that substantial differences existed in the distribution of Cr in plant materials after exposure to either of the chemical forms: roots were the major site for accumulation in both willow species amended with Cr (VI). In contrast, more Cr was accumulated in lower stems of weeping willows and roots of hankow willows exposed to Cr (III), respectively. This suggests that different transporting pathways for either of the chemical forms exist in willow species.
Although detached leaves of both willow species can efficiently take up Cr (III) from the hydroponic solution, negligible change of Cr (VI) concentration was detected in the vessels with leaves, implying that Cr (VI) rather than Cr (III) is unable to actively pass through the biomembrane of the leaves. This contradicted with early findings in which the sulfate carrier is responsible for actively taking up Cr (VI) (Zayed et al. 1998), while Cr (III) is taken up passively, being retained by the cation-exchange sites of the cell walls (Skeffington et al. 1976; Shanker et al. 2005). In contrast, roots of willows showed a more remarkable capacity to remove Cr (III) from the hydroponic solution than Cr (VI). This provided additional evidence to support that dissimilar uptake mechanisms for Cr (VI) and Cr (III) by roots of willows were exist in roots of willows.
Conclusion
The two willow species are able to eliminate Cr (VI) and Cr (III) from the hydroponic solution. Although willows showed a faster removal rate for Cr (III) than for Cr (VI), the distribution of both chemical species in the plant biomass was quite different. A significant decrease in the removal rate of Cr (VI) and Cr (III) was detected due to the absence of leaves of willows. Roots and lower stems were the major sites for accumulation in weeping willows exposed to Cr (VI) and Cr (III), respectively, while Cr was accumulated mostly in roots of hankow willows amended with either of the chemical forms. The information suggests that different uptake, assimilation and translocation of Cr (VI) and Cr (III) exist in willow species and phytoextraction of Cr needs to consider the differences in species for effective treatments.
References
Banks MK, Schwab AP, Henderson C (2006) Leaching and reduction of chromium in soil as affected by soil organic content and plants. Chemosphere 62:255–264
Cheung KH, Gu J-D (2003) Reduction of chromate (CrO 2−4 ) by an enrichment consortium and an isolate of marine sulfate-reducing bacteria. Chemosphere 52:1523–1529
Cheung KH, Gu J-D (2005) Chromate reduction by Bacillus megaterium TKW3 isolated from marine sediments. World J Microbiol Biotechnol 21:213–219
Cheung KH, Lai HY, Gu J-D (2006) Membrane-associated hexavalent chromium reductase of Bacillus megaterium TKW3 with induced expression. J Microbiol Biotechnol 16:855–862
Dixit V, Pandey V, Shyam R (2002) Chromium ions inactivate electron transport and enhance superoxide generation in vivo in pea (Pisum sativum L.cv. Azad) root mitochondria. Plant Cell Environ 25:687–693
Katz SA, Salem H (1994) The biological and environmental chemistry of chromium. VCH Publishers, New York
Khan AG (2001) Relationships between chromium biomagnification ratio, accumulation factor, and mycorrhizae in plants growing on tannery effluent-polluted soil. Envinon Int 26:417–423
Kimbrough DE, Cohen Y, Winer AM, Creelam L, Mabuni C (1999) A critical assessment of chromium in the environment. Crit Rev Environ Sci Technol 29:1–46
Kuffner M, Puschenreiter M, Wieshammer G, Gorfer M, Sessitsch A (2008) Rhizosphere bacteria affect growth and metal uptake of heavy metal accumulating willows. Plant Soil 304:35–44
Licina V, Antic-Mladenovic S, Kresovic M (2007) The accumulation of heavy metals in plants (Lactuca sativa L. Fragaria vesca L.) after the amelioration of coalmine tailing soils with different organo-mineral amendments. Arch Agric Soil Sci 53:39–48
McIntyre T (2003) Phytoremediation of heavy metals from soils. Adv Biochem Eng Biotechnol 78:97–123
Meers E, Hopgood M, Lesge E, Vervake P, Tack FMG, Verloo MG (2004) Enhanced phytoextraction: in search of EDTA alternatives. Int J Phytorem 6:95–109
Overesch M, Rinklebe J, Broll G, Neue HU (2007) Metals and arsenic in soils and corresponding vegetation at Central Elbe river floodplains (Germany). Environ Pollut 145:800–812
Quaggiotti S, Barcaccia G, Schiavon M, Nicole S, Galla G, Rossignolo V, Soattin M, Malagoli M (2007) Phytoremediation of chromium using Salix species: cloning ESTs and candidate genes involved in the Cr response. Gene 402:68–80
Ryan MP, Williams DE, Chater RJ, Hutton BM, McPhail DS (2002) Why stainless steel corrodes. Nature (London) 415:770–774
Shahandeh H, Hossner LR (2001) Plant screening for chromium phytoremediation. Int J Phytorem 2:31–51
Shanker AK, Cervantes C, Loza-Tavera H, Avudainayagam S (2005) Chromium toxicity in plants. Environ Int 31:739–753
Skeffington RA, Shewry PR, Petersen PJ (1976) Chromium uptake and transport in barley seedlings Hordeum vulgare. Planta 132:209–214
Trapp S, Zambrano KC, Kusk KO, Karlson U (2000) A phytotoxicity test using transpiration of willows. Arch Environ Contam Toxicol 39:154–160
Vajpayee P, Tripathi RD, Rai UN, Ali MB, Singh SN (2000) Chromium(VI) accumulation reduces chlorophyll biosynthesis, nitrate reductase activity and protein content in Nymphaea alba L. Chemosphere 41:1075–1082
Xu XR, Li HB, Gu J-D (2005a) Reduction of hexavalent chromium by ascorbic acid in aqueous solutions. Chemosphere 57:609–613
Xu XR, Li HB, Gu J-D, Li XY (2005b) Kinetics of the reduction of chromium (VI) by vitamin C. Environ Toxicol Chem 24:1310–1314
Yu XZ, Gu J-D (2006) Uptake, metabolism and toxicity of methyl tert-butyl ether (MTBE) in weeping willows. J Hazard Mater 137:1417–1423
Yu XZ, Gu J-D (2007a) Accumulation and distribution of trivalent chromium and effects on hybrid willow (Salix matsudana Koidz × alba L.) metabolism. Arch Environ Contam Toxicol 52:503–511
Yu XZ, Gu J-D (2007b) Metabolic responses of weeping willows to selenate and selenite. Env Sci Pollut Res 14:510–517
Yu XZ, Gu J-D (2008a) The role of EDTA in phytoextraction of hexavalent and trivalent chromium by two willow trees. Ecotoxicology 17:143–152
Yu XZ, Gu J-D (2008b) Effect of available nitrogen on photoavailability and bioaccumulation of hexavalent and trivalent chromium in hankow willows (Salix matsudana Koidz). Ecotoxicol Environ Saf 70:216–222
Yu XZ, Gu J-D, Huang SZ (2007) Hexavalent chromium induced stress and metabolic responses of in hybrid willows. Ecotoxicology 16:299–309
Yu XZ, Zhou PH, Yang YM (2006) The potential for phytoremediation of iron cyanide complex by willows. Ecotoxicology 15:461–467
Zayed AM, Lytle CM, Qian JH, Terry N (1998) Chromium accumulation, translocation and chemical speciation in vegetable crops. Planta 206:293–299
Acknowledgements
This work was supported in part by CAG HKUST 3/04C from Hong Kong Research Grant Council.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yu, XZ., Gu, JD. & Xing, LQ. Differences in uptake and translocation of hexavalent and trivalent chromium by two species of willows. Ecotoxicology 17, 747–755 (2008). https://doi.org/10.1007/s10646-008-0224-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-008-0224-y