Abstract
The aim of the present study was to examine the ability of I. pseudacorus L., an ornamental macrophyte of great potential for phytoremediation, to tolerate and accumulate Cr and Zn. Plants were grown in nutritive solution with ZnCl2 or CrCl3·6H2O at 0, 10, 50, 100, and 200 μg ml−1 for 5 weeks; all survived and continued growing. The accumulation of Cr and Zn increased with increasing supply in all plant tissues, to reach 59.97 mg Cr and 25.64 mg Zn in roots. Leaves retained a remarkable amount of Zn (14.2 mg). Growth inhibition reached 65% and 31% (dry weight) in response to Cr and Zn, respectively. The root:shoot dry matter partitioning (R/S) increased 80% at 100 μg ml−1 CrCl3. The most marked alterations in mineral content were in roots, where both metals decreased Al, Ca, Mg, Mn and S, and increased P concentration. No effect was noted on either leaf chlorophyll fluorescence kinetics (F v /F m and ΦPSII), or photosynthetic pigment content, signifying that the light phase of photosynthesis was not impaired. Carbon isotope composition (δ13C) was only slightly heavier, indicating that the reduction of carbon fixation was not the main cause for growth decrease. This was attributed to the restricted mineral uptake and to the increased demand of carbohydrates of damaged roots. Biomass allocation to rhizomes (Cr) or roots (Zn) contributes to heavy metal tolerance by limiting transpiration and increasing metal–storing tissues and the surface for water and cation uptake. This species is a good candidate for Cr rhizofiltration and Zn phytoextraction.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Fresh water resources have been steadily reduced in recent decades as a result of increasing human consumption, contamination, and climatic change. Anthropogenic pollution of water is currently a major environmental concern as it poses a serious hazard for humans and other organisms, and dramatically limits the uses of water. Among the toxic substances found in water bodies, heavy metals deserve special attention. They are highly toxic at low doses, strongly persistent in the environment and living tissues, and easily transferred to food chain. In addition, their monitoring and removal is costly. Cr and Zn, two of the most relevant heavy metals, are included in the US Environmental Protection Agency list of priority pollutants (USEPA 2005). Symptoms of Cr and Zn phytotoxicity include chlorosis, inhibited germination, stunted growth, reduced leaf number and area, reduced yield and flower production, inhibited photosynthesis, dysfunction of relevant enzymes, impaired nutrient uptake, plant wilting and altered water relations (Deng et al. 2006; Dhir et al. 2008; Prasad 2004; Shanker et al. 2005). The excess of metals has deleterious effects on the content and functionality of the photosynthetic pigments (Broadley et al. 2007; Shanker et al. 2005). This can be caused by the inhibition of the pigment synthesis (Prasad and Prasad 1987), the formation of metal-substituted chlorophylls of reduced functionality (Küpper et al. 1996), or the direct oxidative damage of the pigments (Oláh et al. 2010). Several authors have reported damages on the reaction centres or the peripheral antennae complexes of PSII in response to high concentrations of metals (Janik et al. 2010; Vernay et al. 2007; Paiva et al. 2009). Todeschini et al. (2011) recently described the reduction of D1 and D2 expression in poplar exposed to high levels of Zn.
The phytoremediation of heavy metals by means of constructed wetlands constitutes a low cost, environmentally friendly alternative to conventional cleanup techniques (Salt et al. 1998). Furthermore, as metals accumulate mainly in roots, part of the biomass harvested from such wetlands has many potential uses in non-food industries. Some of these side-products that could yield substantial economic benefits for affected communities are biogas and compost (Malik 2007), fibres (Kuzovkina and Quigley 2005), and ornamental plants (Belmont and Metcalfe 2003). At present, few plant species with ornamental flowers have been evaluated for heavy metal removal in spite of their high market value. Several studies have revealed that Iris lactea var. chinensis (Fisch.) Koidz. Rank accumulates Cd in leaves and roots (Han et al. 2007), and Lythrum salicaria L. tolerates Pb (Uveges et al. 2002). However, greater research efforts are required to screen the performance of other suitable species.
Iris pseudacorus L. is native to Northern Africa, Western Asia and Europe, naturalized in Australia, New Zealand and North and South America, and cultivated worldwide as an ornamental plant. This plant displays a high rate of biomass production, tolerates polluted environments and is useful for water treatment purposes. Compared with Acorus gramineus Sol. in Aiton, Acorus calamus L., L. salicaria and Reineckea carnea (Andrews) Kunth, I. pseudacorus shows better performance in removing total nitrogen and phosphorus, COD, BOD, and heavy metals (Cr, Pb, Cd, Fe, Cu, and Mn) from sewage. In addition, it shows a high stress-tolerance response, which includes low lipid peroxidation, and increased proline levels and catalase activity (Zhang et al. 2007). I. pseudacorus plants exposed to high levels of Cd or Pb show decreased growth and chlorophyll content (Zhou et al. 2010), increased peroxidase, catalase, superoxide dismutase, and ascorbate peroxidase activity, and increased concentration of proline and malondialdehyde (Qiu and Huang 2008; Zhou et al. 2010). The roots are also able to form Cu nanoparticles in response to high levels of Cu (Manceau et al. 2008). I. pseudacorus shows a higher phenol concentration in roots than Phragmites australis (Cav.) Trin. ex Steud. and Typha latifolia L, which makes it more suitable for the treatment of metal polluted waters (Larue et al. 2010). However, there is insufficient information about heavy metal accumulation and distribution in I. pseudacorus and the effects of other metals on the parameters that condition biomass production, such as growth, chlorophyll synthesis, photosynthetic performance and plant nutritional status. These data are determinants in establishing the potential of this promising species for phytoremediation purposes. Here we assessed the physiological response of I. pseudacorus to a range of Cr or Zn concentrations, and evaluated the accumulation of these metals throughout the plant.
Materials and methods
Plant material and treatments
Iris pseudacorus L. plants were purchased from a local nursery (Bioriza, Breda, Spain) in 300-ml multipot containers holding a peat–perlite 50/50 substrate. Plants were then root-washed in tap water to remove the original substrate, weighed, and placed in a pure hydroponics system in individual 4-L pots containing diluted Hoagland nutritive solution at pH 6.5. This solution comprised 130.25 mg l−1 NO3−, 5.5 mg l−1 NH4+, 28.5 mg l−1 PO4 2−, 35.5 mg l−1 K+, 24.5 mg l−1 Ca2+, 4 mg l−1 Mg2+, 14.25 mg l−1 SO4 2−, 0.325 mg l−1 Fe, 0.240 mg l−1 Mn, 0.09 mg l−1 Zn, 0.030 mg l−1 B, 0.090 mg l−1 Cu, 0.028 mg l−1 Mo, and 0.005 mg l−1 Co. After an acclimation period of 2 weeks, individual plants were selected within a small range of initial fresh weight (104.0 ± 5.2 g expressed as average ± standard error). The nutritive solution was then amended with ZnCl2 or CrCl3·6H2O at 0, 10, 50, 100, and 200 μg ml−1, which correspond to Zn ion concentrations of 0.07, 0.4, 0.7 and 1.5 mM, and to Cr ion concentrations of 0.04, 0.2, 0.4, and 0.8 mM. The Cr ion concentrations were approximately half those of Zn, to compensate for the higher toxicity of Cr(III) for plants (Hara and Sonoda 1979). Five replicates (plants) of each treatment were randomly distributed and grown under glasshouse conditions for 5 weeks in June and July. The average temperature was 36–18°C (day/night), the relative humidity 31–59%, the maximum global solar irradiance 1,353 W m−2, and the transmission of the greenhouse covers 51%. Nutritive solution was renewed regularly.
In vivo measurements
Before collecting the plants, in vivo measurements were taken. Chlorophyll content on leaf area basis was measured at the base, centre and tip of four representative mature pre-bloom leaves per plant using a portable chlorophyll meter (SPAD-502 Minolta, Illinois, USA), following Krugh et al. (1994). A reading checker of 72.4 ± 0.3 was used to calibrate the apparatus. Chlorophyll fluorescence was measured with a modulated fluorometer (Hansatech Fluorescence Monitoring System FMS2, Norfolk, UK) to obtain estimates of maximum quantum yield (F v /F m ) after 30 min of dark adaptation and of relative quantum yield (ΦPSII) measured at environmental light (Genty et al. 1989). Plants were then thoroughly washed in tap water, gently wrapped in absorbent paper to remove excess water and weighed to record the increase in biomass. Each plant was divided into leaves, rhizomes and roots, and each section was weighed separately. The underground organs were not desorbed to preserve the fraction of metal adsorbed to cell walls, which would also be collected after harvest in phytoremediation systems. A portion of each fresh sample was ground in liquid nitrogen and stored at −80°C until analysis. The remaining fresh sample was oven-dried at 60°C until constant weight, ground in an agate mortar and passed through a 0.05-mm sieve.
Photosynthetic pigment content
The chlorophyll and carotenoid concentration of leaves was measured on extracts of frozen leaf samples in 80% acetone. Pigment contents were calculated from absorbance at wavelengths 663.2, 646.8, and 470.0 nm, as described by Lichtenthaler (1987). The absorbance values were measured in the extracts by means of a UV-160 spectrophotometer.
Element composition
Two replicates of each frozen sample were digested overnight at 90°C in a HNO3–H2O2 mixture 1:1 v/v. The Al, Ca, Cu, Fe, K, Mg, Mn, S and P content of the extracts was determined by inductively coupled plasma optical emission spectrometry (ICP-OES) and by inductively coupled plasma mass spectrometry (ICP-MS) using a Perkin Elmer Optima-3200RL and a Perkin Elmer Elan-6000 apparatus, respectively. A blank and a sample of aquatic plant (Trapa natans L, CRM 596) or sea lettuce (Ulva lactuca L, CRM 279) certified reference material from the Community Bureau of Reference (BCR®), were processed in the same way and analysed per 12 samples. Element content determination was performed in the technical services of the University of Barcelona (Serveis Científicotècnics). Ash content was determined by furnacing samples at 500°C for 6 h or until constant weight.
Stable isotope composition
For each plant, a sample of dried leaf, rhizome, and root tissue was ground into a fine powder, and 1 mg was weighed in tin cups. The total C and N content of samples was analysed using an Elemental Analyser (EA, Carlo Erba 2100, Milan, Italy), which was interfaced with an Isotope Ratio Mass Spectrometer (IRMS, Thermo-Finnigan Deltaplus Advantage, Bremen, Germany) to analyse 13C/12C and 15N/14N ratios. Results were expressed as δ13C and δ15N values, using a secondary standard calibrated against Vienna Pee Dee Belemnite calcium carbonate (VPDB) for C, and air for N. Analytical precision was of 0.1‰. All analyses were undertaken at the Colorado Plateau Stable Isotope Laboratory (CPSIL, Northern Arizona University). δ13C and δ15N were calculated as:
Statistical methods
ANOVA (analysis of variance) was performed on the basis of a one-factor design using SPSS (Statistical Package for the Social Sciences) version 14.0 for Windows. Logarithmic transformation was used when data did not meet the assumption of equal variances. Student–Newman–Keuls post hoc tests were performed to assess the differences between groups. Sigma Plot version 10.0 was used for graphic edition. Cluster analysis was done using Gene Cluster (Standford University, USA) on standardized averages, and distances between clusters were established by average linkage clustering.
Results
Biomass, water content and chlorophyll
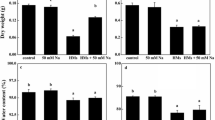
Plant growth was strongly impaired by heavy metal stress. Both Cr and Zn decreased fresh weight increment (ΔFW) and dry weight increment (ΔDW) of the whole plant after 5 weeks of treatment (Table 1). The treatment with ZnCl2 did not disturb plant growth at low concentrations, but at 100 μg ml−1 ΔFW and ΔDW fell dramatically (59 and 65%, respectively). The effect of CrCl3 was gradual, to reach a decrease of 61% (ΔFW) and 44% (ΔDW) at 200 μg ml−1.
To determine whether growth was equally inhibited in all plant organs, final fresh weight (FW) and final dry weight (DW) of leaves, rhizomes and roots were recorded separately (Table 1). Increasing Zn and Cr reduced the FW of leaves (Table 1) up to 48% (200 μg ml−1 ZnCl2) and 56% (100 μg ml−1 CrCl3). The FW and the DW of all organs showed strong decrease when supplied with high concentrations of metals, but these variations were only significant in leaves due to the high variability of the response. Nevertheless, it is of note that high Zn (100 or 200 μg ml−1 ZnCl2) reduced DW up to 48% (leaves), 37% (rhizomes) and 22% (roots). In contrast, DW was 33% lower in roots and 24% lower in leaves, but 14% greater in rhizomes treated with high concentrations of Cr, than in controls. Water content (WC) calculated as a percentage was 5% lower in leaves and rhizomes treated with 200 μg ml−1 CrCl3, and remained stable in all the other treatments (results not shown).
The biomass allocation was altered (Fig. 1) as a result of the marked reduction of leaf DW, which accounts for most of the total biomass of I. pseudacorus. The root:shoot dry matter partitioning (R/S) was affected from 100 μg ml−1 CrCl3 upwards (Fig. 1b). ANOVA performed on the same data confirmed the effect of Cr treatment on R/S (p value = 0.03). The R/S was proportional to the external Zn concentration (Fig. 1a), but the tendency was not significant (p value = 0.18).
Effect of treatments on biomass distribution ratio. Plants were grown in nutritive solution containing ZnCl2 or CrCl3·6H2O at 0, 10, 50, 100, and 200 μg ml−1. Root to shoot ratio (R/S) was calculated from final DW data, where “Shoot” designates the biomass of the emerged tissues, and “Root” the biomass of submerged tissues, with rhizomes and roots summed together. Values are the average of n = 5 replicates; error bars indicate the standard errors. The respective ANOVA p values were of 0.18 for a, and of 0.03 for b
No significant effect of metal concentration in growth media was found on either F v /F m , ΦPSII or photosynthetic pigment content. The means of plants treated with the highest metal concentrations scarcely differed from those of control plants (Table 2).
Metal concentration and extraction
Metal concentration in leaf, rhizome and root tissues increased with increasing concentration in the growth media, both for Cr and Zn treatments (Fig. 2). The roots achieved the highest concentrations (4.8 mg g−1 Zn and 10.1 mg g−1 Cr), followed by rhizomes (2.1 mg g−1 Zn and 0.7 mg g−1 Cr). Leaves showed a remarkable capacity to retain Zn, reaching 0.6 mg g−1. Chromium concentration was lower than Zn concentration in leaves and rhizomes, but higher in roots.
Concentration of metals in I. pseudacorus leaves (a), rhizomes (b) and roots (c). Values are the average of n = 5 replicates. Different letters indicate significant differences between groups according to Student–Newman–Keuls post hoc test. Data transformation log(y + 1) was conducted to meet the equal variances assumption
Despite the reduction of plant growth at high metal concentrations (Table 1), the amount of Cr and Zn extracted (calculated as the amount of metal extracted per unit of biomass) continued to increase as a result of the rising concentration in tissues. Chromium extraction (Table 3) was greater in roots than in rhizomes and leaves. The portion extracted by leaves was very small. The average total extraction per individual plant at the maximum Cr supply was 70.6 mg. Similarly, Zn extraction was greater in roots than in rhizomes and leaves, which extracted a similar amount. The average total extraction per individual plant at the highest Zn supply was 56.8 mg, thus 24% lower than in the highest Cr treatment. This in spite of the Zn ion concentration supplied being twice as much as that of Cr (1.5 mM Zn vs. 0.08 mM Cr for 200 μg ml−1 treatments). However, the amount of Zn extracted by leaves was 10-fold that of Cr (1.4 mg Cr vs. 14.2 mg Zn).
C and N content and isotopic composition
In response to the addition of either of the two metals, but particularly Cr, leaves, rhizomes and roots became isotopically heavier (Table 4). However, the increment of δ13C did not attain significance in rhizomes due to the high variability between samples. Increasing Cr also augmented the C/N ratio, more markedly in roots (32.4%) than in rhizomes (21.7%). In agreement, %N decreased 32.1% in roots and 19.4% in rhizomes of Cr-exposed plants. The %C and δ15N remained stable (results not shown).
Element content
The trees generated from element content cluster analysis (Fig. 3) clearly separated controls from treatments, and Zn from Cr treatments. Except for leaves, treatments with high concentrations of metals (100 and 200 μg ml−1) were closer to each other than to those applying low concentrations (10 and 50 μg ml−1), which grouped together.
Both Cr and Zn stress induced diverse changes in the elemental composition and ash content (m a) of plants (Tables 5, 6, 7). Chromium and Zn stress had a similar effect on some elements. Both caused an increment in Mn (leaves), and P content (roots), together with reduced Al (rhizomes) and Cu (rhizomes and leaves) content and Al, S, Mn, Mg and Ca content (roots). The other effects detected were of an opposite sign under Cr and Zn stress. In leaves, Cr decreased Fe, S, and Ca content, whereas Zn increased P and Ca. The quantification of Mg, Fe, S, and Al in leaves in the 100 μg ml−1 ZnCl2 treatment was inconsistent with the other Zn treatments, and must thus be interpreted with caution. In rhizomes, high Cr diminished the concentration of Ca, Fe, Mg and K, whereas Zn had the opposite effect on Ca and K. In roots, high Zn decreased Fe, whereas all the Zn treatments increased Cu and K. High Cr decreased Cu and K.
The response of m a to Zn varied. This parameter increased in leaves (Table 5) while in rhizomes and roots (Tables 6, 7) it decreased; however, none of these deviations were higher than 12%. In contrast, Cr decreased the m a of leaves (5.4%), rhizomes (38.2%) and roots (23.7%).
Discussion
Iris pseudacorus was highly tolerant to Zn and Cr stress, as all plants survived the high metal concentrations supplied. Both metals were accumulated preferentially in roots, especially Cr, but Zn was also exported to leaves, in agreement with the literature (Deng et al. 2006; Mazej and Germ 2009; Qian et al. 1999). According to the definition by Baker and Brooks (1989), a plant must concentrate Cr to a minimum 1,000 μg g−1, and Zn to a minimum 10,000 μg g−1 in its leaves to be considered a hyperaccumulator. These levels are much higher than that observed in the present experiment. Samecka-Cymerman and Kempers (2001) analysed leaves of I. pseudacorus naturally growing in a Polish anthropogenic lake with 120 μg g−1 Cr and 11 μg g−1 Zn in the sediment. These levels are comparable to our treatments with 10–50 μg ml−1 ZnCl2 (which contain 4.8–24.0 μg ml−1 Zn, respectively), but are approximately threefold our highest Cr treatment (39.0 μg ml−1 Cr). The concentration of Zn in leaves in those conditions reached 21 μg g−1, which is comparable to our 35 μg g−1 at 10 μg ml−1 ZnCl2. However, the concentration of Cr was clearly inferior, only 4 μg g−1 versus our 41 μg g−1. This discrepancy is possibly the result of the limited solubility of Cr in lake sediments, as described by Polyák and Hlavay (1999).
Our results demonstrated a specific response of the biomass acquisition and water content of plant sections to Zn and Cr, owing to their being essential and nonessential nutrient, respectively. The pattern shown by Zn-treated plants is consistent with the typical growth response to essential nutrients, which enhance growth at a sub-optimal or optimal concentration, but become toxic above a critical level (Marschner 1995). This threshold would lie between 50 and 100 μg ml−1 ZnCl2 for I. pseudacorus. In contrast, Cr is a nonessential element that promotes growth only at very low doses (Bonet et al. 1991). At the Cr concentrations used in our study, growth was not enhanced but gradually inhibited as the external Cr concentration increased.
The greater growth reduction observed in Zn-treated leaves is coherent with the higher amount of Zn transported to leaves. Zinc molar concentration is also twice as high as that of Cr in equivalent treatments (1.5 vs. 0.8 mM at 200 μg ml−1). The reduction of growth caused by Cr was due both to poor dry matter acquisition, which affected the roots more severely, and to reduced plant water content. Both effects are derivable from root damage and consistent with the preeminent role of roots in Cr retention and the restricted exportation of this metal to leaves. The decrease in m a in Cr-stressed roots was lower than expected; most probably because the high amounts of Cr accumulated there (Table 3) partially compensated the decrease of other elements. A similar process might have occurred in Zn-stressed plants.
There is ample evidence of the deleterious effects of Cr and Zn at various stages of photosynthesis and biosynthesis of chlorophyll (Ali et al. 2006; Chandra and Kulshreshtha 2004; Küpper et al. 1996; Oláh et al. 2010; Prasad and Strzałka 2002; Todeschini et al. 2011). Nevertheless, our results showed no harmful effect of Cr or Zn either on photosynthetic pigment content or on chlorophyll fluorescence. This observation implies that the efficiency of the light phase of photosynthesis was preserved. Despite the high concentration of metals supplied, PSII appeared fully functional: ΦPSII values were high, in agreement with the low intensity of environmental light, and F v /F m values were optimal. F v /F m is widely accepted as a rigorous measure of photo-inhibition, whereas ΦPSII is a measure of photochemistry, which is related to electron transport (effective quantum yield) and thus to photosynthesis (Maxwell and Johnson 2000). The correlation of ΦPSII with CO2 fixation in C3 plants is not always linear, but can be modified depending on the electron fractionation between photosynthesis and photorespiration (Krall and Edwards 1992). Dhir et al. (2008) studied the photosynthetic performance of Salvinia natans L. in response to Cr and Zn stress, and reported that pigment content and RuBisCo activity was decreased by both metals, while F v /F m was decreased only by Zn. A reduction in CO2 fixation as a result of heavy metal stress is therefore not necessarily reflected in chlorophyll fluorescence, and a decay of assimilation cannot be excluded from our results, even if pigment content was not affected.
The correlation of δ13C with intercellular CO2 concentration (C i) has been extensively demonstrated (Farquhar 1983). The mild increase of δ13C in response to the high metal concentrations supplied indicates that the stomatal aperture was restricted to some extent, which could affect intrinsic CO2 fixation. However, the changes in δ13C were too subtle, in our opinion, to be the only cause of the notable reduction in growth detected. Wei et al. (2008) observed that δ13C was slightly affected by Cd exposure in mangrove (Aegiceras corniculatum (L.) Blanco) and roots were more sensitive than leaves. These authors observed that δ13C also differed between plant parts, with assimilating organs showing lower values than non-assimilating or storage organs. In our study, leaves were isotopically lighter than rhizomes and roots, but δ13C was equally responsive in all tissues. The δ13C values were between −30.58 and −25.88‰, which is within the range of C3 plants (Boutton et al. 1998). Average values across all plant parts and growing conditions ranged from 29.5 to −27.4‰, which again is usual for C3 plants. The WC and m a reduction noted in response to Cr is therefore best attributed to other causes than the subtle inhibition of transpiration, such as restrained water and nutrient uptake induced by severe root damage. Water uptake is directly connected to the deposition and absorption of minerals (Bakker and Elbersen 2005).
Zinc and Cr disturbed not only plant growth, but also biomass allocation. The most plausible explanation for this is a source-to-sink carbohydrate relocation from leaves to non-assimilating tissues. The growth decrease per plant section was ranked leaf > rhizome > root, which caused the constant increase of R/S with increasing Zn concentration. This finding is consistent with the Zn accumulation pattern and suggests that roots were the most relevant sink tissue. In contrast, although roots accumulated higher amounts of Cr and showed a stronger growth inhibition, R/S increased abruptly at high Cr treatments as a result of the weight increase of the rhizomes. This observation points to rhizomes as the most demanding sink tissue in Cr-stressed plants. The roots treated with Cr accumulated a significantly higher amount of metal and showed more symptoms of damage, which may explain a restricted unload of carbohydrates.
The carbohydrate requirements of the roots and rhizomes of a plant under heavy metal stress might increase as a result of active detoxification mechanisms, such as ROS scavenging, compartmentalization, damage repair, cell wall thickening, or the synthesis of secondary metabolites. Some authors report increased dark respiration and ATP in response to heavy metal exposure, which may exert a protective role (Pavlovič et al. 2006; Romanowska et al. 2002). This notion is also in agreement with the reduced growth and increased P concentration, especially in roots, where the demand for ATP in order to neutralize the negative effects of excess Zn or Cr should be most augmented. P is assimilated as ATP, the chemical energy storage of the cell. The decrease in P content in Cr-treated leaves could be interpreted as relocation to P-demanding roots. Stobrawa and Lorenc-Plucińska (2007) found no evidence of increased respiration in the fine roots of Populus nigra L. growing in a site polluted by multiple metals; however, sucrose breakdown was activated and the level of soluble carbohydrates lowered. Those authors proposed that sucrose is used for the synthesis of cell wall polysaccharides (callose or cellulose) or secondary metabolites.
A high R/S has also been described in response to nutrient or water deficiency (Hermans et al. 2006; Price et al. 2002), as a tolerance mechanism that reduces transpiration and redirects carbohydrates to increase the surface available for water and nutrient uptake. Chromium (III) and Zn are passively taken up and retained by cation exchange sites in cell walls (Marschner 1995; Skeffington et al. 1976). An excessive concentration of these metals can compete with other polyvalent cations such as Mg, Mn, and Ca for the formation of coordination complexes, and induce mineral nutrient deficiency. This would explain the decreased levels of Al, Mg, Mn, and Ca in Zn-treated roots and of Al, Mg, Mn, Ca, and K in Cr-treated roots in our study, and is in agreement with the response of m a. Increased P concentration and decreased S concentration have been described in cauliflower plants under Cr stress (Chatterjee and Chatterjee 2000). Sulphur is absorbed by plant roots as sulphate by means of a proton symporter (like nitrate and phosphate), and stored in vacuoles (Buchner et al. 2004). Sulphur may be displaced from metal-occupied vacuoles of roots and rhizomes, where Cr, Zn and other heavy metals are compartmentalized to prevent their toxic effects on cell metabolism. This notion is coherent with previous data from TEM microanalysis of the vacuoles of Cr-exposed I. pseudacorus rhizomes (Caldelas et al. 2012), which showed high of S and Cr contents. In summary, a greater number of elements showed a tendency to reduce their contents in Cr-stressed plants than in Zn-stressed plants, and roots were more affected by this decrease than leaves and rhizomes. However, when considering the previous results, that WC was lower in Cr–stressed rhizomes and roots must be taken into account. This lower WC might have caused the concentration of some elements to be higher in these tissues, and does not imply that uptake has increased or relocation taken place. Moreover, reductions in the element contents of these samples might pass unnoticed if they are small, or appear less relevant than they truly are.
Conclusions
Iris pseudacorus shows a great capacity to tolerate and accumulate both Zn and Cr, which displayed distinct distribution patterns, thereby leading to specific physiological responses. Chromium was retained mainly in roots, causing greater root damage than Zn. Zinc was partially exported to the rest of the plant, and consequently caused a higher decrease in the growth of photosynthetic tissues, but less root malfunction. The functionality of the PSII persisted in all the treatments, and the stomatal aperture was only partially limited by Cr.
We conclude that the reduction of growth in I. pseudacorus in response to exposure to Cr and Zn is due to the restricted mineral and water uptake and to the increased demand of carbohydrates of damaged roots, rather than to the direct effects of these metals on photoassimilating tissues. Biomass allocation to rhizomes (Cr) or roots (Zn) may contribute to heavy metal tolerance in this species by reducing transpiration and increasing metal-accumulating tissues and/or the surface for water and mineral uptake.
The high biomass production and metal extraction capacity makes this species a good candidate for Cr rhizofiltration and Zn phytoextraction, as reflected by the level of exportation of each metal to leaves. The reduced exportation of Cr to leaves can be advantageous for flower production, yield of emerged parts, and human safety. Metal extraction would also be higher than in environments polluted by Zn, as long as the whole plant is collected. After harvest the metal-enriched biomass must be disposed of safely, a technical issue which remains partially unsolved. A variety of techniques under development, i.e. composting, compacting, pyrolysis, or biogas production (Ghosh and Singh 2005; Rai 2009), will remove this limitation in future and allow for a wider use of phytoremediation.
Author contribution
C. Caldelas designed and performed the experiments, obtained the analytical data, interpreted the results and wrote the manuscript. J.L. Araus, J. Bort and A. Febrero, acting as thesis advisors, supervised the conception and development of the experiments, discussed the interpretation of the results, and reviewed the proofs of the manuscript.
References
Ali NA, Dewez D, Didur O, Popovic R (2006) Inhibition of photosystem II photochemistry by Cr is caused by the alteration of both D1 protein and oxygen evolving complex. Photosynth Res 89:81–87. doi:10.1007/s11120-006-9085-5
Baker AJM, Brooks RR (1989) Terrestrial higher plants which hyperaccumulate metallic elements—a review of their distribution, ecology and phytochemistry. Biorecovery 1:81–126
Bakker RR, Elbersen HW (2005) Managing ash content and quality in herbaceous biomass: an analysis from plant to product. In: 14th European biomass conference and exhibition, 17–21 October 2005, Paris, France
Belmont MA, Metcalfe CD (2003) Feasibility of using ornamental plants (Zantedeschia aethiopica) in subsurface flow treatment wetlands to remove nitrogen, chemical oxygen demand and nonylphenol ethoxylate surfactants—a laboratory-scale study. Ecol Eng 21:233–247. doi:10.1016/j.ecoleng.2003.10.003
Bonet A, Poschenrieder C, Barceló J (1991) Chromium III - Iron Interaction in Fe-deficient and Fe-sufficient bean plants. 1. Growth and nutrient content. J Plant Nutr 14:403–414. doi:10.1080/01904169109364211
Boutton TW, Archer SR, Milwood AJ, Zitzer SF, Bol R (1998) δ13C values of soil organic carbon and their use in documenting vegetation change in a subtropical savanna ecosystem. Geoderma 82:5–41. doi:10.1016/S0016-7061(97)00095-5
Broadley MR, White PJ, Hammond JP, Zelko I, Lux A (2007) Zinc in plants. New Phytol 173:677–702
Buchner P, Takahashi T, Hawkesford J (2004) Plant sulphate transporters: co-ordination of uptake, intracellular and long-distance transport. J Exp Bot 55:1765–1773. doi:10.1093/jxb/erh206
Caldelas C, Bort J, Febrero A (2012) Ultrastructure and subcellular distribution of Cr in Iris pseudacorus L. using TEM and X-ray microanalysis. Cell Biol Toxicol 28:57–68. doi:10.1007/s10565-011-9205-7
Chandra P, Kulshreshtha K (2004) Chromium accumulation and toxicity in aquatic vascular plants. Bot Rev 70:313–327. doi:10.1663/0006-8101(2004)070[0313:CAATIA]2.0.CO;2
Chatterjee J, Chatterjee C (2000) Phytotoxicity of cobalt, chromium and copper in cauliflower. Environ Pollut 109:69–74. doi:10.1016/S0269-7491(99)00238-9
Deng H, Ye ZH, Wong MH (2006) Lead and zinc accumulation and tolerance in populations of six wetland plants. Environ Pollut 141:69–80. doi:10.1016/j.envpol.2005.08.015
Dhir B, Sharmila P, Pardha Saradhi P (2008) Photosynthetic performance of Salvinia natans exposed to chromium and zinc rich wastewater. Braz J Plant Physiol 20:61–70. doi:10.1590/S1677-04202008000100007
Farquhar GD (1983) On the nature of isotope discrimination in C4 species. Aust J Plant Physiol 9:205–226. doi:10.1146/annurev.pp.40.060189.002443
Genty B, Briantais JM, Baker NR (1989) The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim Biophys Acta 990:87–92. doi:10.1016/S0304-4165(89)80016-9
Ghosh M, Singh SP (2005) A review on phytoremediation of heavy metals and utilization of its byproducts. Appl Ecol Environ Res 3:1–18
Han Y, Yuan H, Huang S, Guo Z, Xia B, Gu J (2007) Cadmium tolerance and accumulation by two species of Iris. Ecotoxicol 16:557–563. doi:10.1007/s10646-007-0162-0
Hara T, Sonoda Y (1979) Comparison of the toxicity of heavy metals to cabbage growth. Plant Soil 51:127–133
Hermans C, Hammond JP, White PJ, Verbruggen N (2006) How do plants respond to nutrient shortage by biomass allocation? Trends Plant Sci 11:610–615. doi:10.1016/j.tplants.2006.10.007
Janik E, Maksymiec W, Mazur R, Garstka M, Gruszecki WI (2010) Structural and functional modifications of the major light-harvesting complex II in cadmium- or copper-treated Secale cereale. Plant Cell Physiol 51:1330–1340. doi:10.1093/pcp/pcq093
Krall JP, Edwards G (1992) Relationship between photosystem II activity and CO2 fixation in leaves. Physiol Plant 86:180–187. doi:10.1111/j.1399-3054.1992.tb01328.x
Krugh B, Bischham L, Miles D (1994) The solid-state chlorophyll meter, a novel instrument for rapidly and accurately determining the chlorophyll concentration in seedling leaves. Maize Genet Coop News Lett 68:25–27
Küpper H, Küpper F, Spiller M (1996) Environmental relevance of heavy metal-substituted chlorophylls using the example of water plants. J Exp Bot 47:259–266
Kuzovkina YA, Quigley MF (2005) Willow beyond wetlands: uses of Salix L. species for environmental projects. Water Air Soil Pollut 162:183–204. doi:10.1016/j.ecoleng.2009.03.010
Larue C, Korboulewsky N, Wang RY, Mévy JP (2010) Depollution potential of three macrophytes: exudated, wall-bound and intracellular peroxidase activities plus intracellular phenol concentrations. Bioresour Technol 101:7951–7957. doi:10.1016/j.biortech.2010.05.010
Lichtenthaler HK (1987) Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. Methods Enzymol 148:350–382
Malik A (2007) Environmental challenge vis a vis opportunity: the case of water hyacinth. Environ Int 33:122–138. doi:10.1016/j.envint.2006.08.004
Manceau A, Nagy KL, Marcus MA, Lanson M, Geoffroy N, Jacquet T, Kirpichtchikova T (2008) Formation of metallic copper nanoparticles at the soil–root interface. Environ Sci Technol 42:1766–1772. doi:10.1021/es072017o
Marschner H (1995) Mineral nutrition of higher plants. Academic Press, London
Maxwell K, Johnson GN (2000) Chlorophyll fluorescence—a practical guide. J Exp Bot 51:659–668. doi:10.1093/jexbot/51.345.659
Mazej Z, Germ M (2009) Trace element accumulation and distribution in four aquatic macrophytes. Chemosphere 74:642–647. doi:10.1016/j.chemosphere.2008.10.019
Oláh V, Lakatos G, Bertók C, Kanalas P, Szőllősi E, Kis J, Mészáros I (2010) Short-term chromium (VI) stress induces different photosynthetic responses in two duckweed species, Lemna gibba L. and Lemna minor L. Photosynthetica 48:513–520. doi:10.1007/s11099-010-0068-6
Paiva L, Oliveira J, Azevedo R, Ribeiro D, Silva M, Vitoria A (2009) Ecophysiological responses of water hyacinth exposed to Cr3+ and Cr6+. Environ Exp Bot 65:403–409. doi:10.1016/j.envexpbot.2008.11.012
Pavlovič A, Masarovičová E, Král’ová K, Kubová J (2006) Response of chamomile plants (Matricaria recutita L.) to cadmium treatment. Bull Environ Contam Toxicol 77:763–771. doi:10.1007/s00128-006-1129-1
Polyák K, Hlavay J (1999) Environmental mobility of trace metals in sediments collected in the Lake Balaton. Fresenius J Anal Chem 363:587–593
Prasad MNV (2004) Heavy metal stress in plants. From biomolecules to ecosystems. Springer, Berlin
Prasad DDK, Prasad ARK (1987) Altered delta-aminolevulinic-acid metabolism by lead and mercury in germinating seedlings of bajra (Pennisetum typhoideum). J Plant Phys 127:241–249
Prasad MNV, Strzałka K (2002) Physiology and biochemistry of metal toxicity and tolerance in plants. Kluwer, Dordrecht
Price AH, Steele KA, Gorham J, Bridges JM, Moore BJ, Evans JL, Richardson P, Jones RGW (2002) Upland rice grown in soil-filled chambers and exposed to contrasting water-deficit regimes. I. Root distribution, water use and plant water status. Field Crops Res 76:11–24. doi:10.1016/S0378-4290(02)00012-6
Qian JH, Zayed A, Zhu YL, Yu M, Terry N (1999) Phytoaccumulation of trace elements by wetland plants: III. Uptake and accumulation of ten trace elements by twelve plant species. J Environ Qual 28:1448–1455
Qiu S, Huang S (2008) Study on growth and Cd accumulation of root system of Iris pseudacorus seedling under Cd stress. J Plant Res Environ 17:33–38. doi:CNKI:SUN:ZWZY.0.2008-03-007
Rai PK (2009) Heavy metal phytoremediation from aquatic ecosystems with special reference to macrophytes. Crit Rev Environ Sci Technol 39:697–753. doi:10.1080/10643380801910058
Romanowska E, Igamberdiev AU, Parys E, Gardestrom P (2002) Stimulation of respiration by Pb2+ in detached leaves and mitochondria of C-3 and C-4 plants. Physiol Plant 116:148–154. doi:10.1034/j.1399-3054.2002.1160203.x
Salt DE, Smith RD, Raskin I (1998) Phytoremediation. Annu Rev Plant Physiol Plant Mol Biol 49:643–648
Samecka-Cymerman A, Kempers AJ (2001) Concentrations of heavy metals and plant nutrients in water, sediments and aquatic macrophytes of anthropogenic lakes (former open cut brown coal mines) differing in stage of acidification. Sci Total Environ 281:87–98. doi:10.1016/S0048-9697(01)00838-5
Shanker AK, Cervantes C, Loza-Tavera H, Avudainayagem S (2005) Chromium toxicity in plants. Environ Int 31:739–753. doi:10.1016/j.envint.2005.02.003
Skeffington RA, Shewry PR, Peterson PJ (1976) Chromium uptake and transport in barley seedlings (Hordeum vulgare L.). Planta 132:209–320
Stobrawa K, Lorenc-Plucińska G (2007) Changes in carbohydrate metabolism in fine roots of the native European black poplar (Populus nigra L.) in a heavy-metal-polluted environment. Sci Total Environ 373:157–165. doi:10.1016/j.scitotenv.2006.11.019
Todeschini V, Lingua G, D’Agostino G, Carniato F, Roccotiello E, Berta G (2011) Effects of high zinc concentration on poplar leaves: a morphological and biochemical study. Env Exp Bot 71:50–56. doi:10.1016/j.envexpbot.2010.10.018
USEPA (2005) Priority pollutants. Code of federal regulations. Title 40: protection of environment, chap I. Appendix A to 40 CFR Part 423. 1st July 2005. Environmental Protection Agency
Uveges JL, Corbett AL, Mal TK (2002) Effects of lead contamination on the growth of Lythrum salicaria (purple loosestrife). Environ Pollut 120:319–323. doi:10.1016/S0269-7491(02)00144-6
Vernay P, Gauthier-Moussard C, Hitmi A (2007) Interaction of bioaccumulation of heavy metal chromium with water relation, mineral nutrition and photosynthesis in developed leaves of Lolium perenne L. Chemosphere 68:1563–1575. doi:10.1016/j.chemosphere.2007.02.052
Wei L, Yan C, Wu G, Guo X, Ye B (2008) Variation of δ13C in Aegiceras corniculatum seedling induced by cadmium application. Ecotoxicol 17:480–484. doi:10.1007/s10646-008-0201-5
Zhang X, Liu P, Yang Y, Chen W (2007) Phytoremediation of urban wastewater by model wetlands with ornamental hydrophytes. J Environ Sci 19:902–909
Zhou YQ, Huang SZ, Yu SL, Gu JG, Zhao JZ, Han YL, Fu JJ (2010) The physiological response and sub-cellular localization of lead and cadmium in Iris pseudacorus L. Ecotoxicol 19:69–76. doi:10.1007/s10646-009-0389-z
Acknowledgments
This study was part of the International Cooperation European Project MEDINDUS, EC Contract No INCO-CT-2004-509159. Experiments were conducted in the experimental field services (Servei de Camps Experimentals) of the Universitat de Barcelona. Sample digestion and determination of element content were performed in the technical services (Serveis Científicotècnics) of the Universitat de Barcelona. We wish to thank their personnel for their collaboration and advice.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by K. Strzalka.
Rights and permissions
About this article
Cite this article
Caldelas, C., Araus, J.L., Febrero, A. et al. Accumulation and toxic effects of chromium and zinc in Iris pseudacorus L.. Acta Physiol Plant 34, 1217–1228 (2012). https://doi.org/10.1007/s11738-012-0956-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11738-012-0956-4