Abstract
The key players of the CKD–MBD are calcium, phosphorus, PTH, FGF23/Klotho, and the vitamin D hormonal system. The progressive reduction of kidney function greatly modified the tightly interrelated mechanisms controlling these parameters. As a result, important changes occurred in the bone and mineral hormonal axis leading to changes in bone turnover with consequences in relevant clinical outcomes, such as decrease in bone mass with increased bone fragility and bone fractures and increased vascular and valvular calcification with great impact in the cardiovascular outcomes.
So far, knowledge about mineral and bone disorders in CKD and the increased variety of efficacious therapies should lead to a better prevention and management of CKD–MBD.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
FormalPara Before You Start: Facts You Need to Know-
After 60 years of use, the term “renal osteodystrophy” was changed to “chronic kidney disease–mineral and bone disorders” (CKD–MBD).
-
CKD–MBD group is a complex clinical syndrome including biochemical parameters and other surrogated markers.
-
“Renal osteodystrophy” is reserved to describe the bone histological lesions.
-
The key regulators of CKD–MBD are calcium, phosphorus, PTH, FGF23/Klotho, and the vitamin D hormonal system.
-
The prevention and treatment of CKD–MBD needs to be done integrating all the components of the syndrome.
16.1 Mineral and Bone Disorders in CKD
16.1.1 General Aspects, Epidemiology, and Pathophysiology
In healthy individuals, kidneys regulate calcium and phosphorus homeostasis modifying their tubular resorption. Patients with CKD experience a progressive compromise of the homeostatic mechanisms, giving rise to different adaptive changes in calcium, phosphorus, parathyroid hormone (PTH), vitamin D, and fibroblast growth factor 23 (FGF23)/Klotho levels. These elements and hormones exert their effects on several tissues, but they act mainly on their principal targets: the bone, kidney, and intestine.
For six decades, the mineral and bone abnormalities of CKD patients were known as “renal osteodystrophy.” However, in 2006, the new term “chronic kidney disease–mineral and bone disorders” (CKD–MBD) was proposed by KDIGO guidelines (Box 16.1) to group and describe a more ample and complex clinical syndrome which includes not only biochemical and bone histological abnormalities but also other bone and cardiovascular complications such as fractures and cardiovascular abnormalities occurring in CKD patients. The term “renal osteodystrophy” was reserved to describe the bone abnormalities associated with CKD which require a bone biopsy for the diagnosis. For the latter, a new classification system was proposed based on parameters of bone turnover, mineralization, and volume (TMV) [1].
Box 16.1 Relevant Guidelines
-
1.
KDIGO Guidelines.
-
(a)
KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease-mineral and bone disorder (CKD-MBD). Kidney Int Suppl. 2009;76(113):S1–130 [1].
-
(b)
KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;3(1):S1–150 [17].
-
(c)
KDIGO 2017 Clinical Practice Guideline Update for the Diagnosis, Evaluation, Prevention, and Treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Kidney Int Suppl. 2017;7(1):1–59 [20].
-
(a)
-
2.
KDOQI Guidelines.
-
(a)
K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39(2 Suppl 1):S1–266 [18].
-
(a)
-
3.
Spanish Society of Nephrology Guidelines.
-
(a)
Spanish Society of Nephrology recommendations for controlling mineral and bone disorder in chronic kidney disease patients (S.E.N.-M.B.D.). Nefrologia. 2011;31 Suppl 1:3–32 [19].
-
(a)
-
4.
Japanese Society for Dialysis Therapy Guidelines.
-
(a)
Clinical practice guideline for the management of chronic kidney disease-mineral and bone disorder. Ther Apher Dial. 2013;17(3):247–88 [58].
-
(a)
The mineral and endocrine functions disrupted in CKD are critically important in the regulation of bone modeling during growth and bone remodeling during adulthood. These CKD–MBD are found almost universally in patients requiring dialysis but also in the majority of patients in CKD stages 3–5. In recent years, there has been an increased concern with the non-skeletal calcification which increases early in the course of CKD, due to the deranged mineral and bone metabolism, but it might also occur as a result of therapies used to correct the CKD–MBD themselves. Numerous cohort studies have shown associations between several CKD–MBD, such as bone fractures, vascular calcification, and cardiovascular disease with increased mortality.
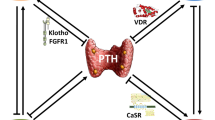
As mentioned, the key regulators of bone and mineral metabolism are calcium, phosphorus, PTH, FGF23, Klotho, and the vitamin D hormonal system [2]. Even though there is still some debate concerning the chronology of changes, it is currently accepted that the increments in FGF23 and the reduction of serum Klotho are possibly the earliest events in the pathogenesis of CKD–MBD; both factors favor the reduction of 1-alpha-hydroxylase in the kidney, which in turn results in low levels of the active form of vitamin D (1,25-dihydroxyvitamin D3-[1,25(OH)2D3] or calcitriol), impairing calcium absorption in the intestine favoring the reduction in serum calcium. The decrease in serum calcium stimulates PTH synthesis and release, which in turn increases bone turnover, increases bone resorption, and stimulates 1-alpha-hydroxylase (see Fig. 16.1). All these mechanisms lead to compensatory increases in serum calcium [3].
In addition, in non-advanced phases of CKD, the increments of FGF23 and PTH increase urinary phosphorus excretion in order to avoid phosphorus accumulation [3]. Despite FGF23 and PTH featuring synergic effects to increase phosphorus excretion, both have opposite effects on calcitriol synthesis: FGF23 inhibits 1-alpha-hydroxylase, decreasing calcitriol synthesis, whereas PTH stimulates 1-alpha-hydroxylase production, thus increasing calcitriol synthesis (see Fig. 16.1). It has recently shown that phosphorus could also exert its action via calcium-sensing receptor (CaSR) [4]. FGF23 exerts its tubular effect binding to its Klotho co-receptor and activating FGFR-1 and FGFR-3 receptors, while PTH does so by binding to its specific receptor. Both increase phosphate excretion by reducing apical abundance of sodium-coupled cotransporters NaPi2a and NaPi2c via both PKA- and PKC-dependent pathways [5].
Both calcium and calcitriol act on the parathyroid cells through their specific receptors, the CaSR and the vitamin D receptor (VDR), respectively (see Fig. 16.1). While CaSR is a cell membrane receptor member of the G protein-coupled receptor family, VDR is a nuclear receptor that, when bound to vitamin D, acts as a transcription factor. The differences in the nature of the two ligands and their receptors lead to two different mechanisms of action with complementary functions on the parathyroid cells.
On the one hand, small decreases in extracellular calcium concentrations are rapidly sensed by the CaSR, triggering within seconds or minutes increments in PTH release. Small increases in calcium are also sensed by the CaSR, yielding opposite results. If the stimulus persists for longer periods (hours, days), calcium is able to regulate PTH synthesis post transcriptionally by modifying the mRNA stability through differences in binding of the parathyroid proteins to an element in its 3′-untranslated region. As a result, the decreases in serum calcium reduce mRNA degradation by increasing its stability and the half-life of mRNA PTH. On the contrary, the active form of vitamin D (calcitriol) acts at the transcriptional level and inhibits PTH gene transcription resulting in a reduction of PTH synthesis [3].
When kidney function decreases, all these complex and tightly interrelated mechanisms fail to adequately control the mineral metabolism. As a result, a progressive trend to reduce serum levels of calcitriol and increase phosphorus and calcium retention begins, ending at later stages of CKD, despite the permanent and progressive parathyroid hormone stimulation, in a manifest incapacity to control the mineral metabolism. As a result, in advanced stages of CKD–MBD, patients show severe forms of secondary hyperparathyroidism with diffuse and nodular hyperplasia and a significant reduction in CaSR, VDR, and FGFR/Klotho expression with a poor response of the parathyroid glands to the effect of calcium, VDR activators (VDRAs), and FGF23 (see Fig. 16.2) [2, 6].
Progression of secondary hyperparathyroidism: Initially, the parathyroid glands respond by increasing the number of secretory cells; this results in diffuse hyperplasia of the gland where cell growth is polyclonal and is accompanied by downregulation of CaSR, VDR, and FGFR/Klotho. As CKD progresses to end-stage renal disease (CKD stage 5), parathyroid hyperplasia evolves even further; monoclonal abnormalities lead to nodular hyperplasia of the glands associated with significant under-expression of CaSR, VDR, and FGFR/Klotho. (Modified from Tominaga et al. [6], with permission from John Wiley and Sons)
Cross-sectional studies have shown the pattern of abnormalities in serum calcium, phosphorus, PTH, 25(OH)D3 (calcidiol), and calcitriol at different stages of CKD. As Fig. 16.3 shows, serum calcium and phosphorus values did not become abnormal until the glomerular filtration rate (GFR) fell around 30–40 mL/min. The abnormalities progressively increase starting from these values. By contrast, calcitriol started to decrease early in the course of CKD (GFR between 70 and 80 mL/min) and PTH increased a bit later (GFR between 60 and 70 mL/min) (see Fig. 16.3) [7].
Prevalence of abnormal mineral metabolism in CKD. (a) The prevalence of hyperparathyroidism, hypocalcemia, and hyperphosphoremia by GFR levels at 10 mL/min per 1.73 m2 intervals. (b) Median values of 1,25(OH)2D3, 25(OH)D3, and intact PTH by GFR levels. (Republished by permission from Macmillan Publishers Ltd.: Levin et al. [7])
Even though all the above discussed changes lead to the stimulation of the parathyroid gland and high bone turnover, the latter is not the most frequent histological finding of renal osteodystrophy in CKD patients. Due to the combination of several factors, such as aging, diabetes, and the medical management of CKD–MBD (calcium overload, high dose of VDRAs, aluminum salts), throughout the recent decades, the more frequent pattern of bone lesions has changed from high to low bone turnover forms of renal osteodystrophy (see Table 16.1, [8,9,10,11,12,13,14]). Recent studies also suggest that an early inhibition of the Wnt/ßcatenin pathway may play a role in the pathogenesis of low bone turnover [15, 16]. Despite high and low bone turnover being quite different and also opposite extremes of the CKD bone abnormalities, they have been associated with similar clinical outcomes, such as a higher prevalence of vascular calcification and bone fragility fractures leading to a higher mortality risk.
16.2 Diagnosis of CKD–MBD
16.2.1 Biochemical Abnormalities
The changes in the biochemical parameters of CKD–MBD currently begin in CKD stage 3b, but the rate of change and the severity of abnormalities vary greatly among patients. Therefore, assessment should begin in stages 2-3a, and the frequency of assessments, the type and duration of the identified abnormalities, the degree and rate of change of GFR, and the concomitant therapy need to be taken into account to adapt the frequency of the assessments and the non-pharmacological and pharmacological interventions.
The diagnosis of CKD–MBD includes the use of laboratory testing of calcium, phosphorus, PTH, calcidiol, alkaline phosphatase (ALP) (total or bone specific), and the acid-base status together with other serum and urinary parameters used in the follow-up of patients with CKD. Although much progress has been made in the mechanisms involved in the role of FGF23/Klotho, its usefulness in routine clinical practice is still limited. The recommended frequency of assessment of these biochemical markers is detailed in Table 16.2 [1]. One important limitation of the biochemical markers used to diagnose, treat, and monitor CKD–MBD is the inter-assay variability and other variations as well (postprandial, diurnal, seasonal). The interpretation of values calls for the careful analysis of the type and precision of the assay used in order to avoid overemphasizing the role of minimal or inconsistent laboratory changes in the clinical decision-making process. The importance of one single abnormal value of any one bone and mineral serum biochemical markers should not be determinant. By contrast, the diagnosis and the management of CKD–MBD should be based mainly on the trend of changes; this aspect is even more relevant in the interpretation of serum PTH and ALP values.
In the last three decades, there has been a debate to better define the normal or acceptable upper and lower limits of these biochemical markers and thus guide managerial and therapeutical decisions. The KDOQI (Box 16.1), the European, and more recently the KDIGO and national guidelines have established different cutoff levels [1, 17,18,19]. Table 16.3, which considers mainly the 2017 KDIGO recommendations, summarizes what we should consider as adequate or acceptable values of the main serum bone and mineral markers according to the degree of CKD [20]. Most of the recommendations are backed by a reasonable scientific rationale, but, unfortunately, the degree of evidence based on randomized clinical trials is very low. At present, most of the best available evidence comes from population-based or cohort-based prevalence studies.
16.2.2 Bone Abnormalities
Bone tissue has excellent biomechanical properties: it possesses a great mechanical tension to tensile stress, which is lower than that of iron, but it is at least three times lighter and ten times more flexible than iron. This outstanding property of bone explains why during long periods of life, only a reduced number of bone fractures occur despite the remarkable number of falls suffered by most people. Bone has such clinically relevant biomechanical properties thanks to the activity of the bone remodeling units, which during the young adult life allow for the renewal of a mean of 5–10% of the skeleton per year. However, the capacity to renew bone tissue progressively decreases after age 50. Apart from the changes due to aging and gender differences, two bone disorders, osteoporosis and renal osteodystrophy, greatly influence bone turnover, since they exert an important impact on bone mass and bone quality.
The rate of bone turnover impacts both cancellous (trabecular) and compact (cortical) bone, and it depends on the activity of the bone remodeling units which are regulated by several factors; among them, PTH plays a key role. Cortical bone is the most abundant type (85% of the skeleton), but the most metabolically active one is trabecular bone. Trabecular bone decreases in CKD patients, but due to the quantity of cortical bone and also the sustained major effect of PTH on the latter, in the current long-term evolution of the CKD–MBD, there is a predominant loss of cortical bone, which after several years of CKD leads to a generalized thin cortex with trabecular aspects due to insidious bone cortical erosions.
High serum PTH levels are associated with hyperdynamic bone. PTH stimulates bone cell proliferation and activity but also bone turnover. In secondary hyperparathyroidism, the cycles of the bone remodeling units are more rapid and active, but also more bone remodeling units are activated; as a result of these two mechanisms, an abnormal, immature, non-lamellar bone matrix is formed. At the end, a woven and less resistant bone is produced, which yields an increased fracture risk. Very high serum PTH levels (>450 pg/mL) have a good predictive value for high bone turnover, but moderately high serum PTH values (300–450 pg/mL) do not exhibit a good correlation with bone turnover; in fact, normal or low bone turnover can be found within these ranges of serum PTH values.
Low PTH levels are associated with adynamic bone, low bone cellular activity, and bone turnover [21]. Consequently, the inadequate renewal of bone increases its fragility. In practice, adynamic bone is currently suspected when serum PTH levels are below the normal values. PTH levels lower than 150 pg/mL have a good predictive value for low bone turnover and adynamic bone, but PTH between 150 and 450 pg/mL, currently considered normal or adequate serum PTH values, can be associated with adynamic bone [22]. Then, despite the measurement of serum PTH levels in CKD patients being the current noninvasive method to assess bone turnover, its specificity within the previous mentioned ranges is limited. Overall, the clinical consequences of low bone turnover observed in adynamic bone are similar to those observed in osteoporosis, with a higher prevalence of bone fractures and more frequent and severe vascular calcification compared with patients in whom bone turnover remains close to normal [21].
For the precise diagnosis of high or low bone turnover (mainly for the latter), it is necessary to perform a bone biopsy. An increased number of osteoclasts and osteoblasts, a non-laminar osteoid, woven bone, high bone formation rate with high activation frequency, normal or high mineralization rate (double tetracycline labeling), and increased marrow fibrosis are typically found in high bone turnover states. By contrast, the reduction or absence of osteoblasts and osteoclasts, a decreased or null osteoid formation, and low or no bone mineralization rate (low or absent tetracycline labeling) with low activation frequency are found in adynamic bone [21]. Another histological diagnosis associated with low bone turnover is osteomalacia, which was the most common form of low bone turnover in the 1970s and 1980s and it was associated with aluminum overload. The proper control of the sources of aluminum exposure (aluminum in dialysis fluids and the reduction of the massive use of aluminum-containing phosphate binders) has drastically reduced the incidence of osteomalacia in CKD patients.
A decrease in BMD and changes in the bone microarchitecture occur early in CKD and worsen as the disease progresses. As a result, CKD patients present flaws in the quantity and quality of bone, resulting in a higher risk of bone fracture, mainly of non-vertebral bone fractures [1, 21, 23,24,25]. The measurement of bone mass as bone mineral density (BMD) using dual-energy X-ray absorptiometry (DXA) is the best noninvasive marker to predict bone fractures in the general population, as well as in CKD patients as it has recently described [20, 26,27,28,29,30,31].
Bone strength is determined by the density and quality of the bone, but the BMD measured using DXA is not able to capture bone quality (cortical and trabecular microarchitecture). Changes in quality are better studied using high-resolution peripheral quantitative computed tomography (HR-pQCT).
16.2.3 Diagnosis and Type of Vascular Calcification
The predisposition of patients with CKD toward developing vascular calcification was already mentioned in the nineteenth century; but now it has great interest in nephrology because it is closely related to cardiovascular disease and mortality. The presence of vascular calcifications was included in KDIGO guidelines for the classification of mineral and bone disorders of chronic kidney diseases (CKD).
Vascular calcification was initially thought to be a passive, degenerative process; however, the evidence now suggests that it is an active process, a dysregulation of the equilibrium between promoters and inhibitors of calcification. Several uremic factors, including abnormalities in the mineral metabolism, age, diabetes, dyslipidemia, hypertension, smoking, inflammatory process, oxidative stress, genetic factors, are implicated [32]. Table 16.4 summarizes the traditional and nontraditional uremia-related risk factors for vascular calcification in CKD patients.
Despite the debate still exists somewhat, it has been suggested that a lateral abdominal X-ray and an echocardiogram, both simple and inexpensive procedures, can be effectively used to detect vascular and valvular calcification, respectively.
Most studies examining calcification have been performed using CT-based techniques (electron beam tomography and multi-slice computed tomography, EBCT and MSCT, respectively), which are quite sensitive methods for the detection of and quantification of calcium in the vessels. However, these more precise techniques are not widely available. The localization and extension of vascular calcification can be scored in a reproducible manner using X-ray. Several available methods such as the Kauppila, Adragao, and others are able to quantify and score vascular calcification, featuring a good correlation with the CT-based gold standard techniques and also with outcomes such as mortality.
An association of higher mineralized bone volume evaluated in bone biopsies, with a lower vascular calcification score assessed by plain X-ray was showed [33]. In addition, valvular calcification detected by echocardiography is a good predictor of coronary artery calcium. The information provided by these studies should help not only to evaluate risk and prognosis but also to guide the therapeutic management of CKD patients [34,35,36].
There are three types of arteries which differ according to their size and structure: elastic or large-caliber arteries, muscular or medium-caliber arteries, and small-caliber arteries.
The elastic or large-caliber arteries are responsible of conducting the blood to the distribution arteries; they show a relatively thin wall in proportion to their diameter and a rather thick tunica media containing more elastic fibers than smooth muscle with a fairly thin adventitia. The aorta, the subclavia, and the common carotid arteries belong to this group. The muscular or medium-caliber arteries are capable of withstanding further vasodilatation and vasoconstriction to adjust the volume of blood to the perfusion requirements; they have a tunica media which contains a high proportion of smooth muscle. The axillary, brachial, radial, coronary, femoral, and tibial arteries are included in this group. Finally, the small-caliber arteries are responsible for regulating the local blood flow and perfusion pressure through luminal size variations caused by vasoconstriction and vasodilatation; they are less than 2-mm thick, and their tunica media contains only smooth muscle. This group includes, among others, the palmar arch and the digital arteries [34].
Vascular calcification can occur in the intima and the media layers. Intimal calcification begins and progresses throughout lifetime mainly under the influence of genetic and lifestyle circumstances. Intimal calcification is associated with atherosclerosis including endothelial dysfunction, intimal edema, lipid cell formation, and blood cell migration that may cause a plaque rupture, leading to the formation of a thrombus. It is currently associated with chronic arterial inflammation exacerbated by well-characterized risk factors, such as hypertension, diabetes, hypercholesterolemia, obesity, smoking, and a family history of heart disease.
Calcification of the media occurs in the elastic lamina of large-caliber and medium/small-sized arteries. It seems to be independent of atherosclerosis but can coexist with it. This type of calcification was known initially as “Monckeberg sclerosis,” and it has been radiographically described as railroads. It affects the arteries that are less likely to develop atherosclerosis, such as visceral abdominal, thyroid, lung, limb, and femoral arteries, but it is also common in the aorta.
16.2.4 Epidemiology and Pathophysiology of Vascular Calcification
CKD patients exhibit a very high prevalence of vascular calcifications exceeding the percentage observed in the general population of the same age, sex, and region (see Fig. 16.4), leading to cardiovascular disease, decreased life expectancy, and mortality, even in the early phases of CKD. Moreover, calcification of the cardiac valves involves a high risk of cardiovascular dysfunction. A study in patients in CKD stage 5D has shown that vascular calcifications are frequently localized in high-caliber arteries, such as the aorta (around 80%); medium-caliber arteries, including coronary arteries (around 60–70%); and small-caliber arteries (20–30%), reflecting the heterogeneity of the three categories of arteries previously described [34]. Time on hemodialysis has been positively associated with vascular calcification, particularly in medium-caliber arteries. Each year on dialysis increased the risk of developing vascular calcifications by approximately 15% [37]. The mechanisms by which vascular and valvular calcification are produced are complex. It is not a mere precipitation of calcium and phosphate; it involves active and modifiable processes. This regulated process involves several changes, such as a decrease of vascular calcification inhibitors, an increase of vascular calcification promoters (see Fig. 16.5), and the formation of calcification vesicles; the result is the induction of a cellular phenotypic change of vascular smooth muscle cells which are turned into bone-like cells. The outcome is the formation of bone tissue inside the artery wall [38,39,40].
Differences in the prevalence of aortic calcifications in hemodialysis (HD) patients and in a randomly selected general population of the same age and region (Asturias, Spain) (control). p < 0.001 HD patients (N = 92) compared to general population (N = 245). (Adapted with permission of the American Society of Nephrology, from Cannata-Andia et al. [59])
Promoters and inhibitors of vascular calcification. RANKL, receptor activator of nuclear factor-kappa B Ligand; LDL low-density lipoprotein, ALP alkaline phosphatase, Ca calcium, BMP bone morphogenetic proteins, P phosphate, TNF-α tumor necrosis factor-alpha, Vit D3 calcitriol, MGP matrix GLA protein, HDL high-density lipoprotein, OPG osteoprotegerin, OPN osteopontin, FGF23 fibroblast growth factor 23. (Modified with permission of Oxford University Press from Nephrol Dial Transplant. 2011; 26, 3429–3436) [60])
Among the promoters of vascular calcification, high serum phosphorus is considered the most important uremia-related, nontraditional risk factor associated to vascular calcification in CKD patients. Phosphorus is capable of acting as a secondary intracellular messenger, activating several molecular pathways related to bone formation. It reaches the intracellular space via a specific Na-dependent channel called Pit1 and exerts some important actions, such as the blockade of Pit1 which prevents vascular calcification. In vitro experiments have demonstrated that high intracellular phosphorus levels may directly increase Cbfa1, the bone-specific transcription factor, resulting in the activation of several osteogenic pathways and factors, including bone morphogenetic proteins (BMPs) which lead to the phenotypic changes of vascular smooth muscle cells into bone-like cells. Phosphorus also promotes the expression of osteocalcin and ALP in the vasculature.
Among the inhibitors of vascular calcification, pyrophosphates, fetuin-A, osteoprotegerin (OPG), and matrix Gla protein (MGP) are the most studied either in tissue or in serum. In the former, pyrophosphates are located in the vascular matrix to preserve the vascular smooth muscle cells phenotype inhibiting calcium phosphate crystal formation and the change of vascular smooth muscle cells into bone-like cells. In serum, the most abundant inhibitors of vascular calcification are fetuin-A, OPG, and MGP. Fetuin-A, a known inhibitor of osteogenesis, is capable of hampering vascular calcification. OPG holds back osteoclast differentiation, modulating bone resorption through its action as a decoy receptor of RANKL, but it may also act as inhibitor of vascular calcification [41].
Players such as FGF23 and its co-receptor Klotho have been also related with vascular calcification. FGF23 and Klotho knockout mice showed low bone mass and accelerated aging with widespread tissue calcification [42, 43]. The mechanisms by which FGF23/Klotho affects bone health and vascular calcifications may involve phosphorus excretion, vitamin D synthesis, and also PTH regulation. More recently also the microRNA have been implicated in the process of vascular calcification [38, 44] (see Fig. 16.5).
16.2.5 Vascular Calcification and Bone Health
Most of the previously discussed factors, either promoters or inhibitors of the vascular calcification process (see Fig. 16.5), have been related not only with vascular calcification but also with bone health, a fact which suggests there might be several links and common pathways between bone and vascular disorders. Vascular calcification, bone loss, and fragility fractures are very common disorders associated with aging, both in CKD patients and in the general population. Several studies have drawn attention to the fact that apart from aging, there might be other common factors linking vascular calcification and bone health. Even though these factors are not still fully understood, in CKD patients and in animal models, there is evidence that the progression of vascular calcification is directly associated with the reduction of bone mass and an increased risk of fragility fractures. The more severe the vascular calcification, the greater the loss of bone mass [35, 38].
16.2.6 Calciphylaxis
Calciphylaxis, also called calcific uremic arteriolopathy when it affects patients with CKD, is a clinical syndrome characterized by necrotic ulceration of the skin due to calcification of the media, with fibrosis of the arteriolar intima and subsequent cutaneous ischemia due to thrombosis. It usually occurs in patients receiving renal replacement therapy, either dialysis or kidney transplantation, and in patients with deficient glomerular filtration rate. Although abnormalities in mineral bone metabolism seem to be the main cause, other factors can contribute to its pathogenesis. Thus, calciphylaxis can also occur in patients with normal glomerular filtration rate, especially those who are elderly or with a vascular disorder [45].
Irrespectively on their renal function, calciphylaxis patients share certain histologic features (arteriolar calcification that leads to vessel narrowing, ischemia and microthrombosis), that suggest a common final pathway for the disease [46].
Calciphylaxis lesions exhibit two types of manifestations: - The disease may have an insidious onset, in which patients are asymptomatic, although they may experience pruritus and present with cutaneous lamellar erythema. - Alternatively, the disease may have a rapid evolution, with very painful ischemic purpura, in which the pain is disproportionate to the skin lesion and there is subsequent progression to ulceration and skin necrosis. Both types of lesions can occur simultaneously. The first type has a mortality rate of about 30%, and the second type has a mortality rate of approximately 80% [47].
The distribution of skin lesions is heterogeneous, and there are two typical patterns [48]. A distal pattern occurs in approximately 90% of cases. These patients present with lesions in the lower extremities, especially between the ankle and calf, although there are reports of lesions in the fingers, hands and even the genitals. A proximal pattern occurs in approximately 10% of cases. These patients present with lesions in areas with more adipose tissue such as the trunk, inner thighs, buttocks and occasionally the breasts. In addition, both patterns can coexist in the same patient. A small number of patients (<1%) have atypical lesions, in that they appear in unusual locations. In general, the proximal pattern is associated with a worse prognosis.
The pathogenesis of this form of severe vascular calcification is not yet fully understood, and apart from the uncontrolled mineral metabolism, dysregulation of some calcification inhibitors such as fetuin-A, MGP, and vitamin K have been implicated in its pathogenesis.
16.3 Management of CKD–MBD
16.3.1 Management of Biochemical Abnormalities
Even though for didactic reasons we shall describe individually the management of the main mineral and bone disorders, it is important to emphasize that in CKD–MBD, all the parameters need to be evaluated and put together in an integrated manner as they all are very tightly interrelated players [22, 49].
In CKD stages 3–5, there is a clear trend to have a positive phosphate balance; thus, therapeutic strategies aim to avoid the accumulation of phosphorus. The rationale behind the importance of controlling serum phosphorus is based on epidemiological and solid experimental studies which have shown that hyperphosphoremia is an important risk factor, not only for secondary hyperparathyroidism but also for cardiovascular disease and mortality [3]. In addition, despite the lack of evidence from randomized controlled trials that lowering serum phosphorus levels can improve clinical outcomes, most strategies aim to reduce serum phosphorus in CKD in patients with hyperphosphoremia. The approaches to achieve this goal include three levels of action: reduction of dietary phosphorus intake, use of phosphate-binding agents, and increasing phosphorus removal by adding more hours of dialysis.
The control of serum phosphorus through dietary phosphorus restriction merits specific and important comments. The factors affecting gastrointestinal phosphorus absorption include vitamin D levels and phosphorus food content and bioavailability. Currently, the sources of dietary phosphorus are protein-rich foods, which in a non-vegetarian Western diet may represent around 60% of the dietary phosphorus. Foods rich in phosphorus include dairy products, meat, fish, legumes, nuts, and chocolates. In addition, a great amount of phosphorus (e.g., inorganic phosphate) with a high bioavailability are found in food additives and preservatives. The phosphate content of plants is high in phosphorus, but its bioavailability and gastrointestinal absorption is low. The reduction of dietary phosphorus intake has a clear limitation: the need to ensure an adequate protein intake to avoid under nutrition. In fact, a restrictive prescription of dietary phosphorus has been associated with poorer indices of nutritional status, and a stepwise trend toward greater survival with more liberal phosphorus prescription has been postulated.
To obtain a higher removal of phosphorus, increasing the hours of dialysis, either by prescribing prolonged nocturnal dialysis or short daily dialysis, has become a useful approach to control hyperphosphoremia and to reduce serum PTH levels and the dose of phosphate binders prescription in CKD 5D patients. However, despite the progressive use of the two aforementioned useful strategies, still most patients in CKD stage 5D (between 80 and 90%) need the use of phosphate-binding agents to control hyperphosphatemia.
There is recent evidence that lowering serum phosphorus would lead to improved clinical outcomes [50]. As a safe strategy, the recent review of the KDIGO guidelines suggest that serum phosphorus should be maintained as close as possible to the normal range at all stages of CKD [1, 17]. The use of moderate phosphate-restricted diets in combination with phosphate-binding agents has become a reasonable approach to avoid phosphorus accumulation in patients in CKD stages 3–5D. This strategy allows a more liberal diet which leads to a better nutritional status which can positively impact survival, as it has been shown in recent large-scale epidemiological studies.
Several observational studies and clinical trials have shown that all available phosphate-binding agents are effective in reducing serum phosphorus, but to date, the available data do not support a strong superiority of the novel non-calcium-containing phosphate-binding agents on outcomes such as cardiovascular and mortality endpoints. In addition, the great differences in dietary phosphorus intake and availability of phosphate binders around the world, the different economic and social scenarios, and the individual clinical circumstances of each CKD patient are enough reasons to avoid making general and conclusive recommendations for the generalized use of one specific phosphate-binding agent [51, 52].
However, based on the analyses of the individual circumstances of CKD patients, it is reasonable that the choice of the phosphate binder would take into account several aspects, such as the stage of CKD, the presence of other components of CKD–MBD, and any concomitant therapies. In CKD stages 3–5D patients with persistent or recurrent hypercalcemia, arterial calcification, adynamic bone disease, and persistent low serum PTH levels, the use and dose of calcium-based phosphate binders, calcitriol, or other less hypercalcemic and hyperphosphoremic VDRAs should be carefully and individually evaluated. It is recommended to avoid or restrict the long-term use of aluminum-containing phosphate binders to prevent aluminum overload.
Regarding calcium, apart from the limitations already discussed about the use of calcium-based phosphate agents and the need to individualize the therapy, the most appropriate approach for CKD stage 5D patients is to use a dialysate calcium concentration between 1.25 and 1.50 mmol/L (2.5 and 3.0 mEq/L). It is important to stress that the combined use of a high calcium concentration in the dialysate (>1.50 mmol/L) together with calcium-based phosphate binders should be avoided as they would increase the risk of calcium overload in CKD 5D patients.
In the case of CKD stages 3–5 patients not on dialysis, it is suggested that if PTH levels are above the upper normal limit of the assay, they should be first evaluated for hyperphosphoremia, hypocalcemia, and calcidiol deficiency and correct them if they are present. If serum PTH increases progressively and remains persistently above the upper limit despite having corrected the abovementioned factors, treatment with VDRAs could be initiated but the risk of hypercalcemia should be carefully evaluated. There are several VDRAs in the market (see Table 16.5); all of them are effective in PTH suppression even though they may have a differential effect in calcium and phosphorus absorption [53].
The approach is different for CKD stage 5D patients, for whom the KDIGO guidelines suggest to maintain serum PTH levels within the range of approximately two to nine times the upper normal limit for the assay [1]. Accordingly, changes in therapy should be based on the observed trends of changes, and therapy should be initiated or modified to avoid any progression to serum PTH levels outside this range in either direction always evaluating and correcting modifiable factors such as hyperphosphoremia and hypocalcemia, phosphate intake and vitamin D status [20]. This recommendation makes it difficult to be implemented in clinical practice due to the wide range of PTH normality, then the KDOQI ranges (serum PTH levels between 150 and 300 pg/dL) and similar ranges from national guidelines are also currently used in practice [18, 19]. Despite the absence of definitive evidence, several large-scale observational studies released after the CKD–MBD KDIGO guidelines were published have confirmed that in CKD patients, the better outcomes are associated with serum PTH values around 150–300 pg/dL [54, 55].
To reduce PTH in CKD stage 5D patients, the suggested management is the use of calcimimetics and/or VDRAs. The selection of the initial drug for the treatment should be based on serum calcium and phosphorus levels and other aspects of CKD–MBD, such as the presence of vascular calcification. When using calcimimetics, if hypocalcemia is present, they should be reduced by adding VDRAs, if these changes are not enough calcimimetics should be stopped. Likewise, when using VDRAs, if either hyperphosphoremia or hypercalcemia is present, they should be reduced or stopped. The association of both drugs currently renders benefits. All changes in the therapy of secondary hyperparathyroidism should take into account other aspects, signs, symptoms, severity of the disorders, and concomitant medications.
If PTH levels fall and reach the range of low bone turnover, the use of VDRAs and/or calcimimetics should be reduced or stopped. In patients with severe hyperparathyroidism who did not respond with a clinically meaningful reduction of serum PTH levels after following the previous recommendations, parathyroidectomy should be considered. Even though there is no agreement regarding at which serum PTH level a parathyroidectomy should be indicated, the most current practice is to perform any type of parathyroidectomy when patients maintain PTH levels above 800 pg/mL despite an adequate medical treatment. Subtotal parathyroidectomy and total parathyroidectomy with parathyroid implants are the two techniques more currently used.
16.3.2 Osteoporosis
The CKD–MBD constellation also includes the study of bone fragility fractures, which may appear due to high and low bone turnover states but also due to the combination with osteoporosis, an age-dependent and highly prevalent bone disorder whose importance has greatly increased due to aging of the CKD population [20, 26,27,28].
In osteoporosis there is a reduction in bone mass with no specific defect in bone formation. This occurs because the balance between bone formation and bone resorption is lost, favoring the latter. As a result, less new bone is formed to replace bone loss. The DXA definition of osteoporosis and the bone mass criteria followed for its diagnosis were adopted for the first time by the World Health Organization (WHO) in 1993. It stands as “a disease characterized by low bone mineral density and micro architectural deterioration leading to low bone strength and increased risk of fractures.” Strictly speaking, the definition applied only to Caucasian postmenopausal women, and it was conceived to be used for diagnostic purposes, but not for treatment. However, its use progressively expanded to include men and also to help in the treatment decision process. The WHO definition of osteoporosis never included the CKD condition.
The T-score of the DXA measurement is used for the assessment of BMD and for the definition of osteoporosis. Each T-score difference in BMD represents 1 standard deviation (SD) from the peak bone mass. Values up to −1 SD BMD below the mean peak bone mass are considered normal; values between −1 SD and − 2.5 SD BMD are indicative of osteopenia, and values below −2.5 SD BMD are indicative of osteoporosis (see Fig. 16.6) [21]. BMD measurement plays an important diagnostic, preventive, and managerial role in the general population and also in CKD patients [20, 26,27,28, 30].
The WHO osteoporosis diagnostic criteria: T-score values for normality, osteopenia, and osteoporosis using DXA. (Adapted with permission of Società Italiana di Nefrologia, from Cannata-Andia et al. [21])
In addition to the aforementioned limitations in the interpretation of BMD, restrictions exist in the treatment of osteoporosis in CKD patients. Apart from the possibility of using calcium supplements and VDRAs (drugs which are also used in the management of osteoporosis), due to the complexity of CKD–MBD, the use of the currently available antiosteoporotic compounds also presents additional limitations. There are two main reasons for such limitations: the first is the fact that all large-scale, long-term clinical trials carried out to register active antiosteoporotic drugs have specifically excluded patients with low kidney function, particularly CKD stages 4–5; the second reason is the fact that kidneys play a key role in the clearance of some of these compounds (e.g., bisphosphonates and strontium ranelate). Thus, the available evidence comes from the post hoc analysis of the studies, selecting patients with reduced kidney function in whom the drug was administered. In some of these studies, there were enough CKD stage 3 patients, but that was not the case with CKD stages 4–5 patients.
Despite the mentioned limitations [1, 17, 20, 27,28,29,30], CKD stages 1–3 patients should be managed as the general population; CKD stage 3 patients should be individually evaluated taking into account other important biochemical parameters such as PTH values. In CKD stage 3B, since GFR is low (45–30 mL/min), it is necessary to carefully monitor the progression of kidney failure and the serum PTH levels. The prescription of bisphosphonates is still not recommended in patients with GFR <30 mL/min without a strong clinical indication. In general, patients with biochemical anomalies, such as PTH or other serum bone parameter abnormalities, should be managed differently, and the treatment choices should take into account the magnitude and reversibility of those biochemical abnormalities as well as the progression of CKD. Before using any osteoporotic drug, all the abnormalities of Ca, P and PTH should be connected as much as possible. A bone biopsy can be always considered for this type of patients, and a greater caution needs to be taken when considering the use of antiresorptive agents when PTH levels are normal/low or low. In the latter, bisphosphonates is not indicated because the risk of further reduction of bone turnover and bone fragility due its long-term deposition in bone. Denosumab has been the drug more used because it is not cleared by the kidney and its long-term action greatly decreases after 6 months.
16.3.3 Vascular Calcification
Another important aspect of CKD–MBD is to follow strategies to minimize or avoid the progression of vascular calcification. Any strategy designed to reduce the impact of vascular calcification needs to take into account primary prevention measures to control cardiovascular risk factors. It is crucial to promote a healthy lifestyle, a balanced diet, regular physical exercise, smoking cessation, and a low alcohol intake. Once vascular calcifications appear, secondary prevention must aim to reduce their complications, intensifying the measures and treatments previously described. Most strategies to reduce vascular calcifications focus on several risk factors such as hyperphosphoremia, hypercalcemia, secondary hyperparathyroidism, smoking, dyslipidemia, hypertension, diabetes, inflammation, and to stop the use of warfarin [56].
16.3.4 Calciphylaxis
Despite calciphylaxis is an infrequent form of vascular calcification, its management remains a challenge. The treatment of calciphylaxis is based on three complementary levels [57]: proper medical-surgical management of ulcers (analgesia, surgical debridement, antibiotic therapy, hyperbaric oxygen therapy); modification of all possible factors that could precipitate ectopic calcification (adjust or normalize diet, stop use of calcium-based phosphate binders, stop use of vitamin D active metabolites, normalize PTH blood levels, and overall stop use of vitamin K antagonists); use of one or more alternative therapies to inhibit the cutaneous calcification process (sodium thiosulfate, bisphosphonates).
Before You Finish: Practice Pearls for the Clinician
-
The changes in biochemical parameters of CKD–MBD currently begin in CKD stage 3, with important variations among patients. Assessment should start at this stage.
-
The optimal or normal values for each of the serum biochemical markers of CKD–MBD have been obtained from population- or cohort-based studies. A single value can alert, but the diagnosis and management should be guided considering also the trend of changes.
-
In CKD, both high and low bone turnover and osteoporosis are associated with vascular calcification, bone fractures, and increased mortality.
-
Calcium overload should be avoided at all stages of CKD. Excess of calcium and phosphorus are two potent but avoidable promoters of vascular calcification with negative impact in outcomes.
-
In CKD stages 3–5D, there is a clear trend to a positive phosphate balance that needs to be avoided using the available strategies but taking into account that aggressive dietary restriction of phosphorus may lead to undernutrition with possible negative effect on survival.
References
KDIGO clinical practice guideline for the diagnosis. Evaluation, prevention, and treatment of chronic kidney disease-mineral and bone disorder (CKD-MBD). Kidney Int Suppl. 2009;113:S1–130.
Cannata-Andía JB, Martín-Carro B, Martín-Vírgala J, Rodríguez-Carrio J, Bande-Fernández JJ, Alonso-Montes C, et al. Chronic kidney disease-mineral and bone disorders: pathogenesis and management. Calcif Tissue Int. 2020;108:410.
Olgaard K, Salusky I, Silver J, editors. The spectrum of mineral and bone disorders in chronic kidney disease. Oxford: Oxford University Press; 2010.
Centeno PP, Herberger A, Mun HC, Tu C, Nemeth EF, Chang W, et al. Phosphate acts directly on the calcium-sensing receptor to stimulate parathyroid hormone secretion. Nat Commun. 2019;10(1):4693.
Gattineni J, Bates C, Twombley K, Dwarakanath V, Robinson ML, Goetz R, et al. FGF23 decreases renal NaPi-2a and NaPi-2c expression and induces hypophosphatemia in vivo predominantly via FGF receptor 1. Am J Physiol Renal Physiol. 2009;297(2):F282–91.
Tominaga Y, Tanaka Y, Sato K, Nagasaka T, Takagi H. Histopathology, pathophysiology, and indications for surgical treatment of renal hyperparathyroidism. Semin Surg Oncol. 1997;13(2):78–86.
Levin A, Bakris GL, Molitch M, Smulders M, Tian J, Williams LA, et al. Prevalence of abnormal serum vitamin D, PTH, calcium, and phosphorus in patients with chronic kidney disease: results of the study to evaluate early kidney disease. Kidney Int. 2007;71(1):31–8.
Lorenzo V, Torres A, Gonzalez-Posada JM, Pestaña M, Rodriguez A, Diaz-florez L. Prevalencia de las distintas formas histológicas de osteodistrofia renal, con especial referencia a la osteomalacia. Nefrologia. 1986;6(2):25–33.
Moriniere P, Cohen-Solal M, Belbrik S, Boudailliez B, Marie A, Westeel PF, et al. Disappearance of aluminic bone disease in a long term asymptomatic dialysis population restricting A1(OH)3 intake: emergence of an idiopathic adynamic bone disease not related to aluminum. Nephron. 1989;53(2):93–101.
Sherrard DJ, Hercz G, Pei Y, Maloney NA, Greenwood C, Manuel A, et al. The spectrum of bone disease in end-stage renal failure—an evolving disorder. Kidney Int. 1993;43(2):436–42.
Hercz G, Pei Y, Greenwood C, Manuel A, Saiphoo C, Goodman WG, et al. Aplastic osteodystrophy without aluminum: the role of "suppressed" parathyroid function. Kidney Int. 1993;44(4):860–6.
Torres A, Lorenzo V, Hernandez D, Rodriguez JC, Concepcion MT, Rodriguez AP, et al. Bone disease in predialysis, hemodialysis, and CAPD patients: evidence of a better bone response to PTH. Kidney Int. 1995;47(5):1434–42.
Ferreira A, Frazao JM, Monier-Faugere MC, Gil C, Galvao J, Oliveira C, et al. Effects of sevelamer hydrochloride and calcium carbonate on renal osteodystrophy in hemodialysis patients. J Am Soc Nephrol. 2008;19(2):405–12.
Asci G, Ok E, Savas R, Ozkahya M, Duman S, Toz H, et al. The link between bone and coronary calcifications in CKD-5 patients on haemodialysis. Nephrol Dial Transplant. 2011;26(3):1010–5.
Carrillo-Lopez N, Panizo S, Alonso-Montes C, Roman-Garcia P, Rodriguez I, Martinez-Salgado C, et al. Direct inhibition of osteoblastic Wnt pathway by fibroblast growth factor 23 contributes to bone loss in chronic kidney disease. Kidney Int. 2016;90(1):77–89.
Moyses RM, Schiavi SC. Sclerostin, osteocytes, and chronic kidney disease—mineral bone disorder. Semin Dial. 2015;28(6):578–86.
KDIGO. 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;3(1):S1–150.
K/DOQI clinical practice guidelines for chronic kidney disease. Evaluation, classification, and stratification. Am J Kidney Dis. 2002;39(2 Suppl 1):S1–266.
Torregrosa JV, Bover J, Cannata Andia J, Lorenzo V, de Francisco AL, Martinez I, et al. Spanish Society of Nephrology recommendations for controlling mineral and bone disorder in chronic kidney disease patients (S.E.N.-M.B.D.). Nefrologia. 2011;31(Suppl 1):3–32.
KDIGO. 2017 clinical practice guideline update for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease-mineral and bone disorder (CKD-MBD). Kidney Int Suppl. 2017;7(1):1–59.
Cannata-Andia JB, Rodriguez Garcia M, Gomez AC. Osteoporosis and adynamic bone in chronic kidney disease. J Nephrol. 2013;26(1):73–80.
Bover J, Ureña-Torres P, Cozzolino M, Rodríguez-García M, Gómez-Alonso C. The non-invasive diagnosis of bone disorders in CKD. Calcif Tissue Int. 2021;108:512.
Naylor KL, Garg AX, Zou G, Langsetmo L, Leslie WD, Fraser LA, et al. Comparison of fracture risk prediction among individuals with reduced and normal kidney function. Clin J Am Soc Nephrol. 2015;10(4):646–53.
West SL, Lok CE, Langsetmo L, Cheung AM, Szabo E, Pearce D, et al. Bone mineral density predicts fractures in chronic kidney disease. J Bone Miner Res. 2015;30(5):913–9.
Hong N, Kim BJ, Kim CH, Baek KH, Min YK, Kim DY, et al. Low plasma level of leucine-rich repeat-containing 17 (LRRc17) is an independent and additive risk factor for osteoporotic fractures in postmenopausal women. J Bone Miner Res. 2016;31(12):2106–14.
Evenepoel P, Cunningham J, Ferrari S, Haarhaus M, Javaid MK, Lafage-Proust MH, et al. Diagnosis and management of osteoporosis in chronic kidney disease stages 4 to 5D: a call for a shift from nihilism to pragmatism. Osteoporos Int. 2021;32(12):2397–405.
Evenepoel P, Cunningham J, Ferrari S, Haarhaus M, Javaid MK, Lafage-Proust MH, et al. European consensus statement on the diagnosis and management of osteoporosis in chronic kidney disease stages G4-G5D. Nephrol Dial Transplant. 2021;36(1):42–59.
Ketteler M, Block GA, Evenepoel P, Fukagawa M, Herzog CA, McCann L, et al. Executive summary of the 2017 KDIGO chronic kidney disease-mineral and bone disorder (CKD-MBD) guideline update: what's changed and why it matters. Kidney Int. 2017;92(1):26–36.
Pimentel A, Ureña-Torres P, Bover J, Luis Fernandez-Martín J, Cohen-Solal M. Bone fragility fractures in CKD patients. Calcif Tissue Int. 2020;108:539.
Jørgensen HS, David K, Salam S, Evenepoel P. Traditional and non-traditional risk factors for osteoporosis in CKD. Calcif Tissue Int. 2021;108(4):496–511.
Fusaro M, Cianciolo G, Evenepoel P, Schurgers L, Plebani M. Vitamin K in CKD bone disorders. Calcif Tissue Int. 2021;108:476.
Mazzaferro S, Bagordo D, De Martini N, Pasquali M, Rotondi S, Tartaglione L, et al. Inflammation, oxidative stress, and bone in chronic kidney disease in the Osteoimmunology era. Calcif Tissue Int. 2021;1-9:452.
Adragao T, Ferreira A, Frazao JM, Papoila AL, Pinto I, Monier-Faugere MC, et al. Higher mineralized bone volume is associated with a lower plain X-ray vascular calcification score in hemodialysis patients. PloS One. 2017;12(7):e0179868.
Rodriguez Garcia M, Naves Diaz M, Cannata Andia JB. Bone metabolism, vascular calcifications and mortality: associations beyond mere coincidence. J Nephrol. 2005;18(4):458–63.
Roman-Garcia P, Rodriguez-Garcia M, Cabezas-Rodriguez I, Lopez-Ongil S, Diaz-Lopez B, Cannata-Andia JB. Vascular calcification in patients with chronic kidney disease: types, clinical impact and pathogenesis. Med Princ Pract. 2011;20(3):203–12.
Ketteler M, Floege J. Clinical management of vascular and soft tissue calcifications in chronic kidney disease patients. In: Olgaard K, Salusky I, Silver J, editors. The spectrum of mineral and bone disorders in chronic kidney disease. Oxford: Oxford University Press; 2010. p. 317–31.
Rodriguez-Garcia M, Gomez-Alonso C, Naves-Diaz M, Diaz-Lopez JB, Diaz-Corte C, Cannata-Andia JB. Vascular calcifications, vertebral fractures and mortality in haemodialysis patients. Nephrol Dial Transplant. 2009;24(1):239–46.
Cannata-Andía JB, Carrillo-López N, Messina OD, Hamdy NAT, Panizo S, Ferrari SL, et al. Pathophysiology of vascular calcification and bone loss: linked disorders of ageing? Nutrients. 2021;13(11):3835.
Lee SJ, Lee IK, Jeon JH. Vascular calcification-new insights into its mechanism. Int J Mol Sci. 2020;21(8):2685.
Zununi Vahed S, Mostafavi S, Hosseiniyan Khatibi SM, Shoja MM, Ardalan M. Vascular calcification: an important understanding in nephrology. Vasc Health Risk Manag. 2020;16:167–80.
Carrillo-López N, Martínez-Arias L, Fernández-Villabrille S, Ruiz-Torres MP, Dusso A, Cannata-Andía JB, et al. Role of the RANK/RANKL/OPG and Wnt/β-catenin systems in CKD bone and cardiovascular disorders. Calcif Tissue Int. 2021;108(4):439–51.
Hanazaki A, Ikuta K, Sasaki S, Sasaki S, Koike M, Tanifuji K, et al. Role of sodium-dependent pi transporter/Npt2c on pi homeostasis in klotho knockout mice different properties between juvenile and adult stages. Physiol Rep. 2020;8(3):e14324.
Kuro-o M. Klotho and aging. Biochim Biophys Acta. 2009;1790(10):1049–58.
Panizo S, Naves-Diaz M, Carrillo-Lopez N, Martinez-Arias L, Fernandez-Martin JL, Ruiz-Torres MP, et al. MicroRNAs 29b, 133b, and 211 regulate vascular smooth muscle calcification mediated by high phosphorus. J Am Soc Nephrol. 2016;27(3):824–34.
Brandenburg VM, Evenepoel P, Floege J, Goldsmith D, Kramann R, Massy Z, et al. Lack of evidence does not justify neglect: how can we address unmet medical needs in calciphylaxis? Nephrol Dial Transplant. 2016;31(8):1211–9.
Chen TY, Lehman JS, Gibson LE, Lohse CM, El-Azhary RA. Histopathology of Calciphylaxis: cohort study with clinical correlations. Am J Dermatopathol. 2017;39(11):795–802.
Weenig RH. Pathogenesis of calciphylaxis: Hans Selye to nuclear factor kappa-B. J Am Acad Dermatol. 2008;58(3):458–71.
Hayashi M. Calciphylaxis: diagnosis and clinical features. Clin Exp Nephrol. 2013;17(4):498–503.
Komaba H, Ketteler M, Cunningham J, Fukagawa M. Old and new drugs for the Management of Bone Disorders in CKD. Calcif Tissue Int. 2021;108:486.
Fernandez-Martin JL, Martinez-Camblor P, Dionisi MP, Floege J, Ketteler M, London G, et al. Improvement of mineral and bone metabolism markers is associated with better survival in haemodialysis patients: the COSMOS study. Nephrol Dial Transplant. 2015;30(9):1542–51.
Jamal SA, Vandermeer B, Raggi P, Mendelssohn DC, Chatterley T, Dorgan M, et al. Effect of calcium-based versus non-calcium-based phosphate binders on mortality in patients with chronic kidney disease: an updated systematic review and meta-analysis. Lancet. 2013;382:1268.
Cannata-Andia JB, Fernandez-Martin JL, Locatelli F, London G, Gorriz JL, Floege J, et al. Use of phosphate-binding agents is associated with a lower risk of mortality. Kidney Int. 2013;84:998.
Lund RJ, Andress DL, Amdahl M, Williams LA, Heaney RP. Differential effects of paricalcitol and calcitriol on intestinal calcium absorption in hemodialysis patients. Am J Nephrol. 2010;31(2):165–70.
Naves-Diaz M, Passlick-Deetjen J, Guinsburg A, Marelli C, Fernandez-Martin JL, Rodriguez-Puyol D, et al. Calcium, phosphorus, PTH and death rates in a large sample of dialysis patients from Latin America. The CORES Study. Nephrol Dial Transplant. 2011;26(6):1938–47.
Floege J, Kim J, Ireland E, Chazot C, Drueke T, de Francisco A, et al. Serum iPTH, calcium and phosphate, and the risk of mortality in a European haemodialysis population. Nephrol Dial Transplant. 2011;26(6):1948–55.
Singh A, Tandon S, Tandon C. An update on vascular calcification and potential therapeutics. Mol Biol Rep. 2021;48(1):887–96.
Cucchiari D, Torregrosa JV. Calciphylaxis in patients with chronic kidney disease: a disease which is still bewildering and potentially fatal. Nefrologia (Engl Ed). 2018;38(6):579–86.
Fukagawa M, Yokoyama K, Koiwa F, Taniguchi M, Shoji T, Kazama JJ, et al. Clinical practice guideline for the management of chronic kidney disease-mineral and bone disorder. Ther Apher Dial. 2013;17(3):247–88.
Cannata-Andia JB, Rodriguez-Garcia M, Carrillo-Lopez N, Naves-Diaz M, Diaz-Lopez B. Vascular calcifications: pathogenesis, management, and impact on clinical outcomes. J Am Soc Nephrol. 2006;17(12 Suppl 3):S267–73.
Cannata-Andia JB, Roman-Garcia P, Hruska K. The connections between vascular calcification and bone health. Nephrol Dial Transplant. 2011;26(11):3429–36.
Acknowledgments
The authors wish to thank the support of Instituto de Salud Carlos III (ISCIII) through the project PI19/00532 (Co-funded by European Regional Development Fund/European Social Fund “A way to make Europe”/“Investing in your future”), the ISCIII Retic REDinREN (RD16/0009/0017) and RICORS2040 (Kidney Disease; RD21/0005/0019), Plan de Ciencia, Tecnología e Innovación 2013-2017 y 2018-2022 del Principado de Asturias (GRUPIN14-028, IDI-2018-000152, IDI-2021-000080), Fundación Renal Iñigo Álvarez de Toledo (FRIAT). NC-L was supported by IDI-2018-000152 and IDI-2021-000080.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Cannata-Andía, J.B., Carrillo-López, N., Rodriguez-García, M., Torregrosa, JV. (2023). Mineral and Bone Disorders in Chronic Kidney Disease. In: Arıcı, M. (eds) Management of Chronic Kidney Disease. Springer, Cham. https://doi.org/10.1007/978-3-031-42045-0_16
Download citation
DOI: https://doi.org/10.1007/978-3-031-42045-0_16
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-42044-3
Online ISBN: 978-3-031-42045-0
eBook Packages: MedicineMedicine (R0)