Abstract
The recent viral disease COVID-19 has attracted much attention. The disease is caused by SARS-CoV-19 virus which has different variants and mutations. The mortality rate of SARS-CoV-19 is high and efforts to establish proper therapeutic solutions are still ongoing. Inflammation plays a substantial part in the pathogenesis of this disease causing mainly lung tissue destruction and eventually death. Therefore, anti-inflammatory drugs or treatments that can inhibit inflammation are important options. Various inflammatory pathways such as nuclear factor Kappa B (NF-κB), signal transducer of activators of transcription (STAT), nod-like receptor family protein 3 (NLRP), toll-like receptors (TLRs), mitogen-activated protein kinase (MAPK), and mammalian target of rapamycin (mTOR) pathways and mediators, such as interleukin (IL)-6, IL-1β, tumor necrosis factor-α (TNF-α), and interferon-γ (INF-γ), cause cell apoptosis, reduce respiratory capacity and oxygen supply, eventually inducing respiratory system failure and death. Statins are well known for controlling hypercholesterolemia and may serve to treat COVID-19 due to their pleiotropic effects among which are anti-inflammatory in nature. In this chapter, the anti-inflammatory effects of statins and their possible beneficial effects in COVID-19 treatment are discussed. Data were collected from experimental and clinical studies in English (1998–October 2022) from Google Scholar, PubMed, Scopus, and the Cochrane Library.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
The global outburst of infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), a strain of coronavirus that causes COVID-19 (coronavirus disease 2019), began in China. Global high mortality, constant mutations, lack of knowledge about the nature of the virus, and uncertain treatment options made the disease a worldwide concern. SARS-CoV-2 acts via angiotensin-converting enzyme 2 (ACE2) and transmembrane protease serine 2 (TMPRSS2) expressing epithelial cell receptors leading to extensive synthesis and release of inflammatory agents inducing immune cells and acute respiratory distress syndrome (ARDS) (Fig. 25.1) [1]. The increased rate of mortality in COVID-19 patients is attributed to immune dysregulation resulting in a cytokine storm. This results from over-activation of complex inflammatory networks interconnecting different cells, signaling pathways, and cytokines [2]. Activation of nuclear factor kappa light chain enhancer of activated B cells (NF-κB), signal transducer and activator of transcription 3 (STAT3), Janus kinase (JAK), protein kinase B (AKT), mammalian target of rapamycin (mTOR) signaling pathways causes elevated levels of pro-inflammatory cytokines such as interleukin (IL)-1, IL-6, IL-18, IL-33, IL-37, IL-1β, tumor necrosis factor-alpha (TNF-α), and interferon-gamma (INF-γ) in COVID-19 patients, while anti-inflammatory cytokines i.e. IL-10 are downregulated by NF-κB [3, 4]. These mediators cause deleterious effects on respiratory, cardiovascular, and digestive system. For COVID-19 prevention and treatment vaccines, immune-based treatments and drugs are used.
For instance, remdesivir, an anti-viral drug, is prescribed for COVID-19 patients with respiratory symptoms leading to a faster recovery. Hydroxychloroquine was found to prevent viral replication in SARS-CoV and was used in Middle East respiratory syndrome coronavirus (MERS-CoV) patients a decade ago [5]. Lopinavir/ritonavir was used as an anti-viral agent, and corticosteroids, such as dexamethasone, methylprednisolone, are recommended for their anti-inflammatory properties. Tocilizumab is used in patients with ARDS, and it reduces elevated levels of IL-6. Besides chemical medicines, herbal medicines such as curcumin and quercetin are also used as a complementary treatment to decrease COVID-19 symptoms by suppressing inflammatory signaling pathways and mediators [6].
Statins have been used for more than three decades as drugs of choice in preventing cardiovascular disease, both in terms of efficiently decreasing plasma low-density lipoproteins cholesterol (LDL-C) and due to their cost-effectiveness. Besides their LDL-C lowering effects, statins have different pleiotropic properties, such as their anti-inflammatory and immunomodulatory effects, which are beneficial in managing inflammatory conditions [7,8,9,10,11,12,13,14,15,16,17]. Statins are either fungal derivatives (e.g., lovastatin, mevastatin, pravastatin, pitavastatin, and simvastatin) or they are synthetic drugs (e.g., atorvastatin, fluvastatin, and rosuvastatin) [7]. Here, we present a detailed review of the possible use of statins in treating COVID-19 patients. The relevant anti-inflammatory properties of these drugs are discussed in detail.
2 Search Methods
Data were collected from experimental and clinical studies published in English between 1998 and October 2022, from Google Scholar, PubMed, Scopus, and the Cochrane library. Search terms were as follows “SARS-CoV-19” or “COVID-19” and “Statins” and “Cytokine storm” or “Inflammation” and “Novel therapeutic approach.”
3 SARS-CoV-2 and COVID-19
3.1 Biology
Coronaviruses are an extremely diverse group of ribonucleic acid (RNA) viruses which cause diseases in mammalian and avian species. They are composed of a positive-sense, single-stranded RNA (+ssRNA) genome varying from 26.4 to 31.7 kilobases. The genome has a 5′ methylated cap and a 3′ polyadenylated tail [18]. The large genome enables this family of viruses to adapt and modify achieving better virulence [19]. Coronaviruses such as SARS-CoV, MERS-CoV, and SARS-CoV-2 can cause several life-threatening infections [20]. SARS-CoV-2 is the coronavirus strain which has caused the ongoing COVID-19 pandemic.
3.2 Structure
Coronavirus virions consist of the RNA genome, helical nucleocapsid, and the viral membrane containing spike protein, membrane protein, and envelope protein [21]. All coronaviruses share a similar structure. The first two-thirds of the genome are open reading frames (ORFs) 1a and 1b encoding 16 nonstructural proteins [18]. The structural proteins, such as spike (S), envelope (E), membrane (M), and nucleocapsid (N), are encoded by the later reading frames [22]. Coronaviruses differ in the number and function of accessory proteins. The reading frames between the nonstructural and structural proteins encode the accessory proteins.
The S protein controls the virus activity and virulence and different accessory proteins that attack the host immune functions [23, 24]. The S protein is composed of S1 and S2 subunits. The S1 subunit has a receptor-binding domain (RBD) that binds with the receptor-binding motif (RBM) to the host surface. S2 subunit mediates receptor attachment and the host membrane fusion [25, 26]. The primary host receptor for SARS-CoV and SARS-CoV-2 is angiotensin-converting enzyme 2 (ACE2), while for MERS-CoV this is dipeptidyl peptidase 4 (DPP4) [27,28,29,30].
Coronaviruses are large with an average diameter of 80–120 nm and molecular mass of 40,000 kDa. They are roughly spherical and relatively pleiomorphic viruses with surface spikes [31]. Their RNA genome is situated in the center of the virus and protected by the N and M proteins and lipid bilayer envelope [32, 33]. The S protein is crucial for interaction with the host cell. In addition to S protein, the viral surface also has hemagglutinin-esterase dimer (HE), which is not necessary for replication but is important for viral entry [34, 35]. The E protein is a minor structural protein and is different in different coronaviruses [36]. The M protein is the primary structural protein and shapes the envelope [37]. The N protein is tied to the RNA and enables the virus to take over the host cells [38, 39]. The genome of coronaviruses contains various ORFs. The gene order in all members is 5′-leader-UTR-replicase (ORF1ab)-S-E-M-N-3′UTR-poly (A) tail [40]. Their genomes seem to have a bias against cytosine (C) and guanine (G) nucleotides, with the highest composition of uracil (U) and adenosine (A) [41]. In addition to these components, 16 nonstructural proteins (NSP1 to NSP16) differ between different groups of coronaviruses [18]. These NSPs have important roles in assembling the replication–transcription complex, RNA polymerization, RNA proofreading, mRNA capping, allosteric activation, and repression of the host immune system [42, 43].
To enter the cells, the S protein anchors the virus to ACE2 receptors which are expressed on surface. Transmembrane protease serine 2 (TMPRSS2) and lysosomal proteases also have a significant role in enabling SARS-CoV-2 entry into the cells [44]. After entering into the cytoplasm, the virus induces spatial alteration in the endosome resulting in its uncoating. Finally, the viral genome is released within the cytoplasm and the RTC initiates [45]. A unique characteristic of SARS-CoV-2 among the coronaviruses is the integration of furin-mediated cleavage of the S protein at the polybasic site that amplifies its virulence. It has been proposed that this site in SARS-CoV-2 S protein is necessary to enable the virus to infect humans as well as animals [46].
3.3 Variants
Coronaviruses are members of sub-family of Orthocoronavirinae in the family Coronaviridae order Nidovirales and realm Riboviria [47, 48]. Based on the latest International Committee of Taxonomy of Viruses (ICTV) classification, coronaviruses are sorted into four genera: Alphacoronavirus, Betacoronavirus, Gammacoronavirus, and Deltacoronavirus. However, the number of species is large and many coronaviruses are unspecified [47, 49]. The Alphacoronavirus and Betacoronavirus infect only mammalian species, while Gammacoronavirus and Deltacoronavirus infect mammalian and avian species. Coronavirus infection mostly causes respiratory, gastrointestinal, and neurologic disorders [50, 51]. Several variants of concern have been recognized so far [52, 53]. These include: (1) the Alpha (B.1.1.7) variant which was first detected in the United Kingdom in September 2020; (2) Beta (B.1.351) which appeared originally in South Africa in May 2020; (3) Gamma (P.1, B.1.1.28.1) which arose in Brazil in November 2020; (4) Delta (B.1.617.2) which appeared as multiple forms in India in October 2020; and (5) the highly infectious Omicron (B.1.1.529) which arose in Botswana and South Africa in November 2021 and has since given rise to multiple sub-variants (BA.1–BA.5).
4 Pathogenesis of COVID-19: The Role of Inflammation
COVID-19 has often severe respiratory and gastrointestinal manifestations. In addition, extensive hyperinflammatory responses and inflammatory cytokine release have been reported in different organs. COVID-19 disease activates several inflammatory pathways, leading to immune system imbalance and impairment in the renin-angiotensin system (RAS), thus reducing expression of ACE2 and induction of the “cytokine storm.” Extensive cytokine (i.e., TNF-α, IL-1β, IL-2R, IL-6, IFN)-γ and chemokine (i.e., C-motif chemokine ligands; CCL-2, CCL-3, CCL-10) release exacerbates the systemic inflammation and worsens patient prognoses. Also, ACE2 downregulation stimulates angiotensin II receptor1 (AT1R), leading to more severe disease [44, 54]. Molecular analyses have demonstrated the involvement of multiple signaling pathways in this inflammatory response, including IL-6-Janus kinase (JAK)-signal transducer and activator of transcription (STAT) pathway, TNF-α-nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) pathway, and toll-like receptor (TLR)-myeloid differentiation primary response 88 (MYD88)-NF-кB pathway. TNF-α is one of the main pro-inflammatory cytokines that plays a significant role in initiating and propagating the inflammatory signaling transduction. TNF-α activates IL-6 and contributes to activation of the JAK-STAT kinase pathway. TNF-α also stimulates NF-κB signaling. Simultaneously, toll-like receptors (TLRs) and IFN-γ also actively participate in stimulating the inflammatory response. TLRs trigger myeloid differentiation primary response 88 (MYD88) overexpression and activates NF-κB. Furthermore, IFN-γ stimulates JAK-STAT signaling [55, 56]. Activation of these inflammatory pathways can cause acute lung injury, ARDS, thrombosis, organ failure, and an increased morbidity and mortality [55, 57]. Therefore, as mentioned earlier, treatment with medications which have anti-inflammatory effects which suppress these signaling pathways can result in favorable outcomes of COVID-19 and/or decrease mortality.
5 Statins
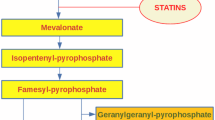
Statins are potent inhibitors of cholesterol synthesis and the use of these compounds has revolutionized the treatment of hypercholesterolemia [58]. Cholesterol is synthesized from acetyl coenzyme A, in a mechanism that occurs over 30-steps in which the rate-limiting step is modulated by 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase. This enzyme transforms HMG into mevalonate [59] and statins are competitive, reversible inhibitors of HMG-CoA reductase in the mevalonate pathway [60]. This inhibition results in the lowering of plasma LDL-C concentrations, which is a beneficial effect [61]. The statins family includes atorvastatin, fluvastatin, lovastatin, pitavastatin, pravastatin, rosuvastatin, and simvastatin. Regarding the origin, simvastatin, lovastatin, and pravastatin are extracted during fungal fermentation, while atorvastatin, fluvastatin, and cerivastatin are chemically synthesized. Lovastatin is produced from Aspergillus terreus strains, and simvastatin is a semisynthetic derivative of lovastatin [62]. Rosuvastatin has been synthesized more recently and is more potent than the older statins.
Pravastatin and rosuvastatin are less lipophilic and more hydrophilic in comparison with the other members of the statin family, while atorvastatin, fluvastatin, lovastatin, and simvastatin are more lipophilic. This property is important since lipophilic drugs have greater ability to diffuse into cell membranes, including those of hepatic cells, and water solubility is important for diminishing cytochrome P450 enzyme metabolism. Bioavailability is also an important pharmacological variable. Fluvastatin has a 24% bioavailability, while that of rosuvastatin is 20%, pravastatin 17%, atorvastatin 14%, and simvastatin less than 5% [63]. Regarding elimination half-life, rosuvastatin with 20 h, and atorvastatin with 14 h have a highly prolonged profiles. The elimination half-life of simvastatin, pravastatin, and fluvastatin are 1–2 h. The plasma half-life indicates their first-pass metabolism [64]. Lovastatin, simvastatin, and atorvastatin are metabolized by cytochrome P 450 3A4, while fluvastatin metabolism depends upon CYP2C9. Pravastatin is not significantly metabolized by the CYP family of enzymes [65]. Statins have many pleiotropic effects including modulation of anti-inflammatory responses (Fig. 25.1) [66] and antioxidant pathways [67, 68]. Therefore, statins have additional benefits besides their effects on serum lipoproteins [69].
Statins also change the function of platelets thereby significantly affecting atherosclerosis and thrombosis [70]. Vascular endothelial function is enhanced mostly by the increase of nitric oxide (NO) [71]. Statins can also play a crucial neuroprotective role in neurodegenerative disorders including Parkinson’s disease, Alzheimer’s disease, multiple sclerosis, and ischemic stroke, due to their anti-inflammatory, anti-oxidative, and anti-excitotoxic properties [72]. The most known adverse effects of statins concern those regarding muscle and liver tissue [73]. Muscle pain, fatigue and weakness, as well as rare rhabdomyolysis, are most common side effects related to statins, particularly if they are applied in high doses [74]. For example, myopathy can occur in 1–2000 patients and abnormalities in liver are seen in 1–2% of patients per year. However, these effects are mostly reversible and cease when the drug is reduced or stopped [75].
5.1 Anti-Inflammatory Effects of Statins
Experimental and clinical trials have showed that statins provide cardiovascular benefits beyond their lipid-lowering effects. These effects include (1) improvement of endothelial function; (2) modulation of inflammation and oxidative stress; (3) increasing plaque stability; and (4) inhibition of the thrombogenesis response [76,77,78,79,80]. These properties of statins are caused by intracellular isoprenoid inhibition and modulation of the reductive-oxidative (REDOX) state and nitric oxide pathway that eventually drive reduced levels of C-reactive protein (CRP) and pro-inflammatory cytokines (Fig. 25.1) [81, 82]. Moreover, statins can intensify ACE2 expression and suppress the TLR-MYD88-NF-кB pathway [83]. On the other hand, statin discontinuation in patients with coronary heart disease can cause adverse cardiovascular events, even without changes of lipid levels [84, 85]. Because of their anti-viral, immunomodulatory, anti-thrombotic, and anti-inflammatory effects, statins may have beneficial roles as adjuvant therapy in COVID-19. MYD88 is one of the host genes stimulated by SARS-CoV-2 infection. Stimulation of MYD88 triggers the NF-кB signaling transduction, reduces IFNs, and amplifies inflammation. Moreover, oxidized LDLs bind to TLR receptors and initiate inflammation via the TLR-MYD88-NF-кB pathway, eventually increasing inflammatory cytokine levels [86, 87]. Statins may exert their protective effects by maintaining the regular activity of the MYD88 pathway and its subsequent downstream products. This effect might be beneficial against COVID-19 by suppressing the beginning of the inflammatory cascade and subsequent release of inflammatory cytokines [88,89,90,91].
Administration of lovastatin (20 and 40 mg/day) in 284 intensive care unit (ICU) patients significantly decreased IL-6, IL-8, and CRP levels. The results of this study also showed that the hospitalization duration was reduced in patients who received lovastatin in comparison with control patients [92]. However, studies which evaluated the effect of pravastatin in COVID-19 patients did not find any significant improvements in prognosis. Although decreased mortality rates in patients who received simvastatin and atorvastatin were reported, patients who were treated with pravastatin and rosuvastatin did not show such an improvement [93].
The potential therapeutic effects of fluvastatin against SARS-CoV-2 infection have been studied in vitro and ex vivo. Fluvastatin at a concentration of 5 μM significantly reduced viral proteins, viral replication, and viral protein translation in human lung cells. The outcomes also suggested a slight inhibitory activity of lovastatin, pravastatin, and rosuvastatin in infected human lung cells but this effect was not as potent as that caused by fluvastatin [94]. A retrospective study of 87 COVID-19 patients admitted to ICU showed that atorvastatin treatment caused slower progression of the disease and a slower progression to death but, given the observational nature of this study, these results should be interpreted with caution [95]. Another retrospective cohort study which enrolled 421 confirmed cases of hospitalized COVID-19 patients showed that treatment with atorvastatin was associated with reduced mortality and lower endotracheal intubation rates [96]. A double-blind, randomized clinical trial also showed that adjunct therapy with atorvastatin was more effective in hospitalized COVID-19 patients compared to the standard antiviral (lopinavir/ritonavir) treatment alone [97]. In contrast, a similar randomized control trial that compared the effect of atorvastatin (20 mg/day) versus placebo in 605 patients failed to confirm any significant beneficial effects of atorvastatin therapy [98], and another randomized clinical trial reported that addition of atorvastatin (20 mg/day) to the standard treatment (hydroxychloroquine + lopinavir/ritonavir) was associated with adverse outcomes in hospitalized COVID-19 patients [99]. This discrepancy in clinical trial results may be due to variations in the standard treatment of COVID-19, duration of treatment, or even the clinical stage of the disease. Another explanation might be that the effects of statins may be restricted to the early phases of inflammatory responses in COVID-19 [98]. Finally, an in silico molecular docking study which evaluated the interactions between statins (lovastatin, fluvastatin, pravastatin, atorvastatin, simvastatin, rosuvastatin, and pitavastatin) and the SARS-CoV-2 main protease (Mpro) suggested that statins may act as inhibitors of this enzyme. However, additional confirmations from experimental studies are needed concerning this issue [100].
6 Statins in COVID-19: New Possibility for COVID-19 Treatment
Several pieces of evidence support the anti-inflammatory effect of statins (Fig. 25.1) [101]. It is also known that statins have anti-viral [102], anti-inflammatory, and antithrombotic characteristics, suggesting their potential use as complementary drugs in COVID-19 therapeutics [90, 91, 103, 104]. Furthermore, statins have effects on reducing viral transmission by effects on cellular membranes [105].
6.1 Clinical Evidence
Retrospective cohort study, including patients who were hospitalized with confirmed diagnosis of severe COVID-19. Baseline characteristics and related clinical data of patients were recorded. Clinical outcomes consist of in-hospital mortality, need for invasive mechanical ventilation, and hospital length of stay. COX regression analysis models were used to assess the association of independent factors to outcomes. Atorvastatin was administered for 421 of 991 patients. The mean age was 61.640 ± 17.003 years. Older age, higher prevalence of hypertension, and coronary artery disease reported in patients who received atorvastatin. These patients have shorter hospital length of stay (P = .001). Based on COX proportional hazard model, in-hospital use of atorvastatin was associated with decrease in mortality (HR = 0.679, P = .005) and lower need for invasive mechanical ventilation (HR = 0.602, P = .014). Atorvastatin add-on therapy in patient with severe COVID-19 was associated with lower in-hospital mortality and reduced the risk of need for invasive mechanical ventilation which supports to continue the prescription of the medication (Table 25.1) [96].
Atorvastatin is one of the most commonly used statins in treatment of hypercholesterolemia. Many studies have confirmed its pleiotropic effect on inflammation. A double-blind, parallel group, randomized clinical trial by Davoodi et al. analyzing the outcomes of atorvastatin treatment on COVID-19 patients. Forty patients were included in the study, and they were divided into two groups. Half of the patients received lopinavir/ritonavir (400/100 mg twice daily) and were the control group while the rest were treated with lopinavir/ritonavir (400/100 mg twice daily) + atorvastatin (40 mg daily) for 5 days. The hospitalization rate was shorter in the group treated additionally with atorvastatin (9.75 ± 2.29 vs. 7.95 ± 2.04 days; p = 0.012) and invasive mechanical ventilation was mandatory only for one patient in the lopinavir/ritonavir group. In addition, the CRP level was decreased, and O2 saturation (O2sat) increased significantly on the sixth day in comparison with the first day in the atorvastatin group. In the control group, the O2 sat was not changed while CRP was increased (Table 25.1) [97].
Another study carried out by Karampoor et al. analyzed the anti-inflammatory effect of lovastatin on COVID-19 patients. The case control study included 284 ICU patients who were randomized into three different groups: (1) 92 patients received no lovastatin; (2) 99 patients were treated with 20 mg lovastatin per day; and (3) 93 patients received 40 mg lovastatin per day for 1 week. The results showed that CRP, IL-6, and IL-8 biomarkers were decreased in patients who received lovastatin in comparison with the control group and the decrease of IL-6 and IL-8 was dose-dependent. Also, IL-6 showed a greater decrease in the group who received 40 mg/day lovastatin than in those who received 20 mg/day. Moreover, IL-8 was higher in the control group than in the two intervention groups (p < 0.05). Finally, duration of hospitalization was significantly shorter in lovastatin-treated patients (p < 0.05) and the mortality rate was reduced although this effect was not significant (Table 25.1) [92].
Other studies have reported minimal or no effects of statin treatment on COVID-19 outcomes. A randomized controlled trial was done to evaluate the effect of atorvastatin on COVID-19 patients. Out of 587 patients suffering from COVID-19, 290 were assigned to be treated with atorvastatin, and 297 received placebo. Atorvastatin was administered orally (20 mg) or by a naso- or oro-gastric route to those patients who were mechanically ventilated and unable to take the drug orally. The study lasted for 30 days from randomization until the primary efficacy outcome was observed (a composite of venous or arterial thrombosis, treatment with extracorporeal membrane oxygenation, or all-cause mortality within 30 days from randomization). The primary outcome occurred in 33% patients assigned to atorvastatin and 36% assigned to placebo after 30 days follow-up (odds ratio 0.84, 95% confidence interval 0.58–1.21, p = 0.35). The median duration of ICU hospitalization was 5 days (interquartile range 3–9 days) in the atorvastatin group and 5 days (2–10) in the control group. No significant difference was found between the two groups concerning atrial fibrillation, venous thromboembolism, and arterial thrombosis. Liver enzyme levels were increased in five atorvastatin-treated patients and in six placebo-treated patients (odds ratio 0.85, 95% confidence interval 0.25–2.81; p = 0.79) while venous thromboembolism occurred in six patients in the atorvastatin group and nine in the placebo group (odds ratio 0.71, 95% confidence interval 0.24–2.06) but myopathy was not clinically diagnosed in either group and the treatment was safe (Table 25.1) [106].
The effect of rosuvastatin plus colchicine, emtricitabine/tenofovir, and combinations of these were evaluated in 633 COVID-19 patients in a randomized, open parallel group multi-center-controlled trial. The patients received either: (1) usual care (n = 162; control group); (2) emtricitabine + tenofovir + colchicine + rosuvastatin (n = 163); (3) colchicine + rosuvastatin (n = 161); or (4) emtricitabine + tenofovir (n = 163). The results showed that need for invasive mechanical ventilation and 28-day mortality was significantly lower in the emtricitabine + tenofovir + colchicine + rosuvastatin group than in the standard care group. The results supported the idea that combination therapy with anti-viral and anti-inflammatory drugs can be useful in decreasing the damage in COVID-19 disease and over-activation of the innate immune system (Table 25.1) [107].
It should be stressed again that statin use can have adverse effects on muscle tissues and cause elevations in the levels of creatine kinase, liver enzymes, and serum glucose levels, all of which may already be elevated in severe COVID-19 disease. Some authors have also raised concerns as to whether statins might interfere with response to COVID-19 vaccines, although there has been no evidence shown thus far to confirm this. Also, concomitant administration of statins and some antiviral therapeutics might exacerbate the risk of adverse effects of statins because most statins are metabolized mainly through CYP3A4, and this CYP enzyme is potently inhibited by the antiviral drug Paxlovid [110,111,112,111].
6.2 In Vivo/In Vitro Evidence
An in vivo study testing the effects of statin administration were performed on K18-hACE2-transgenic mice infected with either a medium (mock) control or a 105 tissue culture infective dose of SARS-CoV-2 gamma strain [112]. In the mice that received 20 mg/kg of simvastatin as pre-treatment and throughout the study, the functional capillary density was higher and adhesion of leukocytes to inflamed endothelium lower than in control mice. In addition, there was a lower number of viral genome copies in the lungs, and less edema, tissue hemorrhage, inflammation, and oxidation in the simvastatin-treated compared to the control animals. In addition, both pre-treatment and post-treatment with 10 μM of simvastatin prevented monocyte death induced by SARS-CoV-2 infection. However, this effect was greater in the pre- compared to the post-protocol suggesting that the simvastatin treatment may be more effective if administered in the early stages of viral infection. At the molecular biomarker level, intracellular adhesion molecule-1 (ICAM-1) and integrin alpha M mRNA levels were decreased, and there were lower levels of inflammatory biomarkers such as TNF-α, IL-6, monocyte chemoattractant protein 1 (MCP1), IFN-α, chemokine (C-C motif) ligand 5 CCL5, and chemokine (C-X-C motif) ligand 1 (Table 25.2).
In an in vitro study on human lung microvascular endothelial cells, Qian et al. showed that administration of the SARS-CoV-2 N protein led to activation of the NF-kB and MAPK signaling pathways, with increased expression of cellular adhesion and inflammatory molecules [113]. They also found that simvastatin treatment blocked this endothelial activation in a dose-dependent manner, suggesting that this compound might help to ameliorate SARS-CoV-2-induced vasculopathy and coagulopathy in COVID-19 patients (Table 25.3).
Zapatero-Belinchón et al. performed an in vitro study to investigate the effect of statin pre-treatment on lung cells infected with the human coronaviruses, CoV-229E and SARS-CoV-2 [94] (Table 25.3). The statin pre-treatment with 5 mM fluvastatin led to a dose-dependent reduction in the susceptibility of these cells to coronavirus infection. The researchers followed this up by testing the effects of pre-treatment with either 10 or 50 mM fluvastatin on SARS-CoV-2 infected human primary bronchial epithelial cells. This showed that the 10 mM fluvastatin dose decreased viral release moderately and the 50 mM dosage decreased viral release in samples from all donors. In addition, label-free mass spectrometry proteomic profiling showed that the 35 proteins were significantly decreased by the fluvastatin treatment. Many of these proteins were associated with RNA degradation, protein translation, and viral replication processes (Table 25.3).
7 Conclusions and Future Perspectives
COVID-19 is a worldwide pandemic causing often mild symptoms including fatigue, dry cough, dyspnea, myalgia, chills, and fever but also severe symptoms that can cause organ failure. COVID-19 can affect several organs, including the respiratory, digestive, and central nervous system. Inflammation plays a pivotal role in COVID-19 and therefore anti-inflammatory medications might suppress the harmful effects of the virus on organs and tissues. Statins are currently the most often prescribed and effective LDL-cholesterol lowering drugs that are used to prevent atherosclerotic cardiovascular disease. Statins decrease total and LDL-cholesterol, they slightly reduce triglycerides and slightly increase HDL-cholesterol, therefore decreasing the risk of adverse cardiovascular events. In addition to their effects on cholesterol metabolism, statins reduce the circulating isoprenoid and inactivation of signaling proteins. Statins also have anti-inflammatory, antioxidant, antiproliferative, and immunomodulatory effects. Statins can also stabilize atherosclerotic plaques and prevent platelet aggregation on the plaques. Because of their proven anti-inflammatory effects statins, this review focused on their potential use as an adjuvant therapy in the treatment of COVID-19. Statins are safe drugs without many adverse effects but their musculoskeletal adverse effects should be taken into consideration.
References
Saengsiwaritt W, Jittikoon J, Chaikledkaew U, et al (2022) Genetic polymorphisms of ACE1, ACE2, and TMPRSS2 associated with COVID-19 severity: A systematic review with meta-analysis. Rev Med Virol 32(4):e2323. https://doi.org/10.1002/rmv.2323
Pearce L, Davidson SM, Yellon DM (2020) The cytokine storm of COVID-19: a spotlight on prevention and protection. Expert Opin Ther Targets 24(8):723–730
Altable M, de la Serna JM (2021) Protection against COVID-19 in African population: Immunology, genetics, and malaria clues for therapeutic targets. Virus Res 299:198347. https://doi.org/10.1016/j.virusres
Datta C, Bhattacharjee A (2020) Cytokine Storm and its Implication in Coronavirus disease 2019 (COVID-19). J Immunol Sci 4(3). https://doi.org/10.1002/jmv.26232
Cascella M, Rajnik M, Aleem A, et al (2022) Features, evaluation, and treatment of coronavirus (COVID-19). In: StatPearls [Internet]; StatPearls Publishing; Treasure Island, FL, USA)
Azmi NU, Puteri MU, Lukmanto D (2020) Cytokine storm in COVID-19: An overview, mechanism, treatment strategies, and stem cell therapy perspective. Pharm Sci Res 7(1). https://doi.org/10.7454/psr.v7i4.1092
Daniels LB, Kumar K (2022) The role of statin therapy in COVID-19. Expert Rev Cardiovasc Ther 20(6):415–417
Khalifeh M, Penson PE, Banach M, et al (2021) Statins as anti-pyroptotic agents. Arch Med Sci 17(5):1414–1417
Sahebkar A, Serban C, Mikhailidis DP, et al (2015) Association between statin use and plasma d-dimer levels: A systematic review and meta-analysis of randomised controlled trials. J Thromb Haemost 114(3):546–557
Serban C, Sahebkar A, Ursoniu S, et al (2015) A systematic review and meta-analysis of the effect of statins on plasma asymmetric dimethylarginine concentrations. Sci Rep 5:9902. https://doi.org/10.1038/srep09902
Sohrevardi SM, Nasab FS, Mirjalili MR, et al (2021) Effect of atorvastatin on delirium status of patients in the intensive care unit: A randomized controlled trial. Arch Med Sci 17(5):1423. https://doi.org/10.5114/aoms.2019.89330
Bahrami A, Bo S, Jamialahmadi T, et al (2020) Effects of 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors on ageing: Molecular mechanisms. Ageing Res Rev 58:101024. https://doi.org/10.1016/j.arr.2020.101024
Bland AR, Payne FM, Ashton JC, et al (2022) The cardioprotective actions of statins in targeting mitochondrial dysfunction associated with myocardial ischaemia-reperfusion injury. J Pharm Res 175:105986. https://doi.org/10.1016/j.phrs.2021.105986.
Dehnavi S, Kiani A, Sadeghi M, et al (2021) Targeting AMPK by Statins: A Potential Therapeutic Approach. Drugs 81(8):923–933
Gorabi AM, Kiaie N, Bianconi V, et al (2021) Statins Attenuate Fibrotic Manifestations of Cardiac Tissue Damage. Curr Mol Pharmacol 14(5):782–797
Hosseini A, Sahranavard T, Reiner Ž, et al (2022) Effect of statins on abdominal aortic aneurysm. Eur J Pharm Sci 178:106284. https://doi.org/10.1016/j.ejps.2022.106284.
Kouhpeikar H, Delbari Z, Sathyapalan T, et al (2020) The Effect of Statins through Mast Cells in the Pathophysiology of Atherosclerosis: a Review. Curr Atheroscler Rep 22(5):19. https://doi.org/10.1007/s11883-020-00837-9
Fehr, A.R., Perlman, S. (2015) Coronaviruses: An Overview of their Replication and Pathogenesis. Methods Mol Biol 1282:1–23
Woo PCY, Huang Y, Lau SKP, et al (2010) Coronavirus genomics and bioinformatics analysis. Viruses 2(8):1804–1820
Chathappady House NN, Palissery S, Sebastian H (2021) Corona Viruses: A Review on SARS, MERS and COVID-19. Microbiol Insights 14:11786361211002481. https://doi.org/10.4103/jfmpc.jfmpc_839_21.
Cherry J, Demmler-Harrison GJ, Kaplan SL, et al (2018) Feigin and Cherry’s Textbook of Pediatric Infectious Diseases. Elsevier Health Sciences; Amsterdam, Netherlands. ISBN-13: 0323376921-978
Snijder EJ, Bredenbeek PJ, Dobbe JC, et al (2003) Unique and conserved features of genome and proteome of SARS-coronavirus, an early split-off from the coronavirus group 2 lineage. J Mol Biol 331(5):991–1004
Neuman BW, Kiss G, Kunding AH, et al (2011) A structural analysis of M protein in coronavirus assembly and morphology. J Struct Biol 174(1):11–22
Cruz CAK, Medina PMB (2021) Diversity in the Accessory Proteins of SARS-CoV-2, SARS-CoV, and MERS-CoV Betacoronaviruses. Curr Protein Pept Sci 22(10):695–715
Rastogi M, Pandey N, Shukla A, et al (2020) SARS coronavirus 2: from genome to infectome. Respir Res 21(1):318. https://doi.org/10.1186/s12931-020-01581-z.
Tortorici MA, Veesler D (2019) Structural insights into coronavirus entry. Adv Virus Res 105:93–116
Zhu Z, Lian X, Su X, et al (2020) From SARS and MERS to COVID-19: a brief summary and comparison of severe acute respiratory infections caused by three highly pathogenic human coronaviruses. Respir Res 21(1):224. https://doi.org/10.1186/s12931-020-01479-w
Samukawa K (1982) [Use of stable isotopes in life science (III). Measurement of 15N abundance in amino acids with gas chromatography-mass spectrometry (author’s transl)]. Radioisotopes 31(3):166–174
Dooley DC (1982) Glycerol permeation of the human granulocyte. Exp Hematol 10(5):413–422
Lu G, Hu Y, Wang Q, et al (2013) Molecular basis of binding between novel human coronavirus MERS-CoV and its receptor CD26. Nature 500(7461):227–231
Sender R, Bar-On YM, Gleizer S, et al (2021) The total number and mass of SARS-CoV-2 virions. Proc Natl Acad Sci 118(25):e2024815118. https://doi.org/10.1073/pnas.2024815118
Goldsmith CS, Tatti KM, Ksiazek TG, et al (2004) Ultrastructural characterization of SARS coronavirus. Emerg Infect Dis 10(2):320–326
Kumar S, Nyodu R, Maurya VK, et al. Morphology, Genome Organization, Replication, and Pathogenesis of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2). Coronavirus Disease 2019 (COVID-19):23–31. https://doi.org/10.1007/978-981-15-4814-7_3
Ke Z, Oton J, Qu K, et al (2020) Structures and distributions of SARS-CoV-2 spike proteins on intact virions. Nature 588(7838):498–502
Hurdiss DL, Drulyte I, Lang Y, et al (2020) Cryo-EM structure of coronavirus-HKU1 haemagglutinin esterase reveals architectural changes arising from prolonged circulation in humans. Nat Commun 11(1):4646. https://doi.org/10.1038/s41467-020-18440-6
Mandala VS, McKay MJ, Shcherbakov AA, et al (2020) Structure and drug binding of the SARS-CoV-2 envelope protein transmembrane domain in lipid bilayers. Nat Struct Mol Biol 27(12):1202–1208
Hu Y, Wen J, Tang L, et al (2003) The M protein of SARS-CoV: basic structural and immunological properties. Genomics Proteomics Bioinformatics 1(2):118–130
Lissenberg A, Vrolijk MM, van Vliet AL, et al (2005) Luxury at a cost? Recombinant mouse hepatitis viruses expressing the accessory haemagglutinin esterase protein display reduced fitness in vitro. J Virol 79(24):15054–15063
Boopathi S, Poma AB, Kolandaivel P (2021) Novel 2019 coronavirus structure, mechanism of action, antiviral drug promises and rule out against its treatment. J Biomol Struct Dyn 39(9):3409–3418
de Haan CAM, Volders H, Koetzner CA, et al (2002) Coronaviruses maintain viability despite dramatic rearrangements of the strictly conserved genome organization. J Virol 76(24):12491–12502.
Kandeel M, Ibrahim A, Fayez M, et al (2020) From SARS and MERS CoVs to SARS-CoV-2: Moving toward more biased codon usage in viral structural and nonstructural genes. J Med Virol 92(6):660–666
Gorkhali R, Koirala P, Rijal S, et al (2021) Structure and Function of Major SARS-CoV-2 and SARS-CoV Proteins. Bioinform Biol 15:11779322211025876. https://doi.org/10.1177/11779322211025876.
Romano M, Ruggiero A, Squeglia F, et al (2020) A Structural View of SARS-CoV-2 RNA Replication Machinery: RNA Synthesis, Proofreading and Final Capping. Cells 9(5):1267. https://doi.org/10.3390/cells9051267.
Shang J, Wan Y, Luo C, et al (2020) Cell entry mechanisms of SARS-CoV-2. Proc Natl Acad Sci USA 117(21):11727–11734
van Hemert MJ, van den Worm SH, Knoops K, et al (2008) SARS-coronavirus replication/transcription complexes are membrane-protected and need a host factor for activity in vitro. PLoS Pathog 4(5):e1000054. https://doi.org/10.1371/journal.ppat.1000054.
Hu B, Guo H, Zhou P, et al (2021) Characteristics of SARS-CoV-2 and COVID-19. Nat Rev Microbiol 19(3):141–154
Fan Y, Zhao K, Shi ZL, et al (2019) Bat Coronaviruses in China. Viruses 11(3):210. https://doi.org/10.3390/v11030210.
Payne S. Family Coronaviridae. Viruses. 2017:149–158. https://doi.org/10.1016/B978-0-12-803109-4.00017-9
International Committee on Taxonomy of Viruses (ICTV). https://ictv.global/. Accessed Oct 14, 2022
Corman VM, Muth D, Niemeyer D, et al (2018) Hosts and Sources of Endemic Human Coronaviruses. Adv Virus Res 100:163–188
Pal M, Berhanu G, Desalegn C, et al (2020) Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2): An Update. Cureus 2(3):e7423. https://doi.org/10.7759/cureus.7423
World Health Organization; Tracking SARS-CoV-2 variants. https://www.who.int/activities/tracking-SARS-CoV-2-variants. Accessed October 24, 2022
Konings F, Perkins MD, Kuhn JH, et al (2021) SARS-CoV-2 Variants of Interest and Concern naming scheme conducive for global discourse. Nat Rev Microbiol 6(7):821–823
Silhol F, Sarlon G, Deharo JC, et al (2020) Downregulation of ACE2 induces overstimulation of the renin-angiotensin system in COVID-19: should we block the renin-angiotensin system? Hypertens Res 43(8):854–856
Choudhary S, Sharma K, Silakari O (2021) The interplay between inflammatory pathways and COVID-19: A critical review on pathogenesis and therapeutic options. Microb Pathog 150:104673. https://doi.org/10.1016/j.micpath.2020.104673.
Farahani M, Niknam Z, Mohammadi Amirabad L, et al (2022) Molecular pathways involved in COVID-19 and potential pathway-based therapeutic targets. Biomed Pharmacother 145:112420. https://doi.org/10.1016/j.biopha.2021.112420.
Yarmohammadi A, Yarmohammadi M, Fakhri S, et al (2021) Targeting pivotal inflammatory pathways in COVID-19: A mechanistic review. Eur J Pharmacol 890:173620. https://doi.org/10.1016/j.ejphar.2020.173620.
Vuorio A, Kuoppala J, Kovanen PT, et al (2019) Statins for children with familial hypercholesterolemia. Cochrane Database Syst Rev (11):CD006401. https://doi.org/10.1002/14651858.CD006401.pub5.
Toth PP, Banach M (2019) Statins: Then and Now. Methodist Debakey Cardiovasc J 15(1):23–31
Istvan E (2003) Statin inhibition of HMG-CoA reductase: a 3-dimensional view. Atheroscler Suppl 4(1):3–8
Grundy SM (1988) HMG-CoA reductase inhibitors for treatment of hypercholesterolemia. N Engl J Med 319(1):24–33
Barrios-González J, Miranda RU (2005) Biotechnological production and applications of statins. Appl Microbiol Biotechnol 85(4):869–883
Schachter M (2005) Chemical, pharmacokinetic and pharmacodynamic properties of statins: an update. Fundam Clin Pharmacol (1):117–125
Desager JP, Horsmans Y (1996) Clinical pharmacokinetics of 3-hydroxy-3-methylglutaryl-coenzyme A reductase inhibitors. Clin Pharmacokinet (5):348–371
Lennernäs H, Fager G (1997) Pharmacodynamics and pharmacokinetics of the HMG-CoA reductase inhibitors. Similarities and differences. Clin Pharmacokinet 32(5):403–425
Bu DX, Griffin G, Lichtman AH (2011) Mechanisms for the anti-inflammatory effects of statins. Curr Opin Lipidol (3):165–170
Davignon J, Jacob RF, Mason RP (2004) The antioxidant effects of statins. Coron Artery Dis (5):251–258
Parizadeh SMR, Azarpazhooh MR, Moohebati M, et al (2011) Simvastatin therapy reduces prooxidant-antioxidant balance: Results of a placebo-controlled cross-over trial. Lipids 46(4):333–340
Antonopoulos AS, Margaritis M, Lee R, et al (2012) Statins as anti-inflammatory agents in atherogenesis: molecular mechanisms and lessons from the recent clinical trials. Curr Pharm Des 18(11):1519–1530
Sikora J, Kostka B, Marczyk I, et al (2013) Effect of statins on platelet function in patients with hyperlipidemia. Arch Med Sci 9(4):622–628
O’Driscoll G, Green D, Taylor RR (2017) Simvastatin, an HMG-coenzyme A reductase inhibitor, improves endothelial function within 1 month. Circulation 95(5):1126–1131
Wang Q, Yan J, Chen X, et al (2011) Statins: multiple neuroprotective mechanisms in neurodegenerative diseases. Exp Neurol 230(1):27–34
Golomb BA, Evans MA (2008) Statin adverse effects: a review of the literature and evidence for a mitochondrial mechanism. Am J Cardiovasc Drugs 8(6):373–418
Wierzbicki AS, Lumb PJ, Semra Y, Chik G, et al (1999) Atorvastatin compared with simvastatin-based therapies in the management of severe familial hyperlipidaemias. QJM 92(7):387–94.
Gotto Jr AMJC (2002) Statins: powerful drugs for lowering cholesterol: advice for patients. Am Heart J 105(13):1514–1516
Liao JK, Laufs U (2005) Pleiotropic effects of statins. Annu Rev Pharmacol Toxicol 45:89–118
Blum A, Shamburek R (2009) The pleiotropic effects of statins on endothelial function, vascular inflammation, immunomodulation and thrombogenesis. Atherosclerosis 203(2):325–330
Nissen SE, Nicholls SJ, Sipahi I, et al (2006) Effect of very high-intensity statin therapy on regression of coronary atherosclerosis: the ASTEROID trial. JAMA 295(13):1556–1565
Ridker PM, Danielson E, Fonseca FA, et al (2008) Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med 359(21):2195–2207
Kones R (2010) Rosuvastatin, inflammation, C-reactive protein, JUPITER, and primary prevention of cardiovascular disease--a perspective. Drug Des Devel Ther 4:383–413
Ascer E, Bertolami MC, Venturinelli ML, et al (2004) Atorvastatin reduces proinflammatory markers in hypercholesterolemic patients. Atherosclerosis 177(1):161–166
van de Ree MA, Huisman MV, Princen HM, et al (2003) Strong decrease of high sensitivity C-reactive protein with high-dose atorvastatin in patients with type 2 diabetes mellitus. Atherosclerosis 166(1):129–135
Chansrichavala P, Chantharaksri U, Sritara P, et al (2009) Atorvastatin attenuates TLR4-mediated NF-kappaB activation in a MyD88-dependent pathway. Asian Pac J Allergy Immunol 27(1):49–57
Spencer FA, Fonarow GC, Frederick PD, et al (2004) Early withdrawal of statin therapy in patients with non-ST-segment elevation myocardial infarction: national registry of myocardial infarction. Arch Intern Med 164(19):2162–2168
Heeschen C, Hamm CW, Laufs U, et al (2002) Withdrawal of statins increases event rates in patients with acute coronary syndromes. Circulation 105(12):1446–1452
DeDiego ML, Nieto-Torres J, Regla-Nava JA, et al (2013) Inhibition of NF- B-Mediated Inflammation in Severe Acute Respiratory Syndrome Coronavirus-Infected Mice Increases Survival. J Virol 88(2):913–24
Cimato TR, Palka BA (2015) Effects of statins on TH1 modulating cytokines in human subjects. PeerJ;3:e764. https://doi.org/10.7717/peerj.764.
Yuan S (2015) Statins May Decrease the Fatality Rate of Middle East Respiratory Syndrome Infection. mBio 6(4):e01120. https://doi.org/10.1128/mBio.01120-15.
Jammal M, Riachy M, Haddad F (2022) Can statins be beneficial in Covid 19 patients? Int J Diabetes Clin Res 8:138. https://doi.org/10.23937/2377-3634/1410138
Reiner Ž, Hatamipour M, Banach M, et al (2020) Statins and the Covid-19 main protease: In silico evidence on direct interaction. Arch Med Sci 16(2):490–496
Vahedian-Azimi A, Mohammadi SM, Beni FH, et al (2021) Improved COVID-19 ICU admission and mortality outcomes following treatment with statins: A systematic review and meta-analysis. Arch Med Sci 17(3):579–595
Karampoor S, Hesamizadeh K, Shams Z, et al (2021) The role of lovastatin in the attenuation of COVID-19. Int Immunopharmacol 101(Pt A):108192. https://doi.org/10.1016/j.intimp.2021.108192
Vahedian-Azimi A, Mohammadi SM, Heidari Beni F, et al (2021) Improved COVID-19 ICU admission and mortality outcomes following treatment with statins: a systematic review and meta-analysis. Arch Med Sci 17(3):579–95
Zapatero-Belinchón FJ, Moeller R, Lasswitz L, et al (2021) Fluvastatin mitigates SARS-CoV-2 infection in human lung cells. iScience 24(12):103469. https://doi.org/10.1016/j.isci.2021.103469
Rodriguez-Nava G, Trelles-Garcia DP, Yanez-Bello MA, et al (2020) Atorvastatin associated with decreased hazard for death in COVID-19 patients admitted to an ICU: a retrospective cohort study. Crit Care 24(1):429. https://doi.org/10.1186/s13054-020-03154-4.
Haji Aghajani M, Moradi O, Azhdari Tehrani H, et al (2021) Promising effects of atorvastatin on mortality and need for mechanical ventilation in patients with severe COVID-19; a retrospective cohort study. Int J Clin Pract 75(9):e14434. https://doi.org/10.1111/ijcp.14434
Davoodi L, Jafarpour H, Oladi Z, et al (2021) Atorvastatin therapy in COVID-19 adult inpatients: A double-blind, randomized controlled trial. Int J Cardiol Heart Vasc 36:100875. https://doi.org/10.1016/j.ijcha.2021.100875
INSPIRATION Investigators (2022) Atorvastatin versus placebo in patients with covid-19 in intensive care: randomized controlled trial. 2022;376. BMJ 376:e068407. https://doi.org/10.1136/bmj-2021-068407
Ghafoori M, Saadati H, Taghavi M, et al (2022) Survival of the hospitalized patients with COVID-19 receiving atorvastatin: A randomized clinical trial. J Med Virol 94(7):3160–3168
Reiner Ž, Hatamipour M, Banach M, et al (2020) Statins and the COVID-19 main protease: in silico evidence on direct interaction. Arch Med Sci 16(3):490–496
Sapey E, Patel JM, Greenwood HL, et al. Pulmonary Infections in the Elderly Lead to Impaired Neutrophil Targeting, Which Is Improved by Simvastatin. Am J Respir Crit Care Med 196(10):1325–1336
Shrivastava-Ranjan P, Flint M, Bergeron É, et al (2018) Statins suppress Ebola virus infectivity by interfering with glycoprotein processing. mBio 9(3):e00660–18. https://doi.org/10.1128/mBio.00660-18
Guo H, Huang M, Yuan Q, et al (2017) The Important Role of Lipid Raft-Mediated Attachment in the Infection of Cultured Cells by Coronavirus Infectious Bronchitis Virus Beaudette Strain. PLoS One;12(1):e0170123. https://doi.org/10.1371/journal.pone.0170123.
Shakour N, Ruscica M, Hadizadeh F, et al (2020) Statins and C-reactive protein: In silico evidence on direct interaction. Arch Med Sci 16(6):1432–1439
Wang, H, Yang, P, Liu, K, et al (2008) SARS coronavirus entry into host cells through a novel clathrin- and caveolae-independent endocytic pathway. Cell Res 18(2):290–301
INSPIRATION-S Investigators (2022) Atorvastatin versus placebo in patients with covid-19 in intensive care: randomized controlled trial. BMJ 376:e068407. https://doi.org/10.1136/bmj-2021-068407
Gaitán-Duarte HG, Álvarez-Moreno C, Rincón-Rodríguez CJ, et al (2021) Effectiveness of Rosuvastatin plus Colchicine, Emtricitabine/Tenofovir and a combination of them in Hospitalized Patients with SARS Covid-19. medRxiv https://doi.org/10.1101/2021.07.06.21260085
Hayden MR (2020) An Immediate and Long-Term Complication of COVID-19 May Be Type 2 Diabetes Mellitus: The Central Role of beta-Cell Dysfunction, Apoptosis and Exploration of Possible Mechanisms. Cells 9(11). https://doi.org/10.3390/cells9112475
Banach M, Penson PE, Fras Z, et al (2020) Brief recommendations on the management of adult patients with familial hypercholesterolemia during the COVID-19 pandemic. Pharmacol Res 158:104891. https://doi.org/10.1016/j.phrs.2020.104891
Couzin-Frankel J (2021) Antiviral pills could change pandemic’s course. Science 374(6569):799–800, https://doi.org/10.1126/science.acx9605
Menni C, Klaser K, May A, et al (2021) Vaccine side-effects and SARS-CoV-2 infection after vaccination in users of the COVID Symptom Study app in the UK: a prospective observational study. Lancet Infect Dis 21(7):939–949
Teixeira L, Temerozo JR, Pereira-Dutra FS, et al (2022) Simvastatin Downregulates the SARS-CoV-2-Induced Inflammatory Response and Impairs Viral Infection Through Disruption of Lipid Rafts. Front Immunol 13:820131. https://doi.org/10.3389/fimmu.2022.820131
Qian Y, Lei T, Patel PS, et al (2021) Direct Activation of Endothelial Cells by SARS-CoV-2 Nucleocapsid Protein Is Blocked by Simvastatin. J Virol 95(23):e0139621. https://doi.org/10.1128/JVI.01396-21.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Lashgari, NA. et al. (2023). Statins: Beneficial Effects in Treatment of COVID-19. In: Guest , P.C. (eds) Application of Omic Techniques to Identify New Biomarkers and Drug Targets for COVID-19. Advances in Experimental Medicine and Biology(), vol 1412. Springer, Cham. https://doi.org/10.1007/978-3-031-28012-2_25
Download citation
DOI: https://doi.org/10.1007/978-3-031-28012-2_25
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-28011-5
Online ISBN: 978-3-031-28012-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)