Abstract
Oxidative stress is thought to play an important role in atherogenesis. The statin group of cholesterol-lowering drugs have been shown to reduce cardiovascular events and possess antioxidant properties. We aimed to assess the effects of simvastatin on a novel measure of prooxidant–antioxidant balance (PAB) in dyslipidemic patients. The PAB assay can measure the prooxidant burden and the antioxidant capacity simultaneously in one assay, thereby giving a redox index. We treated 102 dyslipidemic individuals with simvastatin, or a placebo in a double-blind, cross-over, placebo-controlled trial. PAB values were measured before and after each treatment period. Seventy-seven subjects completed the study. We found that statin therapy was associated with a significant reduction in PAB values (P < 0.001). This effect appeared to be independent of the cholesterol-lowering effects of statins. We conclude that serum PAB values are decreased by simvastatin therapy. Regarding previous reports on the elevation of PAB in conditions associated with oxidative stress, the PAB assay, along with other markers of oxidative stress, may be applied to estimate the extent of oxidative stress in patients, assessment of the antioxidative efficacy of medication such as statins and perhaps also for the identification of those individuals who need antioxidant therapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Oxidative stress is an imbalance between the production of pro-oxidants and antioxidant defenses in favor of pro-oxidants. Oxidative stress is usually related to the increased formation of reactive oxygen species (ROS), and is thought to play an important role in the pathogenesis of cardiovascular disease (CVD) and its complications. Recently oxidative stress [1] and inflammation [2] have been proposed to be significant risk factors for CVD, and the lipid oxidation hypothesis provides one mechanism by which oxidative stress may be implicated [3].
It is also suggested that oxidative stress may be a strong and independent prognostic predictor of cardiovascular events [4].
Low density lipoprotein cholesterol (LDL-C) has been associated with several pro-atherothrombotic processes through the development of endothelial dysfunction, inflammation and foam cell formation [5]. Indeed, individuals with relatively normal LDL levels but high exposure to oxidative stress such as those with hypertension [6], are at increased risk of developing CVD via the formation of pro-inflammatory and pro-atherogenic molecules associated with the formation of oxidized-LDL (ox-LDL). Ox-LDL has been shown to accumulate in the arterial wall and this is associated with the development of endothelial dysfunction [7, 8]. Thus, reduction in plasma levels of LDL and ox-LDL may represent a useful approach for preventing from atherosclerotic diseases.
Statins are a group of lipid-lowering agents which block the rate limiting step in cholesterol biosynthesis, the conversion of 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) to mevalonic acid. The LDL-cholesterol lowering property of statins is associated with a reduction of cardiovascular endpoints including definite coronary events (specified as nonfatal myocardial infarction or death from coronary heart disease), definite nonfatal myocardial infarctions; death from definite plus suspected coronary heart disease and death from all cardiovascular causes [9]. However, it has been argued that the benefits obtained with statin therapy in patients with a wide range of cholesterol levels may be due to their “pleiotropic” non-cholesterol-lowering effects. These pleiotropic effects of statins include: improving endothelial function, decreasing oxidative stress (by lowering ROS production and increasing the resistance of LDL-C to oxidation), inhibiting platelet adhesion, reducing inflammation, and enhancing the stability of atherosclerotic plaques [10].
Until now, many methods have been developed that can separately determine the total pro-oxidant and antioxidant capacities and are therefore hard, time consuming, expensive, and imprecise. We have recently used a simple, rapid and inexpensive method [11] to measure the PAB directly, by using 3,3′,5,5′-tetramethylbenzidine (TMB) and two different kinds of reaction, an enzymatic reaction where the chromogen TMB is oxidized to a colored cation by peroxides, and a chemical reaction in which the colored TMB cation is reduced to a colorless compound by antioxidants. A redox index is thereby derived from these two reactions. The assay has been calibrated against the most significant known oxidants and antioxidants and its response has been found to be a linear decrease against antioxidants and a linear increase against oxidants. The method was also, validated in depth by other known oxidative stress markers [11]. In this study, we aimed to evaluate the effect of simvastatin on PAB values by a modified PAB assay [12] in a randomized, double blind, cross-over trial in dyslipidemic patients.
Methods
Subjects
One hundred and two men and women, aged 20–88 who were not originally taking lipid-lowering agents were recruited from the lipid clinics at the Qaem hospital, Mashhad, Iran. In addition to a history of not taking statins, other inclusion criteria were any of the following conditions (based on the NCEP-ATP III guidelines [13]): (1) patients with <2 risk factors (except diabetes mellitus) for coronary heart disease (CHD) and 160 mg/dL < LDL-C < 190 mg/dL, or (2) patients with ≥2 risk factors (except diabetes mellitus) for coronary heart disease (CHD) and 130 mg/dL < LDL-C < 160 mg/dL. Cardiovascular risk factors were defined as age >65 years, hypertension (defined as taking any anti-hypertensive medication; or systolic blood pressure ≥140 mmHg or diastolic blood pressure ≥90 mmHg), diabetes mellitus (defined as fasting blood sugar (FBS) ≥ 126 mg/dL), positive family history of CVD, smoking, male sex and obesity [defined as body mass index (BMI) ≥ 30 kg/m2]. The exclusion criteria were; malignancy or history of malignancy, infections, connective tissue disorders or treatment with immunomodulatory drugs (e.g. corticosteroids), liver or renal disease, leukocytosis (white blood cell count >10,000 109/L), thrombocytosis (platelet count >450,000 109/L) and anemia (hematocrit <40%). Each subject gave informed written consent to participate in the study, which had previously been approved by the Mashhad University of Medical Science Ethics Committee. In addition, subjects were advised to continue their normal medication schedule.
Study Design
This study was designed as a randomized, double blind, cross-over trial in which each patient received simvastatin or a placebo and then crossed over to the alternate regimen. Each treatment period was 30 days and there was a 2-week washout interval in between the regimens. The dose of simvastatin and all other medication remained unchanged during the experimental period, and the patients were advised not to change their lifestyle during the study. At the first visit, patients were randomized for one of two treatment regimens, 51 patients were provided with simvastatin 40 mg/day for 30 days and other 51 patients received a placebo (simply prepared by filling empty capsules—which were matched for size and color with simvastatin capsules—with starch instead of simvastatin) for 30 days. After another 2-week wash-out period, patients crossed over to the other form of treatment.
Anthropometric Measurements
Anthropometric parameters including weight, height, and BMI were measured. Weight was measured with the subjects dressed in light clothing after an overnight fasting using a standard scale. BMI was calculated as weight (kg) divided by height squared (m2).
Blood Sampling
Blood samples were collected four times for each subject (before and after starting each period). Blood samples for laboratory assays were obtained on the day of sampling after 12 h of fasting. Following venipuncture, blood samples were collected in Vacutainer® tubes and centrifuged at 10,000g for 15 min at 4 °C. After separation, aliquots of serum were frozen at −80 °C until analysis.
Routine Biochemical Analysis
A full fasted lipid profile comprising total cholesterol, triglycerides, high-density lipoprotein cholesterol (HDL-C) and LDL-C was determined for each subject. Serum lipid and FBS concentrations were measured enzymatically with the use of commercial kits.
Chemicals
TMB powder (3,3′,5,5′-tetramethylbenzidine, Fluka), peroxidase enzyme (Applichem: 230 U/mg, A3791,0005, Darmstadt, Germany), chloramine T trihydrate (Applichem: A4331, Darmstadt, Germany), hydrogen peroxide (30%) (Merck). These chemicals and all the other reagents used were reagent grade and were prepared in double distilled water.
Prooxidant–Antioxidant Balance (PAB) Assay
A modified PAB assay was applied based on a previously described method [11, 12]. The standard solutions were prepared by mixing varying proportions (0–100%) of 250 μM hydrogen peroxide with 3 mM uric acid (in 10 mM NaOH). TMB powder (60 mg) was dissolved in 10 mL DMSO. For preparation of the TMB cation, 400 μL of the TMB/DMSO solution was added to 20 mL of acetate buffer (0.05 M buffer, pH 4.5), and then 70 μL of fresh chloramine T (100 mM) solution was added to this 20 mL. The solution was mixed well and incubated for 2 h at room temperature in a dark place. Then 25 U of peroxidase enzyme solution was added to 20 mL of TMB cation solution, dispensed in 1 mL and stored at −20 °C. In order to prepare the TMB solution 200 μL of TMB/DMSO was added to 10 mL of acetate buffer (0.05 M buffer, pH 5.8) and the working solution was prepared by mixing 1 mL TMB cation with 10 mL of TMB solution. This working solution was incubated for 2 min at room temperature in a dark place and used immediately. Ten microliters of each sample, standard or blank (distilled water) were mixed with 200 μL of working solution in each well of a 96-well plate, which was then incubated in a dark place at 37 °C for 12 min. At the end of the incubation time, 100 μL of 2 N HCl was added to each well, and the optical density (OD) was measured in an ELISA reader at 450 nm with a reference wavelength of 620 or 570 nm. A standard curve was provided from the values relative to the standard samples. The values of the PAB are expressed in arbitrary units, this is the percentage of hydrogen peroxide in the standard solution. The values of the unknown samples were then calculated based on the values obtained from the above standard curve.
Statistical Analysis
Values were expressed as means ± SD or, in the case of non-normally distributed data, as median and inter-quartile range. The comparison between pre and post treatments was done using the paired t test or the Wilcoxon signed rank test. Data obtained from independent variables analyzed using Student’s t test (for those with normal distribution) or Mann–Whitney U test (for those without normal distribution). Categorical data were compared using χ2 test. Correlations between changes in serum PAB and LDL-C levels were assessed using the Pearson correlation coefficient. Mixed model analysis of variance for 2 × 2 cross-over studies were fitted when assumption for normality were met. All analysis were performed with the Statistical Analysis Software (SAS version 8). A two-sided P value of <0.05 was considered statistically significant.
Results
From 102 subjects who entered our study, 25 (24.5%) did not complete the study, leading to a final sample size of 77 (78.18%). The reasons for drop-outs were non-compliance with the study protocol (n = 21), drug intolerance (n = 2) and moving to another city (n = 2) (Fig. 1). To rule out the possibility of a carryover effect from one treatment period to the other treatment period, we compared baseline values before the first treatment period to those before the second treatment period. No significant difference was found in the analysis (P > 0.05). The mean age and BMI of subjects were 46.61 ± 13.95 and 29.94 ± 6.08, respectively, with 72.1% being female. The prevalence of smoking, diabetes mellitus and hypertension were 7.0, 14.0 and 9.3%, respectively.
Effect of Administration of Simvastatin Versus Placebo on Weight, BMI and FBS
FBS was not significantly affected by simvastatin nor by the placebo (P > 0.05). However, mean baseline values for BMI and weight were significantly different between the first and second periods of treatment (P = 0.019 and P = 0.003, respectively) (Table 1).
Effect of Administration of Simvastatin Versus Placebo on Lipid Parameters
As expected, total cholesterol, LDL-C and triglycerides were reduced significantly after 4-weeks of treatment with simvastatin (P < 0.001). However, HDL-C did not change significantly with either treatment (P > 0.05) (Table 1).
Effect of Administration of Simvastatin Versus Placebo on PAB Values
Treatment with simvastatin 40 mg/day for 4 weeks caused a statistically significant reduction in the mean PAB values (P < 0.001) (Fig. 2).
Correlation Between Changes in PAB Values with Changes in LDL-C Levels
Statistical analysis showed that there was no significant correlation between changes in PAB values and serum LDL-C levels in any of the periods, neither in the placebo-statin nor in the statin-placebo group (P > 0.05, Table 2). The only exception was a significant correlation between PAB changes and serum total cholesterol changes in the placebo-statin group (P < 0.05, Table 2).
Discussion
In the present study we found that simvastatin therapy for 4 weeks caused a statistically significant reduction in mean PAB values, showing that statin therapy may be associated with a reduction in levels of oxidative stress. Our finding confirms the results of other studies evaluating the effect of statins on plasma measures of oxidation status. Fluvastatin therapy was found to reduce superoxide radical generation and the susceptibility of LDL to oxidation in cholesterol-fed rabbits [14]. Moreover, simvastatin was reported to lower superoxide generation in human macrophages [15]. Furthermore, oxidative stress of different patients has been shown to decrease after treatment with atorvastatin, simvastatin, pravastatin, fluvastatin, and lovastatin [16–18]. In addition, treatment with these statins was followed by a prolonged lag time of LDL oxidation [19–21]. Statin therapy also causes a significant reduction in plasma levels of ox-LDL [17] and it has also been reported that in hypercholesterolemia or mixed type hyperlipidemia, atorvastatin can increase total plasma antioxidant status, leading to a lower LDL oxidation capacity [19].
There appear to be several mechanisms whereby statins may reduce oxidative stress. Apart from their hypocholesterolemic effects and the subsequent reduction of oxidation substrate, statins are known to exert radical scavenging activity and decrease the generation and release of ROS through different mechanism such as inhibition of Rac1GTPase and NADPH-oxidase [22].
Nicotinamide adenine dinucleotide phosphate (NADPH) oxidases are as one of the important sources of superoxide in human coronary artery, and their activities have been reported to be increased in patients with CVD [23]. Statins have been reported to reduce NADPH dependant superoxide formation by monocyte-derived cell lines in culture [24]. Atorvastatin has been reported to inhibit angiotensin II-induced superoxide formation by NADPH oxidase in rats in vivo [25]. Statin therapy has been also suggested to inhibit ox-LDL induced NADPH oxidase expression and superoxide anion formation [26]. The anti-inflammatory properties of statins make them able to inhibit macrophage growth and foam cell formation stimulated with ox-LDL [27], leading to a reduction in influx of inflammatory cells, which consecutively results in a decreased release of ROS and the LDL oxidation.
Statins may also act as an antioxidant via different mechanisms, for instance, there are some reports, though not consistent, indicating that statins are able to increase the activity and/or expression of antioxidant enzymes such as catalase, paraoxonase and glutathione peroxidase, while decreasing those of NADPH oxidase [28–31]. It is also reported that statins can increase the release of NO [32], and also several statins have the ability to increase eNOS expression in the blood vessels of treated animals [33], resulting in the restoration of endothelial function. Moreover, atorvastatin has been reported to increase paraoxonase activity and decrease the enhanced cellular uptake of ox-LDL of monocytes differentiating into macrophages [34]. Finally some statin metabolites are considered to possess antioxidant properties and prevent lipid peroxidation [35] (Fig. 3).
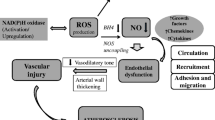
Some antioxidant mechanisms of statins. Statins inhibit the assembly of NAD(P)H oxidase in both vascular cells and phagocytes, thus preventing the formation of superoxide (O ·−2 ). Bi-directional arrows associated with gene expression indicate upregulation of expression of antioxidant enzymes (eNOS and catalase) and downregulation of oxidants (NAD[P]H components and cyclooxygenase-2). Permission from Drugs of Today [41] [Drugs of Today 2004;40(12):975–989. Copyright © 2004 Prous Science, S.A. All rights reserved.]
In our study, we did not find a significant correlation between changes in PAB values with changes in serum levels of LDL-C, implying that antioxidant activity of statins may be at least partly due to other pleiotropic properties rather than just lipid-lowering effects of these drugs. It is suggested that the underlying cholesterol independent effects relates to the inhibition of isoprenoid intermediates of the cholesterol synthesis pathway [36].
The aforementioned studies together with the findings of the present study support the notion that oxidative stress could be used as a significant risk predictor in the atherosclerotic process. Statins have pleiotropic properties as endothelial protective, anti-inflammatory, and antioxidative agents, and suggest that other markers such as C-reactive protein, oxidative biomarkers and isoprenoid intermediates might be used in conjunction with serum cholesterol levels to assess the therapeutic benefits of statin therapy better.
In previous studies using the PAB assay, it was reported to be elevated in a number of conditions associated with oxidative stress including diabetes mellitus [11], coronary artery disease [12], acute coronary syndrome [37], exfoliative glaucoma [38], and stroke [39]. PAB values have been also reported to decrease following antioxidant vitamins (E and C) [11] and selenium consumption [40]. The PAB assay may be useful as a CVD risk predictor [12, 37] and could help to identify patients with higher oxidative stress in order to introduce interventions for the prevention of vascular disease earlier. In the present study, we showed that this assay may serve as a useful method to assess the effect of statin therapy in CVD, indicating that the PAB assay, along with other known markers of oxidative stress, may be used to estimate the extent of oxidative stress in high-risk groups, identify subjects who need antioxidant therapy, and evaluate the antioxidative efficacy of different supplements and medications. Finally, further clinical research is required based on a larger healthy population, as well as on various physiological and pathological states associated with oxidative stress, and by multiple laboratories in order to substantiate the potency of the assay to become a clinical laboratory test.
Study Limitations
The present study had several limitations. First, 25 subjects did not complete the study due to non-compliance with the study protocol or drug intolerance. Second, simvastatin was administered at a dose of 40 mg/day for a limited period (30 days), and longer term studies are necessary to show that this effect is sustained. Finally, using several doses of statins could have been useful to determine whether the observed effects of simvastatin are dose-dependent and whether higher doses exert more dramatic effects.
Abbreviations
- BMI:
-
Body mass index
- CVD:
-
Cardiovascular disease
- eNOS:
-
Endothelial nitric oxide synthase
- FBS:
-
Fasting blood sugar
- HDL-C:
-
High-density lipoprotein cholesterol
- HMG-CoA:
-
3-Hydroxy-3-methylglutaryl-coenzyme A
- LDL-C:
-
Low density lipoprotein cholesterol
- NADPH:
-
Nicotinamide adenine dinucleotide phosphate
- NF-κB:
-
Nuclear factor kappa-light-chain-enhancer of activated B cells
- NO:
-
Nitric oxide
- ox-LDL:
-
Oxidized low density lipoprotein cholesterol
- PAB:
-
Prooxidant–antioxidant balance
- ROS:
-
Reactive oxygen species
- SAS:
-
Statistical analysis software
- SD:
-
Standard deviation
- TC:
-
Total cholesterol
- TG:
-
Triglycerides
- TMB:
-
3,3′,5,5′-Tetramethylbenzidine
References
Cai H, Harrison DG (2000) Endothelial dysfunction in cardiovascular diseases: the role of oxidant stress. Circ Res 87:840–844
Osterud B, Bjorklid E (2003) Role of monocytes in atherogenesis. Physiol Rev 83:1069–1112
Singh U, Jialal I (2006) Oxidative stress and atherosclerosis. Pathophysiology 13:129–142
Walter MF, Jacob RF, Jeffers B, Ghadanfar MM, Preston GM, Buch J et al (2004) Serum levels of thiobarbituric acid reactive substances predict cardiovascular events in patients with stable coronary artery disease: a longitudinal analysis of the PREVENT study. J Am Coll Cardiol 44:1996–2002
Rosenson RS, Tangney CC (1998) Antiatherothrombotic properties of statins: implications for cardiovascular event reduction. JAMA 279:1643–1650
Giugliano D, Ceriello A, Paolisso G (1995) Diabetes mellitus, hypertension, and cardiovascular disease: which role for oxidative stress? Metabolism 44:363–368
Yla-Herttuala S, Palinski W, Rosenfeld ME, Parthasarathy S, Carew TE, Butler S et al (1989) Evidence for the presence of oxidatively modified low density lipoprotein in atherosclerotic lesions of rabbit and man. J Clin Invest 84:1086–1095
Thorne SA, Abbot SE, Winyard PG, Blake DR, Mills PG (1996) Extent of oxidative modification of low density lipoprotein determines the degree of cytotoxicity to human coronary artery cells. Heart 75:11–16
Shepherd J, Cobbe SM, Ford I, Isles CG, Lorimer AR, MacFarlane PW et al (1995) Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. West of Scotland Coronary Prevention Study Group. N Engl J Med 333:1301–1307
Ray KK, Cannon CP (2005) The potential relevance of the multiple lipid-independent (pleiotropic) effects of statins in the management of acute coronary syndromes. J Am Coll Cardiol 46:1425–1433
Alamdari DH, Paletas K, Pegiou T, Sarigianni M, Befani C, Koliakos G (2007) A novel assay for the evaluation of the prooxidant-antioxidant balance, before and after antioxidant vitamin administration in type II diabetes patients. Clin Biochem 40:248–254
Alamdari DH, Ghayour-Mobarhan M, Tavallaie S, Parizadeh MR, Moohebati M, Ghafoori F et al (2008) Prooxidant–antioxidant balance as a new risk factor in patients with angiographically defined coronary artery disease. Clin Biochem 41:375–380
Third Report of the National Cholesterol Education Program (NCEP) (2002) Expert Panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult treatment panel III). Final report. Circulation 106:3143
Rikitake Y, Kawashima S, Takeshita S, Yamashita T, Azumi H, Yasuhara M et al (2001) Anti-oxidative properties of fluvastatin, an HMG-CoA reductase inhibitor, contribute to prevention of atherosclerosis in cholesterol-fed rabbits. Atherosclerosis 154:87–96
Giroux LM, Davignon J, Naruszewicz M (1993) Simvastatin inhibits the oxidation of low-density lipoproteins by activated human monocyte-derived macrophages. Biochim Biophys Acta 1165:335–338
van den Akker JM, Bredie SJ, Diepenveen SH, van Tits LJ, Stalenhoef AF, van LR (2003) Atorvastatin and simvastatin in patients on hemodialysis: effects on lipoproteins, C-reactive protein and in vivo oxidized LDL. J Nephrol 16:238–244
Inami S, Okamatsu K, Takano M, Takagi G, Sakai S, Sano J et al (2004) Effects of statins on circulating oxidized low-density lipoprotein in patients with hypercholesterolemia. Jpn Heart J 45:969–975
Aviram M, Dankner G, Cogan U, Hochgraf E, Brook JG (1992) Lovastatin inhibits low-density lipoprotein oxidation and alters its fluidity and uptake by macrophages: in vitro and in vivo studies. Metabolism 41:229–235
Orem C, Orem A, Calapoglu M, Baykan M, Uydu HA, Erdol C (2002) Plasma fibronectin level and its relationships with lipids, lipoproteins and C-reactive protein in patients with dyslipidaemia during lipid-lowering therapy. Acta Cardiol 57:421–425
Sobal G, Sinzinger H (2005) Effect of simvastatin on the oxidation of native and modified lipoproteins. Biochem Pharmacol 70:1185–1191
Portal VL, Moriguchi EH, Vieira JL, Schio S, Mastalir ET, Buffe F et al (2003) Comparison of the effect of two HMG CoA reductase inhibitors on LDL susceptibility to oxidation. Arq Bras Cardiol 80:156–161
Stancu C, Sima A (2001) Statins: mechanism of action and effects. J Cell Mol Med 5:378–387
Guzik TJ, Mussa S, Gastaldi D, Sadowski J, Ratnatunga C, Pillai R et al (2002) Mechanisms of increased vascular superoxide production in human diabetes mellitus: role of NAD(P)H oxidase and endothelial nitric oxide synthase. Circulation 105:1656–1662
Delbosc S, Morena M, Djouad F, Ledoucen C, Descomps B, Cristol JP (2002) Statins, 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors, are able to reduce superoxide anion production by NADPH oxidase in THP-1-derived monocytes. J Cardiovasc Pharmacol 40:611–617
Wassmann S, Laufs U, Baumer AT, Muller K, Ahlbory K, Linz W et al (2001) HMG-CoA reductase inhibitors improve endothelial dysfunction in normocholesterolemic hypertension via reduced production of reactive oxygen species. Hypertension 37:1450–1457
Rueckschloss U, Galle J, Holtz J, Zerkowski HR, Morawietz H (2001) Induction of NAD(P)H oxidase by oxidized low-density lipoprotein in human endothelial cells: antioxidative potential of hydroxymethylglutaryl coenzyme A reductase inhibitor therapy. Circulation 104:1767–1772
Sakai M, Kobori S, Matsumura T, Biwa T, Sato Y, Takemura T et al (1997) HMG-CoA reductase inhibitors suppress macrophage growth induced by oxidized low density lipoprotein. Atherosclerosis 133:51–59
Wassmann S, Laufs U, Müller K, Konkol C, Ahlbory K, Bäumer AT et al (2002) Cellular antioxidant effects of atorvastatin in vitro and in vivo. Arterioscler Thromb Vasc Biol 22:300–305
Luo JD, Zhang WW, Zhang GP, Zhong BH, Ou HJ (2002) Effects of simvastatin on activities of endogenous antioxidant enzymes and angiotensin-converting enzyme in rat myocardium with pressure-overload cardiac hypertrophy. Acta Pharmacol Sin 23:124–128
Deakin S, Leviev I, Guernier S, James RW (2003) Simvastatin modulates expression of the PON1 gene and increases serum paraoxonase: a role for sterol regulatory element-binding protein-2. Arterioscler Thromb Vasc Biol 23:2083–2089
Li J, Sun YM, Wang LF, Li ZQ, Pan W, Cao HY (2010) Comparison of effects of simvastatin versus atorvastatin on oxidative stress in patients with coronary heart disease. Clin Cardiol 33:222–227
Kaesemeyer WH, Caldwell RB, Huang J, Caldwell RW (1999) Pravastatin sodium activates endothelial nitric oxide synthase independent of its cholesterol-lowering actions. J Am Coll Cardiol 33:234–241
Laufs U, Gertz K, Dirnagl U, Bohm M, Nickenig G, Endres M (2002) Rosuvastatin, a new HMG-CoA reductase inhibitor, upregulates endothelial nitric oxide synthase and protects from ischemic stroke in mice. Brain Res 942:23–30
Fuhrman B, Koren L, Volkova N, Keidar S, Hayek T, Aviram M (2002) Atorvastatin therapy in hypercholesterolemic patients suppresses cellular uptake of oxidized-LDL by differentiating monocytes. Atherosclerosis 164:179–185
Adam O, Laufs U (2008) Antioxidative effects of statins. Arch Toxicol 82:885–892
Liao JK, Laufs U (2005) Pleiotropic effects of statins. Annu Rev Pharmacol Toxicol 45:89–118
Ghayour-Mobarhan M, Alamdari D, Moohebati M, Sahebkar A, Nematy M, Safarian M et al (2009) Determination of pro-oxidant-antioxidant balance after acute coronary syndrome using a rapid assay: a pilot study. Angiology 60:657–662
Koliakos GG, Befani CD, Mikropoulos D, Ziakas NG, Konstas AG (2008) Prooxidant-antioxidant balance, peroxide and catalase activity in the aqueous humour and serum of patients with exfoliation syndrome or exfoliative glaucoma. Graefes Arch Clin Exp Ophthalmol 246:1477–1483
Parizadeh SMR, Azarpazhooh MR, Mobarra N, Nemati M, Alamdari DH, Tavallaie S et al (2011) Prooxidant-antioxidant balance in stroke patients and 6-month prognosis. Clin Lab (Accepted)
Tara F, Rayman MP, Boskabadi H, Ghayour-Mobarhan M, Sahebkar A, Alamdari DH et al (2010) Prooxidant-antioxidant balance in pregnancy: a randomized double-blind placebo-controlled trial of selenium supplementation. J Perinat Med (Epub ahead of print). doi:10.1515/JPM.2010.068
Stoll LL, McCormick ML, Denning GM, Weintraub NL (2004) Antioxidant effects of statins. Drugs Today 40:975–989
Acknowledgments
We are particularly grateful to the patients and their family members who volunteered to participate in this study. This work was financially supported by the Iran National Science Foundation and Mashhad University of Medical Science (MUMS), Mashhad, Iran.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Parizadeh, S.M.R., Azarpazhooh, M.R., Moohebati, M. et al. Simvastatin Therapy Reduces Prooxidant-Antioxidant Balance: Results of a Placebo-Controlled Cross-Over Trial. Lipids 46, 333–340 (2011). https://doi.org/10.1007/s11745-010-3517-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11745-010-3517-x