Abstract
Polyhydroxyalkanoates (PHAs) are polyesters synthetized by microorganisms or a chemical synthetic route with inherent chemical and physical properties comparable to conventional non-biodegradable polymers but a less environmental impact. Furthermore, the new generations of PHAs have found engineering and specialties applications in biotechnological sector, biomedical for tissue engineering, drug delivery, etc. Similarly, synthesis, processing and recycle of PHAs involves processes that helps to change until a circular and green economy. Nevertheless, the low cost-effectiveness associated with fermentation and downstream processing for recovery and purification of PHAs after biopolymerization are one of the issues that remains. Additionally, PHAs offer several mechanical behaviors from hard to elastic due to partial crystallinity, wide values in glass transition temperature, variety of structures of repeating units, as well as several additives and fillers to design tailor-made properties. Moreover, PHAs are usually blended with other biodegradable polymers searching synergistic interactions (e.g., in mechanical, biodegradability, barrier properties, etc.) through miscibility modification and microdomains interactions for the diversification of their applications. Eventually, single use products of PHAs for packing could improve the managing plastics waste through reach short times of biodegradation, a carbon neutrality and the use of some residues and contaminants sources as raw materials for PHAs synthesis.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
In the last years, the production rate of plastics has reached above 360 million tons per year [1] even during the economy contraction by COVID-19 pandemic. Considering the substantial quantities of plastic waste generated, only 9% is recycled, 19% is incinerated, 50% reaches landfills and 22% remains as uncollected litter or mismanaged [2]. Additionally, approximately of 0.02% of plastic waste leaked into aquatic environments and 0.005% flowed into oceans [2]. While a build international willingness and participation to curb plastic pollution through summits, agendas, social and individual strategies [3, 4] and governmental regulations [5, 6] the plastic waste exceeds the efforts to mitigate plastic pollution [7, 8].
Green polymers are produced using green chemistry, and IUPAC defined the latter as the invention, design and application of chemical products and processes to reduce or to eliminate the use or generation of substances hazardous to humans, animals, plants, and the environment [9]. Polyhydroxyalkanoates (PHAs) are one of the most attractive types of green polymers due to biodegradability [10], compostability [11], biocompatibility [12, 13], hydrophobicity [14], wide availability in mechanical behavior [15], barrier properties (for single-use products) [16], etc. [17]., in medical, environmental, energy and other areas [18].
The PHAs are polyesters synthetized by a wide variety of microorganisms (generally by bacteria) or via pure chemical synthesis with inherent chemical and physical properties so useful as the single-use and non-biodegradable polymers showing a less environmental impact compared with conventional polymers [17]. Furthermore, the new generations of PHAs have found specialties and engineering applications as in biotechnological sector, for example, in biomedical for tissue engineering, drug delivery, cosmetic surgery, etc. [19].
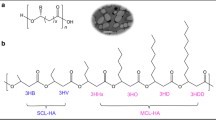
For the most part, the PHAs are synthetized by microorganisms producing lipid inclusions for energy storage in granular form inside the microorganisms’ structure. The advantage of PHAs considering other biopolymers is based on mechanical and thermal response because are natural polyesters of 3-, 4-, 5-, 6- and m-hydroxyalkanoic acids which produces generally thermoplastic polymers. In the backbone of polymer chain there is a –O–C– chemical bond that it makes sensitive (due to polarity of the covalent bond) to chain scission via hydrolysis using a biotic and/or abiotic degradation, see Fig. 1.
Compared to other biopolymers, polyhydroxyalkanoates have more than 150 different repeating units [22, 23], with a general structure that is shown in Fig. 1. If the group R = CH3 the polymer is named polyhydroxybutyrate of poly (3-hydroxyalkanoate) (P3HB). Nowadays, there are available several chemical functionalizations of the side groups of PHA searching for specific activities and interactions to diversify their applications. The molecular weight of PHAs is from hundreds to several million Daltons according to type of microorganisms, metabolic route, the type of reactor operation and carbon sources fed for the PHAs obtained with microbes and stability of polymerization mechanism, as well as conditions and type of reactor for a pure chemical synthesis.
According to the size of repeating units the PHAs are classified into three main groups:
-
Short chain length (scl-PHA): if the PHAs have 3–5 carbon atoms into repeating unit, leading to a mechanical behavior rigid and brittle, not recommended for biomedical and packing film applications.
-
Medium chain length (mcl-PHA): for PHAs having 6–14 carbon atoms, shows an elastomeric response to mechanical tests but a lower mechanical stress that limits the applications.
-
Long chain length (lgl-PHA): the repeating unit with more than 15 carbon atoms with elastomeric properties [15].
The mechanical properties and green characteristics depends or their pathways synthesis [24], chemical structure [25] and the hierarchy structure at nano and micro level. Because of chemical and structure difference of repeating units in PHAs, the physical, chemical, physicochemical properties as well as biodegradation differs from each other.
The PHAs have several disadvantages of microbial biopolymers in comparison to synthetic polymers, one of them are the high costs involved in the fermentation process, the carbon source, the efficiency of PHAs yield, the productivity of the process and down-stream processing [26, 27]. The final cost of PHA is directly related to PHA accumulation capacity of microorganism and productivity of the process. Furthermore, the economy of the process is still governed by the final application of the end products [28]. Nevertheless, these are eco-friendlier materials for production of single-use products, and these have several applications in medical fields. In addition to the advantages mentioned, the life cycle analysis has shown that PHAs are more sustainable that conventional synthetic polymers [17]. The big challenges are focus on segregate the pure monomers from complex mixtures and the biotechnologies of genetic engineering of microorganisms that allows the develop of new strains with higher accumulation capacities.
2 Synthesis of Polyhydroxyalkanoates
The synthesis of PHAs has been focused in two main routes: (1) biosynthesis to obtain semiprecision/speciality/engineering polymers [29, 30] and (2) precision polymers obtained by chemical routes for advanced applications searching stereoregularity [31], tacticity [32], sequence [33] and specificity [15] as well as incorporation of new side groups or functional groups [34]. Nevertheless, both ideas contribute to develop of mechanisms for diversification of structures’ polyhydroxyalkanoates and with this an increase of applications with a variety of chemical, physical and physicochemical properties.
2.1 Natural and Synthetic Synthesis of Polyhydroxyalkanoates
Biological and bioinspired polymerizations make use of the key feature in biology, the sequence control during biopolymerization. Here, the high complexity, and advanced properties of proteins of proteinaceous (e.g., enzymes) or polynucleotide (as in ribosomes) in nature involved in these polymerization mechanisms is simplify through metabolic pathways to obtention of PHAs.
The PHAs can be obtained by microorganisms are from ten thousands to several million Dalton controlling the PHA synthase structures and activities [35] and metabolic pathways that is not easily by a chemical synthesis approaches [36]. Besides, is easy to understand the lower cost in production at industry level because of diversification of raw materials. On the other hand, a higher control in the structure, sequence of repeating units, stereoregularity and chain’s architecture of the polymer can be expected using ring-opening polymerizations (ROP), among other polymerization types.
2.1.1 Natural Synthesis of Polyhydroxyalkanoates
2.1.1.1 Natural Microorganisms
The P3HB was the first PHA and was found by Maurice Lemoige in 1926 as intracellular granules in bacterium Bacillus megaterium [37]. Since that time, the P3HB has been the most studied and well characterized to be used as reserve material in bacteria above 80% of the cell dry weight (CDW) [38]. A biological polymerization is commonly described through metabolized mechanisms normally catalyzed via enzymatic process. However, natural biosynthesis of PHAs regularly does not allows a high control over the polymer chain [30] and isotactic polymers, as well as random copolymers are the usual PHAs produced.
A key factor for the structure of PHAs synthetized inside of microorganisms is the carbon sources fed. Thus, the molecules supplied as raw materials to the enzyme PHA synthase define the ended structure of PHAs. Considering the requirement of nutrients, nutrient stress (i.e., the deficiency or excess of nutrients along the time), and their growth pattern, PHA accumulating bacteria have been classified into two main groups: (G1) those that needs limited nutrients such as phosphorous, nitrogen, oxygen, sulfur and magnesium to store PHAs and are not able biosynthesize PHAs during their growth periods [39, 40] (e.g., A. eutrophus, Protomonas extorques, Pseudomonas oleovorans, and Pseudomonas) and (G2) the bacteria not affected by nutrient limitation, and it can accumulate PHAs during its growth phase [41] (e.g., Alcaligenes latus, Promotomonas extorquens, and recombinant Escherichia coli) [17].
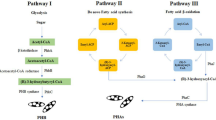
Heretofore, there are three primary metabolic pathways (see in Fig. 2) reported to synthetize PHAs:
Mainly metabolic pathways to synthetize polyhydroxyalkanoates [42]
Pathway 1: To obtain scl-PHAs between 3 and 5 carbon atoms, generally starts with the production of 3HB monomers by the Krebs cycle, involving the acetyl-CoA in presence of 3-ketothiolase enzyme (or PhaA) from sugars, fatty acids, oils or amino acids that are converted to acetoacetyl-CoA reductase to hydroxybutyryl-CoA and polymerized by scl-PHA synthase to produce P3HB [17, 43, 44].
Pathway 2: Initiate with the fatty acids as raw material by way of the β-oxidation in presence of 3-hydroxyacyl-CoA enzyme and mcl-PHA synthase [45] or lcl-PHA synthase [46], which preference of size of the repeating unit is due to proteins in the β-oxidation pathway for each one microorganism. Here, the fatty acids are transformed into enoyl-CoA and then by R-3-hydroxyacyl-CoA hydrates to R-3-hydroxyacyl-CoA to produce mcl-PHA polymers through mcl-PHA synthase. Moreover, a few PHA synthases in natural (e.g., Thermus thermophilus HB8, see Table 1) and engineered microorganisms (e.g., Transgenic arabidopsis thaliana [36], see Table 2) can produce copolymers of scl-mcl-PHAs, simultaneously utilizing pathways I and II.
Pathway 3: The in situ fatty acid synthesis or the use of substrates as glucose, sucrose and fructose pathway starts with R-3-hydroxyacyl-(acyl carrier protein) ACP dehydratase in presence of 3-hydroxyl-ACP-transacylase (PhaG enzyme) to produce R-3-hydroxyacyl-CoA and with mcl-PHA synthase finally obtain mcl-PHAs [17, 84].
Considering the activities of PHA synthase and depolymerase can affects the size of molecular-weight, Mn, the effect can be minimized in presence of chain transfer agents (as in traditional polymerizations) being helpful for the control using poly(ethylene glycol) (PEG), methanol, ethanol and isopropanol in the culture or via mutations in the N-terminus of PHA synthase [85]. Nevertheless, small changes during biopolymerization normally leads to variations in final molecular weight between a batch and another even at the same conditions.
2.1.1.2 Extremophile Microorganisms
Extremophiles, which concept is different from extremotolerant, are highly adapted and metabolically active under uncommon environmental. Their use for the synthesis of PHA increases the biological conditions to production of biopolymers at high temperatures, extreme pH conditions, salinity, radiation, desiccation, man-made toxic environments (such as toxic metals, surfactants, etc.) and other unfavorable environmental conditions to overcome, for example, in landfills, ocean, vinasses and toxic waste.
Extremophilic archaea could be an economically viable option in conventional aerobic processes [86]. However, their pathways and PHA accumulation capacities are less-know, even so, specific adaptive mechanisms towards extreme environments by extremophiles and specific role of PHAs are grow up [87]. Additionally, genetic engineering and process engineering approaches are required for high-rate PHA production using extremophilic archaea [86].
Haloarchaea and halophilic bacteria are considered a promising cell factories for PHA synthesis due to its several unique characteristics as high salinity requirement to avoid microbial contamination, high intracellular osmotic pressure allowing easy cell lysis for PHA recovery, and the use of a wide variety of low-cost substrates [64]. Within these, Hfx. mediterranei can produce PHAs from various organic substrates up to 70% of dry weight biomass [64].
The actinobacteria Rubrobacter xylanophilus has been exposed to multi-extremophilic growth conditions shown highly radiation-resistant, halotolerant, thermotolerant or even thermophilic with accumulation of PHAs [65], see Table 1. A wide spectrum of thermophilic microorganisms had been reported for the production of PHAs as Chelatococcus daeguensis TAD1, Zobellella denitrificans MW1, Bacillus licheniformis and B. licheniformis M2–12 with a higher content of dry weight over 60% of biomass [88].
The metabolic pathways do not exist in all extremophiles and the specific production of PHAs in extremophiles are not well-understood yet. Nevertheless, a necessity to develop genetic engineering approaches might improve their capacity for their commercialization and application in real scenarios.
2.1.2 Synthetic Biosynthesis of Polyhydroxyalkanoates
The production of PHAs can also be obtained via biosynthetic pathways, via genetic engineering, metabolic engineering, or synthetic biology approaches. In addition, genetically engineered polymers are designed by sequence-specific polymers by genetic engineering [89] as the case of proteins and polynucleotides used in genetic tools, technologies, processes, and other methods. With the development of genome editing and molecular biology approaches and modifying the PHA synthase enzyme, tailor PHAs can be synthetized with some degree of control in sequence of repeating units (random or block copolymers) and composition of repeating unit, changing the biodegradability, biocompatibility, as well as the thermal/mechanical and other properties [36].
In natural biosynthesis of PHAs the molecules fed to microorganism are structurally related to structure of repeating units of PHAs. However, the enzyme PhaC synthase is a key to create new metabolic pathways to use new molecules as raw materials as sugar that are low-cost [36, 90, 91]. PhaC is the most important element to determine the PHA composition because in bacteria depends not only on monomer supply, but also on specificity of PHA synthases [92]. Other advantage on the use of genetic modified microorganisms is for the search of a high production of PHA by microorganism or design tailor-made and robust microbes to produce PHA even for waste.
The case reported of the fatty acid in β-oxidation pathway and the 3-hydroxyacyl-ACP:CoA transacylase (PhaG) in Pseudomonas putida, increases the fatty acid flux and obtaining a higher accumulation of mcl-PHA syntheses [93]. The genetic engineering of genes to modify the PhaC encoding a lees-specific PHA synthase in pathway 2 allows the synthesis of PHAs as homopolymers, random or block copolymers, and functional polymers [94, 95], see Table 3. Some PHA synthases have been reported to polymerize scl and mcl repeating units as enzymes with the feature of low-specify PHA synthases which can be modified by molecular evolution or by chimera formation [36].
Taking into consideration one of the tools of genetic engineering, the system clustered regularly interspaced short palindromic repeats (CRISPR) associated to protein 9 (Cas9) has been used to edit eukaryotic genomes [98]. CRISPR Cas9 has been applied to control PHA biosynthesis pathway flux and to adjust PHA composition changing the composition of PHA synthase [99].
Clustered regularly interspaced short palindromic repeats interference (CRISPRi) has been employed to control the PhaC transcription and thus PhaC activity to maximize the P3HB contents [100]. A higher PhaC activity leads to higher accumulation with a less molecular weight and a wider molecular weight dispersity. PHB controlled in the intervals of 2.0 for 75.0% of CDW [85].
Finally, the extraction methods of PHAs from inside of microorganisms at the end of bioprocess can be classify in: solvent extraction, floatation, supercritical fluid extraction or aqueous two-phase extraction [17]. The PHA extraction is one of the costliest procedures in PHA production, it requires the separation of PHA-containing cells and the cell lysis to release intracellular PHA granules. Such as other synthesis process, the chemical and physical separation process needs more develop searching efficiently to reduces and grow up to industrial scales.
A high production of PHAs is obtained by fed batch or continuous fermentation. In fed-batch culture of bacteria belonging to G1, a two-step cultivation method is often employed. A desired concentration of biomass without nutrient limitation in the first stage after and in the second stage a limiting concentration of nutrients. In second stage, the residual cell concentration remains almost constant, and the cell concentration increases only because of the intracellular accumulation of PHA. Other bacteria belonging to G1 produce PHA more efficiently when a nutrient is limited but not completely depleted. For a bioreactor working in fed-batch of G2, the nutrient feeding strategy to produce yield of PHAs must be applied along with the selection of microorganism based on cell’s ability to use an inexpensive carbon source, growth rate, polymer synthesis rate, and the maximum accumulation of PHAs [40].
2.2 Chemical Synthesis of Polyhydroxyalkanoates
PHAs produced by microorganisms have limitations in advances applications by the isotactic and not sequence controlled of repeating units along polymer chain. However, PHAs produced by bacteria commonly are purely isotactic polymers containing a chiral site in each repeating unit leading to thermal and mechanical properties as a function of length of the side group (R-group in Fig. 1), making them more useful for a wide range of applications [31]. Unlike of natural synthesis of PHA, the chemical mechanisms have the aim of incorporation of atypical side groups as aromatic pendant groups to increase the glass transition temperature (Tg) or long branches as side groups to increase the flexibility of the chain and with that modify the crystallinity [34]. Additionally, the molecular weight and molecular weight dispersity are reasonably more controllable using chemical synthesis in comparison with microbes because even using the same microorganism and the same conditions and raw materials during the microorganism growth and reproduction are several changes at molecular level. Other advantage to synthesis using metabolic pathways, a pure chemical polymerization might provide a better scalability and faster reaction kinetics [31].
The PHAs has been obtained via ring-opening polymerization of cyclic esters since 1960s, adding up PHA with different chemical structures and stereoregularities. Some bespoke polyesters can be obtained through an organocatalyzed mechanism via ring-opening polymerization of cyclic esters as stereoselective synthesis of isotactic and syndiotactic P3HB by racemic 4-alkoxymethylene-β-propiolactones (BPLORs) [101] as shown in Fig. 3. Using catalytic activity of alkoxy-functional poly(3-hydroxyalkanoate) and the simple modification of o, p-R’ substituents on [ONXOR’2]2− plataform of yttrium catalyst for the switching syndioselective to isoselective polymerization of β-lactones.
Ring-opening polymerization from racemic β-lactones to produce isotactic and syndiotactic poly(3-hydroxybutyrate) [101]
Ligny R. et al., has demonstrated that o, p-dichloro-substituted ligand deliver highly isotactic polyesters (Pi > 0.90) from three racemic BPLOR monomers (where R: allyl, benzyl and methyl groups). Yttriumisopropoxide catalysts/initiators were generated during the reaction from the activity of 1 equiv. of iPrOH from precursors 1a-g differing by the nature of the R’ phenolate substituents. The final number molecular weight was until to 18,300 Da and a molecular weight dispersity about 1.1 [101] similar to other ring-opening polymerizations utilizing other catalyzers [34].
3 Physical, Chemical and Physicochemical Properties and Characterization
PHAs shows properties analogue to conventional polymers such as hydrophobicity, resistance to UV, high degree of polymerization. They are usually thermoplastic polymers, exhibiting a wide variety of mechanical properties from hard to elastomeric response with the advantage of biodegradability and biocompatibility.
3.1 Physical Properties
Glass transition temperatures of PHAs is about 0 °C, triggering the flexibility of the polymer chain and a melting point from 50 to 179 °C [96], see Table 3. For other hand, thermal degradability starting at 260 °C for copolymer with 3HV, 4HB and 3HHx repeating units until 300 °C for P3HB [96].
The crystalline structure of P3HB determined by X-ray indicated an orthorhombic unit cell with dimensions a = 0.576 nm, b = 1.320 nm, and c = 0.596 nm. Typically, P3HB forms lath-shape crystals with dimensions of around 0.3–2 µm for the short and 5–10 µm for the long axes. The ability of PHA to crystallize is determined by the inner properties and the structure of repeating units that for P3HB a 70% or higher of crystallinity degree it is found. For random copolymers with 3HB monomer is from 49 to 60% for mcl and 2% to 50% for scl. For copolymers with 3HB/3HV, 3HB/3HP and 3HB/3HHx the degrees of crystallinities measured by X-ray shown a slightly increased (50–70%) for the first because of isodimorphism, i.e., co-crystallization of the two repeating units of the homopolymer crystal lattices of P3HB and the second monomer. This leads to a reduction near to 50/50% for the rest of copolymers due to defects in the P3HB crystal lattice by the repeating units of 3HB [102].
3.1.1 Mechanical Properties
The wide variety of mechanical properties from hard to elastic is a function on composition, crystallinity degree and structure of repeating unit [40]. The maximum stress at break point is achieve at 45 MPa for P3HB, which has the shorter repeating unit and decrease as is expected for longer repeating units, see Fig. 1. A higher toughness and an elastic behavior are expected for mcl-PHA in comparison to scl-PHA because of long branches or long repeating units made of –C–C– bonds trigger a flexible polymer chain. Furthermore, a low crystallinity degree by side groups for chain folded and a glass transition temperature below of 0 °C (around −80 °C for monomers with more than 6 carbon atoms) for mcl-PHA and lgl-PHAs usually shows an elastomeric response.
The PHAs of high molecular weight (> 40 kDa) obtaining via fermentative processes exhibits mechanical properties which can be grouped into three subcategories: scl-PHA, which short chain length and repeating units until five carbon atoms with thin crystals and the higher melting point. Young modulus, tensile strength, impact strength and, UV resistance and oxygen permeability, due to this, the P3HB has a similar behavior to isotactic polypropylene, useful as packaging material that can increases with the copolymerization with soft repeating units or blending with elastic and partial o completely miscible polymer to avoid the brittleness that appears after several days. The mcl-PHA are amorphous macromolecules with decreasing glass transition temperature with increasing side groups length [103]. For lcl-PHA an higher crystallinity degree is expected with longer side groups and similar flexibility on the chain to mcl-PHA.
The length and mechanical flexibility of the side chain and its functional group modify considerably the properties like melting point, glass transition temperature, crystallinity, and the biodegradability. The Tg of the amorphous domain can be related to the maximum deformation in PHA along with the crystallinity degree according with the temperature of use of polymer material. However, a higher crystallinity degree leads to poor mechanical properties and PHAs need to be tailored to achieve a better performance and tunable mechanical properties blending with different types of PHAs, other synthetic biodegradable polymers or a chemical modification of PHA.
Some PHA processing methods as the incorporation of additives as plasticizers can lead to modulate the mechanical behavior changing the glass transition temperature in the final product. The PHA as commodity material can be added: poly (ethylene glycol), oligomeric lactic acid, glycerol diglycidyl ether or soybean oil as plasticizers.
3.2 Chemical Properties
Some post-polymerization modification to expand the structural variety of PHAs are focus on the chemical modification of unsaturated bonds. Here, the desired functional groups are attached to the side groups of the polymer chains to crosslinking [104].
The PHA modification add new properties as an enhanced hydrophilicity and thermoresponsibility in PHA-graft-PNIPAm [105], temperature controllable protein adsorption in PHA-graft-PDMAEMA [106], higher thermal degradation temperature and electrical conductivity for PHA-graft-graphene [107], intense photoluminescence under UV laser excitation with PHA modified with rare-earth [108], formation of hydrogels as in PHA modified with PDT [109], and superhydrophobicity in physical surface modification [110].
The carboxylation is the addition of carboxylic (–COOH) functional group to the substrate. These groups are an active binding site for biologically active moieties like hydrophilic as the phase transfer and dissociating agent [15, 111]. The halogenation enhances the properties, functions, and applications of polymers for the addition and substitution reactions. For other hand, hydroxylation makes easier acid or base catalyzed reactions for PHA modification in the presence of low molecular weight mono or diol compounds by the process of hydroxylation [112]. In addition of previous chemical modifications, the epoxidation increases the reactivity attaching epoxy groups that response as anionic and polar groups. These kinds of groups can be used for crosslinked polyhydroxyalkanoates for self-healing applications.
3.3 Properties for Single-Use Products of Polyhydroxyalkanoates
The use of PHAs for bottles, disposables, coatings with cardboard boxes, films, animal feed supplements and biofuels as hydroxyalkanoates methyl esters are some of their single-use applications. The general properties for food packaging material are:
-
(a)
Antimicrobial function.
-
(b)
Mechanical properties.
-
(c)
Optical properties.
-
(d)
Thermal properties.
-
(e)
Eco-friendly.
-
(f)
Barrier properties: gas barrier, aroma barrier, vapor barrier.
-
(g)
UV-resistance.
The polyhydroxyalkanoates shown excellent barrier properties to gases [113]. The permeability of water vapor values of P3HB and its copolymer with 3HV in films are comparable to opponents such as PET and poly (vinyl chloride) [114, 115]. Evaluation of water vapor barrier properties of the packaging material is one of the most importance as physical or chemical deterioration of the packed food and important to maintain and extend the shelf-life of packaged food [116].
The oxygen permeability value of a packaging material is a decisive key to preserve fresh foods (e.g., fruits, vegetables, meats, etc.). The oxidative deterioration affects its color, flavor, and microbial stability for lacteous products. If the oxygen transmission rate is below of 2 nmol ms−1GPa−1; the material is often labeled as “Barrier polymers” [116]. The CO2 is another gas of interest to conserve fresh vegetables and food sensible to oxidation. For these barrier properties, a low CO2 permeability of P3HB exhibits a diffusion coefficient value comparable to poly(vinylidene chloride) [114]. However, the water and gas transport properties of commercial PHAs films shows a higher water and gas permeability values for solvent cast films compared to compressed counterparts [117].
The nature acidic or basic of foods may cause hydrolytic reactions to the packaging material. Unfortunately, the chemical resistance to several values of pH in PHAs are known to undergo acid-catalyzed hydrolytic degradation [114]. This kind of degradation change drastically the mechanical properties of films’ polymer. An easy solution can be found in the composite materials or blending to modify the susceptibility to chemical degradation.
Finally, the migration of subproducts as monomers or additives added for the processing plays an important role for food safety. The P3HB using for food packaging, commercial grade BIOCYCLE, obtained through injection process shows a performance compared to polypropylene [118].
The PHA exhibit greater UV-resistance than compounds such as propylene and the addition of UV stabilizer led to improvement in the retention of mechanical properties final products.
4 Processing, Blends and Composites of Polyhydroxyalkanoates
The processing of PHAs usually is leading by extrusion or injecting process with extrusion temperature profiles (140–160 °C) about 100 °C below the degradation temperatures (240–270 °C) [119]. For this reason, the residence time distribution in melting processing must be careful in extruders and injector to minimizing the thermal degradation. The rheological properties depend on the chemical structure and molecular weight because of melting regime the viscosity always increases with the size of molecular weight of the polymers. Some additives for manufacturing for conventional polyesters can be used for PHAs but green additives for are not completely extended for PHA.
Electrospinning is also used to fabricate nanofibers with the selection of an appropriate and non-hazardous solvent o solvent system that determines the rheological properties. The electrospin nanofibers have several applications such filtration sysrems, chemical and optical sensors, tissue engineering, drug release due to their high surface are to volume ratio, small pore size and high porosity.
Blending is a simple and effective approach to produce new polymeric materials with improved properties. Polymer blending has attracted the attention because of polymers with extraordinary properties can be obtained via chemical synthesis are more expensive than existing polymers and blending operations. To achieve this aim, a high or partial miscibility must be searched to ensure at least a micro or nano segregation of phases to achieve a synergistic interaction between domains. On the contrary, a compatibilizer needs to be added to increase the interaction between polymeric phases.
The properties of blends using PHAs with starch, poly (lactic acid), poly (ε-caprolactone) and cellulose can be improved and adding the new properties as homopolymers if a phase segregation at micro or nano level is ensure. The blending of PHA with natural raw materials usually help to reduce the cost and increase the biodegradability for single-use product in packaging products. The cellulose derivatives are of great interest as blending components with PHA because of their compatibility with and the enhance of rate degradation. The PHA blending with starch allows the compostability of materials due to inherent biodegradability and abundance. The PHA blending with lignin makes the blend valuable for the high functional groups presents in lignin. Besides, the thermal analysis indicated that lignin can improve the total thermal stability of P3HB [15].
The blending of PHA with other biodegradable polymers as poly (lactic acid) is environmentally friendly. The P3HB/PLA blend is one of the most studied blends, due to mechanical properties shows an intermediate response following the blend law between the individual components. PCL/PHAs shows miscibility with a PCL content between 0 and 30 w/w% with high flexibility and good biodegradability to be applied in packaging products [120].
Blends of PHBV and polyurethane (TPU) improves mechanical and barrier properties, at highest concentration of TPU and increased water vapor permeability[121].
4.1 Nanocomposites and Fillers
The application of nanoparticles and nanofillers as composite is attractive because of the nanofillers not only enhance the polymer crystallization, the gas-barrier, thermo-mechanical and physicochemical properties [122]. The mainly nanofillers are: silylated kaolinite, carbon nanotubes, graphene, organophilic montmorillonite, nanoclay and cellulose nanocrystal [122], which properties added require a minimum concentration to incorporate the partial characteristics of material, e.g., electrical o thermal conductivity with the addition of carbon nanotubes or graphene.
An option to reduce significantly the oxygen transmission rate was obtained with composite of P3HB/cellulose nanocrystal (CNC) by the positive effect on crystallinity of CNC [97].
5 Biotechnological Applications of Polyhydroxyalkanoates
5.1 Drug Delivery Carriers
The PHAs are biodegradable and biocompatible polymers [13] reason for which are used as nano and micro particles to release drugs. The PHAs have been applied in several and in humans to treat the gingivitis [123, 124]. The PHB have also been used in transdermal tissue as vehicle to increase the transdermal permeability of tamsulosin drug [125]. The P3HB and its copolymers in nanoparticles have been used for the release of molecular drugs able to cross intracellular membranes in applications as anticancer drugs, immunomodulatory agents, antibiotics, and hormones [126].
Different processing routes for fabrication of PHAs for drug delivery can be chosen as: spherical or worm-like particles obtained by emulsion or supercritical fluids that normally has been studied until an optimal rate and dose regimen in order to minimize side effects and toxicity.
For in vivo therapy poly (3-hydroxybutyrate-co-4- hydroxybutyrate) are used as antibiotic in Sulperazone® and Duocid® to the controlled release for chronic osteomyelitis [127]. To release an hormone for osteoporosis therapy the P3HB3HHx is used [128]. For cancer of colon, the release of 5-fluorouracil is leading with blend spheres of P3HB3HV and cellulose acetate phthalate [129].
5.2 Scaffolds and Medical Devices
The aforementioned properties for drug delivery and low inflammatory response makes attractive their use in tissue regeneration for human body ad bio-implants [130]. For this, PHAs are used as matrices in vitro for proliferation of human cells as endothelium cell, liver cells, and fibroblasts as they show adhesive properties to PHAs [131]. Endothelium cells, isolated hepatocytes and fibroblast show similar adhesion to P3HB and P3HV when are used as matrices for regeneration or cellular growth [131]. Microspheres of PHBV have been used in brain tissue engineering to support primary neurons [132].
Porous materials can be design via 3D printing, gas forming, phase separation/evaporation as scaffolds in tissue engineering for bone tissue regeneration, tissue engineering blood vessels and cardiac valves. The application of PHA, especially PHA, and biodegradable scaffolds are used to replace defective valves in human heart [133]. Fibers can be obtained through extrusion or electrospinning.
In vivo implants as the poly (3-hydroxyoctanoate)/poly (3-hydroxybutyrate) blends have been used as highly tensile wires for sutures [134]. Poly(3-hydroxybutyrate-co-3- hydroxyhexanoate) has been applied in peripheral nerve tissue engineering for rat model experiments for nerve regeneration [135]. Blends of poly (3-hydroxyoctanoate)/poly (3-hydroxybutyrate) for preparation of blood vessels stents [136].
6 Degradation and Biodegradability of Polyhydroxyalkanoates
The biodegradability of PHAs is well-known due to PHA hydrolases and depolymerases enzymes produced by microorganisms which assist the degradation of PHA. The key factors involving the structure of PHAs in biodegradability are chemical composition, molecular weight, and crystallinity degree. For other hand, the environmental conditions as temperature, pH, moisture, and the oxygen content are the most important factor in biodegradation.
The PHAs are degraded to H2O and CO2 under aerobic conditions and to methane under anaerobic conditions by microorganisms in soil, sea, lake water and sewage. The PHAs are solid polymers with a high molecular weight and before to be transported through the cell wall usually the microorganisms excrete extracellular PHA depolymerases to hydrolyze PHA into water-soluble oligomers and monomers to be used as nutrients [40].
The abiotic degradation of PHA and their blends is through hydrolytic mechanism by the chain scission in the –O–C– chemical bond that is accelerated to 60 °C. To increase the degradation rate, carboxylic groups, amine groups or any polar groups promote the water penetration into the polymer making attractive for degradation in water as in oceans, lakes, rivers, etc.
The PHA are high resistant to chemical decomposition by strong agents such as NaOH but easily degraded by strong acids and soluble in chloroform and other chlorinated hydrocarbons.
The enzymatic activities depend on the composition of the polymer chain and the environmental conditions during degradation [42], see Table 4. The biodegradation of PHAs polymers in anaerobic sewage is from months to years in saline water and ultraviolet light increases the fate of degradation [137]. Additionally, inside of mammalian systems, the hydrolysis and degradation are very slow [138].
Finally, the PHA and their copolymers are readily degraded in a several environmental conditions, with faster degradation rates in anaerobic sewage and slowest in sea water. Nevertheless, the locations for biotic (biodegradation) and abiotic degradation always requires be tested due to diverse microorganisms and the local conditions.
7 Conclusions
Polyhydroxyalkanoates can be synthetized by microorganisms as a reserve for energy and carbon (under stressful conditions where there is an excess of carbon and an absence of an essential nutrient) or a pure chemical route to achieve a higher control on the structure and composition of polymer chain. Biobased plastics will certainly be an effective way to reduce the carbon footprint when gradually replace the conventional plastics.
The reduction of the production cost of PHAs can introduce a new platform for their applications in biomedical fields. With the advances in genetic engineering and synthetic biology, the PHAs will be produced with a comparative price compared with petroleum-based polymers.
Cost-efficient extraction method is also considered a crucial factor, which determines the economy of these green polymers. However, The PHA nanocomposites produced with nanosized fillers, have reached the level at which they can compete with the properties of conventional plastics, making attractive in the packaging industry. Searching create green polymers, additives, fillers and reinforcements need to be developed to improve the physical and mechanical properties for the diversification of applications.
Although biodegradation studies of PHA in soils, aquatic, and atypical environments (as in human body) need a wider geographical reach, a great part of evidence shows a full degradation for long periods of time decreasing the contamination impact. The PHA are becoming attractive in the future to replace at least the conventional plastics and for advanced applications as the medical fields, for example, in drug release and tissue engineering making use of some polymer tools.
References
Nicholson, S.R., Rorrer, N.A., Carpenter, A.C., Beckham, G.T.: Manufacturing energy and greenhouse gas emissions associated with plastics consumption. Joule 5, 673–686 (2021). https://doi.org/10.1016/j.joule.2020.12.027
OECD Global Plastics Outlook Database: Plastic pollution is growing relentlessly as waste management and recycling fall short, says OECD. https://doi.org/10.1787/de747aef-en
Kumar, R.V., Hari, B.S., Ved, P.R., Biswajit, S., Jayanta, B., Brajesh, K.D., Goel, S.: Challenges and strategies for effective plastic waste management during and post COVID-19 pandemic. Sci. Total Environ. 750 (2021). https://doi.org/10.1016/j.scitotenv.2020.141514
Dey, A., Dhumal, C.V., Sengupta, P., Kumar, A., Pramanik, N.K., Alam, T.: Challenges and possible solutions to mitigate the problems of single-use plastics used for packaging food items: a review. J. Food Sci. Technol. 58, 3251–3269 (2021). https://doi.org/10.1007/s13197-020-04885-6
Lam, C.-S., Ramanathan, S., Carbery, M., Gray, K., Vanka, K.S., Maurin, C., Bush, R., Palanisami, T.: A comprehensive analysis of plastics and microplastic legislation worldwide. Water Air Soil Pollut. 229, 19 (2018). https://doi.org/10.1007/s11270-018-4002-z
Filiciotto, L., Rothenberg, G.: Biodegradable plastics: standards, policies, and impacts. Chemsuschem 14, 56–72 (2021). https://doi.org/10.1002/cssc.202002044
Borelle, S.B., Ringma, J., Law-Lavender, K., Monnahanc, C., Lebreton, L., MC Givern, A., Murphy, E., Jambeck, J., Leonard, G.H., Hilleary, M.A., Eriksen, M., Possingham, H.P., De Frond, H., Gerber, L.R., Polidoro, B., Tahira, A., Bernard, M., Barnes, M., Rochman, C.M.: Predicted growth in plastic waste exceeds efforts to mitigate plastic pollution. Science 369, 1515–1518 (2020). https://doi.org/10.1126/science.aba3656
Dauvergne, P.: Why is the global governance of plastic failing the oceans? Glob. Enviromental Chang. 51, 22–31 (2018). https://doi.org/10.1016/j.gloenvcha.2018.05.002
Tundo, P., Anastas, P., Black, D.S., Breen, J., Collins, T., Memoli, S., Miyamoto, J., Polyakoff, M., Tumas, W.: Special topic issue on green chemistry. Pure Appl. Chem. 72, 1207–1208 (2000). https://doi.org/10.1351/pac200072071207
Meereboer, K.W., Misra, M., Mohanty, A.K.: Review of recent advances in the biodegradability of polyhydroxyalkanoate (PHA) bioplastics and their composites. Green Chem. 22, 5519–5558 (2020). https://doi.org/10.1039/D0GC01647K
Byrom, D.: The synthesis and biodegradation of polyhydroxyalkanoates from bacteria. Int. Biodeterior. Biodegrad. 31, 199–208 (1993). https://doi.org/10.1016/0964-8305(93)90005-M
Butt, F.I., Muhammad, N., Hamid, A., Moniruzzaman, M., Sharif, F.: Recent progress in the utilization of biosynthesized polyhydroxyalkanoates for biomedical applications—review. Int. J. Biol. Macromol. 120, 1294–1305 (2018). https://doi.org/10.1016/j.ijbiomac.2018.09.002
Elmowafy, E., Abdal-Hay, A., Skouras, A., Tiboni, M., Csettari, L., Guarino, V.: Polyhydroxyalkanoate (PHA): applications in drug delivery and tissue engineering. Expert Rev. Med. Devices. 16, 467–482 (2019). https://doi.org/10.1080/17434440.2019.1615439
Patil, P.B., Sarkar, D., Poddar, K., Sarkar, A.: Synthesis and characterization of polyhydroxyalkanoates from soil bacterium Bacillus sp. PhNs9. J. Chem. Technol. Biotechnol. (2022). https://doi.org/10.1002/jctb.7093
Sharma, V., Sehgal, R., Gupta, R.: Polyhydroxyalkanoate (PHA): properties and modifications. Polymer (Guildf). 212, (2021). https://doi.org/10.1016/j.polymer.2020.123161
Xu, P., Yang, W., Niu, D., Yu, M., Du, M., Dong, W., Chen, M., Lemstra, P.J., Ma, P.: Multifunctional and robust polyhydroxyalkanoate nanocomposites with superior gas barrier, heat resistant and inherent antibacterial performances. Chem. Eng. J. 382, (2020). https://doi.org/10.1016/j.cej.2019.122864
Kumar, M., Rathour, R., Singh, R., Sun, Y., Pandey, A., Gnansounou, E., Lin, K.Y.A., Tsng, D.C.W., Thakur, I.S.: Bacterial polyhydroxyalkanoates: opportunities, challenges, and prospects. J. Clean. Prod. 263 (2020). https://doi.org/10.1016/j.jclepro.2020.121500
Philip, S., Keshavarz, T., Roy, I.: Polyhydroxyalkanoates: biodegradable polymers with a range of applications. J. Chem. Technol. Biotechnol. Int. Res. Process. Environ. clean Technol. 82, 233–247 (2007). https://doi.org/10.1002/jctb.1667
Kalia, V.C., Ray, S., Patel, S.K.S., Singh, M., Singh, G.P.: The dawn of novel biotechnological applications of polyhydroxyalkanoates. In: Biotechnological Applications of Polyhydroxyalkanoates, pp. 1–11. Singapore (2019). https://doi.org/10.1007/978-981-13-3759-8_1
Ojumu, T., Yu, J., Solomon, B.: Production of polyhydroxyalkanoates, a bacterial biodegradable polymers. Afr. J. Biotechnol. 3, 18–24 (2004). https://doi.org/10.4314/ajb.v3i1.14910
Lee, S.Y.: Bacterial polyhydroxyalkanoates. Biotechnol. Bioeng. 49, 1–14 (1996). https://doi.org/10.1002/(SICI)1097-0290(19960105)49:1%3c1::AID-BIT1%3e3.0.CO;2-P
Zhang, B., Carlson, R., Srienc, F.: Engineering the monomer composition of polyhydroxyalkanoates synthesized in Saccharomyces cerevisiae. Appl. Environ. Microbiol. 72, 536–543 (2006). https://doi.org/10.1128/AEM.72.1.536-543.2006
Tang, X.: Chemical synthesis of polyhydroxyalkanoates via metal-catalyzed ring-opening polymerization of cyclic esters. In: In: Advances in Polymer Science, pp. 1–14. Springer, Heidelberg, Berlin (2022). https://doi.org/10.1007/12_2022_119
Masood, F., Yasin, T., Hameeed, A.: Comparative oxo-biodegradation study of poly-3-hydroxybutyrate-co-3-hydroxyvalerate/polypropylene blend in controlled environments. Int. Biodeterior. Biodegradation. 87, 1–8 (2014). https://doi.org/10.1016/j.ibiod.2013.09.023
Luzi, F., Torre, L., Kenny, J.M., Puglia, D.: Materials: bio-and fossil-based polymeric blends and nanocomposites for packaging: structure–property relationship. Materials 12, 471–520 (2019). https://doi.org/10.3390/ma12030471
Koller, M., Braunegg, G.: Advanced approaches to produce polyhydroxyalkanoate (PHA) biopolyesters in a sustainable and economic fashion. EuroBiotech. J. 2, 89–103 (2018). https://doi.org/10.2478/ebtj-2018-0013
Zahari, M.A.K.M., Abdullah, S.S.S., Roslan, A.M., Ariffin, H., Shirai, Y., Hassan, M.A.: Efficient utilization of oil palm frond for bio-based products and biorefinery. J. Clean. Prod. 65, 252–260 (2014). https://doi.org/10.1016/j.jclepro.2013.10.007
Chen, S.S., Maneerung, T., Tsang, D.C., Ok, Y.S., Wang, C.H.: Valorization of biomass to hydroxymethylfurfural, levulinic acid, and fatty acid methyl ester by heterogeneous catalysts. Chem. Eng. J. 328, 246–273 (2017). https://doi.org/10.1016/j.cej.2017.07.020
Alves, A.A., Siqueira, E.C., Barros, M.P.S., Silva, P.E.C., Houllou, L.M.: Polyhydroxyalkanoates: a review of microbial production and technology application. Int. J. Environ. Sci. Technol. 1–12 (2022). https://doi.org/10.1007/s13762-022-04213-9
Chen, G.Q.: Plastics completely synthesized by bacteria: polyhydroxyalkanoates. In: Chen, G.Q. (ed.) Plastics from Bacteria: Natural Functions and Applications, pp. 17–37. Springer, Heidelberg, Berlin (2010). https://doi.org/10.1007/978-3-642-03287-5_2
Tang, X., Westlie, A.H., Caporaso, L., Cavallo, L., Falivene, L., Chen, E.Y.X.: Biodegradable polyhydroxyalkanoates by stereoselective copolymerization of racemic diolides: stereocontrol and polyolefin-like properties. Angewandte 130, 7955–7964 (2020). https://doi.org/10.1002/ange.201916415
Bouyahyi, M., Ajellal, N., Kirillov, E., Thomas, C.M., Carpentier, J.F.: Exploring electronic versus steric effects in stereoselective ring-opening polymerization of lactide and β-butyrolactone with amino-alkoxy-bis (phenolate)–yttrium complexes. Chem. Eur. J. 17, 1872–1883 (2011). https://doi.org/10.1002/chem.201002779
Luo, Z., Wu, Y.L., Li, Z., Loh, X.J.: Recent progress in polyhydroxyalkanoates-based copolymers for biomedical applications. Biotechnol. J. 14, (2019). https://doi.org/10.1002/biot.201900283
Westlie, A.H., Chen, E.Y.X.: Catalyzed chemical synthesis of unnatural aromatic polyhydroxyalkanoate and aromatic–aliphatic phas with record-high glass-transition and decomposition temperatures. Macromolecules 53, 9906–9915 (2020). https://doi.org/10.1021/acs.macromol.0c02110
Sindhu, R., Madhavan, A., Arun, K.B., Pugazhendhi, A., Reshmy, R., Awasthi, M.K., Sirohi, R., Tarafdar, A., Binod, P.: Metabolic circuits and gene regulators in polyhydroxyalkanoate producing organisms: intervention strategies for enhanced production. Bioresour. Technol. 327 (2021). https://doi.org/10.1016/j.biortech.2021.124791
Chen, G.Q., Hajnal, I., Wu, H., Lv, L., Ye, J.: Engineering biosynthesis mechanisms for diversifying polyhydroxyalkanoates. Trends Biotechnol. 33, 565–574 (2015). https://doi.org/10.1016/j.tibtech.2015.07.007
Lemoigne, M.: Produits de deshydration et de polymerisation de l’acide β-oxybutyrique. Bull. Soc. Chim. Biol. 8, 770–782 (1926)
Keshavarz, T., Roy, I.: Polyhydroxyalkanoates: bioplastics with a green agenda. Curr. Opin. Microbiol. 13, 321–326 (2010). https://doi.org/10.1016/j.mib.2010.02.006
Guzik, M.W., Kenny, S.T., Duane, G.F., Casey, E., Woods, T., Babu, R.P., Nikodinovic-Runic, J., Murray, M., O’Conner, K.E.: Conversion of post consumer polyethylene to the biodegradable polymer polyhydroxyalkanoate. Appl. Microbiol. Biotechnol. 98, 4223–4232 (2014). https://doi.org/10.1007/s00253-013-5489-2
Khanna, S., Srivastava, A.K.: Recent advances in microbial polyhydroxyalkanoates. Process Biochem. 40, 607–619 (2005). https://doi.org/10.1016/j.procbio.2004.01.053
Rai, R., Roy, I.: Polyhydroxyalkanoates: the emerging new green polymers of choice. In: Sharma, S.K., Mudhoo, A. (ed.) A Handbook of Applied Biopolymer Technology, Synthesis, Degradation and Application, pp. 79–101. RSC Publishing (2011). https://doi.org/10.1039/9781849733458-00079
Verlinden, R.A., Hill, D.J., Kenward, M.A., Williams, C.D., Radecka, I.: Bacterial synthesis of biodegradable polyhydroxyalkanoates. J. Appl. Microbiol. 102, 1437–1449 (2007). https://doi.org/10.1111/j.1365-2672.2007.03335.x
Wang, Q., Tappel, R.C., Zhu, C., Nomura, C.T.: Development of a new strategy for production of medium-chain-length polyhydroxyalkanoates by recombinant Escherichia coli via inexpensive non-fatty acid feedstocks. Appl. Environ. Microbiol. 78, 519–527 (2012). https://doi.org/10.1128/AEM.07020-11
Tamaki, A., Kunioka, M., Soga, K.: Production of copolyesters of 3-hydroxybutyrate and 3-hydroxyvalerate by Alcaligenes eutrophus from butyric and pentanoic acids. Appl. Microbiol. Biotechnol. 28, 330–334 (1988). https://doi.org/10.1007/BF00268190
Sudesh, K., Abe, H., Doi, Y.: Synthesis, structure and properties of polyhydroxyalkanoates: biological polyesters. Prog. Polym. Sci. 25, 1503–1555 (2000). https://doi.org/10.1016/S0079-6700(00)00035-6
Guzik, M.W., Narancic, T., Ilic-Tomic, T., Vojnovic, S., Kenny, S.T., Casey, W.T., Duane, G.F., Casey, E., Woods, T., Babu, R.P., Nikodinovic-Runic, J., O’Connor, K.E.: Identification and characterization of an acyl-CoA dehydrogenase from Pseudomonas putida KT2440 that shows preference towards medium to long chain length fatty acids. Microbiology 160, 1760–1771 (2014). https://doi.org/10.1099/mic.0.078758-0
Sonnleitner, B., Heinzle, E., Braunegg, G., Lafferty, R.M.: Formal kinetics of poly-β-hydroxybutyric acid (PHB) production in Alcaligenes eutrophus H 16 and Mycoplana rubra R 14 with respect to the dissolved oxygen tension in ammonium-limited batch cultures. Eur. J. Appl. Microbiol. Biotechnol. 7, 1–10 (1979). https://doi.org/10.1007/BF00522473
Smit, A.M., Strabala, T.J., Peng, L., Rawson, P., Lloyd-Jones, G., Jordan, T.W.: Proteomic phenotyping of Novosphingobium nitrogenifigens reveals a robust capacity for simultaneous nitrogen fixation, polyhydroxyalkanoate production, and resistance to reactive oxygen species. Appl. Environ. Microbiol. 78, 4802–4815 (2012). https://doi.org/10.1128/AEM.00274-12
Gomez, J.G.C., Rodrigues, M.F.A., Alli, R.C.P., Torres, B.B., Netto, C.L., Oliveira, M.S., Da Silva, L.F.: Evaluation of soil gram-negative bacteria yielding polyhydroxyalkanoic acids from carbohydrates and propionic acid. Appl. Microbiol. Biotechnol. 45, 785–791 (1996). https://doi.org/10.1007/s002530050763
Yu, P.H., Chua, H., Huang, A.L., Lo, W., Chen, G.Q.: Conversion of food industrial wastes into bioplastics. Appl. Biochem. Biotechnol. 70, 603–614 (1998). https://doi.org/10.1007/BF02920172
Valentin, H.E., Dennis, D.: Production of poly (3-hydroxybutyrate-co-4-hydroxybutyrate) in recombinant Escherichia coli grown on glucose. J. Biotechnol. 58, 33–38 (1997). https://doi.org/10.1016/S0168-1656(97)00127-2
Morya, R., Kumar, M., Thakur, I.S.: Utilization of glycerol by Bacillus sp. ISTVK1 for production and characterization of polyhydroxyvalerate. Bioresour. Technol. Reports. 2, 1–6 (2018). https://doi.org/10.1016/j.biteb.2018.03.002
Kumar, M., Gupta, J., Thakur, I.S.: Production and optimization of polyhydroxyalkanoate from oleaginous bacteria Bacillus sp. ISTC1. RRJMB. 5, 80–89 (2016)
Kumar, M., Gupta, A., Thakur, I.S.: Carbon dioxide sequestration by chemolithotrophic oleaginous bacteria for production and optimization of polyhydroxyalkanoate. Bioresour. Technol. 213, 249–256 (2016). https://doi.org/10.1016/j.biortech.2016.02.038
Jiang, Y., Song, X., Gong, L., Li, P., Dai, C., Shao, W.: High poly (β-hydroxybutyrate) production by Pseudomonas fluorescens A2a5 from inexpensive substrates. Enzyme Microb. Technol. 42, 167–172 (2008). https://doi.org/10.1016/j.enzmictec.2007.09.003
Cavalheiro, J.M., de Almeida, M.C.M., Grandfils, C., Da Fonse, M.M.R.: Poly (3-hydroxybutyrate) production by Cupriavidus necator using waste glycerol. Process Biochem. 44, 509–515 (2009). https://doi.org/10.1016/j.procbio.2009.01.008
Wen, Q., Chen, Z., Tian, T., Chen, W.: Effects of phosphorus and nitrogen limitation on PHA production in activated sludge. J. Environ. Sci. 22, 1602–1607 (2010). https://doi.org/10.1016/S1001-0742(09)60295-3
Cromwick, A.M., Foglia, T., Lenz, R.W.: The microbial production of poly(-hydroxyalkanoates) from tallow. Appl. Microbiol. Biotechnol. 46, 464–469 (1996). https://doi.org/10.1007/s002530050845
Sun, Z., Ramsay, J.A., Guay, M., Ramsay, B.A.: Carbon-limited fed-batch production of medium-chain-length polyhydroxyalkanoates from nonanoic acid by Pseudomonas putida KT2440. Appl. Microbiol. Biotechnol. 74, 69–77 (2007). https://doi.org/10.1007/s00253-006-0655-4
Gumel, A.M., Annuar, M.S.M., Heildelberg, T.: Growth kinetics, effect of carbon substrate in biosynthesis of mcl-PHA by Pseudomonas putida Bet001. Brazilian J. Microbiol. 45, 427–438 (2014). https://doi.org/10.1590/S1517-83822014000200009
Pantazaki, A.A., Papaneophytou, C.P., Pritsa, A.G., Liakopoulou-Kyriakides, M., Kyriakidis, D.A.: Production of polyhydroxyalkanoates from whey by Thermus thermophilus HB8. Process Biochem. 44, 847–853 (2009). https://doi.org/10.1016/j.procbio.2009.04.002
Bhattacharyya, A., Pramanik, A., Maji, S.K., Haldar, S., Mukhopadhyay, U.K., Mukherjee, J.: Utilization of vinasse for production of poly-3-(hydroxybutyrate-co-hydroxyvalerate) by Haloferax mediterranei. AMB Express 2, 1–10 (2012). https://doi.org/10.1186/2191-0855-2-34
Koller, M., Hesse, P., Bona, R., Kutschera, C., Atlić, A., Braunegg, G.: Potential of various archae-and eubacterial strains as industrial polyhydroxyalkanoate producers from whey. Macromol. Biosci. 7, 218–226 (2007). https://doi.org/10.1002/mabi.200600211
Mitra, R., Xu, T., Xiang, H., Han, J.: Current developments on polyhydroxyalkanoates synthesis by using halophiles as a promising cell factory. Microb. Cell Fact. 19, 1–30 (2020). https://doi.org/10.1186/s12934-020-01342-z
Kouřilová, X., Schwarzerová, J., Pernicová, I., Sedlář, K., Mrázová, K., Krzyžánek, V., Nebesářová, J., Obruča, S.: The first insight into polyhydroxyalkanoates accumulation in multi-extremophilic Rubrobacter xylanophilus and Rubrobacter spartanus. Microorganisms 9, 909–922 (2021). https://doi.org/10.3390/microorganisms9050909
Cui, B., Huang, S., Xu, F., Zhang, R., Zhang, Y.: Improved productivity of poly (3-hydroxybutyrate)(PHB) in thermophilic Chelatococcus daeguensis TAD1 using glycerol as the growth substrate in a fed-batch culture. Appl. Microbiol. Biotechnol. 99, 6009–6019 (2015). https://doi.org/10.1007/s00253-015-6489-1
Ibrahim, M.H., Steinbüchel, A.: High-cell-density cyclic fed-batch fermentation of a poly (3-hydroxybutyrate)-accumulating thermophile, Chelatococcus sp. strain MW10. Appl. Environ. Microbiol. 76, 7890–7895 (2010). https://doi.org/10.1128/AEM.01488-10
Pernicova, I., Novackova, I., Sedlacek, P., Kourilova, X., Koller, M., Obruca, S.: Application of osmotic challenge for enrichment of microbial consortia in polyhydroxyalkanoates producing thermophilic and thermotolerant bacteria and their subsequent isolation. Int. J. Biol. Macromol. 144, 698–704 (2020). https://doi.org/10.1016/j.ijbiomac.2019.12.128
Sangkharak, K., Prasertsan, P.: The production of polyhydroxyalkanoate by Bacillus licheniformis using sequential mutagenesis and optimization. Biotechnol. Bioprocess Eng. 18, 272–279 (2013). https://doi.org/10.1007/s12257-012-0615-z
Jung, Y.K., Kim, T.Y., Park, S.J., Lee, S.Y.: Metabolic engineering of Escherichia coli for the production of polylactic acid and its copolymers. Biotechnol. Bioeng. 105, 161–171 (2010). https://doi.org/10.1002/bit.22548
Wang, H.H., Zhou, X.R., Liu, Q., Chen, G.Q.: Biosynthesis of polyhydroxyalkanoate homopolymers by Pseudomonas putida. Appl. Microbiol. Biotechnol. 89, 1497–1507 (2011). https://doi.org/10.1007/s00253-010-2964-x
Wang, Q., Yang, P., Liu, C., Xue, Y., Xian, M., Zhao, G.: Biosynthesis of poly (3-hydroxypropionate) from glycerol by recombinant Escherichia coli. Bioresour. Technol. 131, 548–551 (2013). https://doi.org/10.1016/j.biortech.2013.01.096
Meng, D.C., Chen, G.Q.: Synthetic biology of polyhydroxyalkanoates (PHA). In: Zhao, H., Zeng, A. (ed.) Synthetic Biology-Metabolic Engineering, pp. 147–154. Advances in Biochemical Engineering/Biotechnology, 162. Springer, Cham (2017). https://doi.org/10.1007/10_2017_3
Zhou, X.Y., Yuan, X.X., Shi, Z.Y., Meng, D.C., Jiang, W.J., Wu, L.P., Chen, J.C., Chen, G.Q.: Hyperproduction of poly (4-hydroxybutyrate) from glucose by recombinant Escherichia coli. Microb. Cell Fact. 11, 1–8 (2012). https://doi.org/10.1186/1475-2859-11-54
Meng, D.C., Wang, Y., Wu, L.P., Shen, R., Chen, J.C., Wu, Q., Chen, G.Q.: Production of poly (3-hydroxypropionate) and poly (3-hydroxybutyrate-co-3-hydroxypropionate) from glucose by engineering Escherichia coli. Metab. Eng. 29, 189–195 (2015). https://doi.org/10.1016/j.ymben.2015.03.015
Wang, Q., Liu, X., Qi, Q.: Biosynthesis of poly (3-hydroxybutyrate-co-3-hydroxyvalerate) from glucose with elevated 3-hydroxyvalerate fraction via combined citramalate and threonine pathway in Escherichia coli. Appl. Microbiol. Biotechnol. 98, 3923–3931 (2014). https://doi.org/10.1007/s00253-013-5494-5
Wong, Y.M., Brigham, C.J., Rha, C., Sinskey, A.J., Sudesh, K.: Biosynthesis and characterization of polyhydroxyalkanoate containing high 3-hydroxyhexanoate monomer fraction from crude palm kernel oil by recombinant Cupriavidus necator. Bioresour. Technol. 121, 320–327 (2012). https://doi.org/10.1016/j.biortech.2012.07.015
Wang, Q., Yang, P., Xian, M., Liu, H., Cao, Y., Yang, Y., Zhao, G.: Production of block copolymer poly (3-hydroxybutyrate)-block-poly (3-hydroxypropionate) with adjustable structure from an inexpensive carbon source. ACS Macro Lett. 2, 996–1000 (2013). https://doi.org/10.1021/mz400446g
Pederson, E.N., McChalicher, C.W., Srienc, F.: Bacterial synthesis of PHA block copolymers. Biomacromolecules 7, 1904–1911 (2006). https://doi.org/10.1021/bm0510101
Hu, D., Chung, A.L., Wu, L.P., Zhang, X., Wu, Q., Chen, J.C., Chen, G.Q.: Biosynthesis and characterization of polyhydroxyalkanoate block copolymer P3HB-b-P4HB. Biomacromolecules 12, 3166–3173 (2011). https://doi.org/10.1021/bm200660k
Shen, R., Cai, L., Meng, D., Wu, L., Guo, K., Dong, G., Li, L., Chen, J.C., Wu, Q., Chen, G.: Benzene containing polyhydroxyalkanoates homo-and copolymers synthesized by genome edited Pseudomonas entomophila. Sci. China Life Sci. 57, 4–10 (2014). https://doi.org/10.1007/s11427-013-4596-8
Abraham, G.A., Gallardo, A., San, R.J., Olivera, E.R., Jodra, R., García, B., Garcia, J.L., Luengo, J.M.: Microbial synthesis of poly (β-hydroxyalkanoates) bearing phenyl groups from pseudomonas p utida: chemical structure and characterization. Biomacromol 2, 562–567 (2001). https://doi.org/10.1021/bm010018h
Li, S., Cai, L., Wu, L., Zeng, G., Chen, J., Wu, Q., Chen, G.Q.: Microbial synthesis of functional homo-, random, and block polyhydroxyalkanoates by β-oxidation deleted Pseudomonas entomophila. Biomacromolecules 15, 2310–2319 (2014). https://doi.org/10.1021/bm500669s
Wang, H.H., Li, X.T., Chen, G.Q.: Production and characterization of homopolymer polyhydroxyheptanoate (P3HHp) by a fadBA knockout mutant Pseudomonas putida KTOY06 derived from P. putida KT2442. Process Biochem. 44, 106–111 (2009). https://doi.org/10.1016/j.procbio.2008.09.014
Chen, G.Q., Chen, X.Y., Wu, F.Q., Chen, J.C.: Polyhydroxyalkanoates (PHA) toward cost competitiveness and functionality. Adv. Ind. Eng. Polym. Res. 3, 1–7 (2020). https://doi.org/10.1016/j.aiepr.2019.11.001
Obulisamy, P.K., Mehariya, S.: Polyhydroxyalkanoates from extremophiles: a review. Bioresour. Technol. 325, 124653 (2021). https://doi.org/10.1016/j.biortech.2020.124653
Obruča, S., Dvořák, P., Sedláček, P., Koller, M., Sedlář, K., Pernicová, I., Šafránek, D.: Polyhydroxyalkanoates synthesis by halophiles and thermophiles: towards sustainable production of microbial bioplastics. Biotechnol. Adv. 58, (2022). https://doi.org/10.1016/j.biotechadv.2022.107906
Chavan, S., Yadav, B., Tyagi, R.D., Drogui, P.: A review on production of polyhydroxyalkanoate (PHA) biopolyesters by thermophilic microbes using waste feedstocks. Bioresour. Technol. 341 (2021). https://doi.org/10.1016/j.biortech.2021.125900
Mozhdehi, D., Luginbuhl, K.M., Dzuricky, M., Costa, S.A., Xiong, S., Huang, F.C., Lewis, M., Zelenetz, S., Colby, C.D., Chilkoti, A.: Genetically encoded cholesterol-modified polypeptides. J. Am. Chem. Soc. 141, 945–951 (2019). https://doi.org/10.1021/jacs.8b10687
Wang, Q., Zhuang, Q., Liang, Q., Qi, Q.: Polyhydroxyalkanoic acids from structurally-unrelated carbon sources in Escherichia coli. Appl. Microbiol. Biotechnol. 97, 3301–3307 (2013). https://doi.org/10.1007/s00253-013-4809-x
Neoh, S.Z., Chek, M.F., Tan, H.T., Linares-Pastén, J.A., Nandakumar, A., Hakoshima, T., Sudesh, K.: Polyhydroxyalkanoate synthase (PhaC): the key enzyme for biopolyester synthesis. Curr. Res. Biotechnol. 4, 87–101 (2022). https://doi.org/10.1016/j.crbiot.2022.01.002
Tan, D., Yin, J., Chen, G.Q.: Production of polyhydroxyalkanoates. In: Pandey, A., Negi, S., S.C.R. (ed.) Current Developments in Biotechnology and Bioengineering, pp. 655–692. Elsevier (2017). https://doi.org/10.1016/B978-0-444-63662-1.00029-4
Liu, Q., Luo, G., Zhou, X.R., Chen, G.Q.: Biosynthesis of poly (3-hydroxydecanoate) and 3-hydroxydodecanoate dominating polyhydroxyalkanoates by β-oxidation pathway inhibited Pseudomonas putida. Metab. Eng. 13, 11–17 (2011). https://doi.org/10.1016/j.ymben.2010.10.004
Wang, Q., Luan, Y., Cheng, X., Zhuang, Q., Qi, Q.: Engineering of Escherichia coli for the biosynthesis of poly (3-hydroxybutyrate-co-3-hydroxyhexanoate) from glucose. Appl. Microbiol. Biotechnol. 99, 2593–2602 (2015). https://doi.org/10.1007/s00253-015-6380-0
Tripathi, L., Wu, L.P., Dechuan, M., Chen, J., Wu, Q., Chen, G.Q.: Pseudomonas putida KT2442 as a platform for the biosynthesis of polyhydroxyalkanoates with adjustable monomer contents and compositions. Bioresour. Technol. 142, 225–231 (2013). https://doi.org/10.1016/j.biortech.2013.05.027
Volova, T., Kiselev, E., Nemtsev, I., Lukyanenko, A., Sukovatyi, A., Kuzmin, A., Ryltseva, G., Shishatskaya, E.: Properties of degradable polyhydroxyalkanoates with different monomer compositions. Int. J. Biol. Macromol. 182, 98–114 (2021). https://doi.org/10.1016/j.ijbiomac.2021.04.008
Avella, M., La Rota, G., Martuscelli, E., Raimo, M.: Review properties of blends and composites based on poly(3-hydroxy)butyrate (PHB) and poly(3-hydroxybutyrate-hydroxyvalerate) (PHBV) copolymers. J. Mater. Sci. 35, 523–545 (2000). https://doi.org/10.1023/A:1004740522751
Qi, L.S., Larson, M.H., Gilbert, L.A., Doudna, J.A., Weissman, J.S., Arkin, A.P., Lim, W.A.: Repurposing CRISPR as an RNA-guided platform for sequence-specific control of gene expression. Cell 152, 1173–1183 (2013). https://doi.org/10.1016/j.cell.2013.02.022
Lv, L., Ren, Y.L., Chen, J.C., Wu, Q., Chen, G.Q.: Application of CRISPRi for prokaryotic metabolic engineering involving multiple genes, a case study: controllable P (3HB-co-4HB) biosynthesis. Metab. Eng. 29, 160–168 (2015). https://doi.org/10.1016/j.ymben.2015.03.013
De-Chuang, M., Chen, G.Q.: Synthetic biology of polyhydroxyalkanoates (PHA). In: Synthetic Biology—Metabolic Engineering, pp. 147–174. Springer (2018). https://doi.org/10.1007/10_2017_3
Ligny, R., Hänninen, M.M., Guillaume, S.M., Carpentier, J.F.: Highly syndiotactic or isotactic polyhydroxyalkanoates by ligand-controlled yttrium-catalyzed stereoselective ring-opening polymerization of functional racemic β-lactones. Angew. Chemie. 129, 10524–10529 (2017). https://doi.org/10.1002/ange.201704283
Doi, Y.: Microbial synthesis, physical properties, and biodegradability of polyhydroxyalkanoates. Basel Hüthig Wepf Verlag. 98, 585–599 (1995). https://doi.org/10.1002/masy.19950980150
Winnacker, M.: Polyhydroxyalkanoates: recent advances in their synthesis and applications. Eur. J. Lipid Sci. Technol. 121, 1900101 (2019). https://doi.org/10.1002/ejlt.201900101
Taguchi, S., Matsumoto, K.I.: Evolution of polyhydroxyalkanoate synthesizing systems toward a sustainable plastic industry. Polym. J. 53, 67–79 (2021). https://doi.org/10.1038/s41428-020-00420-8
Ma, Y.M., Yao, H., Wei, D.X., Wu, L.P., Chen, G.Q.: Synthesis, characterization and application of comb-like polyhydroxyalkanoate-graft-poly(N-isopropylacrylamide). Biomacromolecules 17, 2680–2690 (2016). https://doi.org/10.1021/acs.biomac.6b00724
Yao, H., Wei, D., Che, X., Cai, L., Tao, L., Liu, L., Wu, G.Q., Chen, G.Q.: Comb-like temperature-responsive polyhydroxyalkanoate-graft-poly(2dimethylaminoethylmethacrylate) for controllable protein adsorption. Polym. Chem. 7, 5957–5965 (2016). https://doi.org/10.1039/C6PY01235C
Yao, H., Wu, L.P., Chen, G.Q.: Synthesis and characterization of electroconductive PHA-graft-graphene nanocomposites. Biomacromolecules 20, 645–652 (2018). https://doi.org/10.1021/acs.biomac.8b01257
Yu, L.P., Zhang, X., Wei, D.X., Wu, Q., Jiang, X.R., Chen, G.Q.: Highly efficient fluorescent material based on rare-earth-modified polyhydroxyalkanoates. Biomacromolecules 20, 3233–3241 (2019). https://doi.org/10.1021/acs.biomac.8b01722
Zhang, X., Li, Z., Che, X., Yu, L., Jia, W., Shen, R., Chen, J., Ma, Y., Chen, G.Q.: Synthesis and characterization of polyhydroxyalkanoate organo/hydrogels. Biomacromolecules 20, 3303–3312 (2019). https://doi.org/10.1021/acs.biomac.9b00479
Che, X.M., Wei, D.X., Chen, G.Q.: Superhydrophobic polyhydroxyalkanoates: preparation and applications. Biomacromolecules 20, 618–624 (2018). https://doi.org/10.1021/acs.biomac.8b01176
Kai, D., Loh, X.J.: Polyhydroxyalkanoates: chemical modifications toward biomedical applications. ACS Sustain. Chem. Eng. 2, 106–119 (2014). https://doi.org/10.1021/sc400340p
Timbart, L., Renard, E., Langlois, V., Guerin, P.: Novel biodegradable copolyesters containing blocks of poly(3-hydroxyoctanoate) and poly(ε-caprolactone): synthesis and characterization. Macromol. Biosci. 4, 1014–1020 (2004). https://doi.org/10.1002/mabi.200400104
Raza, Z.A., Abid, S., Banat, I.M.: Polyhydroxyalkanoates: characteristics, production, recent developments and applications. Int. Biodeterior. Biodegradation 126, 45–56 (2018). https://doi.org/10.1016/j.ibiod.2017.10.001
Miguel, O., Fernandez-Berridi, M.J., Iruin, J.J.: Survey on transport properties of liquids, vapors, and gases in biodegradable poly (3-hydroxybutyrate)(PHB). J. Appl. Polym. Sci. 64, 1849–1859 (1997). https://doi.org/10.1002/(SICI)1097-4628(19970531)64:9%3c1849::AID-APP22%3e3.0.CO;2-R
Miguel, O., Iruin, J.J.: Evaluation of the transport properties of poly (3‐hydroxybutyrate) and its 3‐hydroxyvalerate copolymers for packaging applications. In: Macromolecular Symposia, pp. 427–438 (1999). https://doi.org/10.1002/masy.19991440140
Israni, N., Shivakumar, S.: Polyhydroxyalkanoates in packaging. In: Biotechnological Applications of Polyhydroxyalkanoates, pp. 363–388. Springer, Singapore (2019). https://doi.org/10.1007/978-981-13-3759-8_14
Follain, N., Chappey, C., Dargent, E., Chivrac, F., Crétois, R., Marais, S.: Structure and barrier properties of biodegradable polyhydroxyalkanoate films. J. Phys. Chem. C. 118, 6165–6177 (2014). https://doi.org/10.1021/jp408150k
Bucci, D.Z., Tavares, L.B.B., Sell, I.: Biodegradation and physical evaluation of PHB packaging. Polym. Test. 26, 908–915 (2007). https://doi.org/10.1016/j.polymertesting.2007.06.013
Vanheusden, C., Samyn, P., Goderis, B., Hamid, M., Reddy, N., Ethirajan, A., Peeters, R., Buntinx, M.: Extrusion and injection molding of poly (3-hydroxybutyrate-co-3-hydroxyhexanoate)(PHBHHx): influence of processing conditions on mechanical properties and microstructure. Polymers (Basel) 13, 4012–4035 (2021). https://doi.org/10.3390/polym13224012
Ramachandran, H., Kannusamy, S., Huong, K.H., Mathava, R., Amirul, A.A.: Blends of polyhydroxyalkanoates (PHAs). In: Polyhydroxyalkanoate (PHA) Based Blends, Composites and Nanocomposites, pp. 66–97 (2014). https://doi.org/10.1039/9781782622314-00066
Martínez-Abad, A., González-Ausejo, J., Lagarón, J.M., Cabedo, L.: Biodegradable poly (3-hydroxybutyrate-co-3-hydroxyvalerate)/thermoplastic polyurethane blends with improved mechanical and barrier performance. Polym. Degrad. Stab. 132, 52–61 (2016). https://doi.org/10.1016/j.polymdegradstab.2016.03.039
Gumel, A.M., Annuar, M.S.M.: Nanocomposites of polyhydroxyalkanoates (PHAs). In: Polyhydroxyalkanoate (PHA) Based Blends, Composites and Nanocomposites, pp. 98–118 (2014). https://doi.org/10.1039/9781782622314-00098
Valappil, S.P., Boccaccini, A.R., Bucke, C., Roy, I.: Polyhydroxyalkanoates in gram-positive bacteria: insights from the genera Bacillus and Streptomyces. Antonie Van Leeuwenhoek 91, 1–17 (2007). https://doi.org/10.1007/s10482-006-9095-5
Valappil, S.P., Misra, S.K., Boccaccini, A.R., Roy, I.: Biomedical applications of polyhydroxyalkanoates, an overview of animal testing and in vivo responses. Expert Rev. Med. Devices. 3, 853–868 (2006). https://doi.org/10.1586/17434440.3.6.853
Gursel, I., Yagmurlu, F., Korkusuz, F.E.Z.A., Hasirci, V.A.S.I.F.: In vitro antibiotic release from poly (3-hydroxybutyrate-co-3-hydroxyvalerate) rods. J. Microencapsul. 19, 153–164 (2002). https://doi.org/10.1080/02652040110065413
Zhang, C., Zhao, L., Dong, Y., Zhang, X., Lin, J., Chen, Z.: Folate-mediated poly (3-hydroxybutyrate-co-3-hydroxyoctanoate) nanoparticles for targeting drug delivery. Eur. J. Pharm. Biopharm. 76, 10–16 (2010). https://doi.org/10.1016/j.ejpb.2010.05.005
Türesin, F., Gürsel, I., Hasirci, V.A.S.I.F.: Biodegradable polyhydroxyalkanoate implants for osteomyelitis therapy: in vitro antibiotic release. J. Biomater. Sci. Polym. Ed. 12, 195–207 (2001). https://doi.org/10.1163/156856201750180924
Scheithauer, E.C., Li, W., Ding, Y., Harhaus, L., Roether, J.A., Boccaccini, A.R.: Preparation and characterization of electrosprayed daidzein—loaded PHBV microspheres. Mater. Lett. 158, 66–69 (2015). https://doi.org/10.1016/j.matlet.2015.05.133
Chaturvedi, K., Kulkarni, A.R., Aminabhavi, T.M.: Blend microspheres of poly (3-hydroxybutyrate) and cellulose acetate phthalate for colon delivery of 5-fluorouracil. Ind. Eng. Chem. Res. 50, 10414–10423 (2011). https://doi.org/10.1021/ie2011005
Ali, I., Jamil, N.: Polyhydroxyalkanoates: current applications in the medical field. Front. Biol. (Beijing) 11, 19–27 (2016). https://doi.org/10.1007/s11515-016-1389-z
Sevastianov, V.I., Perova, N.V., Shishatskaya, E.I., Kalacheva, G.S., Volova, T.G.: Production of purified polyhydroxyalkanoates (PHAs) for applications in contact with blood. Polym. Ed. 14, 1029–1042 (2003). https://doi.org/10.1163/156856203769231547
Chen, W., Tong, Y.W.: PHBV microspheres as neural tissue engineering scaffold support neuronal cell growth and axon–dendrite polarization. Acta Biomater. 8, 540–548 (2012). https://doi.org/10.1016/j.actbio.2011.09.026
Hong, H., Dong, N., Shi, J., Chen, S., Guo, C., Hu, P., Qi, H.: Fabrication of a novel hybrid heart valve leaflet for tissue engineering: an in vitro study. Artif. Organs. 33, 554–558 (2009). https://doi.org/10.1111/j.1525-1594.2009.00742.x
Basnett, P., Ching, K.Y., Stolz, M., Knowles, J.C., Boccaccini, A.R., Smith, C., Locke, I.C., Keshavarz, T., Roy, I.: Novel poly (3-hydroxyoctanoate)/poly (3-hydroxybutyrate) blends for medical applications. React. Funct. Polym. 73, 1340–1348 (2013). https://doi.org/10.1016/j.reactfunctpolym.2013.03.019
Bian, Y.Z., Wang, Y., Aibaidoula, G., Chen, G.Q., Wu, Q.: Evaluation of poly (3-hydroxybutyrate-co-3-hydroxyhexanoate) conduits for peripheral nerve regeneration. Biomaterials 30, 217–225 (2009). https://doi.org/10.1016/j.biomaterials.2008.09.036
Basnett, P., Knowles, J.C., Pishbin, F., Smith, C., Keshavarz, T., Boccaccini, A.R., Roy, I.: Novel biodegradable and biocompatible poly (3-hydroxyoctanoate)/bacterial cellulose composites. Adv. Eng. Mater. 14, B330–B343 (2012). https://doi.org/10.1002/adem.201180076
Shangguan, Y.Y., Wang, Y.W., Wu, Q., Chen, G.Q.: The mechanical properties and in vitro biodegradation and biocompatibility of UV-treated poly (3-hydroxybutyrate-co-3-hydroxyhexanoate). Biomaterials 27, 2349–2357 (2006). https://doi.org/10.1016/j.biomaterials.2005.11.024
Volova, T., Shishatskaya, E., Sevastianov, V., Efremov, S., Mogilinaya, O.: Results of biomedical investigations of PHB and PHB/PHV fibers. Biochem. Eng. J. 16, 125–133 (2003). https://doi.org/10.1016/S1369-703X(03)00038-X
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Rivera Gálvez, F.J. (2023). Diversifying Polyhydroxyalkanoates: Synthesis, Properties, Processing and Applications. In: Avalos Belmontes, F., González, F.J., López-Manchado, M.Á. (eds) Green-Based Nanocomposite Materials and Applications. Engineering Materials. Springer, Cham. https://doi.org/10.1007/978-3-031-18428-4_11
Download citation
DOI: https://doi.org/10.1007/978-3-031-18428-4_11
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-18427-7
Online ISBN: 978-3-031-18428-4
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)