Abstract
Pseudomonas putida KT2440 grew on glucose at a specific rate of 0.48 h−1 but accumulated almost no poly-3-hydroxyalkanoates (PHA). Subsequent nitrogen limitation on nonanoic acid resulted in the accumulation of only 27% medium-chain-length PHA (MCL-PHA). In contrast, exponential nonanoic acid-limited growth (μ = 0.15 h−1) produced 70 g l−1 biomass containing 75% PHA. At a higher exponential feed rate (μ = 0.25 h−1), the overall productivity was increased but less biomass (56 g l−1) was produced due to higher oxygen demand, and the biomass contained less PHA (67%). It was concluded that carbon-limited exponential feeding of nonanoic acid or related substrates to cultures of P. putida KT2440 is a simple and highly effective method of producing MCL-PHA. Nitrogen limitation is unnecessary.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Poly-3-hydroxyalkanoates (PHAs) have attracted extensive commercial interest due to their inherent biocompatibility and biodegradability (Zinn et al. 2001). In particular, medium-chain-length PHAs (MCL-PHAs), which contain 6 to 14 carbons in their repeating units, show great promise as thermoelastomers for biomedical applications, such as drug delivery (Pouton and Akhtar 1996) and tissue engineering (Williams et al. 1999; Chen and Wu 2005). Efficient production of MCL-PHAs is a prerequisite for the extensive study of these polyesters. Although it was observed that 8-carbon and 9-carbon alkanes or alkanoates result in the highest MCL-PHA accumulation (Brandl et al. 1988; Gross et al. 1989; Kang et al. 2001), and octanoic acid was used as the carbon source in pH-stat processes with nitrogen limitation to obtain 47 g l−1 biomass containing 55% PHA (Dufresne and Samain 1998) and 63 g l−1 containing 62% PHA (Kim 2002), almost no research into the development of production processes using nonanoic acid as the principle carbon source was reported. While octanoic acid is derived from coconut and palm kernel oils, nonanoic acid can be produced from oleic, linoleic and erucic and other carboxylic acids produced in temperate zone plants such as canola. For a variety of reasons, nonanoic acid has potential as a commodity feedstock for ‘biorefinery’ processes in industrialized countries.
Nitrogen (N) or phosphate (P) limitation stimulates rapid PHA synthesis in most of the well-studied SCL (short-chain-length)-PHA-synthesizing bacteria, such as Ralstonia eutropha. Therefore most SCL-PHA production processes employ a stage of rapid cell growth followed by a PHA accumulation stage, which is almost always N-limited or P-limited. Possibly due to an assumption that MCL-PHA production physiology is similar to that of most SCL-PHA accumulating bacteria, almost all publications dealing with MCL-PHA production have incorporated N or P limitation (Preusting et al. 1993; Huijberts and Eggink 1996; Dufresne and Samain 1998; Jung et al. 2001; Kim 2002). Other than continuous plug-flow processes, which are impractical at a commercial scale, fed-batch fermentation is theoretically and practically the best method of achieving a high density of biomass containing the highest possible amount of PHA. High PHA content is critical to the reduction of PHA separation cost in commercial processes. Because the rate of substrate demand is difficult to measure or predict, the design and control of a N-limited or P-limited accumulation phase in a fed-batch process is difficult when it involves the feeding of inhibitory substrates such as short-chain-length and medium-chain-length carboxylic acids. While a pH-stat approach is feasible, as mentioned above, simple exponential feeding of substrate is more reliable and potentially more productive. However, exponential feeding is only possible when N or P limitation is not imposed.
The metabolic regulation of SCL-PHA was well-studied (Senior and Dawes 1971; Steinbuchel and Lutke-Eversloh 2003), but less is known about the factors controlling MCL-PHA synthesis. While the rate of MCL-PHA production in P. resinovorans is greatly stimulated by N-limitation (Ramsay et al. 1992), this did not occur in P. oleovorans GPo1 ATCC 29347 (Ramsay et al. 1991) now recognized to be a strain of P. putida (Diard et al. 2002). Significant MCL-PHA accumulation during the exponential growth phase of P. putida KT2442 (Huisman et al. 1992), P. putida U (Carnicero et al. 1997) and P. putida GPo1 (Durner et al. 2001) was reported, indicating little need for N or P limitation. We found that high MCL-PHA content (>50%) occurred under carbon-limited conditions in a chemostat study of P. putida KT2440 using nonanoic acid as carbon source (unpublished data). P. putida strains are known to produce large amounts of MCL-PHA (Lee et al. 2000; Diniz et al. 2004), and strain KT2440 (ATCC 47054) has no plasmid load (Timmis 2002). Based on these observations, the aim of the present study was to compare a single-stage carbon-limited process with a 2-stage process incorporating an N-limited stage for the production of MCL-PHAs by P. putida KT2440. Production from nonanoic acid and glucose was also compared.
Materials and methods
Microorganism and growth medium
P. putida KT2440 (ATCC 47054) was maintained in lyophiles and on nutrient agar plates at 4°C. For the two-stage fermentation using glucose as sole substrate during the growth phase, the inoculum medium, initial and feeding medium for the growth phase of the fermentation was described previously (Sun et al. 2006). For all other fermentations, the inoculum medium contained per liter: 4.70 g (NH4)2SO4, 0.80 g MgSO4·7H2O, 12.00 g Na2HPO4·7H2O, 2.70 g KH2PO4, 3.00 g nutrient broth. The initial culture medium contained per liter: 4.70 g (NH4)2SO4, 0.80 g MgSO4·7H2O, 9.00 g Na2HPO4·7H2O, 2.03 g KH2PO4, 1–3 g nonanoic acid and 10 ml trace element solution. Each liter of trace element solution contained 10 g FeSO4·7H2O, 3 g CaCl2·2H2O, 2.2 g ZnSO4·7H2O, 0.5 g MnSO4·4H2O, 0.3 g H3BO3, 0.2 g CoCl2·6H2O, 0.15 g Na2MoO4·2H2O, 0.02 g NiCl2·6H2O and 1.00 g CuSO4·5H2O. Nitrogen was supplied using 14% (w/v) NH4OH to control the pH during growth and was replaced by 2 M KOH during N-limited stages. Nonanoic acid (96%, Sigma Aldrich) was filter-sterilized. To avoid precipitation, all the needed MgSO4·7H2O was not added to the initial medium. Additional MgSO4·7H2O was fed at a ratio of 0.033 g MgSO4·7H2O g−1 nonanoic acid addition assuming a Y X/Mg of 240 g g−1 (Sun et al. 2006) and a YX/NA of 0.80 g g−1.
Fermentation conditions
Inoculum culture was cultivated in 500 ml shake flasks (100 ml medium) at 30±1°C and 200 rpm overnight. Fed-batch fermentations were all carried out at 31±1°C with a 3.5 l initial working volume in a Minifors 5 l stirred tank bioreactor (Infors HT, Bottmingen, Switzerland). Data acquisition (dissolved oxygen, outlet gas CO2, carbon source and base addition, and pH) and control was conducted with LabVIEW 6.1 (National Instruments). pH was controlled at 6.85 ± 0.05. Dissolved oxygen was measured with an Ingold polarographic probe and maintained at or above 40% air saturation (except where indicated) by adjusting the agitation speed up to 1,200 rpm and by automatically adjusting the mixture of air and pure oxygen flow via mass flow controllers while maintaining total gas flow at 1 vvm. Exit gas CO2 content was measured with an infrared CO2 monitor (Guardian Plus, Topac Inc. Hingham, MA, USA). Feeding of nonanoic acid with peristaltic pumps was automatically controlled based on the mass of the reservoirs.
Substrate feeding and control methods
Nutrient-unlimited growth on glucose followed by an N-limited accumulation phase on nonanoic acid (two-stage fed-batch)
A continuous feeding strategy based on glucose estimation was applied to first obtain about 40 g l−1 of biomass, as previously described (Sun et al. 2006). N-limitation was then imposed by replacing ammonium hydroxide with KOH. During this N-limited stage, a pulse of 3 g l−1 nonanoic acid was first added, followed by linear feeding at 0.47 g l−1 h−1 until 17 h, then 1.01 g l−1 h−1 until the end of the fermentation.
Single-stage, nonanoic acid-limited fed-batch
The initial nonanoic acid concentration was 1 g l−1. Additional substrate was fed at the rate shown in Eq. 1.
where S t is total nonanoic acid required to produce biomass X t at time t, Y X/S is the yield of biomass from nonanoic acid (0.80 g g−1), X 0 is the initial biomass, obtained by measuring the OD650 nm of the inoculum culture, and μ is the desired specific growth rate.
Nonanoic acid-limited growth followed by an N-limited accumulation phase on nonanoic acid (two-stage fed-batch)
With 3 g l−1 of initial nonanoic acid, exponential feeding was applied according to Eq. 1 with a target μ = 0.25 h−1. Shortly before reaching the maximum oxygen transfer capacity, nitrogen limitation was imposed by replacing ammonium hydroxide with KOH. During this N-limited stage, nonanoic acid was fed at a rate of 1.31 g l−1 h−1 until 41.5 h, then 1.82 g l−1 h−1 until the end of the fermentation.
Analytical procedures
Cell dry weight was determined after lyophilization of biomass obtained by centrifugation of 5 ml culture broth at 17,000×g for 15 min and washing twice with 5 ml distilled water. The supernatant of the sample and the distilled water wash were saved for analysis of the nonanoic acid and key nutrients. Nonanoic acid concentration was determined by methylation followed by GC analysis with decanoic acid as internal standard (Ramsay et al. 1991). Phosphate was measured via reduction of phosphomolybdate to molybdene blue (Clesceri et al. 1999). Ammonium was determined by the phenol–hypochlorite method (Weatherburn 1967). PHA content and composition was determined by modification of the Braunegg et al. (1978) and Lageveen et al. (1988) methodologies. Lyophilized cell samples were suspended with 2 ml of methanol containing 15% (v/v) H2SO4 and 0.2% (w/v) benzoic acid (internal standard) and 2 ml of chloroform. Methanolysis was carried out in a 100°C water bath for 3.5 h with periodic vigorous mixing. After cooling to room temperature, 2 ml distilled water was added and vortexed for 1 min. The resulting emulsion was left overnight for phase separation. The organic phase was used for GC analysis. The PHA standard for this assay was prepared by repeated cycles of solvent extraction followed by precipitation in cold methanol. Its composition was determined by NMR. Residual biomass (Xr) was defined as the total cell concentration minus the PHA concentration. The specific PHA synthesis rate based on the amount of residual biomass (q PHA(Xr)) was defined as (dP/dt)/Xr (g of PHA, g of Xr−1 h−1). The specific PHA synthesis rate based on the amount of PHA (q PHA(PHA)) was defined as (dP/dt)/PHA (g of PHA, g of PHA−1 h−1). They were calculated using the slope of the PHA concentration curve and the measured Xr and PHA concentration values, respectively, at that point.
Results
Growth on glucose followed by nonanoic acid linear feeding under N-limitation
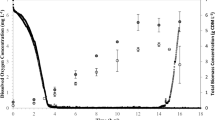
In SCL-PHA fermentations with bacteria such as Ralstonia eutropha or Burkholderia cepacia, glucose is typically fed to produce dense biomass (growth phase). N or P is then allowed to fall to a growth-limiting value, which stimulates PHA synthesis. At the point of N or P limitation or slightly before, co-substrates such as propionic acid are fed to produce the desired copolymer composition during the accumulation phase (Ramsay et al. 1990). High-cell-density cultivation of P. putida KT2440 on glucose had already been established (Sun et al. 2006). Based on this knowledge, 40 g l−1 of biomass was produced using glucose as the sole source of carbon and energy. Almost no PHA synthesis was detected in P. putida grown on glucose without N or P limitation (Fig. 1). The specific growth rate throughout this phase was 0.48 h−1. Although some PHA was initially produced during the N-limited stage, the nonanoic acid feed rate of 0.47 g l−1 h−1 proved to be insufficient. Increasing the feed rate to 1.01 g l−1 h−1 enabled the cells to accumulate more PHA. The nonanoic acid concentration in the reactor remained below 2 g l−1 during this period. Eventually 26.8% PHA was accumulated in 46.1 g l−1 biomass giving a cumulative PHA productivity of 0.25 g l−1 h−1.
Growth of P. putida KT2440 on glucose followed by N-limited growth on nonanoic acid (NA). The division between growth phase and N-limited phase is indicated by the dashed line. The nonanoic acid feeding rate (F NA) was increased from 0.47 g l−1 h−1 to 1.01 g l−1 h−1 at 27 h during the N-limited phase, as indicated by the dash-dot line
Single-stage, exponential feeding of nonanoic acid (target μ=0.15 h−1)
To further assess whether N or P limitation is required for substantial MCL-PHA synthesis in P. putida, a nonanoic acid single-stage fed-batch process was conducted. To avoid overfeeding, the μ in the feeding equation (Eq. 1) was set at a conservative value of 0.15 h−1. The nonanoic acid concentration was maintained well below 1 g l−1 until 41 h (Fig. 2). The exponential rates of consumption and growth indicate that nonanoic acid was the only limiting nutrient until the end of the fermentation. Control of pH with ammonium hydroxide allowed the ammonium concentration to be maintained between 1 and 2 g l−1 during the entire fermentation. Phosphate concentration was not controlled but it never decreased to a level that limited the growth rate. Although little lag phase was observed by gas analysis, growth was initially very slow due to the imposed low rate of feeding. However, the total cell concentration eventually increased to 70.2 g l−1 and the measured μ, based on dry biomass samples, was the predicted value of 0.15 h−1. The inoculum culture (grown on nutrient broth) contained no measurable MCL-PHA but PHA content increased throughout the fermentation to a maximum of 75.4%, giving a cumulative PHA productivity of 1.11 g l−1 h−1. Only hydroxynonanoate (C9) and hydroxyheptanoate (C7) PHA subunits were detected. The ratio of C9/C7 was about 2.4:1 (mol/mol) throughout the fermentation. Although agitation was maximal and 1 vvm of pure oxygen was supplied to the fermentor, after 41 h the dissolved oxygen concentration dropped to zero. Oxygen limited cell growth caused nonanoic acid to rapidly accumulate in the medium (to above 3 g l−1). This resulted in growth inhibition, cell lysis, and excessive foaming.
Single-stage, exponential feeding of nonanoic acid (target μ=0.25 h−1)
To improve productivity and evaluate the effects of a higher growth rate on PHA accumulation, the single-stage carbon-limited fermentation was repeated but at a higher target μ (0.25 h−1) in the feeding equation (Eq. 1). Key results were compared to those obtained at μ = 0.15 h−1 (Fig. 3). Very similar patterns were observed in the two fermentations despite the difference in feed rate. The final total biomass, total PHA, and % PHA of the fermentation at μ = 0.25 h−1 were 56.0 g l−1, 34.7 g l−1, and 66.9%, respectively, which were slightly lower than the final values at the lower μ (Fig. 3a). However, the final cumulative PHA productivity (Fig. 3b) increased to 1.44 g l−1 h−1 at μ = 0.25 h−1 because the total cultivation time decreased significantly. The specific rate of PHA synthesis based on residual biomass (q PHA(Xr)) increased steadily at both feed rates up to 0.48 g g−1 h−1 at μ = 0.25 h−1 and 0.53 g g−1 h−1 at μ = 0.15 h−1.
Single-stage, exponential feeding of nonanoic acid (target μ=0.25 h−1) followed by N-limited culture on nonanoic acid
Single-stage, exponential feeding of nonanoic acid resulted in high PHA productivity and content but perhaps N-limitation could further increase PHA synthesis. Therefore an experiment was conducted in which N-limitation was imposed after exponential feeding (target μ = 0.25 h−1) of nonanoic acid (Fig. 4). Cell growth and PHA synthesis during the growth stage were very similar to the single-stage fed-batch where both were fed to achieve μ = 0.25 h−1 but nitrogen limitation was imposed at 27 h by replacing ammonium hydroxide with KOH. Linear feeding after this point allowed the nonanoic acid concentration to be maintained just below the 3 g l−1 level. However, although the total amount of biomass increased, there was little increase in the percentage of PHA in the biomass produced during the N-limited stage. The final PHA concentration was 52.4 g l−1, corresponding to an overall (i.e. cumulative) productivity of 0.91 g l−1 h−1.
Two-stage, fed-batch using nonanoic acid (NA) as the sole carbon source. Exponential feeding (μ = 0.25 h−1) was used during the growth phase with all other nutrients in excess. The division between the growth and N-limited phases is indicated by the dashed line. The nonanoic acid feed rate was increased from 1.31 g l−1 h−1 to 1.82 g l−1 h−1 at 41.5 h during the N-limited phase as indicated by the dash-dot line
Biomass and PHA yield
The total biomass, PHA, and residual biomass (Xr) data from both single-stage (carbon-limited) fed-batch fermentations were plotted against the total nonanoic acid consumption (Fig. 5). There was a linear relationship between total biomass and nonanoic acid consumption demonstrating a yield of 0.83 g biomass g−1 nonanoic acid (R 2 = 0.995) throughout both fermentations. In contrast, the yield of PHA and residual biomass varied during the growth. The yield of PHA increased gradually throughout the growth phase as more carbon was directed to PHA synthesis and less towards residual biomass. The overall PHA yield was about 0.60 g g−1 nonanoic acid while the overall yield of residual biomass was 0.24 g g−1 nonanoic acid.
Discussion
Process design implications
Relatively little PHA was accumulated whether the N-limited PHA accumulation phase on nonanoic acid was preceded by growth on glucose or on nonanoic acid. Although better control of nonanoate concentration during N-limited production would increase PHA production, simple exponential feeding of nonanoic acid with no N or P limitation was clearly more productive. Similar results are likely with octanoic acid. In shake flasks, nonanoic acid concentrations higher than 3 g l−1 inhibit growth of P. putida KT2440. This has led process development researchers to work with less toxic substrates such as oleic acid. Although remarkable progress was made in the production of PHA from oleic acid (Huijberts and Eggink 1996; Lee et al. 2000), its use limits the type of PHA that can be made and can lead to downstream processing difficulties. The standard approach to PHA production exemplified by the Biopol process (Byrom 1990) consists of dense biomass production followed by an accumulation phase. The Biopol process is relatively easy to control because the toxic co-substrate (propionic acid) used in the accumulation phase is typically mixed at low concentration with a high concentration of glucose. Use of a single toxic substrate such as nonanoic acid in an accumulation phase requires more sophisticated control. Fortunately, an accumulation phase does not appear to be required or even beneficial to MCL-PHA synthesis in P. putida KT2440. The Biomer process (Hänggi 1990) currently used for PHB production employs a single-stage fermentation with no accumulation phase. The organism used in the Biomer process, A.latus, exhibits a growth-associated PHA production pattern (Braunegg and Bogensberger 1985). Co-substrates may be fed to produce copolymers in such a single-stage process (Ramsay et al. 1990). Although the kinetics of PHA synthesis in P. putida KT2440 are not directly linked to growth, we propose a similar single-stage process in which nonanoic acid or related substrates are fed exponentially. Co-substrates may be mixed with the major substrate to produce the desired polymer subunit composition.
Since a lower specific growth rate demands less oxygen transfer, a higher final PHA content (75%) was achieved than when μ was controlled at 0.25 h−1 (67%). However, the cumulative PHA productivity decreased from 1.44 g l−1 h−1 to 1.11 g l−1 h−1 when operating at a lower μ due to the longer culturing time. Thus, there is a trade-off between the amount of PHA in the biomass and fermentation productivity. An optimal approach can be calculated based on economic considerations such as substrate and separation costs, as well as oxygen transfer and mixing properties of the fermentor to be employed.
Yield
Yields of total biomass and PHA from nonanoic acid were 0.83 g g−1 and 0.60 g g−1, respectively (Fig. 5). These are relatively high when compared to 0.40 g PHA g octanoate−1 under glucose co-feeding conditions (Kim et al. 1997), or 0.63 g PHA g octane−1 under optimized PHA accumulating conditions in the second stage of a continuous process (Hazenberg and Witholt 1997). The consistency of the yield values (Fig. 5) and C9/C7 ratio of fermentations at different growth rates indicate that this is a highly repeatable process.
There appears to be a very low maintenance energy requirement in this strain because the biomass yield values were essentially the same at both growth rates. Much more PHA is being synthesized during latter parts of the fermentation yet the yield remained the same (Fig. 5). This indicates that the true yields for Y X/S and Y P/S are very similar. Since the % PHA, PHA concentration and specific PHA synthesis rate increased continuously at both growth rates until oxygen transfer limited production, higher final PHA content and productivity could be achieved in a bioreactor system with better oxygen transfer capacity. Extended cultivation using linear feeding of nonanoic acid may further increase PHA accumulation.
Kinetics of MCL-PHA synthesis
Although nutrient (mainly N and P) limitation was commonly applied to MCL-PHA production processes, some controversial results were reported. Limitation of N or P was shown to be stimulatory to the synthesis of PHA in P. oleovorans ATCC 29347 growing on n-alkanes (Lageveen et al. 1988), P. resinovorans and P. putida BM01 on octanoate (Ramsay et al. 1992; Kim et al. 1997) and P. putida KT2442 on oleic acid (Huijberts and Eggink 1996; Lee et al. 2000). In contrast, it was also reported that nutrient limitation is unnecessary for the production of significant amounts of PHA in P. putida KT2442 (Huisman et al. 1992), P. oleovorans ATCC 29347 (Ramsay et al. 1991; Durner et al. 2001 ) or P. putida U (Carnicero et al. 1997) on octanoate. In the present study, high PHA accumulation was achieved during nonanoic acid-limited growth with no other apparent nutrient limitation. The link between MCL-PHA accumulation and growth limitation by key nutrients such as nitrogen or phosphate seems to differ depending on the strain, carbon sources, the cultivation conditions, or possibly a combination of these factors.
There are many possible explanations for the production of substantial amounts of PHA during carbon-limited growth. Some deficiency in the TCA cycle, blockage in β-oxidation, a detoxification mechanism or, as in the case of Azotobacter vinelandii UWD, an ineffective NADH oxidase (Page and Knosp 1989) may divert substrate and energy toward PHA synthesis to cause this effect. Although it was not our purpose to study the mechanisms governing PHA production in this organism, data analysis suggests that unlike A. latus (Braunegg and Bogensberger 1985), the kinetics of MCL-PHA synthesis during the growth of P. putida KT2440 are not strictly linked to growth kinetics. Rather than remaining constant, the PHA content increased from close to zero in the inoculum to above 70%. The continually increasing q PHA(Xr) (Fig. 3b) demonstrated that the growing cells were synthesizing PHA at a rate higher than the growth rate of the other biomass components (residual biomass). After a certain point in the fermentation process, the specific rate of PHA synthesis remains constant only if calculated based on the amount of PHA in the cells (Fig. 6). This indicates that the rate of PHA synthesis is controlled by a property of the granules themselves, possibly related to their surface area. Much more kinetic, physiological, and biochemical study is required to understand the mechanisms of MCL-PHA synthesis during the growth of P. putida KT2440 and other MCL-PHA accumulating bacteria.
References
Brandl H, Gross RA, Lenz RW, Fuller RC (1988) Pseudomonas oleovorans as a source of poly(beta-hydroxyalkanoates) for potential applications as biodegradable polyesters. Appl Environ Microbiol 54:1977–1982
Braunegg G, Bogensberger B (1985) About kinetics of growth and accumulation of poly-d(−)-3-hydroxybutyric acid in Alcaligenes latus strains. Acta Biotechnol 5:339–345
Braunegg G, Sonnleitner B, Lafferty RM (1978) Rapid gas-chromatographic method for determination of poly-beta-hydroxybutyric acid in microbial biomass. Eur J Appl Microb Biotechnol 6:29–37
Byrom D (1990) Industrial production of copolymer from Alcaligenes eutrophus. In: Dawes EA (eds) Novel biodegradable microbial polymers, Kluwer, Netherlands, pp 113–117
Carnicero D, FernandezValverde M, Canedo LM, Schleissner C, Luengo JM (1997) Octanoic acid uptake in Pseudomonas putida U. FEMS Microbiol Lett 149:51–58
Chen GQ, Wu Q (2005) The application of polyhydroxyalkanoates as tissue engineering materials. Biomaterials 26:6565–6578
Clesceri LS, Greenberg AE, Eaton AD (1999) Standard methods for the examination of water and wastewater, 20th edition, American Public Health Association, Washington, DC
Diard S, Carlier JP, Ageron E, Grimont PAD, Langlois V, Guerin P, Bouvet OMM (2002) Accumulation of poly(3-hydroxybutyrate) from octanoate, in different Pseudomonas belonging to the rRNA homology group I. Syst Appl Microbiol 25:183–188
Diniz SC, Taciro MK, Gomez JG, da Cruz Pradella JG (2004) High-cell-density cultivation of Pseudomonas putida IPT 046 and medium-chain-length polyhydroxyalkanoate production from sugarcane carbohydrates. Appl Biochem Biotechnol 119:51–70
Dufresne A, Samain E (1998) Preparation and characterization of a poly(beta-hydroxyoctanoate) latex produced by Pseudomonas oleovorans. Macromolecules 31:6426–6433
Durner R, Zinn M, Witholt B, Egli T (2001) Accumulation of poly[(R)-3-hydroxyalkanoates] in Pseudomonas oleovorans during growth in batch and chemostat culture with different carbon sources. Biotechnol Bioeng 72:278–288
Gross RA, Demello C, Lenz RW, Brandl H, Fuller RC (1989) Biosynthesis and characterization of poly(beta-hydroxyalkanoates) produced by Pseudomonas oleovorans. Macromolecules 22:1106–1115
Hänggi UJ (1990) Pilot scale production of PHA with A. latus In: Dawes EA (eds), Novel biodegradable microbial polymers, Kluwer, Netherlands, pp 65–70
Hazenberg W, Witholt B (1997) Efficient production of medium-chain-length poly(3-hydroxyalkanoates) from octane by Pseudomonas oleovorans: economic considerations. Appl Microbiol Biotechnol 48:588–596
Huijberts GNM, Eggink G (1996) Production of poly(3-hydroxyalkanoates) by Pseudomonas putida KT2442 in continuous cultures. Appl Microbiol Biotechnol 46:233–239
Huisman GW, Wonink E, De Koning G, Preusting H, Witholt B (1992) Synthesis of poly(3-hydroxyalkanoates) by mutant and recombinant Pseudomonas strains. Appl Microbiol Biotechnol 38:1–5
Jung K, Hazenberg W, Prieto M, Witholt B (2001) Two-stage continuous process development for the production of medium-chain-length poly(3-hydroxyalkanoates). Biotechnol Bioeng 72:19–24
Kang HO, Chung CW, Kim HW, Kim YB, Rhee YH (2001) Cometabolic biosynthesis of copolyesters consisting of 3-hydroxyvalerate and medium-chain-length 3-hydroxyalkanoates by Pseudomonas sp. DSY-82. Antonie Van Leeuwenhoek 80:185–191
Kim BS (2002) Production of medium chain length polyhydroxyalkanoates by fed-batch culture of Pseudomonas oleovorans. Biotechnol Lett 24:125–130
Kim GJ, Lee IY, Yoon SC, Shin YC, Park YH (1997) Enhanced yield and a high production of medium-chain-length poly(3-hydroxyalkanoates) in a two-step fed-batch cultivation of Pseudomonas putida by combined use of glucose and octanoate. Enzyme Microb Technol 20:500–505
Lageveen RG, Huisman GW, Preusting H, Ketelaar P, Eggink G, Witholt B (1988) Formation of polyesters by Pseudomonas oleovorans—effect of substrates on formation and composition of poly-(R)-3-hydroxyalkanoates and poly-(R)-3-hydroxyalkenoates. Appl Environ Microbiol 54:2924–2932
Lee SY, Wong HH, Choi JI, Lee SH, Lee SC, Han CS (2000) Production of medium-chain-length polyhydroxyalkanoates by high-cell-density cultivation of Pseudomonas putida under phosphorus limitation. Biotechnol Bioeng 68:466–470
Page WJ, Knosp O (1989) Hyperproduction of poly-beta-hydroxybutyrate during exponential growth of Azotobacter vinelandii UWD. Appl Environ Microbiol 55:1334–1339
Pouton CW, Akhtar S (1996) Biosynthetic polyhydroxyalkanoates and their potential in drug delivery. Adv Drug Deliv Rev 18:133–162
Preusting H, Vanhouten R, Hoefs A, Vanlangenberghe EK, Favrebulle O, Witholt B (1993) High-cell-density cultivation of Pseudomonas oleovorans—growth and production of poly (3-hydroxyalkanoates) in 2-liquid phase batch and fed-batch systems. Biotechnol Bioeng 41:550–556
Ramsay BA, Ramsay JA, Lomaliza K, Chavarie C, Bataille P (1990) Production of poly-(beta-hydroxybutyric-co-beta-hydroxyvaleric) acid copolymers. Appl Environ Microbiol 56:2093–2098
Ramsay BA, Saracovan I, Ramsay JA, Marchessault RH (1991) Continuous production of long-side-chain poly-beta-hydroxyalkanoates by Pseudomonas oleovorans. Appl Environ Microbiol 57:625–629
Ramsay BA, Saracovan I, Ramsay JA, Marchessault RH (1992) Effect of nitrogen limitation on long-side-chain poly-beta-hydroxyalkanoate synthesis by Pseudomonas resinovorans. Appl Environ Microbiol 58:744–746
Senior PJ, Dawes EA (1971) Poly-beta-hydroxybutyrate biosynthesis and regulation of glucose metabolism in Azotobacter beijerinckii. Biochem J 125(1):55–66
Steinbuchel A, Lutke-Eversloh T (2003) Metabolic engineering and pathway construction for biotechnological production of relevant polyhydroxyalkanoates in microorganisms. Biochem Eng J 16:81–96
Sun Z, Ramsay JA, Guay M, Ramsay BA (2006) Automated feeding strategies for high-cell-density fed-batch cultivation of Pseudomonas putida KT2440. Appl Microbiol Biotechnol 71 (4):423–431
Timmis KN (2002) Pseudomonas putida: a cosmopolitan opportunist par excellence. Environ Microbiol 4:779–781
Weatherburn MW (1967) Phenol–hypochlorite reaction for determination of ammonia. Anal Chem 39:971–974
Williams SF, Martin DP, Horowitz DM, Peoples OP (1999) PHA applications: addressing the price performance issue: I. Tissue engineering. Int J Biol Macromol 25:111–121
Zinn M, Witholt B, Egli T (2001) Occurrence, synthesis and medical application of bacterial polyhydroxyalkanoate. Adv Drug Deliv Rev 53:5–21
Acknowledgement
This research was supported by the Natural Science and Engineering Research Council of Canada and a grant from BIOCAP Canada.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sun, Z., Ramsay, J.A., Guay, M. et al. Carbon-limited fed-batch production of medium-chain-length polyhydroxyalkanoates from nonanoic acid by Pseudomonas putida KT2440. Appl Microbiol Biotechnol 74, 69–77 (2007). https://doi.org/10.1007/s00253-006-0655-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-006-0655-4