Abstract
Polyhydroxyalkanoates (PHAs) that contain varied monomers with different chain lengths/structures were normally synthesized when a structurally-related precursor was present. The biosynthesis of PHAs from unrelated carbon sources in microorganisms including Escherichia coli met many challenges in the past. Recently, with the development of metabolic engineering and synthetic biology, the production of PHAs from unrelated carbon sources obtained a breakthrough. Polyesters containing 2-hydroxypropionate, 3-hydroxypropionate, 4-hydroxybutyrate, 3-hydroxyvalarate, and medium-chain-length 3-hydroxyalkanoate monomers can all be synthesized in E. coli by integrating exogenous or endogenous pathways and/or genes. This review will summarize the progresses in this area. In addition, the strategies that lead to the production of PHAs with varied monomers and high polymer content in the cell are discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Polyhydroxyalkanoates (PHAs) are carbon and energy storage materials synthesized by a variety of bacteria that grows in the presence of excess carbon sources and if another macroelement (N, O, P, S) is depleted at the same time (Anderson and Dawes 1990; Steinbüchel et al. 1995). These polymers have attracted extensive interest as environmentally friendly, biodegradable alternatives to petroleum-based plastics (Chen 2009). More than 150 types of PHAs with various monomer constituents have been reported till now (Hazer and Steinbüchel 2007; Steinbüchel and Valentin 1995).
Although Escherichia coli do not accumulate PHAs under natural conditions, it was supposed to be an ideal host for the production of PHAs due to the absence of an intracellular depolymerization system and the presence of convenient metabolic engineering tools (Li et al. 2007a). The most typical polyester, poly-β-3-hydroxybutyrate (P3HB), was first heterologously synthesized in E. coli in 1988 (Schubert et al. 1988; Slater et al. 1988). Since then, many strategies have been developed to improve the polymer content in the host and to facilitate the isolation of the polymers (Li et al. 2007b). P3HB is naturally accumulated by microorganisms from structurally unrelated carbon sources, while most other polyesters (either homopolymers or copolymers) that contain the monomers such as 3-hydroxypropionate (3HP), 4-hydroxybutyrate (4HB), and 5-hydroxyvalarate etc. cannot be accumulated under natural conditions from unrelated carbon sources (Steinbüchel and Valentin 1995). Structurally-related precursors which were used to synthesize PHAs are usually high cost, toxic to cells, and insoluble in water. Therefore, scientists have made many efforts to create new metabolic pathways for such monomers synthesis in E. coli from unrelated carbon source by combining genes from various organisms. This review summarized the efforts that have been done in this area (Table 1).
Polyesters containing the 3HV monomers
Besides P3HB, polyesters containing the 3HV monomers were also found in the early days. In initial P(3HB-co-3HV) (PHBV) production, propionic acid or pentanoic acid had to be supplied together with glucose (Byrom 1987; Fidler and Dennis 1992; Law et al. 2004). Since propionic acid is toxic to cells, researchers tried to find a propionate-independent pathway. Early efforts on Cupriavidus necator proved that the propionate-independent pathway is possible but with a very low 3HV fraction (Eschenlauer et al. 1996; Steinbüchel and Pieper 1992). In 2002, Aldor et al. constructed a recombinant Salmonella enterica serovar Typhimurium strain (Aldor et al. 2002). In this strain, a methylmalonyl-CoA mutase gene sbm and a methylmalonyl-CoA decarboxylase gene ygfG from E. coli were subcloned therefore, succinyl-CoA that derived from the tricarboxylic acid (TCA) cycle, can be converted to propionyl-CoA. The resulting S. enterica strain was proved to accumulate PHBV with a 30 mol% 3HV fraction in the copolymer from glycerol. However, in recombinant E. coli, only a 4 % 3HV fraction was obtained in the copolymer even when threonine was added to the medium (Eschenlauer et al. 1996). We recently engineered a metabolic pathway in E. coli via threonine to synthesize PHBV from glucose. By deregulating the feedback inhibition of the threonine and overexpressing three threonine synthesis genes thrA C1035T BC, this strain can first accumulate threonine, then convert the threonine to 2-ketobutyrate by threonine deaminase IlvA, which was incorporated from Cornybacterium glutamicum. To increase the propionyl-CoA content, the competitive pathways of catalytic conversion of propionyl-CoA to 3-hydroxyvaleryl-CoA were blocked (Chen et al. 2011). To further increase the 3HV fraction in the copolymer, the threonine biosynthesis pathway should be optimized. In addition, new pathways leading to the formation of 3HV is also valuable. Nevertheless, it seems that it is not possible to synthesize the PHV homopolymer in E. coli.
Polyesters containing the 4HB monomers
In 1988, Doi et al. discovered that C. necator (formerly known as Ralstonia eutropha, Alcaligenes eutrophus, Wautersia eutropha, and Hydrogenomonas eutropha) was able to synthesize P(3HB-co-4HB) when 4-hydroxybutyric or 4-chlorobutyric acid was fed as the second carbon source in the medium (Doi et al. 1988). Later, researchers found that many bacteria including Comamonas acidovorans (Lee et al. 2004; Park et al. 2005), Alcaligenes latus (Kang et al. 1995), Comamonas testosteroni (Renner et al. 1996), and Hydrogenophaga pseudoflava (Choi et al. 1999) can all accumulate P(3HB-co-4HB) and/or P4HB homopolymer. The capability of polyesters containing the 4HB monomers formation in wild-type microorganisms inspired researchers to explore the possibility of P4HB synthesis in recombinant E. coli. In 1996, a 4-hydroxybutyrate CoA transferase gene orfZ was discovered from Clostridium kluyveri, a non PHAs producer, that can transfer the CoA to 4-hydroxybutyrate (Söhling and Gottschalk 1996), which made it possible for the heterologous P4HB biosynthesis in E. coli. By cloning orfZ gene from C. kluyveri, researchers first synthesized P4HB homopolymer in recombinant E. coli in the presence of precursor substrate 4HB (Hein et al. 1997; Song et al. 1999). Then, by introducing the whole succinate degradation pathway genes and P3HB biosynthesis genes phaCAB from C. necator, recombinant E. coli was proved to accumulate P(3HB-co-4HB) directly from glucose (Valentin and Dennis 1997). In this recombinant E. coli succinate, a TCA cycle intermediate was converted to 4-hydroxybutyryl-CoA via succinyl-CoA: CoA transferase, succinic semialdehyde dehydrogenase (SucD), 4-hydroxybutyrate dehydrogenase (4hbD), and 4-hydroxybutyryl-CoA via: CoA transferase. This was the first example that a copolymer was heterologously synthesized in E. coli from unrelated carbon source. However, the 4HB fraction in the initial synthesized polymer was very low. Inactivation of E. coli native succinate semialdehyde dehydrogenase genes sad and gabD improved the 4HB content up to 11 % in the copolymer, resulting to the highest P(3HB-co-4HB) production in E. coli from glucose to this day (Li et al. 2010). Meanwhile, the biosynthesis of 4HB homopolymer from unrelated carbon source was achieved. In 2005, Song et al. engineered a 4HB biosynthesis pathway via glutamate in E. coli using glucose as a sole carbon source with 0.78 g/L yield of P4HB (Song et al. 2005). Significant level of P4HB can now be obtained from glucose via succinate degradation pathway in sad and gabD genes deficient strain of E. coli JM109 that coexpressed with four PHA binding proteins PhaP1, PhaP2, PhaP3, and PhaP4, respectively. Over 68 % P4HB (11.5 g/L) of the cell dry weight was produced in a fed-batch fermentation process (Zhou et al. 2012).
Polyesters containing the LA monomers
Polylactic acid (PLA), the unnatural polyester, is usually synthesized by chemical polymerization of fermented product, lactic acid (2-hydroxypropionic acid, 2HP). Due to its promising market and drawbacks during the chemical synthesis process (Södergård and Stolt 2002), people had tried to synthesize this polymer directly by microbial fermentation. Taking advantage of the wide substrate specificity of PHA synthase, enzymes/mutants that can incorporate LA monomer were created and selected. Recombinant E. coli expressing the mutated propionate CoA-transferase gene pct from Megasphaera elsdenii, which allows generation of (D)-lactyl-CoA in the cell, and the mutated type II PHA synthase gene phaC1 from Pseudomonas sp. 61-3 was proved to synthesize LA copolymer directly by fermentation from glucose. The polymer composition is 94 mol% 3HB and 6 mol% LA, and the polymer content in the cell is 19 % of the dry cell weight. In the following experiments, this group enriched the LA fraction in the copolymer from the previous 6 mol% to 47 mol% by anaerobic cultivation (Taguchi et al. 2008; Yamada et al. 2009). Employing a pct gene from Clostridium propionicum and phaC1 from Pseudomonas sp. MBEL6-19, Lee Sang Yup group reported that PLA homopolymer could be produced up to 11 wt% from glucose (Jung et al. 2010). Several PhaC1 Ps6-19 variants with mutations were investigated with respect to their PLA biosynthesis capability in wild-type E. coli XL1-Blue (Yang et al. 2011). Together with β-ketothiolase and acetoacetyl-CoA reductase genes phbAB, the recombinant strain can accumulate P(3HB-co-LA) with 6 ∼ 64 mol% LA fraction in the copolymer from glucose (Park et al. 2008; Yang et al. 2010). Then, they further engineered the host by knocking out the ackA (acetate kinase), ppc (phosphoenolpyruvate carboxylase), and adhE (acetaldehyde/alcohol dehydrogenase) genes and by replacing the promoters of the ldhA (D-lactate dehydrogenase) and acs (acetyl-CoA synthetase) genes with the trc promoter to increase the metabolic fluxes at the systems level. The final P(3HB-co-LA) copolymer contains up to 70 mol% LA fraction with 46 wt% polymer content (Jung et al. 2010). Using this in vivo PLA biosynthesis system, they can also synthesize PHAs containing 2-hydroxybutyrate monomer (Park et al. 2012). The conversion of chemical synthesis process to fermentative process opened up a new way for PLA production, and this process can also generated polyesters that contains both LA and 3HB fractions. The synthesis of copolymer containing LA and other types of monomers is expected.
Polyesters containing the 3HP monomers
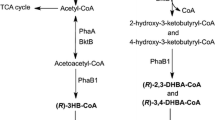
Polyesters containing the 3HP monomers could be produced when 3HP, α,ω-alkanediols, or acrylate was added as carbon source (Green et al. 2002; Nakamura et al. 1991; Valentin et al. 2000). Since this kind of polymer was supposed to have higher rigidity, ductility, and stability, many efforts have been made in E. coli for the production of P3HP or its copolymer with high content and controllable compositions (Meng et al. 2012; Zhou et al. 2011). In 2008, P(3HP-co-3HB) synthesis from unrelated carbon sources was first achieved in engineered C. necator by introducing malonyl-CoA reductase (Mcr) and the 3HP-CoA synthetase domain of trifunctional propionyl-CoA synthase (Acs) from Chloroflexus aurantiacus. Strains harboring the two heterologous genes synthesized P(3HB-co-3HP) only with 0.2–2.1 mol% of 3HP fraction (Fukui et al. 2009). In 2010, an engineered E. coli was developed by introducing the genes of glycerol dehydratase (DhaB1) from Clostridium butyricum, propionaldehyde dehydrogenase (PduP) from S. enterica serovar Typhimurium LT2, and PHA synthase (PhbC) of C. necator. This recombinant E. coli accumulated up to 1.42 g/L P3HP homopolymer in fed-batch fermentation from unrelated carbon source, glycerol (Andreeßen et al. 2010). In the same year, production of P3HP from glucose in recombinant E. coli was achieved by cloning the genes accABCD encoding acetyl-CoA carboxylase and genes for Mcr, propionyl-CoA synthetase (PrpE), and PhbC from C. necator (Wang et al. 2012a). However, the polymer content was 1.32 g/L and only 0.98 % wt/wt of the cell dry weight. Nevertheless, this attempt shows the feasibility of engineering a P3HP biosynthetic pathway using a structurally-unrelated carbon source in E. coli. Further effort towards the improvement of the P3HP production should be made. Since several 3HP synthetic pathways were designed and large quantity of 3HP was produced from unrelated carbon sources, many experiences involved in 3HP production in E. coli should be used for reference (Cho et al. 2010; Jiang et al. 2009; Rathnasingh et al. 2009; Rathnasingh et al. 2012).
Polyesters containing the 3HA monomers
Medium-chain-length PHAs (mcl-PHA) are naturally synthesized through fatty acid de novo biosynthesis pathway or β-oxidation pathway from Pseudomonads (Timm and Steinbüchel 1990). In 1997, recombinant E. coli expressed the phaC1 gene from Pseudomonas aeruginosa and was found to produce mcl-PHA from related carbon source fatty acid through a β-oxidation pathway by knocking out the fadB gene (Langenbach et al. 1997; Qi et al. 1997). Later, by introducing with a transacylase gene phaG from Pseudomonas putida, E. coli was proved to accumulate 2 to 3 % mcl-PHA of cellular dry weight through fatty acid de novo biosynthesis from glucose (Rehm et al. 2001). The transacylase phaG catalyzes the transfer of the (R)-3-hydroxy-acyl moiety from the acyl-carrier-protein (ACP) thioester to CoA making the intermediates from de novo fatty acid biosynthesis into the substrates for mcl-PHA biosynthesis. However, due to the low efficiency of PhaG, further improvement of mcl-PHA production in this pathway was made. A recent study suggested that PhaG is not a 3-hydroxyacyl-ACP: CoA transferase as reported but a 3-hydroxyacyl-ACP thioesterase. Based on this, they overexpressed a predicted mcl-fatty acid CoA ligase PP0763 from P. putida together with P. putida PhaG and the engineered Pseudomonas sp. 61-3 PhaC1 in E. coli. The new-generated strains can accumulate 11.6 % mcl-PHA of the cell dry weight (about 400 mg/L mcl-PHA) when grown on glucose as a sole carbon source (Wang et al. 2012b). Meanwhile, some researchers tried to combine fatty acid de novo biosynthesis with β-oxidation by using an acyl-ACP thioesterase, which hydrolyzes acyl-ACPs and produces enhanced intracellular free fatty acid. Then, fatty acid is channeled into β-oxidation pathway to form (R)-3-hydroxyacyl-CoA. Coexpression of the cytosolic thioesterase I gene tesA and a PHA synthase gene (phaC2 from Pseudomonas oleovorans) in E. coliΔfadBΔfadR resulted in the synthesis of mcl-PHA composed mainly of 3-hydroxyoctanoate from the glucose (Klinke et al. 1999). Another acyl-ACP thioesterase from Umbellularia californica can also lead to the formation of mcl-PHA in E. coli fad mutants (Rehm and Steinbüchel 2001). To improve the production of mcl-PHA, a deep-going reinvent was carried out. They found that E. coli ΔfadRABIJ expressed with the same acyl-ACP thioesterase (named by BTE), P. aeruginosa PhaC2, and enoyl-CoA hydratase (phaJ3), CoA ligase PP0763 from P. putida produced mcl-PHA with over 15 % CDW, (Agnew et al. 2012). Mutated 3-ketoacyl-ACP synthase III genes fabH and 3-ketoacyl-ACP reductases (FabG) genes from E. coli can channeled the de novo fatty acid to mcl-PHA biosynthesis but via a different point (Nomura et al. 2004). In this case, FabH channeled the 3-ketoacyl-ACP to 3-ketoacyl-CoA, while FabG enhanced the conversion of 3-ketoacyl-CoA to (R)-3-hydroxyacyl-CoA (Nomura et al. 2005).
Some PHAs such as P(3HB-co-3HHx) can be synthesized from unrelated carbon source in wild-type bacteria (Fukui et al. 2002; Qiu et al. 2005) but not in recombinant E. coli yet. To realize this, the metabolic pathways in wild-type bacteria should be made clear first.
Perspective
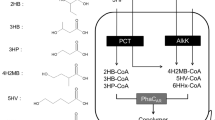
Since the PHA synthase has wide-substrate specificity, which enabled the incorporation of various monomers into PHA polymer, many efforts have been done to synthesize various PHAs with different properties in E. coli. However, most of the natural microorganism can only synthesize the PHAs with varied monomers under laboratory conditions by adding structurally-related precursors (Steinbüchel and Lütke-Eversloh 2003). These structurally-related precursors are usually high cost, poorly miscible with water, toxic to bacteria at relatively low concentrations, hard control for fed batch, and/or higher oxygen demand than carbohydrates. Therefore, more and more researchers have moved their interest onto inexpensive and renewable unrelated carbon sources such as glucose. Increasing the efficiency of their use in PHAs biosynthesis is critical to the overall economics. (Figure 1 shows the known metabolic pathways so far which is constructed for PHAs biosynthesis in recombinant E. coli from unrelated carbon source.)
Therefore, the principal task in this area is to exploit the metabolic potential of E. coli for the production of PHAs with tailor-made monomer composition from unrelated carbon source. Pathways could be constructed via heterologous and/or combinatorial expression of genes from different organisms. In this case, synthetic biology offered us a conceptual and technological framework to speed up the creation of new metabolic enzymes and/or pathways (Lee et al. 2012). Some new creating noninherent pathways for the synthesis of fuels or chemicals (Dellomonaco et al. 2011; Felnagle et al. 2012) could be used as a bridge to link unrelated carbon source with PHAs production. Second, improvement of the PHAs content in the cell is also important. The metabolic flux which leads to the PHAs synthesis can be maximized by optimizing the physiological state of the cell at the systems level. For this purpose, omics technology and/or systems biology provide many tools. Third, most of unrelated carbon sources used for PHAs production is glucose and employment of other cheap, renewable, unrelated carbon source for PHAs production is necessary.
References
Agnew DE, Stevermer AK, Youngquist JT, Pfleger BF (2012) Engineering Escherichia coli for production of C(12)-C(14) polyhydroxyalkanoate from glucose. Metab Eng 14:705–713. doi:10.1016/j.ymben.2012.08.003
Aldor IS, Kim SW, Prather KL, Keasling JD (2002) Metabolic engineering of a novel propionate-independent pathway for the production of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) in recombinant Salmonella enterica serovar typhimurium. Appl Environ Microbiol 68:3848–3854
Anderson AJ, Dawes EA (1990) Occurrence, metabolism, metabolic role, and industrial uses of bacterial polyhydroxyalkanoates. Microbiol Rev 54:450–472
Andreeßen B, Lange AB, Robenek H, Steinbüchel A (2010) Conversion of glycerol to poly(3-hydroxypropionate) in recombinant Escherichia coli. Appl Environ Microbiol 76:622–626. doi:10.1128/AEM.02097-09
Byrom D (1987) Polymer synthesis by microorganisms: technology and economics. Trends Biotechnol 5:246–250
Chen GQ (2009) A microbial polyhydroxyalkanoates (PHA) based bio- and materials industry. Chem Soc Rev 38:2434–2446. doi:10.1039/b812677c
Chen Q, Wang Q, Wei G, Liang Q, Qi Q (2011) Production in Escherichia coli of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) with differing monomer compositions from unrelated carbon sources. Appl Environ Microbiol 77:4886–4893. doi:10.1128/AEM.00091-11
Cho A, Yun H, Park JH, Lee SY, Park S (2010) Prediction of novel synthetic pathways for the production of desired chemicals. BMC Syst Biol 4:35. doi:10.1186/1752-0509-4-35
Choi MH, Yoon SC, Lenz RW (1999) Production of poly(3-hydroxybutyric acid-co-4-hydroxybutyric acid) and poly(4-hydroxybutyric acid) without subsequent degradation by Hydrogenophaga pseudoflava. Appl Environ Microbiol 65:1570–1577
Dellomonaco C, Clomburg JM, Miller EN, Gonzalez R (2011) Engineered reversal of the beta-oxidation cycle for the synthesis of fuels and chemicals. Nature 476:355–359. doi:10.1038/nature10333
Doi Y, Kunioka M, Nakamura Y, Soga K (1988) Nuclear magnetic resonance studies on unusual bacterial copolyesters of 3-hydroxybutyrate and 4-hydroxybutyrate. Macromolecules 21:2722–2727
Eschenlauer AC, Stoup SK, Srienc F, Somers DA (1996) Production of heteropolymeric polyhydroxyalkanoate in Escherichia coli from a single carbon source. Int J Biol Macromol 19:121–130
Felnagle EA, Chaubey A, Noey EL, Houk KN, Liao JC (2012) Engineering synthetic recursive pathways to generate non-natural small molecules. Nat Chem Biol 8:518–526. doi:10.1038/nchembio.959
Fidler S, Dennis D (1992) Polyhydroxyalkanoate production in recombinant Escherichia coli. FEMS Microbiol Rev 9:231–235
Fukui T, Abe H, Doi Y (2002) Engineering of Ralstonia eutropha for production of poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) from fructose and solid-state properties of the copolymer. Biomacromolecules 3:618–624
Fukui T, Suzuki M, Tsuge T, Nakamura S (2009) Microbial synthesis of poly((R)-3-hydroxybutyrate-co-3-hydroxypropionate) from unrelated carbon sources by engineered Cupriavidus necator. Biomacromolecules 10:700–706. doi:10.1021/bm801391j
Green PR, Kemper J, Schechtman L, Guo L, Satkowski M, Fiedler S, Steinbuchel A, Rehm BH (2002) Formation of short-chain length/medium chain length polyhydroxyalkanoate copolymers by fatty acid beta-oxidation inhibited Ralstonia eutropha. Biomacromolecules 3:208–213
Hazer B, Steinbüchel A (2007) Increased diversification of polyhydroxyalkanoates by modification reactions for industrial and medical applications. Appl Microbiol Biotechnol 74:1–12. doi:10.1007/s00253-006-0732-8
Hein S, Söhling B, Gottschalk G, Steinbüchel A (1997) Biosynthesis of poly(4-hydroxybutyric acid) by recombinant strains of Escherichia coli. FEMS Microbiol Lett 153:411–418
Jiang X, Meng X, Xian M (2009) Biosynthetic pathways for 3-hydroxypropionic acid production. Appl Microbiol Biotechnol 82:995–1003. doi:10.1007/s00253-009-1898-7
Jung YK, Kim TY, Park SJ, Lee SY (2010) Metabolic engineering of Escherichia coli for the production of polylactic acid and its copolymers. Biotechnol Bioeng 105:161–171. doi:10.1002/bit.22548
Kang CK, Kusaka S, Doi Y (1995) Structure and properties of poly (3-hydroxybutyrate-co-4-hydroxybutyrate) produced by Alcaligenes latus. Biotechnol Lett 17:583–588
Klinke S, Ren Q, Witholt B, Kessler B (1999) Production of medium-chain-length poly(3-hydroxyalkanoates) from gluconate by recombinant Escherichia coli. Appl Environ Microbiol 65:540–548
Langenbach S, Rehm BH, Steinbüchel A (1997) Functional expression of the PHA synthase gene phaC1 from Pseudomonas aeruginosa in Escherichia coli results in poly(3-hydroxyalkanoate) synthesis. FEMS Microbiol Lett 150:303–309
Law KH, Chan PL, Lau WS, Cheng YC, Leung YC, Lo WH, Lawford H, Yu HF (2004) Construction of recombinant Escherichia coli strains for production of poly-(3-hydroxybutyrate-co-3-hydroxyvalerate). Appl Biochem Biotechnol 113–116:361–372
Lee JW, Na D, Park JM, Lee J, Choi S, Lee SY (2012) Systems metabolic engineering of microorganisms for natural and non-natural chemicals. Nat Chem Biol 8:536–546. doi:10.1038/nchembio.970
Li R, Chen Q, Wang PG, Qi Q (2007a) A novel-designed Escherichia coli for the production of various polyhydroxyalkanoates from inexpensive substrate mixture. Appl Microbiol Biotechnol 75:1103–1109
Li R, Zhang H, Qi Q (2007b) The production of polyhydroxyalkanoates in recombinant Escherichia coli. Bioresour Technol 98:2313–2320. doi:10.1016/j.biortech.2006.09.014
Li ZJ, Shi ZY, Jian J, Guo YY, Wu Q, Chen GQ (2010) Production of poly(3-hydroxybutyrate-co-4-hydroxybutyrate) from unrelated carbon sources by metabolically engineered Escherichia coli. Metab Eng 12:352–359. doi:10.1016/j.ymben.2010.03.003
Meng DC, Shi ZY, Wu LP, Zhou Q, Wu Q, Chen JC, Chen GQ (2012) Production and characterization of poly (3-hydroxypropionate-co-4-hydroxybutyrate) with fully controllable structures by recombinant Escherichia coli containing an engineered pathway. Metab Eng 14:317–324
Nakamura S, Kunioka M, Doi Y (1991) Biosynthesis and characterization of bacterial poly (3-hydroxybutyrate-co-3-hydroxypropionate). Macromol Rep 28:15–24
Nomura CT, Taguchi K, Gan Z, Kuwabara K, Tanaka T, Takase K, Doi Y (2005) Expression of 3-ketoacyl-acyl carrier protein reductase (fabG) genes enhances production of polyhydroxyalkanoate copolymer from glucose in recombinant Escherichia coli JM109. Appl Environ Microbiol 71:4297–4306. doi:10.1128/AEM.71.8.4297-4306.2005
Nomura CT, Taguchi K, Taguchi S, Doi Y (2004) Coexpression of genetically-engineered 3-ketoacyl-ACP synthase III (fabH) and polyhydroxyalkanoate synthase (phaC) genes leads to short-chain-length-medium-chain-length polyhydroxyalkanoate copolymer production from glucose in Escherichia coli JM109. Appl Environ Microbiol 70:999–1007
Park SJ, Choi J, Lee SY (2005) Engineering of Escherichia coli fatty acid metabolism for the production of polyhydroxyalkanoates. Enzym Microb Technol 36:579–588
Park SJ, Lee TW, Lim SC, Kim TW, Lee H, Kim MK, Lee SH, Song BK, Lee SY (2012) Biosynthesis of polyhydroxyalkanoates containing 2-hydroxybutyrate from unrelated carbon source by metabolically engineered Escherichia coli. Appl Microbiol Biotechnol 93:273–283. doi:10.1007/s00253-011-3530-x
Park SJ, Yang TH, Kang HO, Lee SH, Lee EJ, Kim TW, Lee SY (2008) Mutants of PHA synthase from Pseudomonas sp. 6-19 and method for preparing lactate homopolymer or copolymer using the same. WO Patent 2,008,062,999
Qi Q, Rehm BH, Steinbüchel A (1997) Synthesis of poly(3-hydroxyalkanoates) in Escherichia coli expressing the PHA synthase gene phaC2 from Pseudomonas aeruginosa: comparison of PhaC1 and PhaC2. FEMS Microbiol Lett 157:155–162
Qiu YZ, Han J, Guo JJ, Chen GQ (2005) Production of poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) from gluconate and glucose by recombinant Aeromonas hydrophila and Pseudomonas putida. Biotechnol Lett 27:1381–1386. doi:10.1007/s10529-005-3685-6
Rathnasingh C, Raj SM, Jo JE, Park S (2009) Development and evaluation of efficient recombinant Escherichia coli strains for the production of 3-hydroxypropionic acid from glycerol. Biotechnol Bioeng 104:729–739. doi:10.1002/bit.22429
Rathnasingh C, Raj SM, Lee Y, Catherine C, Ashok S, Park S (2012) Production of 3-hydroxypropionic acid via malonyl-CoA pathway using recombinant Escherichia coli strains. J Biotechnol 157:633–640. doi:10.1016/j.jbiotec.2011.06.008
Rehm BH, Mitsky TA, Steinbüchel A (2001) Role of fatty acid de novo biosynthesis in polyhydroxyalkanoic acid (PHA) and rhamnolipid synthesis by pseudomonads: establishment of the transacylase (PhaG)-mediated pathway for PHA biosynthesis in Escherichia coli. Appl Environ Microbiol 67:3102–3109. doi:10.1128/AEM.67.7.3102-3109.2001
Rehm BH, Steinbüchel A (2001) Heterologous expression of the acyl-acyl carrier protein thioesterase gene from the plant Umbellularia californica mediates polyhydroxyalkanoate biosynthesis in recombinant Escherichia coli. Appl Microbiol Biotechnol 55:205–209
Renner G, Pongratz K, Braunegg G (1996) Production of poly (3-hydroxybutyrate-co-4-hydroxybutyrate) by Comamonas testosteronii A3. Food Technol Biotech 34:2–3
Södergård A, Stolt M (2002) Properties of lactic acid based polymers and their correlation with composition. Prog Polym Sci 27:1123–1163
Schubert P, Steinbüchel A, Schlegel HG (1988) Cloning of the Alcaligenes eutrophus genes for synthesis of poly-beta-hydroxybutyric acid (PHB) and synthesis of PHB in Escherichia coli. J Bacteriol 170:5837–5847
Slater SC, Voige WH, Dennis DE (1988) Cloning and expression in Escherichia coli of the Alcaligenes eutrophus H16 poly-beta-hydroxybutyrate biosynthetic pathway. J Bacteriol 170:4431–4436
Söhling B, Gottschalk G (1996) Molecular analysis of the anaerobic succinate degradation pathway in Clostridium kluyveri. J Bacteriol 178:871–880
Song S, Hein S, Steinbüchel A (1999) Production of poly (4-hydroxybutyric acid) by fed-batch cultures of recombinant strains of Escherichia coli. Biotechnol Lett 21:193–197
Song SS, Ma H, Gao ZX, Jia ZH, Zhang X (2005) Construction of recombinant Escherichia coli strains producing poly (4-hydroxybutyric acid) homopolyester from glucose. Wei Sheng Wu Xue Bao 45:382–386
Steinbüchel A, Lütke-Eversloh T (2003) Metabolic engineering and pathway construction for biotechnological production of relevant polyhydroxyalkanoates in microorganisms. Biochem Eng J 16:81–96
Steinbüchel A, Pieper U (1992) Production of a copolyester of 3-hydroxybutyric acid and 3-hydroxyvaleric acid from single unrelated carbon sources by a mutant of Alcaligenes eutrophus. Appl Microbiol Biotechnol 37:1–6
Steinbüchel A, Valentin HE (1995) Diversity of bacterial polyhydroxyalkanoic acids. FEMS Microbiol Lett 128:219–228
Steinbüchel A, Aerts K, Babel W, Follner C, Liebergesell M, Madkour MH, Mayer F, Pieper-Furst U, Pries A, Valentin HE, Roman W (1995) Considerations on the structure and biochemistry of bacterial polyhydroxyalkanoic acid inclusions. Can J Microbiol 41(Suppl 1):94–105
Taguchi S, Yamada M, Matsumoto K, Tajima K, Satoh Y, Munekata M, Ohno K, Kohda K, Shimamura T, Kambe H, Obata S (2008) A microbial factory for lactate-based polyesters using a lactate-polymerizing enzyme. Proc Natl Acad Sci U S A 105:17323–17327. doi:10.1073/pnas.0805653105
Timm A, Steinbüchel A (1990) Formation of polyesters consisting of medium-chain-length 3-hydroxyalkanoic acids from gluconate by Pseudomonas aeruginosa and other fluorescent pseudomonads. Appl Environ Microbiol 56:3360–3367
Valentin HE, Dennis D (1997) Production of poly(3-hydroxybutyrate-co-4-hydroxybutyrate) in recombinant Escherichia coli grown on glucose. J Biotechnol 58:33–38
Valentin HE, Mitsky TA, Mahadeo DA, Tran M, Gruys KJ (2000) Application of a propionyl coenzyme A synthetase for poly(3-hydroxypropionate-co-3-hydroxybutyrate) accumulation in recombinant Escherichia coli. Appl Environ Microbiol 66:5253–5258
Wang Q, Liu C, Xian M, Zhang Y, Zhao G (2012a) Biosynthetic pathway for poly(3-hydroxypropionate) in recombinant Escherichia coli. J Microbiol 50:693–697. doi:10.1007/s12275-012-2234-y
Wang Q, Tappel RC, Zhu C, Nomura CT (2012b) Development of a new strategy for production of medium-chain-length polyhydroxyalkanoates by recombinant Escherichia coli via inexpensive non-fatty acid feedstocks. Appl Environ Microbiol 78:519–527. doi:10.1128/AEM.07020-11
Yamada M, Matsumoto K, Nakai T, Taguchi S (2009) Microbial production of lactate-enriched poly[(R)-lactate-co-(R)-3-hydroxybutyrate] with novel thermal properties. Biomacromolecules 10:677–681. doi:10.1021/bm8013846
Yang TH, Jung YK, Kang HO, Kim TW, Park SJ, Lee SY (2011) Tailor-made type II Pseudomonas PHA synthases and their use for the biosynthesis of polylactic acid and its copolymer in recombinant Escherichia coli. Appl Microbiol Biotechnol 90:603–614. doi:10.1007/s00253-010-3077-2
Yang TH, Kim TW, Kang HO, Lee SH, Lee EJ, Lim SC, Oh SO, Song AJ, Park SJ, Lee SY (2010) Biosynthesis of polylactic acid and its copolymers using evolved propionate CoA transferase and PHA synthase. Biotechnol Bioeng 105:150–160
Zhou Q, Shi ZY, Meng DC, Wu Q, Chen JC, Chen GQ (2011) Production of 3-hydroxypropionate homopolymer and poly(3-hydroxypropionate-co-4-hydroxybutyrate) copolymer by recombinant Escherichia coli. Metab Eng 13:777–785. doi:10.1016/j.ymben.2011.10.002
Zhou XY, Yuan XX, Shi ZY, Meng DC, Jiang WJ, Wu LP, Chen JC, Chen GQ (2012) Hyperproduction of poly(4-hydroxybutyrate) from glucose by recombinant Escherichia coli. Microb Cell Fact 11:54. doi:10.1186/1475-2859-11-54
Acknowledgments
This research was financially supported by a grant from the National Basic Research Program of China (2011CB707405, 2012CB725202) and grants from the National Natural Science Foundation of China (31200033) and the promotive research fund for excellent, young, and middle-aged scientists of Shandong Province (BS2012SW005).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, Q., Zhuang, Q., Liang, Q. et al. Polyhydroxyalkanoic acids from structurally-unrelated carbon sources in Escherichia coli . Appl Microbiol Biotechnol 97, 3301–3307 (2013). https://doi.org/10.1007/s00253-013-4809-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-013-4809-x