Abstract
A particularly successful polyhydroxyalkanoate (PHA) in industrial applications is poly (3-hydroxybutyrate) (PHB). However, one of the major obstacles for wider application of PHB is the cost of its production and purification. Therefore, it is desirable to discover a method for producing PHB in large quantities at a competitive price. Glycerol is a cheap and widely used carbon source that can be applied in PHB production process. There are numerous advantages to operating fermentation at elevated temperatures; only several thermophilic bacteria are able to accumulate PHB when glycerol is the growth substrate. Here, we report on the possibility of increasing PHB production at low cost using thermophilic Chelatococcus daeguensis TAD1 when glycerol is the growth substrate in a fed-batch culture. We found that (1) excess glycerol inhibited PHB accumulation and (2) organic nitrogen sources, such as tryptone and yeast extract, promoted the growth of C. daeguensis TAD1. In the batch fermentation experiments, we found that using glycerol at low concentrations as the sole carbon source, along with the addition of mixed nitrate (NH4Cl, tryptone, and yeast extract), stimulated PHB accumulation in C. daeguensis TAD1. The results showed that the PHB productivity decreased in the following order: two-stage fed-batch fermentation > fed-batch fermentation > batch fermentation. In optimized culture conditions, a PHB amount of 17.4 g l−1 was obtained using a two-stage feeding regimen, leading to a productivity rate of 0.434 g l−1 h−1, which is the highest productivity rate reported for PHB to date. This high PHB biosynthetic productivity could decrease the total production cost, allowing for further development of industrial applications of PHB.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Polyhydroxyalkanoates (PHAs) are natural polyesters that are of special interest in industrial applications due to their biodegradability and biocompatibility (Brandl et al. 1995; Madison and Huisman 1999; Zinn et al. 2001; Chen 2009). PHAs can be synthesized and stored as intracellular reserve materials in a wide variety of microorganisms. They are accumulated when the bacterial cells experience growth-limiting conditions of nutrients other than carbon. To date, more than a hundred different monomers have been reported to be incorporated in bacterial PHAs, resulting in a range of polymers with different material properties (Steinbüchel and Valentin 1995; Ward and O’Connor 2005; Chen and Wu 2005; Ren et al. 2010).
The production of PHAs from renewable resources, coupled with their complete biodegradability, give PHAs a number of advantages from an environmental point of view (Braunegg et al. 1998). One of the most promising PHAs for use in green packaging, biomedical devices, and tissue engineering applications is poly(3-hydroxybutyrate) (PHB) (Chen and Wu 2005; Xu et al. 2014), which is very similar to thermoplastic polypropylene (Holmes 1985). Many studies have investigated the use of glucose as carbon source for the biosynthesis of PHAs (Lee. 1996; Borah et al. 2002; Ibrahim and Steinbüchel 2010a, b; Gahlawat and Srivastava 2013). However, the high cost of glucose would hinder the commercial use of such methods.
Glycerol would be an attractive alternative carbon source for PHB biosynthesis, because it is less expensive and readily available as a waste by-product during biodiesel production (Thompson and He 2006). A recent study demonstrated that crude and refined glycerol from the biodiesel industry could be used as a carbon substrate for Pseudomonas mediterranea and Pseudomonas corrugate, which accumulated medium-chain-length PHAs (Palmeri et al. 2012). Glycerol has also been tested as growth substrate for Escherichia coli in fed-batch processes and shown to achieve a high cell density (Le Meur et al. 2014). In our previous research (Liang et al. 2012; Yang et al. 2012; Liu et al. 2014; Xu et al. 2014), Chelatococcus daeguensis TAD1 was identified as a thermophilic strain of bacteria that can effectively use glucose for PHB production at 50 °C. The next step is to demonstrate the biosynthesis of PHB from glycerol using thermophilic C. daeguensis TAD1.
The current work is aimed at developing a strategy to improve the production of PHB by thermophilic C. daeguensis TAD1 at 50 °C, using glycerol as the inexpensive carbon source. First, flask cultures were used to investigate the most relevant factors, such as inoculum size, initial pH, nitrogen sources, the concentration of glycerol, and NaCl, which would influence the biosynthesis. Second, optimized conditions, based on these results, were used in batch fermentation in a 5-L bioreactor to evaluate different feeding regimens that could be employed in the production of PHB from glycerol by C. daeguensis TAD1.
Materials and methods
Microorganism and culture conditions
C. daeguensis TAD1, an isolated and characterized bacterium accumulating PHB (Liang et al. 2012; Yang et al. 2012; Xu et al. 2014), was used in this study. C. daeguensis TAD1 had been deposited in China General Microbiological Culture Collection Center (CGMCC no. 5226). The 16S ribosomal DNA gene sequence of C. daeguensis TAD1 had been submitted to the DNA Data Bank of Japan (DDBJ)/European Molecular Biology Laboratory (EMBL)/GenBank databases under accession no. HM000004 (Liang et al. 2012; Xu et al. 2014).
The growth media and their corresponding compositions (Xu et al. 2014) were as follows: Luria-Bertani (LB) medium (g l−1): tryptone, 10.0; yeast extract, 5.0; NaCl, 5.0. Mineral salt medium (MSM) (g l−1) (Ibrahim and Steinbüchel 2010b): Na2HPO4 · 2H2O, 9.0; KH2PO4, 1.5; MgSO4 · 7H2O, 0.2; NH4Cl, 1.0; CaCl2 · 2H2O, 0.02. The MSM also included 1 mL of trace element solution containing the following (g l−1): EDTA, 50.0; FeCl3, 8.3; ZnCl2, 0.84; CuCl2 · 2H2O, 0.13; CoCl2 · 6H2O, 0.1; MnCl2 · 6H2O, 0.016; H3BO3, 0.1.
Preculture was grown in 250-mL Erlenmeyer flasks containing 50-mL LB medium. The flasks were incubated at 45 °C and 180 rpm for 12 h. Five milliliters of this seed medium was inoculated to 100-mL MSM medium with glycerol (18.5 g l−1) as sole carbon source in 300-mL flask, and then grown for 36 h at 45 °C, and 180 rpm. The pH of the medium was adjusted to 7.3 before sterilization. These cells were harvested by centrifugation for 20 min at 1200 × g and 4 °C, washed with distilled water twice, and then frozen and lyophilized. Cell concentration, defined as cell dry weight (CDW) per liter of culture broth, was determined by weighing the lyophilized cells.
Optimization of the amount of PHB produced from glycerol by C. daeguensis TAD1
Effects of the inoculum size
The influences of the inoculum size on the PHB accumulation of TAD1 were determined by testing at different inoculation quantities (3, 5, 10, 15, and 20 %) in MSM medium at 50 °C. Other culture conditions were the same as described in the “Microorganism and culture conditions” section.
Effects of initial pH
The influences of the initial pH on growth and PHB accumulation by C. daeguensis TAD1 were determined by testing at different PH (5.5, 6, 6.5, 7, 7.5, 8, 8.5, and 9) in MSM medium. The other culture conditions were as described in “Microorganism and culture conditions,” except for the inoculum size, which was as optimized in the “Effects of the inoculum size” section.
Effect of nitrogen sources
The ability of C. daeguensis TAD1 to utilize different nitrogen sources for PHB accumulation was investigated in MSM at 50 °C. Nitrogen sources were divided into NH4Cl, KNO3, tryptone, yeast extract, and the mixed nitrogen sources containing NH4Cl with various concentrations of tryptone and yeast extract. The culture conditions were as described in “Microorganism and culture conditions,” with the optimized inoculum size and PH stated above.
Effects of the concentration of glycerol
The influences of the glycerol concentration on growth and PHB accumulation by C. daeguensis TAD1 were determined by testing at various concentrations (10, 20, 30, 40, and 50 g l−1) in MSM medium. The other culture conditions were as described in “Microorganism and culture conditions,” with the optimizations given above.
Effects of the concentration of sodium chloride
To estimate the effects of sodium chloride on growth and PHB accumulation by C. daeguensis TAD1, different concentrations of NaCl (0, 5, 10, 15, 20, 25, and 30 g l−1) were added to MSM medium. The other culture conditions were as described in “Microorganism and culture conditions,” with the above-mentioned optimizations.
Batch and fed-batch fermentation of C. daeguensis TAD1 in a 5-L bioreactor
One loop of cells from a fresh slant was transferred to 50-mL seed culture medium and cultured on a rotary shaker at 180 rpm and 45 °C for 12 h. Ten milliliters of this seed medium was inoculated to 100-mL medium with the above optimizations in a 300-mL flask, and then grown for 24 h at 50 °C and 180 rpm.
After preculture in the optimization medium, the following processes were carried out: (1) batch fermentation, (2) fed-batch fermentation, and (3) two-stage fed-batch fermentation, using a 5.0-L Biostat Aplus O2-Enrichment reactor (B. Braun Biotech International, Melsungen, Germany) with a working volume of 3 L. This was done to obtain the cell densities and PHB contents of cell of C. daeguensis TAD1, using glycerol as the sole carbon source. The contents of the fermentation medium were as described in “Microorganism and culture conditions,” with the optimizations set out above.
Cultivations were done at 50 °C and at a dissolved oxygen (pO2) saturation between 0 and 100 % in the medium. Unless stated otherwise, the pH in the medium was maintained at 7.5 by controlled addition of 5 M HCl or NaOH. Foam was removed by a mechanical foam destroyer; if this was not sufficient, an antifoaming agent was added. Small samples were withdrawn from the culture fluid for analytical purposes.
The fed-batch culture with constant feeding rate was initiated as a batch culture with an initial glycerol concentration of 20 g l−1. After 2 h of batch culture, additional medium I was pumped into the bioreactor by a peristaltic pump coupled with a computer. The feeding rate was selected to make the total glycerol concentration in the fed-batch culture 10 g l−1 or above, and maintain the nitrogen source concentration.
In the two-stage culture strategy, the glycerol concentration was maintained with the additional medium I until the cell concentration reached the maximum cell concentration of the batch culture at 25 h. After 25 h, the batch culture of C. daeguensis TAD1 in the second stage was fed with the additional medium II.
Aliquots of 40 mL were taken at 5-h time intervals to estimate glycerol concentration, cell dry weight, and nitrogen concentration.
The fermentation medium contained the following (g l−1): glycerol, 20; yeast extract, 0.7; peptone, 0.7; Na2HPO4 · 12H2O, 9.0; KH2PO4, 1.5; MgSO4 · 7H2O, 0.2; NH4Cl, 1.0; CaCl2 · 2H2O, 0.02 and 1-mL trace element solution.
Additional medium I contained the following (g l−1): glycerol, 50 % (v/v); yeast extract, 1.4; peptone, 1.4; Na2HPO4 · 12H2O, 18.0; KH2PO4, 3.0; MgSO4 · 7H2O, 0.4; NH4Cl, 1.0; CaCl2 · 2H2O, 0.04 and 2-mL trace element solution.
Additional medium II contained (g l−1): glycerol, 50 % (v/v); Na2HPO4 · 12H2O, 18.0; KH2PO4, 3.0; MgSO4 · 7H2O, 0.4; NH4Cl, 1.0; CaCl2 · 2H2O, 0.04 and 2-mL trace element solution.
Analytical methods
The presence of cytoplasmic PHB inclusions was investigated by staining the biopolymer with Nile red [0.25 mg dissolved in 1 mL dimethyl sulfoxide (DMSO)] and observing the cells under a fluorescence microscope (Ibrahim and Steinbüchel 2010a, b). The observations were carried out using a Nikon ECLIPSE 90i equipped with a Nikon JAPAN Plan Apo VC 100×/1.40 oil immersion Plan-Apochromat objective lens. The images were initially collected by a Nikon DIGITAL CAMAERA DXM 1200C, and then processed through an image software package called Nikon NIS-Elements License. Nile red fluorescence was excited using a Nikon INTENSILIGHT C-HGFI high-pressure mercury arc lamp, and the recording of the fluorescence images was performed using emission band-pass filters at EX 510-560 nm/DM 575 nm/BA 590 nm for Nile red.
Lyophilized cells were treated with acetone, dried, and then stirred in 50 volumes of chloroform for 48 h at 30 °C. After filtration, the extracted PHB was concentrated by partially evaporating the solvent, and five volumes of cold methanol were used for precipitation. The precipitated PHB was filtered and dried at 37 °C for 24 h (Ibrahim and Steinbüchel 2010b). The PHB was then repurified by the same procedure.
The samples were subjected to methanolysis in the presence of 15 % (v/v) sulfuric acid to determine the PHB content of the cells and the composition; samples were subjected to methanolysis in the presence of 15 % (v/v) sulfuric acid. The resulting 3-hydroxybutyric acid methyl esters were analyzed by gas chromatography (Rodgers and Wu 2010). PHB homopolymer (Sigma) was used as the standard for calibration. The content % (w/w) was defined as a percentage of PHB from CDW. The residual cell dry weight (RCDW) standing for cell growth was calculated as the total CDW minus the mass of PHB.
The concentrations of ammonium in cell-free supernatants were determined by employing a gas-sensitive type 1 5252303000 ammonium electrode (Mettler Toledo GmbH, Greifensee, Switzerland) (Ibrahim and Steinbüchel 2010b). The analysis of glycerol and organic acids was monitored via high-performance liquid chromatography (Shimadzu, LC-20A, Japan) using a JADE-PAKTM OA column (300 × 7.8 mm, 10 μm) with a UV detector (Shimadzu, SPD-20AV) at 210 nm. Aliquots of 10 μL were injected and eluted with 2.5 mM sulfuric acid in double-distilled water at a flow rate of 0.5 mL/min and operation temperature of 60 °C.
Results
Time course of growth and PHB accumulation on glycerol by C. daeguensis TAD1
According to the variation of CDW shown in Fig. 1a, the growth of C. daeguensis TAD1 was divided into two phases, the logarithmic phase (7–32 h) and stationary phase (32–120 h). During the logarithmic phase, the PHB content and concentration of RCDW both increased with the cultivation time. The highest PHB content (76.2 %) occurred at 32 h; at that time, the amount of PHB and the RCDW were 2.02 and 0.63 g l−1, respectively. From 32 h onward, the amount of PHB and the PHB content decreased slightly during the stationary phase, being 1.84 g l−1 and 71.7 % at the end of the experiment, respectively. In contrast, the concentration of RCDW increased during the stationary phase, and its peak (0.76 g l−1) occurred at 60 h. This means that the autolysis of C. daeguensis TAD1 was not obvious during the experiment. The curves of NH4Cl in Fig. 1 during the logarithmic phase show that these were inversely proportional to the proliferation of C. daeguensis TAD1. After exhaustion of NH4Cl at 48 h, C. daeguensis TAD1 stopped proliferating. This shows that the concentration of NH4Cl would play an important role in the accumulation of PHB.
Time course of growth and PHB accumulation on glycerol by C. daeguensis TAD1. According to the variation of RCDW, the growth of C. daeguensis TAD1 was divided into two phases by the cutoff line (vertical dashed lines), including logarithmic and stationary phases. a The concentrations of cell dry weight (CDW) (closed circle), NH4Cl (opened circle), residual cell dry weight (RCDW) (closed triangle), and PHB (opened triangle); b PHB content (%, w/w) (closed square) and the concentrations of glucose (opened square). A total of 5 % TAD1 was inoculated in a 1000-mL flask containing 300-mL mineral salt medium with 18.5 g l−1 glycerol. Cultures were incubated at 45 °C and 180 rev min−1
Effects of the inoculum size
Based on the time course of growth and PHB accumulation on glycerol by C. daeguensis TAD1 obtained above, we determined that the harvest time for C. daeguensis TAD1 cells was 32 h, when the highest PHB amount occurred. The effects of inoculum size on the growth and PHB accumulation of C. daeguensis TAD1 were investigated, and the results are shown in Fig. 2.
Effects of inoculum size on the growth and PHB accumulation of Chelatococcus daeguensis TAD1. Cultures were inoculated in 250-mL flasks containing 50-mL mineral salt medium with 18.5 g l−1 glycerol at pH 7.0 and were incubated for 32 h at 50 °C and 180 rev min−1 at different inoculum sizes. Values are means ± SD (error bars) for three replicates
As shown in Fig. 2, the amount of PHB and PHB content was initially positively related to the inoculum size from 3 to 10 % and exhibited the maximum values at the size of 10 %, which were 2.66 g l−1 and 78.3 %, respectively. Meanwhile, the CDW was 3.4 g l−1. As the inoculum size continued to rise, the peak of CDW (3.6 g l−1) occurred at 15 %, while the concentration of PHB and PHB content decreased from their previous values. This would indicate that the relative lack of nutrient in the medium affects the PHB accumulation by C. daeguensis TAD1.
Effects of initial pH
The impacts of the initial pH on growth and PHB accumulation by C. daeguensis TAD1 were further investigated. For this, the pH values of the culture medium were adjusted to 5.5, 6.0, 6.5, 7.0, 7.5, 8.0, 8.5, and 9.0 with concentrated HCl or NaOH solutions prior to sterilization. As shown in Fig. 3, an initial pH of 7.5 was optimal for CDW and the amount of PHB (3.87 and 3.12 g l−1, respectively). The most negative effects on the growth and PHB accumulation occurred at pH 5.5. In contrast, alkalinity just slightly decreased the PHB accumulation. This is because the higher pH could be more capable of neutralizing the acidity, which was produced during PHB accumulation (Cavalheiro et al. 2012; Juszczyk et al. 2013; Chen et al. 2013; Xu et al. 2014). But, it could be expected that high alkalinity would hinder the CDW of C. daeguensis TAD1 (Ibrahim and Steinbüchel 2010b).
Effects of initial pH value on the growth and PHB accumulation of Chelatococcus daeguensis TAD1. Cultures were inoculated in 250-mL flasks containing 50-mL mineral salt medium with 10 % inoculum size and 18.5 g l−1 glycerol, and were incubated for 32 h at 50 °C and 180 rev min−1 at different initial pH. Values are means ± SD (error bars) for three replicates
Effects of nitrogen sources
Ammonium chloride (1.0 g l−1), potassium nitrate (2.0 g l−1), yeast extract (2.0 g l−1), tryptone (2.0 g l−1), and mixed nitrate at different concentrations were used in MSM containing glycerol to identify an optimum nitrogen source for both growth and PHB accumulation (Table 1). According to the data for a sole nitrogen source in Table 1, the highest CDW (5.63 g l−1) was obtained when 2.0 g l−1 yeast extract was used, followed by tryptone and then inorganic nitrogen sources (NH4Cl and KNO3). This shows that organic nitrogen sources were more suitable for the growth of C. daeguensis TAD1. However, the amounts of PHB obtained with the sole organic nitrogen sources were lower than those seen with NH4Cl. This means that more of the glycerol was used for the growth of C. daeguensis TAD1 than for PHB accumulation, while only a sole organic nitrogen sources was used in the MSM.
On the other hand, comparing the date for a sole nitrogen source and that for mixed nitrate, higher CDW and higher PHB yields were obtained with the latter. The best PHB amount (5.07 g l−1) was obtained when 0.8 g l−1 yeast extract and 0.8 g l−1 tryptone were used. Meanwhile, the residual glycerol was decreased to 0.2 g l−1. This thus proved that the use of a mixed nitrate produced more PHB.
Effects of the glycerol concentration
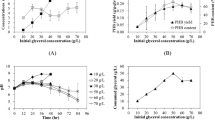
The effects of the concentration of glycerol on PHB accumulation were also investigated. As shown in Table 2, the CDW (8.47 g l−1) and amount of PHB (6.59 g l−1) were maximized at 30.0 g l−1 glycerol, but the best PHB content (78.3 % of CDW, w/w) occurred at 40.0 g l−1 glycerol. When increasing the glycerol concentration to 50.0 g l−1, the CDW, PHB content, and amount of PHB started to decrease. It thus seems that excessive glycerol would greatly affect the process of PHB accumulation by C. daeguensis TAD1 (Liang et al. 2012; Yang et al. 2012; Xu et al. 2014). The best productivity (0.162 g l−1 h−1) was recorded at 20.0 g l−1 glycerol. And, this is better than that obtained with from Zobellella denitrificans MW1 (0.044 g l−1 h−1) (Ibrahim and Steinbüchel 2010b) and recombinant Escherichia coli (0.100 g l−1 h−1) (Mahishi et al. 2003), which worked biomanufacturing PHB from glycerol.
Effects of the concentration of sodium chloride
To estimate the effects of sodium chloride on growth and PHB accumulation by C. daeguensis TAD1, different concentrations of NaCl were added to MSM containing 20.0 g l−1 glycerol. Sodium chloride at a concentration of 15.0 g l−1 enhanced both growth and polymer contents of the cells. In comparison to a control culture without additional NaCl, the CDW and PHB amount increased from 7.0 to 8.12 g l−1 and from 5.0 to 6.56 g l−1, respectively. Negative effects were observed at higher concentrations of sodium chloride. For example, in the presence of 30.0 g l−1 NaCl, only 2.06 g l−1 PHB amount was obtained (Fig. 4). This shows that sodium chloride improves the activity of C. daeguensis TAD1 at low concentrations.
Effects of NaCl concentration on the growth and PHB accumulation of Chelatococcus daeguensis TAD1. Cultures were inoculated in 250-mL flasks containing 50-mL mineral salt medium with 10 % inoculum size, 20 g l−1 glycerol and mixed nitrate (1 g l−1 NH4Cl, 0.8 g l−1 yeast extract and 0.8 g l−1 tryptone) at pH 7.5, and were incubated for 32 h at 50 °C and 180 rev min−1 at different concentrations of NaCl. Values are means ± SD (error bars) for three replicates
Production of PHB by batch cultivation of C. daeguensis TAD1
In the first fermentation (Fig. 5), nutrients were added at the beginning of the experiment. After 5-h cultivation, the CDW and PHB content increased sharply. The glycerol was exhausted at 20 h, when the maximum CDW (9.28 g l−1) and PHB amount (2.94 g l−1) were recorded. Thereafter, the CDW and PHB content decreased slightly until the end of experiment. In the next fed-batch fermentation (Fig. 6), the feeding regimen was designed to provide a sufficient amount of glycerol (≥10 ~ 15 g l−1) to support both cell growth and PHB accumulation during growth of C. daeguensis TAD1. The maximum productivity recorded was 0.384 g l−1 h−1 at the time of 30 h, while the CDW, PHB content, and PHB amount were 24.7 g l−1, 46.6 %, and 11.5 g l−1, respectively (Table 3). At the end of this fermentation (40 h), a high CDW and also high PHB amount were obtained (27.8 and 12.4 g l−1, respectively), although the PHB content (44.7 %) was relatively low at 40 h. In comparison with the batch fermentation, the feeding regimen of fed-batch fermentation could greatly improve the growth of C. daeguensis TAD1 and PHB accumulation (Table 3). In the third fermentation (Fig. 7), there were two stages of feeding, and in the first, the additional medium I, which contained mixed nitrate, was used for 25-h fermentation. After that, the additional medium II, which only contained NH4Cl, was applied between 25 and 40 h. In the first stage of fermentation (Figs. 6 and 7), similar values were observed for the CDW, PHB amount, and PHB content to those seen with fed-batch fermentation. After the 25 h, the PHB content increased sharply, with no significant changes in the CDW in the second feeding stage. The highest PHB content (68.3 % of CDW, w/w) was obtained at 40 h, meanwhile the high CDW, PHB amount, product yield (YP/S, yield gram PHB per gram substrate used), and productivity of C. daeguensis TAD1 were 25.4 g l−1, 17.4 g l−1, 0.26, and 0.434 g l−1 h−1, respectively.
Time course of the growth and PHB accumulation by Chelatococcus daeguensis TAD1 under batch fermentation. Batch fermentation was done by cultivating Chelatococcus daeguensis TAD1 in a 5-L bioreactor. The fermentation medium and cultivation conditions were as described in the text. Batch fermentation was supplied no additional medium, and operated with 30 % DO at 50 °C and pH 7.5. Closed circles indicate the concentrations of PHB amount (PHB, g l−1); opened triangles indicate the concentrations of cell dry weight (CDW, g l−1); opened square indicate PHB content (%, w/w); opened diamond indicates the concentrations of glycerol (g l−1)
Time course of the growth and PHB accumulation by Chelatococcus daeguensis TAD1 under fed-batch fermentation. Fed-batch fermentation was done by cultivating Chelatococcus daeguensis TAD1 in a 5-L bioreactor. The additional medium I, feeding solutions, and cultivation conditions were as described in the text. Fed-batch fermentation was supplied with the additional medium I, and operated with 30 % DO at 50 °C and pH 7.5. Closed circles indicate the concentrations of PHB amount (PHB, g l−1); opened triangles indicate the concentrations of cell dry weight (CDW, g l−1); opened squares indicate PHB content (%, w/w); opened diamonds indicate the concentrations of glycerol (g l−1)
Time course of the growth and PHB accumulation by Chelatococcus daeguensis TAD1 under two-stage fed-batch fermentation. Two-stage fed-batch fermentation was done by cultivating Chelatococcus daeguensis TAD1 in a 5-L bioreactor. The additional medium II, feeding solutions, and cultivation conditions were as described in the text. Two-stage fed-batch fermentation was operated with 30 % DO at 50 °C and pH 7.5. The additional medium I was fed during the first stage (0–25 h) of fed-batch fermentation; then, the additional medium II was fed in the second stage (25–40 h). Closed circles indicate the concentrations of PHB amount (PHB, g l−1); opened triangles indicate the concentrations of cell dry weight (CDW, g l−1); opened squares indicate PHB content (%, w/w); opened diamonds indicate the concentrations of glycerol (g l−1)
Discussion
To date, Alcaligenes latus (Yamane et al. 1996; Grothe and Chisti 2000), Methylobacterium sp. GW2 (Yezza et al. 2006) and Zobellella denitrificans strain MW1 (Ibrahim and Steinbüchel 2010b) and C. daeguensis TAD1 (Liang et al. 2012; Yang et al. 2012; Xu et al. 2014) have been used as model organisms for growth-associated PHB accumulation, and the behaviors of these strains with glucose in fed-batch culture have been studied in detail. These results revealed that the PHB production by C. daeguensis TAD1 exhibits strong tolerance to high heat stress when compared to that of other PHB-accumulating bacteria.
In our previous study, we investigated the ability of C. daeguensis TAD1 to utilize glucose and glycerol for PHB accumulation in MSM at 50 °C, respectively. Table 4 shows the CDW, PHB content, PHB amount, as well as the product yield (YP/S, gram PHB per gram substrate used) after 28-h cultivation of C. daeguensis TAD1 on glucose and glycerol (Xu et al. 2014). Although glucose exhibited significantly higher PHB amount (3.44 ± 0.3 g l−1), it was not a viable raw material for large-scale PHB production due to its higher price (Lee et al. 2013). In Table 4, the product yield of glycerol (0.26 ± 0.03 g g−1) is 1.5-fold higher than that of glucose, and the PHB content is slightly higher than that of glucose. This is attributed to the fact that almost all the glucose added in the medium used up at 28 h (Xu et al. 2014), meanwhile more than half glycerol still existed in the medium. These results are also in well accordance with those of other PHB-accumulating microorganisms (Ibrahim and Steinbüchel 2010a, b, c), indicating that glycerol would be more suitable for PHB accumulation rather than the growth of C. daeguensis TAD1 in contrast to that of glucose. Moreover, glycerol could be tremendously produced by the biodiesel industry as a by-product (Solaiman et al. 2006). Glycerol may be a cheap alternative to the conventional fermentation substrates for large-scale PHB production by C. daeguensis TAD1.
In this work, PHB accumulation on glycerol by C. daeguensis TAD1 is associated with cell growth (Fig. 1) and occurs in parallel with the consumption of glycerol and NH4Cl during the logarithmic phase. Our results show that it is capable of accumulating PHB at up to 76.2 % of cell dry weight (CDW, w/w) within 32 h at 45 °C. Therefore, Chelatococcus daeguensis TAD1 is a thermophilic PHA-accumulating bacterium capable of utilizing glycerol to accumulate PHB (a short-chain-length PHA) at elevated temperatures. This means that it is possible to obtain high cell densities accompanied by high polymer contents in a simple one-step fermentation process without the need to apply an obvious nutrient limitation. Most important of all, it can reduce the cost of PHB accumulation effectively (Yamane et al. 1996).
Based on a study using a 250-mL flask on the optimization of the amount of PHB produced by C. daeguensis TAD1 from glycerol, we found that increasing the inoculum size or adding mixed nitrate could enhance productivity. We obtained the optimal product yield of 0.33 and productivity of 0.21 g l−1 h−1 (Table 3) under the culture of 10 % inoculum size, mixed nitrate (1.0 g l−1 NH4Cl, 0.8 g l−1 yeast extract, and 0.8 g l−1 tryptone), initial pH at 7.5, temperature at 50 °C, 20 g l−1 glycerol, 15 g l−1 NaCl, and for 32 h. The optimal productivity of C. daeguensis TAD1 with a flask (0.21 g l−1 h−1) was better than that seen with the Zobellella denitrificans strain MW1 (0.044 g l−1 h−1) (Ibrahim and Steinbüchel 2010b), recombinant Escherichia coli harboring Streptomyces aureofaciens (0.10 g l−1 h−1) (Mahishi et al. 2003) and Yarrowia lipolytica (0.005 g l−1 h−1) (Juszczyk et al. 2013), as listed in Table 3.
We obtained the curve of the time course of growth and PHB accumulation on glucose by C. daeguensis TAD1 in our previous research (Xu et al. 2014). After 4-h cultivation, we found that C. daeguensis TAD1 showed logarithmic growth and PHB accumulation. But, in this research (Fig. 1), we recorded the logarithmic phase of C. daeguensis TAD1 and PHB accumulation at the time of 10 h of cultivation. This means that glycerol was less favorable for the growth of C. daeguensis TAD1, and thus more enzymes, such as glycerol facilitator (GlpF), glycerol kinase (GlpK), or G-3-P dehydrogenase (GlpD) (Escapa et al. 2013), need to be involved in the process. This was also reflected at 32-h cultivation after the NH4Cl had been exhausted (Fig. 1). Due to the lack of nitrogen source to synthesize enzyme, the growth of C. daeguensis TAD1 ceased. This finding is similar to those of other studies worked on biomanufacturing PHB from glycerol (Mahishi et al. 2003; Ibrahim and Steinbüchel 2010b; Andreessen et al. 2010; López et al. 2012).
Based on the optimal conditions for the growth of C. daeguensis TAD1 and accumulation of the PHB from flask culture, the high CDW was obtained from the three batch fermentations (fermentation, fed-batch fermentation, and two-stage fed-batch fermentation of C. daeguensis TAD1) (Table 3). This means that the constant pH and DO in bioreactor was beneficial to the growth of microorganisms (Nath et al. 2008). During the fed-batch fermentation, maximum product yield (YP/S, 0.22 g PHB per gram substrate used) as well as PHB content (46.6 % of CDW, w/w) occurred in 30 h. During the two-stage fed-batch fermentation, in which the inorganic nitrogen source was used in the second stage for PHB production, the optimal product yield (YP/S, 0.26 g PHB per gram substrate used) as well as PHB content (68.3 % of CDW, w/w) occurred within 40 h. With the two-stage fed-batch fermentation, PHB amount increased to 17.4 g l−1 compared with 2.94 g l−1 in the batch fermentation. However, the optimal PHB contents from batch fermentations were far below those of from flask fermentation. This may be because the DO was mostly engaged in the tricarboxylic acid cycle, which promoted the growth of microorganisms, rather than synthesizing acetyl coenzyme A, which contributed to PHB accumulation (Reddy and Mohan 2012; Ciggin et al. 2013). Although the optimal productivity of 0.384 g l−1 h−1 was obtained in fed-batch fermentation, the low PHB content (46.6 %) would be against the extraction of PHB from C. daeguensis TAD1. Therefore, we changed the feeding regimen in the two-stage fed-batch fermentation, and fed only NH4Cl instead of mixed nitrate source during 25–40 h. The results (Table 3) show that adding NH4Cl in the appropriate concentration would improve PHB productivity in C. daeguensis TAD1. In addition, it should be pointed out that C. daeguensis TAD1 is thermophilic, while other strains used for PHB biomanufacturing are mesophilic. The results in Table 3 show that C. daeguensis TAD1 can reduce the PHB fermentation time. This indicates the efficiency of PHB production by two-stage fed-batch fermentation > fed-batch fermentation > batch fermentation. Fed-batch fermentation was also proved to be a highly efficient process for producing PHB on a large scale, utilizing glycerol as the sole carbon source.
References
Andreessen B, Lange AB, Robenek H, Steinbüchel A (2010) Conversion of Glycerol to Poly(3-Hydroxypropionate) in Recombinant Escherichia coli. Appl Environ Microbiol 76(2):622–626
Ashby RD, Solaiman DKY, Foglia TA (2004) Bacterial poly(hydroxyalkanoate) polymer production from the biodiesel co-product stream. J Polym Environ 12(3): 105-112
Borah B, Thakur PS, Nigam JN (2002) The influence of nutritional and environmental conditions on the accumulation of poly-β-hydroxybutyrate in Bacillus mycoides RLJ B-017. J Appl Microbiol 92(4):776–783
Bormann EJ, Roth M (1999) The production of polyhydroxybutyrate by Methylobacterium rhodesianum and Ralstonia eutropha in media containing glycerol and casein hydrolysates. Biotechnol Lett 21(12): 1059–1063
Brandl H, Bachofen R, Mayer J, Wintermantel E (1995) Degradation and applications of polyhydroxyalkanoates. Can J Microbiol 41:143–153
Braunegg G, Lefebvre G, Genser KF (1998) Polyhydroxyalkanoates, biopolyesters from renewable resources: physiological and engineering aspects. J Biotechnol 65:127–161
Cavalheiro JMBT, Raposo S, Almeida MCMD, Cesário MT, Sevrin C, Grandfils C, Fonseca MMR (2012) Effect of cultivation parameters on the production of poly (3-hydroxybutyrate-co-4-hydroxybutyrate) and poly (3-hydroxybutyrate-4-hydroxybutyrate-3-hydroxyvalerate) by Cupriavidus necator using waste glycerol. Bioresour Technol 111:391–397
Chen GQ (2009) A microbial polyhydroxyalkanoates (PHA) based bio- and materials industry. Chem Soc Rev 38(8):2434–2446
Chen GQ, Wu Q (2005) Microbial production and applications of chiral hydroxyalkanoates. Appl Microbiol Biotechnol 67:592–599
Chen SK, Chin WC, Tsuge K, Huang CC, Li SY (2013) Fermentation approach for enhancing 1-butanol production using engineered butanologenic Escherichia coli. Bioresour Technol 145:204–209
Ciggin AS, Orhon D, Capitanid D, Miccheli A, Puccetti C, Majone M (2013) Aerobic metabolism of mixed carbon sources in sequencing batch reactor under pulse and continuous feeding. Bioresour Technol 129:118–126
Escapa IF, Delcerro C, Garcia JL, Prieto MA (2013) The role of GlpR repressor in Pseudomonas putida KT2440 growth and PHA production from glycerol. Environ Microbiol 15(1):93–110
Gahlawat G, Srivastava AK (2013) Development of a mathematical model for the growth associated Polyhydroxybutyrate fermentation by Azohydromonas australica and its use for the design of fed-batch cultivation strategies. Bioresour Technol 137:98–105
Grothe E, Chisti Y (2000) Poly(beta-hydroxybutyric acid) thermoplastic production by Alcaligenes latus: behavior of fed-batch cultures. Bioprocess Biosyst Eng 22:441–449
Holmes PA (1985) Applications of PHB - a microbially produced biodegradable thermoplastic. Phys Technol 16:32–36
Ibrahim MHA, Steinbüchel A (2009) Poly(3-hydroxybutyrate) production from glycerol by Zobellella denitrificans MW1 via high-cell-density fed-batch fermentation and simplified solvent extraction. Appl Environ Microbiol 75(19): 6222–6231
Ibrahim MHA, Steinbüchel A (2010a) Isolation and characterization of new poly (3HB)-accumulating star-shaped cell-aggregates-forming thermophilic bacteria. J Appl Microbiol 109:1579–1590
Ibrahim MHA, Steinbüchel A (2010b) High-cell-density cyclic fed-batch fermentation of a poly(3-hydroxybutyrate)-accumulating thermophile, Chelatococcus sp. strain MW10. Appl Environ Microbiol 76:7890–7895
Ibrahim MHA, Steinbüchel A (2010c) Zobellella denitrificans strain MW 1, a newly isolated bacterium suitable for poly(3-hydroxybutyrate) production from glycerol. J Appl Microbiol 108:214–225
Juszczyk P, Tomaszewska L, Kita A, Rymowicz W (2013) Biomass production by novel strains of Yarrowia lipolytica using raw glycerol, derived from biodiesel production. Bioresour Technol 137:124–131
Le Meur S, Zinn M, Egli T, Thöny-Meyer L, Ren Q (2014) Improved productivity of poly (4-hydroxybutyrate)(P4HB) in recombinant Escherichia coli using glycerol as the growth substrate with fed-batch culture. Microb Cell Factories 13:131
Lee SY (1996) Bacterial polyhydroxyalkanoates. Biotechnol Bioeng 49(1):1–14
Lee JS, Chi WJ, Hong SK, Yang JW, Chang YK (2013) Bioethanol production by heterologous expression of Pdc and AdhII in Streptomyces lividans. Appl Microbiol Biotechnol 97:6089–6097
Liang W, Huang S, Liu J, Zhang R, Yan F (2012) Removal of nitric oxide in a biotrickling filter under thermophilic condition using Chelatococcus daeguensis. J Air Waste Manag Assoc 62:509–516
Liu Y, Huang SB, Zhang YQ, Xu FQ (2014) Isolation and characterization of a thermophilic Bacillus shackletonii K5 from a biotrickling filter for the production of polyhydroxybutyrate. J Environ Sci China 26:1453–1462
López JA, Naranjo JM, Higuita JC, Cubitto MA, Cardona CA, Villar MA (2012) Biosynthesis of PHB from a new isolated Bacillus megaterium strain: Outlook on future developments with endospore forming bacteria. Biotechnol Bioproc Eng 17(2):250–258
Madison LL, Huisman GW (1999) Metabolic engineering of poly(3-Hydroxyalkanoates): from DNA to plastic. Microbiol Mol Biol Rev 63(1):21–53
Mahishi LH, Tripathi G, Rawal SK (2003) Poly(3-hydroxybutyrate) (PHB) synthesis by recombinant Escherichia coli harbouring Streptomyces aureofaciens PHB biosynthesis genes: effect of various carbon and nitrogen sources. Microbiol Res 158(1):19–27
Nath A, Dixit M, Bandiya A, Chavda S, Desai AJ (2008) Enhanced PHB production and scale up studies using cheese whey in fed batch culture of Methylobacterium sp. ZP24. Bioresour Technol 99(13):5749–5755
Palmeri R, Pappalardo F, Fragalà M, Tomasello M, Damigella A, Catara AF (2012) Polyhydroxyalkanoates (PHAs) production through conversion of glycerol by selected strains of Pseudomonas mediterranea and Pseudomonas corrugata. Chem Eng Trans 27:121–126
Reddy MV, Mohan SV (2012) Influence of aerobic and anoxic microenvironments on polyhydroxyalkanoates (PHA) production from food waste and acidogenic effluents using aerobic consortia. Bioresour Technol 103(1):313–321
Ren Q, Ruth K, Thöny-Meyer L, Zinn M (2010) Enatiomerically pure hydroxycarboxylic acids: current approaches and future perspectives. Appl Microbiol Biotechnol 87:41–52
Rodgers M, Wu G (2010) Production of polyhydroxybutyrate by activated sludge performing enhanced biological phosphorus removal. Bioresour Technol 101:1049–1053
Solaiman DKY, Ashby RD, Foglia TA, Marmer WN (2006) Conversion of agricultural feedstock and coproducts into poly (hydroxyalkanoates). Appl Microbiol Biotechnol 71:783–789
Steinbüchel A, Valentin HE (1995) Diversity of bacterial polyhydroxyalkanoic acids. FEMS Microbiol Lett 128(3):219–228
Thompson JC, He BB (2006) Characterization of crude glycerol from biodiesel production from multiple feedstocks. Appl Eng Agric 22(2):261–265
Ward PG, O’Connor KE (2005) Bacterial synthesis of polyhydroxyalkanoates containing aromatic and aliphatic monomers by Pseudomonas putida CA-3. Int J Biol Macromol 35:127–133
Xu FQ, Huang SB, Liu Y, Zhang YQ, Chen SW (2014) Comparative study on the production of poly(3-hydroxybutyrate) by thermophilic Chelatococcus daeguensis TAD1: a good candidate for large-scale production. Appl Microbiol Biotechnol 98:3965–3974
Yamane T, Fukunaga M, LEE YW (1996) Increased PHB productivity by high-cell-density fed-batch culture of Alcaligenes latus, a growth-associated PHB producer. Biotechnol Bioeng 50(2):197–202
Yang YL, Huang SB, Liang W, Zhang YQ, Huang HX, Xu FQ (2012) Microbial removal of NOX at high temperature by a novel aerobic strain Chelatococcus daeguensis TAD1 in a biotrickling filter. J Hazard Mater 203–204:326–332
Yezza A, Fournier D, Halasz A, Hawari J (2006) Production of polyhydroxyalkanoates from methanol by a new methylotrophic bacterium Methylobacterium sp. GW2. Appl Microbiol Biotechnol 73:211–218
Zinn M, Witholt B, Egli T (2001) Occurrence, synthesis and medical application of bacterial polyhydroxyalkanoate. Adv Drug Deliv Rev 53(1):5–21
Acknowledgments
The authors would like to acknowledge the financial support for this work provided by the National Natural Science Foundation of China (Grants No. 51378217 and No. U1360101) and Guangdong Natural Science and Foundation (No. S2012020010887).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cui, B., Huang, S., Xu, F. et al. Improved productivity of poly (3-hydroxybutyrate) (PHB) in thermophilic Chelatococcus daeguensis TAD1 using glycerol as the growth substrate in a fed-batch culture. Appl Microbiol Biotechnol 99, 6009–6019 (2015). https://doi.org/10.1007/s00253-015-6489-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-015-6489-1