Abstract
Several etiologies of kidney disease may result in renal dysfunction including direct or indirect insults to the glomerulus responsible for filtration function, or renal epithelial cells responsible for many of the regulatory and secretory functions of the kidney. Acute kidney injury (AKI) arising from acute renal tubule cell injury may be due to direct insult or through ischemic and/or nephrotoxic processes. Although AKI can arise from hyperinflammation, most commonly resulting from sepsis or tissue trauma, acute tubule cell injury without underlying hyperinflammation has been demonstrated to initiate and promote a systemic hyperinflammatory process that potentiates not only AKI but injury and dysfunction of other organs. Chronic kidney disease (CKD) such as end stage renal disease (ESRD) may be the result of long-term injury induced by previous occurrence of AKI, or progressive due to chronic inflammation. Conventional therapies to treat AKI such as hemofiltration and hemodialysis to treat CKD have been used to replace the reduced or lost filtration function of the kidney, while some sorbent therapies have focused on the removal of pathogens and secondary immunological mediators such as cytokines thought to be responsible for worsening hyperinflammation. This review centers on a review of emerging extracorporeal device treatment interventions using renal epithelial cell-based devices, bioartificial kidneys (BAK), and leukocyte processing therapies, such as the selective cytopheretic device (SCD). New technologies generally face regulatory hurdles, and a fully implantable bioartificial kidney is still years away. Some technologies such as the SCD which process a patient’s own cells may have fewer regulatory hurdles to overcome. Clinical data is mounting and suggests an effect of SCD to diminish ongoing organ injury and speed the recovery of organ function, most likely due to SCD induced modulation of the immunologic responses controlling hyperinflammation as well as the regenerative repair processes responsible for recovery of kidney function.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Acute kidney injury (AKI)
- Chronic kidney disease (CKD)
- End stage renal disease (ESRD)
- Bioartificial kidney
- Selective cytopheretic device (SCD)
- Renal assist device (RAD)
- Bioartificial renal epithelial cell system
14.1 Introduction
Several etiologies of kidney injuries may result in renal dysfunction including direct or indirect insults to the glomerulus responsible for filtration function, or renal epithelial cells responsible for many of the regulatory and secretory functions of the kidney. Rabb et al. provides a good primer on inflammatory processes associated with acute kidney injury (AKI), including the initial response, features of acute inflammation, and reparative phases of kidney injury [1]. Other comprehensive, systematic reviews of septic shock-associated AKI discuss pathogen-associated molecular pattern molecules (PAMPs) such as endotoxin, and damage-associated molecular pattern molecules (DAMPs) such as cytokines [2,3,4]. AKI arising from acute renal tubule cell injury, also referred to acute tubular necrosis (ATN), may be due to direct insult from PAMPs, or through ischemic and/or nephrotoxic processes via DAMPs. Although AKI can arise from hyperinflammation, most commonly resulting from sepsis or tissue trauma, acute tubule cell injury without underlying hyperinflammation has been demonstrated to initiate and promote a systemic hyperinflammatory process that potentiates not only AKI but injury and dysfunction of other organs [5]. The resulting hyperinflammatory process is characterized by excessive activation of innate immune cells, namely neutrophils and monocytes, as well as activation of capillary microvasculature resulting in dysregulated levels of cytokines heavily weighted toward pro-inflammatory mediators. Immune dysregulation and hyperinflammatory, leukocyte-driven processes may lead to tissue damage, organ injury, and consequently, progressive organ dysfunction. Multiple organ dysfunction syndrome (MODS) may result due to inflammatory processes involving both activated leukocytes in circulation and activated endothelial cells in organ microvasculature. Poor tissue perfusion resulting from deleterious interactions of these activated cell populations has ischemic and toxic consequences, including tissue edema, systemic hypovolemia, hypotension, and cardiovascular instability a negative impact on organ function. In the kidney, sequestration and aggregation of activated neutrophils in the peritubular capillaries and tissue infiltration of these cells can lead to necrosis of renal tubule cells, which promotes AKI. Conventional therapies such as hemofiltration strategies have been used to replace the reduced or lost filtration function of the kidney, while some sorbent therapies have focused on the removal of pathogens and secondary immunological mediators such as cytokines thought to be responsible in MODS. This review centers on a review of emerging extracorporeal device treatment interventions using renal epithelial cell-based devices, bioartificial kidneys, and leukocyte processing therapies.
14.2 Overview of Conventional Devices in the Treatment of Kidney Disease: Dialysis, Filtration, and Hemopurification
The most established device-based strategies in the treatment of AKI are filtration technologies including hemodiafiltration and peritoneal dialysis, which attempt to replace lost filtration function of the kidneys. The aim of these filtration-based renal replacement therapies (RRT) is the removal of low molecular weight (LMW) uremic toxins, middle molecular weight cytokines, and other inflammatory molecules through porous membranes via diffusive (dialysis) or convective (filtration) processes, while retaining beneficial molecules such as albumin through size exclusion. Dialysis is commonly utilized for ESRD to maximize uremic toxin clearance during intermittent treatments. For a thorough, systematic review of peritoneal dialysis in AKI, see Chionh et al. [6]. Extracorporeal blood purification using devices to capture and/or remove inflammatory mediators and both endogenous and exogenous toxins particularly during sepsis and sepsis associated AKI is an evolving area that has been comprehensively reviewed [7]. Extracorporeal cytokine removal using extracorporeal devices is a mature concept focused on the belief that reducing peak cytokine serum levels during the hyperinflammatory period would ameliorate detrimental actions of these molecules [8], potentially also altering tissue cytokine gradients and facilitating restoration of immunologic homeostasis. Many cytokines fall in a molecular weight (MW) range of 8 kDa to 70 kDa (Table 14.1), and endotoxin fragments associated with sepsis are 1 kDa to 15 kDa, which are generally removed by conventional hemodialysis (HD) and hemofiltration (HF) membranes, with a MW-cut off (MWCO) of between 45 and 65 kDa. While measurable cytokine removal is achieved using various modalities including use of high molecular weight cut-off membranes and high volume hemofiltration schemes [8], therapies solely aimed at reducing cytokine load have been largely ineffective in improving clinical outcomes [9,10,11,12,13]. Ineffective filtration treatments may be due to in part, failure to remove blood protein bound toxins, cytokines, and inflammatory molecules [14]. Higher MWCO membranes which may remove protein bound cytokines also result in blood protein loss [15, 16] and require relatively expensive protein administration, entailing albumin supplementation at a minimum. Additional hemopurification strategies using newly developed membranes and alternative techniques such as apheresis or selective plasma exchange have seen some utility for cytokine removal, but still have limited data regarding efficacy in sepsis associated AKI [17]. Devices such as plasma fractionators with precise MWCO along with methods such as double/cascade filtration and coupled plasma filtration adsorption have been used for very precise removal of molecules within a specific MW window [18, 19], but have not seen wide-spread adoption.
14.3 Introduction to Sorbents and Immunomodulatory Devices for AKI and Multiple Organ Dysfunction Syndrome (MODS)
Citing the difficulty in removing middle molecular weight PAMPs and DAMPs via filtration, the use of sorbents attempts to specifically adhere these molecular targets with high potential therapeutic impact. Use of sorbent materials in conjunction with hemofiltration or as stand-alone devices are relatively mature technologies that attempt to adhere inflammatory molecules to specialized device surfaces through adsorption or through binding via various mechanisms. Sorbent technologies to date have mainly focused on removing specific PAMPs such as endotoxin, or DAMPs including a myriad of cytokines, and are exemplified by devices such as Cytosorb®. Sorbent technologies have been well reviewed by Winchester et al. [20,21,22]. In brief, sorbent columns or cartridges can be packed with various media in order to adsorb specific molecular targets, many times utilizing immobilized antibodies, functionalized surfaces, or specialized porous resins for molecular interaction and capture. PAMP targets have included endotoxin, also known as lipopolysaccharide (LPS), typified by devices utilizing Polymixin B [23]. Clinical studies involving these devices have been heterogenous, lacking adequate randomization, blinding and incomplete outcomes [23]. Due to the low quality of evidence provided to date, therapeutic use of Polymyxin B-based devices may only be conditionally recommended for very high-risk patients [23]. DAMP molecules have been targeted by sorbent systems such as Cytosorb® [24], finding utility in sepsis, cardiac surgery, organ transplant, and liver failure among other contexts. Specific DAMPs targets of sorbent technologies have included β2-microglobulin, angiogenin, complement factor D, leptin, IL-1, IL-6, IL-8, IL-10, IL-13, IL-18, interferon-α, TGF-β, and TNF-α [22, 24]. However, to date, Sorbent-based blood-purifying technologies have not shown selectivity and durability in lowering blood cytokine levels nor robust efficacy to improve clinical outcomes [2]. Like many technologies in the AKI/sepsis field, additional large, randomized, controlled, clinical trials are still required to provide evidence of clear therapeutic benefit [23].
With the limitations of filtration and sorbent technologies, there is an unmet medical need to develop advanced therapies that address hyperinflammation in kidney disease. Immunomodulatory device treatments in development cover a wide range of approaches to recapitulate the immunologic functions of the kidney from bioartificial kidney and extracorporeal cell bioreactors to leukocyte processing devices. These emerging technologies, which utilize admittedly less well-understood mechanisms of action, attempt to harness the innate characteristics of cells and their cellular feedback mechanisms involved with immune system regulation in order to correct immune dysregulation. Exogenous, extracorporeal bioreactor strategies such as the renal assist device (RAD) and the bioartificial renal epithelial cell system (BRECS) employ metabolically active cells maintained in an extracorporeal circuit in order to process body fluids such as blood or plasma, to both remove inflammatory molecules and supplement beneficial molecules such as secreted factors, leveraging both anabolic and catabolic functions of exogenous renal cells. Leukocyte processing devices, such as the selective cytopheretic device (SCD), attempt to ameliorate immune dysregulation through a multifactorial approach of sequestration of activated leukocyte subpopulations including neutrophils and monocytes, while modulating the circulating leukocyte populations.
14.4 Exogenous Cell-Based Devices: Renal Assist Device (RAD), Bioartificial Kidney (BAK), and Bioartificial Renal Epithelial Cell System (BRECS)
In order to replace renal tubule immunomodulatory activity during AKI, an extracorporeal device containing human renal tubule cells, the RAD, was used in intensive care unit (ICU) patients with AKI requiring continuous RRT (CRRT). Incorporation of the bioreactor into the CRRT circuit was associated with a decrease in the high plasma levels of cytokines in these patients, amelioration of the AKI promoted hyperinflammatory condition and improved survival [25]. This cell-based strategy replaces some of the cellular functions lost or compromised during AKI by supplementing with exogenous renal cells grown in an immuno-isolating device with blood access, which allows for metabolic and secretory support from the applied cells. The premise of the RAD was based upon perceived shortcomings of established renal replacement therapies: HD, HF, HDF, etc., which are centered on small solute and toxin removal of molecules below the MWCO of porous membranes and volume control. However, these therapies fail to replace the many additional aspects of renal function outside of small toxin clearance, including the secretion of hormones: renin, prostaglandins, angiotensin, endothelin, bradykinin, erythropoietin and calcitriol, as well as ignore the important immunomodulatory role held by the kidney, which is still being elucidated. Part of the regulatory strategy in development of a fully implantable bioartificial kidney (BAK), was based on proving the safety and efficacy of extracorporeal cell-based therapy while concurrently working on the miniaturization of required technology and biocompatibility of components [26, 27]. Different BAK technology approaches, engineering challenges, and related regulatory hurdles are presented in several full-length reviews [28,29,30].
In the RAD IIa clinical study, safety and initial efficacy was demonstrated in subsets of patients that were treated with a cell containing RAD over a sham device without renal cells [31]. However, in the Phase IIb RAD clinical study, additional cohorts were added, allowing the enrollment of patients being treated with regional citrate anticoagulation (RCA). Surprisingly, improved patient survival rate was demonstrated in the sham device group receiving RCA, in addition to the RAD cell treatment group, in comparison to the sham acellular device during systemic heparin anticoagulation [32]. These cohorts were scrutinized in a retrospective analysis, and the 28-day survival rate in the RCA sham group was observed to be 75% vs. 50% for the heparin anticoagulation sham group (n = 12 for each treatment arm). Similarly, at the 90-day mark, survival rate was 67% for sham device with RCA vs. 25% for sham device with heparin anticoagulation. Demographics for the two patient subsets were comparable, having similar Sequential Organ Failure Assessment (SOFA) scores (12.2 ± 0.9 vs. 13.4 ± 1.1), organ failure number (3.93 ± 0.36 vs. 4.17 ± 0.46), and identical incidence of sepsis (58%) for the heparin versus RCA sham groups, respectively [32]. This unexpected clinical result identified the potential benefit of using an acellular fiber-based device in conjunction with RCA to treat AKI. This approach later became known as selective cytopheretic device (SCD) therapy, which is detailed below.
The halted RAD clinical trial effectively stopped the development and commercialization of the RAD, but led to the development of both the acellular, SCD and a second generation cell-based device called the bioartificial renal epithelial cell system (BRECS), which was designed to be a cryopreservable bioreactor populated with allogenic renal cells for potential “off-the-shelf” use in an extracorporeal therapy for AKI. BRECS cell device recapitulated many aspects of the metabolic support of the RAD in a more practical form factor for on-demand, acute use. Unfortunately, the regulatory hurdles facing the BRECS, including requirement of an extensive Pre-Market Approval (PMA) study and expensive manufacturing requirements led to prioritize the development of the SCD, an acellular device with fewer regulatory hurdles. Review articles of the medical device development process recognize the slow clinical translation process moving from benchtop to bedside, especially so for FDA Class III devices with added regulatory requirements, which tend to take longer to develop [33, 34].
14.5 Leukocyte Processing Strategies: Selective Cytopheretic Device (SCD) in the Treatment of AKI
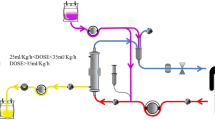
Mounting evidence for clinical benefit from targeting modulation of the leukocytes themselves to directly modulate hyperinflammation, utilizing innate characteristics of cellular machinery which are effectors of secondary factors (e.g., cytokines), has arisen from serendipitous discovery of the acellular SCD during the RADIIb trial. The SCD is an extracorporeal, immunomodulatory, device containing hemocompatible fibers enclosed in a housing with inlet and outlet blood flow ports. SCD can be incorporated into a patient’s established CRRT circuit (Fig. 14.1), where the blood flow is directed along the outer surface of the fibers where blood cells can interact with membrane surfaces under a low shear stress blood flow and low ionized calcium environment. When SCD were examined microscopically after patient treatment, a significant population of leukocytes were found to be adhered on to the outer membrane surface of the fibers at the interface with the blood flow path [31]. Sequestered leukocytes were dominated by cells of myeloid lineage, namely neutrophils and monocytes. Leukocyte adhesion is not routinely observed in the inner lumen of hollow fiber membranes, the blood flow path for standard hemofilters and dialyzers. This is likely due to the high shear stresses involved at the fiber interface with blood, with blood flowing at high flow rates through small-diameter hollow fibers (on the order of a hundred to hundreds of dynes/cm2). However, in SCD, with blood flow directed along the outside surfaces of fibers, the shear forces are on the same order as capillary force (<1 dyne/cm2), favorable dynamics for LE–material interactions such as adhesion are achieved. Some leukocytes are adhered and sequestered over long periods of time in SCD, while some leukocytes may adhere and subsequently release from SCD [35]. Capture and release after potential alteration within the SCD microenvironment and return of these cells to the patient, seems to have biofeedback implications that results in amelioration of many deleterious effects of hyperinflammation during AKI.
The precise mechanism of action of the SCD is becoming better understood and appears to be an immunomodulatory process which inhibits leukocyte activation, a critical component of the systemic inflammatory response syndrome (SIRS) leading to multi-system organ failure. The modulation of the pro-inflammatory state also allows recovery of renal function in AKI and other associated organ failures. The cartridge in the presence of citrate anticoagulant acts as a selective cytopheretic device to bind and immunomodulate potentially damaging circulating leukocytes. This perspective is based upon evolving data from in vitro bench studies, preclinical animal models, and human clinical trials (Tables 14.2 and 14.3) utilizing measurements of biomarkers and leukocyte cell sorting by cytometric analysis.
The low ionized calcium (iCa) environment during regional citrate anticoagulation and the low shear stress along the blood pathway within the SCD promotes a selective binding of the most activated neutrophils and monocytes to the membranes of the device [35, 39, 41]. This selectivity is due to the calcium dependency of leukocyte binding processes. It is postulated that once bound, the activated neutrophils are promoted in the low iCa environment to transition from delayed apoptosis to an apoptotic program and released back to the systemic circulation [44,45,46]. The transition of these neutrophils to apoptosis and release from the SCD results in the clearance of these previously highly activated inflammatory cells via well-described pathways of phagocytosis and digestion within macrophages in the bone marrow and liver [47]. A continuous process of binding, apoptotic conversion, release, and clearance from the circulation of the most activated circulating neutrophils results in immunomodulation of the systemic inflammatory process to a less proinflammatory state [41]. For monocytes, the most activated, proinflammatory circulating monocyte pool is selectively bound to the SCD. The binding and sequestration of this monocyte subset promotes a shift of the circulating pool of the proinflammatory monocytes to a patrolling, reparative phenotype. This shift thereby promotes immunomodulation of circulating monocytes from a degradative phenotype to a reparative, recovery subset [35, 48], enhancing tissue repair and functional recovery.
Leukocytes that adhere more avidly to the SCD appear to be more highly activated based upon analysis of the expression of the CD11b integrin on the surface of these cells. In an animal model of sepsis associated AKI, an immunomodulatory shift in the leukocyte population to a less inflammatory state is observed with SCD treatment, evidenced by reduced expression of the CD11b integrin marker on the surface of neutrophils in circulation, lower serum levels of myeloperoxidase, lower secretion of proinflammatory cytokines by isolated mononuclear cells and reduced emergence of immature neutrophils in the circulation [35]. Furthermore, in SCD-treated animals, the degree of both cardiovascular and renal dysfunction during sepsis was significantly reduced [35]. SCD therapy aims to treat AKI by ameliorating the degree of renal tubule cell injury through these immunomodulatory processes, whether the damage stems from a non-renal process (e.g., sepsis) or a renal derived hyperinflammatory condition initiated and potentiated from primary ischemic or nephrotoxic insult (Fig. 14.2). SCD intervention diminishes the feedback cycle of continuing inflammatory injury to the kidney, interrupting worsening hyperinflammation. This process elicits a systemic immunomodulatory effect different than simplistic leukocyte trapping and removal achieved by leukoreduction filters.
First in man studies for SCD were unintentionally completed during the acellular cohort testing in the RAD IIb study. However, since then, SCD has been used in several clinical studies in patients undergoing dialytic support for severe AKI as well as other acute indications (Table 14.2), including a Phase I/II study in AKI patients in China [36] and a Phase II multi-center trial in the USA [37]. Once integrated, SCD therapy is administered continuously with sequential replacement of the SCD every 24 h, and generally used for up to 7 consecutive days, but to date, has been used safely as long as 17 consecutive days. This continuous treatment is required to maintain the immunomodulation of circulating neutrophils due to the short half-life of circulating neutrophils, which is less than 24 h [49].
In a Phase III, multi-center, randomized, controlled, pivotal study to assess the safety and efficacy of SCD. In patients with AKI, the study was designed with two-arms: SCD-therapy integrated into CRRT circuits compared to contemporaneous patients being treated with CRRT alone (control). Both groups received RCA. The primary outcome measure was 60-day all-cause mortality. Secondary endpoints included RRT dependency at Day 60, and ventilator free days at Day 28. This trial enrolled 134 patients at 21 U.S centers. Each clinical site used their approved RCA protocol for anticoagulation of the extracorporeal circuit. The per protocol recommended ionized calcium (RiCa) intracircuit levels of 0.25–0.4 mm. Unfortunately, before the study had enrolled only 134 patients which was substantially less than half of the planned target, a national injectable calcium shortage occurred in the United States due to FDA concerns regarding the manufacturing procedures in the major supplier. This shortage resulted in some sites reducing the infusion rate of citrate to minimize calcium solutions to prevent hypocalcemia in patients, thereby resulting in RiCa above per protocol requirements below 0.4 mm and losing efficacy in SCD therapy. The calcium shortage was so severe that nine sites were not able to enroll due to lack of injectable calcium. Accordingly, the clinical trial was paused, and an interim analysis undertaken on 134 enrolled patients. This analysis demonstrates no significant difference in any of the primary or secondary endpoints between SCD-treated subjects and controls. A post hoc analysis was undertaken to see if the key variable of RiCa had an impact on the endpoints of the study. Those patients whose circuit RiCa below 0.4 mm 90% of the therapy time had a substantially improved 60-day mortality rate in SCD-treated subjects compared to controls treated with conventional CRRT therapy: 16% (3/19) vs. 41% (11/27). The 60-day dialysis dependency was also improved with 0/16 survivors in the SCD-treated group versus 4/16 (25%) in the control non-treated group. A composite endpoint consisting of 60-day mortality or dialysis dependency between the two groups of patients was statistically significant (p < 0.01). Dialysis dependency following dialysis requiring AKI is considered a poor outcome due to the high probability of progression to end stage renal disease. In fact, large prospective trials in these types of patients have demonstrated an incidence of greater than 20% of survivors being dialysis dependent after 60 days or more of follow-up [50, 51]. In this regard, a supplemental IDE has been approved by the FDA for a follow-up on pivotal clinical trial with this composite endpoint as the primary outcome.
SCD has also been tested in pediatric patients with AKI (>15 kg, age up to 22 years) in a Phase II clinical trial. A multi-center trial of the SCD therapy to treat children with AKI and MODS receiving CRRT as part of standard of care was initiated under FDA-approved IDE G150179. In pediatric patients, mortality rates have historically approached 50% for those with AKI and MODS requiring CRRT [40, 52, 53].
The first adolescent patient treated with SCD was written up as a case study, which reports on the treatment course for an 11-year-old female with a severe reaction to propofol during an elective surgery that resulted in MODS [39]. After SCD treatment for only 24 h, improvements were seen with regard to the degree of liver injury and hematologic failure. After only 4 days of SCD therapy, lung function improved markedly, allowing for extubation. After SCD therapy for 7 days, kidney function was significantly improved. The patient was later discharged from the hospital with normal renal function [39] and did not require any follow-up dialysis treatments.
In this pediatric clinical trial, 16 pediatric patients have been treated with SCD. Favorable results have been observed with 12 of the 16 SCD treated patients surviving at 60 days. No deaths were associated with device treatment but were due to underlying illness or treatment interventions (post-op complications, extracorporeal membrane oxygenation (ECMO), viral myocarditis). All 12 surviving patients were dialysis independent. Treated patients ranged from 5 to 20 years old; admission diagnoses included: severe rhabdomyolysis (case study presented below), shigatoxin-associated hemolytic-uremic syndrome, community acquired pneumonia, multiple patients with AKI and/or septic shock. Patients generally received 3–7 days of SCD therapy, with one patient withdrawn from care after 11 h of SCD therapy. No SCD-related SAE were recorded [54]. Of importance, the FDA has recently designated the SCD as a Humanitarian Use Device with a pathway for Humanitarian Device Exemption for the treatment of pediatric patients with AKI.
In summary, the SCD has been tested in a number of clinical trials in adult patients with AKI requiring CRRT [32, 36, 37] and pediatric patients [39, 54], demonstrating an excellent safety profile with strong efficacy signals. However, despite promising preclinical data and early clinical data in pilot studies, clinical translation of the SCD has been hampered by the setback encountered during the Phase III multicenter, randomized, controlled, pivotal trial where injectable calcium shortage impacted the intended RCA protocol. This underscores an acknowledgment of the need for better designed clinical trials in the AKI/sepsis field including functional outcome measures that may be decoupled from mortality in heterogenous patient populations, and secondly, the importance of understanding the mechanism of action for proper device function, to ensure the proposed therapy may be beneficial to the sub-group of sepsis patients treated; common issues in clinical trial design in sepsis have been emphasized in reviews by Vincent et al. and Gomez and Kellum [55,56,57]. In the case of the SCD, maintaining a low iCa environment in the SCD therapy circuit in order to immunomodulate hyperinflammation and achieve efficacious treatment was demonstrated by mortality reduction and elimination of dialysis dependence at 60 days in the subset of patients maintaining RiCa. The combined functional outcome measure including dialysis dependence at 60 days is a more robust clinical measure and is already approved in the SCD-004 IDE protocol as an IDE supplement for Phase III trial. The immunomodulatory effect of the SCD on neutrophils and monocytes appears to have a critical role in reducing acute inflammation resulting in more rapid improvement of cardiovascular, pulmonary, and renal functions in SCD-treated multiorgan failure patients compared to controls. This effect also translates into a regulated repair and recovery of renal function as reflected in the lack of 60-day dialysis dependency in surviving patients after SCD therapy in trials to date. These clinical results, when viewed together with preclinical large animal studies (Table 14.3), reviewed more comprehensively by Pino et al. [41], suggest a beneficial treatment effect in multiple acute inflammatory conditions, as well as chronic inflammatory conditions involved with chronic organ dysfunction, suggesting utility of leukocyte processing in several other inflammatory disease indications.
14.6 Beyond AKI: Potential Impact of Immunomodulatory Devices for Other Kidney-Related Diseases
A focal non-renal inflammatory process, such as acute MI or pneumonia or pulmonary embolism, results in a localized inflammatory response but generally not a systemic inflammatory process with far-reaching sequelae. In contrast, when an AKI insult occurs, it results in loss of tubule immunoregulatory function so that a localized kidney injury develops into a systemic process due to a vicious cycle of incremental worsening inflammation. If a non-renal injury or infectious process is severe or secondary complications occur, such as developing hypotension, AKI often results. The progression to AKI requiring RRT is a reflection of failure of standard medical care to limit a local inflammatory process such that peripheral organ injury ensues and continues propagating with extension to MODS. An immunomodulatory therapy may then be required for more complete RRT, attempting to halt the downward spiral of progressive inflammation, and worsening renal injury. Despite clinical observations that generally AKI patients have demonstrated a predisposition to go on to develop CKD, clinical trials utilizing the SCD in ICU patients with AKI have demonstrated that all survivors treated with the SCD were dialysis independent 60 days after treatment. In an Investigator-initiated trial of SCD used in ESRD patients for safety and bio-inflammatory assessment, no device-related SAEs were found due to SCD. The bio-inflammatory assay portion of testing showed SCD therapy promoted a shift in circulating monocyte population from predominantly CD14hi expressing MO at baseline/pre-SCD therapy to CD14low expressing MO post-SCD therapy [48].
Treating the hyperinflammatory process with the SCD has been demonstrated in preclinical large animal models (Table 14.3) to reduce the degree of AKI as hyperinflammation develops from various insults, including septic shock and cardiopulmonary bypass (CPB), common disorders associated with AKI [36, 43]. In CPB, leukoreduction filters have been previously utilized to help reduce cell-based hyperinflammation [58]. In SCD preclinical studies, reduction in injury biomarkers during treatment of organ dysfunction including heart and lung failure have been observed [35, 43] and have prompted several investigator initiated pilot clinical trials to assess impact of SCD therapy in patients with various types of organ dysfunction such as cardiorenal syndrome and hepatorenal syndrome. Related chronic indication clinical trials are listed herein (Table 14.4).
Recently, the FDA approved an IDE for SCD treatment of ICU patients with AKI and/or acute respiratory distress syndrome (ARDS)-associated COVID-19 infection. Evidence suggests that hyperinflammation with high concentrations of cytokines plays a critical role in the development of respiratory insufficiency and ARDS in COVID-19. SCD immunomodulatory therapy has been used in emergency/expanded use treatment of patients with refractory COVID-19 ARDS requiring ECMO. Two severely ill patients were selected for treatment based upon their declining clinical criteria and IL-6 levels greater than 100 pg/mL, a biomarker used to assess severity of hyperinflammation. In patient 1, the elevated IL-6 level before treatment was 231 pg/mL, which was reduced to 3.32 pg/mL within 52 h of SCD treatment initiation [59]. For patient 2, cytokine profile was greatly elevated with a pretreatment IL-6 level of 598 pg/mL, which was reduced to 116 pg/mL within 50 h of SCD therapy initiation [59]. Improved IL-6 levels corresponded with improvements in other inflammatory indices in both patients, including procalcitonin, D-dimers, lactate dehydrogenase, ferritin, C-reactive protein, and IL-10 [59]. Pulmonary edema was rapidly reduced, and vasopressors were discontinued within 30 h for both patients, after the start of SCD therapy. Patient 1 received a total of 17 days of SCD therapy and was taken off ECMO 20 days after initiation. Patient 2 was taken off both SCD therapy and ECMO after 16 days of therapy [59]. Both patients were subsequently extubated and discharged alive from the hospital.
14.7 Summary
Conventional filtration and sorbent therapies will continue to see utility in the management of AKI and MODS, including treating a vast number of cases that arise from sepsis, viral and toxic causes. However, emerging technologies, such as extracorporeal renal cell bioreactor-based bioartificial kidneys, and leukocyte-processing devices such as the SCD may see increased utility as they are developed further and become commercially available. Immunomodulatory therapies aim to treat AKI by reducing the degree of renal tubule cell injury, whether this damage stems from the non-renal hyperinflammation process or a renal derived hyperinflammatory condition, initiated and potentiated from primary ischemic or nephrotoxic renal tubule cell injury. Despite clinical observations that generally AKI patients have demonstrated a predisposition to go on to develop CKD, clinical trials utilizing the SCD in ICU patients with AKI have demonstrated that all survivors treated with the SCD were dialysis independent 60-days after treatment. This is in sharp contrast with the observed 25% incidence of ongoing renal support requirements in survivors receiving intensive dialytic therapy without SCD during these trials. Mechanistic details of immunomodulatory device impact on inflammatory diseases are still being fully elucidated; however, SCD has been demonstrated to impact the activity of neutrophils, which are key cellular players in acute inflammatory processes. Clinical data is mounting and suggests an effect of SCD to diminish ongoing organ injury and speed the recovery of organ function. This effect is most likely related to SCD-induced modulation of the immunologic responses controlling hyperinflammation as well as the regenerative repair processes responsible for functional recovery of organs, and specifically, kidney function. However, like many developing technologies for the treatment of AKI and sepsis, additional large, randomized, controlled, clinical trials with functional outcome measures are still required to provide evidence of clear therapeutic benefit.
Abbreviations
- AKI:
-
Acute kidney injury
- ALI:
-
Acute lung injury
- AMI:
-
Acute myocardial infarction
- ARDS:
-
Acute respiratory distress syndrome
- ARF:
-
Acute renal failure
- ATN:
-
Acute tubular necrosis
- BAK:
-
Bioartificial kidney
- BRECS:
-
Bioartificial Renal Epithelial Cell System
- CD:
-
Cluster of differentiation
- CHF:
-
Chronic heart failure
- CKD:
-
Chronic kidney disease
- CO:
-
Cardiac output
- COVID-19:
-
Coronavirus disease 2019
- CPB:
-
Cardiopulmonary bypass
- CRRT:
-
Continuous renal replacement therapy
- CRS:
-
Cardiorenal syndrome
- DAMP:
-
Damage-associated molecular pattern molecules
- ECMO:
-
Extracorporeal membrane oxygenation
- ESRD:
-
End stage renal disease
- FDA:
-
Food and drug administration
- FiO2:
-
Fraction of inspired oxygen
- HD:
-
Hemodialysis
- HDF:
-
Hemodiafiltration
- HF:
-
Hemofiltration
- HRS:
-
Hepatorenal syndrome
- iCa:
-
Ionized calcium
- ICH:
-
Intracerebral hemorrhage/hemorrhagic stroke
- ICU:
-
Intensive care unit
- IDE:
-
Investigational device exemption
- IHC:
-
Immunohistochemistry
- IL-:
-
Interleukin
- IRB:
-
Institutional review board
- LMW:
-
Low molecular weight
- LPS:
-
Lipopolysaccharide
- MELD:
-
Model for end-stage liver disease
- MO:
-
Monocyte
- MODS:
-
Multiple organ dysfunction syndrome
- MW:
-
Molecular weight
- MWCO:
-
Molecular weight cutoff
- PAMP:
-
Pathogen-associated molecular pattern molecules
- RAD:
-
Renal assist device
- RCA:
-
Regional citrate anticoagulation
- riCA:
-
Recommended ionized calcium
- RRT:
-
Renal replacement therapy
- SAE:
-
Serious adverse events
- SCD:
-
Selective cytopheretic device
- SIRS:
-
Systemic inflammatory response syndrome
- SOFA:
-
Sequential organ failure assessment
- SVR:
-
Systemic vascular resistance
- T2D:
-
Type 2 diabetes
- TBI:
-
Traumatic brain injury
- TNFα:
-
Tumor necrosis factor alpha
- US:
-
United States
- VFD:
-
Ventilator-free days
- WBC:
-
White blood counts
References
Rabb H, Griffin MD, McKay DB, Swaminathan S, Pickkers P, Rosner MH, et al. Inflammation in AKI: current understanding, key questions, and knowledge gaps. J Am Soc Nephrol. 2016;27(2):371–9.
Honore PM, Hoste E, Molnar Z, Jacobs R, Joannes-Boyau O, Malbrain M, et al. Cytokine removal in human septic shock: where are we and where are we going? Ann Intensive Care. 2019;9(1):56.
Rimmer E, Houston BL, Kumar A, Abou-Setta AM, Friesen C, Marshall JC, et al. The efficacy and safety of plasma exchange in patients with sepsis and septic shock: a systematic review and meta-analysis. Crit Care. 2014;18(6):699.
Gomez H, Ince C, De Backer D, Pickkers P, Payen D, Hotchkiss J, et al. A unified theory of sepsis-induced acute kidney injury: inflammation, microcirculatory dysfunction, bioenergetics, and the tubular cell adaptation to injury. Shock. 2014;41(1):3–11.
Lee SA, Cozzi M, Bush EL, Rabb H. Distant organ dysfunction in acute kidney injury: a review. Am J Kidney Dis. 2018;72(6):846–56.
Chionh CY, Soni SS, Finkelstein FO, Ronco C, Cruz DN. Use of peritoneal dialysis in AKI: a systematic review. Clin J Am Soc Nephrol. 2013;8(10):1649–60.
House AA, Ronco C. Extracorporeal blood purification in sepsis and sepsis-related acute kidney injury. Blood Purif. 2008;26(1):30–5.
Ronco C, Tetta C, Mariano F, Wratten ML, Bonello M, Bordoni V, et al. Interpreting the mechanisms of continuous renal replacement therapy in sepsis: the peak concentration hypothesis. Artif Organs. 2003;27(9):792–801.
Bonavia A, Groff A, Karamchandani K, Singbartl K. Clinical utility of extracorporeal cytokine Hemoadsorption therapy: a literature review. Blood Purif. 2018;46(4):337–49.
Villa G, Zaragoza JJ, Sharma A, Neri M, De Gaudio AR, Ronco C. Cytokine removal with high cut-off membrane: review of literature. Blood Purif. 2014;38(3–4):167–73.
Cavaillon JM, Munoz C, Fitting C, Misset B, Carlet J. Circulating cytokines: the tip of the iceberg? Circ Shock. 1992;38(2):145–52.
Clark E, Molnar AO, Joannes-Boyau O, Honore PM, Sikora L, Bagshaw SM. High-volume hemofiltration for septic acute kidney injury: a systematic review and meta-analysis. Crit Care. 2014;18(1):R7.
Joannes-Boyau O, Honore PM, Perez P, Bagshaw SM, Grand H, Canivet JL, et al. High-volume versus standard-volume haemofiltration for septic shock patients with acute kidney injury (IVOIRE study): a multicentre randomized controlled trial. Intensive Care Med. 2013;39(9):1535–46.
Niwa T. Removal of protein-bound uraemic toxins by haemodialysis. Blood Purif. 2013;35(Suppl 2):20–5.
Villa G, Neri M, De Rosa S, Samoni S, Chelazzi C, Romagnoli S, et al. Albumin loss and citrate load in pre-dilution high cut-off-CVVHDF with regional citrate (18 mmol/L) and high cut-off CVVHD with systemic heparin: an in vitro study. Blood Purif. 2018;46(3):205–13.
Yasuda N, Goto K, Yamamoto S, Hidaka S, Hagiwara S, Noguchi T. Removal of 17 cytokines, HMGB1, and albumin by continuous hemofiltration using a cellulose triacetate membrane: an ex vivo study. J Surg Res. 2012;176(1):226–31.
Honore PM, Jacobs R, Joannes-Boyau O, De Regt J, De Waele E, van Gorp V, et al. Newly designed CRRT membranes for sepsis and SIRS—a pragmatic approach for bedside intensivists summarizing the more recent advances: a systematic structured review. ASAIO J. 2013;59(2):99–106.
La Manna G, Donati G. Coupled plasma filtration adsorption: a multipurpose extracorporeal detoxification therapy. Blood Purif. 2018;46(3):228–38.
Falkenhagen D, Strobl W, Vogt G, Schrefl A, Linsberger I, Gerner FJ, et al. Fractionated plasma separation and adsorption system: a novel system for blood purification to remove albumin bound substances. Artif Organs. 1999;23(1):81–6.
Winchester JF, Kellum JA, Ronco C, Brady JA, Quartararo PJ, Salsberg JA, et al. Sorbents in acute renal failure and the systemic inflammatory response syndrome. Blood Purif. 2003;21(1):79–84.
Winchester JF, Ronco C, Brady JA, Cowgill LD, Salsberg J, Yousha E, et al. The next step from high-flux dialysis: application of sorbent technology. Blood Purif. 2002;20(1):81–6.
Winchester JF, Salsberg JA. Sorbents in the treatment of renal failure. Minerva Urol Nefrol. 2004;56(3):215–21.
Terayama T, Yamakawa K, Umemura Y, Aihara M, Fujimi S. Polymyxin B Hemoperfusion for sepsis and septic shock: a systematic review and meta-analysis. Surg Infect (Larchmt). 2017;18(3):225–33.
Ankawi G, Xie Y, Yang B, Xie Y, Xie P, Ronco C. What have we learned about the use of Cytosorb adsorption columns? Blood Purif. 2019;48(3):196–202.
Humes HD, Weitzel WF, Bartlett RH, Swaniker FC, Paganini EP, Luderer JR, et al. Initial clinical results of the bioartificial kidney containing human cells in ICU patients with acute renal failure. Kidney Int Rep. 2004;66(4):1578–88.
Fissell WH, Fleischman AJ, Humes HD, Roy S. Development of continuous implantable renal replacement: past and future. Transl Res. 2007;150(6):327–36.
Tiranathanagul K, Dhawan V, Lytle IF, Zhang W, Borschel GH, Buffington DA, et al. Tissue engineering of an implantable bioartificial hemofilter. ASAIO J. 2007;53(2):176–86.
Humes HD, Buffington D, Westover AJ, Roy S, Fissell WH. The bioartificial kidney: current status and future promise. Pediatr Nephrol. 2014;29(3):343–51.
Kim S, Fissell WH, Humes DH, Roy S. Current strategies and challenges in engineering a bioartificial kidney. Front Biosci (Elite Ed). 2015;7:215–28.
Legallais C, Kim D, Mihaila SM, Mihajlovic M, Figliuzzi M, Bonandrini B, et al. Bioengineering organs for blood detoxification. Adv Healthc Mater. 2018;7(21):e1800430.
Tumlin J, Wali R, Williams W, Murray P, Tolwani AJ, Vinnikova AK, et al. Efficacy and safety of renal tubule cell therapy for acute renal failure. J Am Soc Nephrol. 2008;19(5):1034–40.
Humes HD, Sobota JT, Ding F, Song JH. A selective cytopheretic inhibitory device to treat the immunological dysregulation of acute and chronic renal failure. Blood Purif. 2010;29(2):183–90.
Barlas S. Critics assail FDA medical device approval process: slow review time and safety are at issue. P T. 2011;36(7):395–409.
Mishra S. FDA, CE mark or something else? Thinking fast and slow. Indian Heart J. 2017;69(1):1–5.
Ding F, Song JH, Jung JY, Lou L, Wang M, Charles L, et al. A biomimetic membrane device that modulates the excessive inflammatory response to sepsis. PLoS One. 2011;6(4):e18584.
Ding F, Yevzlin AS, Xu ZY, Zhou Y, Xie QH, Liu JF, et al. The effects of a novel therapeutic device on acute kidney injury outcomes in the intensive care unit: a pilot study. ASAIO J. 2011;57(5):426–32.
Tumlin JA, Chawla L, Tolwani AJ, Mehta R, Dillon J, Finkel KW, et al. The effect of the selective cytopheretic device on acute kidney injury outcomes in the intensive care unit: a multicenter pilot study. Semin Dial. 2013;26(5):616–23.
Tumlin JA, Galphin CM, Tolwani AJ, Chan MR, Vijayan A, Finkel K, et al. A multi-center, randomized, controlled, pivotal study to assess the safety and efficacy of a selective cytopheretic device in patients with acute kidney injury. PLoS One. 2015;10(8):e0132482.
Selewski DT, Goldstein SL, Fraser E, Plomaritas K, Mottes T, Terrell T, et al. Immunomodulatory device therapy in a pediatric patient with acute kidney injury and multiorgan dysfunction. Kidney Int Rep. 2017;2(6):1259–64.
Symons JM, Chua AN, Somers MJ, Baum MA, Bunchman TE, Benfield MR, et al. Demographic characteristics of pediatric continuous renal replacement therapy: a report of the prospective pediatric continuous renal replacement therapy registry. Clin J Am Soc Nephrol. 2007;2(4):732–8.
Pino CJ, Westover AJ, Johnston KA, Buffington DA, Humes HD. Regenerative medicine and immunomodulatory therapy: insights from the kidney, heart, brain, and lung. Kidney Int Rep. 2018;3(4):771–83.
Pino CJ, Lou L, Smith PL, Ding F, Pagani FD, Buffington DA, et al. A selective cytopheretic inhibitory device for use during cardiopulmonary bypass surgery. Perfusion. 2012;27(4):311–9.
Johnston KA, Westover AJ, Rojas-Pena A, Haft JW, Toomasian JM, Johnson T, et al. Novel leukocyte modulator device reduces the inflammatory response to cardiopulmonary bypass. ASAIO J. 2019;65(4):401–7.
Westover AJ, Johnston KA, Buffington DA, Humes HD. An immunomodulatory device improves insulin resistance in obese porcine model of metabolic syndrome. J Diabetes Res. 2016;2016:3486727.
Ayub K, Hallett MB. Ca2+ influx shutdown during neutrophil apoptosis: importance and possible mechanism. Immunology. 2004;111(1):8–12.
Whyte MK, Hardwick SJ, Meagher LC, Savill JS, Haslett C. Transient elevations of cytosolic free calcium retard subsequent apoptosis in neutrophils in vitro. J Clin Invest. 1993;92(1):446–55.
Christopher MJ, Link DC. Regulation of neutrophil homeostasis. Curr Opin Hematol. 2007;14(1):3–8.
Szamosfalvi B, Westover A, Buffington D, Yevzlin A, Humes HD. Immunomodulatory device promotes a shift of circulating monocytes to a less inflammatory phenotype in chronic hemodialysis patients. ASAIO J. 2016;62(5):623–30.
Tak T, Tesselaar K, Pillay J, Borghans JA, Koenderman L. What's your age again? Determination of human neutrophil half-lives revisited. J Leukoc Biol. 2013;94(4):595–601.
Bellomo R, Cass A, Cole L, Finfer S, Gallagher M, Lo S, et al. Intensity of continuous renal-replacement therapy in critically ill patients. N Engl J Med. 2009;361(17):1627–38.
VA NIH Acute Renal Failure Trial Network, Palevsky PM, Zhang JH, O'Connor TZ, Chertow GM, Crowley ST, et al. Intensity of renal support in critically ill patients with acute kidney injury. N Engl J Med. 2008;359(1):7–20.
Modem V, Thompson M, Gollhofer D, Dhar AV, Quigley R. Timing of continuous renal replacement therapy and mortality in critically ill children. Crit Care Med. 2013;42(4):943–53.
Goldstein SL, Somers MJ, Baum MA, Symons JM, Brophy PD, Blowey D, et al. Pediatric patients with multi-organ dysfunction syndrome receiving continuous renal replacement therapy. Kidney Int. 2005;67(2):653–8.
Goldstein S, Selewski D, Asskenazi D, Brophy P, Mottes T, Terrell T, et al. Multicenter evaluation of the selective cytopheteric device (SCD) in critically ill children requiring CRRT: report from the first 4 patients. Am Soc Nephrol Renal Week 2017. New Orleans, LA. Abstract: FR-PO089.
Gomez H, Kellum JA. Sepsis-induced acute kidney injury. Curr Opin Crit Care. 2016;22(6):546–53.
Vincent JL, Sakr Y. Clinical trial design for unmet clinical needs: a spotlight on sepsis. Expert Rev Clin Pharmacol. 2019;12(9):893–900.
Vincent JL, Van Nuffelen M. Septic shock: new pharmacotherapy options or better trial design? Expert Opin Pharmacother. 2013;14(5):561–70.
Olivencia-Yurvati AH, Ferrara CA, Tierney N, Wallace N, Mallet RT. Strategic leukocyte depletion reduces pulmonary microvascular pressure and improves pulmonary status post-cardiopulmonary bypass. Perfusion. 2003;18(Suppl 1):23–31.
Yessayan L, Szamosfalvi B, Napolitano L, Singer B, Kurabayashi K, Song Y, et al. Treatment of cytokine storm in COVID-19 patients with immunomodulatory therapy. ASAIO J. 2020;66(10):1079–83.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Pino, C.J., Humes, H.D. (2022). Bioartificial Kidneys, Renal Epithelial Cell Systems, and Biomimetic Membrane Devices. In: Bezerra da Silva Junior, G., Nangaku, M. (eds) Innovations in Nephrology. Springer, Cham. https://doi.org/10.1007/978-3-031-11570-7_14
Download citation
DOI: https://doi.org/10.1007/978-3-031-11570-7_14
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-11569-1
Online ISBN: 978-3-031-11570-7
eBook Packages: MedicineMedicine (R0)