Abstract
End-stage kidney disease (ESKD) is one of the most prevalent diseases in the world with significant morbidity and mortality. Current modes of renal replacement therapy include dialysis and renal transplantation. Although dialysis is an acceptable mode of renal replacement therapy, it does have its shortcomings, which include poorer life expectancy compared with renal transplantation, risk of infections and vascular thrombosis, lack of vascular access and absence of biosynthetic functions of the kidney. Renal transplantation, in contrast, is the preferred option of renal replacement therapy, with improved morbidity and mortality rates and quality of life, compared with dialysis. Renal transplantation, however, may not be available to all patients with ESKD. Some of the key factors limiting the availability and efficiency of renal transplantation include shortage of donor organs and the constant risk of rejection with complications associated with over-immunosuppression respectively. This review focuses chiefly on the potential roles of bioengineering in overcoming limitations in renal transplantation via the development of cell-based bioartificial dialysis devices as bridging options before renal transplantation, and the development of new sources of organs utilizing cell and organ engineering.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
End-stage kidney disease (ESKD), defined as a glomerular filtration rate of less than 15 ml/min/1.73m2 or upon initiation of renal replacement therapy [1], is one of the most prevalent diseases in the world.
In the USA, the point prevalence of ESKD in the pediatric population was 9,721 patients as of 31 December 2014, compared with 9,921 in the preceding year [2, 3]. Although the incidence of ESKD in the pediatric population in 2014 was comparatively lower compared with 2013, almost 1,400 children, nevertheless, had new onset ESKD in 2014 [2]. Likewise, in Europe, based on the data from 24 registries in 15 countries, the point prevalence of pediatric patients on renal replacement therapy was 1,430 patients as of 31 December 2014 and was similar to that of the preceding year [4]. This represented an incident rate of 8.2 per million age-related population (pmarp) [4]. In the Asia–Pacific region, there is also a notable prevalence of ESKD in the pediatric population. In 2008, the prevalence of children on renal replacement therapy varied from 34 pmarp in Japan to 65 pmarp in Australia and Malaysia [5]. The incidence of children on renal replacement therapy in the same year varied from 4.3 pmarp in Japan [5] to about 8 pmarp in Australia and New Zealand [6].
Current available modalities of renal replacement therapy include dialysis and renal transplantation. Both modalities, however, have their limitations.
Although dialysis is life-saving, it cannot replicate the biosynthetic and metabolic activities of the normal kidney and there are several risks and limitations associated with dialysis, including risk of infections [2, 7,8,9,10], vascular thrombosis [11, 12] with subsequent loss of vascular access in hemodialysis patients.
Renal transplantation seems to be a better alternative than renal dialysis in terms of quality of life [13,14,15], morbidity and mortality rates [16,17,18], and financial expenditure [19, 20]. However, the scarcity of donor kidneys (secondary to increasing prevalence of ESKD, together with stable or declining rates of organ donation) [21], limited graft survival [22, 23], and complications associated with immunosuppression (opportunistic infections [24], post-transplant lymphoproliferative disorder [25]) and the immunosuppressants themselves (e.g., calcineurin inhibitor- and corticosteroid-associated new onset of diabetes after transplantation [26]), limit the availability of this option.
In recent years, tremendous progress has been made in terms of the development of novel bridging options before transplantation and potential new sources of transplantable kidneys secondary to technological advancements and breakthroughs in the fields of tissue engineering and regenerative medicine. This review focuses on the progress and potential solutions offered by bioengineering in resolving the key issues facing renal transplantation.
Bioartificial dialysis devices as a bridging option before renal transplantation
The word “dialysis” is a direct borrowing from the Greek word for “loosening” and describes a process of separation of solutes from the blood, which is essentially the underlying principle of dialysis. The first dialysis machine, or “artificial kidney,” was built by Willem Johan Kolff, a Dutch physician, during the Second World War in 1943 [27]. The crude machine, built from salvaged car and washing machine parts, orange juice cans, and sausage skins was used to treat 16 patients with acute renal failure, but without much success, until 1945, when a 67-year-old woman in uremic coma regained consciousness following 11 h of hemodialysis [28,29,30]. Since the inception of dialysis as a modality of renal replacement therapy, the technology involved has progressed tremendously.
As previously discussed, dialysis does not offer the endocrine, metabolic, and immunomodulatory functions compared with a native kidney. Another chief limitation of dialysis is its restriction on mobility. The decline in mobility after initiating dialysis has been demonstrated to be associated with increased short-term mortality [31]. The restriction in mobility is of even greater consequence in the pediatric population as that could lead to frequent school absences and impair the learning process in pediatric ESKD patients. To overcome the above, dialysis machines need to be more compact and recapitulate the functions of the native kidneys. This may be achieved via the development of a self-sufficient bioartificial kidney or a more compact portable bioartificial dialysis device.
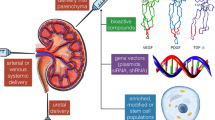
The renal assist device (RAD) is one of the potential solutions that may be utilized to solve the above challenge. The device was developed by Humes and team and consisted of living human renal tubule cells seeded into the fibers of a standard hemofilter to simulate the solute clearance, fluid homeostasis, endocrine and metabolic functions of the native kidney [32]. The hollow fibers of the hemofilter acted as an immunological barrier and provided a mechanical scaffold for the living renal tubule cells. In a phase II multicenter, randomized controlled open-label trial involving 58 patients with acute kidney injury, of which 40 patients received both continuous venovenous hemofiltration (CVVH) + RAD and 18 patients received CVVH alone, it was demonstrated that patients in the RAD arm had a lower mortality rate than patients in the CVVH only group at 28 days (33% vs 61% respectively) [33]. In the same study, patients with RAD also demonstrated faster renal recovery rates than patients who received CVVH alone [33]. Unexpectedly, a follow-up phase IIb study was discontinued after an interim analysis revealed a surprising high survival rate in patients treated with sham RAD without the living renal cells [34]. The RAD remains, thus far, the only bioartificial renal device that has been successfully tested in humans.
The successful utilization of the RAD in humans has prompted the search for and development of a miniaturized version of the RAD that can be implanted into the human body—the implantable renal assist device (iRAD). The use of microelectromechanical system (MEMS) technology has allowed the production of silicon nanopore membranes (SNMs) for use as a hemofilter and a scaffold for immunoisolation of renal cells [35,36,37,38]. The SNMs have a slit pore design that closely resembles that of the glomerular filtration barrier and the pores can be as small as 5 nm [39]. The SNMs also have higher performance with greater selectivity compared with the standard hemofilter membranes [40]. The high permeability of these membranes allows for filtration to occur utilizing only the arterial–venous pressure difference, obviating the additional need for a pump. The other chief component of the iRAD is the renal cells seeded on the SNM scaffold that provide the endocrine, metabolic, and immunomodulatory functions of the native kidney. A step forward in the application of these SNMs as hemofilters involved the successful implantation of these SNMs in dog models [41]. Further mechanistic studies, however, are required to further define the ideal membrane characteristics and limitations before trialing them in humans.
A key limitation preventing the widespread use of allogeneic renal epithelial cells within an extracorporeal environment is the lack of a cryopreservable system and the need to maintain an anticoagulated blood circuit, which is the conventional modality for solute clearance and supporting cell viability. This prohibited large-scale device manufacturing, storage, and delivery. An important breakthrough in an attempt to overcome the above limitation is the development of the bioartificial renal epithelial cell system (BRECS), whereby cells and cell function could be sustained by perfusion fluids aside from blood [42]. The system consisted of porous carbon disks seeded with renal epithelial cells in porous polycarbonate housing. Perfusion fluid flows through the disks to maintain cell viability and remove cell products [42]. In a demonstration of the potential utility of BRECS in ESKD, anephric sheep were attached to a continuous flow peritoneal dialysis circuit that included a BRECS, and cell viability was noted to be sustained by the extracorporeal peritoneal fluid at the end of the study [43]. In another separate study, BRECS seemed to confer a survival advantage in a porcine model of septic shock [44].
Another portable dialysis device of interest is the portable artificial kidney, jointly developed by Debiotech of Switzerland, AWAK of Singapore, and Neokidney Development (an initiative of the Dutch Kidney Foundation), with clinical trials stipulated to be carried out in 2017 [45].
Cell and organ engineering in the development of novel sources of cells and organs for renal replacement therapy
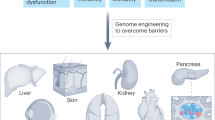
As highlighted above, renal transplantation offers the best option for patients with ESKD, but is, however, limited by the scarcity of organs, which remains the greatest limitation to widespread transplantation in these patients. In addition, although current immunosuppressive regimens have kept acute rejection rates low, the long-term graft survival in renal transplant recipients remains a significant problem [46]. In addition, in low-income countries, cost, lack of infrastructure, infectious diseases, and malnutrition further complicate and limit organ transplantation.
The above issues may be solved with the development of a bioengineered kidney. However, the challenges that face the development of a bioengineered kidney lie in the anatomical complexity [47] in addition to the need to replicate the myriad kidney functions, which include fluid and electrolyte homeostasis, endocrine functions via secretion of erythropoietin and hydroxylation of vitamin D and immunomodulatory functions. Cellular engineering, which is of less complexity than organ engineering, may provide a bridging solution between ESKD and organ transplantation/implantation of a bioengineered kidney. A number of different approaches in cell and organ engineering have been investigated as alternatives to dialysis and kidney transplantation.
Stem cell transplantation as a potential mode of renal reparative and replacement therapy
Stem cells are inherently involved in the reparative process during kidney injury [48, 49]. Augmentation of the reparative process through direct stem cell supplementation seemed like a promising and possible approach in the therapy of kidney injury, as demonstrated in several studies [50,51,52,53,54,55,56,57]. A key limitation in this renal stem cell-based therapy is the limited source of renal stem cells. Several strategies have been developed to overcome this limitation.
Transdifferentiation of stem cells or reprogramming of stem cells from one lineage to another may be a plausible approach to obtaining more renal stem cells. In a study by Jia et al. [58], it was reported that bone marrow stem cells when transplanted into mice with acute kidney injury acquired properties similar to those of renal stem cells.
Mesenchymal stem cells (MSCs) may be another source of stem cells that can be utilized in acute and chronic renal injury therapy. MSCs have been noted to transdifferentiate to renal cells, together with a host of other properties beneficial to renal repair, when applied to in vivo models of both acute kidney injury and chronic ischemic kidney disease [59].
Embryonic stem (ES) cells hold great promise as a potential source of renal stem cells. Mouse ES cells were noted to differentiate into renal tubular cells when transfected with specific transcription factors or cultured with certain growth factors [60,61,62,63]. ES cells, however, have experienced little progress in view of underlying ethical considerations.
Induced pluripotent stem cells (iPSCs), terminally differentiated cells reprogrammed to become pluripotent stem cells, as first illustrated by Takahashi and Yamanaka [64], are another potential source of stem cells that are able to undergo differentiation into kidney-related cells. An advantage of iPSCs is that they do not face the ethical constraints encountered with ES cells. The major limitation in iPSCs lies with their low induction efficiency. Strategies to overcome the low induction efficiency include improvements in reprogramming, for example, the addition of vitamin C during iPSC generation, and have increased induction efficiency and enhanced the progression of cells to pluripotency [65].
Organ engineering as a potential mode of renal replacement therapy
As previously discussed, the major obstacle in the development of a bioengineered kidney lies in the anatomical complexity of the kidney with various kidney-related cellular subtypes. Several technological advances have emerged that may potentially enable the fabrication of an organ ex vivo, namely:
-
1.
De novo renal organogenesis
-
2.
Organogenesis via decellularization and recellularization
-
3.
Three-dimensional (3D) bioprinting
De novo renal organogenesis involves the transplantation of the metanephros, which is the embryonic renal tissue, into the recipient for in situ organ development. Embryologically, the metanephric (permanent) mammalian kidney starts development around gestational weeks 4–5 [66]. The metanephros consists of cells derived from the metanephric blastema (giving rise to the nephron) and the ureteric bud (giving rise to the collecting ducts, renal pelvis, and ureter). Unlike pluripotent stem cells, these are embryonic cells committed to the development of the mature kidney [66]. The plausibility of utilizing metanephroi to supplement the function of a kidney was initially demonstrated via the implantation of metanephroi into the cortex of newborn mice with subsequent growth and development of the donor embryonic cells into glomeruli and tubules, with demonstrable glomerular filtration function [67]. In another study that examined the potential role of metanephroi in augmenting the renal function of the recipient rat, in addition to similar findings from the preceding study by Woolf et al. [67], it was also demonstrated that transplanted metanephroi had a decreased immune response, possibly attributable to poor human leukocyte antigen expression in these cells [68]. Besides the implantation of metanephroi into the kidneys for growth and differentiation, the transplantation of metanephroi into the omentum has also shown some promise, the metanephroi developing into mature glomeruli and tubules, with vascularization of the tissue from the omentum [69].
Despite the promising nature of metanephroi transplantation as a mode of renal replacement therapy, a key challenge facing this option is the availability and source of embryonic renal stem cells for transplantation, for which the currently available option is aborted human fetuses. The development of new sources of embryonic cells via novel cell engineering techniques may hold the key to overcoming this major limitation.
In the process of organogenesis via decellularization and recellularization, the native cells are first removed via a mixture of physical, chemical, and enzymatic means, leaving behind only the extra-cellular matrix [70]. The resultant scaffold is subsequently repopulated with cells creating the reseeded organ. The ability of the extra-cellular matrix to direct differentiation of pluripotent stem cells to renal cells was illustrated in a study by Ross et al. [71]. In another study by Nakayama et al., it was further verified that the 3D decellularized scaffolds could indeed provide the signaling and attachment for cellular repopulation [72]. The feasibility of application of decellularization and recellularization processes in organogenesis for utility in the clinical setting was further demonstrated in a breakthrough study by Song et al., which showed the ability of reseeded decellularized kidney scaffold to produce urine and basic solute transport function after orthotropic implantation into rats [73]. The urine produced by the regenerated kidneys was, however, lesser, and of lower urinary urea and creatinine concentrations, compared with native kidneys [73]. This may be attributable to the partial seeding of the scaffold and the immature state of the repopulated cells. More work, however, is required to improve the cell seeding efficiency, increase the scale of organ culture, and improve solute transport characteristics.
Chief considerations in the widespread application of this method include the source of stem cells and the source of the scaffolds. Although the supply of stem cells may be limited, the advent of iPSC may be the underlying solution to overcoming this limitation. Hence, the key limitation for organogenesis using the decellularization and recellularization technique lies with the scaffold. There are a few options pertaining to scaffold availability.
One possible source of scaffold involves the harvesting and decellularization of the patient’s own kidney, followed by repopulation of the organ using the patient’s own iPSCs in a bioreactor and finally implantation back into the patient, without the need for immunosuppression. A similar feasible choice involves the decellularization of discarded cadaveric kidneys deemed unsuitable for transplant, followed by repopulation with autologous cells [74]. The use of kidney scaffolds derived from nonhuman primates (semi-xenotransplantation) is another interesting strategy for overcoming the shortage of scaffolds. As these animal scaffolds are decellularized, they are almost antigen-free, hence minimizing the risk of sensitization and the need for immunosuppression [75, 76]. If this option is indeed feasible, the organ supply for scaffold derivation will almost certainly be limitless. In addition, with the inception of 3D bioprinting (see below), scaffold shortage for organ synthesis may also be a thing of the past.
Additive manufacturing, also known as 3D printing, has been a major breakthrough innovation in many areas, including engineering, manufacturing industry, research, medicine, and education. Printing technology has evolved from conventional two-dimensional (2D) printing to an additive process in which successive layers are formed into a 3D shape [77]. 3D printing was first introduced in 1986 by Charles W. Hull, whereby layers are added by curing photopolymers with ultraviolet light lasers. The method was termed “stereolithography.” 3D printing is the ideal method for the production of customized and precise medical devices or prosthetics, hence bringing “personalized medicine” a step forward. An example of the clinical applicability of 3D printing is the recent creation of an airway splint using the technology for use in an infant with tracheobronchomalacia [78]. Compared with nonbiological printing, 3D bioprinting is more sophisticated with additional complexities, such as the need for precise 3D imaging of the organ to be printed, the type of bioink required (choice of biomaterials, cellular subtypes, various growth and differentiation factors to be incorporated), and other associated challenges. 3D bioprinting is increasingly used in organ engineering to address the unmet need for tissues and organs available for transplantation, whether in terms of production of acellular scaffolds for cell-seeding or the synthesis of an entire organ. Although there are as yet no available published studies on the development of a functional kidney produced entirely from 3D bioprinting, there has been encouraging news of an early-stage kidney prototype synthesized using microextrusion bioprinting by the Wake Forest Institute for Regenerative Medicine [79], suggesting the plausibility of a 3D bioprinting-synthesized kidney.
Conclusion
Dialysis and renal transplantation are the only available, clinically viable options for renal replacement therapy. Numerous innovative research studies are ongoing to address the various shortcomings of current renal replacement therapy and to eventually develop a fully functional, bioengineered kidney capable of reproducing the metabolic, endocrine, and immunomodulatory functions of the native kidney. Early work on cell-based therapies and organ engineering has shown promise by developing functional kidney tissue. Despite these early encouraging results, there are obstacles that remain to be overcome before a bioengineered kidney will become standard care. A major issue for the field is the limited source of cells. There are ethical concerns over using ES cells from human embryos for organ development, which fortunately have been circumvented with the advent of iPSCs. Additionally, logistical concerns involving large-scale cell-sourcing, large-scale scaffold design and production, large-scale organ culture, cryopreservation, storage, and distribution remain to be solved before gaining generalized acceptance. The solutions created by bioengineering in the field of renal replacement will also need to extend beyond validation in small animal models and demonstrate their practicality in larger animals and eventually humans. Although still in its juvenile stage, the current landscape for the development of a bioengineered kidney remains encouraging. Numerous obstacles, however, remain to be surmounted before the dream of having a bioengineered kidney for ESKD patients can be realized.
References
National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification
United States Renal Data System 2016 (2016) USRDS annual data report: epidemiology of kidney disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda
United States Renal Data System 2015 (2015) USRDS annual data report: epidemiology of kidney disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda
ERA-EDTA Registry (2016) ERA-EDTA Registry Annual Report 2014. Academic Medical Center, Department of Medical Informatics, Amsterdam, the Netherlands
Harambat J, van Stralen KJ, Kim JJ, Tizard EJ (2012) Epidemiology of chronic kidney disease in children. Pediatr Nephrol 27:363–373
Orr NI, McDonald SP, McTaggart S, Henning P, Craig JC (2009) Frequency, etiology and treatment of childhood end-stage kidney disease in Australia and New Zealand. Pediatr Nephrol 24:1719–1726
Zaritsky JJ, Salusky IB, Gales B, Ramos G, Atkinson J, Allestead A, Brandt ML, Goldstein SL (2008) Vascular access complications in long-term pediatric hemodialysis patients. Pediatr Nephrol 23:2061–2065
Hayes WN, Watson AR, Callaghan N, Wright E, Stefanidis CJ, European Pediatric Dialysis Working Group (2012) Vascular access: choice and complications in European paediatric haemodialysis units. Pediatr Nephrol 27:999–1004
Schaefer F, Feneberg R, Aksu N, Donmez O, Sadikoglu B, Alexander SR, Mir S, Ha IS, Fischbach M, Simkova E, Watson AR, Moller K, von Baum H, Warady BA (2007) Worldwide variation of dialysis-associated peritonitis in children. Kidney Int 72:1374–1379
Sethna CB, Bryant K, Munshi R, Warady BA, Richardson T, Lawlor J, Newland JG, Neu A, SCOPE Investigators (2016) Risk factors for and outcomes of catheter-associated peritonitis in children: the SCOPE collaborative. Clin J Am Soc Nephrol 11:1590–1596
Wartman SM, Rosen D, Woo K, Gradman WS, Weaver FA, Rowe V (2014) Outcomes with arteriovenous fistulas in a pediatric population. J Vasc Surg 60:170–174
Regus S, Almási-Sperling V, Lang W (2016) Pediatric patients undergoing arteriovenous fistula surgery without intraoperative heparin. J Vasc Access 17:494–498
Goldstein SL, Graham N, Burwinkle T, Warady B, Farrah R, Varni JW (2006) Health-related quality of life in pediatric patients with ESRD. Pediatr Nephrol 21:846–850
Goldstein SL, Graham N, Warady BA, Seikaly M, McDonald R, Burwinkle TM, Limbers CA, Varni JW (2008) Measuring health-related quality of life in children with ESRD: performance of the generic and ESRD-specific instrument of the Pediatric Quality of Life Inventory (PedsQL). Am J Kidney Dis 51:285–297
Riaño-Galán I, Málaga S, Rajmil L, Ariceta G, Navarro M, Loris C, Vallo A (2009) Quality of life of adolescents with end-stage renal disease and kidney transplant. Pediatr Nephrol 24:1561–1568
McDonald SP, Craig JC, Australian and New Zealand Paediatric Nephrology Association (2004) Long-term survival of children with end-stage renal disease. N Engl J Med 350:2654–2662
Groothoff JW (2005) Long-term outcomes of children with end-stage renal disease. Pediatr Nephrol 20:849–853
Mitsnefes MM (2008) Cardiovascular complications of pediatric chronic kidney disease. Pediatr Nephrol 23:27–39
Laupacis A, Keown P, Pus N, Krueger H, Ferguson B, Wong C, Muirhead N (1996) A study of the quality of life and cost-utility of renal transplantation. Kidney Int 50:235–242
Loubeau PR, Loubeau JM, Jantzen R (2001) The economics of kidney transplantation versus hemodialysis. Prog Transplant 11:291–297
Wolfe RA, Roys EC, Merion RM (2010) Trends in organ donation and transplantation in the United States, 1999–2008. Am J Transplant 10:961–972
Saeed B (2012) Pediatric renal transplantation. Int J Organ Transplant Med 3:62–73
Fletcher JT, Nankivell BJ, Alexander SI (2009) Chronic allograft nephropathy. Pediatr Nephrol 24:1465–1471
Smith JM, Dharnidharka VR (2015) Viral surveillance and subclinical viral infection in pediatric kidney transplantation. Pediatr Nephrol 30:741–748
Mynarek M, Hussein K, Kreipe HH, Maecker-Kolhoff B (2014) Malignancies after pediatric kidney transplantation: more than PTLD? Pediatr Nephrol 29:1517–1528
Garro R, Warshaw B, Felner E (2015) New-onset diabetes after kidney transplant in children. Pediatr Nephrol 30:405–416
Kolff WJ, Berk HTJ (1943) De kunstmatige nier. Een dialysator met groot oppervlak. Ned Tijdschr Geneeskd 87:1684
Broers H (2006) Inventor for life, the story of W. J. Kolff, father of artificial organs. B&Vmedia, Kampen
Kolff W (1946) De kunstmatige nier. Kok, Kampen
Vienken J (2009) “Bioengineering for life”: a tribute to Willem Johan Kolff. Nephrol Dial Transplant 24:2299–2301
Arai Y, Kanda E, Kikuchi H, Yamamura C, Hirasawa S, Aki S, Inaba N, Aoyagi M, Tanaka H, Tamura T, Sasaki S (2014) Decreased mobility after starting dialysis is an independent risk factor for short-term mortality after initiation of dialysis. Nephrology (Carlton) 19:227–233
Humes HD, Buffington DA, MacKay SM, Funke AJ, Weitzel WF (1999) Replacement of renal function in uremic animals with a tissue-engineered kidney. Nat Biotechnol 17:451–455
Tumlin J, Wali R, Williams W, Murray P, Tolwani AJ, Vinnikova AK, Szerlip HM, Ye J, Paganini EP, Dworkin L, Finkel KW, Kraus MA, Humes HD (2008) Efficacy and safety of renal tubule cell therapy for acute renal failure. J Am Soc Nephrol 19:1034–1040
Pino CJ, Yevzlin AS, Tumlin J, Humes HD (2012) Cell-based strategies for the treatment of kidney dysfunction: a review. Blood Purif 34:117–123
Fissell WH, Fleischman AJ, Humes HD, Roy S (2007) Development of continuous implantable renal replacement: past and future. Transl Res 150:327–336
Fissell WH, Dubnisheva A, Eldridge AN, Fleischman AJ, Zydney AL, Roy S (2009) High-performance silicon nanopore hemofiltration membranes. J Memb Sci 326:58–63
Conlisk AT, Datta S, Fissell WH, Roy S (2009) Biomolecular transport through hemofiltration membranes. Ann Biomed Eng 37:722–736
Fissell WH, Roy S (2009) The implantable artificial kidney. Semin Dial 22:665–670
Fissell WH, Manley S, Westover A, Humes HD, Fleischman AJ, Roy S (2006) Differentiated growth of human renal tubule cells on thin-film and nanostructured materials. ASAIO J 52:221–227
Kanani DM, Fissell WH, Roy S, Dubnisheva A, Fleischman A, Zydney AL (2010) Permeability—selectivity analysis for ultrafiltration: effect of pore geometry. J Memb Sci 349:405
Kensinger C, Karp S, Kant R, Chui BW, Goldman K, Yeager T, Gould ER, Buck A, Laneve DC, Groszek JJ, Roy S, Fissell WH (2016) First implantation of silicon nanopore membrane hemofilters. ASAIO J 62:491–495
Buffington DA, Pino CJ, Chen L, Westover AJ, Hageman G, Humes HD (2012) Bioartificial renal epithelial cell system (BRECS): a compact, cryopreservable extracorporeal renal replacement device. Cell Med 4:33–43
Johnston KA, Westover AJ, Rojas-Pena A, Buffington DA, Pino CJ, Smith PL, Humes HD (2016) Development of a wearable bioartificial kidney using the bioartificial renal epithelial cell system (BRECS). J Tissue Eng Regen Med. doi:10.1002/term.2206
Westover AJ, Buffington DA, Johnston KA, Smith PL, Pino CJ, Humes HD (2017) A bio-artificial renal epithelial cell system conveys survival advantage in a porcine model of septic shock. J Tissue Eng Regen Med 11:649–657
Http://awak.com/news/press/AWAK_PRESS_RELEASE_23052014.pdf. Accessed on 26 September 2016.
Magee CC, Pascual M (2004) Update in renal transplantation. Arch Intern med 164:1373–1388
Al-Awqati Q, Oliver JA (2002) Stem cells in the kidney. Kidney Int 61:387–395
Safirstein R (1999) Renal regeneration: reiterating a developmental paradigm. Kidney Int 56:1599–1600
Nony PA, Schnellmann RG (2003) Mechanisms of renal cell repair and regeneration after acute renal failure. J Pharmacol Exp Ther 304:905–912
Herrera MB, Bussolati B, Bruno S, Fonsato V, Romanazzi GM, Camussi G (2004) Mesenchymal stem cells contribute to the renal repair of acute tubular epithelial injury. Int J Mol Med 14:1035–1041
Kunter U, Rong S, Djuric Z, Boor P, Müller-Newen G, Yu D, Floege J (2006) Transplanted mesenchymal stem cells accelerate glomerular healing in experimental glomerulonephritis. J Am Soc Nephrol 17:2202–2212
Bussolati B, Hauser PV, Carvalhosa R, Camussi G (2009) Contribution of stem cells to kidney repair. Curr Stem Cell Res Ther 4:2–8
Li L, Black R, Ma Z, Yang Q, Wang A, Lin F (2012) Use of mouse hematopoietic stem and progenitor cells to treat acute kidney injury. Am J Physiol Renal Physiol 302:F9–F19
He J, Wang Y, Sun S, Yu M, Wang C, Pei X, Zhu B, Wu J, Zhao W (2012) Bone marrow stem cells-derived microvesicles protect against renal injury in the mouse remnant kidney model. Nephrology (Carlton) 17:493–500
Dorronsoro A, Robbins PD (2013) Regenerating the injured kidney with human umbilical cord mesenchymal stem cell-derived exosomes. Stem Cell Res Ther 4:39
He J, Wang Y, Lu X, Zhu B, Pei X, Wu J, Zhao W (2015) Micro-vesicles derived from bone marrow stem cells protect the kidney both in vivo and in vitro by microRNA-dependent repairing. Nephrology (Carlton) 20:591–600
Li Q, Tian SF, Guo Y, Niu X, Hu B, Guo SC, Wang NS, Wang Y (2015) Transplantation of induced pluripotent stem cell-derived renal stem cells improved acute kidney injury. Cell Biosci 5:45
Jia X, Xie X, Feng G, Lű H, Zhao Q, Che Y, Zheng Y, Han Z, Xu Y, Li Z, Kong D (2012) Bone marrow-derived cells can acquire renal stem cells properties and ameliorate ischemia-reperfusion induced acute renal injury. BMC Nephrol 13:105
Zhu XY, Lerman A, Lerman LO (2013) Concise review: mesenchymal stem cell treatment for ischemic kidney disease. Stem Cells 31:1731–1736
Steenhard BM, Isom KS, Cazcarro P, Dunmore JH, Godwin AR, St John PL, Abrahamson DR (2005) Integration of embryonic stem cells in metanephric kidney organ culture. J Am Soc Nephrol 16:1623–1631
Kobayashi T, Tanaka H, Kuwana H, Inoshita S, Teraoka H, Sasaki S, Terada Y (2005) Wnt4-transformed mouse embryonic stem cells differentiate into renal tubular cells. Biochem Biophys Res Commun 336:585–595
Kim D, Dressler GR (2005) Nephrogenic factors promote differentiation of mouse embryonic stem cells into renal epithelia. J Am Soc Nephrol 16:3527–3534
Morizane R, Monkawa T, Itoh H (2009) Differentiation of murine embryonic stem and induced pluripotent stem cells to renal lineage in vitro. Biochem Biophys Res Commun 390:1334–1339
Takahashi K, Yamanaka S (2006) Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126:663–676
Esteban MA, Wang T, Qin B, Yang J, Qin D, Cai J, Li W, Weng Z, Chen J, Ni S, Chen K, Li Y, Liu X, Xu J, Zhang S, Li F, He W, Labuda K, Song Y, Peterbauer A, Wolbank S, Redl H, Zhong M, Cai D, Zeng L, Pei D (2010) Vitamin C enhances the generation of mouse and human induced pluripotent stem cells. Cell Stem Cell 6:71–79
Horster MF, Braun GS, Huber SM (1999) Embryonic renal epithelia: induction, nephrogenesis, and cell differentiation. Physiol Rev 79:1157–1191
Woolf AS, Palmer SJ, Snow ML, Fine LG (1990) Creation of a functioning chimeric mammalian kidney. Kidney Int 38:991–997
Rogers SA, Lowell JA, Hammerman NA, Hammerman MR (1998) Transplantation of developing metanephroi into adult rats. Kidney Int 54:27–37
Hammerman MR (2003) Therapeutic promise of embryonic kidney transplantation. Nephron Exp Nephrol 93:e58
Gilbert TW, Sellaro TL, Badylak SF (2006) Decellularization of tissues and organs. Biomaterials 27:3675–3683
Ross EA, Williams MJ, Hamazaki T, Terada N, Clapp WL, Adin C, Ellison GW, Jorgensen M, Batich CD (2009) Embryonic stem cells proliferate and differentiate when seeded into kidney scaffolds. J Am Soc Nephrol 20:2338–2347
Nakayama KH, Batchelder CA, Lee CI, Tarantal AF (2010) Decellularized rhesus monkey kidney as a three-dimensional scaffold for renal tissue engineering. Tissue Eng Part A 16:2207–2216
Song JJ, Guyette JP, Gilpin SE, Gonzalez G, Vacanti JP, Ott HC (2013) Regeneration and experimental orthotopic transplantation of a bioengineered kidney. Nat Med 19:646–651
Ott HC (2015) Perfusion decellularization of discarded human kidneys: a valuable platform for organ regeneration. Transplantation 99:1753
Mirmalek-Sani SH, Sullivan DC, Zimmerman C, Shupe TD, Petersen BE (2013) Immunogenicity of decellularized porcine liver for bioengineered hepatic tissue. Am J Pathol 183:558–565
Salvatori M, Peloso A, Katari R, Soker S, Lerut JP, Stratta RJ, Orlando G (2015) Semi-xenotransplantation: the regenerative medicine-based approach to immunosuppression-free transplantation and to meet the organ demand. Xenotransplantation 22:1–6
Hull CW (1986) Apparatus for production of three-dimensional objects by stereolithography. US4575330 A (Google Patents, 1986)
Zopf DA, Hollister SJ, Nelson ME, Ohye RG, Green GE (2013) Bioresorbable airway splint created with a three-dimensional printer. N Engl J Med 368:2043–2045
Murphy SV, Atala A (2014) 3D bioprinting of tissues and organs. Nat Biotechnol 32:773–785
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interests
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Yeo, WS., Zhang, YC. Bioengineering in renal transplantation: technological advances and novel options. Pediatr Nephrol 33, 1105–1111 (2018). https://doi.org/10.1007/s00467-017-3706-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-017-3706-4