Abstract
More than 30 years ago, extracorporeal treatment was used in the intensive care unit (ICU) for the first time in order to deliver hemodialysis. Due to improvements in medical and surgical standards of care and an increase in the average age of patients, clinicians are today facing a significant increase in patients’ severity of illness at ICU admission and the frequent occurrence of multiple organ failure (MOF) with the simultaneous dysfunction of two or more organs. Extracorporeal treatments to support different organs (kidneys, liver, lungs, heart, septic blood) are now common in the ICU. However, paralleling the evolution of continuous dialytic treatments over the last two decades, in recent years, multiple organ support therapy (MOST) has been delivered as a “Christmas-tree like” addition of one system on another (e.g., continuous renal replacement therapy [CRRT] on extracorporeal membrane oxygenation [ECMO] or molecular adsorbent recycling system [MARS] on CRRT). Current developments and the future of MOST foresee the application of dedicated and integrated multipurpose advanced platforms for the support of patients with MOF. This review will detail extracorporeal blood purification treatments that can be delivered for dysfunction of organs other than the kidneys.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Acute Kidney Injury
- Multiple Organ Failure
- Continuous Renal Replacement Therapy
- Acute Heart Failure
- Acute Liver Failure
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Introduction
More than 30 years ago, extracorporeal treatment was used in the intensive care unit (ICU) for the first time in order to deliver hemodialysis. Due to improvements in medical and surgical standards of care and an increase in the average age of patients, clinicians are today facing a significant increase in patients’ severity of illness at ICU admission and the frequent occurrence of multiple organ failure (MOF) with the simultaneous dysfunction of two or more organs. Extracorporeal treatments to support different organs (kidneys, liver, lungs, heart, septic blood) are now common in the ICU. However, paralleling the evolution of continuous dialytic treatments over the last two decades, in recent years, multiple organ support therapy (MOST) has been delivered as a “Christmas‐tree like” addition of one system on another (e.g., continuous renal replacement therapy [CRRT] on extracorporeal membrane oxygenation [ECMO] or molecular adsorbent recycling system [MARS] on CRRT). Current developments and the future of MOST foresee the application of dedicated and integrated multipurpose advanced platforms for the support of patients with MOF. This review will detail extracorporeal blood purification treatments that can be delivered for dysfunction of organs other than the kidneys.
Sepsis
Although mortality related to sepsis has significantly decreased throughout the last decade, it still represents a major challenge for healthcare systems from clinical and economical points of view [1, 2]. The broad heterogeneity of clinical presentations and the complex interplay of host pro‐inflammatory and anti‐inflammatory processes are among the principal obstacles for further reduction in sepsis‐related morbidity and mortality. Since the imbalance between hyper‐inflammation and immunosuppression is supposed to be a key factor in determining outcomes, during recent years most research on sepsis has been focused on re‐establishing an equilibrium in the cytokine‐mediated inflammation by clearing them from the blood using CRRT‐based techniques [3].
High‐Volume Hemofiltration
It has been speculated that CRRT ‘pushed’ to doses higher than usual (> 45 ml/kg/h; high volume hemofiltration, HVHF) may play a role in blood purification in septic patients by clearing the bloodstream of cytokines. Animal models of severe sepsis demonstrated a beneficial effect of HVHF on hemodynamics, proportional to the intensity of ultrafiltration [4] and, based on this hypothesis, different protocols have been developed and tested [5]. A Cochrane analysis published in 2013, including randomized controlled trials (RCTs) and quasi‐randomized trials, that compared HVHF to standard dialysis in adult ICU patients, concluded that the evidence was still insufficient to recommend its use in septic critically ill patients [6]. A systematic review and meta‐analysis of studies performed between 1966 and 2013 was recently published [7]. The analysis included four RCTs (470 participants) that compared HVHF (effluent rate > 50 ml/kg/h) and standard hemofiltration (HF) in patients with sepsis and septic shock. Pooled analysis for 28‐day mortality did not show any difference between HVHF and HF in kidney recovery, improvement in hemodynamics or reduction in ICU or hospital lengths of stay. In addition, significant side effects, including hypophosphatemia and hypokalemia, were more common in the HVHF group. Based on the current literature, HVHF cannot be recommended for routine use in sepsis and septic acute kidney injury (AKI). Moreover, adverse effects should be carefully monitored when more intense doses of CRRT are used in septic and non‐septic patients.
Polymyxin B Hemoperfusion
High levels of endotoxin activity, a basic component of the outer membrane of Gram‐negative bacteria, have been associated with worse clinical outcomes [8]. Polymyxin B hemoperfusion (PMX‐HP) is a technique based on the high affinity for polymyxin B of endotoxin, which, during extracorporeal hemoperfusion, remains bound to the filter. In 2009, a multicenter randomized controlled study [9] showed, in 64 patients with severe sepsis or septic shock, that PMX‐HP, added to conventional therapy, significantly improved hemodynamics and organ dysfunction and reduced 28‐day mortality. More recently, a larger multicenter randomized controlled study including 232 patients with septic shock (119 vs 113 controls) did not confirm the previous findings [10]: there was no difference in 28‐day mortality between the study group and the control patients. By contrast, a recent retrospective analysis, part of an ongoing study (EUPHAS2; PMX‐HP in abdominal vs non‐abdominal sepsis), showed that the sequential organ failure assessment (SOFA) score decreased significantly 72 h after PMX‐HP in patients with abdominal sepsis (p < 0.001), 28‐day mortality was 35% in abdominal sepsis vs 49% in patients with non‐abdominal sepsis, and in‐hospital mortality was 44% in abdominal sepsis vs 55% in non‐abdominal sepsis [11]. Based on the current evidence, the efficacy of PMX‐HP is currently being questioned and new data are absolutely necessary to clarify its role in abdominal and non‐abdominal septic patients.
High Cut‐Off Hemofiltration
Because of the role of the humoral mediators of the immune system in the pathogenesis of sepsis, many attempts have been made to remove the cytokines from the bloodstream [12]. High cut‐off (HCO) membranes have pore diameters (> 0.01 µm) that allow molecules up to 60 kDa to pass [13]. A review that included 23 publications on HCO hemofiltration (HCO‐HF), showed that a reduction in inflammatory and anti‐inflammatory cytokines (interleukin [IL]‐4, IL‐6, IL‐1 receptor antagonist [IL‐1ra], IL‐8, IL‐10, IL‐12, tumor necrosis factor [TNF]‐α) was common in all the studies [13]. Moreover, cytokine removal was associated with significant improvement in hemodynamics, oxygenation, and organ dysfunction [13]. A 16‐ICU multicenter observational study, designed to evaluate changes in inflammatory biomarkers and tissue oxygenation/perfusion indexes in septic ICU patients with AKI during HCO‐CVVHD, has recently been concluded and preliminary results released [14]. A significant improvement in organ function was demonstrated but there was no reduction in mortality. Before drawing definitive conclusions on the effects of HCO‐HF in sepsis, some factors need to be considered: first, the current literature is biased by the lack of a standardized definition and classification of HCO dialysis membranes making comparison among studies difficult [13, 15]; second, heterogeneity of the clinical picture of septic patients requires strict patient selection; third, which mediators should be removed and which should not during different phases of sepsis has not been established [16]; and, finally, technology that can select specific molecules has not been developed so far.
Coupled Plasma Filtration Adsorption
Coupled plasma filtration adsorption (CPFA) is a complex extracorporeal blood purification technique: plasma, separated from blood by a plasma‐filter, is run through a synthetic resin cartridge (with adsorption capacity for inflammatory mediators) and then returned to the blood circuit where a hemofilter removes excess fluid and allows renal replacement [17, 18]. In other words, CPFA is a sorbent technology based on RRT for removal of endotoxin, bacterial products and both pro‐ and anti‐inflammatory endogenous substances in septic patients with AKI [19]. In 2014, Livigni and colleagues, published the results from a multicenter, randomized trial comparing CPFA with standard care in the treatment of critically ill patients with septic shock [20]. There were no statistically significant differences in hospital mortality secondary endpoints (occurrence of new organ failure), or free‐ICU days during the first 30 days. The authors suggested that the technical difficulties occurring during the treatments (early clotting) may have biased the results. A new trial, including only patients achieving the prescribed CPFA treatment is currently ongoing: in order to limit clotting‐related treatment failure, only citrate is now allowed as anticoagulant [20]. Technical difficulties, frequently met during CPFA application, should be carefully weighed with potential, theoretical advantages. New generation machines, implementing default citrate anticoagulation, have recently been released into the market. In a recent study, including 15 critically ill patients with septic shock, citrate pharmacokinetics during CPFA were evaluated [21]. The study showed that high doses of citrate (needed for this complex treatment) can be safely managed with predilution hemodiafiltration and pre/postdilution hemofiltration.
Although promising, the results from studies in septic patients undergoing blood purification therapies are totally inconclusive. It is possible that key factors to improve extracorporeal treatment for sepsis will be the identification of a selected population of patients, targeting the clearance of a specific molecular pattern, and improved understanding of the optimal timing for such treatments.
Lung
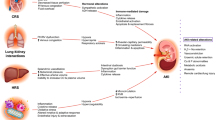
The majority of ICU patients with AKI are affected by MOF and many patients need respiratory and renal support [22–25]. Patients who develop severe AKI are burdened by extrarenal complications and respiratory function is frequently impaired because of pulmonary congestion and increased vascular permeability [26]. At the same time, mechanical ventilation itself causes negative effects on kidney function through hemodynamic impairment, biohumoral mediators, blood gas disorders, and biotrauma (one of the components of ventilator‐induced lung injury [VILI]) highlighting a clear negative organ crosstalk [27, 28]. Barotrauma (high volumes leading to high transpulmonary pressures), volutrauma (alveolar overdistention), atelectrauma (repetitive opening and closing of alveoli) represent the other components of VILI often associated with mechanical ventilation [28]. All these physical and biochemical stresses, mutually and simultaneously interacting, lead to local and systemic inflammatory reactions that negatively impact distal organs and promote the development of MOF [29, 30]. A landmark study published in 2000 [31] in patients with acute respiratory distress syndrome (ARDS), strongly contributed to highlight that mechanical ventilation may further worsen already injured lungs and stimulated the practice of protective ventilation [32]. The concept of protective ventilation relies on the observation that ‘less aggressive’ ventilation – low tidal volumes and airway pressures – limits pulmonary damage and respiratory complications [28, 33, 34]. On the other hand, when protective ventilation cannot maintain normal values of partial pressure of carbon dioxide in the arterial blood (PaCO2), so‐called ‘permissive hypercapnia’ is usually tolerated as a “lesser evil” [35]. Although permissive hypercapnia has proved advantages and benefits [36], it may also exert multiple negative effects [37–39]. In order to limit excessive CO2 accumulation, devices for extracorporeal CO2 removal (ECCO2R), have been developed. The general concept of an ECCO2R system is very close to the hemofiltration circuit: the blood is drained from the patients, CO2 is cleared by a membrane and blood is then re‐injected into the systemic circulation. Apart from the first attempts to remove CO2 through hemodialysis [40, 41], currently ECCO2R is mainly applied in two clinical conditions: chronic obstructive pulmonary disease (COPD) patients with hypercapnic respiratory failure who fail non‐invasive ventilation (NIV) [42, 43] and ARDS patients with excessive hypercapnia [44]. From a technical point of view, two principal ECCO2R systems can be considered (Table 1): the arterio‐venous (AV) and the veno‐venous (VV) configurations. The AV modality does not need an artificial pump because it uses the AV pressure gradient to generate flow through a low resistance membrane [45]. Two main drawbacks limit this pumpless system: the need for an AV pressure gradient, which is unsuitable for hemodynamically unstable patients, and the cannulation of a major artery, which can result in distal ischemia [46]. The VV configuration, adopted in newer ECCO2R devices, uses 14–18 French (Fr) venous double cannulas in a pumped system (which generates a blood flow rate of 300–500 ml/min) equipped with a membrane (gas exchanger) that allows the elimination of CO2 [47]. In order to clear CO2, ECCO2R requires higher pump flow rates than RRT. By contrast, during ECMO, blood oxygenation is delivered with blood flows exceeding 3 l/min [48, 49]. ECCO2R (low‐flow) may be integrated into a CRRT system, possibly using the same extracorporeal circuit (Table 1). Although pioneering attempts date to about 25 years ago [50], modern applications with (easy‐to‐use) standard pump‐driven RRT‐circuits are very recent: Godet and coworkers [51] instrumented five adult female healthy pigs with a low flow CO2 removal device (PrismaLung®, Hospal®) integrated on a CRRT platform (a device based on a Prismaflex® system). The gas exchanger membrane was connected into the CRRT circuit, in place of the hemofilter. Satisfactory CO2 clearance was obtained (in vivo mean decrease of 14%) demonstrating the applicability of the system. Forster and colleagues, in a pilot study, applied a CRRT system, with a ECCO2R device, in 10 critically ill patients with combined respiratory‐renal failure [52]. A standard CRRT system was integrated, in series, with a hollow‐fiber gas. This “lung‐assisting renal replacement system” gave encouraging results since the treatment allowed a mean 28.1% decrease in PaCO2 with a blood flow of 378 ml/min. Finally, Quintard and colleagues, in 16 mechanically ventilated patients with respiratory acidosis and AKI requiring ongoing CRRT, applied a gas exchanger originally designed for pediatric use [53]. The system significantly reduced the PaCO2 (−31% at 6 h and −39% at 12 h) and increased arterial pH (+0.16 at 6 h and +0.23 at 12 h) without complications.
Liver
Acute liver dysfunction in the ICU is not a rare occurrence although its incidence may vary significantly, depending on whether or not liver disease referral centers are considered. The main cause of acute liver failure (ALF) is acetaminophen toxicity [54]. Interestingly, in critically ill patients, liver disease is frequently at the pyramid of the development of MOF, being the trigger of several pathological pathways, eventually involving lungs, kidneys and brain [54]. Essentially two kinds of hepatic syndrome can be treated in the ICU: ALF and acute‐on‐chronic liver failure (ACLF). ALF is defined as the development of hepatic encephalopathy within 26 weeks of jaundice and coagulopathy with an international normalized ratio (INR) > 1.5 in a patient with no previous liver disease. ACLF is an acute worsening of hepatic function in cirrhotic patients, either secondary to deterioration of initial liver injury (alcoholic hepatitis, superimposed viral hepatitis, portal vein thrombosis, drug‐induced liver injury) or caused by secondary liver involvement in the context of trauma, surgery, sepsis or, more generically, MOF.
The kidneys are involved in about 50% of overall liver failure cases and AKI is an independent risk factor for mortality. In cirrhotic patients with renal involvement, a very well studied and peculiar disease has been described: hepato‐renal syndrome (HRS) [55]. The definition of HRS has been recently updated to “a potentially reversible syndrome that occurs in patients with cirrhosis, ascites and liver failure that is characterized by impaired kidney function, marked alterations in cardiovascular function, and over‐activity of the sympathetic nervous system and renin‐angiotensin system. Severe renal vasoconstriction leads to a decrease of glomerular filtration rate. HRS may appear spontaneously or can follow a precipitating event” [55].
When extracorporeal treatments for liver or combined liver‐kidney support are indicated, several options have been described: no conclusive evidence can be currently recommended since no specific extracorporeal treatment has shown a consistent increase in survival with respect to liver transplantation. Interestingly, Naka and coworkers [56], in a retrospective study, compared the effects of CVVH in liver failure patients admitted to the ICU and in patients who had received liver transplantation. The authors showed that CVVH achieved very good outcomes in transplanted patients, but was not effective in improving blood chemistry (creatinine, lactate, acidosis) or mortality in patients with persistent liver dysfunction. Important considerations on the dialytic support of ALF patients are that: coagulation derangements have to be taken into account and that generally a low heparin approach should be considered [56]; citrate may not be well tolerated even though several authors have described satisfactory use even in ALF patients [57]; and intermittent techniques should not be recommended due to the risk of increasing intra‐cranial pressure [58]. Very recently, with a similar rationale but using an (11 year long) RCT design, Larsen and coworkers showed that treatment with high volume plasma exchange (1–2 l/h up to 8–10 l per day for three consecutive days) improved hospital survival in ALF patients compared to standard medical treatment (59 vs 48%) [59]. By contrast with Naka’s observations, this improvement was not particularly evident in patients who underwent liver transplantation but in those who were not listed to receive a transplantation: this study has the merit of being one of the first to show the benefit of extracorporeal therapy other than after liver transplantation. The authors elegantly showed that high volume plasma exchange was able to significantly blunt the inflammatory syndrome of treated patients and to correct INR, bilirubin, alanine transaminase (ALT) and ammonia levels. Interestingly plasma exchange also prevented the occurrence of AKI and need for RRT [59].
Unlike hemodialysis for the kidneys, specific liver support systems able to effectively support liver function until either recovery or transplant remain elusive [60]. Specific extracorporeal liver substitution can currently be achieved by albumin dialysis (MARS), fractionated plasma separation and adsorption (Prometheus) – classified as artificial systems‐ and bioartificial systems that combine plasma separation with perfusion of bio‐reactors filled with human or animal hepatocytes (such as the extracorporeal liver assist device [ELAD], Vital Therapies, San Diego, California, USA). MARS and Prometheus are available commercially and have been repeatedly tested in large case series and randomized trials: neither has been shown to decrease mortality in patients with ACLF [60]. A recent important trial that compared MARS with medical therapy in patients with ALF and listed for liver transplant failed to demonstrate benefit because the median time to transplantation was too short to allow MARS enough time to exert any significant beneficial effect [61]. Improved survival of ALF and ACLF patients treated with bioartificial liver support systems has also never been clearly demonstrated [60]. At present, intense research into artificial liver support is ongoing even though there is currently little evidence to support routine clinical use in ALF.
Heart
Systemic congestion in the context of acute heart failure includes pulmonary insufficiency but also impairment of renal function. Pharmacological decongestion with loop diuretics is fundamental but has been commonly associated with AKI and type 1 cardiorenal syndrome [62]. On this background (injured kidneys due to the primary cardiac illness and to pharmacologic management) slow continuous ultrafiltration (SCUF) was described many years ago in order to artificially remove plasma water and relieve cardiopulmonary symptoms, thus bypassing the need for intense diuresis [63]. In case of a reno‐cardiac syndrome (acute heart failure secondary to acute or chronic renal failure), application of artificial fluid balance control and volume unloading for pulmonary edema management to a dialytic session is a common clinical picture encountered by nephrologists [64]. SCUF is currently considered as a last chance therapy by the European Society of Cardiology guidelines for management of patients with acute heart failure that is refractory to diuretic therapy [65]. Several randomized trials have been conducted in order to verify whether extracorporeal water removal might be beneficial compared to diuretic therapy. The UNLOAD (Ultrafiltration Versus Intravenous Diuretics for Patients Hospitalized for Acute Decompensated Heart Failure) Study showed that ultrafiltration allowed a greater amount of plasma water to be removed than did furosemide [66]. Interestingly, however, more patients in the ultrafiltration group than in the diuretic arm experienced an increase in creatinine levels of 0.3 mg/dl. Of note, the timing, dose, duration and clinical target of ultrafiltration remains to be investigated. By contrast, the CARRESS‐HF (Cardiorenal Rescue Study in Acute Decompensated Heart Failure) showed that ultrafiltration was not associated with a significant difference in weight loss at 96 h and, again, was associated with a significantly greater increase in creatinine levels [67]. Another small recent randomized trial (Continuous Ultrafiltration for cOngestive heaRt failure, CUORE), showed that extracorporeal ultrafiltration was associated with prolonged clinical stabilization and a greater freedom from re‐hospitalization for acute heart failure [68].
Given the apparently controversial results of these trials, which are probably due to different therapeutic algorithms, clinical targets and severity of included patients, it seems reasonable to reserve ultrafiltration for use in the most severely ill patients with initial signs of diuretic resistance. Furthermore, pharmacologic and extracorporeal removal of water should not be seen as alternative approaches but may be considered as synergistic. Finally, institutional expertise with extracorporeal devices should always be taken into account, because it may have a significant impact on final outcomes. The results of the large Aquapheresis versus Intravenous Diuretics and Hospitalizations for Heart Failure (AVOID‐HF) trial will likely clarify the safety and effectiveness of ultrafiltration therapy [69]. As far as worsening of renal function during decongestion therapies is concerned, it must be noted that loop diuretics and ultrafiltration have both been associated with increased creatinine levels. Extracorporeal water removal, however, has not been associated with neuro‐hormonal activation and it should not activate tubulo‐glomerular feedback as diuretics do [70]. Excessive intravascular depletion due to aggressive ultrafiltration prescription or a severely decreased glomerular filtration rate before ultrafiltration start may be possible reasons for the described increase in creatinine levels [70]. Furthermore, regardless of whether decongestion is achieved with drugs or ultrafiltration, a recent post hoc analysis of patients enrolled in the Diuretic Optimization Strategy Evaluation in Acute Decompensated Heart Failure (DOSE‐AHF) and CARRESS‐HF trials showed that only patients free of signs of orthodema at discharge had lower 60‐day rates of death, rehospitalization, or unscheduled visits compared to those having residual orthodema [71]. The authors hypothesize that despite congestion relief, therapies might be ineffective in definitively treating orthodema during hospitalization. It can be speculated that tools, such as biomarkers, bioimpedance, echocardiography, and minimally invasive hemodynamic monitors, should also be implemented in order to improve patient care.
Conclusions
New extracorporeal therapies are conceived to provide supportive treatment beyond the classic renal indications: today, consistent artificial support can be provided to multiple organs simultaneously. Ideally, new machines will include multiple platforms in which different circuits and filters can be used in combination to support renal, heart, liver, and lung function according to increasing patient needs and severity of MOF. Similarly to what happened about 30 years ago for hemofiltration use in critically ill patients, which was initially seen as cumbersome and reserved for a few patients and has now become a routine tool, MOST may today appear somewhat naïve and burdened by an excessive rate of treatment failures: technological improvements, increased clinical experience and marked improvements in clinical results are certainly expected in the next few years.
References
Vincent J, Opal S, Marshall J, Tracey K (2013) Sepsis definitions: time for change. Lancet 381:774–775
Kaukonen K-M, Bailey M, Suzuki S et al (2014) Mortality related to severe sepsis and septic shock among critically ill patients in Australia and New Zealand, 2000–2012. JAMA 311:1308–1316
Remick DG (2011) The pathogenesis of sepsis. Annu Rev Pathol 6:19–48
Lonneman G (1999) Tumor necrosis factor-alpha during continuous high-flux hemodialysis in sepsis with acute renal failure. Kidney Int 56:S84–S87
Lehner GF, Wiedermann CJ, Joannidis M (2014) a systematic review and meta-analysis. Minerva Anestesiol 80:595–609
Borthwick E, Hill C, Rabindranath K et al (2013) High-volume haemofiltration for sepsis. Cochrane Database Syst Rev 1:CD008075
Clark E, Molnar AO, Joannes-Boyau O et al (2014) High-volume hemofiltration for septic acute kidney injury: a systematic review and meta-analysis. Crit Care 18:R7
Marshall JC, Foster D, Vincent JL et al (2004) Diagnostic and prognostic implications of endotoxemia in critical illness: results of the MEDIC study. J Infect Dis 190:527–534
Cruz DN, Antonelli M, Fumagalli R, Foltran F (2009) Early use of polymyxin b hemoperfusion in abdominal septic shock. JAMA 301:2445–2452
Payen DM, Guilhot J, Launey Y et al (2015) Early use of polymyxin B hemoperfusion in patients with septic shock due to peritonitis: a multicenter randomized control trial. Intensive Care Med 41:975–984
Early Use of Polymyxin B Hemoperfusion in the Abdominal Sepsis 2 Collaborative Group (2014) Polymyxin B hemoperfusion in clinical practice: The picture from an unbound collaborative registry. Blood Purif 37:22–25
Connolly A, Vernon D (2000) Manipulations of the metabolic response for management of patients with severe surgical illness: review. World J Surg 24:696–704
Villa G, Zaragoza JJ, Sharma A et al (2014) Cytokine removal with high cut-off membrane: review of literature. Blood Purif 38:167–173
Villa G, Chelazzi C, Valente S et al (2014) Hemodialysis with high cutoff membranes improves tissue perfusion in severe sepsis: preliminary data of the Sepsis in Florence sTudy (SIFT). Crit Care 18:401
Ronco C (2014) Standard nomenclature for renal replacement therapy in acute kidney injury: very much needed! Blood Purif 38:37–38
Hotchkiss RS, Monneret G, Payen D (2013) Sepsis-induced immunosuppression: from cellular dysfunctions to immunotherapy. Nat Rev Immunol 13:862–874
Ronco C, Brendolan A, Lonnemann G et al (2002) A pilot study of coupled plasma filtration with adsorption in septic shock. Crit Care Med 30:1250–1255
Formica M, Olivieri C, Livigni S et al (2003) Hemodynamic response to coupled plasmafiltration-adsorption in human septic shock. Intensive Care Med 29:703–708
Bellomo R, Tetta C, Ronco C (2003) Coupled plasma filtration adsorption. Intensive Care Med 29:1222–1228
Livigni S, Bertolini G, Rossi C et al (2014) Efficacy of coupled plasma filtration adsorption (CPFA) in patients with septic shock: A multicenter randomised controlled clinical trial. BMJ open 4:e003536
Mariano F, Morselli M, Holló Z et al (2015) Citrate pharmacokinetics at high levels of circuit citratemia during coupled plasma filtration adsorption. Nephrol Dial Transplant 30:1911–1919
Bone R, Balk R, Cerra F et al (2009) Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. 1992. Chest 136(5 Suppl):e28
Liu KD, Matthay M (2008) Advances in critical care for the nephrologist: Acute lung injury/ARDS. Clin J Am Soc Nephrol 3:578–586
Esteban A, Alía I, Gordo F et al (2000) Prospective randomized trial ventilation and volume-controlled ventilation in ARDS. Chest 117:1690–1696
Dasta JF, Mclaughlin TP, Mody SH, Piech CT (2005) Daily cost of an intensive care unit day: The contribution of mechanical ventilation. Crit Care Med 33:1266–1271
Vieira JM, Castro I, Curvello-Neto A et al (2007) Effect of acute kidney injury on weaning from mechanical ventilation in critically ill patients. Crit Care Med 35:184–191
Kuiper JW, Groeneveld BJ, Slutsky AS, Plötz FB (2005) Mechanical ventilation and acute renal failure. Crit Care Med 33:1408–1415
Slutsky A, Ranieri V (2013) Ventilator-induced lung injury. N Engl J Med 369:2126–2136
Tremblay L, Slutsky A (1998) Ventilator-induced injury: from barotrauma to biotrauma. Proc Assoc Am Physicians 110:482–488
Kuiper JW, Vaschetto R, Della Corte F et al (2011) Bench-to-bedside review: Ventilation-induced renal injury through systemic mediator release – just theory or a causal relationship? Crit Care 15:228
ARDSNetwork (2000) Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med 342:1301–1308
Fitzgerald M, Millar J, Blackwood B et al (2014) Extracorporeal carbon dioxide removal for patients with acute respiratory failure secondary to the acute respiratory distress syndrome: a systematic review. Crit Care 18:222
Serpa Neto A, Simonis FD, Barbas CSV et al (2015) Lung-protective ventilation with low tidal volumes and the occurrence of pulmonary complications in patients without acute respiratory distress syndrome. Crit Care Med 28:1
Futier E, Jaber S (2014) Lung-protective ventilation in abdominal surgery. Curr Opin Crit Care 20:426–430
Feihl F, Perret C (1998) Permissive hypercapnia. European Respiratory Monograph 3:162–173
Contreras M, Masterson C, Laffey J (2015) Permissive hypercapnia: what to remember. Curr Opin Crit Care 18:26–37
Brian JJ (1998) Carbon dioxide and the cerebral circulation. Anesthesiology 88:1365–86
Doerr CH, Gajic O, Berrios JC et al (2005) Hypercapnic acidosis impairs plasma membrane wound reseating in ventilator-injured lungs. Am J Respir Crit Care Med 171:1371–1377
Curley G, Contreras M, Nichol A et al (2010) Hypercapnia and acidosis in sepsis: a double-edged sword? Anesthesiology 112:462–472
Isobe J, Mizuno H, Matsunobe S et al (1989) A new type of low blood flow ECCO2R using a hemodialysis system in apneic states. ASAIO Trans 35:638–639
Nolte SH, Jonitz WJ, Grau J et al (1989) Hemodialysis for extracorporeal bicarbonate/CO2 removal (ECBicCO2R) and apneic oxygenation for respiratory failure in the newborn. Theory and preliminary results in animal experiments. ASAIO Trans 35:30–34
Quinnell TG, Pilsworth S, Shneerson JM, Smith IE (2006) Prolonged invasive ventilation following acute ventilatory failure in COPD: weaning Results, survival, and the role of noninvasive ventilation. Chest 129:133–139
Menzies R, Gibbons W, Goldberg P (1989) Determinants of weaning and survival among patients with COPD who require mechanical ventilation for acute respiratory failure. Chest 95:398–405
Bein T, Weber-Carstens S, Goldmann A et al (2013) Lower tidal volume strategy (≈3 ml/kg) combined with extracorporeal CO2 removal versus “conventional” protective ventilation (6 ml/kg) in severe ARDS: The prospective randomized Xtravent-study. Intensive Care Med 39:847–856
Cove ME, MacLaren G, Federspiel WJ, Kellum J (2012) Bench to bedside review: Extracorporeal carbon dioxide removal, past present and future. Crit Care 16:232
Bein T, Weber F, Philipp A et al (2006) A new pumpless extracorporeal interventional lung assist in critical hypoxemia/hypercapnia. Crit Care Med 34:1372–1377
Del Sorbo L, Pisani L, Filippini C et al (2013) Extracorporeal CO2 removal in Hypercapnic patients at risk of noninvasive ventilation failure: a matched cohort study with historical control. Crit Care Med 43:120–127
Ricci Z, Romagnoli S, Ronco C (2014) Extracorporeal support therapies. In: Miller R (ed) Miller’s Anesthesia, 2-Volume Set, 8th edn. Elsevier, Philadelphia, pp 3158–3181
MacLaren G, Combes A, Bartlett R (2011) Respiratory dialysis is not extracorporeal membrane oxygenation. Crit Care Med 39:2787–2788
Young J, Dorrington K, Blake G, Ryder W (1992) Femoral arteriovenous extracorporeal carbon dioxide elimination using low blood flow. Crit Care Med 20:805–809
Godet T, Combes A, Zogheib E et al (2015) Novel CO2 removal device driven by a renal-replacement system without hemofilter. A first step experimental validation. Anaesth Crit Care Pain Med 34:135–140
Forster C, Schriewer J, John S et al (2013) Low-flow CO2 removal integrated into a renal-replacement circuit can reduce acidosis and decrease vasopressor requirements. Crit Care 17:R154
Quintard JM, Barbot O, Thevenot F, de Matteis O, Benayoun L, Leibinger F (2014) Partial extracorporeal carbon dioxide removal using a standard continuous renal replacement therapy device. ASAIO J 60:564–569
Siddiqui MS, Stravitz RT (2014) Intensive care unit management of patients with liver failure. Clin Liver Dis 18:957–978
Fagundes C, Ginès P (2012) Hepatorenal syndrome: A severe, but treatable, cause of kidney failure in cirrhosis. Am J Kidney Dis 59:874–885
Naka T, Wan L, Bellomo R et al (2004) Kidney failure associated with liver transplantation or liver failure: the impact of continuous veno-venous hemofiltration. Int J Artif Organs 27:949–955
Patel S, Wendon J (2012) Regional citrate anticoagulation in patients with liver failure – time for a rethink? Crit Care 16:153
Leventhal TM, Liu KD (2015) What a nephrologist needs to know about acute liver failure. Adv Chronic Kidney Dis 22:376–381
Larsen FS, Schmidt LE, Bernsmeier C et al (2016) High-volume plasma exchange in patients with acute liver failure: An open randomised controlled trial. J Hepatol 64:69–78
Willars C (2014) Update in intensive care medicine: acute liver failure. Initial management, supportive treatment and who to transplant. Curr Opin Crit Care 20:202–209
Saliba F, Camus C, Durand F et al (2013) Albumin dialysis with a noncell artificial liver support device in patients with acute liver failure. Ann Intern Med 159:522–531
Palazzuoli A, Ruocco G, Ronco C, McCullough P (2015) Loop diuretics in acute heart failure: beyond the decongestive relief for the kidney. Crit Care 19:296
Nalesso F, Garzotto F, Ronco C (2010) Technical aspects of extracorporeal ultrafiltration: Mechanisms, monitoring and dedicated technology. Contrib Nephrol 164:199–208
Ronco C, Haapio M, House A (2008) Cardiorenal syndrome. J Am Coll Cardiol 52:1527–1539
McMurray JJV, Adamopoulos S, Anker SD et al (2012) ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Eur Heart J 33:1787–1847
Costanzo MR, Guglin ME, Saltzberg MT et al (2007) Ultrafiltration versus intravenous diuretics for patients hospitalized for acute decompensated heart failure. J Am Coll Cardiol 49:675–683
Bart BA, Goldsmith SR, Lee KL et al (2012) Ultrafiltration in decompensated heart failure with cardiorenal syndrome. N Engl J Med 367:2296–2304
Marenzi G, Muratori M, Cosentino ER et al (2014) Continuous ultrafiltration for congestive heart failure: The CUORE trial. J Card Fail 20:9–17
Krishnamoorthy A, Felker GM (2014) Fluid removal in acute heart failure: diuretics versus devices. Curr Opin Crit Care 20:478–483
Goldsmith SR, Bart BA, Burnett J (2014) Decongestive therapy and renal function in acute heart failure: Time for a new approach? Circ Heart Fail 7:531–535
Lala A, McNulty SE, Mentz RJ et al (2015) Relief and recurrence of congestion during and after hospitalization for acute heart failure. Circ Heart Fail 8:741–748
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Ricci, Z., Romagnoli, S., Ronco, C. (2016). (Multiple) Organ Support Therapy Beyond AKI. In: Vincent, JL. (eds) Annual Update in Intensive Care and Emergency Medicine 2016. Annual Update in Intensive Care and Emergency Medicine. Springer, Cham. https://doi.org/10.1007/978-3-319-27349-5_11
Download citation
DOI: https://doi.org/10.1007/978-3-319-27349-5_11
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-27348-8
Online ISBN: 978-3-319-27349-5
eBook Packages: MedicineMedicine (R0)