Abstract
The mainstay of treatment for sinonasal and skull base malignancies includes surgical resection with or without adjuvant therapy. Depending on the extent of the tumor, surgical removal can be extremely challenging because of the proximity of the paranasal sinuses to the orbit, intracranial cavity, and major neurovascular structures. Traditionally, an open approach was used to achieve surgical resection of these malignancies. Over the last 30 years, there have been significant advances in sinonasal and skull base surgery. With improvements in anatomical knowledge, surgical technique, and skull base reconstruction, the endoscopic endonasal approach has now become an accepted surgical approach in the management of certain sinonasal malignancies. This has added to the armamentarium of options available for surgical control of these complex tumors.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Neurovascular complications
- Orbital complications
- CSF leak
- Pneumocephalus
- Chronic rhinosinusitis
- Mucocele
- Infection
- Smell disturbance
- Infection
- Wound healing
Introduction
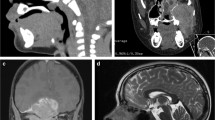
Sinonasal and skull base malignancies are rare tumors, representing 3–5% of all cancers affecting the head and neck, and less than 1% of all malignant tumors [1,2,3,4,5]. Because of the rarity, the literature related to surgical complications in the management of these conditions is often reflective of smaller, retrospective institutional series. Complications of surgical resection of sinonasal malignancies can range from relatively minor, such as wound infection and adhesion formation, to severe or even devastating, including blindness, severe bleeding, and intracranial injury (Fig. 10.1). Based on reports from the literature, there are no significant differences in complication rates when comparing the endoscopic endonasal versus open approach [6]. The overall complication rate of traditional craniofacial resection ranges from 25% to 65%, [7] as compared to 8.5% to 26% [6, 8,9,10] for the endoscopic endonasal approach. Factors predictive of increased risk of complication include medical comorbidity, prior radiation therapy, dural and brain involvement, as well as tumor staging.
Surgical planning is extremely important because of the close proximity of surrounding major neurovascular structures and the orbit. A thorough preoperative clinical evaluation and imaging review is essential to identify critical structures that will potentially be encountered during surgery in order to minimize morbidity. Skull base surgery for sinonasal malignancy can be technically complex, and even in the hands of a skilled operator complications can arise. Thus, surgeons should anticipate possible complications that may occur and create a surgical resection and reconstructive plan that includes an algorithm for managing anticipated complications (Table 10.1). The remainder of this chapter highlights the specific complications that can occur, the relative likelihood of these complications, and management techniques.
Intraoperative Complications
Injury to Major Neurovascular Structures
Vascular injury during surgery for sinonasal malignancy can be devastating, leading to major morbidity and mortality. Injury to the internal carotid artery (ICA) and its branches are correspondingly a common concern [10, 11]. However, injuries to small vessels that are adjacent to the brain or brainstem also carry a significant risk of neurological deficit if violated [10]. Overall, the incidence of major vascular injury ranges from 0.3% to14% in endoscopic endonasal skull base surgery, depending on the approach, tumor extension, and complexity of the case [11,12,13,14,15,16,17,18,19]. In a review of 800 patients with various skull base pathology, major vascular complication occurred in 0.9% of cases, including injuries to the ICA, ophthalmic artery, and other major intracranial vessels, as well as intracranial hemorrhage and subdural hematoma [10]. The risk of vascular complications during endoscopic endonasal surgery for sinonasal malignancy is determined by the location of the tumor and the anatomic relationship to surrounding major neurovascular structures. Other factors include the invasive nature of the tumor, its adherence to the ICA, prior adjuvant therapy, and the extent of planned oncologic resection [11]. Vascular complications are known to occur more frequently with increasing level of complexity of the surgery and with surgical resection requiring craniotomy, indicating an increased need for exposure [10].
ICA injury during surgery carries the potential risk of significant neurologic sequelae; anticipation of the potential risk of this injury can allow for pre-operative planning, and review of an algorithm for management if ICA injury occurs. If there is concern for ICA involvement by the tumor, a pre-operative balloon occlusion test can be performed to evaluate if the patient can tolerate ICA sacrifice should that become necessary. Multiple options for control of intra-operative ICA injury have been described. When managing bleeding from an ICA injury, it is important to communicate with the entire operating room team so that adequate fluid resuscitation is provided and cerebral perfusion is maintained while temporary packing is applied to stop the bleeding. For repair of the ICA injury, an endovascular clip can be applied if the exposure is adequate. However, given the large volume of bleeding often encountered, the visualization needed for endovascular clipping is often difficult to maintain. Placement of a crushed muscle patch has also been described for control of ICA injury. Muscle from the sternocleidomastoid, temporalis, abdominal rectus, or longus capitis can be harvested, crushed, and held in direct contact with the area of ICA injury. Crushing the muscle allows for release of calcium which in turn activates the clotting cascade [11, 17, 18]. If surgical control is not feasible, another option for control is endovascular intervention. The availability of the neurointerventional team should be considered during surgical planning if there is a high risk of ICA injury. Given the complexity of controlling an ICA injury, training models have been developed to allow surgical teams to practice management of this rare complication [19,20,21].
The risk of transient or permanent neurological deficits encountered during surgical intervention depends on the location of the tumor and the surgical approach used for access. In one review, transient neurological deficits occurred in 2.5% and permanent neurological deficit in 1.8% of patients who underwent endonasal approaches to the skull base [10]. These ranged from transient visual deficits and cranial nerve deficits to hemiplegia, ataxia, and quadraplegia [10]. Cranial nerve injury has been reported in 0.4–5% of extended endoscopic endonasal skull base surgeries [10, 22]. Depending on the tumor location, different approaches to the skull base will have varying levels of risk for neurological complications. All cranial nerves have the potential for injury during skull base surgery.
Postoperative Complications
Pneumocephalus
Pneumocephalus is the presence of air within the intracranial vault (Fig. 10.2). It can be seen with surgery for sinonasal malignancy that requires skull base resection and reconstruction, and is usually asymptomatic and managed expectantly, with resolution over a period of 1–2 weeks. This is a commonly reported finding if imaging is obtained in the immediate postoperative period and is an expected finding if the skull base has been entered during surgical resection. In contrast, the development of postoperative pneumocephalus is a rare and potentially devastating complication that can occur in conjunction with postoperative cerebrospinal fluid (CSF) leak and can also result in tension pneumocephalus. Tension pneumocephalus can lead to rapid neurological decline secondary to large amounts of intracranial air that causes compression of intracranial structures, leading to mass effect, and eventual herniation. This complication typically manifests as a reduced level of consciousness, focal neurological symptoms, seizures, followed by herniation, and ultimately death if not recognized and treated [23, 24]. Because this is a rare complication, most reports on diagnosis and management are based on case series or case reports. There are two ways that tension pneumocephalus can develop. The first is the “ball-valve” mechanism that allows air to be trapped in the intracranial cavity with a forceful movement, such as a sneeze or cough. The second is the “inverted pop bottle” mechanism where air enters the intracranial cavity to equalize the pressure difference as CSF exits the defect [23].
Symptoms of pneumocephalus include headache and focal neurological deficits. Loss of consciousness and rapid neurological decline signify the development of tension pneumocephalus and potential herniation. This condition requires immediate recognition and urgent intervention [25]. Conservative management, including bed rest, head of bed elevation, hyperosmolar therapy, avoiding Valsalva maneuvers, placement of nasal packing, and use of normobaric hyperoxia with 100% inspired oxygen, can be used to temporize pneumocephalus if it is not significant [23, 26,27,28]. Surgical intervention, including placement of a burr hole, ventriculostomy, or craniotomy, can be utilized to provide rapid decompression if tension pneumocephalus is present [23]. Ultimately, a careful evaluation of the skull base repair is needed to identify and repair the area of dehiscence that allowed the pneumocephalus to develop.
Cerebrospinal Fluid Leak
When skull base resection is performed during resection of sinonasal malignancy, the goal of surgical skull base defect reconstruction is to separate the cranial cavity from the sinonasal cavity, eliminate dead space, create a watertight seal, while preserving neurovascular structures, ocular function, and cosmesis [29]. Robust reconstruction of the skull base defect is extremely important if concurrent radiation therapy will be used postoperatively [30]. CSF leaks can occur secondary to failure of complete closure of the defect, graft migration (Fig. 10.2), and wound healing issues. Postoperative CSF leak is the most common complication after endoscopic endonasal skull base surgery, with a wide range reported from 3.8% to 69% of cases depending on the series [8, 10, 30,31,32,33,34,35]. However, many of these reports include a wide variety of skull base pathology, including sinonasal malignancy as well as benign tumors and meningoencephaloceles. The advent of the vascularized flap reconstruction with the pericranial or nasoseptal flap has significantly decreased the incidence of postoperative CSF leaks to 5–6.5% [30, 36,37,38]. A meta-analysis comparing open resection and endoscopic resection of sinonasal malignancy did not find a significant difference in complication rates, including that of CSF leak [6, 9, 35]. Based on data from the last 20 years, a multilayer closure, preferably with vascularized tissue, has been successful, especially if radiation therapy is necessary.
Depending on the size of the tumor, extent of surgery, and repair technique, there should be routine surveillance and a high index of suspicion in the immediate postoperative period for the development of CSF rhinorrhea. Extent of surgery, especially entry into the arachnoid cisterns or ventricles, patient BMI, location of the defect, size of the defect, lack of vascularized reconstruction, and other comorbidities that affect poor tissue healing, increases the risk for postoperative CSF leak [30].
Symptoms suggestive of CSF leak include clear nasal drainage, salty taste, and dependent rhinorrhea. The diagnosis is confirmed by the presence of Beta-2 transferrin in the collected nasal fluid. CSF leaks occurring 2–6 weeks postoperatively are usually low flow leaks; however, they can develop acutely following an episode of Valsalva maneuver or nose blowing. This can be accompanied by pneumocephalus with onset of immediate headache. For patients presenting with this history, a computed tomography (CT) of the head should be performed to assess for pneumocephalus. If an active CSF leak is present but not identified, the risk of subsequent meningitis or other intracranial complication can be as high as 40% [39]. Therefore, early diagnosis and treatment is crucial. CSF rhinorrhea as a delayed complication can occur many weeks to months after surgery and is often associated with adjuvant radiation therapy [40].
Historically, CSF diversion with lumbar drains has been used to allow controlled, low resistance egress of CSF in the immediate postoperative period following skull base surgery, with the goal of reducing tension on the repair and decreasing the likelihood of postoperative CSF leak [41]. However, in recent meta-analyses and systematic reviews, there was no statistically significant difference in rates of CSF leak with respect to the use of lumbar drains [41, 42]. Furthermore, CSF leak rate was higher in the group with lumbar drains, which is likely explained by the propensity to place a lumbar drain in higher risk cases. Lumbar drains are associated with a 5% risk of minor complications, including meningitis, headache, cellulitis at the puncture site, and pneumocephalus, and a reported rate of 5% for major complications, including tension pneumocephalus, subdural hemorrhage, and uncal herniation [41, 43]. Additionally, the placement of a lumbar drain requires bedrest while in place, leading to a risk of deep vein thrombosis, as well as longer hospitalization and costs [42, 44]. Therefore, the current literature does not provide adequate evidence to support the routine use of lumbar drains following surgery requiring skull base resection. Anecdotally, lumbar drains should be considered in patients with a high risk for postoperative CSF leak, such as those with high-flow CSF leaks and intracranial hypertension. Patients with intracranial hypertension may ultimately require long-term management with acetazolamide or ventriculoperitoneal shunting [45]. Further studies to stratify patients’ risk of postoperative CSF leak and the benefit of CSF diversion would help clarify which patients are likely to benefit from placement of a lumbar drain at the time of surgery.
Once identified, postoperative CSF leaks should be definitively managed. Conservative treatment with bedrest and lumbar drain diversion can be considered, especially in cases of intracranial hypertension, early postoperative leaks, or to augment a poor or limited reconstruction [46]. Surgical exploration and definitive repair should be highly considered in all other cases and if conservative treatment fails. If the site of the CSF leak is not immediately obvious, intrathecal fluorescein can be utilized for assistance with localization with 93% sensitivity and 100% specificity. This intervention is especially useful if the CSF leak occurred in a delayed fashion [47].
Infection
The rates of meningitis and postoperative infection after endoscopic endonasal surgery ranges from 0.7% to 3.1%, as compared to 1.5% in open approaches [48,49,50,51]. The rate of postoperative meningitis after skull base surgery is correlated with CSF leak, which indicates a communication between the sinonasal cavity and the intracranial cavity through the surgical defect [52]. The risk of meningitis in a patient with postoperative CSF leak has been reported as 66%, as compared to a 4.5% risk in those patients without postoperative CSF leak [53]. This emphasizes the importance of proper skull base reconstruction after resection of sinonasal malignancy.
Postoperative infection in skull base surgery, whether performed through an open or endoscopic endonasal approach, can lead to poor healing of the reconstruction, flap dehiscence, wound infection, and necrosis. It has been well documented that prophylactic antibiotics decrease the risk of postoperative wound infection and the frequency of meningitis. There are many reports evaluating the best choice for antibiotic prophylaxis; however, no guidelines have been established because of the variability in surgical approach, surgeon and institution preference, and the overall low rates of infection associated with skull base surgery. In general, most surgeons use preoperative intravenous (IV) antibiotics [54] and continue them for 24–48 hours to help decrease rates of postoperative infection and meningitis. The type of IV antibiotic used is highly variable across institutions. The goal of prophylactic antibiotic use includes broad spectrum coverage of the common bacteria of the sinonasal cavity that could seed the intracranial space, while minimizing the unwanted sequelae often associated with antibiotics, including cost, development of multi-drug resistant organisms, and side effects of long-term antibiotic use, such as Clostridium difficile proliferation. There is variability in the use of antibiotics in the postoperative period, with a reported 51% using antibiotics where no nasal packing was placed and 88% using antibiotics with nonabsorbable packing [54]. While a 2017 meta-analysis of prophylactic systemic antibiotic use for patients with nasal packing did not show a significant reduction in infection rates associated with antibiotic use [55], in practice, many groups continue to use them due to the elevated risk of intracranial infection after surgery for sinonasal malignancy specifically [56]. Intracranial infection is a major consideration after skull base surgery, since there is a significant morbidity and mortality concern. Multi-institutional studies are needed to determine optimal guidelines for the use of prophylactic antibiotics.
Orbital Complications
Orbital involvement increases the staging of the sinonasal malignancy and increases the complexity of surgical planning and postoperative adjuvant therapy. Tumors with orbital involvement may require orbital exenteration for complete resection. Studies have shown that cases with orbital infiltration were likely to require an open surgical approach and were associated with higher in-hospital costs [9, 57]. Furthermore, orbital involvement reduced 5-year disease-specific survival from 78.0% to 44.4% and orbital soft tissue infiltration predicted significantly worse overall survival as compared to orbital bone infiltration [9, 58].
The orbit and optic nerve can be injured in the management of sinonasal malignancy because of their close proximity to the sinonasal cavity. In the last three decades of sinonasal malignancy management, there has been significant reduction in surgical complication rates; however, ocular complications have remained largely stable [59]. Ocular complications immediately after surgical management of sinonasal malignancies include orbital hematoma, diplopia due to transient and permanent cranial nerve III, IV, and VI palsy, and blindness. Surgical resection can also result in nasolacrimal duct dysfunction, resulting in the delayed development of nasolacrimal obstruction, epiphora, and dacryocystitis. Surgical resection with preservation of the orbit may also lead to secondary long-term complications associated with distortion of the anatomy, including enophthalmos and diplopia. Lid ectropion, epiphora from lid malposition, or canthal dystopia, ptosis, exposure keratitis and eventual loss of visual acuity can also develop in a delayed fashion [60]. Malposition and distortion of the eyelid anatomy can lead to trichiasis and blepharitis which can in turn have serious long-term consequences on eye health, including keratitis and corneal irritation, and eventual loss of visual acuity and chronic pain requiring orbital exenteration.
Orbital hematoma is a rare but potentially emergent complication of sinonasal and skull base surgery if not recognized immediately [60,61,63]. This occurs after vascular injury, either venous or arterial, leading to bleeding within the orbit, increase in intraocular pressure within the closed orbital space, and ischemic damage to the optic nerve and retina leading to vision changes and blindness [61]. The incidence of orbital hematoma in management of sinonasal malignancy may actually be lower than that encountered in routine endoscopic sinus surgery because surgery for sinonasal malignancy usually requires wide surgical exposure, and often removal of the lamina papyracea, which eliminates the closed space of the orbit [61]. Additionally, there may be preemptive ligation of the anterior and posterior ethmoid arteries, which are a common source of orbital hematoma. During surgery, there should be continuous monitoring of the orbit with vigilance for the development of an orbital hematoma. If suspected, the orbit can be examined and palpated for proptosis and firmness for early diagnosis, and an elevation in intraocular pressure (normally 10–20 mmHg) is confimatory [61]. Urgent conservative management includes orbital massage, administration of mannitol, and intravenous steroids can be considered. Ultimately, surgical intervention is usually necessary, especially with a rapidly expanding hematoma. Immediate management with lateral canthotomy and cantholysis can be performed, followed by surgical exploration, control of the bleeding source, and orbital decompression [61]. If recognized immediately, an orbital hematoma can be decompressed, avoiding the devastating complication of visual loss.
Delayed Complications
Orbital Complications
After traditional craniofacial resection of malignant sinonasal tumors, orbital complications were the most common type of delayed complication, defined as occurring at least 6 months after the end of treatment, and most frequently seen in patients who received postoperative radiation therapy [40]. Therefore, much of the literature on ocular complications after sinonasal malignancy treatment does not delineate whether the causative etiology is surgery, radiation therapy, or a combination of both. Additionally, this is a difficult distinction to make, especially with late onset complications and most patients receiving bimodality treatment.
Epiphora is the most common delayed orbital complication in patients receiving treatment for sinonasal and skull base malignancy, occurring at a rate of 22–36% [64]. This can occur as a direct complication of surgery. Transection of the nasolacrimal duct without stenting or other management carries a risk of epiphora of approximately 11% [65]. However, a larger portion of postoperative nasolacrimal duct dysfunction is observed at least 6 months following treatment in patients that also received radiation therapy [66]. The nasolacrimal duct and sac are lined with stratified squamous and pseudostratified columnar epithelium, similar to the oral cavity mucosa and upper respiratory tract, respectively [69]. Radiation therapy leads to thinning of the epithelium, loss of mucosal pliability, submucosal edema, generalized inflammation, and eventual desquamation. Thus, the changes in the nasolacrimal duct and sac epithelium secondary to radiation therapy can lead to obstruction and/or infection. Several options exist for the management of the nasolacrimal system. If transection of the nasolacrimal duct is anticipated, primary dacryocystorhinostomy (DCR) can be performed at the time of surgical resection. DCR along with other procedures, such as distal stenting, transcanalicular stenting, and marsupialization of the nasolacrimal duct, are additional options in the management of delayed epiphora [67, 68].
Beyond epiphora, other orbital complications exacerbated by radiation therapy include diplopia, optic neuropathy, retinopathy, keratopathy, and retinal hemorrhage. Radiation therapy causes injury to the lacrimal gland, like that of the salivary gland, leading to xerophthalmia, which can progress to foreign body sensation, corneal ulceration, and eventually decreased vision. This may in turn be severe enough to require orbital exenteration. Corneal complications can range from mild dry eye to perforation and blindness [70, 71]. Retinal and optic nerve complications are secondary to radiation-induced vitreous hemorrhage, neovascularization, exudates, nerve atrophy, and arterial thrombosis leading to hypoperfusion, which can develop as a delayed complication 1–3 years postirradiation [69]. Most orbital complications are well tolerated; however, it is important to counsel patients on potential development of these complications many years after the completion of treatment, even if the orbit is spared from surgery.
Chronic Rhinosinusitis
There are limited studies describing the development of chronic rhinosinusitis (CRS) after treatment for sinonasal malignancy. Depending on the size and location of the tumor, there may be evidence of rhinosinusitis prior to treatment as a direct result of secondary obstruction by the sinonasal mass. Additionally, surgical resection of the sinonasal malignancy can lead to distortion of the anatomy, scarring, poor mucociliary clearance, crusting, impaired olfaction, and nasal obstruction and congestion, which can significantly affect sinonasal health and quality of life [72]. All patients undergoing surgical resection of sinonasal malignancy will experience some degree of postoperative sinonasal symptoms and nasal crusting, with improvement over the course of 3 months or longer [71,72,74]. In order to minimize long-term sinonasal compromise, preservation of uninvolved sinonasal structures and mucosa should be considered when possible [75]. If the nasoseptal flap is utilized for repair, the donor site also comes with potential morbidity, including potential nasal crusting, necrosis of septal cartilage, loss of external nasal support, and flap necrosis, and careful surgical planning should be utilized to avoid this [76, 77].
Radiation therapy also results in sinonasal mucosal changes that can precipitate the development of CRS. Postradiation rhinosinusitis develops from epithelial cell degeneration and impairment and loss of mucociliary function leading to poor clearance of secretions, predisposing patients to upper respiratory infections and sinusitis [77,78,80]. The stratified rearrangement of the epithelial cells with loss of cytoplastic volume, ciliary loss, and dysmorphism can be observed even after 23 years in an irradiated patient [80]. The histopathology of radiation-induced CRS has been found to be different than that of other subtypes of CRS, exhibiting greater squamous metaplasia and subepithelial edema, decreased eosinophilia and basement membrane thickening [81].
In addition to postradiation changes of the targeted field and the immediate surrounding structures, the contralateral sinuses can also be affected. The incidence and degree of mucosal thickening are also significantly increased at 3 months postradiation in the contralateral sinuses, with the maxillary and anterior ethmoid sinus being the most affected [80, 82]. Patients with a history of treatment of sinonasal malignancy can develop secondary symptoms of nasal obstruction, purulent rhinorrhea, smell disturbance, facial pain/headache, with associated findings of significant crusting and sinonasal adhesions. If medical treatment fails to manage the CRS secondary to sinonasal malignancy treatment, these patients can benefit from endoscopic sinus surgery [83]. Thus, patient counseling and knowledge of delayed complications are essential in the comprehensive management of sinonasal malignancies .
Mucocele
Mucocele of the sinuses can develop as a rare, but late complication after treatment for sinonasal and skull base malignancy, ranging from 0% to 3.6% [84,85,86,87,88,89,90]. Mucocele is defined as an expansile collection of mucous within the paranasal sinuses with bony distortion secondary to distension, with possible compression of nearby neurovascular structures or the orbit [91]. Depending on the location of the mucocele, compression of the optic nerve, cranial nerves III, IV, VI, and within the cavernous sinus, with resultant cranial nerve palsies can occur [92,93,94]. Orbital compression can lead to facial deformity and visual changes, secondary to displacement of the globe. The most commonly involved sinuses are the frontal and ethmoid; however, sphenoid mucoceles have also been reported [91, 95,96,97]. Mucocele formation can also occur after sinonasal reconstruction at the site of flap inset if there is incomplete removal of the mucosa leading to entrapped mucosal glands between the reconstruction layers. Mucocele formation has been reported 2–6 years after surgery, and as early as the first 2 months postoperatively in patients with a history of sinonasal malignancy [84, 86]. Imaging is the optimal modality for the diagnosis of a mucocele. Postoperative mucocele formation can potentially be prevented by avoiding obstruction of sinus ostia and carefully removing all of the mucosa before the flap inset at the time of primary surgery [77, 98]. Wide surgical marsupialization of the mucocele cavity, either endoscopic or open, is the gold standard of treatment, allowing adequate drainage and minimizing recurrence [99,100,101].
Smell and Taste Disturbance
Olfactory dysfunction can significantly impact quality of life by decreasing food enjoyment, leading to nutritional deficits, and may pose a safety hazard when smoke or spoiled foods cannot be detected. Sinonasal surgery can directly traumatize olfactory structures, depending on the location of the primary tumor, but can also cause associated changes in airflow and the inflammatory milieu that can have indirect effects on olfaction [102]. While studies have shown conflicting long-term outcomes for skull base surgery patients, a large study performed by Kim et al. reviewed 226 patients undergoing skull base surgery for neoplasm and found significant decreases in all smell scoring systems tested [103].
In order for patients to perceive smell, chemical compounds in odorants need to reach the olfactory fibers at the olfactory groove. Therefore, physical obstruction of this area caused by scarring and synechiae secondary to surgery can lead to hyposmia or anosmia [104]. As a result of surgery, there may also be resection of the olfactory epithelium and bulb, which would also lead to anosmia secondary to loss of nerve fibers. Reconstruction of the skull base with nasoseptal flap has been shown to affect smell identification test scores temporarily in the immediate postoperative period, however, with return of scores to baseline at 3 and 6 months [89, 105]. Another study reported a difference in preoperative smell function as compared postoperatively in midline skull base tumors including craniopharyngiomas and pituitary tumors [106]. This reflects the variability in tumor pathology that may diminish olfaction preoperatively and the degree of olfactory cleft and skull base resection, which would affect olfaction postoperatively. In an attempt to preserve olfaction, preserving 1 cm of septal mucosa superiorly when raising the nasoseptal flap for skull base reconstruction may preserve the olfactory epithelium [103, 107, 108]. Radiation therapy can further affect olfactory and taste function, and this has been well-studied in head and neck cancer patients [109]. Those treated with radiation suffer from smell disturbance that ranges from partial to complete smell loss, poor odor identification and discrimination, and phantosmia. There is a decrease in odor detection threshold with cumulative radiation of 0.8 Gy and 50% of patients have smell impairment when reaching a dose of 40 Gy to the olfactory area [109].
Wound Healing
Late-stage wound complications can occur as a consequence of both surgical management and adjuvant radiation for sinonasal malignancy. As nasoseptal flaps are commonly employed for the closure of skull base defects, nasal synechiae can form in the postoperative period arising from the donor area of the septum and attaching to the lateral nasal wall and inferior turbinate. These synechiae occur with a reported incidence of 9–20% [110, 111]. Regular postoperative use of nasal saline irrigations, in addition to nasal splinting in the postoperative period, can potentially ameliorate this complication. Choanal stenosis can also occur, especially if the posterior choana is adjacent to or involved by disease and requires maximum radiation dosage to be delivered to this area [112]. Circumferential scarring of the choana due to fibrosis results in acquired choanal stenosis, and can be managed surgically followed by stent placement.
Nasocutaneous fistula , a severe wound complication, occur in 3.6% of patients treated for sinonasal malignancy [113]. This complication occurs primarily in patients with a transfacial surgical incision; patients receiving radiation alone do not often develop fistulas [114]. Squamous cell carcinoma pathology, increased tumor stage, and adjuvant radiation therapy all increase the risk of nasocutaneous fistula formation. When a fistula develops, surgical treatment is often necessary to correct the defect. However, surrounding tissues have often been radiated as well, which can pose unique challenges when considering reconstructive options.
A rare but important delayed complication in patients with sinonasal malignancy is the development of osteoradionecrosis (ORN) of the skull base, which is most commonly reported in patients requiring multiple courses of radiation [115]. Accumulated dose of radiation is typically over 100 Gy in this patient population [116]. Rates of ORN are significantly higher in larger tumors due to the increased size of the radiation field during adjuvant therapy [117]. Skull base ORN most frequently is reported in the sphenoid area, followed by the clivus and surrounding the internal carotid canal. Patients typically present with headache, intermittent epistaxis, nasal crusting, and foul odor emanating from the nose. Chronic rhinosinusitis may exist both as a comorbid condition and as a possible exacerbating factor in the etiology of ORN development [116, 118]. When ORN is suspected in a patient treated for sinonasal malignancy, a thorough evaluation with imaging and possibly biopsy may be required to rule out recurrence before the final diagnosis of ORN is made.
Conclusion
Surgical intervention for sinonasal and skull base malignancies has evolved tremendously over the past several decades, as increased access with novel tools and techniques has allowed for a more comprehensive approach. The proximity of the sinonasal cavity to critical neurovascular structures and the orbit makes treatment with surgery and radiation therapy challenging. The surgeon should have a thorough understanding of the possible complications that may arise after treatment and preserve a high index of suspicion. Early diagnosis of most complications and prompt management can prevent permanent serious sequelae. Patients should be thoroughly counseled on the potential complications that may arise with surgery for sinonasal malignancy and adjuvant therapy. Because of the rare nature of sinonasal malignancies and the variability in the tumor, management of complications after treatment should be individualized to the patient. However, general principles should be used to guide management, including clearance of infection, creating a barrier between the intracranial and the sinonasal cavity, and preserving and restoring function of the sinonasal cavity.
References
Silverberg E, Grant R. Cancer statistics, 1970. CA Cancer J Clin. 1970;20:11–23.
Roush G. Epidemiology of cancer of the nose and paranasal sinuses: current concepts. Head Neck Surg. 1979;2:3–11.
Kuijpens J, Louwman M, Peters R, et al. Trends in sinonasal cancer in the Netherlands: more squamous cell cancer, less adenocarcinoma. A population-based study 1973–2009. Eur J Cancer. 2012;48:2369–74.
Haerle S, Gullane P, Witterick I, Zweifel C, Gentili F. Sinonasal carcinomas: epidemiology, pathology, and management. Neurosurg Clin N Am. 2013;24(1):34–49.
Ayiomamitis A, Parker L, Havas T. The epidemiology of malignant neoplasms of the nasal cavities, the paranasal sinuses and the middle ear in Canada. Arch Otorhinolaryngol. 1988;244:367–71.
Lu VM, Ravindran K, Phan K, et al. Surgical outcomes of endoscopic versus open resection for primary sinonasal malignancy: a meta-analysis. Am J Rhinol Allergy. 2019;33(5):608–16.
Ganly I, Patel S, Bilsky M, Shah J, Kraus D. Complications of craniofacial resection for malignant tumors of the skull base: report of an international collaborative study. Otolaryngol Head Neck Surg. 2009;140:218–23.
Kutlay M, Durmaz A, Özer İ, et al. Extended endoscopic endonasal approach to the ventral skull base lesions. Clin Neurol Neurosurg. 2018;167:129–40.
Hagemann J, Roesner J, Helling S, et al. Long-term outcome for open and endoscopically resected sinonasal tumors. Otolaryngol Head Neck Surg. 2019;160(5):862–9.
Kassam AB, Prevedello DM, Carrau RL, et al. Endoscopic endonasal skull base surgery: analysis of complications in the authors’ initial 800 patients. J Neurosurg. 2010;114(6):1544–68.
Gardner PA, Tormenti MJ, Pant H, Fernandez-Miranda JC, Snyderman CH, Horowitz MB. Carotid artery injury during endoscopic endonasal skull base surgery: incidence and outcomes. Neurosurgery. 2013;73(SUPPL. 2):261–70.
Cappabianca P, Cavallo L, Colao A, de Divitiis E. Surgical complications associated with the endscopic endonasal transsphenoidal approach for pituitary adenomas. J Neurosurg. 2002;97:293–8.
Dehdashti A, Ganna A, Karabatsou K, Gentili F. Pure endoscopic endonasal approach for pituitary adenomas: early surgical results in 200 patients and comparison with previous microsurgical series. Neurosurgery. 2008;62:1006–17.
Fatemi N, Dusick J, de Paiva NM, Kelly D. The endonasal microscopic approach for pituitary adenomas and other parasellar tumors: a 10-year experience. Neurosurgery. 2008;63:244–56.
Feiz-Erfan I, Han P, Spetzler R, et al. The radical transbasal approach for resec- tion of anterior and midline skull base lesions. J Neurosurg. 2005;103(3):485–90.
Sekhar L, Pranatartiharan R, Chanda A, Wright D. Chordomas and chondrosarcomas of the skull base: results and complications of surgical management. Neurosurg Focus. 2001;10(3):E2.
Gardner P, Snyderman C, Fernandez-Miranda J, Jankowitz B. Management of major vascular injury during endoscopic endonasal skull base surgery. Otolaryngol Clin N Am. 2016;49:819–28.
Wang W-H, Lieber S, Lan M. Nasopharyngeal muscle patch for the management of internal carotid artery injury in endoscopic endonasal surgery. J Neurosurg. 2019;18:1–6.
Padhye V, Valentine R, Sacks R, et al. Coping with catastrophe: the value of endoscopic vascular injury training. Int. 2015;5(3):247–52.
Valentine R, Wormald P. A vascular catastrophe during endonasal surgery: an endoscopic sheep model. Skull Base. 2011;1(212):109–14.
Valentine R, Wormald P. Controlling the surgical field during a large endoscopic vascular injury. Laryngoscope. 2011;121(3):562–6.
Couldwell WT, Weiss M, Rabb C, Liu J, Apfelbaum R, Fukushima T. Variations on the standard transsphenoidal approach to the sellar region, with emphasis on the extended approaches and parasellar approaches: surgical experience in 105 cases. Neurosurgery. 2004;55(3):539–50.
Yin C, Chen B. Tension pneumocephalus from skull base surgery: a case report and review of the literature. Surg Neurol Int. 2018;9:128.
Chou S, Ning M, Buonanno F. Focal intraparenchymal tension pneumocephalus. Neurology. 2006;67:1465.
Horowitz B. An unusual complication following mastoid surgery. J Laryngol Otol. 1964;78:128–34.
Aksoy F, Dogan R, Ozturan O, Tu S. Tension pneumocephalus: an extremely small defect leading to an extremely serious problem. Am J Otolaryngol. 2013;34(6):749–52.
Goldmann R. Pneumocephalus as a consequence of barotrauma. JAMA. 1986;255:3154–6.
Hong B, Biertz F, Raab P, et al. Normobaric hyperoxia for treatment of pneumocephalus after posterior fossa surgery in the semisitting position: a prospective randomized controlled trial. PLoS One. 2015;10:e0125710.
Neligan P, Mulholland S, Irish J, et al. Flap selection in cranial base reconstruction. Plast Reconstr Surg. 1996;98(7):1159–66.
Zanation AM, Thorp BD, Parmar P, Harvey RJ. Reconstructive options for endoscopic Skull Base surgery. Otolaryngol Clin N Am. 2011;44(5):1201–22.
Naunheim M, Goyal N, Dedmon M. An algorithm for surgical approach to the anterior skull base. J Neurol Surg B. 2016;77:364–70.
Shemesh R, Alon EE, Gluck I, Yakirevitch A. Endoscopic surgery for delayed sinonasal complications of radiation therapy for nasopharyngeal carcinoma: a subjective outcome. Int J Radiat Oncol Biol Phys. 2018;100(5):1222–7.
Gallia GL, Asemota AO, Blitz AM, et al. Endonasal endoscopic resection of olfactory neuroblastoma: an 11-year experience. J Neurosurg. 2018;131:1–7.
Ganly I, Pagel S, Singh B, et al. Complications of craniofacial ressection for malignant tumors of the skull base: report of an international collaborative study. Head Neck. 2005;27(6):445–51.
Hanna E, Ibrahim S, Roberts D, Kupferman M, DeMonte F, Levine N. Endoscopic resection of sinonasal cancers with and without craniotomy: oncologic results. Arch Otolaryngol Head Neck Surg. 2009;135(12):1219–24.
Jackson I, Adham M, Marsh W. Use of the galeal frontalis myofascial flap in craniofacial surgery. Plast Reconstr Surg. 1986;77(6):905–10.
Synderman C, Janecka I, Sekhar L, Sen C, Eibling D. Anterior cranial base reconstruction: role of galeal and pericranial flaps. Laryngoscope. 1990;100(6):607–14.
Hadad G, Bassagasteguy L, Carrau R, et al. A novel reconstructive technique after endoscopic expanded endonasal approaches: vascular pedicle nasoseptal flap. Laryngoscope. 2006;116(10):1882–6.
Harvey R, Smith J, Wise S, et al. Intracranial complications before and after endoscopic skull base reconstruction. Am J Rhinol. 2008;22(5):516–21.
Gray ST, Lin A, Curry WT, et al. Delayed complications after anterior craniofacial resection of malignant skull base tumors. J Neurol Surg B Skull Base. 2014;75(2):110–6.
D’Anza B, Tien D, Stokken J, Recinos P, Woodard T, Sindwani R. Role of lumbar drains in contemporary endonasal skull base surgery: meta-analysis and systematic review. Am J Rhinol Allergy. 2016;30(6):430–5.
Ahmed OH, Marcus S, Tauber JR, Wang B, Fang Y, Lebowitz RA. Efficacy of perioperative lumbar drainage following endonasal endoscopic cerebrospinal fluid leak repair: a meta-analysis. Otolaryngol Head Neck Surg. 2017;156(1):52–60.
Governale L, Fein N, Logsdon J, Black P. Techniques and complicationsof external lumbar drainage for normal pressure hydrocephalus. Neurosurgery. 2008;63:379–84.
Zuckerman J, DelGaudio J. Utility of preoperative high- resolution CT and intraoperative image guidance in identification of cerebrospinal fluid leaks for endoscopic repair. Am J Rhinol. 2008;22:151–4.
Woodworth BA, Prince A, Chiu AG, et al. Spontaneous CSF leaks: a paradigm for definitive repair and management of intracranial hypertension. YMHN. 2008;138(6):715–20.
Stokken J, Recinos P, Woodard T, Sindwani R. The utility of lumbar drains in modern endoscopic skull base surgery. Curr Opin Otolaryngol Head Neck Surg. 2015;23(1):78–82.
Raza SM, Banu MA, Donaldson A, Patel KS, Anand VK, Schwartz TH. Sensitivity and specificity of intrathecal fluorescein and white light excitation for detecting intraoperative cerebrospinal fluid leak in endoscopic skull base surger: a prospective study. J Neurosurg. 2016;124(3):621–6.
Dumont A, Nemergut EC, Jane JA, Laws ER. Postoperative care following pituitary surgery. J Intensive Care Med. 2005;20:127–40.
van Aken M, Feelders R, de Marie S, et al. Cerebrospinal fluid leakage during transsphenoidal surgery: postoperative external lumbar drainage reduces the risk for meningitis. Pituitary. 2004;7:89–93.
Korinek A, Baugnon T, Golmard J, et al. Risk factors for adult nosocomial meningitis after craniotomy role of antibiotic prophylaxis. Neurosurgery. 2006;59:126–33.
Kono Y, Prevedello D, Snyderman C, et al. One thousand endoscopic skull base surgical procedures demystifying the infection potential: incidence and description of postoperative meningitis and brain abscesses. Infect Control Hops Epidemiol. 2011;32:77–83.
Johans S, Burkett D, Swong K, et al. Antibiotic prophylaxis and infection prevention for endoscopic endonasal skull base surgery: our protocol, results, and review of the literature. J Clin Neurosci. 2018;47:249–53.
Horowitz G, Fliss D, Margalit N, et al. Association between cerebrospinal fluid leak and meningitis after skull base surgery. Otolaryngol Head Neck Surg. 2011;145:689–93.
Roxbury C, Lobo B, Kshettry V, et al. Perioperative management in endoscopic endonasal skull-base surgery: a survey of the North American Skull Base Society. Int Forum Allergy Rhinol. 2018;8(5):631–40.
Lange J, Peeden E, Stringer S. Are prophylactic systemic antibiotis necessary with nasal packing? A systematic review. Am J Rhinol Allergy. 2017;31(4):240–7.
Deng ZY, Tang AZ. Bacteriology of postradiotherapy chronic rhinosinusitis in nasopharyngeal carcinoma patients and chronic rhinosinusitis. Eur Arch Oto-Rhino-Laryngol. 2009;266(9):1403–7.
Fu T, Monteiro E, Almeida J, et al. Costs and perioperative outcomes associated with open versus endoscopic resection of sinonasal malignancies with skull base involvement. J Neurol Surg B. 2017;78:430–40.
Ganly I, Patel SG, Singh B, et al. Craniofacial resection for malignant melanoma of the skull base: report of an International Collaborative tudy. Arch Otolaryngol Head Neck Surg. 2006;132(1):73–8.
Gil Z, Patel SG, Bilsky M, Shah JP, Kraus DH. Complications after craniofacial resection for malignant tumors: are complication trends changing? Otolaryngol Head Neck Surg. 2009;140(2):218–23.
Neel GS, Nagel TH, Hoxworth JM, et al. Management of orbital involvement in sinonasal and ventral skull base malignancies. Otolaryngol Clin N Am. 2017;50(2):347–64.
Ransom E, Chiu A. Prevention and management of complications in intracranial endoscopic skull base surgery. Otolaryngol Clin NA. 2010;43(4):875–95.
Pelausa E, Smith K, Dempsey I. Orbital complications in functional endoscopic sinus surgery. J Otolaryngol. 1995;24(3):154–9.
May M, Levine H, Mester S, Schaitkin B. Complications of endoscopic sinus surgery: analysis of 2108 patients—incidence and prevention. Laryngoscope. 1994;104(9):1080–3.
Andersen P, Kraus D, Arbit E, Shah J. Management of the orbit during anterior fossa craniofacial resection. Arch Otolaryngol Head Neck Surg. 1996;122(12):1305–7.
Rotsides J, Franco A, Albader A, et al. Nasolacrimal duct management during endoscopic sinus and skull base surgery. Ann Otol Rhinol Laryngol. 2019;128(10):932–7.
Gray S, Lin A, Curry W, et al. Delayed complications after anterior craniofacial resection of malignant skull base tumors. J Neurol Surg B Skull Base. 2014;75:110–6.
Yeo N, Wang J, Chung Y, et al. Contributing factors to prevent prolonged epiphora after maxillectomy. Arch Otolaryngol Head Neck Surg. 2010;136(3):229–33.
Himwich W, Spurgeon H. Pulse pressure contours in cerebral arteries. Acta Neurol Scand. 1968;44(1):43–56.
Nakissa N, Rubin P, Strohl R, et al. Ocular and orbital complications following radiation therapy of paranasal sinus maljgnancies and review of literature. Cancer. 1983;51:980–6.
Blodi F. The late effects of x-radiation on the cornea. Trans Am Opthalmol Soc. 1958;56:413–50.
Durkin S, Roos D, Higgs B, et al. Ophthalmic and adnexal complications of radiotherapy. Acta Ophthalmol Scand. 2006;85:240–50.
Bhenswala P, Schlosser R, Nguyen S, et al. Sinonasal quality-of-life outcomes after endoscopic endonasal skull base surgery. Int Forum Allergy Rhinol. 2019;9(10):1105–18.
Gallagher M, Durnford A, Wahab S, et al. Patient-reported nasal morbidity following endoscopic endonasal skull base surgery. Br J Neurosurg. 2014;28(5):622–5.
de Almeida J, Snyderman C, Gardner P, et al. Nasal morbidity following endoscopic skull base surgery: a prospective cohort study. Head Neck. 2011;33(4):547–51.
Kuhn F, Citardi M. Advances in postoperative care following functional endoscopic sinus surgery. Otolaryngol Clin N Am. 1997;30:479–90.
Chabot J, Patel C, Hughes M, et al. Nasoseptal flap necrosis: a rare complication of endoscopic endonasal surgery. J Neurosurg. 2018;128(5):1463–72.
Lavigne P, Faden D, Wang E, Synderman C. Complications of nasoseptal flap reconstruction: a systematic review. J Neurol Surg B Skull Base. 2018;79(Suppl 4):S291–9.
Huang CC, Huang SF, Lee TJ, Ng SH, Chang JTC. Postirradiation sinus mucosa disease in nasopharyngeal carcinoma patients. Laryngoscope. 2007;117(4):737–42.
Surico G, Muggeo P, Mappa L, et al. Impairment of nasal mucociliary clearance after radiotherapy for childhood head cancer. Head Neck. 2001;23:461–6.
Lou P, Chen W, Tai C. Delayed irradiation effects on nasal epithelium in patients with nasopharyngeal carcinoma. An ultrastructural study. Ann Otol Rhinol Laryngol. 1999;108(5):474–80.
Kuhar H, Tajudeen B, Heilingoetter A, et al. Distinct histopathologic features of radiation-induced chronic sinusitis. Int Forum Allergy Rhinol. 2017;7(10):990–8.
Maxfield A, Chambers K, Sedaghat A, et al. Mucosal thickening occurs in contralateral paranasal sinuses following sinonasal malignancy treatment. J Neurol Surg Part B Skull Base. 2017;78(4):331–6.
Gray ST, Sadow PM, Lin DT, Sedaghat AR. Endoscopic sinus surgery for chronic rhinosinusitis in patients previously treated for sinonasal malignancy. Laryngoscope. 2016;126(February):304–15.
Bleier B, Wang E, Vandergrift W 3rd, Schlosser R. Mucocele rate after endoscopic skull base reconstruction using vascularized pedicled flaps. Am J Rhinol Allergy. 2011;25(3):186–7.
Vaezeafshar R, Hwang P, Harsh G, Turner J. Mucocele formation under pedicled nasoseptal flap. Am J Otolaryngol. 2012;33(5):634–6.
Husain Q, Sanghvi S, Kovalerchik O, et al. Assessment of mucocele formation after endoscopic nasoseptal flap reconstruction of skull base defects. Allergy Rhinol (Providence). 2013;4(1):e27–31.
Nyquest C, Anand V, Singh A, Schwartz T. Janus flap: bilateral nasoseptal flap for anterior skull base reconstruction. Otolaryngol Head Neck Surg. 2010;142:327–31.
McCoul E, Anand V, Singh A, et al. Long-term effectiveness of a reconstructive protocol using the nasoseptal flap after endoscopic skull base surgery. World Neurosurg. 2014;81(1):136–43.
Soudry E, Psaltis A, Lee K, et al. Complication associated with the pedicled nasoseptal flap for skull base reconstruction. Laryngoscope. 2015;125(01):80–5.
Dolci R, Miyake M, Tateno D, et al. Postoperative otorhinolaryngologic complications in transnasal endoscopic surgery to assess the skull base. Braz J Otorhinolaryngol. 2017;83:349–55.
Janakiram T, Karunasagar A. Sphenoid mucocele: a complication of skull base reconstruction with nasoseptal flap – a critical review and our experience. Indian J Otolaryngol Head Neck Surg. 2019;71(Suppl 3):2151–6.
Moriyama H, Hesaka H, Tachibana T, et al. Mucoceles of ethmoid and sphenoid sinus with visual disturbance. Arch Otolaryngol Head Neck Surg. 1992;118:142–6.
Lee L, Huang C, Lee T. Prolonged visual disturbance seconadry to isolated sphenoid sinus disease. Laryngoscope. 2004;114(6):986–90.
Sethi D, Lau D, Chan C. Sphenoid sinus mucocele presenting with isolated oculomotor nerve palsy. J Laryngol Otol. 1997;111:471–3.
Kosling S, Hinter M, Brandt S, et al. Mucoceles of the sphenoid sinus. Eur J Radiol. 2004;51:1–5.
Hantzakos A, Dowley A, Yung M. Sphenoid sinus mucocele: late complication of sphenoidotomy. J Laryngol Otol. 2003;117:561–3.
Delfini R, Missori P, Iannetti G, et al. Mucocles of the paranasal sinuses with intracranial andn intraorbital extension: report of 28 cases. Neurosurgery. 1993;32:901–6.
Wang L, Kim J, Heilman C. Intracranial mucocele as a complication of endoscopic repair of cerebrospinal fluid rhinorrhea: case report. Neurosurgery. 1999;45(5):1243–5.
Har-El G. Endoscopic management of 108 sinus mucoceles. Laryngoscope. 2001;111:2131–4.
Lund V. Endoscopic management of paranasal sinus mucoceles. J Laryngol Otol. 1998;112:36–40.
Benkhatar H, Khettab I, Sultanik P, et al. Mucocele development after endoscopic sinus surgery for nasal polyposis: a long-term analysis. Ear Nose Throat J. 2018;97(9):284–94.
Patel Z, DelGaudio J. Olfaction following endoscopic skull base surgery. Curr Opin Otolaryngol Head Neck Surg. 2016;24(1):70–4.
Kim B, Kang S, Kim S, et al. Olfactory changes after endoscopic endonasal transsphenoidal approach for skull base tumors. Laryngoscope. 2014;124(11):2470–5.
Ho W-k. Change in olfaction after radiotherapy for nasopharyngeal cancer – a prospective study. Am J Otolaryngol Head Neck Med Surg. 2002;23(4):209–14.
Upadhyay S, Buohliqah L, Dolci R, et al. Periodic olfactory assessment in patients undergoing skull base surgery with preservation of the olfactory strip. Laryngoscope. 2017;127(09):1970–5.
Soyka M, Serra C, Regli L, et al. Long-term olfactory outcome after nasoseptal flap reconstructions in midline skull base surgery. Am J Rhinol Allergy. 2017;31(5):334–7.
Rotenberg B, Saunders S, Duggal N. Olfactory outcomes after endoscopic transsphenoidal pituitary surgery. Laryngoscope. 2011;121:1611–3.
Tam S, Duggal N, Rogenberg B. Olfactory outcomes following endoscopic pituitary surgery with or without septal flap reconstruction: a randomized controlled trial. Int Forum Allergy Rhinol. 2013;3:62–5.
Álvarez-Camacho M, Gonella S, Campbell S, Scrimger RA, Wismer WV. A systematic review of smell alterations after radiotherapy for head and neck cancer. Cancer Treat Rev. 2017;54:110–21.
Koren I, Hadar T, Rappaport Z, Yaniv E. Endoscopic transnasal transsphenoidal microsurgery versus the sublabial approach for the treatment of pituitary tumors: endonasal complications. Laryngoscope. 1999;109(11):1838–40.
Pant H, Bhatki A, Snyderman C, et al. Quality of life following endonasal skull base surgery. Skull Base. 2010;20(1):35–40.
Alon E, Lipschitz N, Bedrin L, et al. Delayed sino-nasal complications of radiotherapy for nasopharyngeal carcinoma. Otolaryngol Head Neck Surg. 2014;151(2):354–8.
Waldron J, O’Sullivan B, Gullane P, et al. Carcinoma of the maxillary antrum: a retrospective analysis of 110 cases. Radiother Oncol. 2000;57(2):167–73.
Cianchetti M, Varvares M, Deschler D, et al. Risk of sinonasal-cutaneous fistula after treatment for advanced sinonasal cancer. J Surg Oncol. 2012;105(3):261–5.
Hua Y, Chen M, Qian C, et al. Postradiation nasopharyngeal necrosis in the patients with nasopharyngeal carcinoma. Head Neck. 2009;31(6):807–12.
Huang X, Zheng Y, Zhang X, et al. Diagnosis of management of skull base osteoradionecrosis after radiotherapy for nasopharyngeal carcinoma. Laryngoscope. 2006;116(9):1626–31.
Han P, Wang X, Liang F, et al. Osteoradionecrosis of the skull base in nasopharyngeal carcinoma: incidence and risk factors. Int J Radiat Oncol Biol Phys. 2018;102(3):552–5.
Liu J, Ning X, Sun X, et al. Endoscopic sequestrectomy for skull base osteoradionecrosis in nasopharyngeal carcinoma patients: a 10-year experience. Int J Clin Oncol. 2019;24(3):248–55.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Maxfield, A.Z., Workman, A., Gray, S.T. (2022). Complications of Surgical Management of Skull Base and Sinonasal Malignancies. In: Saba, N.F., Lin, D.T. (eds) Sinonasal and Skull Base Malignancies. Springer, Cham. https://doi.org/10.1007/978-3-030-97618-7_10
Download citation
DOI: https://doi.org/10.1007/978-3-030-97618-7_10
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-97617-0
Online ISBN: 978-3-030-97618-7
eBook Packages: MedicineMedicine (R0)