Abstract
Volcanic soils are widely distributed in the Hokkaido prefecture of Japan, where seasonal freezing and thawing occur. In frozen soils, a portion of pore water remains unfrozen, due to the effects of capillarity, adsorption, and possibly solute. The variation of the amount of unfrozen water in a frozen soil, which is primarily influenced by subzero temperature, has great impacts on the physical and mechanical behavior of the soil. In the present study, the soil-freezing characteristic curve (SFCC) of a typical volcanic soil sampled in Hokkaido, namely, the Komaoka soil, was investigated. The unfrozen water content of the prepared Komaoka soil specimens was measured using a cheap and convenient moisture sensor. The temperature of the specimens was determined by a rugged temperature sensor. Different number of freeze-thaw (F-T) cycles, and different freezing/thawing methods (i.e. one- and three-dimensional) were considered, and their effects on the SFCC were investigated. The experimental results suggest that neither of the F-T cycles nor the freezing/thawing methods had significant influence on the measured SFCC. The present study can contribute to understanding the freezing characteristic of the investigated soil in specific and that of other cold region soils in general.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
The unfrozen water and pore ice coexist within a frozen soil. The unfrozen water exists in small pore spaces and as thin films adsorbed on the surfaces of soil particles in equilibrium with the pore ice at subzero temperatures. The soil-freezing characteristic curve (SFCC) describes the relationship between the amount of unfrozen water and its energy state or subzero temperature in a frozen soil (Koopmans and Miller 1966; Spaans and Baker 1996; Azmatch et al. 2012; Watanabe and Osada 2016; Schafer 2018). This relationship has also been referred to as the phase composition curve (PCC) (Anderson and Tice 1972; Liu and Yu 2014), unfrozen water characteristic curve (UWCC) (Mu et al. 2018), unfrozen water content curve (Kozlowski 2003), soil freezing curve (SFC) (Grant and Sletten 2002), freezing characteristic curve (Smith 1985), and soil freezing retention curve (SFRC) (Mao et al. 2018).
The SFCC links the degree of phase transition to the subzero temperature in a frozen soil. Therefore, many important physical and mechanical properties of frozen soils can be estimated using SFCC. In addition, the constitutive relationships for hydraulic, thermal, and mechanical fields of frozen soils are functions of the quantity of unfrozen water, the SFCC is therefore essential to modeling the transport mechanism of water, heat, and solutes in frozen soils (Spaans and Baker 1996; Zhang et al. 2016; Yu et al. 2018; Xu et al. 2020).
The SFCC of various coarse- and fine-grained soils have been investigated in the literature. The present study focuses on the experimental determination of the SFCC of a volcanic soil. Volcanic soils cover 1% of the Earth’s surface (or more than 124 million hectares) yet support 10% of the world’s population, including some of the highest human population densities (Neall 2009). One of the major areas of volcanic soils rim the Pacific and occur in countries such as Japan, Philippines, United States, Ecuador, and Peru. In the Hokkaido of Japan, there are over 40 Quaternary volcanoes, and pyroclastic materials cover over 40% of these areas. Significant volcanic activity occurred in the Neogene’s Quaternary period, and various pyroclastic materials, such as volcanic ash, pumice and scoria, formed during those eruptions (Kawamura and Miura 2013). The volcanic soils show different behavior from that of clay or sand, and have been classified as problematic soils in 1980s, according to IS-Tohoku98 (Kawamura and Miura 2013). The Komaoka soil, which is a typical volcanic soil found in Hokkaido, is investigated in the present study. The original soil sample was sampled from the ejectas of the Shikotsu calderas near the Sapporo city, as shown in Fig. 1.
2 Experimental Setup for SFCC Measurement
The Komaoka soil contains a considerable amount of highly porous particles. These highly porous particles are weak and easy to break by hands. Many pores can be seen by naked eyes. This largely contributes to the low bulk density of the Komaoka soil. For example, the in-situ dry density of the Komaoka soil is around 0.794 g/cm3 (Nguyen 2017). In the present study, the soil particles with sizes smaller than 2 mm were used for the SFCC test. The basic properties of the Komaoka soil is summarized in Table 1, and its gradation curve is shown in Fig. 2. The Komaoka soil has a very low fraction of clay and is a non-plastic coarse-grained soil. For comparison, the gradation curve of the Japanese standard sand, Toyoura sand, is shown in Fig. 2. The Toyoura sand has a specific gravity of 2.65 and zero percentage of fines (Ishikawa et al. 2013).
2.1 Experimental Setup
The procedures for preparing soil specimen and conducting SFCC measurement are succinctly described below.
-
(1)
A certain amount of dry soils and distilled water were hand mixed, and stored in plastic bags for achieving uniform moisture distribution. The soil specimen was compacted in an acrylic cylinder (Fig. 3(a)), at the gravimetric water content of 30% and to a dry density around 0.915 g/cm3 (see Table 1). The soil specimen was compacted into four layers by volume control method, with each layer of 25 mm thick. The final dimension of the soil specimen was approximately 100 mm in height and 49.6 mm in diameter. After compaction, the mass of the compacted specimen was recorded.
-
(2)
The compacted soil specimen was kept in the acrylic cylinder. Different amounts of distilled water can be added to the specimen for achieving different post-compaction water contents. This facilitate the SFCC measurement on specimens with different initial water contents. Since the Komaoka soil is a non-plastic coarse-grained soil, the volume change during the wetting process was assumed insignificant. A trial test has been performed to saturate the compacted specimen. As schematically shown in Fig. 3(b), filter papers and porous stones were put on the top and bottom of the specimen. The specimen was then transferred to a container with de-aired distilled water and subjected to vacuum for full saturation for 12 h. However, it was found that the specimen collapsed after saturation, due to its weak water retention ability.
-
(3)
The prepared soil specimen was sealed by plastic bag for waterproof (as the specimen will be submerged in antifreeze liquid). One EC-5 moisture sensor (METER Group, Inc. USA) and one RT-1 temperature sensor (METER Group) were inserted into the specimen, and had good contact with the specimen. The two sensors were connected to an EM50 datalogger (METER Group). The time interval for water content and temperature measurements was generally 15 min. Figure 4(a) is an example showing the prepared specimens ready for SFCC test. The EC-5 moisture sensor and RT-1 temperature sensor were calibrated prior to their use, as summarized in the following section. It is assumed that the EC-5 sensor measures the volumetric water content of the bulk specimen. In other words, the sampling volume of the EC-5 sensor is equal to (or within) the volume of the specimen. Therefore, the effect of the surrounding antifreeze liquid on EC-5 reading is insignificant.
-
(4)
The specimen was then submerged in antifreeze liquid inside a low-temperature bath for SFCC measurement, as shown in Fig. 3(c) and Fig. 4(b). The temperature of the antifreeze liquid can be controlled and different temperature paths (e.g., freezing and thawing) were exerted on the soil specimen for determining SFCC. Each controlled temperature was maintained for a sufficient period of time to approximate to thermodynamic equilibrium condition, which facilitates the measurement of SFCC.
-
(5)
After the SFCC test, the total water content of the specimen was determined by oven dry method.
2.2 Sensor Calibration
2.2.1 Temperature Sensor Calibration
The calibration for the RT-1 temperature sensors was fulfilled by referring their measured temperature values to those measured by a thermometer. A couple of different temperature values were selected. The result is shown in Fig. 5. It can be seen that the measured values by the temperature sensors are closely located on or near the 1:1 line. Therefore, it is assumed that the temperature sensors are accurate.
2.2.2 EC-5 Calibration Under Unfrozen Condition
For the reliable measurement of volumetric (unfrozen) water content, the specific calibration of the EC-5 moisture sensor for the Komaoka soil is required. In the present study, the calibration of the EC-5 sensor under room temperature was carried out. More than 20 specimens were prepared for this purpose. The specimens were compacted at the gravimetric water content of 30% and to a dry density around 0.915 g/cm3 (by following the same procedures described above), as shown in Fig. 6(a). After compaction, several specimens were subjected to drying or wetting, by leaving the top/bottom surfaces of the specimen open to air or by adding distilled water to the specimen. The masses of the specimens were monitored during the drying and wetting processes, from which the desired water contents can be approached. As a result, specimens with different water contents were prepared.
The EC-5 sensor was then inserted into those prepared specimens and their volumetric water contents were measured, as shown in Fig. 6(b) and (c). After measurement, the specimens were oven dried, and their actual gravimetric water contents can be obtained. By multiplying by the dry density (i.e. 0.915 g/cm3), their volumetric water contents were calculated. Figure 7 summarizes the calculated and measured volumetric water contents of those specimens. A quadratic relationship (Eq. (1)) can be used to best-fit the data points (as shown in Fig. 7), with the coefficient of determination of 0.98.
where, y is the actual (calculated) volumetric water content, and x is the measured volumetric water content by EC-5.
2.2.3 Accuracy of EC-5 Under Frozen Condition
A lack of reliable equipment prevents the calibration of the EC-5 sensor under frozen condition. In the present study, the accuracy of the EC-5 sensor at sub-freezing temperatures was investigated by comparing the EC-5 measured SFCC with that measured by NMR (nuclear magnetic resonance) on the Toyoura sand.
Watanabe and Wake (2009) measured the SFCC of Toyoura sand by using NMR. The Toyoura sand in their study has a mean particle diameter of 0.21 mm and a uniformity coefficient of 1.44. Although these two values are slightly different from those of the Toyoura sand employed in the present study, the effect of this difference on the measured SFCC is assumed negligible. As in Watanabe and Wake (2009), two Toyoura sand specimens were prepared, by mixing certain amount of dry sand and distilled water and by subsequent compaction in the acrylic cylinder shown in Fig. 3. The two specimens had different initial volumetric water contents (0.26 and 0.17 m3/m3), but the same bulk density (1.43 g/cm3) and solid fraction (0.54 m3/m3). The Toyoura sand was previously washed in distilled water to minimize the effect of salinity, if any, on the SFCC. Following similar steps described in Sect. 2.1, the SFCC of the two Toyoura sand specimens were measured by using EC-5, along thawing path. The results are summarized in Fig. 8, together with the SFCC measured by NMR by Watanabe and Wake (2009).
It can be seen that the SFCC measured by EC-5 shows similar shape or desorption rate to that measured by NMR. In other words, the EC-5 correctly captures the tendency of change in unfrozen water content of the soil specimens. However, the two SFCCs are not completely overlapped with each other. This means that there is difference between the volumetric water content values measured by EC-5 and NMR. Three temperature ranges can be distinguished,
-
(1)
When the temperature of the specimen is relatively high (e.g., higher than 1 °C), the measured values by EC-5 and NMR are relatively stable. In this temperature range, the specimen is completely thawed or unfrozen, and the measured value represents the initial (total) water content of the specimen. The EC-5 sensor is able to give reasonable measurement in this range. However, specific calibration for the Toyoura sand can improve the accuracy of the EC-5 sensor in this temperature range.
-
(2)
When the temperature of the specimen is relatively low (e.g., lower than −1 °C), the values measured by EC-5 and NMR show agreement. In this temperature range, most pore water in the specimen become ice and the unfrozen water content values are very low. The EC-5 sensor gives reasonable measurement in this range. And,
-
(3)
When the temperature approaches 0 °C along the thawing process (e.g., between −1 and 1 °C), the difference between the SFCCs measured by EC-5 and NMR is large, even though their desorption rates are similar.
It is noted from the experimental data that in the first two temperature ranges, i.e. the specimen is either completely thawed or “completely frozen”, the volumetric water content values measured by the EC-5 sensor is stable. In other words, when the measured temperature of the specimen kept constant during a sufficient period of time (which is typically taken as thermodynamic equilibrium is achieved), the measured volumetric water content maintained at a specific value during this time period. Within the third temperature range, however, the measured value by EC-5 continuously increased even though the measured temperature of the specimen kept constant. This may imply that pore ice underwent continuous phase change to water, and thermodynamic equilibrium was not actually achieved under the controlled temperatures.
The EC-5 sensor measures the volumetric water content of the bulk specimen. However, the temperature sensor measures the temperature of soil body that is directly contacted with it. Therefore, if thermodynamic equilibrium is not fully established, the measured temperature only represents the temperature of a portion of soil, rather than the temperature of the bulk specimen (Ren and Vanapalli 2019). In other words, the soil mass or volume sampled by these two types of sensors are not equal. The SFCC measured by EC-5 goes to the left side of the 0 °C line. The reason may be that the soil body that has direct contact with the temperature sensor is thawed and have a positive temperature, yet there is still frozen part present in the bulk specimen.
In summary, the measured SFCC by EC-5 sensor shows good result when no ice is present or most pore water changes phase to ice in the soil specimen. However, the measurements in the temperature range where massive phase change occurs may bear errors. Decrease the size of the specimen can facilitate thermodynamic equilibrium, as was assumed for the small NMR specimen (whose volume is only 3% of that of the specimen in the present study) in Watanabe and Wake (2009). However, this method may not be applicable to the EC-5 sensor because it requires a certain volume of soil to obtain a representative measurement. In addition, one difficulty in measuring SFCC of coarse-grained soils is that the phase change of most pore water occurs within a very narrow range of temperature. For example, this temperature range is 0 to −0.3 °C for the Toyoura sand specimens where the unfrozen water content drops from 0.24 or 0.17 to 0.04 m3/m3. The accuracy of controlling the specimen’s temperature and the accuracy and resolution of typical temperature sensors are not good enough for measuring the SFCC of coarse-grained soils in this narrow temperature range.
3 Experimental Results and Discussion
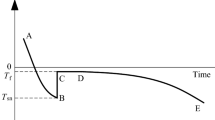
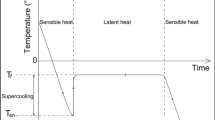
Several Komaoka specimens have been used to conduct the SFCC measurement. Figure 9 shows the temperature-time curves for two specimens, which were subjected to three consecutive freeze-thaw (F-T) cycles. The specimens, which were initially under unfrozen condition, were directly subjected to −10 °C in each F-T cycle. The controlled temperature was then gradually increased to desired subzero temperatures for measuring the thawing branch of SFCC. It can be seen that supercooling was occasionally observed under the testing condition in the present study. The specimens have relatively high freezing temperature (i.e. around −0.2 °C), at which most pore water starts to change phase to ice with a fast rate. This is reasonable since the Komaoka soil is a coarse-grained soil and contains only a small fraction of clayey particles.
Figure 10 summarizes the measured SFCCs of three Komaoka specimens. The three specimens had an initial gravimetric water content of 30.3%, 30.3% and 41.7%, respectively. By multiplying by the dry density of the specimens (i.e. 0.915 g/cm3), their initial volumetric water contents are obtained and shown in the figure with pink lines and numbers. The EC-5 measured water content of the specimen at completely thawed condition was calibrated by using Eq. (1). It can be seen that the measured SFCCs under the three F-T cycles do not show significant differences. In other words, the effect of F-T cycles is not pronounced. This is consistent with the experimental results by Ren and Vanapalli (2020) on five fine-grained Canadian soils. It is also interesting to note that similar to the Toyoura sand, the measured SFCCs of the Komaoka soil specimens spread to the left side of the 0 °C line.
For coarse-grained soils like the Toyoura sand and Komaoka soil, in the narrow temperature range where most phase change occurs, thermodynamic equilibrium is difficult to establish or practically impossible. The continuous phase change in soil pores causes the continuous change of the water content values measured by the EC-5 sensor. In other words, there is gradual increase of water content in the soil volume sampled by the EC-5 sensor, though the measured temperature looks constant. As an example, the SFCCs of two Komaoka soil specimens after two F-T cycles are shown in Fig. 11. The whisker plots shown in this figure indicates the minimum and maximum (i.e. gradual increase) of water content values measured by EC-5 at each specific temperature near 0 °C. The open data points are the average value of the measured water contents at each temperature (the same as the SFCCs shown in Fig. 10). When the temperature becomes more negative below 0 °C, the measured water content by the EC-5 sensor is stable (i.e. no whisker). As explained before, the stable reading of EC-5 can be regarded as that thermodynamic equilibrium was approximately established.
Although the reliable measurement of unfrozen water content and temperature near 0 °C is crucial to determine SFCC, especially for coarse-grained soils, it is practically impossible to stably control a variety of temperatures that are close to 0 °C (e.g., −0.2, −0.15, −0.1, −0.05, and 0 °C) for SFCC measurement. For example, Kong et al. (2020) pointed out that an accuracy of ± 0.1 °C would be the state of the art for temperature control in terms of frozen soil tests. Therefore, the unfrozen water content in this narrow range of temperature is important yet not reliably measured. Since it is generally acknowledged that the unfrozen water content of coarse-grained soils dramatically drops as soil temperature decreases, a practical way could be to linearly connect the initial water content (which is constant under unfrozen condition) to the measured water content value at a temperature lower than 0 °C (where thermodynamic equilibrium can approximately achieves). Examples are the blue and red lines shown in Fig. 11, which can be considered as the first approximation of the SFCCs of the two specimens.
Figure 12 shows the SFCC results of two Komaoka specimens, which were respectively subjected to one- and three-dimensional F-T conditions, as shown in Fig. 4(a). The two specimens, which were initially under unfrozen condition, were directly subjected to −10 °C in each of the F-T cycles. The controlled temperature was then gradually increased to desired subzero temperatures for measuring the thawing branch of SFCC. The average values for the unfrozen water content under each of the temperatures were used to construct the SFCCs in Fig. 12. The measured water content at the completely thawed condition was calibrated by using Eq. (1). It can be seen that under one-dimensional condition, the effect of F-T cycles on SFCC is not significant, similar to the three-dimensional condition. In addition, there is not large difference between the measured SFCCs under the one- and three-dimensional conditions, although it took much longer time for the one-dimensional case to achieve steady state.
The temperature distributions in the specimen under one- and three-dimensional freezing/thawing are different. For example, in one-dimensional freezing condition, the temperature inside the specimen is not uniform. Rather, there is temperature gradient within the specimen. The temperature distribution can be considered linear.
4 Summary
The SFCC describes the relationship between the amount of unfrozen water and its energy state or subzero temperature in a frozen soil. It is essential to estimating the physical and mechanical properties of frozen soils and to modeling the transport mechanism of water, heat, and solutes in cold regions. In the present study, the SFCC of the Komaoka soil, which is a volcanic soil sampled in Sapporo, Hokkaido, Japan, was investigated. The SFCC test is time-consuming as relatively long time is required to approximate to thermodynamic equilibrium condition under each controlled temperature. The EC-5 moisture sensor was used to measure the volumetric water content of the specimens. Good contact between the soil body and the prongs of the EC-5 sensor is generally essential for good results. Great care should also be paid when analyzing the EC-5 measured water content.
By comparing with the SFCCs of two Toyoura sand specimens measured by NMR, it is recognized that the SFCC measured by EC-5 sensor shows good result when no ice is present or most pore water changes phase to ice in the soil specimen. However, the measurements in the temperature range where massive phase change occurs may bear errors. A couple of SFCC tests were carried out on the Komaoka soil, by considering different F-T cycles (up to three) and freezing/thawing methods (i.e. one- and three-dimensional). The measured SFCCs under three F-T cycles do not show significant differences. In other words, the effect of F-T cycles is not pronounced. Under one-dimensional condition, the effect of F-T cycles on SFCC is not significant, similar to the three-dimensional condition. In addition, there is not large difference between the measured SFCCs under the one- and three-dimensional conditions. More investigations however should be conducted to give more credits to the results of the present study.
References
Anderson, D.M., Tice, A.R.: Predicting unfrozen water contents in frozen soils from surface area measurements. Highway Res. Rec. 393, 12–18 (1972)
Azmatch, T.F., Sego, D.C., Arenson, L.U., Biggar, K.W.: Using soil freezing characteristic curve to estimate the hydraulic conductivity function of partially frozen soils. Cold Reg. Sci. Technol. 83, 103–109 (2012)
Grant, S.A., Sletten, R.S.: Calculating capillary pressures in frozen and ice-free soils below the melting temperature. Environ. Geol. 42(2–3), 130–136 (2002). https://doi.org/10.1007/s00254-001-0482-y
Ishikawa, T., Tokoro, T., Nakamura, D., Yamashita, S.: Influence of freeze-thaw action on air-permeability of unsaturated soil ground. In: Third International Conference on Geotechnique, Construction Materials and Environment, Nagoya, Japan, 13–15 November 2013, pp. 71–76 (2013)
Kawamura, S., Miura, S.: Rainfall-induced failures of volcanic slopes subjected to freezing and thawing. Soils Found. 53(3), 443–461 (2013)
Kong, L., Wang, Y., Sun, W., Qi, J.: Influence of plasticity on unfrozen water content of frozen soils as determined by nuclear magnetic resonance. Cold Regions Sci. Technol. 172, 102993 (2020)
Koopmans, R.W.R., Miller, R.D.: Soil freezing and soil water characteristic curves. Soil Sci. Soc. Am. J. 30(6), 680–685 (1966)
Kozlowski, T.: A comprehensive method of determining the soil unfrozen water curves: 1. Application of the term of convolution. Cold Reg. Sci. Technol. 36(1–3), 71–79 (2003)
Liu, Z., Yu, X.: Predicting the phase composition curve in frozen soils using index properties: a physico-empirical approach. Cold Reg. Sci. Technol. 108, 10–17 (2014)
Mao, Y., Romero Morales, E.E., Gens Solé, A.: Ice formation in unsaturated frozen soils. In: Unsaturated Soils: UNSAT 2018: The 7th International Conference on Unsaturated Soils, pp. 597–602. The Hong Kong University of Science and Technology (HKUST) (2018)
Mu, Q.Y., Ng, C.W.W., Zhou, C., Zhou, G.G.D., Liao, H.J.: A new model for capturing void ratio-dependent unfrozen water characteristics curves. Comput. Geotech. 101, 95–99 (2018)
Neall, V.E.: Volcanic soils. Land Use Land Cover Soil Sci. 7, 23–45 (2009)
Nguyen, T.B.: Effect evaluation of grass on infiltration and seepage of volcanic soil ground. Master thesis, Hokkaido University, Sapporo, Japan (2017)
Ren, J., Vanapalli, S.K.: Comparison of soil-freezing and soil-water characteristic curves of two Canadian soils. Vadose Zone J. 18(1), 1–14 (2019)
Ren, J., Vanapalli, S.K.: Effect of freeze–thaw cycling on the soil‐freezing characteristic curve of five Canadian soils. Vadose Zone J. 19(1), e20039 (2020)
Schafer, H.: Freezing characteristics of mine waste tailings and their relation to unsaturated soil properties. Master thesis, University of Alberta, Edmonton, Canada (2018)
Smith, M.: Models of soil freezing. In: Church, M., Slaymaker, O. (eds.) Field and Theory: Lectures in Geocryology, pp. 96–120. University of British Columbia Press, Vancouver (1985)
Spaans, E.J., Baker, J.M.: The soil freezing characteristic: its measurement and similarity to the soil moisture characteristic. Soil Sci. Soc. Am. J. 60, 13–19 (1996)
Watanabe, K., Osada, Y.: Comparison of hydraulic conductivity in frozen saturated and unfrozen unsaturated soils. Vadose Zone J. 15(5), 1–7 (2016)
Watanabe, K., Wake, T.: Measurement of unfrozen water content and relative permittivity of frozen unsaturated soil using NMR and TDR. Cold Reg. Sci. Technol. 59(1), 34–41 (2009)
Xu, J., Lan, W., Li, Y., Wang, S., Cheng, W.C., Yao, X.: Heat, water and solute transfer in saline loess under uniaxial freezing condition. Comput. Geotech. 118, 103319 (2020)
Yu, L., Zeng, Y., Wen, J., Su, Z.: Liquid-Vapor-Air Flow in the frozen soil. J. Geophys. Res. Atmos. 123(14), 7393–7415 (2018)
Zhang, S., et al.: Canopy effect caused by vapour transfer in covered freezing soils. Géotechnique 66(11), 927–940 (2016)
Acknowledgement
The first author gratefully acknowledges the financial support from the Japan Society for the Promotion of Science (JSPS).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this paper
Cite this paper
Ren, J., Zhang, S., Ishikawa, T., Vanapalli, S.K. (2021). The Freezing Characteristic Curve of a Coarse-Grained Volcanic Soil. In: Liu, Y., Cuomo, S., Yang, J. (eds) Advances in Innovative Geotechnical Engineering. GeoChina 2021. Sustainable Civil Infrastructures. Springer, Cham. https://doi.org/10.1007/978-3-030-80316-2_9
Download citation
DOI: https://doi.org/10.1007/978-3-030-80316-2_9
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-80315-5
Online ISBN: 978-3-030-80316-2
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)