Abstract
Determination of freezing point is the prerequisite for any laboratory or field study on the freeze–thaw behaviour of soils. Depression in the freezing point of soil pore water as compared to pure water (which freezes at 0 °C) in a two-phase or a three-phase soil system is associated with several factors such as water content, mineralogical composition, salinity of pore water, pore size, plasticity of soil, etc. An experimental study was conducted on three types of soils of different plasticity and gradation to study the effects of water content, salinity of pore water and plasticity of soil on the freezing point depression. The results showed that the freezing point significantly depresses only when water content decreases beyond a certain value. The freezing point also showed depression with increasing soil plasticity and a linear depression with increasing salinity of pore water. The study is a part of extensive laboratory investigation on frost susceptibility of soils in Kashmir.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
Soils and rocks present in cold regions of the world that experience seasonal frost are required to be tested for freeze–thaw susceptibility. In such regions, the ground freezes in the coldest month of the year and remains in an unfrozen state for the rest. This seasonal freezing and thawing of soils prove detrimental to engineering structures, particularly pavements [1]. This, therefore, demands freeze–thaw testing of soils to device proper measures and modifications for a particular site.
Determination of freezing point is the prerequisite for any laboratory or field study on freeze thaw behaviour of soils [2, 3]. It helps the recognition of the freezing front and determination of total frost penetration in a laboratory freeze–thaw setup. It is also an important design parameter for constructions which involve artificial freezing of grounds like construction of frozen earth walls for deep excavation and for management of ground water seepage at the construction site [4].
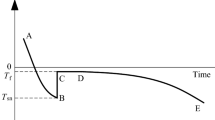
Before actual freezing of soil, it is necessary for the temperature to go down below the freezing point of soil pore water (Tf) to a temperature Tsc, where the super-cooled pore water exists in a metastable state until triggered to turn into ice in a process called nucleation (Fig. 1). Accumulations of water molecules or soil particles may act as nucleation centres that trigger water to ice transformation [5]. The crystallization results in the release of latent heat that raises the temperature of soil to Tf, the actual freezing point of soil. All the free pore water then freezes at this temperature, Tf. The temperature then starts decreasing further depending on the ambient temperature [6, 7].
Unfrozen soil, whether saturated or not, usually freezes at a temperature below 0℃. The depression in the freezing point of soil pore water as compared to pure water in a two-phase or three-phase soil system is primarily because of soil grain matrix effect and osmotic effect [8]. This is associated with the number of factors such as water content, mineralogical composition, salinity of pore water, plasticity of soil, etc. [7, 9, 10].
An experimental study was conducted on three types of soils of different plasticity and gradation to determine the freezing point of soils under different conditions of water contents and pore water salinity. The objective of the study was to recognize the role of water content, salinity of pore water and plasticity on depressing the freezing point of soil. This paper presents the details of the testing procedure, the results obtained and the conclusions drawn therein from the study.
2 Materials and Methodology
For achieving the objectives of the study, three different soils were used of distinctly different composition in terms of their particle size and plasticity. The basic properties of the three soils used in this experiment are shown in Table 1.
The soil S-1 has 42% sand content and is of least plasticity while S-2 and S-3 have <1% coarse-grained fraction with S-3 having the highest plasticity of the three samples. After obtaining the basic properties required for classification of the soils as per Indian system of soil classification, the maximum dry density and the optimum moisture content using light compaction test as per Indian standard code [11] were obtained. The compaction curves of the three soils are shown in Fig. 2. The maximum dry density decreased while the optimum moisture content increased with increasing plasticity of the soil.
2.1 Sample Preparation and Determination of Freezing Point
For the determination of freezing point, a weighed amount of oven dry soil sample was mixed with pre-determined quantity of water as per the desired moisture content and dry density. It was then compacted in a test tube up to a height of 20 mm (Fig. 3).
For obtaining continuous temperature change within this soil sample, a negative temperature coefficient (NTC) thermistor (Fig. 3b) was used. The thermistor was carefully inserted through 10 mm into the soil placed in the test tube (Fig. 3c). For soils prepared on dry side of optimum, a small hole, 10 mm deep, was made using a nail prior to the insertion of thermistor. The thermistor was connected through Arduino Mega 2560 to obtain temperature change directly into Microsoft Excel using Data Streamer Add-in (Fig. 3).
The test tube containing the soil sample and the temperature sensor was then placed in a water bath at 0 ℃ until the temperature of soil stabilized. The test tube was then placed in the water bath at −3 ℃ (in some cases at −5 to −7 ℃). This resulted in the lowering of soil temperature beyond the actual freezing point. In some cases, the super-cooled state did not exist for long, and the temperature jumped up to the actual freezing point without any external instigation, while in most cases the nucleation was initiated by striking the test tube with the wall of water bath while keeping it immersed in the water bath coolant. The freezing point of the soil was obtained from the graph of temperature sensor readings (in °C) with time (t) as shown in Fig. 4.
The different combinations of water contents and solute concentrations tested are shown in Table 2. The test Series 1 covers the tests performed on the soils at different moisture contents without any solute added to the water. Series 2 tests were conducted on the three samples at moisture content specified in Table 2 with solute (NaCl) concentration of 0.5%, 1%, 2% and 5%. For these tests, the salt solution of desired concentration was first prepared, and then the dry sample of soil was mixed with the pre-determined quantity of this solution to obtain desired moisture content.
3 Results and Discussions
Some selected graphs showing variation of temperature, in °C, within the sample as recorded using the NTC thermistor at different water contents and solute concentrations is shown in Fig. 4. Similar graphs were observed for the other combinations (Table 2) considered in this study.
From the graphs in Fig. 4, one can observe the jump in the temperature of the soil sample as the sample is super-cooled to a temperature Tsc. As the spontaneous nucleation takes place at Tsc, the latent heat of crystallization is released, resulting in abrupt rise in temperature to Tf. The latent heat slows down the cooling till most of the free water is frozen [7]. The degree of neutralization between the latent heat release and the effect of outside cooling rate is the deciding factor for the length of equilibrium temperature stage at Tf. Thus, the temperature will not rise to Tf spontaneously until the latent heat is enough to increase the system temperature to Tf [10]. Thus, the supercooling, defined as (Tf – Tsc) has been correlated to cooling rates, in addition to pore water solution [12], plastic limit, mass and water content of the soil [9]. It is important, however, to note here that Tsc, and not Tf, is a function of the cooling rate.
Figure 5 shows the variation of freezing point, in °C, with water content for the three soils. It can be observed that the freezing point for all the three soils showed significant decrease when the water content decreased beyond a certain value, which was close to the optimum moisture content of that soil. It can be inferred that the soils prepared on the wet-side of optimum had no influence of water content on their freezing points.
However, the freezing points of S-1 (having the least plasticity and more than 40% coarse-grained fraction) were higher than S-2 and S-3 for all water contents observed. While S-3, which is of highest plasticity of the three, showed greater depression in freezing point compared to other two soils for the same values of water content.
The reason for lower freezing points in S-3 compared to S-2 and S-1 can be explained by the specific surface area of the soil particles. The nucleation of super-cooled water is a function of water activity [13]. More the specific surface area, greater is the adsorption of water and thicker is the water film (bound water) on the surface of soil particle which results in lower freezing point as amount of free water decreases. As the grain size increases, the adsorption and the bound water film decreases and the soil freezes at a higher temperature. Thus, at the same water content, S-1 showed highest freezing point followed by S-2 and S-3.
The fitting curve for the variation of freezing point with water content is a power function of the form:
where, Tf = the freezing point in degree Celsius and
wo = the percentage of initial water content.
The values of the fitting curve parameters A and B for the three soils are given in Table 3.
Figure 6 shows the variation of freezing point with the solute concentration of the three soils, each having 20% water content. A linear decrease in Tf can be observed with increasing solute concentration (S%). From the values of the slope, m, and the intercept, c, for the three linear fitting curves, it can be inferred that for the same water and solute content, the freezing point is slightly higher for low plastic soil, but this difference decreases with increasing solute concentration.
Using theoretical analysis to understand the effect of pore diameter on freezing point, Xiao et al. [13] demonstrated that the freezing point decreased with decreasing pore diameter as interfacial forces came into play. For the same water content in a soil, the freezing point is influenced by concentration and type of salt [14].
The variation of freezing points, in °C, with solute concentration, S%, for the soil S-1 is shown in Fig. 7. At a particular salt content, the reduction in water content increases the salt concentration thereby reducing the freezing point further. It can be observed from Fig. 7 that for lower water content, the depression in freezing point with increasing salinity was greater compared to that for higher water content. Also, as the water content decreases, water is retained easily in smaller pores than larger pores and the effect of pore size [14] increases, thus reducing the freezing point.
Also, from Figs. 5 and 7, it can be deduced that for higher values of water content, the freezing point is more influenced by solute concentration in pore water than the initial water content of the soil, which almost had no influence when the water content is above the critical water content.
4 Conclusions
An experimental investigation was carried out on three types of soils for the assessment of effects of water content and solute concentration in pore water of the soils on freezing point depression. The following conclusions can be drawn from the results obtained.
-
1.
The freezing points (Tf) of the three soils at their optimum moisture contents (wopt), i.e. 15.5, 19 and 23.8% is −0.35, −0.50 and −0.70 ℃, respectively. When water content increased beyond wopt, there was no significant change in the freezing points of the three soils. However, a decrease in water content beyond optimum resulted in a rapid decrease in freezing points of the soils. For S-3, there was approximately 9 times decrease in the freezing point when water content was lowered to 5%. Similarly, Tf was about 3 times and 5 times lower for S-1 and S-2 at 5% water content compared to that at their respective wopt. It can therefore be concluded that the effect of water content is significant on the depression of freezing point of soils only when the value of water content is less than the wopt of that soil. Increasing the water content beyond this critical value for a particular soil, does not influence the freezing point of that soil.
-
2.
By increasing the solute concentration (S) in pore water, there is a linear decrease in Tf of the three soils. However, the rate of depression (ΔTf /ΔS) increased with decreasing wo of the soil. For S-1, the rate of depression ranges from 0.82–2.5 ℃/% with higher value for lower water content (wo = 5%), that means, 1 ℃ depression in freezing point was caused by almost 1.5% increase in solute concentration at higher water content (20%), but the same level of depression was caused by only 0.4% solute concentration at lower water content (5%).
-
3.
For the similar moisture and solute concentrations, the freezing point was lower for fine-grained, high plastic soil compared to low plastic and coarse-grained soils. This is due to presence of more bounded water in fine-grained soil with higher specific surface area resulting in greater adsorption compared to coarse-grained soils having lesser specific surface and lower adsorption. Lesser adsorption of water results in thinner water film (bounded water) around the soil particle. As the amount of free water is more in a coarse-grained soil, it freezes at a higher temperature. The effect of plasticity and grain size on the freezing point of soil, however, decreased with increasing water content.
References
Simonsen, E., & Isacsson, U. (1999). Thaw weakening of pavement structures in cold regions. Cold RegNs Sci Technol, 29(2), 135–151.
Arenson, L. U., & Sego, D. C. (2006). The effect of salinity on the freezing of coarse-grained sands. Canadian Geotechnical Journal, 43(3), 325–337.
ASTM (2006) International, standard test methods for frost heave and thaw weakening susceptibility of soils D5918. Annual Book of ASTM Standard.
Bell, F. (1993). Grounf freezing. CRC Press.
Andersland OB, Ladanyi B (2013) An introduction to frozen ground engineering. Springer Science & Business Media
Ming F et al. (2020) Investigation into freezing point depression in soil caused by NaCl solution. Water 12(8):2232
Wan, X., Liu, E., & Qiu, E. (2021). Study on ice nucleation temperature and water freezing in saline soils. Permafrost and Periglacial Processes, 32(1), 119–138.
Liyang W et al. (2021) A simplified model for the phase composition curve of saline soils considering the second phase transition. Water Resour Res 57(1) e2020WR028556.
Kozlowski, T. (2009). Some factors affecting supercooling and the equilibrium freezing point in soil–water systems. Cold RegNs Sci Technol, 59(1), 25–33.
Bing, H., & Ma, W. (2011). Laboratory investigation of the freezing point of saline soil. Cold RegNs Sci Technol, 67(1–2), 79–88.
Indian standard (2011) Methods of test for soils. determination of water content—dry density relation using light compaction, 2720 (Part VII). 1980. fifth reprint: Aug 1997
Liu, Z. (1986). Freezing point of wet soil and its measurement. J China Min Technol Chin, 3, 24–31.
Koop T et al. (2000) Water activity as the determinant for homogeneous ice nucleation in aqueous solutions. Nature 406(6796):611–614
Xiao, Z., Lai, Y., & Zhang, M. (2018). Study on the freezing temperature of saline soil. Acta Geotechnica, 13(1), 195–205.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this paper
Cite this paper
Shah, R., Mir, B.A. (2022). The Freezing Point of Soils and the Factors Affecting its Depression. In: Loon, L.Y., Subramaniyan, M., Gunasekaran, K. (eds) Advances in Construction Management. Lecture Notes in Civil Engineering, vol 191. Springer, Singapore. https://doi.org/10.1007/978-981-16-5839-6_14
Download citation
DOI: https://doi.org/10.1007/978-981-16-5839-6_14
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-16-5838-9
Online ISBN: 978-981-16-5839-6
eBook Packages: EngineeringEngineering (R0)