Abstract
Soil freezing characteristic curve (SFCC) represents the relationship between soil temperature and unfrozen water content of soil during freezing and thawing processes. In this study, SFCC of sandy soils was determined in laboratory. Pure sand was mixed with clay at various contents (0, 5, 10, 15, and 20% of the total dry mass), and the mixtures were compacted to their respective maximum dry density. Compacted specimens were then placed in a close and rigid cell, and the soil’s temperature was decreased step-by-step to freeze the soil water and then increased back to thaw it. During this thermal cycle, soil’s temperature and volumetric water content were monitored in order to determine the SFCC. The results show that SFCC was strongly dependent on the fines content: at higher fines content, the temperature of spontaneous nucleation was lower, and the residual unfrozen volumetric water content was higher.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Frozen soil consists of mineral particles, liquid water, ice and gas. It is formed from unfrozen soil during freezing, when a fraction of liquid water solidifies into ice at temperatures sufficiently low below 0 °C [2]. This phase change causes significant modifications of physical–hydraulic–mechanical properties of soils [3]. The freezing–thawing process is encountered in cold regions, seasonal cold regions as well as construction works using artificial ground freezing technique. Two main consequences of this process that need to be mentioned are frost heave and thaw settlement. These phenomena can induce damages to infrastructure [26, 56, 86, 87].
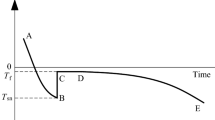
The freezing–thawing process in porous media has been investigated not only in civil engineering and geosciences but also in physics [1, 19, 39, 41, 49]. While bulk water melts at 0 °C, water in porous media melts at temperatures below 0 °C because of physical interactions between water and solid particles [22, 53, 58]. Freezing process of a soil sample (where heat is extracted from the sample with a constant rate) can be divided into three steps (as shown in Fig. 1): (i) supercooling with release of sensible heat; (ii) first water freezing with release of latent heat; (iii) further water freezing with release of sensible heat. In the first step, during cooling (extraction of heat from soil), soil temperature decreases to reach a certain value from that it cannot decrease anymore. This value is called temperature of spontaneous nucleation Tsn where the first ice embryo nucleus forms because it attains the critical size [4, 5]. Formation of ice crystals releases latent heat and thus increases soil temperature. From Tsn, soil temperature increases to reach another value which is called freezing temperature Tf, where it remains on a plateau for a while. During this second step, soil water is gradually frozen along with releasing latent heat. After that, within the third step, soil temperature decreases with further water freezing. Freezing temperature Tf, also considered to be equal to thawing temperature Tt at which soil state changes from frozen to unfrozen, is usually used as a boundary value index to distinguish between frozen soil and unfrozen soil [74, 85, 90]. These characteristic temperatures (Tsn and Tf) were investigated in several studies [4, 11, 90].
Soil freezing characteristic curve (SFCC) represents the relationship between the temperature and the quantity of liquid water in soil. It is one of the most essential data in studying the freezing–thawing process in soils. On the one hand, several SFCC models were empirically developed. From SFCC obtained experimentally, empirical models were proposed using power, piecewise or exponential functions [9, 24, 29, 40, 43, 67, 69, 81]. On the other hand, SFCC can be derived from soil water characteristic curve (SWCC). This approach is based on the theory of similarity between freezing–thawing and drying–wetting processes that is illustrated by Clapeyron equation [20, 35, 46, 59, 64, 66, 88, 89, 95]. More generally, various physical models were developed based on theory of capillarity, sorption or that of interface pre-melting [33, 76, 92]. Most of the existing SFCC models consider the effect of fines content, but this effect is considered in different ways. For instance, some empirical models used specific surface or liquid limit as input data, while physics-based models consider absorption parameters of soil. Due to the diversity of SFCC models, there is no unified standard for choosing SFCC in numerical simulations [12]. In addition, except few models (e.g. [95]), most of the existing ones consider a unique relationship between unfrozen water content and temperature. However, this relation obtained on the freezing path can differ from that of the thawing path; at a given temperature, water content of the freezing path can be higher than at of the thawing path. This hysteresis is usually ignored in the models.
To determine SFCC in the laboratory, a soil specimen is usually subjected to a freeze–thaw cycle, while unfrozen water content is measured. Although controlling specimen’s temperature is technically feasible, measuring unfrozen water content is much more challenging. Several methods and techniques have been developed to evaluate the unfrozen water content at negative temperature, including dilatometry [37, 52], gas dilatometry [61], adiabatic calorimetry [8, 36], isothermal calorimetry [69], differential scanning calorimetry [38, 39, 83], X-ray diffraction [6, 7], time/frequency domain reflectometry (TDR/FDR) [57, 63, 94] and pulsed nuclear magnetic resonance (P-NMR) [45, 70, 76]. Among these methods, TDR and P-NMR are the two most common ones. P-NMR is widely acknowledged as a highly accurate and non-destructive technique. However, the equipment required for this technique is generally expensive [84]. Compared to P-NMR, TDR/FDR can be used in the laboratory as well as in the field, and it is cheaper, quicker, and more portable. With TDR, unfrozen water content is inferred from the measurement of apparent dielectric constant of soil using an empirical equation [60, 71] or dielectric mixing models [55, 63, 77]. It is noted that several factors such as temperature or bound water can affect its accuracy.
Several studies have determined SFCC in the laboratory in both freezing and thawing processes [16, 31, 33, 37, 45, 62, 68, 90]. These studies recognized that hysteresis exists in SFCC in which the unfrozen water content is different in thawing and freezing processes at the same temperature. Hysteresis in freezing–thawing process was believed to be similar to that of wetting–drying process. However, the mechanism inducing hysteresis in SFCC is complex and it may be influenced by several effects such as supercooling, pore blocking, capillarity, free energy barriers, contact angles and electrolytes [16, 45]. It is also noted that hysteresis is significant at temperatures between -2 and 0 °C [32, 37, 44] and that it should not be ignored due to impacts on unfrozen water content on frost heaving [30, 72], creep behaviour of frozen soils [10, 91] as well as thermal regime of frozen ground [21].
Beside hysteresis effect, it is found that the shape of SFCC depends also on several factors, including liquid limit [69], stress condition [50], salt content and solute types [48, 76], initial water content or degree of saturation [34, 65, 80], types of soil [16, 44, 90], pore-size distribution [45], and fines content [45, 68, 69, 90]. Among these factors, fines content can influence others (liquid limit, pore-size distribution and types of soil). As far as fines content is concerned, by determining unfrozen water content of several clays, a silt and a gravel, Tice et al. [69] observed significantly different unfrozen water contents at the same temperature below 0 °C. Tian et al. [68] carried out tests on three soils corresponding to three clay contents and found that unfrozen water degree of saturation also changed in different ways in both freezing and thawing processes. For soils containing higher clay fraction, unfrozen water degree of saturation was higher at any given temperature below freezing point and the hysteresis loop was smaller. The same findings concerning SFCC were obtained in the study of Zhang et al. [90] on silty clay, and silt and in the study of Li et al. [45] on silty clay, fine sand, and medium sand. Some other authors also investigated different soils, but the effect of fines content was out of their focus [16, 66, 67, 70].
The present study aims at systematically investigating the effect of fines content on the SFCC of sandy soils. Clean sand was mixed with clay at dry state firstly and water afterwards to obtain sandy soils with clay content of 0, 5, 10, 15, and 20% prior to compaction at the Proctor maximum dry density followed by a saturation phase. The specimen’s temperature was then decreased progressively to freeze the soil specimen in undrained conditions prior to applying the thawing process. During this freezing–thawing cycle, soil’s temperature and unfrozen water content were measured. After the introduction, the second section of this paper presents the materials and experimental methods. Experimental results are presented in the third section, before being discussed in the fourth section.
2 Materials and experimental methods
2.1 Experimental setup
The experimental setup is shown in Fig. 2, and the details of the sensors used are presented in Table 1. Soil specimen was contained in a rigid metallic cylindrical cell (150 mm in height and 150 mm in diameter). The cell was immerged in a temperature-controlled bath (F38-EH JULABO with ± 0.03 °C accuracy). Soil temperature was measured with a PT100 sensor, soil volumetric water content was measured with a ML2x Thetaprobe sensor, and soil suction was measured with a tensiometer. As Thetaprobe sensor measures soil apparent dielectric constant (\({K}_{a}\)) which is the ratio of the dielectric permittivity of a substance to free space, soil unfrozen volumetric water content (\({\theta }_{u}\)) was estimated from measured \({K}_{a}\) by using empirical equations of Smith and Tice [60] (1) and Topp et al. [71] (2) for frozen and unfrozen states of soil, respectively. Equation (2) is used only for the initial state (before the occurrence of freezing) and for the final state where thawing is complete. Equation (1) is used where ice is expected to exist in soil (i.e. after the occurrence of freezing and before the completion of thawing).
Schematic view of the experimental setup. (1) Temperature-controlled bath; (2) soil specimen; (3) temperature controlling system; (4) temperature-controlled liquid (30% ethylene glycol + 70% water); (5) metallic cylindrical cell; (6) insulating cover; (7) temperature sensor; (8) tensiometer; (9) soil water sensor; (10) thermal conductivity probe (results are not presented in this study); (11) data logger system
2.2 Material
Fontainebleau sand was carefully mixed with Speswhite kaolin clay at dry state using an automatic mortar mixer in order to obtain sandy soils with fines content (dry mass of clay divided by dry mass of soil) of 0, 5, 10, 15, and 20%. The physical properties of sand and clay are shown in Tables 2 and 3, respectively. Figure 3 presents the grain size distribution of these soils. In this study, the name of each soil corresponds to its clay content (for instance, S10 corresponds to a soil having 10% of clay in dry mass). Prior to the preparation of the soil specimens, each soil was carefully mixed with distilled water using the mortar mixer to obtain optimum water content (determined from the Normal Proctor compaction curves obtained on the same soils [17]). Afterwards, wet soil was packed in a plastic bag for at least 24 h to ensure the homogenisation of water content, prior to compaction in the cylindrical cell to reach its maximum dry density.
2.3 Experimental procedure
After soil compaction in the cell, sensors were installed as shown in Fig. 2 and an insulating cover made of expanded polystyrene was placed in order to avoid heat exchange between soil specimen and ambient air. The whole system was then transferred inside the temperature-controlled bath. Prior to the freezing–thawing test, soil specimen was saturated by injecting water from the bottom of the specimen during 0.5–2 days depending on fines content. After the saturation (when a layer of water of 10 mm was visible on the top of the specimen), the temperature of the bath was first set at a temperature between 0 and −1 °C (slightly higher than the expected Tsn). Each test started with the cooling path. The bath temperature was decreased in steps of 0.1 °C to freeze the soil pore water. Once the freezing was triggered, the temperature continued to be decreased in steps of 0.2 °C until −2 or −3 °C to observe the change of liquid water content during further cooling. Afterwards, during the heating path, the bath temperature was increased in steps of 0.2 °C until 0 °C to thaw the frozen soil. During both cooling and heating paths, the bath temperature was changed to the subsequent step only when soil temperature and volumetric unfrozen water content (measured by the sensors) had reached their equilibrium state. The equilibrium state was considered reached when these two quantities did not change (< 0.05 °C for temperature and < 1% for water content) during at least 2 h.
The test programme is shown in Table 4. The test number shows the soil tested (S0 to S20) followed by the number of replicate test (T1 to T4). At least two tests were performed for each soil. Tests T1 were performed following the procedure described above to obtain the complete SFCC curves. For the other tests (T2, T3, T4), only the freezing path of the same procedure was performed in order to replicate the characteristic temperatures.
3 Experimental results
3.1 Typical test (S10-T1)
As an example, the results of test S10-T1 are shown in Fig. 4 where soil temperature, suction, and volumetric unfrozen water content are plotted versus elapsed time for the cooling path.
From −1.2 °C, soil temperature was decreased in steps of 0.1 °C down to −1.6 °C. During this period, soil temperature was controlled through the bath’s temperature, suction remained equal to zero and volumetric water content remained constant. When soil temperature reached −1.6 °C, soil freezing started inducing abrupt changes in the three measured quantities. Results obtained during this stage (elapsed time of 70–86 h) are shown in Fig. 5 for a better view.
As shown in Fig. 5, when the bath temperature was changed from −1.5 to −1.6 °C (at 76 h), soil temperature changed to −1.6 °C after a few minutes. At 77 h, while the bath temperature was still maintained at −1.6 °C, soil temperature increased abruptly to −0.1 °C prior to a progressive decrease and reached the imposed temperature (−1.6 °C) again at 83 h. Soil suction started to increase at 78 h and reached a maximum value of 300 kPa prior to fall down to 100 kPa. At 77 h, soil water content decreased abruptly from 28 to 26% prior to decrease progressively to 3% at 82 h. These results are representative of a freezing process in soil (Fig. 1) where the phase before 77 h corresponds to the supercooling step. At 77 h, soil water started to freeze: soil temperature increased abruptly because of latent heat release prior to decrease because of heat diffusion towards the liquid surrounding the cell; soil suction increased quickly because of the cryogenic suction induced by ice formation in the pore space (the sudden decrease in suction from 300 to 100 kPa corresponded to the cavitation of the tensiometer, after this moment, the sensor did not provide anymore the real soil suction); volumetric water content decreased because of ice formation. From these typical results, the following parameters were defined to characterise the freezing process (see Fig. 5): (i) temperature of spontaneous nucleation, Tsn; (ii) freezing point, Tf; (iii) residual volumetric unfrozen water content, θr (the value recorded at temperature equal to Tsn); (iv) duration of the temperature plateau, tp; and (v) duration of the freezing process, tf.
After the freezing process (from 83 h), decrease in temperature induced slight decrease in volumetric unfrozen water content (see Fig. 4), while soil suction measurement was no longer available because of the cavitation of the tensiometer.
Figure 6 shows the results of test S10-T1 during the heating path. During this path, temperature was increased by steps of 0.2 °C from −2.8 to 0 °C. It induced thawing of frozen water (corresponding to a gradual increase in unfrozen water content).
From the results shown in Figs. 4, 5 and 6, volumetric unfrozen water content obtained at the end of each step is plotted versus the corresponding soil temperature for test S10-T1 in Fig. 7. These results correspond to the SFCC of soil S10 obtained from test S10-T1, which include both freezing and thawing paths.
3.2 Effects of fines content
SFCC of all soils is shown in Fig. 8 where volumetric unfrozen water content was plotted versus temperature. As the initial volumetric water content (which depends on soil dry density) was different from one soil to the others, it is thus difficult to analyse the effect of fines content from these results. For this reason, volumetric unfrozen water content was used to calculate unfrozen water degree of saturation (\({S}_{r}=\theta /{\theta }_{\mathrm{sat}}\); where \({\theta }_{\mathrm{sat}}\) is the volumetric unfrozen water content at saturate state). Figure 9 shows SFCC of all soils where unfrozen degree of saturation was plotted versus temperature. For each soil, from the initial saturated state, when soil temperature decreased from 0 °C, soil remained saturated with unfrozen water. When temperature reached the temperature of spontaneous nucleation, freezing was triggered inducing significant decrease in unfrozen water degree of saturation. After this step, cooling induced only slight decrease in unfrozen water degree of saturation. During the heating path, unfrozen water degree of saturation increased gradually with temperature and the relationship between these two quantities was significantly different from the cooling path for all soils.
In order to quantitatively assess the effects of fines content, temperatures of spontaneous nucleation Tsn and freezing point Tf were plotted versus fines content (Fig. 10). The results show that the temperature of freezing point was close to 0 °C for all soils. The results were quite repeatable (with variation less than 0.1 °C) and only a slight trend of decrease of Tf when fines content increase could be observed. For Tsn, results showed a higher scattering (up to 0.5 °C, except for test at 0% of clay content where this value varied from −0.4 to −1.5 °C). In general, Tsn is lower at a higher clay content.
Figure 11 shows the residual unfrozen water content θr (the value determined at a temperature equal to Tsn, see Fig. 5) versus fines content. A good repeatability (with a scattering of 0.5%) could be observed. The results show that residual unfrozen water content was higher at a higher fines content.
Figure 12 presents the duration of the temperature plateau tp and the duration of the freezing process tf (see the definition on Fig. 5) versus fines content. Results of tp were quite scattering for 0 and 5% of fines content, varying from 0.80 to 4.40 h. They were more repeatable at higher fines contents. A general decrease of this duration when the fines contents increased could be observed. Results of tf varied between 5 and 10 h (except one test, S0-T1 where it was very long, 37.50 h). These results did not show any clear trend.
Table 5 shows the obtained characteristic parameters of all tests for better comparison.
4 Discussion
In this study, in order to determine the relationship between unfrozen water content and temperature during a freezing–thawing cycle, large soil specimens (150 mm in height and 150 mm in diameter) were prepared in order to embed several sensors within the soil mass. To minimise thermal and any other gradients, soil temperature was changed by small steps and equilibrium was checked at the end of each step prior the subsequent step. At equilibrium, the soil temperature and unfrozen water content were thus supposed to be homogeneous within the specimen. Similar large soil specimens were equally used in previous studies investigating SFCC with TDR method for measurement of unfrozen water content [54, 57, 80, 90]. Smaller specimens were used when measurements were performed by pulsed-NMR method [47, 66, 68]. In several previous works, specimens were immerged in a cooling bath with constant cooling rate or at low temperature (between −15 and −30 °C) and kept for several hours [14, 48, 74, 75]. For determining SFCC, unfrozen water content was measured at various controlled temperatures [34, 47, 54, 57, 80, 90]. The difference between two successive controlled temperatures in these studies varies between 0.3 and 5 °C. In the present work, temperature steps of 0.1 and 0.2 °C were chosen before the occurrence of freezing phenomenon and afterwards, respectively, in order to determine more accurately the freezing point, the temperature of spontaneous nucleation and the SFCC.
The measurement of unfrozen water content in the present study was converted from the measurement of apparent dielectric constant. In this study, under the influence of temperature, dielectric constant of each phase in soils changes, particularly those of water and ice [27, 79]. Several models exist to estimate moisture content from unfrozen soil apparent dielectric constant [15, 28, 51, 55, 57, 60, 63, 71, 77]. The most used is Topp’s empirical model [71], but it is not compatible with frozen soils [60, 61, 93]. Otherwise, Smith and Tice [60] proposed a model based on comparison of unfrozen water content measured from NMR and TDR methods for 25 soils covering a wide range of specific surface areas. For this reason, in the present work, the model of Smith and Tice [60], which provides an accuracy of ± 3% compared to measurements from NMR method, was used for frozen soils.
Hysteresis of SFCC (difference between the freezing and the thawing curves) is usually attributed to the same factors inducing hysteresis in SWCC, such as the effect of electrolytes, pore geometry, pore blocking, effect of contact angle and change in pore structure [54]. Actually, in a freezing process, increasing solute concentration by forming ice from water increases the effect of electrolyte. Otherwise, forming ice also changes soil skeleton that affects matric potentials of soils. In addition, the hysteric behaviour is also mainly attributed to supercooling of pore water [16, 68, 70, 90]. Instead of freezing at 0 °C, pore water is necessarily supercooled at lower temperature. In the present work, an insignificant hysteresis of \({\theta }_{u}\) was observed for all soils below Tsn (at frozen state). First, the effect of electrolytes can be ignored. Second, temperature below Tsn of −1 to −2 °C corresponds to a suction of 1 to 2.5 MPa following the Clapeyron equation. This high range of suction corresponds mainly to water in micropore (intra-aggregates) in the clay matrix where SWCC is also reversible. As a result, hysteresis of SFCC observed in the present work could be contributed mainly to supercooling. After the triggering of freezing, SFCC obtained at temperature lower than Tsn was generally reversible (see Figs. 8 and 9).
Results shown in Fig. 9 demonstrate significant effect of fines content on the thawing path of SFCC; at a given temperature, a higher unfrozen water degree of saturation was obtained at a higher fines content. These results are consistent with the findings of previous works [45, 68, 69, 91]. Following these studies, Gibbs–Thompson equation can be used to relate the pore-size distribution and the thawing path of SFCC; a lower temperature corresponds to a smaller pore. In the present work, soil having higher fines content would have a larger volume of micropores (inter-aggregates and intra-aggregates pores) a lower volume of macropores (space between sand particles).
Tsn determined in this study can be associated with supercooling. Figure 10 shows that this parameter generally decreased with an increase in fines content and it was measured with a relatively high scattering. For bulk water, Tsn depends on numerous factors such as sample volume, cooling velocity, the presence and concentration of solutes, the presence of solid impurities, and effects of external fields (impulse waves, electromagnetic radiation, etc.) [23, 41, 73]. In the case of soils, additional factors can be soil components and their fractions. Many studies determined Tsn of various soils and found that increasing clay content in soils decreases temperature of spontaneous nucleation to lower range [4, 90]. These studies focussed on clays or clay and silt, and these results agree with sandy soils in the present study. It is noted that the supercooling is considered as a necessary phase to activate nucleation process and it appears in both cases, either in free pure water or within the porous volume of soils. Because of the high value of released latent heat, about 334 J/g, which appears during nucleation process, water needs to be supercooled at Tsn for equilibrating energy before crystallization. According to Yershov [82], Tsn is remarked as the temperature at which embryo nuclei form and grow to the critical sizes, about 472 H2O corresponding to 10–26 m3. The relatively high scattering of results obtained in the present work can be thus explained by the random behaviour of the crystallization process. The slight effect of fines content on Tsn can be explained by the effect of soil pore-size distribution on the supercooling: soil having a higher fines content would have higher volume of micropores, and Tsn is generally lower in a smaller pore.
Numerous studies investigated Tf and showed that Tf depends on many factors such as salt content [11, 13, 25, 48, 75], salt types [14, 74], initial water content [4], and soil types [18, 39, 42, 45, 66, 90]. In the present study, Tf was found close to 0 °C for all soils. This result can be explained by two main reasons: soils were studied at saturated state, and fines content is sufficiently low. Bing and Ma [14] obtained similar results with saturated sandy soil containing less than 7.5% of clay. Furthermore, freezing point remains constant also above a certain value of water content for all soils [14, 41, 78]. Actually, for the soils considered in the present study, with relatively low contents of low plasticity kaolin clay, the amount of bound water should be negligible and Tf should be similar to that of bulk pure water, i.e. close to 0 °C.
Residual unfrozen content was found higher at a higher fines content (Fig. 11). It is believed that residual unfrozen relates almost directly to the amount of specific surface of soils. According to several studies [45, 66, 68, 69, 90], unfrozen water content remaining in soils at the same temperature decreased in the following order: clay, silts, sands and gravel. Following Bing and Ma [14], only free water was frozen when freezing is triggered. Unfrozen water should then correspond to bound water. According to Tian et al. [68], the amount of bound water in soils is proportional to the thickness of the electric double layer and specific surface area. In the present study, a higher fines content corresponds to a higher specific surface area and then a higher amount of bound water.
The duration of temperature plateau, tp, would depend then on the amount of latent heat released when freezing is triggered. This amount mainly depends on Tsn,as shown in Table 5. As the results of Tsn show significant scattering and a general slight increase when fines content increased (Fig. 10), similar trends were observed with tp (Fig. 12). The duration of the freezing process, tf, which is much longer than tp, corresponds to the thermal diffusion of latent heat released during the whole freezing process. This duration would depend thus mainly on the thermal diffusivity of the frozen soil (which at the same time evolves during freezing).
5 Conclusions
The results obtained in this study show that fines content in sandy soils significantly influenced the soil behaviour under a freezing–thawing cycle. Based on the investigation of five levels of fines content (varying from 0 to 20%), the following conclusions can be addressed:
-
When the temperature decreased from 0 °C, freezing was triggered at Tsn inducing a sudden decrease of \({\theta }_{u}\) from the saturated state to the residual state. Afterwards, \({\theta }_{u}\) continued to decrease but with a lower rate. The subsequent heating induced an increase of \({\theta }_{u}\) (which represents a progressive melting of frozen water).
-
The thawing path of SFCC was strongly dependent on the fines content; at a given temperature, a higher \({\theta }_{u}\) was observed for a higher fines content.
-
Tsn was higher at a higher fines content and varied between −1.0 and −2.0 °C.
-
Tf varied between 0 °C and −0.2 °C, only a slight decrease of Tf with an increase in fines content was observed.
-
\({\theta }_{r}\) (varied from 1 to 7%) was higher at a higher fines content.
-
tp was found scattering and slightly decreased when fines content increased.
-
tf was found independent of fines content.
The findings of the present study would be helpful to predict the soil behaviour under freezing–thawing process. That would imply several applications in cold regions and also in geotechnical engineering ground improvement by artificial ground freezing.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Akyurt M, Zaki G, Habeebullah B (2002) Freezing phenomena in ice–water systems. Energy Convers Manag 43:1773–1789. https://doi.org/10.1016/S0196-8904(01)00129-7
Andersland OB, Ladanyi B (1994) An introduction to frozen ground engineering. Springer, Cham
Andersland OB, Ladanyi B (2004) Frozen ground engineering. Wiley, New York
Anderson DM (1968) Undercooling, freezing point depression, and ice nucleation of soil water. Isr J Chem 6:349–355. https://doi.org/10.1002/ijch.196800044
Anderson DM (1967) Ice nucleation and the substrate-ice interface. Nature 216:563–566. https://doi.org/10.1038/216563a0
Anderson DM, Hoekstra P (1965) Migration of interlamellar water during freezing and thawing of Wyoming bentonite. Soil Sci Soc Am J 29:498–504. https://doi.org/10.2136/sssaj1965.03615995002900050010x
Anderson DM, Morgenstern NR (1973) Physics, chemistry, and mechanics of frozen ground: a review. In: Permafrost: North American Contribution [to The] Second International Conference; National Academies: Washington, DC, USA. p 257
Anderson DM, Tice AR (1973) The unfrozen interfacial phase in frozen soil water systems. In: Physical aspects of soil water and salts in ecosystems, pp 107–124
Anderson DM, Tice AR (1972) Predicting unfrozen water contents in frozen soils from surface area measurements. Highw Res Rec 393:12–18
Arenson LU, Johansen MM, Springman SM (2004) Effects of volumetric ice content and strain rate on shear strength under triaxial conditions for frozen soil samples. Permafr Periglac Process 15:261–271. https://doi.org/10.1002/ppp.498
Ayers AD, Campell RB (1951) Freezing point of water in a soil as related to salt and moisture contents of the soil. Soil Sci 72:201–206
Bai R, Lai Y, Zhang M, Yu F (2018) Theory and application of a novel soil freezing characteristic curve. Appl Therm Eng 129:1106–1114. https://doi.org/10.1016/j.applthermaleng.2017.10.121
Banin A, Anderson DM (1974) Effects of salt concentration changes during freezing on the unfrozen water content of porous materials. Water Resour Res 10:124–128
Bing H, Ma W (2011) Laboratory investigation of the freezing point of saline soil. Cold Reg Sci Technol 67:79–88. https://doi.org/10.1016/j.coldregions.2011.02.008
Birchak JR, Gardner CG, Hipp JE, Victor JM (1974) High dielectric constant microwave probes for sensing soil moisture. Proc IEEE 62:93–98. https://doi.org/10.1109/PROC.1974.9388
Bittelli M, Flury M, Campbell GS (2003) A thermodielectric analyzer to measure the freezing and moisture characteristic of porous media. Water Resour Res, p 39.
Boussaid K (2005) Sols intermédiaires pour la modélisation physique: application aux fondations superficielles. École Centrale de Nantes et Université de Nantes
Cannell GH, Gardner WH (1959) Freezing-point depressions in stabilized soil aggregates, synthetic soil, and quartz sand. Soil Sci Soc Am J 23:418–422. https://doi.org/10.2136/sssaj1959.03615995002300060018x
Chen SL, Lee TS (1998) A study of supercooling phenomenon and freezing probability of water inside horizontal cylinders. Int J Heat Mass Transf 41:769–783. https://doi.org/10.1016/S0017-9310(97)00134-8
Dall’Amico M (2010) Coupled water and heat transfer in permafrost modeling. University of Trento
Darrow MM (2011) Thermal modeling of roadway embankments over permafrost. Cold Reg Sci Technol 65:474–487. https://doi.org/10.1016/j.coldregions.2010.11.001
Enninful HRNB, Schneider D, Kohns R et al (2020) A novel approach for advanced thermoporometry characterization of mesoporous solids: transition kernels and the serially connected pore model. Microporous Mesoporous Mater 309:110534. https://doi.org/10.1016/j.micromeso.2020.110534
Fletcher NH (1970) The chemical physics of ice. Press Cambridge, England, p 111
Ge S, McKenzie J, Voss C, Wu Q (2011) Exchange of groundwater and surface-water mediated by permafrost response to seasonal and long term air temperature variation. Geophys Res Lett 38:1–6. https://doi.org/10.1029/2011GL047911
Han Y, Wang Q, Kong Y et al (2018) Experiments on the initial freezing point of dispersive saline soil. CATENA 171:681–690. https://doi.org/10.1016/j.catena.2018.07.046
Han L, Ye G, Li Y et al (2016) In situ monitoring of frost heave pressure during cross passage construction using ground-freezing method. Can Geotech J 53:530–539. https://doi.org/10.1139/cgj-2014-0486
Haynes WM (2016) CRC handbook of chemistry and physics, 97th edn. CRC Press, Boca Raton
He H, Dyck M (2013) Application of multiphase dielectric mixing models for understanding the effective dielectric permittivity of frozen soils. Vadose Zo J 12. https://doi.org/10.2136/vzj2012.0060
He Z, Teng J, Yang Z et al (2020) An analysis of vapour transfer in unsaturated freezing soils. Cold Reg Sci Technol 169:102914. https://doi.org/10.1016/j.coldregions.2019.102914
Hoekstra P (1966) Moisture movement in soils under temperature gradients with the cold-side temperature below freezing. Water Resour Res 2:241–250
Horiguchi K, Miller RD (1980) Experimental studies with frozen soil in an “ice sandwich” permeameter. Cold Reg Sci Technol 3:177–183. https://doi.org/10.1016/0165-232X(80)90023-3
Hu G, Zhao L, Zhu X et al (2020) Review of algorithms and parameterizations to determine unfrozen water content in frozen soil. Geoderma 368:114277. https://doi.org/10.1016/j.geoderma.2020.114277
Ishizaki T, Maruyama M, Furukawa Y, Dash J (1996) Premelting of ice in porous silica glass. J Cryst Growth 163:455–460
Jia H, Ding S, Wang Y et al (2019) An NMR-based investigation of pore water freezing process in sandstone. Cold Reg Sci Technol 168:102893. https://doi.org/10.1016/j.coldregions.2019.102893
Kebria MM, Na S, Yu F (2022) An algorithmic framework for computational estimation of soil freezing characteristic curves. Int J Numer Anal Methods Geomech 46:1544–1565. https://doi.org/10.1002/nag.3356
Kolaian JH, Low PF (1963) Calorimetric determination of unfrozen water in montmorillonite pastes. Soil Sci 95:376–384
Koopmans RWR, Miller RD (1966) Soil freezing and soil water characteristic curves. Soil Sci Soc Am J 30:680–685
Kozlowski T (2003) A comprehensive method of determining the soil unfrozen water curves. Cold Reg Sci Technol 36:71–79. https://doi.org/10.1016/S0165-232X(03)00007-7
Kozlowski T (2004) Soil freezing point as obtained on melting. Cold Reg Sci Technol 38:93–101. https://doi.org/10.1016/j.coldregions.2003.09.001
Kozlowski T (2007) A semi-empirical model for phase composition of water in clay–water systems. Cold Reg Sci Technol 49:226–236. https://doi.org/10.1016/j.coldregions.2007.03.013
Kozlowski T (2009) Some factors affecting supercooling and the equilibrium freezing point in soil–water systems. Cold Reg Sci Technol 59:25–33. https://doi.org/10.1016/j.coldregions.2009.05.009
Kozlowski T (2016) A simple method of obtaining the soil freezing point depression, the unfrozen water content and the pore size distribution curves from the DSC peak maximum temperature. Cold Reg Sci Technol 122:18–25. https://doi.org/10.1016/j.coldregions.2015.10.009
Kozlowski T, Nartowska E (2013) Unfrozen water content in representative bentonites of different origin subjected to cyclic freezing and thawing. Vadose Zo J 12. https://doi.org/10.2136/vzj2012.0057
Kruse AM, Darrow MM (2017) Adsorbed cation effects on unfrozen water in fine-grained frozen soil measured using pulsed nuclear magnetic resonance. Cold Reg Sci Technol 142:42–54. https://doi.org/10.1016/j.coldregions.2017.07.006
Li Z, Chen J, Sugimoto M (2020) Pulsed NMR measurements of unfrozen water content in partially frozen soil. J Cold Reg Eng 34:04020013. https://doi.org/10.1061/(ASCE)CR.1943-5495.0000220
Liu Z, Yu X (2013) Physically based equation for phase composition curve of frozen soils. Transp Res Rec J Transp Res Board 2349:93–99. https://doi.org/10.3141/2349-11
Ma T, Wei C, Xia X et al (2017) Soil freezing and soil water retention characteristics: connection and solute effects. J Perform Constr Facil 31:1–8. https://doi.org/10.1061/(ASCE)CF.1943-5509.0000851
Ming F, Chen L, Li D, Du C (2020) Investigation into freezing point depression in soil caused by NaCl solution. Water 12:2232. https://doi.org/10.3390/w12082232
Mishima O, Stanley HE (1998) The relationship between liquid, supercooled and glassy water. Nature 396:329–335. https://doi.org/10.1038/24540
Mu QY, Zhou C, Ng CWW, Zhou GGD (2019) Stress effects on soil freezing characteristic curve: equipment development and experimental results. Vadose Zo J 18:1–10. https://doi.org/10.2136/vzj2018.11.0199
Nagare RM, Schincariol RA, Quinton WL, Hayashi M (2011) Laboratory calibration of time domain reflectometry to determine moisture content in undisturbed peat samples. Eur J Soil Sci 62:505–515. https://doi.org/10.1111/j.1365-2389.2011.01351.x
Patterson DE, Smith MW (1981) The measurement of unfrozen water content by time domain reflectometry: results from laboratory tests. Can Geotech J 18:131–144. https://doi.org/10.1139/t81-012
Petrov O, Furó I (2006) Curvature-dependent metastability of the solid phase and the freezing-melting hysteresis in pores. Phys Rev E 73:011608. https://doi.org/10.1103/PhysRevE.73.011608
Ren J, Vanapalli SK (2019) Comparison of soil-freezing and soil-water characteristic curves of two Canadian soils. Vadose Zo J 18:1–14. https://doi.org/10.2136/vzj2018.10.0185
Roth K, Schulin R, Fluhler H, Attinger W (1990) Calibration of time domain reflectometry for water content measurement using a composite dielectric approach. WATER Resour Res 26:2267–2273
Russo G, Corbo A, Cavuoto F, Autuori S (2015) Artificial ground freezing to excavate a tunnel in sandy soil. Measurements and back analysis. Tunn Undergr Sp Technol 50:226–238. https://doi.org/10.1016/j.tust.2015.07.008
Schafer H, Beier N (2020) Estimating soil-water characteristic curve from soil-freezing characteristic curve for mine waste tailings using time domain reflectometry. Can Geotech J 57:73–84. https://doi.org/10.1139/cgj-2018-0145
Schreiber A, Ketelsen I, Findenegg GH (2001) Melting and freezing of water in ordered mesoporous silica materials. Phys Chem Chem Phys 3:1185–1195. https://doi.org/10.1039/b010086m
Sheshukov AY, Nieber JL (2011) One-dimensional freezing of nonheaving unsaturated soils: Model formulation and similarity solution. Water Resour Res 47:1–17. https://doi.org/10.1029/2011WR010512
Smith MW, Tice AR (1988) Measurement of the unfrozen water content of soils: comparison of NMR and TDR methods. CRREL report, pp 88–18.
Spaans EJ, Baker JM (1995) Examining the use of time domain refiectometry for measuring liquid water content in frozen soil. Water Resour Res 31:2917–2925. https://doi.org/10.1029/95WR02769
Spaans EJA, Baker JM (1996) The soil freezing characteristic: its measurement and similarity to the soil moisture characteristic. Soil Sci Soc Am J 60:13–19
Stähli M, Stadler D (1997) Measurement of water and solute dynamics in freezing soil columns with time domain reflectometry. J Hydrol 195:352–369. https://doi.org/10.1016/S0022-1694(96)03227-1
Sun K, Zhou A (2021) A multisurface elastoplastic model for frozen soil. Acta Geotech 16:3401–3424. https://doi.org/10.1007/s11440-021-01391-7
Suzuki S (2004) Dependence of unfrozen water content in unsaturated frozen clay soil on initial soil moisture content. Soil Sci Plant Nutr 50:603–606. https://doi.org/10.1080/00380768.2004.10408518
Teng J, Kou J, Yan X et al (2020) Parameterization of soil freezing characteristic curve for unsaturated soils. Cold Reg Sci Technol 170:102928. https://doi.org/10.1016/j.coldregions.2019.102928
Teng J, Zhong Y, Zhang S, Sheng D (2021) A mathematic model for the soil freezing characteristic curve: the roles of adsorption and capillarity. Cold Reg Sci Technol 181:103178. https://doi.org/10.1016/j.coldregions.2020.103178
Tian H, Wei C, Wei H, Zhou J (2014) Freezing and thawing characteristics of frozen soils: Bound water content and hysteresis phenomenon. Cold Reg Sci Technol 103:74–81. https://doi.org/10.1016/j.coldregions.2014.03.007
Tice AR, Anderson DM, Banin A (1976) The prediction of unfrozen water contents in frozen soils from liquid limit determinations. Department of Defense, Army, Corps of Engineers, Cold Regions Research and Engineering Laboratory
Tice AR, Anderson DM, Sterrett KF (1982) Unfrozen water contents of submarine permafrost determined by nuclear magnetic resonance. In: Developments in Geotechnical Engineering. pp 135–146
Topp GC, Davis JL, Annan AP (1980) Electromagnetic determination of soil water content: Measurements in coaxial transmission lines. Water Resour Reserch 16:574–582
Torrance JK, Schellekens FJ (2006) Chemical factors in soil freezing and frost heave. Polar Rec (Gr Brit) 42:33–42. https://doi.org/10.1017/S0032247405004894
Uzu Y, Sano I (1965) On the freezing of the droplets of aqueous solutions. J Meteorol Soc Japan Ser II 43:290–292
Wan X, Lai Y, Wang C (2015) Experimental study on the freezing temperatures of saline silty soils. Permafr Periglac Process 26:175–187. https://doi.org/10.1002/ppp.1837
Wan X, Liu E, Qiu E (2021) Study on ice nucleation temperature and water freezing in saline soils. Permafr Periglac Process 32:119–138. https://doi.org/10.1002/ppp.2081
Watanabe K, Mizoguchi M (2002) Amount of unfrozen water in frozen porous media saturated with solution. Cold Reg Sci Technol 34:103–110. https://doi.org/10.1016/S0165-232X(01)00063-5
Watanabe K, Wake T (2009) Measurement of unfrozen water content and relative permittivity of frozen unsaturated soil using NMR and TDR. Cold Reg Sci Technol 59:34–41. https://doi.org/10.1016/j.coldregions.2009.05.011
Wen Z, Ma W, Feng W et al (2012) Experimental study on unfrozen water content and soil matric potential of Qinghai-Tibetan silty clay. Environ Earth Sci 66:1467–1476. https://doi.org/10.1007/s12665-011-1386-0
Wraith JM, Or D (1999) Temperature effects on soil bulk dielectric permittivity measured by time domain refiectometry: experimental evidence and hypothesis development. Water Resour Res 35:361–369
Wu M, Tan X, Huang J et al (2015) Solute and water effects on soil freezing characteristics based on laboratory experiments. Cold Reg Sci Technol 115:22–29. https://doi.org/10.1016/j.coldregions.2015.03.007
Ye M, Pan F, Wu Y-S et al (2007) Assessment of radionuclide transport uncertainty in the unsaturated zone of Yucca Mountain. Adv Water Resour 30:118–134. https://doi.org/10.1016/j.advwatres.2006.03.005
Yershov ED (2004) General geocryology. Cambridge University Press
Yong RN, Cheung C, Sheeran DE (1979) Prediction of salt influence on unfrozen water content in frozen soils. In: Developments in Geotechnical Engineering. pp 137–155
Yoshikawa K, Overduin PP (2005) Comparing unfrozen water content measurements of frozen soil using recently developed commercial sensors. Cold Reg Sci Technol 42:250–256. https://doi.org/10.1016/j.coldregions.2005.03.001
Yu F, Guo P, Na S (2022) A framework for constructing elasto-plastic constitutive models for frozen and unfrozen soils. Int J Numer Anal Methods Geomech 46:436–466. https://doi.org/10.1002/nag.3306
Yu W, Zhang T, Lu Y et al (2020) Engineering risk analysis in cold regions: State of the art and perspectives. Cold Reg Sci Technol 171:102963. https://doi.org/10.1016/j.coldregions.2019.102963
Zhang S, Sheng D, Zhao G et al (2016) Analysis of frost heave mechanisms in a high-speed railway embankment. Can Geotech J 53:520–529. https://doi.org/10.1139/cgj-2014-0456
Zhang X, Sun SF, Xue Y (2007) Development and testing of a frozen soil parameterization for cold region studies. J Hydrometeorol 8:690–701. https://doi.org/10.1175/JHM605.1
Zhang S, Teng J, He Z et al (2016) Canopy effect caused by vapour transfer in covered freezing soils. Géotechnique 66:927–940. https://doi.org/10.1680/jgeot.16.P.016
Zhang M, Zhang X, Lai Y et al (2020) Variations of the temperatures and volumetric unfrozen water contents of fine-grained soils during a freezing–thawing process. Acta Geotech 15:595–601. https://doi.org/10.1007/s11440-018-0720-z
Zhang H, Zhang J, Zhang Z et al (2020) Variation behavior of pore-water pressure in warm frozen soil under load and its relation to deformation. Acta Geotech 15:603–614. https://doi.org/10.1007/s11440-018-0736-4
Zhou J, Wei C, Lai Y et al (2018) Application of the generalized clapeyron equation to freezing point depression and unfrozen water content. Water Resour Res 54:9412–9431. https://doi.org/10.1029/2018WR023221
Zhou X, Zhou J, Kinzelbach W, Stauffer F (2014) Simultaneous measurement of unfrozen water content and ice content in frozen soil using gamma ray attenuation and TDR. Water Resour Res 50:9630–9655. https://doi.org/10.1002/2014WR015640
Zhou X, Zhou J, Kinzelbach W, Stauffer F (2014) Simultaneous measurement of unfrozen water content and ice content in frozen soil using gamma ray attenuation and TDR. J Am Water Resour Assoc 5:2–2. https://doi.org/10.1111/j.1752-1688.1969.tb04897.x
Zhou Y, Zhou J, Shi X, Zhou G (2019) Practical models describing hysteresis behavior of unfrozen water in frozen soil based on similarity analysis. Cold Reg Sci Technol 157:215–223. https://doi.org/10.1016/j.coldregions.2018.11.002
Funding
No funding was received for conducting this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors have no competing interests to declare that are relevant to the content of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Vu, Q., Pereira, JM. & Tang, A.M. Effect of fines content on soil freezing characteristic curve of sandy soils. Acta Geotech. 17, 4921–4933 (2022). https://doi.org/10.1007/s11440-022-01672-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11440-022-01672-9