Abstract
The prevalence of obesity is exploding worldwide in our postindustrial era, with increasing morbidity and mortality.
The human gut microbiome exhibits a cardinal role in metabolic, nutritional, physiological, and immunological functions of the human body, and due to this multiplexity some authors consider it as an independent virtual organ by itself. Due to the big progress in phylogenetic investigation and quantification of gut microbiome through modern high-throughput sequencing, our understanding of the gut microbiome in health and diseases is rapidly advancing, and several studies have examined its role in obesity and its changes that occur following bariatric surgery.
There is growing evidence that obesity is associated to a specific gut microbiome profile which confers the host with an augmented ability for calories extraction and reduced gut microbial diversity. However, the mechanism through which the gut microbes and their by-products affect obesity remains mainly undiscovered and therefore more research is required to better comprehend the empirically observed connection between gut microbiome alterations and obesity.
On the other hand, bariatric surgery procedures, such as Roux-en-Y gastric bypass and vertical sleeve gastrectomy, are the most effective interventions for achieving pronounced and sustained weight loss and normalize glucose metabolism in obese patients. Bariatric surgery seems to restore a healthier microbiome with a leaner metabolic profile, and this microbiome rearrangement potentially contributes to the reduced fat mass, increase in lean mass, and resolution of comorbidities such as those observed following bariatric surgery. The exact mechanism is not certain, but it could be mediated by altering the enterohepatic bile acid circulation as well as altering the bile acid structure. Moreover, the bile acid activated farnesoid X transcription factor (FXR) is crucial for the positive effects of bariatric surgery on weight loss and glycemic control improvement. However, recent data showed that the gut microbiota is not fully restored after bariatric surgery. Additionally, unidentified downstream targets such as the gut-derived peptide FGF15/19 may potentially explain the positive metabolic effects of bariatric surgery.
More randomized controlled trials and larger prospective studies including well-defined cohorts are necessary to better identify the associations between the gut microbiome, obesity, and bariatric surgery.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
6.1 Introduction
Obesity is an enormous health problem in our modern society as it is associated with increased morbidity and mortality (Blüher 2019). Recently, research produced a vast amount of evidence of a bidirectional interplay between gut microbiota (GM) and obesity, with the latter considered as both a cause and/or a consequence of gut microbiota disorders (Cӑtoi et al. 2019). In the healthy human, GM is involved in energy intake, adjustment of glucose and lipid homeostasis, as well as in the micronutrients and vitamins composition (Pascale et al. 2018). This GM balance is disrupted in obesity thus presenting with a series of pathological pathways, such as altered insulin resistance, chronic inflammation, and metabolic disturbances (Cӑtoi et al. 2019; Pascale et al. 2018). Furthermore, obesity is accompanied by important deficiencies in vitamins and minerals, which aggravate gut microbiota synthesis and function (Astrup and Bügel 2019; Mohajeri et al. 2018).
Bariatric surgery (BS) is, for the time being, the sole long-term successful therapeutic option treatment of morbid obesity (Buchwald 2014). Several studies report a significant change in the structure and diversity of GM after BS. Additionally, subjects who underwent BS, present some micronutrient deficiencies which could result to serious deficiency-related syndromes (Lupoli et al. 2017; Neylan et al. 2016), the most common being anemia (10–74%) and neurological disfunctions (5–9%) (Xanthakos 2009).
However, except the substantial GM alteration after BS, several other factors coexist impairing the postoperative nutritional status of the bariatric patients: the significantly energy-restricted higher protein intake and adequate nutritional supplementation diet, and the anatomical and physiology impairment of the gastrointestinal tract (GIT) with explicit alterations in food digestion and absorption induced by the type of procedure performed (Buchwald 2014; Lupoli et al. 2017). Therefore, after BS, these patients require a consistent follow-up focused on the prevention of the above side effects, by modulating gut microbiota and prescribing appropriate nutritional supplementation.
The complicated interaction between obesity and GM phylae and the modulation of the gut microbiota and of their by-products balance produced in obese patients that undertake BS as a therapeutic measure represent the main areas of focus in this chapter.
6.2 Obesity
Recent research is showing that each human body hosts a unique set of associated microorganisms which contribute essentially to maintain health and metabolic balance of the subject.
Due to the contemporary modern living style providing easy access to high energy foods and low demanding of physical activity, the prevalence of obesity has exploded. Obesity due to an imbalance of calories ingestion, basal metabolism, and energy expenditure (Wang and Liao 2012). Obesity can be broadly defined as being the result of the discrepancy between calories consumption and energy expenditure. Numerous genetic, behavioral, and environmental factors have been suggested as obesogenic (Cani 2013). Furthermore, obesity is associated with type 2 diabetes (T2DM), hypertension, dyslipidemia, and cardiovascular disease, as well as sleep apnea, musculoskeletal disorders, some forms of cancer, impaired fertility, and with increased incidence of mood disturbances, anxiety, and other psychiatric disorders (Colquitt et al. 2014). Obesity increases mortality and its associated comorbidities, so that today in our modern societies, overweight and obesity associated diseases kill more individuals than undernourishment and starvation (Björklund and Fändriks 2019). Thus, except the burden that obesity provokes to the individual, it also represents a major health and economic load on the healthcare systems into both developed and developing countries (Tremmel et al. 2017).
Commonly, the term Body Mass Index (BMI) is used for classifying obesity and is calculated as body weight (kg) per the square of height (m2). In adults, a “normal” BMI is 18.5–25 kg m−2; overweight is BMI 25–30, while obesity is defined as BMI over 30 kg m−2. The WH O have classified obesity into three classes where class I relates to a BMI 30.00 to 34.99; class II is between 35.00 and 39.99, and BMI >40.00 kg m−2 is regarded as class III obesity (Colquitt et al. 2014). In addition, BMI >50 kg m−2 is sometimes termed superobesity.
Regarding obesity treatment, although substantial weight loss can be achieved by lifestyle interventions such as diet and increased physical activity, it has been shown that those lifestyle changes are hampered on the long term (Stefan et al. 2018). Indeed, the main issue is to keep the reduced body weight on the long term, as it has been reported that within 1–2 years most subjects reclaim the weight lost, and furthermore, they usually exceed the pretreatment levels. Additionally, the antiobesity drugs have several limitations due to adverse events and contraindications especially in cardiac and cerebrovascular diseases. Therefore, for morbidly obese patients, BS is the unique, effective in the long-term procedure to lose weight and to reestablish metabolic health (Miras and le Roux 2014). The term bariatric surgery is introduced and can be defined as a surgical intervention in the GIT for a weight reducing purpose.
6.3 Gut Microbiota in Healthy Subjects
6.3.1 Glossary of Microbiome-Related Terms
Microbes are found in every surface of the body that is exposed to the external environment, including the skin, genitourinary, gastrointestinal, and respiratory tracts (Chen et al. 2018).
The ecological community of symbiotic (promoting the health of the host), commensal (neutral to the host health, without benefit nor negative effects), and pathogenic microorganisms that share our body consists the microbiome (Thomas et al. 2017). The term microbiota comprises the sum of all species which form microbial communities, such as bacteria, archaea, fungi, and protists. When it refers to a specific environment, the term is preceded by the said location, for example, “the gut microbiota” refers to the intestinal tract (Knight et al. 2017).
The term “microbiome” is also commonly referring to the microbiota (i.e., the microorganisms themselves). The study of all microbial DNA of a sample (i.e., the genetic material) directly recovered from a sample such as the gut is called metagenomics . The metagenome, i.e., the collective genome of the microbiota encompasses over 100 times the number of genes of the human genome, thus containing approximately ten-fold more genes in each microbiome (Thomas et al. 2017). The term “shotgun metagenomics ” describes the process during which the total DNA of a sample is fragmented in a random manner and thereafter subjected to next-generation sequencing. This process generates primer-independent and unbiased sequencing data which can then be analyzed by means of various reference-based and/or reference-free methods. Thus, shotgun metagenomics targets all DNA material in a sample and produce relative abundance information for all genes, functions, and organisms (Chen et al. 2018).
In a healthy state, the GM is in a stable equilibrium while any imbalance of the gut bacterial ecosystem is called dysbiosis (Aron-Wisnewsky et al. 2012).
6.3.2 Gut Microbiota Under Normal Conditions
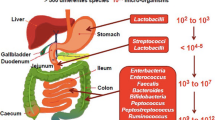
Under healthy conditions in adult humans, the microbial composition appears to remain constant (Li et al. 2016). The human microbiota incorporates all the microorganisms that reside in every surface of the body that is exposed to the external environment, including the skin, genitourinary, gastrointestinal, and respiratory tracts. The largest concentrations of microbes are found in the intestine, the skin, and in the oral cavity (Sender et al. 2016). Among those body sites, the gastrointestinal tract is the most densely colonized organ. It is reported that the gut of a healthy subject contains approximately 1–1.5 kg of microbes, corresponding to about 1014 bacteria, i.e., about 10 times more the number of body cells (Fändriks 2017). There are approximately 1000 species of microbes colonizing the gut, with microbial density increasing along the GI tract from 101 to 104 microbes in the stomach and the duodenum, 104 to 108 cells in the jejunum and ileum, to 1010 to 1012 cells per gram in the colon and feces (Thomas et al. 2017).
Due to the antimicrobial action of hydrochloric acid and nitric oxide, the stomach and the small intestine contain just a small amount of microbes (Lundberg and Weitzberg 2013; Nardone and Compare 2015). On the contrary, the large intestine is presenting better milieu for symbiotic microbes, achieving better conditions to extract energy as well as essential elements from the lumen bulk after digestion/absorption occurring in the small intestine (Mowat and Agace 2014; Woting and Blaut 2016). The bigger number of living microbes is located in the colon but due to the impermeable adherent mucus layer, the direct contact with the epithelium is prevented (Johansson et al. 2008).
The microbiome includes bacteria, fungi, and archaea (Savage 1977). It is estimated that in the gut there are about a 1000 bacterial species which have about 2000 genes per species, yielding to approximately two million genes, which is 100 times the number of nearly 20,000 human genes. The number above is in line with the actual extent of microbial gene catalogues found in MetaHIT and the Human Microbiome Project (Gilbert et al. 2018).
During the whole life, the structure and the function of GM are influenced to a different degree from many factors starting from birth (such as the delivery method) to the diet followed during childhood and adult age as well as the use of antibiotics (Compare et al. 2016). An analysis of the LifeLines DEEP cohort using metagenomic shotgun sequencing of the GM demonstrated a multifactorial involvement among the microbiome and a plethora of extrinsic and intrinsic parameters, including 60 dietary factors, 31 intrinsic factors, 19 drug categories, 12 diseases, and 4 smoking categories, all together accounting for 18.7% of the interindividual variation in the GM. It was also found that diet plays a significant role that alters GM (Zhernakova et al. 2016). It is estimated that about 4.5% of BMI is attributable to the GM (Mohajeri et al. 2018).
The majority of all microorganisms in the human GIT is a diverse community of bacteria, viruses, archaea, fungi, and eukaria (Ejtahed et al. 2018). Gut microbiota are bacteria and belong to two phyla, the Firmicutes (64% encompassing gram-positive genera, e.g., Clostridium, Ruminococcus, Lactobacillus, Butyrivibrio, Anaerostipes, Roseburia, and Faecalibacterium and the Bacteroidetes 23% containing gram-negative genera, e.g., Bacteroides, Porphyromonas, and Prevotella) (Mariat et al. 2009). The other phyla occupying the digestive tract include Proteobacteria (8% including gram-negative genera, e.g., Helicobacter and Escherichia), Actinobacteria (3% encompassing gram-negative genera, e.g., Bifidobacterium), and less of the phyla Fusobacteria, Spirochaetes, Verrucomicrobia (gram-negative species Akkermansia muciniphila), and Lentisphaerae (Zoetendal et al. 2008). The methanogens, Methanobrevibacter and Methanosphaera are the most dominant archaeal groups (Gill et al. 2006; Mihajlovski et al. 2008). Finally, fungi and archaea account for less than 1% of the GM. The two common fungal phyla in the gut include Ascomycota (which includes the genera Candida and Saccharomyces) and Basidiomycota (Scanlan and Marchesi 2008; Ott et al. 2008). Overall, the highest density is located into the colon with the majority of bacteria are anaerobes such as Bacteroides, Porphyromonas, Bifidobacterium, Lactobacillus, and Clostridium (genera that belong to the most abundant phyla: Bacteroidetes, Actinobacteria, and Firmicutes) (Villanueva-Millán et al. 2015). The GM has also its own energy demands and consumes energy from the luminal contents thereby enhancing energy utilization (Tremaroli and Bäckhed 2012). Collectively, the gut microorganisms are considered to constitute a powerful “organ” capable to influence most physiological functions of the human body (Gill et al. 2006; Tremaroli and Bäckhed 2012).
GI microbiota are of crucial importance in the metabolic, nutritional, physiological, and immunological procedures of the entire human body. The GM encompasses different genes involved in carbohydrates metabolism (glucose, galactose, fructose, arabinose, mannose, xylose, starch, and sucrose), thus producing important nutrients which could not be synthesized otherwise, such as short-chain fatty acids (SCFA) (Macfarlane and Macfarlane 2012), vitamins (vitamin K, vitamin B12, folic acid), certain amino acids (Gerritsen et al. 2011; Hamer et al. 2009), neurotransmitters (Cryan and Dinan 2012), and regulation of gastrointestinal hormones (Dockray 2014; Holzer et al. 2012). The above properties of the GM have pushed some authors to regard it as an independent virtual organ by itself (Al-Najim et al. 2018). The microbiome encodes specific enzymes capable to provoke fermentation of the indigestible carbohydrates mentioned above, that is 10–30% approximately of the ingested energy as well as the main fermentation products, i.e., SCFAs (e.g., acetate, propionate, and butyrate), which are at about 90–95% absorbed in the colon representing approximately about 6–10% of the energy needs of the human body (Young 2017).
Between 2013 and 2017, more than 12,900 publications were published studying the GM, a number highlighting that this field of research is blossoming and that a necessity for advancement is underway (Cani 2018). Human microbiome investigations are focusing to understand the underlying mechanisms and to develop novel clinical interventions (Gilbert et al. 2016).
The human microbiome is not constant, but rather changes with age, diet, and health status. It has been reported that the GM interacts in several ways in health and disease with the host, including:
-
1.
Modulating the inflammatory host response to the gut.
-
2.
Synthesizing small molecules and proteins that are absorbed by the host.
-
3.
Changing the amount of available energy in the diet.
The research of GI microbiota has blossomed enormously recently. This is due to the big progress in phylogenetic investigation and quantification of GM through modern high-throughput sequencing. The recent use of cost-effective, culture-independent molecular techniques (i.e., 16 s rDNA sequencing or whole-genome sequencing/metagenomics) on fecal samples enabled for the first time to study accurately and reliably the dynamics of the host–GM interactions. In whole-genome shotgun sequencing , the entire DNA in a given sample is fragmented, sequenced, and then remapped into the original genome (Sweeney and Morton 2013). This information is then compared with preexisting databases to identify species and genes. This method has the advantage of identifying all species and all genes present. The method is computationally intense, requiring a considerable amount of bioinformatic mapping (MetaHIT Consortium et al. 2010; The Human Microbiome Project Consortium 2012). One such freely available knowledge base for systematic analysis of gene functions in terms of the networks of genes and molecules is the Kyoto Encyclopedia of Genes and Genomes (KEGG) (http://www.genome.ad.jp/kegg/). It uses different databases to assign functional meanings to genes and genomes and thus predicts the higher level functional changes as KEGG pathway maps (Ogata et al. 1999). However, these studies are valuable since they may provide the most clinically relevant data because they are able to identify gene networks that may be overexpressed in a particular microbiome, for instance vitamin synthesis or decomposition, giving important clues to the physiology changes of the host. However, basic scientific research is based mainly on rodent models and cell cultures, but their relevance for human physiology and clinical conditions remains unknown as very few studies have validated the translation of rodent-based data to a human context in a “head-to-head” fashion.
In contrast to human genetics which have been unsuccessful to explain the obesity epidemic, the GM can classify individuals as lean or obese with over 90% accuracy, although this result depends on using the correct methods (Sze and Schloss 2016). Also, it is worth to note that recent findings support that GM could be implemented as a new marker of cardiovascular disease (Garcia-Rios et al. 2017).
Additionally, the GM exhibits a significant role in the defense against pathogens as the high microbial content found in the large bowel poses a major challenge to the mucosal immune system. In fact, the intestinal mucosa must tolerate commensal microbiota as well as dietary antigens and eliminate pathogens successfully. Τhe GM products are crucial in order to protect the host from various diseases (Zaneveld et al. 2008) as well as shaping systemic immune homeostasis (Dzutsev et al. 2015). In a healthy state, GM, by producing antimicrobial compounds, keeps the barrier intact and it presents anti-inflammatory action which protects the epithelial cells against pathogens (Compare et al. 2016; Villanueva-Millán et al. 2015). This action is intermediated through Toll-like receptors which can induce the synthesis and delivery of pro-inflammatory factors such as tumor necrosis factor alpha (TNFα) and interleukins 1 and 6 (IL1 and IL6) (Villanueva-Millán et al. 2015). The development of this peripheral production requires the presence of GM in the colon. Although the exact mechanism of this anti-inflammatory action is not well clarified, several microbe components have been detected to increase their expansion and function, including SCFAs (especially butyrate) and polysaccharide A of Bacteroides fragilis (Hoeppli et al. 2015).
The mechanism on how the beneficial bacteria prevent dysbiosis and maintain balance in healthy state is not known. An example is Clostridium difficile which under normal conditions is present in the large intestine in a commensal state not causing any disease. Clostridium difficile colonize and release the exotoxins TcdA and TcdB which can trigger colitis appearance in susceptible subjects (Leffler and Lamont 2015). Recently, a study showed that microcins, which are small size proteins released by numerous favorable bacteria, could restrict the expansion of competing Enterobacteriaceae and thus avoid inflammatory bowel disease (Sassone-Corsi et al. 2016).
GM is both a producer and a consumer of vitamins; Prototrophs (“producers”) are microbes which are able to synthesize vitamins de novo, in contrast to other microbes that require exogenous vitamins provision called auxotroph (“consumers”) (Kim et al. 2017). Some common microbes (i.e., Bacteroides, Enterococcus, Bifidobacterium) have an auxotrophic behavior although they can produce most of the soluble vitamins of the B complex (cobalamin, thiamine, pyridoxine, biotin, folate, nicotinic acid, pantothenic acid) and vitamin K2 (Das et al. 2019). However, it must be noted that the de novo biosynthesis of small micronutrient molecules is demanding a high consumption of energy and that bacteria prefer to uptake these molecules from the environment when they are available (LeBlanc et al. 2013).
As mentioned before, calorie restriction is causing rapid changes in microbial diversity and function. It has been documented in animal studies that diet develops bacterial phylotypes which are positively correlated with longevity. Moreover, it has been shown that bacteria of the Lactobacillus phyla increase in animals on low-fat diet, and this reduces phylotypes which are negatively correlated with life span (Zhang et al. 2013). It has been shown that the GM quickly responds to both directions of weight alterations (gain/reduction) as the structure of the food consumed is of fundamental importance for the composition of GM (David et al. 2014). Notably, it has been shown that short-term consumption of an entirely animal-based diet increased the abundance of bile-tolerant microorganisms, including Alistipes, Bilophila, and Bacteroides while it decreased the levels of Firmicutes that metabolize dietary plant polysaccharides (Roseburia spp, Eubacterium rectale, and Ruminococcus bromii) (David et al. 2014).
In summary, the GM has the capacity to cover the human metabolic needs acting as an energy supplier and as a provider of certain vitamins and micronutrients to the host (Kim et al. 2017). Our understanding of the gut microbiota in health and diseases is advancing rapidly, and several studies have examined the role of the GM in obesity and their change that occurs following BS, although the differences in GM found in obesity and after BS, so far, have been mostly limited to simple comparisons (Sweeney and Morton 2013).
6.4 Gut Microbiota in Obese Subjects
It has been found that the gut microbiome together with host genotype and lifestyle contribute to the pathophysiology of obesity, and therefore, there is an increasing research interest exploring possible associations between obesity and GM (Maruvada et al. 2017; Castaner et al. 2018).
A lot of scientific evidence has been presented during the last decade on the role of GM in obesity. It seems that an amphidromous interrelation exists between obesity and gut microbiota, and obesity being considered as both a cause and a consequence of the gut microbiota shift. However, the question still remains on what comes first, the microbiota shift or the obesity, as well as the magnitude of this bidirectional correlation (Cӑtoi et al. 2019). Several studies performed in mice have shown an interplay between body weight and gut microbiota. It has been demonstrated that this “obese microbiota” pattern is a transferable element, at least in rodents. Thus in a study, a significant increase in body fat of germ-free (GF) mice implanted with microbiota harvested from the cecum of ob/ob mice has been shown, when compared to mice transplanted with a GM from lean rodents (Ley et al. 2006). Specifically, transferring GM from genetically obese mice provoked within 2 weeks a 47% increase of fat mass, while the inoculation from lean mice augmented fat mass just by 26% (Turnbaugh et al. 2006).
It has been reported that GF mice, i.e., mice born and raised in sterile environment without any commensal bacteria, comprise 42% less total body fat when compared to mice with normal GM, although the GF mice daily diet was 29% more than their counterparts. Moreover, GM transfer from conventionally raised mice to GF ones resulted in 60% increase of body fat and insulin resistance despite being on a low food diet (Backhed et al. 2004). Furthermore, the same group reported that the GM of obese mice showed an increased abundance of sensing and digestion of carbohydrate genes, as well as increased SCFA levels. These findings are suggesting that GM is an added factor contributing to the obesity onset (Turnbaugh et al. 2008). The importance of GM composition in the induction of obesity has been proven as a high-fat/high-carbohydrate diet leading to weight/fat gain, induce a GM shift when compared to rodents on a low-fat/high-polysaccharide diet. Additionally, the same authors reported that a low in carbohydrate and fat diet which limits weight gain and reduces obesity can increase Bacteroidetes abundance and reduce fat deposition (Turnbaugh et al. 2008). However, those findings are questioned by Fleissner et al. who found that the absence of GM is not protecting against diet-induced obesity (Fleissner et al. 2010).
Additionally, apart the composition, it is the diversity of GM that has been related to obesity. Comparing obese and normal-weighted Danish subjects, those who had reduced GM diversity, with microbial gene size less than 480,000 (median 600,000), had more adipose tissue, insulin and leptin resistance, and dyslipidemia compared to their counterparts which had huge gene numbers. Also, obese subjects with low gene counts had the tendency to gain more weight over time as compared to those with high gene counts, indicating that a low GM diversity identifies a subset of patients at bigger risk for obesity and related comorbidities (Le Chatelier et al. 2013).
There are still unknown mechanisms of how some factors can influence GM and its association to obesity. For instance we still don’t know the effect of gender (Haro et al. 2016; Santos-Marcos et al. 2019). In addition, sometimes we only have empirical observations: In children before reaching the age of 2 years, the administration of three or more courses of antibiotic therapy that disrupt GM composition, is linked to an augmented risk of early childhood obesity (Scott et al. 2016).
The disruption of the gut microbiota balance observed in obesity is correlated with insulin resistance, chronic inflammation, and metabolic disturbances which further alter GM structure and are increased by the concomitant shift in GM production of vitamins (Astrup and Bügel 2019). For instance, it has been shown that metformin (used for type II diabetes management) changes the rodents’ GM and restore the diminished quantities of Akkermansia muciniphila which decreases the negative effect of the diet on the gut barrier, and therefore reduces metabolic endotoxemia, and improves insulin sensitivity (Compare et al. 2016). It has been shown Akkermansia muciniphila is decreased in obese subjects and administration of those bacteria is beneficial to the host. It is worth to note that for exercising its beneficial effects only the membrane protein Amuc_1100 of the bacterium is needed (Plovier et al. 2017). Moreover, metformin changes several SCFA producing microbiota including Butyrivibrio, Bifidobacterium bifidum, Megasphaera, and Prevotella (de la Cuesta-Zuluaga et al. 2017).
Another beneficial bacterium for weight loss is Christensenella as it has been shown that its abundance into the human intestine reduces BMI, and it can induce weight loss when administered to mice (Goodrich et al. 2014).
It has been reported that 75% of patients with severe obesity have low microbial gene richness (MGR) , a finding which is related with increased BMI, inflammation, and insulin resistance (Debédat et al. 2019). It has been show that in these patients MGR is improved after a short-term energy-restricted diet (Cotillard et al. 2013).
Phylogenetic analysis of GM of three groups (normal weight, obese, and post-RYGB subjects) revealed the presence of six main bacterial phyla. Most of the bacteria were Firmicutes and Bacteroidetes, while the remaining dispersed among Proteobacteria, Actinobacteria, Fusobacteria, and Verrucomicrobia. The distribution of these bacteria in the intestines of the study groups differs greatly. More specifically, Prevotellaceae from the Bacteroidetes family and Erysipelotrichaceae from Firmicutes phyla are mostly abundant in obese subjects. As Prevotellaceae is only found in obese individuals, it is considered “obese specific” while, in contrast, Fusobacteria and the family Enterobacteriaceae within Proteobacteria were found only in the RYGB group (Zhang et al. 2009).
All these data provide evidence that obesity is related to a change of the GM structure and to a disorder deviating from the normal function, with both leading to an augmented energy production from the ingested food. Since this GM dysbiosis is involved from the onset of obesity, it is reasonable to expect that restoring the disturbed GM could result to a metabolic state improvement (Cӑtoi et al. 2019).
Regarding humans, a milestone study showed that 12 obese subjects were initially exhibiting less Bacteroidetes and more Firmicutes than their lean counterparts (Ley et al. 2005). When the subjects assigned to caloric-restricted diet (fat- or carbohydrate-restricted), an increase of Bacteroidetes and a concomitant decrease of Firmicutes occurred, regardless of the type of diet implied. Most importantly, the increased richness of Bacteroidetes correlated with the observed percentage of weight loss and not with the diet switch (Ciobârcă et al. 2020). A recently published study showed that 75% of the candidates to BS displayed a low GM gene abundance and this finding correlated with increased fat mass of the trunk and related comorbidities (T2DM, hypertension, etc.) (Aron-Wisnewsky et al. 2019).
Apart from the decreased diversity of GM observed in obese subjects, it seems that they carry more aerotolerant bacteria, which are capable to produce products which can be easily converted to SCFAs. An imbalanced GM is capable to result in weight gain through its potential to extract calories from nondigestible nutrients which escape from ingestion into the small bowel and can then be transformed to digestible compounds that are finally either excreted in feces or reabsorbed and subsequently transferred and stored to the liver until needed (Cani 2013; Jacobs et al. 2009). Bacterial fermentation of carbohydrate and proteins within the large bowel produces SCFAs mainly butyrate, propionate, and acetate (Krajmalnik-Brown et al. 2012; Rowland et al. 2018). Both butyrate and propionate are used as energy sources of the epithelial cells, and furthermore, they can both activate intestinal gluconeogenesis (IGN) (De Vadder et al. 2014). Additionally, acetate plays a role for the growth of other bacteria which are involved in cholesterol metabolism and lipogenesis. Furthermore, acetate may be engaged in central regulation of appetite (Frost et al. 2014). Therefore, although in normal conditions the involvement of GM in energy supply is small (Turnbaugh et al. 2006), it seems that through SCFA production, it can provide additional energy to the host, thus resulting in the expansion of adipose tissue mass (Cani 2013).
Several studies in obese rodents support the above GM mechanism leading to augmented fermentation and increased SCFA production and therefore to the development of obesity (Turnbaugh et al. 2009). However, the hypothesis of bigger SCFA production acting as a trigger for the onset of obesity is still on debate as some studies showed the opposite, i.e., the increased fermentation produced by the GM plays a protecting role against fat mass increase and obesity appearance (Cӑtoi et al. 2019).
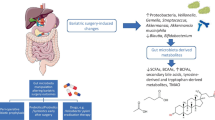
Obesity is also characterized from a low-grade chronic inflammation. It has been found that a high-fat diet for 4 weeks, increased up to two to three times the systemic lipopolysaccharide (LPS) levels and the LPS-containing GM, leading to a condition called as “metabolic endotoxemia.” Thus, the circulating high LPS levels may trigger inflammation which could then be the contributing factor for obesity and T2DM (Villanueva-Millán et al. 2015; Cani et al. 2007).
In obesity and in high-fat diet, because of GM disturbance due to a Bifidobacteria decrease, a markedly increased gut permeability is installed. Due to the break in the intestinal barrier, at first a mucosal inflammation is observed and then follows a migration of bacteria and/or their by-products from the gut lumen to the mesenteric lymph nodes (Compare et al. 2016; Festi et al. 2014). Consequently, the leakage of LPS and bacteria metabolites, as SCFA, and trimethylamine N-oxide (TMAO) result to the induction of “metabolic endotoxemia” followed by further cellular inflammatory responses. Lastly, this produces systemic low-grade inflammation, insulin resistance, and adipocyte hyperplasia (de Punder and Pruimboom 2015). Lately, two more mechanisms have been suggested to be implicated in gut permeability and bacterial translocation: The first implies that the glucagon-like peptide-2 (GLP-2) , an anti-inflammatory as well as an intestinal growth factor, is inhibited by the altered GM. The other one refers to the endocannabinoid system, associated in both maintenance of epithelial barrier integrity and the permeability of the intestine (Compare et al. 2016; Moreira et al. 2012).
Both these mechanisms reveal the link that exists between dysbiotic GM, disruption of the gut barrier function, and “bacterial translocation” associated to a state of low-grade gut inflammation, i.e., “metabolic endotoxemia,” finally leading to systemic inflammation and consequently to the pathogenesis of obesity (de Kort et al. 2011).
Opposite to the previous findings, it has been shown that the Firmicutes/Bacteroidetes ratio changed in favor of Bacteroidetes in overweight and obese subjects (Kasai et al. 2015). Furthermore, other studies reported that the Bacteroidetes and Firmicutes amounts are substantially augmented in the obesity group when compared to the normal-weight one (Ismail et al. 2011). Interestingly enough, some researchers were unable to detect any differences between obese and normal-weighted individuals in the proportion of Bacteroidetes abundance (Duncan et al. 2008). Furthermore, they did not discover any association between BMI and the main phyla population (Finucane et al. 2014).
BS candidate obese patients have impaired nutritional status characterized by poor-quality food choices with a diet with low diversity and essential nutrients intake, thus contributing to intestinal dysbiosis (Al-Mutawa et al. 2018). The most common nutritional deficiencies and their prevalence before BS are Vitamin D (65–93%), Iron (13–47%), and Vitamin B12 (4–13%) (Frame-Peterson et al. 2017). Those results are indicating that diet might be the main contributor in shaping the GM. Some studies reported that diet change accounts for 57% of the total structural shift of GM, while genetic mutation accounts for less than 12%.
Finally, up to now, it is still challenging to answer whether the GM changes are a cause or a consequence of obesity. However, given that obese phenotype can be installed after obese microbiota inoculation, it is logical to assume that GM alterations could be one reason in inducing obesity (Cӑtoi et al. 2019). In summary, there is growing evidence that obesity is attributed to a specific GM profile which confers the host with an increased ability for calories extraction. It seems that GM imbalance contributes to the onset of obesity in tandem with an unhealthy diet. Therefore, the GM should be considered as a set of genetic factors that together with host genotype and lifestyle contribute to the pathophysiology of obesity.
6.5 Bariatric Surgery
6.5.1 Bariatric Surgery Modalities
When the lifestyle and/or medication-based approaches for losing weight in obese patients have proven ineffective, then bariatric surgery is an option, as it has been shown to be a highly effective therapeutic procedure for treating obesity (Tuomi and Logomarsino 2016). Thanks to its capability to encourage substantial and sustainable weight loss, bariatric surgery became an increasingly prevalent intervention for obesity treatment (Al-Najim et al. 2018).
Bariatric surgery (BS) interventions have been developed over the years and can be classified as either being restrictive or malabsorptive, both reducing food intake and promoting weight loss (Andari Sawaya et al. 2012). The different bariatric procedures started from the 1950s with radical small bowel operations such as the jejunal–ileal bypass, to the gastric bypass in the 1960s (Alden 1977; Griffen et al. 1977; Mason and Ito 1967), gastric banding in the 1990s (Kuzmak et al. 1990), and the more recently widely spread vertical sleeve gastrectomy (Almogy et al. 2004). Lately, the whole spectrum of bariatric procedures but especially gastric bypass and sleeve gastrectomy are referred as metabolic surgery procedures, thus emphasizing the health benefits associated with weight loss rather than simply weight loss itself (Santoro 2015).
The armamentarium of metabolic surgery procedures includes laparoscopic adjustable gastric band (LAGB), vertical sleeve gastrectomy (VSG), Roux-en-Y gastric bypass (RYGB), biliopancreatic diversion (BPD), and BPD with duodenal switch (BPD/DS) (Andari Sawaya et al. 2012; Fontana and Wohlgemuth 2010).
From all the abovementioned procedures , the most commonly performed worldwide are RYGB and VSG (Angrisani et al. 2015). Currently, about 50% of the bariatric procedures are VSG and around 40% are RYGB (Angrisani et al. 2017). However, although VSG became more popular during recent years, RYGB has been performed over decades, and therefore it is estimated that millions of RYGB patients are residing worldwide in the general population (Björklund and Fändriks 2019).
Table 6.1 presents a comparison among those two common bariatric procedures.
Today, BS is considered as the only effective treatment for achieving a pronounced and sustained weight loss (Björklund and Fändriks 2019). The Swedish Obese Subject (SOS) trial reports a weight loss following RYGB of 27 ± 12% after 15 years, whereas nonsurgical interventions (lifestyle changes and/or pharmacological treatment) have principally no effect over this time span. Controlled long-term studies (>5–8 years) on the effects of VSG are still few, but weight loss up to 5 years is similar to that occurring after RYGB (Björklund and Fändriks 2019).
Additionally, many studies have reported improvements in obesity-related comorbidities like T2DM, hypertension, metabolic syndrome, sleep apnea, and overall mortality after weight loss (Björklund and Fändriks 2019). It is worth to note that some of these metabolic improvements manifest well before body weight becomes reduced, indicating a direct action on metabolic control by the modified gastrointestinal anatomy and functions (Santoro 2015). As an example, it has been shown that after both RYGB and VSG, glucose levels decrease significantly, well before any considerable weight loss is achieved, due to weight-independent mechanisms (Pucci and Batterham 2019) such as the faster gastric emptying occurring following RYGB and VSG (Melissas et al. 2007; Thaler and Cummings 2009).
In 2016, a joint statement by several international diabetes organizations stated that metabolic surgery should be recommended in patients with class II and III obesity and considered as an option in patients with class I obesity with poor glycemic control (Rubino et al. 2016).
Additionally, after BS, total cholesterol, triglycerides, and LDL were significantly lower, along with increased HDL, implying a normalization of the lipoprotein profile, possibly due to the weight loss (Magouliotis et al. 2017). In a comparison study among RYGB and VSG patients, glucose, triglycerides, and HDL levels were comparable between the two groups, while insulin levels were significantly greater in the VSG group. Therefore, it is evident that both BS procedures are metabolically efficient, a finding parallel with their similar efficiency in weight loss (Magouliotis et al. 2017).
All the above data demonstrate the significant amelioration of metabolic and lipidemic profiles of patients undergoing bariatric surgeries.
6.5.2 The Mechanisms of Gastric Bypass
Gastric bypass procedures are considered as an artificial condition where the intestinal mucosal energy outflow is a physiological variable which can impact both body weight and glycose levels.
Contrary to an old assumption, the weight loss after a BS procedure is not achieved neither by malabsorption nor by a mechanical restriction of food intake. Instead, the main driving force for weight loss is rather a modified eating behavior which reduces energy intake (Makaronidis and Batterham 2016). Also, regarding the old belief that reduced meal size is due to the limited size of the gastric pouch is not valid anymore, as the current surgical procedure leaves a minimum gastric pouch (20–30 mL) but followed by a large caliber gastroenteroanastomosis (GEA) without any outflow restriction. Therefore, the small pouch together with the Roux limb should be considered as a common cavity, so any possibility for the GEA to act as a restriction site can be excluded. Using high-resolution manometry, it has been confirmed that during eating there is no intraluminal pressure gradient between the pouch and the Roux limb (Björklund et al. 2015). However, it has been reported that RYGB exhibits a restrictive element with the restriction site situated to the Roux limb (Björklund et al. 2010). Until now, the actual clearance rate of Roux limb has not been assessed and therefore to what extent such a dynamic flow restriction of the Roux limb plays a food intake regulating significance remains to be investigated.
In addition to regulating energy intake, different studies revealed an expanded energy expenditure in RYGB patients. Interestingly, it appears not to be the basal metabolic rate (BMR) that becomes upregulated, but rather the thermogenesis associated to meal intake is the causative process (Werling et al. 2015). The exact mechanism involved is unknown, but according to experiments in rodents, it might be due to a reprogrammed mucosal metabolism in the Roux limb.
Another two mechanisms of RYGB effect are the changes of circulating bile acids and these of the intestinal microbiota; More specifically, it is hypothesized that bile acids regulate glucose metabolism through the TGR5 receptor acting on L cells, causing release of GLP-1, and also provoke synthesis and secretion of fibroblast growth factor 19 (FGF19) which improves insulin sensitivity, leading to an improved glycemic control (Madsbad et al. 2014).
It has been reported that transferring feces from RYGB-treated to GF mice caused significantly bigger loss of weight as compared to mice receiving feces from sham-surgery treated mice (Makaronidis and Batterham 2016). Additionally, GF mice inoculated with fecal microbiota from BS patients added less fat than mice transplanted with microbiota originating from obese patients (Tremaroli et al. 2015). Theoretically, it is expected that the jejunal mucosa into the Roux limb becomes inflamed by the new intraluminal milieu and, in turn, responds starting an antiingestive signaling. Nevertheless, a thorough examination of the postoperative mucosa did not support this hypothesis, and although some pro-inflammatory signs were present, the Roux limb mucosa did not manifest any inflammation (Spak et al. 2010).
In summary, it seems that the biomechanic properties of the Roux limb wall regulate both food intake and intestinal sensing . Thus, the proposed hypothesis that “big mealers” have a low-threshold for inducing Roux limb clearance motility awaits confirmation (Björklund and Fändriks 2019).
6.5.3 Side Effects of Bariatric Surgery
Bariatric surgery has some unwanted consequences, thus requiring a cost-benefit analysis for every individual candidate. About 4% of patients after BS manifest surgical complications within the first 30 postoperative days (Schulman and Thompson 2017; Sjöström et al. 2004). Typical postoperative complications include anastomotic leakages, bleeding, perforation, and infections, as well as inner herniations (Schulman and Thompson 2017), although the herniation incidence has been dramatically lowered after the closure of any mesenteric defect became a standard routine practice during the BS operation (Stenberg et al. 2016). Late surgical complications are also detected in 15–20% of patients, and they include obstruction of the small bowel, anastomotic stenosis, or marginal ulceration (Franco et al. 2011). Both early and late surgical complications can be diagnosed and treated by means of a surgical or endoscopic intervention. Additionally, except typical surgical complications, there are also procedure-dependent side effects, like excess skin requiring additional cosmetic surgery, dumping symptoms and postprandial hypoglycemia, as well as micronutrients deficiency (Björklund and Fändriks 2019).
Unexplained chronic abdominal pain is a common negative side effect seen in patients after RYGB (Cho et al. 2008). It is reported that 54% of RYGB patients suffer from abdominal pain and in a 5-year follow-up, 34% of these patients still experience abdominal pain (Gribsholt et al. 2016; Høgestøl et al. 2017). It is of paramount importance to elucidate the underlying pathology of chronic abdominal pain following BS but its etiology remains still obscure (Greenstein and O’Rourke 2011). The long-term consumption of morphine or its analogs for pain relief in RYBG patients may provoke to opioid-induced bowel dysfunction which presents with constipation, nausea and vomiting, and to the narcotic bowel syndrome (King et al. 2017a). Furthermore, it is estimated that 4% of patients who were not on opioids before became chronic opioid users after BS (Raebel et al. 2014), and therefore the physician of a RYGB patient with chronic postprandial nausea and pain must be aware of the risk for iatrogenic opioid-associated symptom aggravations.
Hypoglycemia in patients without diabetes appears in 64–82% of patients during the first 5 years of BS (Schauer et al. 2017). The underlying mechanism is not clear, and several theories have been proposed including enhanced B cells mass and function, reduced ghrelin levels, improved insulin sensitivity, and failure of counter regulation (Abdeen and le Roux 2016). The consequent side effects of hypoglycemia often persist throughout the years and can thus worsen the quality of life.
6.6 Gut Microbiota After Bariatric Surgery
Many surgical diseases are related to gut microbiota alterations. So far, obesity, nonalcoholic fatty liver disease, colorectal cancer, intestinal anastomotic leaks, inflammatory bowel disease, and atherosclerosis have been reported (Chen et al. 2018).
As mentioned previously, BS is the treatment of choice to accomplish and maintain in the long term a normal weight to morbidly obese patients. Those patients who undergo BS are losing weight significantly, and they restore their metabolic health regarding T2DM, dyslipidemia, hypertension, and cardiovascular risk (Buchwald et al. 2004; Sjöström et al. 2007).
It has been shown that BS plays a cardinal role by altering the abundance of several microbial species of the GM. However, the available data regarding the changes of GM after BS are highly heterogeneous and insufficient to be included in quantitative analysis (Magouliotis et al. 2017).
The exact mechanisms underlying the postsurgical restructuring of the GM have not yet been elucidated and must yet to be explained. However, it is certain that the dramatic anatomical alterations induced by BS contribute significantly to the substantial metabolic changes observed following BS (Medina et al. 2017). Additionally, several factors coexist that can alter the postoperative status of the BS patients: Caloric restriction (substantially energy-restricted diet with higher protein intake), alterations in the secretion of gut hormones and bile acids, and changes of the GM composition have been proposed as possible mechanisms (Heneghan et al. 2012). Thus, due to the multiple metabolic and hormonal changes which coincide during the early postoperative period, it is rather difficult to establish underlying relationships between factors related to BS and changes in GM composition and function after performing BS (Lakhani et al. 2008).
Several studies have shown that bariatric surgery provokes alterations to the GM which can be installed as early as the first week after surgery and in any case as soon as the first 3 months postoperatively (Tremaroli et al. 2015; Liou et al. 2013; Palleja et al. 2016), and this effect is sustained up to 9 years (Tremaroli et al. 2015).
Additionally, late complications include severe deficiency-related disorders, such as anemia (10–74%) and neurological dysfunctions (5–9%) (Xanthakos 2009). Therefore, the patients who underwent BS are in need of a rigorous follow-up aiming to prevent those side effects through GM modulation and adequate nutritional supplementation (Ciobârcă et al. 2020).
It has been observed that a major alteration in the structure and diversity of GM is taking place after BS. A recent meta-analysis reviewed 22 studies and 562 patients who underwent different types of BS. Despite that different studies reported a considerable variation in the bacterial species, the overall findings support a postoperative shift of the GM (Makaronidis et al. 2016). Therefore, this GM change might not be the result but rather the reason of weight loss after BS, as it has been recently suggested that metabolic regulation is starting from the gut which then is signaling to the brain and other endocrine organs to adapt to this change (Fetissov 2017).
The most common change observed after BS procedures is a decrease of Firmicutes and an increase of Bacteroidetes, Proteobacteria, especially of Gammaproteobacteria (genus Escherichia) abundance (Zmora et al. 2019). In another study, a decrease of the Firmicutes/Bacteroidetes ratio was reported following BS in subjects with morbid obesity, accompanied with a substantial change of the structure and function morbidly of the GM. However, the whole subject is still under debate (Tremaroli et al. 2015). It is also worthwhile to note that additional GM changes following BS have been reported in a study: An increase in the phyla Verrucomicrobia and Fusobacteria and a diminished amount of Actinobacteria (Ulker and Yildiran 2019).
Some articles focused on fecal microbiota transfer experiments. A well-planned study showed that both RYGB and VBG have similar long-term effects on the composition and functional capacity of the gut microbiome. It is worth to note that the GM changes were independent from BMI or from the magnitude of weight and fat mass loss, thus suggesting that BS can cause specific shifts in the GM. In the same study, feces from BS patients were transplanted to GF mice; 2 weeks after transplantation, the mice gained less fat as compared to reciprocal mice transplanted with GM from obese subjects. Those findings suggest a causal relationship between GM and to BS-induced weight loss (Tremaroli et al. 2015). The same results are reported in another study which showed that GM transplantation from mice which underwent RYGB to sham-surgery germ-free mice provoked weight loss and decrease of adipose tissue when compared to recipients of GM from nonoperated mice (Liou et al. 2013).
A similar GM transplantation study was done in a group of females who, 9 years previously, were randomly assigned to undertake RYGB or VSG: Both types of surgery recipients showed similar GM profiles of their fecal samples (as assessed by means of 16S rRNA amplicon sequencing analysis) and furthermore, they were substantially different from the profiles of nonoperated obese women. When feces from BS patients were inoculated to GF mice, the recipients had decreased fat mass as compared to reciprocal mice that received GM from obese, nonoperated subjects. Additionally, the recipient mice which were transplanted with human post-RYGB GM showed the bigger increase of lean body mass. Therefore, it seems that the human GM can directly trigger the reduction of adipose tissue seen after BS (Tremaroli et al. 2015).
In another longitudinal study of obese individuals, it was found that Bacteroidetes were reduced prior to surgery, but 3 months post-RYGB, the Bacteroidetes abundance was returned to presurgery levels, being remarkably similar to that of lean control group. Additionally, the observed abundance in Bacteroidetes following RYGB correlated with a substantial decrease of adipose tissue and an increased serum leptin levels (Furet et al. 2010).
Methanogenesis facilitates the fermentation of dietary fibers through the consumption of hydrogen and acetate, and methanogenic archaea are found in abundance in obese subjects. In a study comparing the 16S rRNA sequences in the feces of three groups, namely normal weight, morbidly obese, and post-RYGB subjects, distinct differences were found in the GM between the three cohorts; Methanogenic archaea were found in abundance in the obese group, but they were found below detection levels in normal weighted or all-but-one post-RYGB patient (Zhang et al. 2009).
The same changes in the GM are also observed after sleeve gastrectomy: In diet-induced obese mice that underwent VSG, a substantial and sustained increase of Bacteroidetes and a relative decrease in Firmicutes is reported. Additionally, GM metabolism is related to that of the host. Thus, 3 months after VSG, several metabolic processes of the patients, such as carbohydrate fermentation, citrate cycle, and amino acids production, as determined by shotgun metagenomic sequencing, became more analogous to those of normally weighted control group (Jahansouz et al. 2017). However, regarding the metabolic improvement or the degree of weight loss, it seems that BS itself is more important factor relatively to the feces transplantation, indicating that apart from GM, BS and other pathways are involved in those positive results (Aron-Wisnewsky et al. 2019).
Several other gut bacteria are proliferating after BS; Due to the increased pH into the lumen and high levels of dissolved oxygen, both been observed after BS, the growth of facultative aerobic microorganisms (such as Proteobacteria) and inhibition of anaerobic microbes is observed (Medina et al. 2017). In tandem, the diminished gastric volume resulting after BS increases the pH of both the stomach and distal intestine, and the resulting gastrointestinal acidity leads to microbial overgrowth and promotes the abundance of Akkermansia muciniphila, E. coli, and Bacteroides spp. or of the oral microbiota bacteria (Anhê et al. 2017).
However, there is a couple of studies using sequencing methods, described a high MGR and bigger GM diversity following both RYGB and VSG as well as a change from “obese” to a “lesser obese” microbial species profile (Debédat et al. 2019; Aron-Wisnewsky et al. 2019). Nevertheless, despite profound weight loss and improvement of metabolic markers after both surgeries, the MGR may not be fully restored 1 year after RYGB and remain unchanged even after 5 years (Aron-Wisnewsky et al. 2019; Anhê et al. 2017). The absence of complete repair of GM after BS could explain the observed delayed regain of weight and the recurrence of obesity related comorbidities observed in some patients after BS. The fact that BS alone cannot reestablish MGR indicates that other contributing mechanisms (i.e., metabolic and inflammatory amelioration, weight loss, or diet) are also involved (Debédat et al. 2019).
However, the two BS surgeries might exhibit different functionality due to the different surgical techniques as well as to resulting different intestinal environmental conditions. With that in mind, one would anticipate more profound changes in the intestine after RYGB as contrasted to VSG, as besides caloric restriction, it involves more radical and complex anatomical changes and more functional modifications of the GI tract (Cӑtoi et al. 2019).
Below are listed some studies exploring the GM-related outcomes of the different surgical BS procedures.
Administration and/or abundance of Akkermansia muciniphila is related to enhanced gut barrier function and diminished metabolic endotoxemia as a result of decrease of the circulating levels of systemic lipopolysaccharide (Everard et al. 2013). Also, the administration of Akkermansia muciniphila rose L cells numbers which, when stimulated, induce GLP-1 release which is involved in glucose homeostasis (Yan et al. 2016) and GLP-2, an important intestinal growth factor (Everard et al. 2011). It has been reported that after RYGB, the Akkermansia muciniphila increases (Graessler et al. 2013) which has been negatively correlated with body mass (Anhê et al. 2015).
Furthermore, following RYGB, Escherichia coli abundance is enhanced and, independently of food intake changes, it is inversely correlated with fat mass and leptin levels, in contrast to Faecalibacterium prausnitzii, which is found to decrease after RYGB (Furet et al. 2010).
Several factors have been advocated to play a role for the vast GM restructuring observed after RYGB as the disrupted anatomy (small gastric remnant and shortened small intestine) results in decreased food ingestion. Additionally those severe anatomic changes also have some physiological consequences like changes in pH, transit time, and input of dissolved oxygen which promotes the relocation of some of the typically residing in the small bowel microbiota, to the large intestine (Zhang et al. 2009). Additionally, the observed GM change after RYGB could also be attributed to altered bile acid metabolism which is regulated by BS as well (Peck and Seeley 2018).
Two recent meta-analyses reported that although after BS the diversity and richness of GM greatly fluctuated across studies, certain bacterial phylae such as Bifidobacteria was strongly correlated with BMI (Magouliotis et al. 2017; Guo et al. 2018).
A study investigated whether the GM changes after RYGB are preserved and whether inoculation of RYGB modified microbiota can provide a transferable weight loss effect on other recipients. Using a mouse RYGB model which resembles many of the metabolic outcomes seen in humans, fecal samples of three groups were collected for 16S ribosomal RNA gene sequencing: after RYGB surgery, sham surgery, or sham surgery coupled to caloric restriction. The sequential analysis showed that distal gastric, ileal, cecal, and colonic microbiota were strongly altered after RYGB. A rapid and sustained increase in the relative abundance of Enterobacteriales and Verrucomicrobiales was found. Three phyla increases are prevailed: In Bacteroidetes, Verrucomicrobia, and Proteobacteria, with resolution to the genus level of Alistipes, Akkermansia, and Escherichia. The observed GM alterations were unbiased of weight alteration and calories restriction and were found along the entire length of the GIT but mostly evident distally from the surgical manipulation site. The recipient lean GF mice transplanted with feces from RYGB-operated rodents had reduction of fat mass which was not observed after inoculation of GM from mice that had lost weight due to food restriction. The above findings provide evidence to the assumption that GM changes contribute to reduced host weight and fat mass following RYGB surgery (Liou et al. 2013).
A study performed in morbidly obese individuals within 3 months after they underwent RYGB found that their GM featured an increased relative abundance of 31 species, including Escherichia coli, Klebsiella pneumoniae, Veillonella spp., Streptococcus spp., and Alistipes spp., while Akkermansia muciniphila and Faecalibacterium prausnitzii decreased in their relative abundance. Furthermore, an augmented potential for oxygen tolerance as well as for microbial utilization of macro- and micronutrients was reported and those changes were still present 1 year after RYGB (Palleja et al. 2016).
The phylogenetic analysis of GM of three groups (healthy, obese, and post-RYGB subjects) showed six main bacterial phyla to be present but distributed differently in the GI of the study groups. Interestingly enough, Prevotellaceae was explicitly detected only in obese subjects, and therefore it is considered as obesity specific bacteria. To the contrary, Fusobacteria and the Enterobacteriaceae within Proteobacteria family were found only in the RYGB group (Al-Najim et al. 2018).
Tremaroli et al. (2015) performed shotgun sequencing of the fecal metagenome to analyze the GM of weight-stable women 9 years post-RYGB. Furthermore, they conducted human-to-mouse GM inoculation. After RYGB, an increased abundance of Gammaproteobacteria was detected, while in contrast lower levels within the Firmicutes phylum of Clostridium difficile, Clostridium hiranonis, and Gemella sanguinis were detected. In contrast, facultative anaerobes within Proteobacteria (Escherichia, Klebsiella, and Pseudomonas) family were found augmented in the RYGB recipient mice. The metabolomic comparisons performed after BS showed an inhibited SCFA/branched-chain fatty acid ratio, a finding suggesting an increased amino acid fermentation. The genetic signatures for microbial enzymes participating in the synthesis of secondary bile acids were enhanced in parallel to a shift of secondary to primary bile acid profiles ratio, suggesting that altered bile acid profiles may participate in reductions in fat mass following BS (Al-Najim et al. 2018).
In a study comparing the impact of both RYGB and VSG on GM, an important increase of Proteobacteria was found. The same altered pattern (a Roseburia abundance) was also shown in T2DM patients who underwent RYGB or VSG when a T2DM remission was achieved. In contrast, 6 months postoperatively, despite similar weight loss, the Bacteroidetes increased in RYGB group of patients, while it decreased in the VSG group (Davies et al. 2019).
Additionally, as RYGB provokes greater rearrangements of the digestive tract than VSG, a significantly lower body weight and a greater shift on GM were produced from RYGB as compared to VSG, 9 weeks postoperatively (Shao et al. 2017). It is postulated that the differences observed between the two techniques could be due to the fact that VSG involves much less intestinal manipulations than RYBG. The above results were also confirmed by a study which revealed that RYGB provoked increased Firmicutes and Actinobacteria but decreased Bacteroidetes, but the later been found increased after VSG. Thus, 1 year following RYGB surgery, more significant functional GM alterations were found as compared to VSG, despite similar diet, weight loss, or remission of T2DM (Murphy et al. 2017).
It has been reported that sleeve gastrectomy provokes both early (1 week after surgery) and prolonged (1 month after surgery) changes of the GM. Furthermore, the same article demonstrated that the altered microbial composition of VSG operated rodents is persisting and does not change even when reexposure to obesity associated GM occurs (Jahansouz et al. 2017). The same findings are also reported regarding the functional capacity of GM after VSG in 23 obese patients. It was found that 3 months post-VSG, the microbial activity was similar to that of lean subjects and a marked increase of B. thetaiotaomicron, an anti-obesogenic substance, was observed (Liu et al. 2017).
In a recent systematic review, Davies et al. summarized 14 clinical studies, with a total of 222 subjects (RYGB = 146, VSG = 25, biliointestinal bypass = 30, vertical banded gastroplasty = 7, and adjustable gastric band = 14). Major switches comprise a reduction of the relative abundance of Faecalibacterium prausnitzii and an increase of E. coli. After VSG, a decrease in the relative abundance of Firmicutes while following RYBG an increase in Bacteroidetes and Proteobacteria was also noticed (Davies et al. 2019).
Their findings are summarized in Table 6.2. It was found that the different types of BS result in dramatic changes of gut bacteria, but the contribution of those alterations to the metabolic benefits achieved is still unclear (Davies et al. 2019).
A systematic review and meta-analysis reviewed the impact of BS in metabolic and GM profiles, of 22 articles published between 2008 and 2016. However, they found that only two studies were randomized, the rest being prospective ones (Tremaroli et al. 2015; Kong et al. 2013). The total sample size was 562; 411 patients had RYGB and 97 underwent VSG (Magouliotis et al. 2017).
As shown in Table 6.3, several microbes are affected by BS. As can be seen from this table, some authors found increased Bacteroides while Firmicutes and Bifidobacterium had lower abundance in the post-RYGB subjects (Graessler et al. 2013; Lips et al. 2014).
More specifically, regarding RYGB , two studies found lower Firmicutes abundance after RYGB (Graessler et al. 2013; Lips et al. 2014) while two other studies showed the opposite (Narath et al. 2016; Trøseid et al. 2016). Additionally, another study showed that Lactobacillus, been part of the Firmicutes family, was in higher abundance after biliointestinal bypass (Papamargaritis et al. 2013). The discrepancies observed among the results of those studies can be explained from the different clinical protocols applied using varying levels of calorie restriction. Furthermore, another couple of studies showed an increased Bacteroides abundance in RYGB patients and the higher was the Bacteroides increase after RYGB, the bigger the decrease in body fat mass and leptin (Graessler et al. 2013; Lips et al. 2014). It is worth to note that the same findings were also reported in less obese subjects (Quercia et al. 2014).
In another study, an increased Bacteroidetes abundance was found after VSG, while after RYGB a decrease for the same phylum was observed (Narath et al. 2016). Regarding E. coli population, it was found enhanced in five studies (Graessler et al. 2013; Lips et al. 2014; Trøseid et al. 2016; Papamargaritis et al. 2013; Gralka et al. 2015). The increase in abundance of Escherichia coli could be due to anatomical readjustments causing higher oxygen concentrations in the distal intestine (Gralka et al. 2015).
In summary, BS seems to restore a healthier microbiome with a leaner metabolic profile, and this realignment of the microbiome potentially contributes to reduced fat mass, increased lean mass, and resolution of BS associated comorbidities. However, the mechanisms by which gut microbes and their by-products affect obesity remain poorly understood and microbiome manipulations that exploit the host–bacteria interaction for the treatment or prevention of obesity still need to be developed (Chen et al. 2018).
6.6.1 Bariatric Surgery–Related Diet on Gut Microbiota
The rearrangement of the gastrointestinal tract following BS leads to alteration of the gut microbial ecology. The postsurgery food intake of patients submitted to RYGB or VSG has major quantitative and qualitative changes; In a matter of days, the calories restriction alters the bacterial structure of the bacterial community (Zmora et al. 2019).
It has been postulated that the observed GM shift after VSG (i.e., the reduction of the Firmicutes/Bacteroidetes ratio) might be the adaptive response of bacteria to the caloric constraint imposed by surgery. More precisely, the Firmicutes decrease results to diminished fermentation, to subsequent reduced energy intake, and, finally, to concomitant SCFAs production, the latter being substrates for gluconeogenesis and lipogenesis. A study showed that VSG, but not a strict dietary regimen with low calories, enhanced the obesity related GM synthesis towards a lean microbiome phenotype (Damms-Machado et al. 2015). Moreover, it has been shown that, in a mouse model, when only food restriction is applied there are no early changes in GM after RYGB, and therefore, weight loss seems to be one among the least important factors involved in the GM shift (Anhê et al. 2017).
Thus, in 45 subjects submitted to either RYGB (n = 23) or VSG (n = 22), GM composition and diversity changes were assessed before following a 2-week crash diet (baseline), by the end of it, as well as 1 week, 3 months, and 6 months postoperatively. A substantial but temporary alteration in GM was noticed after the baseline crash diet, but BS provoked more persistent changes in GM composition and to restoration of microbial diversity well before any significant weight loss, irrespectively of the type of BS performed. Both RYGB and VSG groups exhibited the same magnitude GM changes in all phases of the study (Paganelli et al. 2019).
6.6.2 Bariatric Surgery Effect on Small Intestine Bacteria
Obese patients after bariatric surgery may present small intestine bacterial overgrowth (SIBO) , a condition defined as greater than 105 bacteria (colony forming units) mL−1 of proximal jejunal aspiration (DiBaise 2008). SIBO is a common manifestation of obesity and a recent prospective study, including 378 patients with morbid obesity, reported that 15% of patients before undergoing RYGB had SIBO, and that this figure increased up to 40% after the operation (Paganelli et al. 2019).
In clinical practice, SIBO diagnosis is made from small bowel aspirate test, but this test is invasive and costly so the most practical detection method is the “therapeutic trial,” by empirically administering treatment with antibiotics upon the presence of the clinical manifestations associated with SIBO (Adike and DiBaise 2018).
SIBO interferes to the weight loss process and increases the micronutrient deficiencies risk. It manifests with several gastrointestinal symptoms, including bloating, diarrhea, and nutrients malabsorption, all depending from the specific type of bacteria that overgrow into in the small intestine (Sachdev and Pimentel 2013). Mechanical stasis is frequently associated with RYGB and creation of blind loops. SIBO bacteria bear a resemblance to those normally found in the colon, either gram-negative aerobes and/or anaerobes species, such as E. coli, Enterococcus spp., Klebsiella pneumonia, or Proteus mirabilis, capable to metabolize undigested carbohydrates into SFCA and gas. The disproportionate growth of atypical bacteria in the proximal small intestine permits their competition with the human host for nutrients harvesting . Additionally, the inflammatory response following SIBO provokes alterations of the epithelial cells and provokes villous atrophy and/or stimulates the synthesis of inflammatory cytokines resulting to mucosal injury (Sabate et al. 2017).
It has been shown that SIBO also impairs the absorption of vitamins B1 and B12. In a retrospective analysis of 80 RYGB patients, 39 of them had lower B1 levels than the reference range (Dukowicz et al. 2007). Twenty-eight of these patients had elevated folate levels in plasma, a marker suggesting the SIBO presence, and another 15 were also diagnosed with SIBO by undergoing glucose-hydrogen breath testing (Sachdev and Pimentel 2013). The persistent B1 deficiency rapidly resolved after treating SIBO with antibiotic therapy (Dukowicz et al. 2007). Secondary megaloblastic anemia may be present following RYGB due to impaired B12 absorption. In a case report of two patients submitted to RYGB which were positive for SIBO postoperatively, although antibiotic treatment improved hemoglobin levels, mean cell volume was still increased while B12 level was below the normal range (Sachdev and Pimentel 2013).
The malabsorption of fat-soluble vitamins, like A, E, and D, arises due to the bacterial deconjugation of bile acids by small intestine bacteria leading to the formation of toxic lithocholic acid, which further aggravates the intestinal epithelial cell disfunction and subsidizes carbohydrate and protein malabsorption as well (Sabate et al. 2017). In contrast, in patients with SIBO, the vitamin K levels are within normal limits or even increased since bacteria are capable to synthesize menaquinone (Grace et al. 2013).
The reduced brush border enzyme activity as well as the substrate readiness generate impaired carbohydrate uptake, which small bowel bacteria can metabolize prematurely. Also, increased numbers of small bowel bacteria compete with the host for intraluminal protein, thus disturbing the amino acids and peptides absorption. Furthermore, patients with SIBO demonstrate diminished enterokinases levels which result to impaired proteolytic reactions and subsequently to disturbed activation of pancreatic zymogens (Grace et al. 2013).
6.6.3 Bile Acids and Gut Microbiota Interactions
The bacteria involved in the deconjugation of bile acids are mostly Bacteroides species, which were reported to be decreased in BS patients, and this alteration is correlated with decreased fat mass and improved glucose control (Damms-Machado et al. 2015). The gut bacteria contribution in deconjugation and fermentation of primary bile acids to secondary ones has different impacts on human metabolism; The primary bile acids foster metabolism improvement, while secondary bile acids do not but rather seem to initiate carcinogenic processes (Swann et al. 2011). In addition, GM benefit from the deconjugation of bile acids as it can consume glycine or taurine for its own metabolism (Dawson and Karpen 2015). Also, bile acids shape the GM population through regulation of their growth and colonization and impacting the structure of their cell membrane. It has been reported that bile acids exhibit antimicrobial effects on certain bacteria while they promote the growth of others (Wahlström et al. 2016).
It seems that FXR plays multiple roles in metabolism regulation. FXR is a major regulator of bile acid signaling in both the liver and intestine, controlling the enterohepatic cycle of them by inhibiting hepatic bile acid synthesis and intestinal absorption. Additionally, bile acids serve as a ligand for FXR and appear to control glucose metabolism via FXR-related pathways. In this way, bile acids expand their molecular repertoire as modulators for both glucose and lipids metabolism (Bozadjieva et al. 2018). Finally, genetic and pharmacological mouse models have demonstrated differential roles of liver and intestinal FXR signaling in glucose metabolism and weight management (Bozadjieva et al. 2018).
Bile acid levels are increased in response to BS, and it is suggested that they mediate weight loss and metabolic improvements after BS (Patti et al. 2009; Pournaras et al. 2012). Regarding RYGB, the plasma bile acids are increased due to the fast supply of undiluted bile to the distal L cells and activation of the TGR5 receptors (Peterli et al. 2013). Additionally, a significant increase in the 12a-hydroxylated/non-12a-hydroxylated bile acid ratio has been described following RYGB (Furet et al. 2010). In RYGB, bile acids do not mix with food until the latter part of the jejunum. Therefore, in obese rodents which underwent RYGB, the procedure produced significant weight loss and improvement in glucose tolerance independently from the weight (Kohli et al. 2013). This is also reported in a study where increased bile acid levels were found in T2DM patients who underwent RYGB, but they were decreased after a hypocaloric diet that resulted in similar weight loss in T2DM patients, suggesting that the increase in bile acids after BS is weight independent (Jahansouz et al. 2016).
It has been suggested that FXR is crucial for the positive outcomes of VSG on both weight loss and glycemic control, as FXR-deficient mice despite been submitted to VSG showed reduced ability to decrease body weight and improve glucose tolerance (Ryan et al. 2014). It is worth to note that increased bile acids levels are also found after VSG (Stefater et al. 2011; Nakatani et al. 2009). This implies that this is not simply due to rerouting of bile acid as in the case of RYGB, but rather a physiological change of bile acids regulation than simply an operation-related displacement of the bile acids (Bozadjieva et al. 2018). Moreover, FXR is essential for the positive effects of VSG on weight loss and glycemic control (Bozadjieva et al. 2018; Ryan et al. 2014).
The hypothesis that bile acids exhibit a contributory role in mediating the effects of BS is not always granted. For instance, in a study comprising T2DM and normoglycemic patients who underwent RYGB, glucose metabolism improved shortly after surgery, but the total bile levels did not increase until 3 months postsurgery (Jørgensen et al. 2015). Another study reported decreased bile acid levels shortly after surgery and an increase at 2 years after it (Dutia et al. 2015). These data reveal the possibility that the relationship between the clinically relevant effects of BS procedures and the alterations of bile acid levels may be more complicated.
The gut-derived peptide FGF15/19 is a potential molecular and therapeutic marker to elucidate the positive metabolic effects of BS (Bozadjieva et al. 2018). FGF15/19 is expressed in ileal enterocytes of the small bowel and is released postprandially in response to bile acid absorption. Once released from the ileum, FGF15/19 enters the portal venous circulation and travels to the liver where it binds to its receptor FGFR4 and suppresses the de novo bile acid synthesis via reduction of cholesterol 7a-hydroxylase (CYP7A1) and gallbladder filling.
It has been reported that circulating FGF19 levels increase following BS, indicating FGF15/19 as a potential target to mediate the positive effects of BS. However, how the increased levels of FGF19 in patients following BS directly mediate the beneficial effects of the surgical procedure is still unclear. Future studies that apply BS in combination with animal models with tissue-specific deletion of FGF15 or FGFR1/4 may provide further insight into understanding the direct role of FGF15/19 signaling in mediating the effects of BS. The literature data indicate the need of more studies to fully understand the plethora of FGF15/19-mediated actions. Understanding these complex actions may help researchers to directly link the FGF15/19 increase with specific metabolic benefits of BS (Bozadjieva et al. 2018).
6.6.4 Micronutrient Deficiencies After Bariatric Surgery
Following BS, 30–70% of patients develop nutritional deficiencies which, if severe, can result to edema, hypoalbuminemia , anemia, and hair loss as well as peripheral neuropathy, Wernicke encephalopathy and beriberi, metabolic bone disease, and anemia (Bal et al. 2012). Micronutrient deficiencies are common after RYGB and VSG (Krzizek et al. 2018), and a prevalence up to 50% in mid- and long-term follow-up has been reported (Adike and DiBaise 2018). The underlying causes can be due to either surgery- or patient-related reasons (Alexandrou et al. 2014).
BS may lead to severe postoperative micronutrient deficiencies which persist despite vitamin and mineral supplementation. A variety of factors can contribute to micronutrient deficiency observed after BS including eating behavior, decreased absorption, SIBO, poor compliance to the suggested optimization of diet and to prescribed nutritional supplementation (Sweeney and Morton 2013).
It is well documented that after both RYGB and VSG, the restriction of food intake, the reduced appetite, as well as the changes of gastrointestinal hormones are common mechanisms for the observed weight loss (Patel et al. 2017). Furthermore, the complications observed after BS, such as nausea, vomiting, food intolerance, or SIBO, may result to vitamin and mineral deficiencies (van Rutte et al. 2014).
It is of interest to state that micronutrient deficiencies are manifested in a similar degree after VSG and RYGB, although fewer micronutrient deficiencies are to be expected after VSG, since the small bowel remains intact after this operation (Patel et al. 2017; Aarts et al. 2011). This observation leads to the assumption that BS-related micronutrient deficiencies must be explained by different mechanisms: Namely, VSG accelerates gastric emptying and gastroduodenal transit time and, furthermore, reduces the secretion of hydrochloric acid and of the intrinsic factor. All these changes, due to the gastric fundus resection, affect the gastrointestinal motility, and, therefore, the release and dissolution of several vitamins and minerals is diminished (Aarts et al. 2011).
On the other hand, after RYGB, the bypass of the remnant stomach and of the upper part of the small intestine exclude the exposure of the food bolus to the biliopancreatic secretions and therefore affect the vitamins and minerals absorption. It is worth to note that the degree of malabsorption is related to the length of the common channel (distal jejunum, ileum, and colon) rather than the length of the Roux limb (Ferraz et al. 2018). Additionally, diminished absorption may also occur in the common portion of the small intestine as an asynergia consequence between food bolus, bile acids, and pancreatic enzymes. Finally, following RYGB, the absorption of some micronutrients (especially vitamin B12) can also be reduced due to a lower location of gastric juice output as a result of bypassing the distal stomach (Stefanidis et al. 2011).
Except the abovementioned BS-related variables of micronutrient deficiency , some patient-related causes can alter their postoperative micronutrient status. Thus, it has been reported that patients who underwent BS may exhibit substance and alcohol abuse as well as poor compliance to the nutritional supplementation protocol. Thus, a long-term (up to 7 years) follow-up study of more than 2000 BS patients reported that 20% of patients submitted to RYGB developed alcohol use disorder (King et al. 2017b). In a 2019 questionnaire-based survey on 533 BS patients slightly over half of the respondents reported nonadherence to micronutrient supplementation (Mahawar et al. 2019).
The main micronutrient deficiencies reported after both BS include vitamin B12, folic acid, iron, thiamine (vitamin B1), vitamin D, and calcium (Antoniewicz et al. 2019; Engebretsen et al. 2018). Other reports on nutritional deficiencies after weight loss surgery, particularly following mixed bariatric procedures, are for fat-soluble vitamins (liposoluble), namely, vitamin A (Eckert et al. 2010), vitamin E (Boylan et al. 1988), and vitamin K (Lupoli and Milone 2015), as well as for copper (Boylan et al. 1988), zinc, and selenium (Sallé et al. 2010; Hassan zadeh et al. 2019). Therefore, lifelong nutritional supplementation, especially regarding protein, iron, folate, calcium, vitamins B1, and B12, and D, is a critical part of the postsurgical management of BS-operated patients as those substances are the most affected (Bal et al. 2012).
6.7 Conclusion
Bariatric surgery, being the most effective treatment of severe obesity, has continuously expanding use in our modern era. From the other hand, the role of gut microbiota on the host’s ability to maintain a healthy metabolism and digestion is widely recognized. However, our understanding of the linking mechanisms between obesity and concurrent changes in gut microbiota is not clear as it seems that bariatric surgery cannot fully restore the disrupted microbial balance provoked by obesity. Therefore, there is a growing interest regarding the effects of bariatric surgery on gut microbiota as the weight loss and improvement or remission of obesity related comorbidities after bariatric surgery are associated with significant alterations in gut microbiota composition.
The exact contributing mechanisms which induce the GM alterations after bariatric surgery are not clear as different factors have been suggested namely diet, weight loss, or surgery itself. Moreover, there are some side effects that are triggered from the onset of small intestine bacterial overgrowth, which affect the weight loss process of the patients who underwent bariatric surgery.
Still the impact of bariatric surgery is not well defined, as the microbiota alterations which are detected following surgery are not consistent, and they should be considered in the context of restricted energy intake and altered dietary quality. Moreover, no differences regarding GM modulation were observed among the two most currently performed weight loss surgery techniques, i.e., RYGB and VSG. In general, an increase in members of the phylum Bacteroidetes and Proteobacteria, as well as a decrease in members of the phylum Firmicutes is reported.
In summary, bariatric surgery seems to attempt to restore a healthier gut microbiome with a leaner metabolic profile, and this microbiome realignment potentially contributes to the observed reduced fat mass reduction, the increase of lean mass, as well as resolving the obesity related comorbidities. However, the mechanism by which microbes and microbial by-products restore the gut microbiota remains poorly understood, and microbiome manipulations that exploit the host–bacteria interaction after bariatric surgery still need to be developed.
References
Aarts EO, Janssen IMC, Berends FJ. The gastric sleeve: losing weight as fast as micronutrients? Obes Surg. 2011;21(2):207–11.
Abdeen G, le Roux C. Mechanism underlying the weight loss and complications of Roux-en-Y gastric bypass. Review. Obes Surg. 2016;26(2):410–21.
Adike A, DiBaise JK. Small intestinal bacterial overgrowth. Gastroenterol Clin N Am. 2018;47(1):193–208.
Alden JF. Gastric and jejunoileal bypass: a comparison in the treatment of morbid obesity. Arch Surg. 1977;112(7):799.
Alexandrou A, Armeni E, Kouskouni E, Tsoka E, Diamantis T, Lambrinoudaki I. Cross-sectional long-term micronutrient deficiencies after sleeve gastrectomy versus Roux-en-Y gastric bypass: a pilot study. Surg Obes Relat Dis. 2014;10(2):262–8.
Almogy G, Crookes PF, Anthone GJ. Longitudinal gastrectomy as a treatment for the high-risk super-obese patient. Obes Surg. 2004;14(4):492–7.
Al-Mutawa A, Anderson A, Alsabah S, Al-Mutawa M. Nutritional status of bariatric surgery candidates. Nutrients. 2018;10(1):67.
Al-Najim W, Docherty NG, le Roux CW. Food intake and eating behavior after bariatric surgery. Physiol Rev. 2018;98(3):1113–41.
Andari Sawaya R, Jaffe J, Friedenberg LK, Friedenberg F. Vitamin, mineral, and drug absorption following bariatric surgery. Curr Drug Metab. 2012;13(9):1345–55.
Angrisani L, Santonicola A, Iovino P, Formisano G, Buchwald H, Scopinaro N. Bariatric surgery worldwide 2013. Obes Surg. 2015;25(10):1822–32.
Angrisani L, Santonicola A, Iovino P, Vitiello A, Zundel N, Buchwald H, et al. Bariatric surgery and endoluminal procedures: IFSO worldwide survey 2014. Obes Surg. 2017;27(9):2279–89.
Anhê FF, Roy D, Pilon G, Dudonné S, Matamoros S, Varin TV, et al. A polyphenol-rich cranberry extract protects from diet-induced obesity, insulin resistance and intestinal inflammation in association with increased Akkermansia spp. population in the gut microbiota of mice. Gut. 2015;64(6):872–83.
Anhê FF, Varin TV, Schertzer JD, Marette A. The gut microbiota as a mediator of metabolic benefits after bariatric surgery. Can J Diabetes. 2017;41(4):439–47.
Antoniewicz A, Kalinowski P, Kotulecka KJ, Kocoń P, Paluszkiewicz R, Remiszewski P, et al. Nutritional deficiencies in patients after Roux-en-Y gastric bypass and sleeve gastrectomy during 12-month follow-up. Obes Surg. 2019;29(10):3277–84.
Aron-Wisnewsky J, Doré J, Clement K. The importance of the gut microbiota after bariatric surgery. Nat Rev Gastroenterol Hepatol. 2012;9(10):590–8.
Aron-Wisnewsky J, Prifti E, Belda E, Ichou F, Kayser BD, Dao MC, et al. Major microbiota dysbiosis in severe obesity: fate after bariatric surgery. Gut. 2019;68(1):70–82.
Astrup A, Bügel S. Overfed but undernourished: recognizing nutritional inadequacies/deficiencies in patients with overweight or obesity. Int J Obes. 2019;43(2):219–32.
Backhed F, Ding H, Wang T, Hooper LV, Koh GY, Nagy A, et al. The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci. 2004;101(44):15718–23.
Bal BS, Finelli FC, Shope TR, Koch TR. Nutritional deficiencies after bariatric surgery. Nat Rev Endocrinol. 2012;8(9):544–56.
Björklund P, Fändriks L. The pros and cons of gastric bypass surgery—the role of the Roux-limb. Best Pract Res Clin Gastroenterol. 2019;40–41:101638.
Björklund P, Laurenius A, Een E, Olbers T, Lönroth H, Fändriks L. Is the Roux limb a determinant for meal size after gastric bypass surgery? Obes Surg. 2010;20(10):1408–14.
Björklund P, Lönroth H, Fändriks L. Manometry of the upper gut following Roux-en-Y gastric bypass indicates that the gastric pouch and Roux limb act as a common cavity. Obes Surg. 2015;25(10):1833–41.
Blüher M. Obesity: global epidemiology and pathogenesis. Nat Rev Endocrinol. 2019;15(5):288–98.
Boylan LM, Sugerman HJ, Driskell JA, Vitamin E. vitamin B-6, vitamin B-12, and folate status of gastric bypass surgery patients. J Am Diet Assoc. 1988;88(5):579–85.
Bozadjieva N, Heppner KM, Seeley RJ. Targeting FXR and FGF19 to treat metabolic diseases—lessons learned from bariatric surgery. Diabetes. 2018;67(9):1720–8.
Buchwald H. The evolution of metabolic/bariatric surgery. Obes Surg. 2014;24(8):1126–35.
Buchwald H, Avidor Y, Braunwald E, Jensen MD, Pories W, Fahrbach K, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004;292(14):1724.
Cani PD. Gut microbiota and obesity: lessons from the microbiome. Brief Funct Genomics. 2013;12(4):381–7.
Cani PD. Human gut microbiome: hopes, threats and promises. Gut. 2018;67(9):1716–25.
Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D, et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56(7):1761–72.
Castaner O, Goday A, Park Y-M, Lee S-H, Magkos F, S-ATE S, et al. The gut microbiome profile in obesity: a systematic review. Int J Endocrinol. 2018;2018:1–9.
Cӑtoi AF, Vodnar DC, Corina A, Nikolic D, Citarrella R, Pérez-Martínez P, et al. Gut microbiota, obesity and bariatric surgery: current knowledge and future perspectives. Curr Pharm Des. 2019;25(18):2038–50.
Chen EB, Cason C, Gilbert JA, Ho KJ. Current state of knowledge on implications of gut microbiome for surgical conditions. J Gastrointest Surg. 2018;22(6):1112–23.
Cho M, Kaidar-Person O, Szomstein S, Rosenthal RJ. Emergency room visits after laparoscopic Roux-en-Y gastric bypass for morbid obesity. Surg Obes Relat Dis. 2008;4(2):104–9.
Ciobârcă D, Cătoi AF, Copăescu C, Miere D, Crișan G. Bariatric surgery in obesity: effects on gut microbiota and micronutrient status. Nutrients. 2020;12(1):235.
Colquitt JL, Pickett K, Loveman E, Frampton GK. Surgery for weight loss in adults. Cochrane Database Syst Rev. 2014. Cochrane Metabolic and Endocrine Disorders Group, editor.
Compare D, Rocco A, Sanduzzi Zamparelli M, Nardone G. The gut bacteria-driven obesity development. Dig Dis. 2016;34(3):221–9.
Cotillard A, Kennedy SP, Kong LC, Prifti E, Pons N, Le Chatelier E, et al. Dietary intervention impact on gut microbial gene richness. Nature. 2013;500(7464):585–8.
Cryan JF, Dinan TG. Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat Rev Neurosci. 2012;13(10):701–12.
Damms-Machado A, Mitra S, Schollenberger AE, Kramer KM, Meile T, Königsrainer A, et al. Effects of surgical and dietary weight loss therapy for obesity on gut microbiota composition and nutrient absorption. Biomed Res Int. 2015;2015:1–12.
Das P, Babaei P, Nielsen J. Metagenomic analysis of microbe-mediated vitamin metabolism in the human gut microbiome. BMC Genomics. 2019;20(1):208.
David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE, et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014;505(7484):559–63.
Davies NK, O’Sullivan JM, Plank LD, Murphy R. Altered gut microbiome after bariatric surgery and its association with metabolic benefits: a systematic review. Surg Obes Relat Dis. 2019;15(4):656–65.
Dawson PA, Karpen SJ. Intestinal transport and metabolism of bile acids. J Lipid Res. 2015;56(6):1085–99.
de Kort S, Keszthelyi D, Masclee AAM. Leaky gut and diabetes mellitus: what is the link?: leaky gut in diabetes. Obes Rev. 2011;12(6):449–58.
de la Cuesta-Zuluaga J, Mueller NT, Corrales-Agudelo V, Velásquez-Mejía EP, Carmona JA, Abad JM, et al. Metformin is associated with higher relative abundance of mucin-degrading Akkermansia muciniphila and several short-chain fatty acid–producing microbiota in the gut. Dia Care. 2017;40(1):54–62.
de Punder K, Pruimboom L. Stress induces endotoxemia and low-grade inflammation by increasing barrier permeability. Front Immunol. 2015;6.
De Vadder F, Kovatcheva-Datchary P, Goncalves D, Vinera J, Zitoun C, Duchampt A, et al. Microbiota-generated metabolites promote metabolic benefits via gut-brain neural circuits. Cell. 2014;156(1–2):84–96.
Debédat J, Clément K, Aron-Wisnewsky J. Gut microbiota dysbiosis in human obesity: impact of bariatric surgery. Curr Obes Rep. 2019;8(3):229–42.
DiBaise JK. Nutritional consequences of small intestinal bacterial overgrowth. Pract Gastroenterol. 2008;32(12):15–28.
Dockray GJ. Gastrointestinal hormones and the dialogue between gut and brain: gut-brain signalling. J Physiol. 2014;592(14):2927–41.
Dukowicz AC, Lacy BE, Levine GM. Small intestinal bacterial overgrowth: a comprehensive review. Gastroenterol Hepatol (N Y). 2007;3(2):112–22.
Duncan SH, Lobley GE, Holtrop G, Ince J, Johnstone AM, Louis P, et al. Human colonic microbiota associated with diet, obesity and weight loss. Int J Obes. 2008;32(11):1720–4.
Dutia R, Embrey M, O’Brien S, Haeusler RA, Agénor KK, Homel P, et al. Temporal changes in bile acid levels and 12α-hydroxylation after Roux-en-Y gastric bypass surgery in type 2 diabetes. Int J Obes. 2015;39(5):806–13.
Dzutsev A, Goldszmid RS, Viaud S, Zitvogel L, Trinchieri G. The role of the microbiota in inflammation, carcinogenesis, and cancer therapy. Eur J Immunol. 2015;45(1):17–31.
Eckert MJ, Perry JT, Sohn VY, Boden J, Martin MJ, Rush RM, et al. Incidence of low vitamin A levels and ocular symptoms after Roux-en-Y gastric bypass. Surg Obes Relat Dis. 2010;6(6):653–7.
Ejtahed H-S, Angoorani P, Hasani-Ranjbar S, Siadat S-D, Ghasemi N, Larijani B, et al. Adaptation of human gut microbiota to bariatric surgeries in morbidly obese patients: a systematic review. Microb Pathog. 2018;116:13–21.
Engebretsen KV, Blom-Høgestøl IK, Hewitt S, Risstad H, Moum B, Kristinsson JA, et al. Anemia following Roux-en-Y gastric bypass for morbid obesity; a 5-year follow-up study. Scand J Gastroenterol. 2018;53(8):917–22.
Everard A, Lazarevic V, Derrien M, Girard M, Muccioli GG, Neyrinck AM, et al. Responses of gut microbiota and glucose and lipid metabolism to prebiotics in genetic obese and diet-induced leptin-resistant mice. Diabetes. 2011;60(11):2775–86.
Everard A, Belzer C, Geurts L, Ouwerkerk JP, Druart C, Bindels LB, et al. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc Natl Acad Sci. 2013;110(22):9066–71.
Fändriks L. Roles of the gut in the metabolic syndrome: an overview. J Intern Med. 2017;281(4):319–36.
Federico A, Dallio M, Tolone S, Gravina AG, Patrone V, Romano M, et al. Gastrointestinal hormones, intestinal microbiota and metabolic homeostasis in obese patients: effect of bariatric surgery. In Vivo. 2016;30(3):321–30.
Ferraz ÁAB, Carvalho MRC, Siqueira LT, Santa-Cruz F, Campos JM. Deficiências de micronutrientes após cirurgia bariátrica: análise comparativa entre gastrectomia vertical e derivação gástrica em Y de Roux. Rev Col Bras Cir. 2018;45(6).
Festi D, Schiumerini R, Eusebi LH, Marasco G, Taddia M, Colecchia A. Gut microbiota and metabolic syndrome. World J Gastroenterol. 2014;20(43):16079.
Fetissov SO. Role of the gut microbiota in host appetite control: bacterial growth to animal feeding behaviour. Nat Rev Endocrinol. 2017;13(1):11–25.
Finucane MM, Sharpton TJ, Laurent TJ, Pollard KSA. Taxonomic signature of obesity in the microbiome? Getting to the guts of the matter. PLoS One. 2014;9(1):e84689. Heimesaat MM, editor.
Fleissner CK, Huebel N, Abd El-Bary MM, Loh G, Klaus S, Blaut M. Absence of intestinal microbiota does not protect mice from diet-induced obesity. Br J Nutr. 2010;104(6):919–29.
Fontana MA, Wohlgemuth SD. The surgical treatment of metabolic disease and morbid obesity. Gastroenterol Clin N Am. 2010;39(1):125–33.
Frame-Peterson LA, Megill RD, Carobrese S, Schweitzer M. Nutrient deficiencies are common prior to bariatric surgery. Nutr Clin Pract. 2017;32(4):463–9.
Franco JVA, Ruiz PA, Palermo M, Gagner M. A review of studies comparing three laparoscopic procedures in bariatric surgery: sleeve gastrectomy, Roux-en-Y gastric bypass and adjustable gastric banding. Obes Surg. 2011;21(9):1458–68.
Frost G, Sleeth ML, Sahuri-Arisoylu M, Lizarbe B, Cerdan S, Brody L, et al. The short-chain fatty acid acetate reduces appetite via a central homeostatic mechanism. Nat Commun. 2014;5(1):3611.
Furet J-P, Kong L-C, Tap J, Poitou C, Basdevant A, Bouillot J-L, et al. Differential adaptation of human gut microbiota to bariatric surgery-induced weight loss: links with metabolic and low-grade inflammation markers. Diabetes. 2010;59(12):3049–57.
Garcia-Rios A, Torres-Peña JD, Perez-Jimenez F, Perez-Martinez P. Gut microbiota: a new marker of cardiovascular disease. Curr Pharm Des. 2017;23(22):3233–8.
Gerritsen J, Smidt H, Rijkers GT, de Vos WM. Intestinal microbiota in human health and disease: the impact of probiotics. Genes Nutr. 2011;6(3):209–40.
Gilbert JA, Quinn RA, Debelius J, Xu ZZ, Morton J, Garg N, et al. Microbiome-wide association studies link dynamic microbial consortia to disease. Nature. 2016;535(7610):94–103.
Gilbert JA, Blaser MJ, Caporaso JG, Jansson JK, Lynch SV, Knight R. Current understanding of the human microbiome. Nat Med. 2018;24(4):392–400.
Gill SR, Pop M, DeBoy RT, Eckburg PB, Turnbaugh PJ, Samuel BS, et al. Metagenomic analysis of the human distal gut microbiome. Science. 2006;312(5778):1355–9.
Godon JJ, Zumstein E, Dabert P, Habouzit F, Moletta R. Molecular microbial diversity of an anaerobic digestor as determined by small-subunit rDNA sequence analysis. Appl Environ Microbiol. 1997;63(7):2802–13.
Goodrich JK, Waters JL, Poole AC, Sutter JL, Koren O, Blekhman R, et al. Human genetics shape the gut microbiome. Cell. 2014;159(4):789–99.
Grace E, Shaw C, Whelan K, Andreyev HJN. Review article: small intestinal bacterial overgrowth—prevalence, clinical features, current and developing diagnostic tests, and treatment. Aliment Pharmacol Ther. 2013;38(7):674–88.
Graessler J, Qin Y, Zhong H, Zhang J, Licinio J, Wong M-L, et al. Metagenomic sequencing of the human gut microbiome before and after bariatric surgery in obese patients with type 2 diabetes: correlation with inflammatory and metabolic parameters. Pharmacogenomics J. 2013;13(6):514–22.
Gralka E, Luchinat C, Tenori L, Ernst B, Thurnheer M, Schultes B. Metabolomic fingerprint of severe obesity is dynamically affected by bariatric surgery in a procedure-dependent manner. Am J Clin Nutr. 2015;102(6):1313–22.
Greenstein AJ, O’Rourke RW. Abdominal pain after gastric bypass: suspects and solutions. Am J Surg. 2011;201(6):819–27.
Gribsholt SB, Pedersen AM, Svensson E, Thomsen RW, Richelsen B. Prevalence of self-reported symptoms after gastric bypass surgery for obesity. JAMA Surg. 2016;151(6):504.
Griffen WO, Young VL, Stevenson CCA. Prospective comparison of gastric and jejunoileal bypass procedures for morbid obesity. Ann Surg. 1977;186(4):500–9.
Guo Y, Huang Z-P, Liu C-Q, Qi L, Sheng Y, Zou D-J. Modulation of the gut microbiome: a systematic review of the effect of bariatric surgery. Eur J Endocrinol. 2018;178(1):43–56.
Hamer HM, Jonkers DMAE, Bast A, Vanhoutvin SALW, Fischer MAJG, Kodde A, et al. Butyrate modulates oxidative stress in the colonic mucosa of healthy humans. Clin Nutr. 2009;28(1):88–93.
Haro C, Rangel-Zúñiga OA, Alcalá-Díaz JF, Gómez-Delgado F, Pérez-Martínez P, Delgado-Lista J, et al. Intestinal microbiota is influenced by gender and body mass index. PLoS One. 2016;11(5):e0154090.
Hassan zadeh M, Mohammadi Farsani G, Zamaninour N. Selenium status after Roux-en-Y gastric bypass: interventions and recommendations. Obes Surg. 2019;29(11):3743–8.
Heneghan HM, Nissen S, Schauer PR. Gastrointestinal surgery for obesity and diabetes: weight loss and control of hyperglycemia. Curr Atheroscler Rep. 2012;14(6):579–87.
Hoeppli RE, Wu D, Cook L, Levings MK. The environment of regulatory T cell biology: cytokines, metabolites, and the microbiome. Front Immunol. 2015;6.
Høgestøl IK, Chahal-Kummen M, Eribe I, Brunborg C, Stubhaug A, Hewitt S, et al. Chronic abdominal pain and symptoms 5 years after gastric bypass for morbid obesity. Obes Surg. 2017;27(6):1438–45.
Holzer P, Reichmann F, Farzi A. Neuropeptide Y, peptide YY and pancreatic polypeptide in the gut–brain axis. Neuropeptides. 2012;46(6):261–74.
Ishida RK, Faintuch J, Ribeiro AS, Ribeiro U, Cecconello I. Asymptomatic gastric bacterial overgrowth after bariatric surgery: are long-term metabolic consequences possible? Obes Surg. 2014;24(11):1856–61.
Ismail NA, Ragab SH, ElBaky AA, Shoeib ARS, Alhosary Y, Fekry D. Frequency of Firmicutes and Bacteroidetes in gut microbiota in obese and normal weight Egyptian children and adults. Arch Med Sci. 2011;3:501–7.
Jacobs D, Gaudier E, Duynhoven J, Vaughan E. Non-digestible food ingredients, colonic microbiota and the impact on gut health and immunity: a role for metabolomics. Curr Drug Metab. 2009;10(1):41–54.
Jahansouz C, Xu H, Hertzel AV, Serrot FJ, Kvalheim N, Cole A, et al. Bile acids increase independently from hypocaloric restriction after bariatric surgery. Ann Surg. 2016;264(6):1022–8.
Jahansouz C, Staley C, Bernlohr DA, Sadowsky MJ, Khoruts A, Ikramuddin S. Sleeve gastrectomy drives persistent shifts in the gut microbiome. Surg Obes Relat Dis. 2017;13(6):916–24.
Johansson MEV, Phillipson M, Petersson J, Velcich A, Holm L, Hansson GC. The inner of the two Muc2 mucin-dependent mucus layers in colon is devoid of bacteria. Proc Natl Acad Sci. 2008;105(39):15064–9.
Jørgensen NB, Dirksen C, Bojsen-Møller KN, Kristiansen VB, Wulff BS, Rainteau D, et al. Improvements in glucose metabolism early after gastric bypass surgery are not explained by increases in total bile acids and fibroblast growth factor 19 concentrations. J Clin Endocrinol Metabol. 2015;100(3):E396–406.
Kasai C, Sugimoto K, Moritani I, Tanaka J, Oya Y, Inoue H, et al. Comparison of the gut microbiota composition between obese and non-obese individuals in a Japanese population, as analyzed by terminal restriction fragment length polymorphism and next-generation sequencing. BMC Gastroenterol. 2015;15(1):100.
Kim D, Zeng MY, Núñez G. The interplay between host immune cells and gut microbiota in chronic inflammatory diseases. Exp Mol Med. 2017;49(5):e339.
King WC, Chen J-Y, Belle SH, Courcoulas AP, Dakin GF, Flum DR, et al. Use of prescribed opioids before and after bariatric surgery: prospective evidence from a U.S. multicenter cohort study. Surg Obes Relat Dis. 2017a;13(8):1337–46.
King WC, Chen J-Y, Courcoulas AP, Dakin GF, Engel SG, Flum DR, et al. Alcohol and other substance use after bariatric surgery: prospective evidence from a U.S. multicenter cohort study. Surg Obes Relat Dis. 2017b;13(8):1392–402.
Knight R, Callewaert C, Marotz C, Hyde ER, Debelius JW, McDonald D, et al. The microbiome and human biology. Annu Rev Genom Hum Genet. 2017;18(1):65–86.
Kohli R, Setchell KD, Kirby M, Myronovych A, Ryan KK, Ibrahim SH, et al. A surgical model in male obese rats uncovers protective effects of bile acids post-bariatric surgery. Endocrinology. 2013;154(7):2341–51.
Kong L-C, Tap J, Aron-Wisnewsky J, Pelloux V, Basdevant A, Bouillot J-L, et al. Gut microbiota after gastric bypass in human obesity: increased richness and associations of bacterial genera with adipose tissue genes. Am J Clin Nutr. 2013;98(1):16–24.
Krajmalnik-Brown R, Ilhan Z-E, Kang D-W, DiBaise JK. Effects of gut microbes on nutrient absorption and energy regulation. Nutr Clin Pract. 2012;27(2):201–14.
Krzizek E-C, Brix JM, Herz CT, Kopp HP, Schernthaner G-H, Schernthaner G, et al. Prevalence of micronutrient deficiency in patients with morbid obesity before bariatric surgery. Obes Surg. 2018;28(3):643–8.
Kuzmak LI, Yap IS, McGuire L, Dixon JS, Young MP. Surgery for morbid obesity. AORN J. 1990;51(5):1307–24.
Lakhani SV, Shah HN, Alexander K, Finelli FC, Kirkpatrick JR, Koch TR. Small intestinal bacterial overgrowth and thiamine deficiency after Roux-en-Y gastric bypass surgery in obese patients. Nutr Res. 2008;28(5):293–8.
Le Chatelier E, Nielsen T, Qin J, Prifti E, Hildebrand F, Falony G, et al. Richness of human gut microbiome correlates with metabolic markers. Nature. 2013;500(7464):541–6.
LeBlanc JG, Milani C, de Giori GS, Sesma F, van Sinderen D, Ventura M. Bacteria as vitamin suppliers to their host: a gut microbiota perspective. Curr Opin Biotechnol. 2013;24(2):160–8.
Leffler D, Lamont J. Clostridium difficile infection. N Engl J Med. 2015;373(3):286–8.
Ley RE, Backhed F, Turnbaugh P, Lozupone CA, Knight RD, Gordon JI. Obesity alters gut microbial ecology. Proc Natl Acad Sci. 2005;102(31):11070–5.
Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Human gut microbes associated with obesity. Nature. 2006;444(7122):1022–3.
Li SS, Zhu A, Benes V, Costea PI, Hercog R, Hildebrand F, et al. Durable coexistence of donor and recipient strains after fecal microbiota transplantation. Science. 2016;352(6285):586–9.
Liou AP, Paziuk M, Luevano J-M, Machineni S, Turnbaugh PJ, Kaplan LM. Conserved shifts in the gut microbiota due to gastric bypass reduce host weight and adiposity. Sci Transl Med. 2013;5(178):178ra41.
Lips MA, Van Klinken JB, van Harmelen V, Dharuri HK, PAC ‘t H, JFJ L, et al. Roux-en-Y gastric bypass surgery, but not calorie restriction, reduces plasma branched-chain amino acids in obese women independent of weight loss or the presence of type 2 diabetes. Dia Care. 2014;37(12):3150–6.
Liu R, Hong J, Xu X, Feng Q, Zhang D, Gu Y, et al. Gut microbiome and serum metabolome alterations in obesity and after weight-loss intervention. Nat Med. 2017;23(7):859–68.
Lundberg JO, Weitzberg E. Biology of nitrogen oxides in the gastrointestinal tract. Gut. 2013;62(4):616–29.
Lupoli R, Milone M. Haemostatic and fibrinolytic changes in obese subjects undergoing bariatric surgery: the effect of different surgical procedures. Blood Transfus. 2015;13(3):442–7.
Lupoli R, Lembo E, Saldalamacchia G, Avola CK, Angrisani L, Capaldo B. Bariatric surgery and long-term nutritional issues. World J Diabetes. 2017;8(11):464.
Macfarlane GT, Macfarlane S. Bacteria, colonic fermentation, and gastrointestinal health. J AOAC Int. 2012;95(1):50–60.
Madsbad S, Dirksen C, Holst JJ. Mechanisms of changes in glucose metabolism and bodyweight after bariatric surgery. Lancet Diab Endocrinol. 2014;2(2):152–64.
Magouliotis DE, Tasiopoulou VS, Sioka E, Chatedaki C, Zacharoulis D. Impact of bariatric surgery on metabolic and gut microbiota profile: a systematic review and meta-analysis. Obes Surg. 2017;27(5):1345–57.
Mahawar KK, Clare K, O’Kane M, Graham Y, Callejas-Diaz L, Carr WRJ. Patient perspectives on adherence with micronutrient supplementation after bariatric surgery. Obes Surg. 2019;29(5):1551–6.
Makaronidis JM, Batterham RL. Potential mechanisms mediating sustained weight loss following Roux-en-Y gastric bypass and sleeve gastrectomy. Endocrinol Metab Clin N Am. 2016;45(3):539–52.
Makaronidis JM, Neilson S, Cheung W-H, Tymoszuk U, Pucci A, Finer N, et al. Reported appetite, taste and smell changes following Roux-en-Y gastric bypass and sleeve gastrectomy: effect of gender, type 2 diabetes and relationship to post-operative weight loss. Appetite. 2016;107:93–105.
Manichanh C, Rigottier-Gois L, Bonnaud E, Gloux K, Pelletier E, Frangeul L, et al. Reduced diversity of faecal microbiota in Crohn’s disease revealed by a metagenomic approach. Gut. 2006;55(2):205–11.
Mariat D, Firmesse O, Levenez F, Guimarăes V, Sokol H, Doré J, et al. The Firmicutes/Bacteroidetes ratio of the human microbiota changes with age. BMC Microbiol. 2009;9(1):123.
Maruvada P, Leone V, Kaplan LM, Chang EB. The human microbiome and obesity: moving beyond associations. Cell Host Microbe. 2017;22(5):589–99.
Mason EE, Ito C. Gastric bypass in obesity. Surg Clin N Am. 1967;47(6):1345–51.
Medina DA, Pedreros JP, Turiel D, Quezada N, Pimentel F, Escalona A, et al. Distinct patterns in the gut microbiota after surgical or medical therapy in obese patients. PeerJ. 2017;5:e3443.
Melissas J, Koukouraki S, Askoxylakis J, Stathaki M, Daskalakis M, Perisinakis K, et al. Sleeve gastrectomy—a restrictive procedure? Obes Surg. 2007;17(1):57–62.
MetaHIT Consortium QJ, Li R, Raes J, Arumugam M, Burgdorf KS, et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464(7285):59–65.
Mihajlovski A, Alric M, Brugère J-F. A putative new order of methanogenic Archaea inhabiting the human gut, as revealed by molecular analyses of the mcrA gene. Res Microbiol. 2008;159(7–8):516–21.
Miras AD, le Roux CW. Can medical therapy mimic the clinical efficacy or physiological effects of bariatric surgery? Int J Obes. 2014;38(3):325–33.
Mohajeri MH, Brummer RJM, Rastall RA, Weersma RK, Harmsen HJM, Faas M, et al. The role of the microbiome for human health: from basic science to clinical applications. Eur J Nutr. 2018;57(S1):1–14.
Moreira APB, Texeira TFS, Ferreira AB, do Carmo Gouveia Peluzio M, de Cássia Gonçalves Alfenas R. Influence of a high-fat diet on gut microbiota, intestinal permeability and metabolic endotoxaemia. Br J Nutr. 2012;108(5):801–9.
Mowat AM, Agace WW. Regional specialization within the intestinal immune system. Nat Rev Immunol. 2014;14(10):667–85.
Murphy R, Tsai P, Jüllig M, Liu A, Plank L, Booth M. Differential changes in gut microbiota after gastric bypass and sleeve gastrectomy bariatric surgery vary according to diabetes remission. Obes Surg. 2017;27(4):917–25.
Nakatani H, Kasama K, Oshiro T, Watanabe M, Hirose H, Itoh H. Serum bile acid along with plasma incretins and serum high–molecular weight adiponectin levels are increased after bariatric surgery. Metabolism. 2009;58(10):1400–7.
Narath SH, Mautner SI, Svehlikova E, Schultes B, Pieber TR, Sinner FM, et al. An untargeted metabolomics approach to characterize short-term and long-term metabolic changes after bariatric surgery. PLoS One. 2016;11(9):e0161425.
Nardone G, Compare D. The human gastric microbiota: Is it time to rethink the pathogenesis of stomach diseases? United European Gastroenterol J. 2015;3(3):255–60.
Neylan CJ, Kannan U, Dempsey DT, Williams NN, Dumon KR. The surgical management of obesity. Gastroenterol Clin North Am. 2016;45(4):689–703.
Ogata H, Goto S, Sato K, Fujibuchi W, Bono H, Kanehisa M. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 1999;27(1):29–34.
Ott SJ, Kühbacher T, Musfeldt M, Rosenstiel P, Hellmig S, Rehman A, et al. Fungi and inflammatory bowel diseases: alterations of composition and diversity. Scand J Gastroenterol. 2008;43(7):831–41.
Paganelli FL, Luyer M, Hazelbag CM, Uh H-W, Rogers MRC, Adriaans D, et al. Roux-Y gastric bypass and sleeve gastrectomy directly change gut microbiota composition independent of surgery type. Sci Rep. 2019;9(1):10979.
Palleja A, Kashani A, Allin KH, Nielsen T, Zhang C, Li Y, et al. Roux-en-Y gastric bypass surgery of morbidly obese patients induces swift and persistent changes of the individual gut microbiota. Genome Med. 2016;8(1):67.
Papamargaritis D, le Roux CW, Sioka E, Koukoulis G, Tzovaras G, Zacharoulis D. Changes in gut hormone profile and glucose homeostasis after laparoscopic sleeve gastrectomy. Surg Obes Relat Dis. 2013;9(2):192–201.
Pascale A, Marchesi N, Marelli C, Coppola A, Luzi L, Govoni S, et al. Microbiota and metabolic diseases. Endocrine. 2018;61(3):357–71.
Patel JJ, Mundi MS, Hurt RT, Wolfe B, Martindale RG. Micronutrient deficiencies after bariatric surgery: an emphasis on vitamins and trace minerals. Nutr Clin Pract. 2017;32(4):471–80.
Patil DP, Dhotre DP, Chavan SG, Sultan A, Jain DS, Lanjekar VB, et al. Molecular analysis of gut microbiota in obesity among Indian individuals. J Biosci. 2012;37(4):647–57.
Patrone V, Vajana E, Minuti A, Callegari ML, Federico A, Loguercio C, et al. Postoperative changes in fecal bacterial communities and fermentation products in obese patients undergoing bilio-intestinal bypass. Front Microbiol. 2016;7:20.
Patti M-E, Houten SM, Bianco AC, Bernier R, Larsen PR, Holst JJ, et al. Serum bile acids are higher in humans with prior gastric bypass: potential contribution to improved glucose and lipid metabolism. Obesity. 2009;17(9):1671–7.
Peck BCE, Seeley RJ. How does ‘metabolic surgery’ work its magic? New evidence for gut microbiota. Curr Opin Endocrinol Diab Obesity. 2018;25(2):81–6.
Peterli R, Borbély Y, Kern B, Gass M, Peters T, Thurnheer M, et al. Early results of the swiss multicentre bypass or sleeve study (SM-BOSS): a prospective randomized trial comparing laparoscopic sleeve gastrectomy and Roux-en-Y gastric bypass. Ann Surg. 2013;258(5):690–5.
Plovier H, Everard A, Druart C, Depommier C, Van Hul M, Geurts L, et al. A purified membrane protein from Akkermansia muciniphila or the pasteurized bacterium improves metabolism in obese and diabetic mice. Nat Med. 2017;23(1):107–13.
Pournaras DJ, Glicksman C, Vincent RP, Kuganolipava S, Alaghband-Zadeh J, Mahon D, et al. The role of bile after Roux-en-Y gastric bypass in promoting weight loss and improving glycaemic control. Endocrinology. 2012;153(8):3613–9.
Pucci A, Batterham RL. Mechanisms underlying the weight loss effects of RYGB and SG: similar, yet different. J Endocrinol Investig. 2019;42(2):117–28.
Quercia I, Dutia R, Kotler DP, Belsley S, Laferrère B. Gastrointestinal changes after bariatric surgery. Diabetes Metab. 2014;40(2):87–94.
Raebel MA, Newcomer SR, Bayliss EA, Boudreau D, DeBar L, Elliott TE, et al. Chronic opioid use emerging after bariatric surgery. Pharmacoepidemiol Drug Saf. 2014;23(12):1247–57.
Rowland I, Gibson G, Heinken A, Scott K, Swann J, Thiele I, et al. Gut microbiota functions: metabolism of nutrients and other food components. Eur J Nutr. 2018;57(1):1–24.
Rubino F, Nathan DM, Eckel RH, Schauer PR, Alberti KGMM, Zimmet PZ, et al. Metabolic surgery in the treatment algorithm for type 2 diabetes: a joint statement by international diabetes organizations. Dia Care. 2016;39(6):861–77.
Ryan KK, Tremaroli V, Clemmensen C, Kovatcheva-Datchary P, Myronovych A, Karns R, et al. FXR is a molecular target for the effects of vertical sleeve gastrectomy. Nature. 2014;509(7499):183–8.
Sabate J-M, Coupaye M, Ledoux S, Castel B, Msika S, Coffin B, et al. Consequences of small intestinal bacterial overgrowth in obese patients before and after bariatric surgery. Obes Surg. 2017;27(3):599–605.
Sachdev AH, Pimentel M. Gastrointestinal bacterial overgrowth: pathogenesis and clinical significance. Therap Adv Chronic Dis. 2013;4(5):223–31.
Sallé A, Demarsy D, Poirier AL, Lelièvre B, Topart P, Guilloteau G, et al. Zinc deficiency: a frequent and underestimated complication after bariatric surgery. Obes Surg. 2010;20(12):1660–70.
Santoro S. From bariatric to pure metabolic surgery: new concepts on the rise. Ann Surg. 2015;262(2):e79–80.
Santos-Marcos JA, Haro C, Vega-Rojas A, Alcala-Diaz JF, Molina-Abril H, Leon-Acuña A, et al. Sex differences in the gut microbiota as potential determinants of gender predisposition to disease. Mol Nutr Food Res. 2019;63(7):1800870.
Sassone-Corsi M, Nuccio S-P, Liu H, Hernandez D, Vu CT, Takahashi AA, et al. Microcins mediate competition among Enterobacteriaceae in the inflamed gut. Nature. 2016;540(7632):280–3.
Savage DC. Microbial ecology of the gastrointestinal tract. Annu Rev Microbiol. 1977;31(1):107–33.
Scanlan PD, Marchesi JR. Micro-eukaryotic diversity of the human distal gut microbiota: qualitative assessment using culture-dependent and -independent analysis of faeces. ISME J. 2008;2(12):1183–93.
Schauer PR, Bhatt DL, Kirwan JP, Wolski K, Aminian A, Brethauer SA, et al. Bariatric surgery versus intensive medical therapy for diabetes—5-year outcomes. N Engl J Med. 2017;376(7):641–51.
Schulman AR, Thompson CC. Complications of bariatric surgery: what you can expect to see in your GI practice. Am J Gastroenterol. 2017;112(11):1640–55.
Scott FI, Horton DB, Mamtani R, Haynes K, Goldberg DS, Lee DY, et al. Administration of antibiotics to children before age 2 years increases risk for childhood obesity. Gastroenterology. 2016;151(1):120–129.e5.
Sender R, Fuchs S, Milo R. Are we really vastly outnumbered? Revisiting the ratio of bacterial to host cells in humans. Cell. 2016;164(3):337–40.
Shao Y, Ding R, Xu B, Hua R, Shen Q, He K, et al. Alterations of gut microbiota after Roux-en-Y gastric bypass and sleeve gastrectomy in Sprague-Dawley rats. Obes Surg. 2017;27(2):295–302.
Sjöström L, Lindroos A-K, Peltonen M, Torgerson J, Bouchard C, Carlsson B, et al. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med. 2004;351(26):2683–93.
Sjöström L, Narbro K, Sjöström CD, Karason K, Larsson B, Wedel H, et al. Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med. 2007;357(8):741–52.
Spak E, Björklund P, Helander HF, Vieth M, Olbers T, Casselbrant A, et al. Changes in the mucosa of the Roux-limb after gastric bypass surgery: Roux-limb mucosa after RYGBP. Histopathology. 2010;57(5):680–8.
Stefan N, Häring H-U, Schulze MB. Metabolically healthy obesity: the low-hanging fruit in obesity treatment? Lancet Diab Endocrinol. 2018;6(3):249–58.
Stefanidis D, Kuwada TS, Gersin KS. The importance of the length of the limbs for gastric bypass patients—an evidence-based review. Obes Surg. 2011;21(1):119–24.
Stefater MA, Sandoval DA, Chambers AP, Wilson–Pérez HE, Hofmann SM, Jandacek R, et al. Sleeve gastrectomy in rats improves postprandial lipid clearance by reducing intestinal triglyceride secretion. Gastroenterology. 2011;141(3):939–949.e4.
Stenberg E, Szabo E, Ågren G, Ottosson J, Marsk R, Lönroth H, et al. Closure of mesenteric defects in laparoscopic gastric bypass: a multicentre, randomised, parallel, open-label trial. Lancet. 2016;387(10026):1397–404.
Swann JR, Want EJ, Geier FM, Spagou K, Wilson ID, Sidaway JE, et al. Systemic gut microbial modulation of bile acid metabolism in host tissue compartments. Proc Natl Acad Sci. 2011;108(Supplement_1):4523–30.
Sweeney TE, Morton JM. The human gut microbiome: a review of the effect of obesity and surgically induced weight loss. JAMA Surg. 2013;148(6):563.
Sze MA, Schloss PD. Looking for a signal in the noise: revisiting obesity and the microbiome. MBio. 2016;7(4):e01018-16.
Thaler JP, Cummings DE. Hormonal and metabolic mechanisms of diabetes remission after gastrointestinal surgery. Endocrinology. 2009;150(6):2518–25.
The Human Microbiome Project Consortium. Structure, function and diversity of the healthy human microbiome. Nature. 2012;486(7402):207–14.
Thomas S, Izard J, Walsh E, Batich K, Chongsathidkiet P, Clarke G, et al. The host microbiome regulates and maintains human health: a primer and perspective for non-microbiologists. Cancer Res. 2017;77(8):1783–812.
Tremaroli V, Bäckhed F. Functional interactions between the gut microbiota and host metabolism. Nature. 2012;489(7415):242–9.
Tremaroli V, Karlsson F, Werling M, Ståhlman M, Kovatcheva-Datchary P, Olbers T, et al. Roux-en-Y gastric bypass and vertical banded gastroplasty induce long-term changes on the human gut microbiome contributing to fat mass regulation. Cell Metab. 2015;22(2):228–38.
Tremmel M, Gerdtham U-G, Nilsson P, Saha S. Economic burden of obesity: a systematic literature review. Int J Environ Res Public Health. 2017;14(4):435.
Trøseid M, Hov JR, Nestvold TK, Thoresen H, Berge RK, Svardal A, et al. Major increase in microbiota-dependent proatherogenic metabolite TMAO one year after bariatric surgery. Metab Syndr Relat Disord. 2016;14(4):197–201.
Tuomi K, Logomarsino JV. Bacterial lipopolysaccharide, lipopolysaccharide-binding protein, and other inflammatory markers in obesity and after bariatric surgery. Metab Syndr Relat Disord. 2016;14(6):279–88.
Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444(7122):1027–31.
Turnbaugh PJ, Bäckhed F, Fulton L, Gordon JI. Diet-induced obesity is linked to marked but reversible alterations in the mouse distal gut microbiome. Cell Host Microbe. 2008;3(4):213–23.
Turnbaugh PJ, Hamady M, Yatsunenko T, Cantarel BL, Duncan A, Ley RE, et al. A core gut microbiome in obese and lean twins. Nature. 2009;457(7228):480–4.
Ulker İ, Yildiran H. The effects of bariatric surgery on gut microbiota in patients with obesity: a review of the literature. Biosci Microb Food Health. 2019;38(1):3–9.
van Rutte PWJ, Aarts EO, Smulders JF, Nienhuijs SW. Nutrient deficiencies before and after sleeve gastrectomy. Obes Surg. 2014;24(10):1639–46.
Villanueva-Millán MJ, Pérez-Matute P, Oteo JA. Gut microbiota: a key player in health and disease. A review focused on obesity. J Physiol Biochem. 2015;71(3):509–25.
Wahlström A, Sayin SI, Marschall H-U, Bäckhed F. Intestinal crosstalk between bile acids and microbiota and its impact on host metabolism. Cell Metab. 2016;24(1):41–50.
Wang C-Y, Liao JKA. Mouse model of diet-induced obesity and insulin resistance. In: Weichhart T, editor. mTOR, Methods in molecular biology, vol. 821. Totowa, NJ: Humana Press; 2012. p. 421–33.
Ward EK, Schuster DP, Stowers KH, Royse AK, Ir D, Robertson CE, et al. The effect of PPI use on human gut microbiota and weight loss in patients undergoing laparoscopic Roux-en-Y gastric bypass. Obes Surg. 2014;24(9):1567–71.
Werling M, Fändriks L, Olbers T, Bueter M, Sjöström L, Lönroth H, et al. Roux-en-Y gastric bypass surgery increases respiratory quotient and energy expenditure during food intake. PLoS One. 2015;10(6):e0129784. Covasa M, editor
Woting A, Blaut M. The intestinal microbiota in metabolic disease. Nutrients. 2016;8(4):202.
Xanthakos SA. Nutritional deficiencies in obesity and after bariatric surgery. Pediatr Clin N Am. 2009;56(5):1105–21.
Yan M, Song M-M, Bai R-X, Cheng S, Yan W-M. Effect of Roux-en-Y gastric bypass surgery on intestinal Akkermansia muciniphila. World J Gastrointest Surg. 2016;8(4):301.
Young VB. The role of the microbiome in human health and disease: an introduction for clinicians. BMJ. 2017:j831.
Zaneveld J, Turnbaugh PJ, Lozupone C, Ley RE, Hamady M, Gordon JI, et al. Host-bacterial coevolution and the search for new drug targets. Curr Opin Chem Biol. 2008;12(1):109–14.
Zhang H, DiBaise JK, Zuccolo A, Kudrna D, Braidotti M, Yu Y, et al. Human gut microbiota in obesity and after gastric bypass. Proc Natl Acad Sci. 2009;106(7):2365–70.
Zhang C, Li S, Yang L, Huang P, Li W, Wang S, et al. Structural modulation of gut microbiota in life-long calorie-restricted mice. Nat Commun. 2013;4(1):2163.
Zhernakova A, Kurilshikov A, Bonder MJ, Tigchelaar EF, Schirmer M, Vatanen T, et al. Population-based metagenomics analysis reveals markers for gut microbiome composition and diversity. Science. 2016;352(6285):565–9.
Zmora N, Suez J, Elinav E. You are what you eat: diet, health and the gut microbiota. Nat Rev Gastroenterol Hepatol. 2019;16(1):35–56.
Zoetendal EG, Rajilic-Stojanovic M, de Vos WM. High-throughput diversity and functionality analysis of the gastrointestinal tract microbiota. Gut. 2008;57(11):1605–15.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Georgiou, K. (2021). Gut Microbiota in Obesity and Bariatric Surgery: Where Do We Stand?. In: Gazouli, M., Theodoropoulos, G. (eds) Gut Microbiome-Related Diseases and Therapies. The Microbiomes of Humans, Animals, Plants, and the Environment, vol 1. Springer, Cham. https://doi.org/10.1007/978-3-030-59642-2_6
Download citation
DOI: https://doi.org/10.1007/978-3-030-59642-2_6
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-59641-5
Online ISBN: 978-3-030-59642-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)