Abstract
Purpose of Review
In this review, we summarize what is currently described in terms of gut microbiota (GM) dysbiosis modification post-bariatric surgery (BS) and their link with BS-induced clinical improvement. We also discuss how the major inter-individual variability in terms of GM changes could impact the clinical improvements seen in patients.
Recent Findings
The persisting increase in severe obesity prevalence has led to the subsequent burst in BS number. Indeed, it is to date the best treatment option to induce major and sustainable weight loss and metabolic improvement in these patients. During obesity, the gut microbiota displays distinctive features such as low microbial gene richness and compositional and functional alterations (termed dysbiosis) which have been associated with low-grade inflammation, increased body weight and fat mass, as well as type-2 diabetes. Interestingly, GM changes post-BS is currently being proposed as one the many mechanism explaining BS beneficial clinical outcomes.
Summary
BS enables partial rescue of GM dysbiosis observed during obesity. Some of the GM characteristics modified post-BS (composition in terms of bacteria and functions) are linked to BS beneficial outcomes such as weight loss or metabolic improvements. Nevertheless, the changes in GM post-BS display major variability from one patient to the other. As such, further large sample size studies associated with GM transfer studies in animals are still needed to completely decipher the role of GM in the clinical improvements observed post-surgery.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
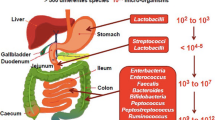
The gut microbiota (GM) colonizes the digestive tract at birth [1, 2] with bacterial compositional changes and diversification until 2 years of age. Although a series of endogenous and exogenous factors (such as diet, drugs and diseases) can impact its composition, the GM is generally stable throughout adolescence and adulthood until individuals reach 70–75 years old [3, 4]. The digestive tract harbors 1014 microorganisms (at least in the colon) which remain mostly unidentified [5]. In humans and rodents, the GM is segmented into two main phyla: Bacteroidetes and Firmicutes [6]. New culture-independent “omics” technologies, mainly metagenomics and metabolomics [7, 8••, 9], have provided major insights into GM composition and functions in both health and diseases [10].
During obesity, a common and frequent fecal microbiota characteristic is reduced microbial gene richness (MGR) and diversity. Low MGR has been observed in obese mice [11] and humans [12, 13] and is more prevalent in populations with a high incidence of obesity [4]. Low MGR is defined using shotgun analysis and is represented by the total number of non-redundant microbial genes below the threshold of 480,000 genes [12, 13] and is associated with increased BMI, low-grade inflammation, and insulin resistance [12, 13]. As such, low MGR can be found in up to 40% of overweight/moderately obese patients. Recently, we have shown that the most extreme forms of obesity (i.e., severe obesity) are characterized by a very high prevalence (75% of the patients) of low MGR [8••]. Beyond corpulence, this decreased MGR is further associated with adverse adipose tissue repartition (i.e., increased trunk-fat mass), type-2 diabetes (T2D), and hypertension and its severity as witnessed by increased polypharmacy [8••]. However, dietary habits are critical in modulating MGR and gut bacterial diversity. Indeed, European children who consume half the fiber intake of their African counterparts display a lower bacterial diversity [14] compared to the African children. Furthermore, high MGR [15] is also observed in moderately obese individuals following a healthy diet. Interestingly, in weight loss intervention programs, obese patients who follow a restrictive diet yet with adequate nutrition display higher gut bacterial diversity as compared to those with self-prescribed dietary restriction and inadequate nutrition [16].
In addition to reduced bacterial richness, the GM undergoes profound compositional and functional changes during obesity. A pioneering study published in 2005 found that the ratio of Bacteroidetes to Firmicutes (the two most common phyla within the GM) is decreased in genetically obese mice (Lep-/-, also known as ob/ob) as compared their heterozygous or wild-type littermates [17, 18]. Although this finding was confirmed in humans shortly afterwards [19, 20], several studies have found diverging results [21] with the current literature, suggesting that this biomarker is probably not universal in obesity. In addition to phylogenetic changes, the GM of obese animals extract more energy from fermentation than that of lean animals [19], and this feature is (at least partially) transmissible via fecal microbiota transfer (FMT), into germ-free animals [19, 22]. FMT from obese individuals into germ-free mice also induces susceptibly to weight gain in germ-free mice when compared to mice transferred with GM from lean donors [23]. Several studies using metagenomic sequencing further assessed GM functional differences between obese and lean controls as well as in individuals with high vs low MGR [12, 13]. These studies reported that subjects with obesity and low MGR harbored less butyrate-producing bacteria, reduced hydrogen and methane production, increased potential to degrade intestinal mucus, and increased oxidative stress management potential [12].
Overall, these studies demonstrate obesity is associated with major GM dysbiosis, which further worsens with increasing BMI and disease aggravation [8••]. Whether this dysbiosis can be reversed upon weight loss has been evaluated using various means, including bariatric surgery (BS), which is the focus of the present review. We here summarize the GM compositional changes after several BS techniques and their link with clinical outcomes. We also discuss the factors potentially involved in major differences and variability observed across studies.
Bariatric Surgeries Techniques and Outcomes
Bariatric surgery is classically recommended for individuals with BMI ≥ 40 kg/m2 or ≥ 35 kg/m2 with associated comorbidities [24]. All BS procedures (adjustable gastric banding (AGB), sleeve gastrectomy (SG), and Roux-en-Y gastric bypass (RYGB)) consist of a reduction of gastric volume by creating a gastric pouch of roughly 30 ml, which drastically reduces food intake [25, 26]. Depending on the surgical technique used (with the exception of AGB), there are also further modifications of the intestinal tract, which have potential consequences on GM composition. For instance, SG induces modifications of pH and gut hormones secretion profiles, whereas, a degree of RYGB adds malabsorption and bile flow diversion (via the exclusion of the duodenum and the proximal jejunum from the intestinal tract), as well as modifications of food taste and macronutrient intake [27]. These mechanisms have been collectively summarized as the BRAVE effect [28] of BS. The gut architecture and digestive ecology is thus deeply modified following BS and leads to a significant pressure on the gut microbial ecosystem (as reviewed in length [5]). To date, BS is an efficient therapeutic option to induce rapid and significant weight loss [29] over time with a variable degree of weight loss maintenance [29]. Because of the progression of severe obesity worldwide, the number of BS intervention has progressed in parallel, reaching a three-fold increase the past 10 years [30]. However, weight loss outcomes display major inter-individual variability. While some patients are considered as good responders [31, 32] (i.e., they lose a large amount of weight and further stabilize this weight loss during follow-up), others lose less weight during the first year [31, 32] or regain weight at mid-term [33]. While several clinical or biological factors including type-2 diabetes [34], surgery conversion [35], and adipose tissue fibrosis [31, 32, 36] are involved in the variability of individuals’ responses, it is suggested that differential changes within the gut microbiota could also contribute to the inter-individual variability observed for post-bariatric surgery outcomes.
Concomitantly to weight loss, patients undergo drastic improvements of their metabolic conditions post-BS [37], due not only to weight loss itself but also to other weight-independent mechanisms extensively described elsewhere [38]. In this context, a growing amount of literature suggests that GM modifications could be associated with or eventually explain BS-induced metabolic and inflammatory improvements as previously reviewed [39]. Indeed, strong evidences have emerged from FMT studies using either mice [40, 41••] or human [42] donors and germ-free mice recipients, which have shown that the modified GM post-BS is able to induce moderate weight loss upon FMT when compared to FMT in sham operated animals or non-operated subjects. However, the precise mechanisms involved in the GM-mediated improvements post-BS remains scarce.
Bariatric Surgery and Gut Microbiota Modulation
Microbial Richness
Bariatric surgery has been shown to increase gut bacterial richness and diversity in different studies with various sequencing techniques (Table 1). Using 16S rRNA pyrosequencing, we previously demonstrated a significant increase in diversity from baseline to 3 months which further remains stable at 6 months post-RYGB. This observation was further confirmed for up to 1 year post-RYGB [43•] using Illumina shotgun sequencing. Recently, Palleja et al. have confirmed this increase in diversity using the same method, yet due to a limited number of patients, it did not reach significance [44]. Furthermore, we confirmed and reinforced this observation showing a significant increase in gut microbial richness (as estimated by bacterial gene count via SOLiD shotgun sequencing) only 1 year post-BS both after RYGB and AGB [8••]. Most interestingly, in another group of patients followed up to 5 years post-RYGB, we observed that the significant increase in MGR obtained at 1 year remains stable thereafter [8••]. Most importantly, BS is not able to completely reverse the initial obesity-associated decrease in MGR, although patients exhibit major weight reductions and metabolic and inflammatory improvements [8••, 45]. Since severely obese patients present with very low MGR at baseline, BS is not sufficient enough to enable a switch from low to high MGR [8••]. Whereas partial, the reason why the bacterial gene richness is improved is not fully understood and could originate from many factors besides gut anatomy modification and could include improvements in metabolism, inflammation, body composition, and weight loss [8••]. Some bacterial genus changes, such as Eubacterium spp., Ruminococcaceae spp. and Faecalibaceterium spp., are associated with the amelioration of metabolic factors, including HbA1c. Moreover, the healthy diet recommended post-BS [24, 46] might also play a role in increasing MGR, as proposed by Griffin et al. [16].
The findings discussed above are reported after AGB and RYGB. However, SG is becoming the most preferred and performed BS intervention worldwide [30], and studies have started assessing gut microbiota modulation post-SG compared to other BS techniques. A recent murine study demonstrated that both SG and RYGB similarly increase diversity as assessed by 16S-pyrosequencing [47]. This significant increase in diversity was confirmed in humans 3 months post-SG [48], using shot gun sequencing; however, diverging results are also reported. Although Murphy et al. observed a significant increase in MGR post-RYGB, no difference was observed post-SG [43•]. More powered studies, with a higher number of patients and including follow-ups, are needed to further assess the effect of BS surgery techniques on gut bacterial richness and diversity and to relate the observed changes with lifestyle and clinical improvements.
Post-BS Evolution of Gut Microbiota Composition
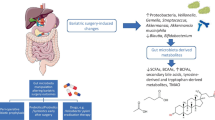
Bariatric surgery modifies GM composition in the short- [49,50,51], mid- [44, 48, 52], and long-term, up to 9 years [8••, 42]. These bacterial compositional changes have been extensively reviewed in the literature [53,54,55,56]. Interestingly, several bacterial and metabolic signatures have been consistently described and are displayed here in Table 1, whereas some bacterial changes have been further associated with clinical parameters, as illustrated in Table 2. Both bacterial changes and their association with clinical parameters are summarized in the Fig. 1.
Summary of the main changes in GM composition across literature and their link with modifications in clinical outcomes. Up arrow: increase; BMI body mass index, CRP C-reactive protein, HbA1c glycated hemoglobin, HOMA-IR homeostasis model assessment of insulin resistance, MCP-1 monocyte chemoattractant protein 1, TNF-α tumor necrosis factor alpha
Gammaproteobacteria [39] represents the class that has been the most consistently described as increased post-BS in animals as well as in both obese and obese diabetic patients [44, 50, 52, 57]. In some studies, this increase is associated with the amount of weight loss [58]. In our previous study using 16S rRNA pyrosequencing, we observed increased Escherichia coli, which is within the Proteobacteria phylum, parallels the decrease in leptin post-BS [49]. Intriguingly, indirect data regarding the mechanism of action of metformin suggest that this increase in Gammaproteobacteria could be involved in the post-BS metabolic improvements [59]. Furthermore, disrupting the GM of rodents with a cocktail of broad spectrum antibiotics induces a major increase in Proteobacteria, which is associated a beneficial phenotype of decreased systemic inflammation and improved glucose homeostasis [60]. Finally, an increase in Proteobacteria, including Escherichia coli, has also been reported in rodents or in drug naïve T2D humans after metformin treatment inducing improved glucose homeostasis, which further suggests that Proteobacteria could be involved in metabolic improvements [61]. However, this beneficial increase of Gammaproteobacteria could be seen as a paradox as an elevation of Proteobacteria and Enterobacteria is generally seen as deleterious in many intestinal diseases, such as inflammatory bowel diseases and colon cancer [62]. The precise mechanisms of this apparent paradox need to be deciphered. Indeed, it is known that Proteobacteria are gram negative bacteria that express lipopolysaccharide (LPS) in their membrane. Since LPS is one of the main drivers of metabolic endotoxemia [63], one could argue whether increasing Proteobacteria should really translate into real clinical benefits. Interestingly, although increased LPS synthesis within the GM has been observed post-BS [42], it is not associated with exacerbated systemic inflammation. This rather suggests that BS might be associated with decreased LPS translocation within the intestine into the systemic circulation, via a potential decreased intestinal permeability post-BS. Murine data have observed that RYGB improves tight-junction integrity and in vivo intestinal permeability while reducing metabolic endotoxemia and systemic inflammation [64]. Yet, such observations in mice following BS remain to be confirmed in humans.
Akkermansia muciniphila has been shown to have an important impact both on improved glucose homeostasis and weight loss as well as on the gut epithelium health in obese mice treated with prebiotics or after oral administration of the live bacteria [51, 65,66,67]. Akkermansia muciniphila also is associated with insulin sensitivity in mice [65] and humans [66]. Indeed, obese individuals with increased A. muciniphila have improved metabolic condition [66]. Studies on small number of patients have also shown that A. muciniphila increases post-BS [44, 50, 51, 68], yet whether it relates to improved glucose homeostasis needs further validation. In an unpublished observation from our group, we did not observe an association between A. muciniphila increase post-BS and glucose metabolism improvement (Dao et al., unpublished).
Impact of Different Bariatric Surgery Techniques
Although SG and RYGB display relatively similar clinical outcomes [69], the gut architecture modification significantly differs between the two procedures, possibly inducing differential GM modulations. Therefore, some, yet still scarce, studies have assessed GM changes after both interventions, after either SG or very low-calorie diet (VLCD), or finally, solely post-SG to assess SG-specific effects.
SG induces specific and distinct GM shifts as seen in a small study comparing VLCD and SG effects on gut microbiota [70]. Bacteroides vulgatus, a bacteria found increased in severe obesity and positively correlated with HbA1c [8••], is reduced significantly post-SG, whereas it is not significantly affected by either post-AGB or RYGB [8••]. Furthermore, SG also increases Faecalibacterium prausnitzii [70], another bacterium found decreased in severely obese individuals with T2D and which increases post-RYGB [49]. Based on these observations, it is tempting to speculate that the change in these bacteria could be involved in glucose improve observed post-SG; however, this has not been clearly described. In another study with small sample size comparing SG and RYGB, Murphy et al. observed that although SG was associated with functional changes in GM, they were fewer than those observed post-RYGB [43•]. Furthermore, whereas both surgery types induce similar clinical improvements and diet intakes, gut microbiota modifications involve distinct pathways according to the surgical technique [43•]. In particular, they observed an increased amino acids biosynthesis capacity post-SG [43•], a mechanism that could be linked to the improvement of glucose control.
A recent human study, including a larger number of individuals undergoing SG, demonstrated a rapid shift of microbial functions 3 months post-SG [48], becoming similar to that of healthy lean controls. Moreover, functions involved in carbohydrate fermentation, citrate cycle, glycosaminoglycan degradation, and LPS synthesis pathway rapidly decreased in these individuals. Most interestingly, Bacteroides thetaiotaomicron, which was found to be decreased in obesity, increased 3 months post-SG and this increase was found to be associated with the decrease in BMI [48]. In this study, A. muciniphila also significantly increased post-SG, a finding concordant with previous data obtained post-RYGB [51]. This study combining metagenomics and metabolomics exploration thus provides a potential link between these GM changes and metabolic improvement post-SG.
Inter-Individual Microbial Modulation
Even though significant shifts in gut microbiome composition and functions are reported in BS cohorts, the reported GM signatures show a major inter-individual variability amongst subjects post-BS that merits consideration. These individual profiles are nevertheless difficult to grasp in published studies as individual data are scarcely presented.
Gut microbial diversity and richness inter-individual variability are observed both pre- and post-BS [8••]. For example, we have reported that the mean baseline MGR is higher in patients who undergo AGB as compared to RYGB, which is likely due to less severe obesity-related comorbidities at baseline in AGB subjects. However, the baseline variance for MGR in both groups is large with the GM of patients undergoing AGB having between 300 k and 600 k genes, while the GM of patients in the RYGB group ranging between 125 k to 550 k genes. Currently, the underlying individual factors explaining this variability are unknown. Moreover, whether we can exploit this inter-individual variability in order to find predictive biomarkers of BS-induced weight loss merit consideration and needs larger-scale studies. Similarly, although the mean MGR significantly increases post-BS, the individual variability remains relatively high, yet lower than that observed at baseline. One could hypothesize that this MGR variability could be due to subjects’ lifestyle (including food patterns) and clinical condition before and after BS. However, it could also be related to differential clinical developments post-BS, including the amount of weight loss and the amplitude of metabolic improvements, and this needs to be examined in dedicated prospective studies.
To date, only one study examined individual relative abundance of GM composition. This study explored three healthy controls as well as in three unpaired obese patients and three patients who underwent RYGB, albeit with variable follow-up duration [51]. The relative abundance of most bacterial classes was found to be highly variable not only between groups of patients but also between patients within the same group; Proteobacteria and Clostridia were the most variable in the GM of obese and RYGB-operated patients, while Verrucomicrobia and Bacteroidetes were the most variable in the healthy controls [51]. We and others [8••, 71] have also recently reported this large inter-individual variability in GM modulation post-BS.
Collectively, the literature thus confirms that bariatric surgery modifies GM composition and function, yet differentially from one individual to the other. This could be related to variable clinical outcomes, which is largely described in bariatric cohorts [32, 33, 37]. Yet, it could also be due to several biases and/or confounding factors discussed below.
Discussion
Although some GM signatures observed post-BS are replicated across studies (as discussed above), this is not always the case as some studies display controversial results. This variability in these findings might originate from the different DNA extraction and sequencing techniques used (DGGE [72], qPCR [49], 16S rRNA pyrosequencing [52], shotgun metagenomics (SOLiD [8••, 70] or Illumina [42, 43•, 44, 48, 50, 73]; see Table 1) across studies, the different bariatric procedures, or different time points of stool collection post-BS (either short- [49, 50, 52], mid- [8••, 44, 48] or long-term [8••, 42] follow-up) where clinical outcomes also differ. Moreover, cohort ethnicity might also play a role and is, in general, not taken into account in these studies. Ethnicity has been shown to influence GM composition [74], and study location (Europe [8••], Asia [48], or Oceania [43•]) could underlie the different BS-induced GM modulations due to different genetic backgrounds and lifestyles. As such, dietary intake [15, 75] is critical in explaining variability in the modulation of GM composition, which also differs from one country to another but also between baseline and post-surgery follow-up [25, 26]. For example, diet drastically changes post-BS, especially fiber intake [25], which is known to have a critical impact on GM composition and function [76]. In a previous study, we observed associations between some bacterial changes and improvements in corpulence, metabolic, or inflammatory markers, yet half of these associations are strongly dependent on food intake [49]. Dietary patterns also differ from one individual to another post-BS [25, 26, 46] and dietary recommendations between clinical centers may differ as well [51]. It is thus necessary to better examine the link between post-BS dietary intake and lifestyle changes (such as physical activity) and gut microbiota modulation to explain the reported variability in GM composition.
Indeed, even though individuals can share broad GM resemblances, as seen with the enterotypes [77], a myriad of environmental factors play a role in this high inter-individual variability [76, 78], including not only lifestyle factors but also medications. In the context of BS, patients are frequently heavily treated for a large set of obesity-associated comorbidities including T2D and dyslipidemia before the intervention [37]. These therapies, such as metformin (the first line of treatment for T2D) or statins, can have profound effects on the GM composition [7, 59, 79, 80]. Since BS induces major metabolic improvement, some, but not all patients, can stop drugs originally taken at baseline, in particular glucose-lowering agents including metformin [81, 82]. Thus, these changes in drug intake, variable from one patient to another, could be involved in the major GM changes seen across individuals.
Finally, although BS induces drastic changes in GM richness and composition [8••, 40, 41••, 42, 43•, 49] some of which are maintained in the longer-term [42], BS does not rescue the GM dysbiosis seen in severe obesity [8••]. While showing some improvement, gut microbial richness remains under the cut-off for low diversity [12, 13]. In studies comparing BS individuals before and after surgery and lean controls, the GM profile at the phylum level does not reach that of lean individuals [49, 51]. It is important to examine whether this partial correction of GM dysbiosis post-BS could be involved in weight regain or the reoccurrence of obesity related comorbidities in some patients [33, 37], which is also associated with a switch towards a less healthy diet and a more sedentary lifestyle. A recent mouse study demonstrated that weight cycling induces GM modulations but with a persistent dysbiotic signature after the first initial weight loss. Most importantly, this dysbiotic GM is associated with increased weight gain when compared to high-fat diet fed mice who never were subjected to the weight loss intervention [83]. Therefore, one could hypothesize that although BS improves GM composition and function, it does not normalize it and this could be linked to adverse clinical outcomes in the long-term, including weight regain and metabolic deterioration [33].
Conclusion
While considered as a useful clinical tool to improve the clinical outcomes of patients with severe obesity, bariatric surgery is also a remarkable model to understand the fundamental mechanisms involved in drastic metabolic and inflammatory amelioration. Amongst the myriad of potential mechanisms, changes in gut microbiota composition and related functional modification have been put forward with the availability of new sequencing tools. While GM changes can be observed and are associated with metabolic improvements in still relatively unpowered human studies, they are not always consistent and vary across population. Given these variations, further research efforts are needed to deepen the understanding of GM changes on improved metabolism post-BS, which may provide evidence for the need to act therapeutically on the GM to improve patient outcomes in the long term.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Penders J, Thijs C, Vink C, Stelma FF, Snijders B, Kummeling I, et al. Factors influencing the composition of the intestinal microbiota in early infancy. Pediatrics. 2006;118:511–21.
Palmer C, Bik EM, DiGiulio DB, Relman DA, Brown PO. Development of the human infant intestinal microbiota. PLoS Biol. 2007;5:e177.
Marchesi JR. Human distal gut microbiome. Environ Microbiol. 2011;13:3088–102.
Yatsunenko T, Rey FE, Manary MJ, Trehan I, Dominguez-Bello MG, Contreras M, et al. Human gut microbiome viewed across age and geography. Nature. 2012;486:222–7.
Aron-Wisnewsky J, Doré J, Clement K. The importance of the gut microbiota after bariatric surgery. Nat Rev Gastroenterol Hepatol. 2012;9:590–8.
Qin J, Li Y, Cai Z, Li S, Zhu J, Zhang F, et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature. 2012;490:55–60.
Pedersen HK, Gudmundsdottir V, Nielsen HB, Hyotylainen T, Nielsen T, Jensen BAH, et al. Human gut microbes impact host serum metabolome and insulin sensitivity. Nature. 2016;535:376–81.
•• Aron-Wisnewsky J, Prifti E, Belda E, Ichou F, Kayser BD, Dao MC, et al. Major microbiota dysbiosis in severe obesity: fate after bariatric surgery. Gut. 2018;68:70–82. https://doi.org/10.1136/gutjnl-2018-316103. This is the first study with multiple time point kinetic follow-ups of patients who underwent two different types of bariatric surgery. It demonstrates that GM dysbiosis is partially rescued at 1 year and further stabilize at 5 years.
Wang Z, Klipfell E, Bennett BJ, Koeth R, Levison BS, DuGar B, et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011;472:57–63.
Shendure J, Ji H. Next-generation DNA sequencing. Nat Biotechnol. 2008;26:1135–45.
Turnbaugh PJ, Bäckhed F, Fulton L, Gordon JI. Diet-induced obesity is linked to marked but reversible alterations in the mouse distal gut microbiome. Cell Host Microbe. 2008;3:213–23.
Le Chatelier E, et al. Richness of human gut microbiome correlates with metabolic markers. Nature. 2013;500:541–6.
Cotillard A, et al. Dietary intervention impact on gut microbial gene richness. Nature. 2013;500:585–8.
De Filippo C, et al. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc Natl Acad Sci U S A. 2010;107:14691–6.
Kong LC, Holmes BA, Cotillard A, Habi-Rachedi F, Brazeilles R, Gougis S, et al. Dietary patterns differently associate with inflammation and gut microbiota in overweight and obese subjects. PLoS One. 2014;9:e109434.
Griffin NW, Ahern PP, Cheng J, Heath AC, Ilkayeva O, Newgard CB, et al. Prior dietary practices and connections to a human gut microbial metacommunity alter responses to diet interventions. Cell Host Microbe. 2017;21:84–96.
Ley RE, Turnbaugh PJ, Klein S, Gordon JIM e. Human gut microbes associated with obesity. Nature. 2006;444:1022–3.
Ley RE, Backhed F, Turnbaugh P, Lozupone CA, Knight RD, Gordon JI. Obesity alters gut microbial ecology. Proc Natl Acad Sci U S A. 2005;102:11070–5.
Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027–31.
Turnbaugh PJ, Hamady M, Yatsunenko T, Cantarel BL, Duncan A, Ley RE, et al. A core gut microbiome in obese and lean twins. Nature. 2009;457:480–4.
Schwiertz A, Taras D, Schäfer K, Beijer S, Bos NA, Donus C, et al. Microbiota and SCFA in lean and overweight healthy subjects. Obes Silver Spring Md. 2010;18:190–5.
Turnbaugh PJ, Backhed F, Fulton L, Gordon JI. Marked alterations in the distal gut microbiome linked to diet-induced obesity. Cell Host Microbe. 2008;3:213–23.
Ridaura VK, Faith JJ, Rey FE, Cheng J, Duncan AE, Kau AL, et al. Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science. 2013;341:1241214.
Fried M, et al. Interdisciplinary European guidelines on metabolic and bariatric surgery. Obes Surg. 2014;24:42–55.
Verger EO, Aron-Wisnewsky J, Dao MC, Kayser BD, Oppert JM, Bouillot JL, et al. Micronutrient and protein deficiencies after gastric bypass and sleeve gastrectomy: a 1-year follow-up. Obes Surg. 2015;26:785–96. https://doi.org/10.1007/s11695-015-1803-7.
Aron-Wisnewsky J, Verger EO, Bounaix C, Dao MC, Oppert JM, Bouillot JL, et al. Nutritional and protein deficiencies in the short term following both gastric bypass and gastric banding. PLoS One. 2016;11:e0149588.
le Roux CW, Bueter M, Theis N, Werling M, Ashrafian H, Löwenstein C, et al. Gastric bypass reduces fat intake and preference. Am J Physiol Regul Integr Comp Physiol. 2011;301:R1057–66.
Ashrafian H, Athanasiou T, Li JV, Bueter M, Ahmed K, Nagpal K, et al. Diabetes resolution and hyperinsulinaemia after metabolic Roux-en-Y gastric bypass. Obes Rev Off J Int Assoc Study Obes. 2011;12:e257–72.
Sjöström L, Peltonen M, Jacobson P, Sjöström CD, Karason K, Wedel H, et al. Bariatric surgery and long-term cardiovascular events. JAMA. 2012;307:56–65.
Angrisani L, Santonicola A, Iovino P, Vitiello A, Zundel N, Buchwald H, et al. Bariatric surgery and endoluminal procedures: IFSO Worldwide Survey 2014. Obes Surg. 2017;27:2279–89.
Divoux A, Tordjman J, Lacasa D, Veyrie N, Hugol D, Aissat A, et al. Fibrosis in human adipose tissue: composition, distribution, and link with lipid metabolism and fat mass loss. Diabetes. 2010;59:2817–25.
Abdennour M, Reggio S, le Naour G, Liu Y, Poitou C, Aron-Wisnewsky J, et al. Association of adipose tissue and liver fibrosis with tissue stiffness in morbid obesity: links with diabetes and BMI loss after gastric bypass. J Clin Endocrinol Metab. 2014;99:898–907.
Courcoulas AP, King WC, Belle SH, Berk P, Flum DR, Garcia L, et al. Seven-year weight trajectories and health outcomes in the Longitudinal Assessment of Bariatric Surgery (LABS) study. JAMA Surg. 2017;153:427–34. https://doi.org/10.1001/jamasurg.2017.5025.
Courcoulas AP, Christian NJ, O’Rourke RW, Dakin G, Patchen Dellinger E, Flum DR, et al. Preoperative factors and 3-year weight change in the Longitudinal Assessment of Bariatric Surgery (LABS) consortium. Surg Obes Relat Dis Off J Am Soc Bariatr Surg. 2015;11:1109–18.
Thereaux J, Corigliano N, Poitou C, Oppert JM, Czernichow S, Bouillot JL. Five-year weight loss in primary gastric bypass and revisional gastric bypass for failed adjustable gastric banding: results of a case-matched study. Surg Obes Relat Dis Off J Am Soc Bariatr Surg. 2015;11:19–25.
Bel Lassen P, Charlotte F, Liu Y, Bedossa P, le Naour G, Tordjman J, et al. The FAT score, a fibrosis score of adipose tissue: predicting weight loss outcome after gastric bypass. J Clin Endocrinol Metab. 2017;102:2443–53. https://doi.org/10.1210/jc.2017-00138.
Debédat J, Sokolovska N, Coupaye M, Panunzi S, Chakaroun R, Genser L, et al. Long-term relapse of type 2 diabetes after Roux-en-Y gastric bypass: prediction and clinical relevance. Diabetes Care. 2018;41:2086–95. https://doi.org/10.2337/dc18-0567.
Laferrère, B. & Pattou, F. Weight-independent mechanisms of glucose control after Roux-en-Y gastric bypass. Front Endocrinol. (2018);9:530.
Aron-Wisnewsky J, Clement K. The effects of gastrointestinal surgery on gut microbiota: potential contribution to improved insulin sensitivity. Curr Atheroscler Rep. 2014;16(454):454.
Liou AP, et al. Conserved shifts in the gut microbiota due to gastric bypass reduce host weight and adiposity. Sci Transl Med. 2013;5:178ra41.
•• Arora T, Seyfried F, Docherty NG, Tremaroli V, le Roux CW, Perkins R, et al. Diabetes-associated microbiota in fa/fa rats is modified by Roux-en-Y gastric bypass. ISME J. 2017;11:2035–46 This is the first study in animal models who evaluated the potential of the GM in post-surgery metabolic improvements using fecal transfer experiments.
Tremaroli V, Karlsson F, Werling M, Ståhlman M, Kovatcheva-Datchary P, Olbers T, et al. Roux-en-Y gastric bypass and vertical banded gastroplasty induce long-term changes on the human gut microbiome contributing to fat mass regulation. Cell Metab. 2015;22:228–38.
• Murphy R, Tsai P, Jüllig M, Liu A, Plank L, Booth M. Differential changes in gut microbiota after gastric bypass and sleeve gastrectomy bariatric surgery vary according to diabetes remission. Obes Surg. 2017;27:917–25 This is the first human study exploring differences in GM composition in patients with or without diabetes remission post-surgery in two different surgical interventions.
Palleja A, Kashani A, Allin KH, Nielsen T, Zhang C, Li Y, et al. Roux-en-Y gastric bypass surgery of morbidly obese patients induces swift and persistent changes of the individual gut microbiota. Genome Med. 2016;8:67.
Cani PD. Severe obesity and gut microbiota: does bariatric surgery really reset the system? Gut. 2018;68:5–6. https://doi.org/10.1136/gutjnl-2018-316815.
Sherf Dagan S, Keidar A, Raziel A, Sakran N, Goitein D, Shibolet O, et al. Do bariatric patients follow dietary and lifestyle recommendations during the first postoperative year? Obes Surg. 2017;27:2258–71.
Guo, Y., Liu C.Q., Shan C.X., Chen Y., Li H.H., Huang Z.P., Zou D.J.. Gut microbiota after Roux-en-Y gastric bypass and sleeve gastrectomy in a diabetic rat model: increased diversity and associations of discriminant genera with metabolic changes. Diabetes Metab Res Rev. (2017); 33:e2857.
Liu R, Hong J, Xu X, Feng Q, Zhang D, Gu Y, et al. Gut microbiome and serum metabolome alterations in obesity and after weight-loss intervention. Nat Med. 2017;23:859–68.
Furet J-P, Kong LC, Tap J, Poitou C, Basdevant A, Bouillot JL, et al. Differential adaptation of human gut microbiota to bariatric surgery-induced weight loss: links with metabolic and low-grade inflammation markers. Diabetes. 2010;59:3049–57.
Graessler J, Qin Y, Zhong H, Zhang J, Licinio J, Wong ML, et al. Metagenomic sequencing of the human gut microbiome before and after bariatric surgery in obese patients with type 2 diabetes: correlation with inflammatory and metabolic parameters. Pharmacogenomics J. 2013;13:514–22.
Zhang H, DiBaise JK, Zuccolo A, Kudrna D, Braidotti M, Yu Y, et al. Human gut microbiota in obesity and after gastric bypass. Proc Natl Acad Sci U S A. 2009;106:2365–70.
Kong L-C, Tap J, Aron-Wisnewsky J, Pelloux V, Basdevant A, Bouillot JL, et al. Gut microbiota after gastric bypass in human obesity: increased richness and associations of bacterial genera with adipose tissue genes. Am J Clin Nutr. 2013;98:16–24.
Davies N, O’Sullivan JM, Plank LD, Murphy R. Altered gut microbiome after bariatric surgery and its association with metabolic benefits: a systematic review. Surg Obes Relat Dis. 2019;15:656–65. https://doi.org/10.1016/j.soard.2019.01.033.
Guo Y, Huang ZP, Liu CQ, Qi L, Sheng Y, Zou DJ. Modulation of the gut microbiome: a systematic review of the effect of bariatric surgery. Eur J Endocrinol. 2018;178:43–56.
Castaner O, Goday A, Park YM, Lee SH, Magkos F, Shiow SATE, et al. The gut microbiome profile in obesity: a systematic review. Int J Endocrinol. 2018;2018:1–9. https://doi.org/10.1155/2018/4095789.
Magouliotis DE, Tasiopoulou VS, Sioka E, Chatedaki C, Zacharoulis D. Impact of bariatric surgery on metabolic and gut microbiota profile: a systematic review and meta-analysis. Obes Surg. 2017;27:1345–57.
Li JV, Ashrafian H, Bueter M, Kinross J, Sands C, le Roux CW, et al. Metabolic surgery profoundly influences gut microbial–host metabolic cross-talk. Gut. 2011;60:1214–23.
Shao Y, Ding R, Xu B, Hua R, Shen Q, He K, et al. Alterations of gut microbiota after Roux-en-Y gastric bypass and sleeve gastrectomy in Sprague-Dawley rats. Obes Surg. 2017;27:295–302.
Guo GL, Xie W. Metformin action through the microbiome and bile acids. Nat Med. 2018;24:1789–90.
Carvalho BM, Guadagnini D, Tsukumo DML, Schenka AA, Latuf-Filho P, Vassallo J, et al. Modulation of gut microbiota by antibiotics improves insulin signalling in high-fat fed mice. Diabetologia. 2012;55:2823–34.
Wu, H. et al. Metformin alters the gut microbiome of individuals with treatment-naive type 2 diabetes, contributing to the therapeutic effects of the drug. Nat. Med. 2017;23:850
Arthur JC, Jobin C. The complex interplay between inflammation, the microbiota and colorectal cancer. Gut Microbes. 2013;4:253–8.
Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D, et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56:1761–72.
Guo Y, Liu C-Q, Liu G-P, Huang Z-P, Zou D-J. Roux-en-Y gastric bypass decreases endotoxemia and inflammatory stress in association with improvement of gut permeability in obese diabetic rats. J Diabetes. 2019. https://doi.org/10.1111/1753-0407.12906.
Plovier H, Everard A, Druart C, Depommier C, van Hul M, Geurts L, et al. A purified membrane protein from Akkermansia muciniphila or the pasteurized bacterium improves metabolism in obese and diabetic mice. Nat Med. 2017;23:107–13.
Dao MC, Everard A, Aron-Wisnewsky J, Sokolovska N, Prifti E, Verger EO, et al. Akkermansia muciniphila and improved metabolic health during a dietary intervention in obesity: relationship with gut microbiome richness and ecology. Gut. 2015;65:426–36. https://doi.org/10.1136/gutjnl-2014-308778.
Everard A, Belzer C, Geurts L, Ouwerkerk JP, Druart C, Bindels LB, et al. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc Natl Acad Sci U S A. 2013;110:9066–71.
Ward EK, Schuster DP, Stowers KH, Royse AK, Ir D, Robertson CE, et al. The effect of PPI use on human gut microbiota and weight loss in patients undergoing laparoscopic Roux-en-Y gastric bypass. Obes Surg. 2014;24:1567–71.
Osland E, Yunus RM, Khan S, Memon B, Memon MA. Weight loss outcomes in laparoscopic vertical sleeve gastrectomy (LVSG) versus laparoscopic Roux-en-Y gastric bypass (LRYGB) procedures: a meta-analysis and systematic review of randomized controlled trials. Surg Laparosc Endosc Percutan Tech. 2017;27:8–18.
Damms-Machado A, et al. Effects of surgical and dietary weight loss therapy for obesity on gut microbiota composition and nutrient absorption. Biomed Res Int. 2015;2015:806248.
Paganelli FL, Luyer M, Hazelbag CM, Uh H-W, Rogers MRC, Adriaans D, et al. Roux-Y gastric bypass and sleeve gastrectomy directly change gut microbiota composition independent of operation type. 2018. https://doi.org/10.1101/395657.
Federico A, Dallio M, Tolone S, Gravina AG, Patrone V, Romano M, et al. Gastrointestinal hormones, intestinal microbiota and metabolic homeostasis in obese patients: effect of bariatric surgery. 2016;30:321–30.
Patrone, V., Vajana E., Minuti A., Callegari M. L., Federico A., Loguercio C., Dallio M., Tolone S., Docimo L., Morelli L.. Postoperative changes in fecal bacterial communities and fermentation products in obese patients undergoing bilio-intestinal bypass. Front Microbiol. (2016):7:200.
Deschasaux M, Bouter KE, Prodan A, Levin E, Groen AK, Herrema H, et al. Depicting the composition of gut microbiota in a population with varied ethnic origins but shared geography. Nat Med. 2018;24:1526–31.
Wu GD, Chen J, Hoffmann C, Bittinger K, Chen YY, Keilbaugh SA, et al. Linking long-term dietary patterns with gut microbial enterotypes. Science. 2011;334:105–8.
Falony G, Joossens M, Vieira-Silva S, Wang J, Darzi Y, Faust K, et al. Population-level analysis of gut microbiome variation. Science. 2016;352:560–4.
Arumugam M, Raes J, Pelletier E, le Paslier D, Yamada T, Mende DR, et al. Enterotypes of the human gut microbiome. Nature. 2011;473:174–80.
Vandeputte D, Kathagen G, D’hoe K, Vieira-Silva S, Valles-Colomer M, Sabino J, et al. Quantitative microbiome profiling links gut community variation to microbial load. Nature. 2017;551:507–11. https://doi.org/10.1038/nature24460.
Bauer PV, Duca FA, Waise TMZ, Rasmussen BA, Abraham MA, Dranse HJ, et al. Metformin alters upper small intestinal microbiota that impact a glucose-SGLT1-sensing glucoregulatory pathway. Cell Metab. 2018;27:101–117.e5. https://doi.org/10.1016/j.cmet.2017.09.019.
Caparrós-Martín JA, Lareu RR, Ramsay JP, Peplies J, Reen FJ, Headlam HA, et al. Statin therapy causes gut dysbiosis in mice through a PXR-dependent mechanism. Microbiome. 2017;5(95):95.
Mingrone G, Panunzi S, de Gaetano A, Guidone C, Iaconelli A, Nanni G, et al. Bariatric-metabolic surgery versus conventional medical treatment in obese patients with type 2 diabetes: 5 year follow-up of an open-label, single-centre, randomised controlled trial. Lancet Lond Engl. 2015;386:964–73.
Schauer PR, Bhatt DL, Kirwan JP, Wolski K, Aminian A, Brethauer SA, et al. Bariatric surgery versus intensive medical therapy for diabetes - 5-year outcomes. N Engl J Med. 2017;376:641–51.
Thaiss CA, Itav S, Rothschild D, Meijer MT, Levy M, Moresi C, et al. Persistent microbiome alterations modulate the rate of post-dieting weight regain. Nature. 2016;540:544–51. https://doi.org/10.1038/nature20796.
Godon JJ, Zumstein E, Dabert P, Habouzit F, Moletta R. Molecular microbial diversity of an anaerobic digestor as determined by small-subunit rDNA sequence analysis. Appl Environ Microbiol. 1997;63:2802–13.
Patil DP, Dhotre DP, Chavan SG, Sultan A, Jain DS, Lanjekar VB, et al. Molecular analysis of gut microbiota in obesity among Indian individuals. J Biosci. 2012;37:647–57.
Manichanh C, Rigottier-Gois L, Bonnaud E, Gloux K, Pelletier E, Frangeul L, et al. Reduced diversity of faecal microbiota in Crohn’s disease revealed by a metagenomic approach. Gut. 2006;55:205–11.
Osto M, Abegg K, Bueter M, le Roux CW, Cani PD, Lutz TA. Roux-en-Y gastric bypass surgery in rats alters gut microbiota profile along the intestine. Physiol Behav. 2013;119:92–6.
Duboc H, Nguyen CC, Cavin JB, Ribeiro-Parenti L, Jarry AC, Rainteau D, et al. Roux-en-Y gastric-bypass and sleeve gastrectomy induces specific shifts of the gut microbiota without altering the metabolism of bile acids in the intestinal lumen. Int J Obes. 2018;1:428–31. https://doi.org/10.1038/s41366-018-0015-3.
Acknowledgments
The authors would like to thank Dr. Tim Swartz for the careful English language review of this work.
Financial Support
Funding to support NutriOmics research unit activity on this review topic was obtained from European Union’s Seventh Framework Program (FP7) for research, technological development, and demonstration under grant agreement HEALTH-F4-2012-305312 (Metacardis) and Metagenopolis grant ANR-11-DPBS-0001 and from the Clinical research program (PHRC Microbaria). JAW received grant from Institut Benjamin Delessert and Société Francophone du Diabète (SFD), and KC received an award from the Fondation de France.
Author information
Authors and Affiliations
Contributions
JD contributed to the research, discussion of content, and writing of this manuscript; J.A.W contributed to the research, discussion of content, writing, and editing of this manuscript; and K.C. contributed to the discussion of content, writing, and reviewing/editing the manuscript before submission. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Conflict of Interest
None of the authors has anything to disclose relevant to this article.
Human and Animal Rights and Informed Consent
All reported studies/experiments with human or animal subjects performed by the authors were performed in accordance with all applicable ethical standards including the Helsinki declaration and its amendments, institutional/national research committee standards, and international/national/institutional guideline.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Obesity Treatment
Rights and permissions
About this article
Cite this article
Debédat, J., Clément, K. & Aron-Wisnewsky, J. Gut Microbiota Dysbiosis in Human Obesity: Impact of Bariatric Surgery. Curr Obes Rep 8, 229–242 (2019). https://doi.org/10.1007/s13679-019-00351-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13679-019-00351-3
Keywords
- Bariatric surgery
- Gut microbiota
- Metagenomics
- Richness
- Obesity
- Metabolism
- Akkermansia muciniphila
- Faecalibacterium prausnitzii
- Microbial gene richness
- Type-2 diabetes
- Roux-en-Y gastric bypass
- Sleeve gastrectomy
- Adjustable gastric banding
- Roseburia intestinalis
- Proteobacteria
- Gammaproteobacteria
- Firmicutes
- Bacteroidetes
- BMI
- HbA1c
- Remission
- Illumina