Abstract
Catheter ablation of tachyarrhythmias without the use of fluoroscopy is, at present, performed only in a minority of laboratories. While the tools exist to allow fluoroless procedures in most instances, experience is lacking. While the learning curve will require time, and may slow the productivity of the lab down for a while, the benefits to both the patients and the staff are worth the investment. If one visits an experienced lab doing fluoroless procedures, they will recognize that, not only is the risk of radiation decreased, but also the comfort level of the staff is increased by the absence of lead protective gear. It is the authors’ personal observation that elimination of lead aprons has been the number one motivation to obtain staff buy-in, making them willing to endure the learning curve. This chapter reviews the experiences of multiple labs, and their approach to overcoming the hurdles of radiation elimination. Hopefully, the reader will find sufficient references to make their own learning curve as smooth as possible.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
Nonfluoroscopic three-dimensional mapping for arrhythmia ablation: tool or toy?
That was the title of a manuscript published in 2000 [1]. Time and experience have proven that three-dimensional mapping is a powerful tool in the world of electrophysiology. This chapter reviews the learning curve through which the profession has evolved since that time.
First, let’s consider why one might want to endure the learning curve. The most apparent reason is to decrease radiation exposure to the patient. According to the National Council on Radiation Protection and Measurement, between 1981 and 2006 there was a 730% increase in medical radiation exposure in the United States [2]. Prior to 1980 medical radiation accounted for only a small fraction of the average annual radiation exposure. By 2006, medical radiation exposure had become the number one source of radiation for Americans. Because of that, the as low as reasonably achievable (ALARA) radiation dose principle is being aggressively pursued. Radiation carries both deterministic and stochastic risks. Deterministic risks are those side effects that are predictable at discrete doses of radiation, such as radiation dermatitis. Stochastic effects have no minimal threshold. These effects are primarily related to the long-term risk of malignancy and birth defects. Stochastic effects are the risks which the ALARA principle is intended to minimize. Other, less apparent, reasons to pursue minimal fluoroscopy include decreased radiation to the medical staff, the ability to perform ablation in the pregnant female, and the ability to make the procedure portable.
Decreasing radiation exposure to the patient has the secondary benefit of decreasing radiation exposure to the staff. We try not to dwell on the fact that our chosen profession has inherent risks, but they are real. A busy interventional cardiologist can have a lifetime risk of occupationally induced cancer as high as 4% [3]. A 2013 publication noted that 85% of head and neck tumors are left sided in physicians who work primarily with fluoroscopy [4]. Eliminating fluoroscopy will eliminate those occupational risks. But eliminating fluoroscopy will also eliminate the need for radiation protective equipment, such as lead aprons, thyroid shields, and lead goggles. Multiple studies have outlined the orthopedic problems related to long-term usage of such equipment [5,6,7]. Some electrophysiology (EP) labs have already achieved such a level of experience with fluoroless procedures that lead aprons are not worn for the majority of cases. The improved staff comfort associated with this is an appealing reason EP labs will be willing to undergo the learning curve.
Pregnancy is an uncommon situation among patients undergoing ablation, but one in which a zero fluoroscopy approach is of great benefit. While a pregnant patient with medical refractory arrhythmias is uncommon, a pregnant staff member is a situation that every lab will deal with over time. There is benefit here also, because the staff member can continue performing her job in the EP lab if it is fluoroscopy free.
Tachycardia-induced cardiomyopathy is a rare situation. These patients will sometimes require ECMO support. In that setting it would be feasible to do the ablation in the ICU rather than transporting the patient to the interventional lab, if fluoroscopy is not needed. For any or all of the above reasons, one may wish to pursue more robust radiation reduction measures in the EP lab. This chapter may help smooth the path and expedite the process as one goes through the learning curve.
History of Fluoroless Technology
To understand the evolution of three-dimensional mapping one must also understand the history of the tools available. There are four three-dimensional navigation systems currently available. These include the EnSite system (Abbott), the CARTO system (Biosense Webster), Mediguide (Abbott), and the Rhythmia system (Boston Scientific). Only two of these systems, the CARTO system and the EnSite system, have played a significant role in shaping the current state of the profession, and will, therefore, be the main points of discussion.
The CARTO system was first released in 1995 as a navigation and ablation tool. It was adopted by most of the major academic institutions and it was found to play a useful role in mapping of complex arrhythmia substrates. The system’s function was dependent on magnetic fields. The patient was positioned lying on a pad containing several magnets. A magnetic detector within the tip of a proprietary catheter could then localize the position of the tip of that catheter three-dimensionally in space. Because the magnetic fields did not change during the case there was a high degree of accuracy and reproducibility regarding the location of the catheter tip [8].
The EnSite system was released in 1999. Its functionality is based on electrical impedances rather than magnetic fields. The system requires placement of six electrode patches on the patient’s torso, setting up three orthogonal electrical fields. By measuring impedances within the three fields, the computer can localize the catheter electrode three-dimensionally within the body relative to a reference electrode. The EnSite system had the benefit of a broader field of view of catheters within the body. However, because lung volume and fluid volume both impacted electrical impedances, the system was more prone to geometrical shift.
In 2002 Drago et al. reported the first ever series of patients undergoing an ablation without fluoroscopy [9]. Their series consisted of 21 children with symptomatic WPW and right-sided accessory pathways. They were able to use the CARTO system to successfully ablate 20 of the 21 pathways. In nine patients no fluoroscopy was used. This sentinel report remains a major milestone in the evolution of electrophysiology.
However, there were no other reports published on this topic for 5 years after Drago’s initial findings. The reason for this long delay can be understood upon closer inspection of what Drago’s group had accomplished.
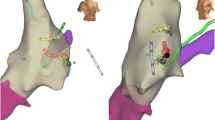
The CARTO system employed in that first report was the first generation. The only catheter that could be localized on that system was the radiofrequency catheter available through Biosense Webster. No mapping catheters and no other radiofrequency or cryoablation catheters could be visualized. So, as a tool for performing zero-fluoroscopy ablation, the original CARTO system was only useful for right-sided manifest accessory pathways. The system was also hindered by a narrow field of view and cumbersome geometry creation. The magnetic fields could not detect the catheter outside of the patient’s thorax and therefore the catheter needed to be advanced from the femoral vein to the heart without any visual data. Creating a three-dimensional geometry required point-by-point three-dimensional marks. This was a slow process and yielded geometry with little correlation to the actual anatomy. Figures 6.1 and 6.2 give examples of the quality of image that could be created using the first version (Fig. 6.1) of CARTO compared to the most current version (Fig. 6.2).
CARTO map with posterior-anterior (PA) view of the right atrium during sinus rhythm. Anatomy reconstruction (left side) and color-coded physiological activation sequence (right side). The sinus node and the physiological activation focus during sinus rhythm are clearly seen in red. CS coronary sinus
In 2007 additional studies were reported. Tuzcu reported his experience of 28 patients with right-sided tachycardia mechanisms undergoing ablation [10]. Twenty-four of the 28 underwent ablation with zero fluoroscopy. Of the four patients requiring fluoroscopy, two were infants, one had typical and atypical atrioventricular nodal reentry tachycardia (AVNRT) , and one had a parahisian pathway. Among the zero-fluoroscopy group, 15 had AVNRT and 9 had accessory pathways. He reported a 92% acute success rate and no complications. That same year, Clark and colleagues reported their experience with a minimal fluoroscopy approach [11]. Their series had 30 patients undergoing ablation for supraventricular tachycardia (SVT) . Twenty-four of the procedures were performed without fluoroscopy. Of the six patients requiring fluoroscopy, five of them needed it only to perform transseptal puncture. Compared to age- and rhythm-matched controls, they noted a 95% reduction in radiation exposure.
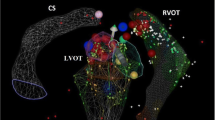
What the two studies from 2007 shared in common was the use of the EnSite system for catheter navigation. At the time, EnSite had several advantages over CARTO for use as a zero-fluoroscopy tool. Being impedance based, it is not restricted to proprietary catheters. It is able to localize any electrode on any mapping or ablation catheter . Also cardiac geometry creation is a faster process with the ability to obtain an appropriate amount of detail needed for an ablation in minutes (Fig. 6.3). In addition, the electrodes can be localized from the moment they exit the sheath into the femoral vein and, therefore, visual guidance of the catheter up the IVC is possible (Fig. 6.4).
From those two studies, it now appeared that electrophysiologists had a tool that could significantly decrease radiation exposure without significant additional work.
Early experience brought to light the fact that transseptal puncture was one of the most common reasons for fluoroscopy use when pursuing a minimal-fluoro procedure . Clark et al. reported the use of transesophageal echocardiography for transseptal puncture [12] and Ferguson reported his group’s use of intracardiac echo to accomplish the same goal [13]. The ability to cross the septum without fluoroscopy paved the way for all arrhythmias to potentially be approached with a zero-fluoroscopy procedure. From 2007 until 2013, all published series of zero-fluoroscopy ablation utilized the EnSite system. This included reports by Pachon et al. [14] addressing atrial flutter, Ferguson et al. [13] and Reddy et al. [15] addressing atrial fibrillation, Miyamoto et al. [16] and Von Bergen et al. [17] looking at ventricular tachycardia, and Casella et al. [18] looking at SVT. There were other publications in addition to these [11, 19,20,21,22,23,24,25].
As experience was rapidly increasing in no-fluoroscopy ablation with the EnSite system , CARTO was less effective in this specific characteristic. That fact was unfortunate because it created a large disparity in the availability of a nonfluoroscopic approach , based solely on the mapping system available at each institution. Since the majority of large academic centers utilized the CARTO system, the organizations that were typically leaders and innovators within the profession found themselves handicapped by technology. They had to wait for the availability of CARTO 3 before they could begin the learning process in earnest.
While only a minority of hospitals were utilizing EnSite for fluoroless procedures, many more hospitals and patients benefited from the experience. The attention being paid to fluoroless procedures with EnSite attracted a new interest in ways to minimize radiation exposure. Those institutions that did not use EnSite began to look at ways to decrease fluoroscopy time and minimize exposure by other means, like altering frame rates, shielding, filtering, and angling of fluoroscopy. Pass et al. were able to decrease radiation exposure by adjusting the fluoro frame rate and consciously being aware of how long the fluoro pedal was activated [26].
In 2009, CARTO 3 was released. It took several years for many centers to transition to the newer system and gain familiarity with its functions. The two major benefits of CARTO 3 were the ability of the system to track nonproprietary catheter electrodes, and to rapidly create a more realistic geometry. Pass’ group was one of the first to embrace the new system. They had done much to minimize radiation exposure and raise awareness of radiation doses during the years when their system did not allow a routine fluoroless procedure. In 2014 they published data of procedures with CARTO 3 and were now leading the field in experience with this system [27]. From 2014 through 2017 there was a steady increase in fluoroless procedures with CARTO 3 [28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44]. Fifteen years after Drago’s first publication, institutions now had more access to functional three-dimensional mapping tools , and the era of fully fluoroless procedures can now advance more rapidly.
Learning Curve of the Physician
Just as there was an evolution in the technology of three-dimensional mapping, there will also be an evolution in the performing physician’s technical skills . Knowledge of the experiences of other physicians can accelerate the pace at which one becomes proficient in these new procedures.
If one has little or no experience with 3D mapping systems , then the first step will be to gain familiarity with how the data is acquired and presented. Fluoroscopy allows for very little image control. The image is always in black and white. There are a few levels of magnification available. There is a broad, but not unlimited, range of angles to view from. And there is only minimal control over image quality. Imaging is not continuous, but rather requires activating the fluoro pedal when catheters need to be seen. With 3D mapping, there is far greater flexibility in data presentation. The images are always in color. The color palate is extensive, and broadly programmable to the operator’s taste.
The amount of information represented on the screen is entirely of the operator’s choosing, from sparse to exquisitely detailed. On one extreme, some labs will mark a catheter at the His location and then in the coronary sinus , and use that as their entire geometry for ablation of AVNRT. While quite minimalistic, it does provide adequate location information, and takes only seconds to generate the image. At the other end of the spectrum, one can import 3D MRI or CT images and “fuse” the images with the 3D mapping system data, thereby creating a minutely detailed geometry. Most EP labs fall somewhere in between the extremes. Our own typical approach is to draw SVC, IVC, right atrium, tricuspid valve, and coronary sinus. That is sufficient for the majority of cases , but more can be added to it if needed.
The geometry and catheters can be viewed from infinite angles and infinite range of magnification. The walls of the geometry can be displayed as transparent, translucent, or opaque. The walls can be “peeled” back to view inside a chamber. Areas of special interest can be marked for later reference. Areas of low voltage, representing scar tissue, can be drawn in. All of the imaging data is visible continuously, in real time, without the need to step on a fluoroscopy pedal. There are some limitations with certain important structures with no visibility on 3D mapping, like implanted pacing or defibrillator leads, and artificial valves. Also, the shaft of the catheter is not visible, only the location of the electrodes. How much geometrical information is acquired is user dependent. The length of time to draw that geometry will depend on experience. To draw our typical amount of geometry mentioned above, it takes an average of 11 min from the time of the first sheath placement until geometry is complete. In our first 30 patients without fluoro, it took an average of 31 min, so we have trimmed off 20 min, but we had over 2 years’ experience with the 3D system before we started doing procedures without fluoro. One should expect that geometry creation will be clumsy at first, but usually within a few months it will be a fairly seamless process.
Once a comfort level has been established with drawing basic geometry and manipulating catheters within it, approximately 70% of SVT cases will be ablatable without fluoroscopy. Atrial flutter, AVNRT, and right-sided accessory pathways can all be addressed with that amount of anatomic detail. Ablating typical atrial flutter involves placing a multipole catheter from the coronary sinus across the tricuspid isthmus, creating a line of lesions through the isthmus, and documenting bidirectional block across the isthmus. All of that is easily done with fluoroscopy. Once one is comfortable with the 3D mapping system, they will be equally comfortable to perform the same tasks without fluoroscopy. A report by Macias et al. compared EnSite and CARTO 3 for this ablation procedure [29]. The group was already accustomed to using EnSite for fluoroless ablation of atrial flutter before they began trialing CARTO 3. In their report, they compared their first 20 EnSite atrial flutter ablations with their most recent 20 EnSite atrial flutter ablations, and then compared their first 20 CARTO 3 atrial flutter ablations. They demonstrated their learning curve with EnSite, noting that the procedure times and fluoro times improved from early cases to late cases. Also, the number of procedures requiring any fluoroscopy improved with time. In their early experience, 85% of cases were completed without fluoro. In their later experience, 95% of cases were fluoroless. Interestingly, their experience from their first 20 cases using CARTO 3 was very similar to their later experience with EnSite. The procedure times and fluoro times were better than their initial experience using EnSite. This suggests that a large part of the learning curve for fluoroless procedures will translate between systems (Table 6.1). Once an EP lab has gained a comfort level of avoiding fluoroscopy with one system, the learning curve for another system will likely be accelerated.
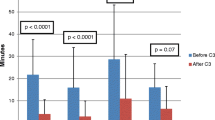
Supraventricular tachycardia due to AVNRT is another rhythm that should be easily ablated without fluoroscopy. Koch’s triangle is readily defined without fluoroscopy. The His electrogram can be recorded and labeled. The coronary sinus can be cannulated, and the tricuspid annulus can be identified. Whether one uses radiofrequency (RF) energy or cryoenergy , the approach is simple. However, the CARTO system still does not allow for simple use of cryoenergy due to proprietary catheter issues. There are ways to work around it [28] and Pass’ group defined a way to “trick” CARTO to allow for cryoablation [38]. But, if your EP lab is one that primarily uses cryoenergy when ablating on the atrial septum, you would be best served by the EnSite system. One of the benefits of either 3D mapping system is that the catheter can be seen continuously. This is of great value when working close to the AV node or His bundle. Solimene et al. reported their fluoroscopy use from a 6-year period. In that timeframe, they performed 433 ablations for AVNRT. They divided the period into three segments of 2 years each. They compared fluoroscopy times for each period. They used CARTO as the primary tool, but also used EnSite at times. Their fluoroscopy time averaged 13 ± 7 min in the early period, and 1 ± 2 min in the late period (P < 0.001). They found similar results for all of the arrhythmias studied (Table 6.2) [33].
Clark and colleagues published results of the use of EnSite and cryoablation for AVNRT ablation in a pediatric lab [20]. There were 27 patients in the early and 35 patients in the late period. There was no fluoro used for either group. Success and complications did not differ between groups. There was a 20% decrease in procedure time between early and late groups (P < 0.01). Both of these studies demonstrate a learning curve with fluoroless procedures. Solimene concludes: “One of the most important factors implied in the fluoroscopy reduction is probably the operator experience . In our series all operators showed a significant reduction in the use of fluoroscopy, regardless their skill level, experience, learning curve, and preference.”
In addition to right-sided tachycardia mechanisms, left-sided accessory pathways with a PFO can be ablated without further geometry creation. However, left-sided pathways without a PFO will require further anatomic detail to address. The options are retrograde arterial, or transseptal puncture. Retrograde arterial is a fairly straightforward process, and is essentially the same as with fluoroscopy. Transseptal puncture , however, will require additional tools to accomplish. This could be either intracardiac echo (ICE) or transesophageal echo (TEE) . Ferguson et al. were the first to report the use of ICE to eliminate fluoroscopy during transseptal puncture [13]. They used rotational ICE through a deflectable sheath to perform double-transseptal puncture. In 19 of 21 patients they were able to complete the ablation without fluoroscopy. There were no procedure-related complications. Reddy et al. reported similar results using nonrotational ICE and single-transseptal puncture [15]. In 20/20 patients, they completed the procedure without fluoroscopy. Figure 6.5 shows the imaging detail related to that procedure. There were no complications. ICE imaging does not require a second operator, and therefore is more time efficient. However, in smaller patients, vascular access can be an issue. For that reason, we have adopted an approach of TEE for transseptal puncture. That process is described in more detail in the pediatric chapter of this textbook, or in the original manuscript [12]. As a means of expediting the procedure, we have added to the TEE the use of the VisionWire guidewire (Biotronik, Inc.). This allows positioning of the transseptal sheath and dilator within the fossa, without the use of TEE.
Once an operator becomes proficient at drawing geometry and performing transseptal puncture without fluoro, the majority of arrhythmias can be treated without radiation exposure. The hurdles remaining beyond that will likely be related to ablation of atrial fibrillation, and ventricular tachycardia . Atrial fibrillation requires transseptal puncture, but also requires more detailed anatomy of the left atrium. It is possible to spend the time drawing the left atrial anatomy with the 3D mapping system, but the systems also allow integration with other imaging modalities. Three-dimensional MRI or CT images can be imported into the system and linked via identifying fiducial points during geometry creation. This allows extremely detailed left atrial geometry without an exhaustive amount of time to create it. CARTOSound can also help with creating extensive anatomy by incorporating ICE images (Fig. 6.6).
Finally, VT ablation is burdened by the fact that long sheaths are often needed for the procedure, but not visible on 3D mapping systems. As discussed in the pediatric chapter of this textbook, electrode distortion can be used as a surrogate to identify the location of the tip of a sheath. This technique can often allow completion of a procedure without fluoroscopy. If all of the abovementioned techniques are employed, fluoroscopy is rarely needed, either in children [45] or adults [46,47,48]. Razminia published his group’s experience of a completely no-fluoro approach to all arrhythmias [49]. In 500 consecutive patients, they performed ablation for 186 atrial fibrillation, 188 atrial flutter, 79 AVNRT, 111 focal atrial tachycardia, 30 ventricular ectopy, and 31 accessory pathways. Despite the variety of arrhythmias presenting for treatment, they used fluoroscopy only once in the 500 patients. This study represents the extreme of what can be accomplished with the current technology. However, the technology is still in its infancy. Many improvements can be made to optimize utilization of the technology. As those technological improvements emanate, results like Razminia’s will become the routine, instead of the extreme.
Committed electrophysiologists and staff are essential to endure the learning curve to minimize radiation exposure and perform consistently fluoroless ablations . As more labs become comfortable with the procedure, more tools will become available to make the process easier. Eventually, fluoroscopy will be phased out. Figure 6.7 shows a graphic trend of the author’s radiation badge readings over a 10-year period surrounding the transition to a fluoroless approach.
There have been tremendous advances in fluoroless ablation over the last 15 years, both in physician skill and technological improvements. As the tools available continue to improve, and physicians continue to gain experience, there will be less need for radiation. By the year 2030, fluoroscopy use for catheter ablation will be mostly of historical interest. By then, the traditional, fluoroscopically based EP lab may also have become obsolete [50].
References
Khongphatthanayothin A, Kosar E, Nademanee K. Nonfluoroscopic three-dimensional mapping for arrhythmia ablation: tool or toy? J Cardiovasc Electrophysiol. 2000;11(3):239–43.
Schauer DA, Linton OW. NCRP report no. 160, ionizing radiation exposure of the population of the United States, medical exposure—are we doing less with more, and is there a role for health physicists? Health Phys. 2009;97(1):1–5.
Limacher MC, Douglas PS, Germano G, et al. ACC expert consensus document. Radiation safety in the practice of cardiology. American College of Cardiology. J Am Coll Cardiol. 1998;31(4):892–913.
Roguin A, Goldstein J, Bar O, Goldstein JA. Brain and neck tumors among physicians performing interventional procedures. Am J Cardiol. 2013;111(9):1368–72.
Goldstein JA, Balter S, Cowley M, Hodgson J, Klein LW. Interventional Committee of the Society of Cardiovascular Interventions. Occupational hazards of interventional cardiologists: prevalence of orthopedic health problems in contemporary practice. Catheter Cardiovasc Interv. 2004;63(4):407–11.
Smilowitz NR, Balter S, Weisz G. Occupational hazards of interventional cardiology. Cardiovasc Revasc Med. 2013;14(4):223–8.
Klein LW, Tra Y, Garratt KN, et al. Occupational health hazards of interventional cardiologists in the current decade: results of the 2014 SCAI membership survey. Catheter Cardiovasc Interv. 2015;86(5):913–24.
Worley SJ. Use of a real-time three-dimensional magnetic navigation system for radiofrequency ablation of accessory pathways. Pacing Clin Electrophysiol. 1998;21(8):1636–45.
Drago F, Silvetti MS, Di Pino A, Grutter G, Bevilacqua M, Leibovich S. Exclusion of fluoroscopy during ablation treatment of right accessory pathway in children. J Cardiovasc Electrophysiol. 2002;13(8):778–82.
Tuzcu V. A nonfluoroscopic approach for electrophysiology and catheter ablation procedures using a three-dimensional navigation system. Pacing Clin Electrophysiol. 2007;30(4):519–25.
Smith G, Clark JM. Elimination of fluoroscopy use in a pediatric electrophysiology laboratory utilizing three-dimensional mapping. Pacing Clin Electrophysiol. 2007;30(4):510–8.
Clark J, Bockoven JR, Lane J, Patel CR, Smith G. Use of three-dimensional catheter guidance and trans-esophageal echocardiography to eliminate fluoroscopy in catheter ablation of left-sided accessory pathways. Pacing Clin Electrophysiol. 2008;31(3):283–9.
Ferguson JD, Helms A, Mangrum JM, et al. Catheter ablation of atrial fibrillation without fluoroscopy using intracardiac echocardiography and electroanatomic mapping. Circ Arrhythm Electrophysiol. 2009;2(6):611–9.
Pachon M, Arias MA, Castellanos E, Puchol A. No fluoroscopy for cavotricuspid isthmus-dependent right atrial flutter ablation. Heart Rhythm. 2009;6(3):433–4.
Reddy VY, Morales G, Ahmed H, et al. Catheter ablation of atrial fibrillation without the use of fluoroscopy. Heart Rhythm. 2010;7(11):1644–53.
Miyamoto K, Tsuchiya T, Narita S, et al. Radiofrequency catheter ablation of ventricular tachyarrhythmia under navigation using EnSite array. Circ J. 2010;74(7):1322–31.
Von Bergen NH, Bansal S, Gingerich J, Law IH. Nonfluoroscopic and radiation-limited ablation of ventricular arrhythmias in children and young adults: a case series. Pediatr Cardiol. 2011;32(6):743–7.
Casella M, Pelargonio G, Dello Russo A, et al. “Near-zero” fluoroscopic exposure in supraventricular arrhythmia ablation using the EnSite NavX mapping system: personal experience and review of the literature. J Interv Card Electrophysiol. 2011;31(2):109–18.
Alvarez M, Tercedor L, Almansa I, et al. Safety and feasibility of catheter ablation for atrioventricular nodal re-entrant tachycardia without fluoroscopic guidance. Heart Rhythm. 2009;6(12):1714–20.
Gist K, Tigges C, Smith G, Clark J. Learning curve for zero-fluoroscopy catheter ablation of AVNRT: early versus late experience. Pacing Clin Electrophysiol. 2011;34(3):264–8.
Giaccardi M, Chiodi L, Del Rosso A, Colella A. ‘Zero’ fluoroscopic exposure for ventricular tachycardia ablation in a patient with situs viscerum inversus totalis. Europace. 2012;14(3):449–50.
Wan G, Shannon KM, Moore JP. Factors associated with fluoroscopy exposure during pediatric catheter ablation utilizing electroanatomical mapping. J Interv Card Electrophysiol. 2012;35(2):235–42.
Tuzcu V. Significant reduction of fluoroscopy in pediatric catheter ablation procedures: long-term experience from a single center. Pacing Clin Electrophysiol. 2012;35(9):1067–73.
Razminia M, Manankil MF, Eryazici PL, et al. Nonfluoroscopic catheter ablation of cardiac arrhythmias in adults: feasibility, safety, and efficacy. J Cardiovasc Electrophysiol. 2012;23(10):1078–86.
Ergul Y, Tola HT, Kiplapinar N, Akdeniz C, Saygi M, Tuzcu V. Cryoablation of anteroseptal accessory pathways in children with limited fluoroscopy exposure. Pediatr Cardiol. 2013;34(4):802–8.
Gellis LA, Ceresnak SR, Gates GJ, Nappo L, Pass RH. Reducing patient radiation dosage during pediatric SVT ablations using an “ALARA” radiation reduction protocol in the modern fluoroscopic era. Pacing Clin Electrophysiol. 2013;36(6):688–94.
Pass RH, Gates GG, Gellis LA, Nappo L, Ceresnak SR. Reducing patient radiation exposure during paediatric SVT ablations: use of CARTO(R) 3 in concert with “ALARA” principles profoundly lowers total dose. Cardiol Young. 2015;25(5):963–8.
Scaglione M, Ebrille E, Caponi D, et al. Single center experience of fluoroless AVNRT ablation guided by electroanatomic reconstruction in children and adolescents. Pacing Clin Electrophysiol. 2013;36(12):1460–7.
Macias R, Uribe I, Tercedor L, Jimenez-Jaimez J, Barrio T, Alvarez M. A zero-fluoroscopy approach to cavotricuspid isthmus catheter ablation: comparative analysis of two electroanatomical mapping systems. Pacing Clin Electrophysiol. 2014;37(8):1029–37.
Christoph M, Wunderlich C, Moebius S, et al. Fluoroscopy integrated 3D mapping significantly reduces radiation exposure during ablation for a wide spectrum of cardiac arrhythmias. Europace. 2015;17(6):928–37.
Scaglione M, Ebrille E, Caponi D, et al. Zero-fluoroscopy ablation of accessory pathways in children and adolescents: CARTO3 electroanatomic mapping combined with RF and cryoenergy. Pacing Clin Electrophysiol. 2015;38(6):675–81.
Bulava A, Hanis J, Eisenberger M. Catheter ablation of atrial fibrillation using zero-fluoroscopy technique: a randomized trial. Pacing Clin Electrophysiol. 2015;38(7):797–806.
Solimene F, Donnici G, Shopova G, et al. Trends in fluoroscopy time during radiofrequency catheter ablation of supraventricular tachycardias. Int J Cardiol. 2016;202:124–5.
Huo Y, Christoph M, Forkmann M, et al. Reduction of radiation exposure during atrial fibrillation ablation using a novel fluoroscopy image integrated 3-dimensional electroanatomic mapping system: a prospective, randomized, single-blind, and controlled study. Heart Rhythm. 2015;12(9):1945–55.
Montgomery JAEC. Zero-fluoroscopy intracardiac echocardiography-guided ablation of atrial fibrillation using a single-catheter technique. J Innov Card Rhythm Manag. 2015;6:2209–15.
Ceresnak SR, Nappo L, Janson CM, Pass RH. Tricking CARTO: cryoablation of supraventricular tachycardia in children with minimal radiation exposure using the CARTO3 system. Pacing Clin Electrophysiol. 2016;39(1):36–41.
Raju H, Whitaker J, Taylor C, Wright M. Electroanatomic mapping and transoesophageal echocardiography for near zero fluoroscopy during complex left atrial ablation. Heart Lung Circ. 2016;25(7):652–60.
Clark BC, Sumihara K, McCarter R, Berul CI, Moak JP. Getting to zero: impact of electroanatomical mapping on fluoroscopy use in pediatric catheter ablation. J Interv Card Electrophysiol. 2016;46(2):183–9.
Kuhne M, Knecht S, Muhl A, et al. Fluoroscopy-free pulmonary vein isolation in patients with atrial fibrillation and a patent foramen ovale using solely an electroanatomic mapping system. PLoS One. 2016;11(1):e0148059.
Nagaraju L, Menon D, Aziz PF. Use of 3D electroanatomical navigation (CARTO-3) to minimize or eliminate fluoroscopy use in the ablation of pediatric supraventricular tachyarrhythmias. Pacing Clin Electrophysiol. 2016;39(6):574–80.
Cano O, Andres A, Osca J, et al. Safety and feasibility of a minimally fluoroscopic approach for ventricular tachycardia ablation in patients with structural heart disease: Influence of the ventricular tachycardia substrate. Circ Arrhythm Electrophysiol. 2016;9(2):e003706.
Lerman BB, Markowitz SM, Liu CF, Thomas G, Ip JE, Cheung JW. Fluoroless catheter ablation of atrial fibrillation. Heart Rhythm. 2017;14(6):928–34.
McCauley MD, Patel N, Greenberg SJ, Molina-Razavi JE, Safavi-Naeini P, Razavi M. Fluoroscopy-free atrial transseptal puncture. Eur J Arrhthymia Electrophysiol. 2016;2(2):57–61.
Clark BC, Sumihara K, Berul CI, Moak JP. Off the pedal: fluoroless transseptal puncture in pediatric supraventricular tachycardia ablation. Pacing Clin Electrophysiol. 2017;40(11):1254–9.
Jan M, Zizek D, Rupar K, et al. Fluoroless catheter ablation of various right and left sided supra-ventricular tachycardias in children and adolescents. Int J Cardiovasc Imaging. 2016;32(11):1609–16.
Fernandez-Gomez JM, Morina-Vazquez P, Morales Edel R, Venegas-Gamero J, Barba-Pichardo R, Carranza MH. Exclusion of fluoroscopy use in catheter ablation procedures: six years of experience at a single center. J Cardiovasc Electrophysiol. 2014;25(6):638–44.
Seizer P, Bucher V, Frische C, et al. Efficacy and safety of zero-fluoroscopy ablation for supraventricular tachycardias. use of optional contact force measurement for zero-fluoroscopy ablation in a clinical routine setting. Herz. 2016;41(3):241–5.
Sanchez JM, Yanics MA, Wilson P, Doshi A, Kurian T, Pieper S. Fluoroless catheter ablation in adults: a single center experience. J Interv Card Electrophysiol. 2016;45(2):199–207.
Razminia M, Willoughby MC, Demo H, et al. Fluoroless catheter ablation of cardiac arrhythmias: a 5-year experience. Pacing Clin Electrophysiol. 2017;40(4):425–33.
Bigelow AM, Smith PC, Timberlake DT, et al. Procedural outcomes of fluoroless catheter ablation outside the traditional catheterization lab. Europace. 2017;19(8):1378–84.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Bigelow, A.M., Clark, J.M. (2019). Learning Curve of Zero Fluoroscopy. In: Proietti, R., Wang, Y., Yao, Y., Zhong, G., Lin Wu, S., Ayala-Paredes, F. (eds) Cardiac Electrophysiology Without Fluoroscopy. Springer, Cham. https://doi.org/10.1007/978-3-030-16992-3_6
Download citation
DOI: https://doi.org/10.1007/978-3-030-16992-3_6
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-16991-6
Online ISBN: 978-3-030-16992-3
eBook Packages: MedicineMedicine (R0)