Abstract
Background
Ablation procedures for arrhythmias have increased in frequency and complexity over the last decade. Improvements in technology have allowed for less reliance on fluoroscopy to guide these procedures. Ablation without fluoroscopy has been reported in small cohorts. We report a single center experience of fluoroless ablation after adoption of this technique for all endovascular ablations.
Methods
This retrospective study evaluated 107 consecutive patients who underwent a catheter ablation procedure for an atrial or ventricular arrhythmias after adoption of a completely fluoroless technique. No fluoroscopy was used in any case. A mapping system was utilized in all cases. Intracardiac echocardiography (ICE) catheters were utilized in 75 of the ablation cases (70.4 %). Of the 107 patients who underwent EP study, three patients did not undergo ablation as they were non-inducible for SVT. Of the remaining 104 patients, 56 patients (53.8 %) underwent ablation for atrial fibrillation, 23 patients (22.1 %) for SVT, 10 patients (9.6 %) for lone atrial flutter, and 16 patients (15.4 %) for a ventricular arrhythmia including PVC, idiopathic VT or ventricular tachycardia.
Results
Catheters were able to be placed in 100 % of patients without complication. Time to placement in the coronary sinus was 2.1 min ± 1.4 min. Mean transseptal time was 3.54 min ± 3 min. Mean procedure time for all ablations was 2 h 6 min ± 50 min. There were no complications in the series of patients.
Conclusions
Fluoroless ablation is feasible and safe with acceptable procedure times. Adoption of this technique is encouraged in order to eliminate unnecessary risk of fluoroscopy.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Electrophysiology catheter ablation procedures have increased over the last decade [1]. Cases have become more complex over time. Multiple modalities including three dimensional (3D) electroanatomical mapping and intracardiac echocardiography (ICE) have been increasingly utilized with improved safety and efficacy. These complimentary technological developments have also allowed for reduced fluoroscopy times. This practice is in keeping with utilizing a radiation dose that is “as low as reasonably achievable,” or ALARA [2]. Physicians working in interventional x-ray laboratories including cardiologists have embraced this concept [3, 4]. Fluoroscopy in ablation procedures continues to be utilized with risks to both patients and operators [5–8]. Catheter ablation without the use of fluoroscopy and guided by 3D mapping systems and ICE has been reported in patients with supraventricular tachycardia (SVT) [9–11] and paroxysmal atrial fibrillation (PAF) [12, 13]. 3D transesophageal echocardiography (TEE) guidance without fluoroscopy has also been reported in ablation procedures [14]. Elimination of fluoroscopy allows for reduction in the total cumulative radiation dose patients receive. The effects of ionizing radiation additionally are a concern for operators who may be at increased risk for head and neck malignancies [15]. Orthopedic concerns for physician and staff continue to be an occupational hazard [16, 17].
We present a large series of consecutive cases performed in a single center after adoption of a completely fluoroless approach to catheter ablation of atrial and ventricular arrhythmias in community setting.
2 Methods
This retrospective study evaluated 107 consecutive patients who underwent a catheter ablation procedure for an atrial or ventricular arrhythmias after adoption of a completely fluoroless technique. IRB approval was obtained for this study. Procedures were performed with conscious sedation for SVT, premature ventricular contraction (PVC) ablation, ventricular tachycardia (VT) ablation, and atrial flutter (AFL) ablation. General anesthesia was utilized with ablation of PAF and persistent atrial fibrillation (PeAF). A single operator (JMS) performed all procedures to avoid inter-operator variability. These procedures were performed in a standard EP lab. No additional pre-procedure radiologic imaging was performed. No computed tomography (CT) or cardiac magnetic resonance imaging (MRI) registration for merger of geometry to a 3D mapping system was performed in any of the cases. No fluoroscopy was utilized in any part of these procedures. Patients excluded from the analysis included those undergoing AV node ablation, those enrolled in an ablation-specific randomized control trial, and those who underwent epicardial ablation requiring epicardial access and therefore requiring fluoroscopy. Access was obtained with modified Seldinger technique with a number of access sites depending on the case type performed.
3 Catheter placement
3.1 SVT ablation
SVT ablations utilized an 8-French sheath, 7-French sheath, and two 5-French sheaths for venous access. A decapolar catheter (Inquiry, St. Jude Medical) was advanced from the right femoral vein under 3D mapping guidance as previously described by Reddy et al. Slow careful advancement was performed while visualizing on the 3D mapping system (NavX, St. Jude Medical.) The 3D mapping system utilized three pairs of skin electrode patches for reference during catheter placement. After catheter placement, operator could choose either a catheter or the skin electrode patches for reference on the 3D mapping system. Any tactile resistance to advancement or deformation on the 3D mapping system resulted in re-evaluation of position. The decapolar catheter was advanced into the right atrium (RA) until atrial signals were visualized from the catheter on the recording system. Once in the RA, the decapolar catheter was deflected while visualizing from a right anterior oblique (RAO) and left anterior oblique (LAO) view on the 3D mapping system. Clockwise torque was applied while visualizing movement in orthogonal views on the 3D mapping system. The decapolar catheter was placed into the coronary sinus (CS) in this manner. Both electrograms and position relative to the chest wall orientation from mapping system confirmed position. A 5-French quadrapolar catheter (CRD™, St. Jude Medical Inc) was advanced in a similar fashion from the left femoral vein and placed at the AV junction until atrial, His, and ventricular signals were noted on the recording system. A 4-French quadrapolar catheter (DAO™, St. Jude Medical Inc) was advanced from the left femoral vein in the same fashion and placed first into the RA. Once in the RA, the catheter was manipulated in RAO and LAO views of the 3D mapping system into the right ventricular (RV) apex with electrocardiographic confirmation (See Fig. 1). If an accessory pathway was diagnosed requiring transseptal puncture for mapping and ablation, then a 9-French phased array ICE catheter (ViewFlex™ Zonare, St. Jude Medical Inc) was utilized through a new access site. ICE navigation was based on ultrasound imaging with careful advancement from the left femoral vein into the inferior vena cava (IVC) and RA. Transseptal puncture with ICE alone is described below when access to the left atrium (LA) was necessary.
3.2 Atrial fibrillation ablation
Both PAF and PeAF ablations were performed. Access was obtained as above with two 8-French sheaths and a 7 French in the right femoral vein. A 7-French sheath and a 9-French sheath were placed in the left femoral vein. A decapolar catheter was placed in the CS, and a His catheter was placed at the AV junction in similar fashion to as described for SVT ablation. The phased array ICE catheter was placed into the RA through the left 9-French venous access sheath, following the other catheters that were visualized during advancement on ICE. The ICE catheter was then placed into the SVC near the RA-SVC junction. Placement of 0.032” exchange wires into the superior vena cava (SVC) was then performed with direct visualization of the wires into the SVC by phased array ICE (See Fig. 2). The two short 8-French sheaths were exchanged for 8-French Preface long sheaths over 0.032” guide wires. Careful attention was given to ensure that no excess wire buckled into the RA or RV as visualized on ICE. Long sheaths were advanced slowly with tip point toward the intra-atrial septum. Advancement was performed with long sheaths being advanced into SVC under direct visualization on ultrasound. Sheaths were then flushed in the usual fashion. Double transseptal puncture was performed as described below. A 20-pole variable radius catheter (Lasso, Biosense Webster) was utilized to create the geometry of the LA (See Fig. 3). After 3D mapping geometry construction, all AF patients underwent pulmonary vein isolation with confirmation of entrance and exit block in all four pulmonary veins. In PAF patients, if the patients were inducible into atypical atrial flutter with burst atrial pacing, then additional stepwise linear ablation was performed with confirmation of bidirectional block by differential pacing. In PeAF patients after PVI, stepwise linear ablation was performed only if the patient did not convert to normal sinus rhythm with PVI alone or if the patient was inducible into an atypical atrial flutter. cavotricuspid (CTI) ablation was performed in all AF patients. CTI ablation was performed under ICE visualization with ablation from the tricuspid valve until the IVC (See Fig. 4). Roof ablation and mitral valve isthmus (MVI) ablation were only performed in those patients with inducible atypical atrial flutter and those patients persistently in AF after PVI. Differential pacing was performed to record delay across CTI line, roof line, and MVI line.
3.3 Transseptal puncture
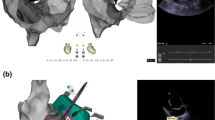
Transseptal punctures were performed with single transseptal in SVT cases when necessary and with double transseptal technique for all PAF and PeAF procedures. A Brockenbrough transseptal needle (BRK-1) was advanced into the long sheath. The long sheath was then brought down from the SVC under ICE visualization until the sheath and needle were placed onto the intra-atrial septum (See Fig. 5). Transseptal puncture was performed after heparinization under ICE visualization with saline confirming puncture into the LA. Then, a 0.032” long wire was then placed out into the left superior pulmonary vein confirmed on ICE. The sheath was then advanced over the wire into the left atrium visualized under ICE directly. Sheaths were appropriately flushed.
3.4 3D electroanatomic mapping
Geometry construction was performed in the usual fashion depending on the type of procedure. SVT ablation did not necessarily require 3D geometry creation and could be performed utilizing 3D mapping system only to visualize catheter location and record lesion location. Ablation of AF, whether PAF or PeAF, as well as PVC/VT ablation required 3D mapping to generate geometry for navigation. In AF ablation, a Lasso was utilized to collect electroanatomical points and construct LA geometry. The Lasso catheter was placed in all four pulmonary veins and confirmed by ICE. Placement in the left atrial appendage was also carefully performed in geometry creation. ICE was utilized to assure the entire LA was included in the created geometry and to maximize contact throughout the LA. A deflectable quadrapolar catheter (St. Jude Medical Inc) was placed into the esophagus in all AF cases. A geometry was created for the esophagus in order to better understand the relationship of the esophagus to the LA. Once esophageal geometry was constructed, the catheter was removed. ICE was utilized to then maneuver the esophageal temperature probe into position. CTI ablation was performed without geometry formation of the RA. Utilization of 3D mapping catheter position in orthogonal views as well as visualization on ICE was sufficient for CTI ablation.
3.5 Ventricular ablation
Outflow tract PVC procedures utilized a similar venous access set as SVT with an ICE catheter to visualize the outflow tract (See Fig. 6). Arterial access was obtained in the right femoral artery with an 8-French sheath when LVOT mapping was necessary. ICE catheter visualization and 3D mapping were utilized to carefully direct the ablation catheter retrograde to the aortic cusps. Ablation within the aortic cusps was performed only when the coronary artery takeoff could be visualized on ICE with doppler flow. VT ablation was performed either with retrograde or transseptal approach in the LV. The ICE catheter was advanced into the RV to visualize left ventricle if ablating within that chamber.
3.6 Statistical analysis
Categorical variables were expressed as a percentage of the total patient population. Continuous variables were expressed as mean values ± standard deviations. Endpoints included time to CS cannulation, time to transseptal puncture, and total case time. Total case time was later stratified by case type. Additionally, acute success in AF ablation was quantified with number of veins demonstrating entrance and exit blocks. Delays across linear ablation lines were also recorded. Average delays were reported to illustrate bidirectional block as measure of success in these procedures. Complications were evaluated within 48 h of the procedure including pericardial effusion, tamponade requiring intervention, groin hematoma requiring intervention, esophageal injury, phrenic nerve injury, CVA, myocardial infarction, stroke, or death.
4 Results
A total of 107 consecutive cases were included in the series from April 2014 to November 2014. Fluoroscopy was not used in any of these cases after adoption of a completely fluoroless ablation technique. The mean age of patients was 58.7 years ± 13.4. There was a majority of male patients with 64 (59.8 %). The majority of patients were overweight or obese with a mean BMI of 30.2 kg/m2 ± 6. Baseline characteristics of the series can be seen in Table 1. All patients underwent venous access and catheter placement. Catheters were able to be placed in 100 % of patients without complication. The CS catheter was successfully placed in 100 % of patients. Assessment of time to cannulation of the CS was estimated from time of insertion of the decapolar CS catheter until confirmation on the 3D mapping system in orthogonal views as well as with electrograms. Time to placement in the CS was 2.1 min ± 1.4 min. An EP study was performed in all patients. No fluoroscopy was utilized. Cardiac implantable electronic devices were present in 11 of patients. Thresholds were checked pre- and post-procedure in these patients. Capture thresholds remained the same in all patients. Cardiac resynchronization therapy (CRT) was present in five patients with LV leads placed within the CS. Thresholds and QRS configuration were tested post-procedure in all cases with no changes to CRT pacing.
Of the 107 patients who underwent EP study, three patients did not undergo ablation as they were non-inducible for SVT. Of the remaining 104 patients, 56 patients (53.8 %) underwent ablation for atrial fibrillation, 23 patients (22.1 %) for SVT, 10 patients (9.6 %) for lone atrial flutter, and 16 patients (15.4 %) for a ventricular arrhythmia including PVC, idiopathic VT or ventricular tachycardia. Note that one patient who underwent PVI also underwent ablation for atypical AVNRT at the same time with slow pathway elimination.
Transseptal puncture was performed in 63 cases (60.6 %) of the 104 requiring ablation. AF ablation accounted for 56 of the 63 cases (88.9 %) requiring transseptal puncture. Double transseptal puncture was performed in all AF procedures. Of the seven remaining cases, three were for SVT (4.8 %) and four for VT (6.3 %). Utilizing passed array ICE, there was 100 % success in performing transseptal puncture. Time from insertion of transseptal needle to successful crossing of the long sheath into the mean transseptal time was 3.54 min ± 3 min. There were no complications including pericardial effusion, tamponade, or air embolism.
ICE catheters were utilized in 75 out of 104 of the ablation cases (72.11 %). ICE use was common in all 56 AF cases (100 %), three out of 31 cases (9.7 %) of SVT , three out of 10 cases (30 %) of lone atrial flutter, as well as utilization of ICE for visualization in 12 out of 16 (75 %) ventricular arrhythmia procedures including PVC ablation and ablation of ventricular tachycardia. ICE was utilized for greater appreciation of positioning as well as contact during ablation.
Standard endpoints of acute success were achieved for all ablation cases. With specific regard to AF ablation, PVI was performed in 56 cases with successful demonstration of entrance and exit block occurring in 224 of 225 pulmonary veins (99.6 %). The single vein that could not be isolated was due to temperature rise in the esophagus during application to the posterior wall. Linear ablation was performed as well in certain cases. CTI was performed in all PVI patients with an average delay of 161 ms ± 37.9 ms when pacing from one side of the CTI to the other. MVI ablation was necessary in 34 of 56 patients (60.7 %). The average delay across the MVI when pacing from the LAA via Lasso catheter to the distal coronary sinus was 161 ms ± 38.3 ms. Roof ablation was necessary in 40 of 56 patients (71.4 %). The average delay when pacing from the LAA via Lasso catheter to the posterior superior wall was 158 ms ± 38.3 ms.
Total procedure time from transvenous access attempt to conclusion of the case was recorded. Mean procedure time for all ablations was 2 h 6 min ± 50 min. For individual mean procedure times, see Table 2. Complications including pericardial effusion, tamponade requiring intervention, groin hematoma, CVA, myocardial infarction, stroke, or death within 48 h of the procedure were recorded. There were no complications in the series of patients.
5 Discussion
Fluoroless ablation has been previously demonstrated to be feasible and safe in select small case series. We report a series of 107 patients who underwent real-world EP study with the majority undergoing ablation after adoption of a completely fluoroless technique utilizing 3D mapping and frequently phased array ICE for guidance and visualization. This series demonstrates that adoption of a completely fluoroless technique in the community setting results in an ALARA dose without compromise of safety, efficacy, or duration of procedures. It also demonstrates the applicability of these techniques in a wide range of differing types of procedures including SVT ablation, PVI, linear ablation, and ablation of ventricular arrhythmias, all without the use of any fluoroscopy or additional merge technologies.
There were no complications in a series of over a hundred patients. Cannulation time for CS is within real-world experience for an operator utilizing fluoroscopy. Total procedure times are also within the times that one would expect with the utilization of fluoroscopy. This series demonstrates that transseptal puncture can be reproducibly performed without increase risk of perforation or tamponade entirely without fluoroscopy. Avoidance of fluoroscopy may lead to increased utilization of ICE which can enhance direct visualization of the catheter against the tissue giving a better sense of contact as well as live assessment of location independent of mapping system.
While patients experience no increase in procedure times or change in acute efficacy, there is a dramatic reduction in radiation exposure to the patient, the physician, and the staff. This may be of consequence with regard to risk of radiation burns, total lifetime radiation dose for patients, lifetime dose of radiation for operators, and increase risk of malignancy. This is especially true among patients undergoing complex catheter ablation for AF or VT. The lifetime risk of excess fatal malignancies normalized to 60 min of fluoroscopy has been reported as 0.07 % for women and 0.1 % for men [8]. Given the increase in the number and complexity of EP procedures, elimination of fluoroscopy certainly would decrease this risk greatly. Obesity is known to double the effective rate of radiation in AF patients [18]. Fluoroless ablation as an option for operators becomes prescient with the epidemic of obesity. Note that in this series the mean patient was obese with mean BMI >30 kg/m2.
Fluoroscopy requires protective apparel that predisposes to orthopedic injury [19]. Adoption of a completely fluoroless technique negates the need for such radiation protective apparel. This decreases the risk of occupational hazard in the form of orthopedic and work-related injuries. No protective lead apparel was necessary during these fluoroless procedures.
5.1 Previous studies
Several studies have been performed utilizing a fluoroless technique. Drago et al. [9] documented the use of fluoroless ablation in nine pediatric patients with right-sided accessory pathways with the Carto (Biosense Webster) system. Grubb et al. [10] was able to completely eliminate fluoroscopy for 90 % of the 76 pediatric right-sided SVT ablations with NavX sytem. Clark et al. [20, 21] demonstrated that use of NavX and TEE could be utilized to perform fluoroless ablation in children for both right and left sided ablations. Ferguson et al. [12] demonstrated the ability to ablate AF without fluoroscopy in 19 of 21 adult patients with utilization of rotational ICE. 3D MRI LA image registration was utilized for geometry creation in this series. There were no complications. Reddy et al. [12] described a series of 20 patients who underwent PVI for PAF without fluoroscopy. CT image integration was utilized in 11 of the 20 patients. NavX and ICE guidance was utilized in all patients. Razminia et al. [22] compared fluoroless (n = 60) to standard ablation (n = 60) with no significant differences between groups for a variety of different ablation procedures.
5.2 Limitations
This study is a retrospective series of clinical experience after adoption of a completely fluoroless ablation technique. It is not a prospective randomized trial and as such is subject to inherent limitations. Further, a single operator (JMS) performed all the procedures in the series. There was no formal evaluation or study of the learning curve for this technique. Initial attempts to place catheters without the aid of fluoroscopy were attempted prior to complete adoption of a fluoroless technique. Nonetheless, formal programs could be developed to instruct new operators on development of these skills allowing operators to learn from experience of those who have developed these techniques. While widespread applicability can be questioned, the previous studies of fluoroless ablation discussed above as well as non-published anecdotal adoption give increased credibility to this as a widely applicable technique without addition of any new and costly additional technology.
6 Conclusions
Fluoroless ablation utilizing 3D mapping and ICE should be pursued by operators to eliminate unnecessary radiation exposure to patients, operators, and staff. Proper utilization of this technique can result in safe and efficacious procedures with no increase in procedure time. Fluoroscopy use in electrophysiology procedures should be limited only those procedures with absolute requirement.
References
Kneeland, P., & Fang, M. (2009). Trends in catheter ablation for atrial fibrillation in the United States. Journal of Hospital Medicine, 4(7), E1–E5.
Title 10, Section 20.1003, of the Code of Federal Regulations(10 CFR 20.1003).
Klein, L. W., Miller, D. L., Balter, S., et al. (2009). Occupational hazards in the interventional laboratory: time for a safer environment. Radiology, 250, 538–544.
Limacher, M. C., Douglas, P. S., Germano, G., Laskey, W. K., Lindsay, B. D., McKetty, M. H., Moore, M. E., Park, J. K., Prigent, F. M., & Walsh, M. N. (1998). ACCexpert consensus document: radiation safety in the practice of cardiology: American College of Cardiology. Journal of the American College of Cardiology, 31, 892–913.
Damilakis, J., Theocharopoulos, N., Perisinakis, K., Manios, E., Dimitriou, P., Vardas, P., & Gourtsoyiannis, N. (2001). Conceptus radiation dose and risk from cardiac catheter ablation. Circulation, 104, 893–897.
Kovoor, P., Ricciardello, M., Collins, L., Uther, J., & Ross, D. (1998). Risk to patients from radiation associated with radiofrequency ablation for supraventricular tachycardia. Circulation, 98, 1534–1540.
Roguin A. Radiation hazards to interventional cardiologists: A report on increased brain tumors among physicians working in the cath lab. SOLACI 2014; April 23, 2014; Buenos Aires, Argentina.
Lickfett, L., Mahesh, M., Vasamreddy, C., Bradley, D., Jayam, V., Eldadah, Z., Dickfeld, T., Kearney, D., Dalal, D., Luderitz, B., Berger, R., & Calkins, H. (2004). Radiation exposure during catheter ablation of atrial fibrillation. Circulation, 110, 3003–3010.
Drago, F., Silvetti, M. S., Di Pino, A., Grutter, G., Belivacqua, M., & Leibovich, S. (2002). Exclusion of fluoroscopy during ablation treatment of right sided accessory pathways in children. Journal of Cardiovascular Electrophysiology, 13, 778–782.
Grubb, N., Petzer, E., Lang, C., Colthart, A., & Elhag, O. (2006). A zero fluoroscopy approach for electrophysiologic studies and catheter ablation for common supraventricular tachycardias. Heart Rhythm, 3, S123.
Ebrille, E., Caponi, D., Siboldi, A., Di Donna, P., Di Clemente, F., Gabbarini, F., Bertero, G., Marasini, M., Gaita, F., & Scaglione, M. (2013). Single center experience of fluoroless AVNRT ablation guided by electroanatomic reconstruction in children and adolescents. Pacing and Clinical Electrophysiology, 36(12), 1460–1467.
Ferguson, J. D., Helms, A., Mangrum, J. M., Mahapatra, S., Mason, P., Bilchick, K., McDaniel, G., Wiggins, D., & DiMarco, J. P. (2009). Catheter ablation of atrial fibrillation without fluoroscopy using intracardiac echocardiography and electroanatomic mapping. Circulation. Arrhythmia and Electrophysiology, 2, 611–619.
Reddy, V. Y., Morales, G., Ahmed, H., Neuzil, P., Dukkipati, S., Kim, S., Clemens, J., & D’Avila, A. (2010). Catheter ablation of atrial fibrillation without the use of fluoroscopy. Heart Rhythm, 7, 1644–1653.
Faletra, F., Regoli, F., Nucifora, G., & Auriccio. (2011). Real-time, fluoroless, anatomic-guided catheter navigation by 3D TEE during ablation procedures. Journal of the American College of Cardiology: Cardiovascular Imaging, 4(2), 203–206.
Finkelstein, M. M. (1998). Is brain cancer an occupational disease of cardiologists? The Canadian Journal of Cardiology, 14, 1385–1388.
Vaño’, E., González, L., Beneytez, F., & Moreno, F. (1998). Lens injuries induced by occupational exposure in non-optimized interventional radiology laboratories. The British Journal of Radiology, 71, 728–733.
Moore, B., van Sonnenberg, E., Casola, G., & Novelline, R. A. (1992). The relationship between back pain and lead apron use in radiologists. AJR. American Journal of Roentgenology, 158, 191–193.
Ector, J., Dragusin, O., Adriaenssens, B., Huybrechts, W., Willems, R., Ector, H., & Heidbüchel, H. (2007). Obesity Is a major determinant of radiation dose in patients undergoing pulmonary vein isolation for atrial fibrillation. Journal of the American College of Cardiology, 50(3), 234–242.
Klein, L. W., Miller, D. L., Balter, S., Laskey, W., Haines, D., Norbash, A., Mauro, M. A., & Goldstein, J. A. (2009). Occupational health hazards in the interventional laboratory: time for a safer environment. Radiology, 250, 538–544.
Clark, J., Bockoven, J. R., Lane, J., Patel, C. R., & Smith, G. (2008). Use of three-dimensional catheter guidance and trans-esophageal echocardiography to eliminate fluoroscopy in catheter ablation of left-sided accessory pathways. Pacing and Clinical Electrophysiology, 31, 283–289.
Smith, G., & Clark, J. M. (2007). Elimination of fluoroscopy use in a pediatric electrophysiology laboratory utilizing three-dimensional mapping. Pacing and Clinical Electrophysiology, 30, 510–518.
Razminia, M., Manankil, M. F., Eryazici, P. L., Arrieta-Garcia, C., Wang, T., D’Silva, O. J., Lopez, C. S., Crystal, G. J., Khan, S., Stancu, M. M., Turner, M., Anthony, J., Zheutlin, T. A., & Kehoe, R. F. (2012). Nonfluoroscopic catheter ablation of cardiac arrhythmias in adults: feasibility, safety, and efficacy. Journal of Cardiovascular Electrophysiology, 23(10), 1078–1086.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Sánchez, J.M., Yanics, M.A., Wilson, P. et al. Fluoroless catheter ablation in adults: a single center experience. J Interv Card Electrophysiol 45, 199–207 (2016). https://doi.org/10.1007/s10840-015-0088-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10840-015-0088-z