Abstract
Background
Conventional catheter ablation of cardiac arrhythmias is associated with radiation risks for patients and laboratory personnel. Widespread use of zero-fluoroscopic catheter ablation in clinical routine is limited by safety concerns. This study investigated the feasibility of zero-fluoroscopy catheter ablation using a three-dimensional mapping system and optional catheter contact force technology for an all-comers collective.
Patients and methods
The study comprised 184 patients; 91 patients, including 29 pediatric patients, underwent a zero-fluoroscopic electrophysiology (EP) study using the EnSite NavX system with real-time visualization of all electrodes. These patients were matched to a control group, which was treated using fluoroscopy in the same period. Inclusion criteria were documented supraventricular tachycardia or a history of symptomatic paroxysmal supraventricular tachycardia. Transseptal access, if necessary, was achieved under transesophageal echocardiographic guidance for ablation of left-sided arrhythmias. Radiofrequency (using optional contact force measurement) or a cryotechnique was used for ablation.
Results
We observed no major acute complications. There were no significant differences between the two groups in the follow-up period.
Conclusion
Zero-fluoroscopic catheter ablation is generally feasible in right-sided cardiac arrhythmias. Safety concerns regarding left atrial substrates or children can be overcome with optional real-time contact force measurement.
Zusammenfassung
Hintergrund
Die konventionelle Katheterablation von Herzrhythmusstörungen impliziert sowohl für den Patienten als auch für das behandelnde Katheterpersonal ein relevantes Strahlenschadensrisiko. Der routinemäßige, breite Einsatz einer strahlenfreien Katheterablation ist allerdings wegen Sicherheitsbedenken limitiert. Ziel dieser Studie ist es, die Durchführbarkeit einer strahlenfreien Katheterablation mittels 3-D-Mapping-System und optionaler Anpresskraftkontrolle in einem breiten Patientenkollektiv zu untersuchen.
Patienten und Methoden
184 Patienten wurden in die Studie eingeschlossen. Bei 91 Patienten, inklusive 29 pädiatrischen Patienten, wurde eine strahlenfreie EPU mittels Ensite-NavX-System durchgeführt. Diese Patienten wurden mit einer Kontrollgruppe verglichen, die mittels Durchleuchtung untersucht wurde. Einschlusskriterien waren eine dokumentierte oder anamnestische paroxysmale supraventrikuläre Tachykardie. Ein transseptaler Zugang für linksatriale Ablationen wurde unter TEE-Führung erzielt. Zur Ablationstherapie wurde entweder Radiofrequenz (mit optionaler Anpresskraftkontrolle) oder Kryotechnik eingesetzt.
Ergebnisse
Es trat keine relevante Akutkomplikation auf. Insgesamt wurde kein signifikanter Unterschied in den beiden Gruppen festgestellt.
Schlussfolgerung
Die komplett strahlenfreie Katheterablation ist generell bei rechtsatrialen Rhythmusstörungen möglich. Die Sicherheit kann bei linksatrialen Eingriffen und bei Kindern durch den Einsatz der Anpresskraftkontrolle erhöht werden.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Conventional catheter ablation of cardiac arrhythmias is associated with radiation risks for patients and laboratory personnel [1–4]. Widespread use of zero-fluoroscopic catheter ablation is limited by safety concerns and economic aspects. However, several studies report successful zero-fluoroscopy procedures especially in right-sided arrhythmias without transseptal puncture [5–8] or with short use of fluoroscopy in left-sided procedures [9]. In addition, there are small-scale studies reporting that zero-fluoroscopy transseptal puncture can be performed under transesophageal echocardiography (TEE) or intracardiac echocardiography (ICE) guidance [10–12]. Since contact force is a recent development [13], there are no larger trials with a zero-fluoroscopy approach using optional contact force technology. Recently, we reported that contact force-controlled zero-fluoroscopy catheter ablation is generally feasible in right-sided and left atrial cardiac arrhythmias in a small collective [14].
To our knowledge, the feasibility of a generally zero-fluoroscopy approach using optional contact force measurement for patients presenting with a history of documented paroxysmal supraventricular tachycardia in a broad all-comers collective has not been reported.
Thus, the aim of the current study was to investigate the feasibility of zero-fluoroscopic catheter ablation using a three-dimensional (3D) mapping system and optional catheter contact force technology in a clinical routine setting.
Patients and methods
Recruitment and follow-up
This retrospective study was approved by the local ethics committee of the University Hospital of Tübingen (project number 230/2014R). The study included 91 patients, who were treated using a zero-fluoroscopy approach from June 2010 to March 2014. These patients were matched with a control group. Matching criteria were location of arrhythmias (left and right sided) and type of arrhythmias. Patient inclusion criteria were documented supraventricular tachycardia or a history of paroxysmal supraventricular tachycardia. Patients with known congenital heart defects were excluded. In addition, patients were included consecutively for zero-fluoroscopy analysis if a second operator with transesophageal standby and a contact force catheter was available. Antiarrhythmic medication was discontinued before catheter ablation. Local anesthesia or intravenous sedation and analgesia were applied. Follow-up of symptomatic arrhythmias was done by phone interview or Holter monitoring. In all patients with manifest Wolff–Parkinson–White (WPW) syndrome, absence of preexcitation was documented by 12-lead surface electrocardiography (ECG) during follow-up.
Electrophysiological study
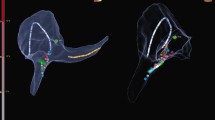
The NavX-Ensite classic and velocity system (St. Jude, Minn.) was used for electroanatomical mapping. Catheters were selected according to the choice of the operator. For cryoablation, a Freezor Xtra (Medtronic, Minn.) was used and for conventional radiofrequency (RF) ablation a solid-tip Marinr (Medtronic). For ablation of the cavotricuspid isthmus, an irrigated-tip catheter was used (Coolflex; St. Jude). If required by the operator owing to safety reasons (extended mapping, left atrial procedures, unclear substrate, difficult anatomy), a catheter with contact force measurement could be used (Tacticath; St. Jude). A steerable decapolar diagnostic catheter (Inquiry; St. Jude Medical) was introduced via the femoral vein and placed in the coronary sinus and a quadripolar woven diagnostic catheter (Bard Electrophysiology; Boston Scientific, Mass.) was positioned in the right ventricle. When needed, left atrial access was attempted via a patent foramen ovale or by transseptal puncture under TEE guidance using a transseptal sheath (Preface; Biosense-Webster Inc., Diamond Bar, Calif.), a Brockenbrough transseptal needle and an atraumatic transseptal guidewire (SafeSept; Pressure Products, San Pedro, Calif.) as described recently [14].
Statistical analysis
All statistical analysis was performed using SPSS version 21.0 (SPSS Inc., Chicago, Ill.). Cumulative event-free survival from recurrences of arrhythmias is presented by Kaplan–Meier curves. The log-rank test was applied to compare recurrence-free survival between patients treated with the use of fluoroscopy vs. zero-fluoroscopy. For comparison of procedure time, Student’s t test was used. Categorical variables were compared using the chi-square test. A p value of < 0.05 was considered statistically significant (two-sided).
Results
In all, 184 patients were analyzed in this study. A zero-fluoroscopy EP study was performed on 91 patients, including 29 pediatric patients and 11 young adults (Table 1). These patients were matched, according to the diagnosed arrhythmia, to 94 patients, who underwent an EP study using fluoroscopy (Table 1). In the nonfluoroscopy group there were more pediatric patients and a higher use of a cryotechnique. In the nonfluoroscopy group use of contact force technology was more frequent.
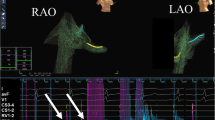
In 11 patients, typical flutter was diagnosed and a cavotricuspid isthmus ablation without fluoroscopy was intended. A complete isthmus blockade was achieved without any complications in all patients. In the mean follow-up of 389 ± 217 days, there was no symptomatic recurrence in any of the groups. Contact force was used in one patient (9.1 %, Fig. 1). Procedure time (door to door) was not significantly different between the two groups (128.3 ± 90.3 min vs. 102.6 ± 35.5 min, p = 0.4).
Use of contact force measurement during zero-fluoroscopy ablation in patients with atrial flutter (Aflutter), atrioventricular nodal reentry tachycardia (AVNRT), Wolff–Parkinson–White syndrome (WPW), and pediatric patients. Bars represent percentage of patients treated with contact force measurement (CFM), if ablation with radiofrequency was indicated
In 48 patients an atrioventricular (AV) nodal reentry tachycardia was diagnosed during zero-fluoroscopy EP study; 29 patients received a slow-pathway modulation via cryotechnique and 19 via RF. Contact force was used in 72.7 % of patients treated with radiofrequency. Acute success was achieved in all patients. In the follow-up period, there were five recurrences, all in the cryotechnique group. One patient in the RF group developed a higher-grade AV block 72 h after the procedure, requiring pacemaker implantation, whereas we observed no major complication in the cryotechnique group. Compared with our matched control group using fluoroscopy, we found no significant differences in the follow-up period (Fig. 2a). Procedure time in the zero-fluoroscopy group was longer; however, it did not reach statistical significance (116.0 ± 49.8 vs. 137.0 ± 61.7, p = 0.08, Table 2).
WPW syndrome was diagnosed in 21 patients; with a left-sided pathway in 14 and a right-sided pathway in seven. In all patients, beside one with a left-sided pathway, contact force measurement was used. Periprocedural success was achieved in all patients with left-sided pathways and in six of seven patients with right-sided pathways (one failed owing to the parahissian position). In one patient a small pericardial effusion was detected by echocardiography requiring no further therapy. In the follow-up there were two recurrences. We found no significant differences compared with patients treated with fluoroscopy (Fig. 2b, p = 0.4). Notably, we observed a significant difference in the procedure time, which was longer in the zero-fluoroscopy group (153.7 ± 78.3 min vs. 210.1 ± 19.3 min, p = 0.02, Table 2).
Finally, a comparison of arrhythmia recurrences in both groups treated with RF revealed no significant difference in the follow-up (Fig. 3, p = 0.1). Notably, we observed a trend for more recurrences in the zero-fluoroscopy group, if patients were treated with the cryotechnique (p = 0.1). Periprocedural data revealed a mean intervention time of 156.4 ± 85.7 min in the zero-fluoroscopy group, which covers the “door-to-door” time including the more time-consuming preparation of patients if using a 3D mapping system. However, procedure time in our control group was significantly shorter (129.5 ± 68.5 vs. 156.4 ± 85.7 min, p < 0.01, Table 2).
Discussion
We provide evidence that zero-fluoroscopy catheter ablation with optional contact force is generally feasible in a clinical routine setting for the diagnosis and therapy of paroxysmal supraventricular tachycardias in an all-comers collective.
Based on our findings, zero-fluoroscopy ablation of typical atrial flutter seems to be a simple and safe procedure, as reported by others [15, 16]. In most of these patients contact force is not needed and may represent an overtreatment.
We treated 48 patients with AV nodal reentry tachycardia without any perforation or vascular complication. However, one patient developed a late-onset AV block more than 72 h after the procedure. By using a cryotechnique in zero-fluoroscopy ablation, the risk of an AV block can be reduced; however, the incidence of recurrence appears to be increased in most studies. Compared with ablation with fluoroscopy, we found no significant difference in the follow-up. In some patients, induction of AV nodal reentry tachycardia was hindered. This could be ascribed to mechanical irritation of the AV node during mapping. However, we found no difference compared with our matched control group (not shown). In this context, we observed that a contact force of 4 g mean and 10 g peak suffices to induce a short AV block during mapping in pediatric patients.
As expected for an all-comers collective most of the diagnosed arrhythmias were AV nodal reentry tachycardias and WPW syndromes with mainly left-sided pathways. The highest challenge in zero-fluoroscopy ablation is ablation of left atrial substrates. We treated 14 patients with left-sided arrhythmia substrates. For our transseptal approach, we used TEE. Notably, in this subgroup we found a significantly longer procedure time, which could mainly be ascribed to the more time-consuming transseptal puncture using TEE. In further studies this may be simplified by intracardiac echocardiography, as described by Reddy et al. [11]. We observed no pericardial tamponade by using contact force measurement. We had six shifts from a normal solid-tip catheter to a contactforce catheter, especially in patients with concealed WPW and atrial tachycardias. Thus, ablation in the left atrium seems to be safe and effective based on our data using optional contact force.
A zero-fluoroscopy diagnostic EP study was performed on 94 patients. In three patients no arrhythmia could be induced and no arrhythmia was documented, thus, no ablation therapy was performed. Our data indicate that beside economic considerations there is no need for fluoroscopy in diagnostic EP, which is an important aspect especially for pediatric patients.
In our study we treated 29 pediatric patients, who may have the highest benefit from zero-fluoroscopy ablation [1, 17]. If RF ablation was indicated, we used contact force measurement in 80.0 % of this sensitive collective without any complications.
As expected, we found a significantly longer procedure time in the zero-fluoroscopy group. However, we observed a learning curve of the interventionalist, as reported by others [6]: Whereas at the beginning of our study a complete electroanatomical mapping was performed using contact force measurement, operators with more experience marked only the hallmarks such as His potential and coronary sinus and used primarily normal diagnostic EP catheters.
Limitations of the study
TEE-guided transseptal puncture without fluoroscopy still requires experienced interventionalists. In addition, we found a significantly longer procedure time over all, which is a limitation of this approach. However, in simple procedures such as for AVNRT or typical atrial flutter, we found no significant difference. Using a 3D mapping system and a contact force catheter can only be fully reimbursed for left atrial procedures in the German DRG system. Further, based on our data, in right atrial procedures contact force is often not necessary and the 3D mapping system can be reimbursed. Our study is a retrospective analysis, which is a clear limitation. In future, randomized controlled trails are necessary to provide further evidence for the non-inferiority of zero-fluoroscopy ablation compared with conventional fluoroscopy. Since we included only patients with symptomatic arrhythmias, we did not have Holter monitoring of all patients in the follow-up.
Conclusion
As far as we know, this is the first study with a zero-fluoroscopy approach using optional contact force in an all-comers collective. We describe an approach that is feasible and economic in a clinical routine setting.
References
Clay MA, Campbell RM, Strieper M, Frias PA, Stevens M, Mahle WT (2008) Long-term risk of fatal malignancy following pediatric radiofrequency ablation. Am J Cardiol 102:913–915
Roguin A, Goldstein J, Bar O, Goldstein JA (2013) Brain and neck tumors among physicians performing interventional procedures. Am J Cardiol 111:1368–1372
Roguin A (2012) Brain tumours among interventional cardiologists: a call for alarm? Eur Heart J 33:1850–1851
Ernst S, Castellano I (2013) Radiation exposure and safety for the electrophysiologist. Curr Cardiol Rep 15:402
Fernandez-Gomez JM, Morina-Vazquez P, Morales Edel R, Venegas-Gamero J, Barba-Pichardo R, Carranza MH (2014) Exclusion of fluoroscopy use in catheter ablation procedures: six years of experience at a single center. J Cardiovasc Electrophysiol 25:638–644
Gist K, Tigges C, Smith G, Clark J (2011) Learning curve for zero-fluoroscopy catheter ablation of avnrt: early versus late experience. Pacing Clin Electrophysiol 34:264–268
Alvarez M, Tercedor L, Almansa I, Ros N, Galdeano RS, Burillo F, Santiago P, Penas R (2009) Safety and feasibility of catheter ablation for atrioventricular nodal re-entrant tachycardia without fluoroscopic guidance. Heart Rhythm 6:1714–1720
Tuzcu V (2007) A nonfluoroscopic approach for electrophysiology and catheter ablation procedures using a three-dimensional navigation system. Pacing Clin Electrophysiol 30:519–525
Earley MJ, Showkathali R, Alzetani M, Kistler PM, Gupta D, Abrams DJ, Horrocks JA, Harris SJ, Sporton SC, Schilling RJ (2006) Radiofrequency ablation of arrhythmias guided by non-fluoroscopic catheter location: a prospective randomized trial. European heart journal 27:1223–1229
Clark J, Bockoven JR Lane J, Patel CR, Patel CR, Smith G (2008) Use of three-dimensional catheter guidance and trans-esophageal echocardiography to eliminate fluoroscopy in catheter ablation of left-sided accessory pathways. Pacing Clin Electrophysiol 31:7
Reddy VY, Morales G, Ahmed H, Neuzil P, Dukkipati S, Kim S, Clemens J, D'Avila A (2010) Catheter ablation of atrial fibrillation without the use of fluoroscopy. Heart Rhythm 7:1644–1653
Ferguson JD, Helms A, Mangrum JM, Mahapatra S, Mason P, Bilchick K, McDaniel G, Wiggins D, DiMarco JP (2009) Catheter ablation of atrial fibrillation without fluoroscopy using intracardiac echocardiography and electroanatomic mapping. Circ Arrhythm Electrophysiol 2:611–619
Kuck KH, Reddy VY, Schmidt B, Natale A, Neuzil P, Saoudi N, Kautzner J, Herrera C, Hindricks G, Jais P, Nakagawa H, Lambert H, Shah DC (2012) A novel radiofrequency ablation catheter using contact force sensing: Toccata study. Heart Rhythm 9:18–23
Kerst G, Weig HJ, Weretka S, Seizer P, Hofbeck M, Gawaz M, Schreieck J (2012) Contact force-controlled zero-fluoroscopy catheter ablation of right-sided and left atrial arrhythmia substrates. Heart Rhythm 9:709–714
Hindricks G, Willems S, Kautzner J, De Chillou C, Wiedemann M, Schepel S, Piorkowski C, Risius T, Kottkamp H, EuroFlutter I (2009) Effect of electroanatomically guided versus conventional catheter ablation of typical atrial flutter on the fluoroscopy time and resource use: a prospective randomized multicenter study. J Cardiovasc Electrophysiol 20:734–740
Macias R, Uribe I, Tercedor L, Jimenez J, Barrio T, Alvarez M (2014) A zero-fluoroscopy approach to cavotricuspid isthmus catheter ablation: comparative analysis of two electroanatomical mapping systems. Pacing Clin Electrophysiol 37:1029–1037
Papagiannis J, Tsoutsinos A, Kirvassilis G, Sofianidou I, Koussi T, Laskari C, Kiaffas M, Apostolopoulou S, Rammos S (2006) Nonfluoroscopic catheter navigation for radiofrequency catheter ablation of supraventricular tachycardia in children. Pacing Clin Electrophysiol 29:971–978
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
P. Seizer, V. Bucher, C. Frische, D. Heinzmann, M. Gramlich, I. Müller, A. Henning, M. Hofbeck, G. Kerst, M. Gawaz, and J. Schreieck state that there are no conflicts of interest.
Rights and permissions
About this article
Cite this article
Seizer, P., Bucher, V., Frische, C. et al. Efficacy and safety of zero-fluoroscopy ablation for supraventricular tachycardias. Herz 41, 241–245 (2016). https://doi.org/10.1007/s00059-015-4358-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00059-015-4358-4