Abstract
Anaerobic digestion (AD) is a widely used technology for the treatment of organic wastes and by-products. Through AD, the organic matter is degraded producing a gaseous stream (biogas, which is a mixture of mainly methane and carbon dioxide) and a liquid/slurry stream (digestates) that contains most of the mineralized elements originating in the feedstock. Digestates are a very interesting source of nutrients for growing microalgae to produce valuable biomass with a simultaneous further treatment of the digestates. The performance of microalgae grown using digestates is influenced by various cultivation parameters, such as the physicochemical characteristics of the digestates (nutrient profile, content of inhibitory compounds, etc.), light penetration (turbidity and colored dissolved compounds), mixing regime, and hydraulic retention time (HRT). Digestates are characterized by their high content in ammoniacal nitrogen, suspended solids, and several inhibitors that might limit growth, and therefore pretreatment of digestates is likely to have a positive effect on biomass production. Microalgal cultivation is proven as an efficient technology for the removal of nitrogen, phosphorus, organic load, and other contaminants (heavy metals, pathogens). The produced biomass could be used as feedstock for the production of various commodities (biofuels, feed, etc.); however there are some concerns about the potential contamination of microalgal biomass with unwanted hazardous pollutants. This book chapter aims to give an overview on the cultivation of microalgae utilizing digestates derived from agro-industrial wastes and by-products, discussing the potentials and the drawbacks of such an approach.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Anaerobic digestion (AD) is a biological technology in which a consortium of microbes breaks down organic material under anoxic conditions producing biogas. Biogas is a mixture of mainly methane (45–65%) and carbon dioxide (45–35%) and is a renewable energy carrier used for the production of thermal/electric energy or as transportation fuel (Weiland 2010). AD is a mature technology and has gained interest as an approach of treating organic wastes (including wastewater) for the production of renewable energy. Worldwide tens of thousands of biogas plants are already operating, treating organic wastes, such as animal manures, crops and food residues, industrial organic wastes, sewage sludge (biosolids), etc. Some biogas plants are also integrated into the production of energy from residues or energy crops, such as maize silage (Lora Grando et al. 2017). AD is a very efficient, when compared to other treatment methods (such as aerobic treatments), at reducing high organic loads and therefore is an option of choice for high strength wastes, such as agro-industrial wastes or by-products. AD can reduce organic loads as high as 80%; however it does not remove inorganic loads, and therefore it does not achieve tertiary treatment. As a consequence, the effluents of AD, which are called digestate, are rich in inorganic nutrients that either require posttreatment in order to reduce their pollutant loads (organic and inorganic, and including excess forms of N and P) before disposal or used as soil amendments and nutrient source for plant production (Möller and Müller 2012).
However, since transportation of digestates over great distances and their spreading over large land areas is not always economically feasible, the extensive application of digestates to lands adjacent to the biogas plants has led to the saturation of soils in N and P, with negative impact on the environment. Moreover, the seasonal application of digestates, i.e., in seasons where crops are at the appropriate growth stages that can use fertilizer nutrients, requires long-term storage, which has its own some drawbacks and can lead to negative impacts on the environment, such as the emission of greenhouse gases like CO2, CH4, and N2O (Monlau et al. 2015). In this context, further strict regulations (see, e.g., the European Union Nitrate Directives) in some jurisdictions create the need for the development of alternative valorization routes for digestate. Among the various alternatives (Monlau et al. 2015), cultivation of microalgae has gain increased attention because of the high potential of producing useful biomass with the simultaneous treatment of waste streams and recycling of valuable nutrients (Cuellar-Bermudez et al. 2017; Salama et al. 2017). This book chapter aims to give an overview on the cultivation of microalgae utilizing digestates derived from agro-industrial wastes and by-products, discussing the potentials and the drawbacks of such an approach.
2 Anaerobic Digestion Process and the Generation of Digestates
2.1 Physicochemical Characteristics and Nutrient Content of Digestates
The digestate is the residual sludge obtained at the end of the AD of organic matter of various sources. The chemistry of the ingestate, the feedstock used in the anaerobic process, does affect the chemistry of the digestate. AD degrades organic compounds and leads to significant losses of volatile solids and total carbon. This lowers the chemical and biological oxygen demands (COD and BOD), an indication of a biologically stable material. Nevertheless, post digestion storage might still lead to further mineralization and accumulation of soluble carbon (Farno et al. 2014). This is treated in more detail in the following sections. Digestion thus leads to mineralization of nitrogen and phosphorus most of which are in chemical forms relatively stable at the usual neutral to slightly alkaline pH. Digestion of animal manure such as pig slurry and cow manure may lead to more accumulation of ammonia and orthophosphate than the digestion of municipal sludge (Risberg et al. 2017; Zuliani et al. 2016), while digestion of high lignin, plant-based waste produces digestates with higher total solids (Lukehurst et al. 2010), possibly with a higher C/N ratio (Eich-Greatorex et al. 2018), and with solids often likely to be present in a hydrated form (Mudryk et al. 2016). Presence of more recalcitrant compounds, such as lignin and lipids, will lead to a more biologically stable digestate (Tambone et al. 2009) with more total solids. The solid phase may be usually separated into colloidal and large particulate matter. Any nutrient that is retained in the solid phase is usually associated with the larger particles, not in the colloidal matter (Akhiar 2017).
The utilization of digestate for algal growth is dependent on the capacity to remove the nutrients in the liquid phase. Separation of solids and liquid may lead to accumulation of mineral nitrogen in the liquid phase, as nitrate and ammonium, but a removal of P in the solid phase. Thus, the proportion of solids to liquid and the charge properties of the solids affect the separation efficiency. Most common separation procedures involve flocculation followed by centrifugation or mechanical separation. Digestates with highly hydrated solids require mechanical separation procedures. The conditions of any storage before the separation step affect the stability of the digestate and the separation efficiency (Oliveira et al. 2015). The most effective separation is a combination of flocculation followed by mechanical separation (Akhiar 2017), a common practice in municipal wastewater treatment plants. One drawback of the separation of solids is that a significant proportion of phosphorus might be retained in the solid phase (Bachmann et al. 2016; Lin et al. 2015).
Another concern when using the liquid phase of the digestate for algal growth is the color. Animal manure-based digestates are notorious for producing colored digestate difficult to remove even through significant dilution (Marcilhac et al. 2014). On the other hand, digestates produced on municipal wastewater are more transparent (Zuliani et al. 2016). For the latter the only significant interference with light is due to colloidal matter, a fraction usually removed easily through flocculation. Nevertheless, co-digestion of municipal wastewater solids with food or manure waste will still produce a highly colored liquid phase digestate difficult to treat (Akhiar et al. 2017).
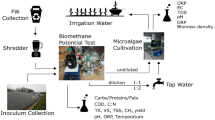
AD is a highly complex and dynamic biological process, which can be divided into four stages: (i) hydrolysis of polymers into soluble monomers, (ii) acidogenesis, (iii) acetogenesis, and (iv) methanogenesis (Deublein and Steinhauser 2008). During AD the organic matter is degraded and mineralized. Macromolecules such as carbohydrates, proteins, and lipids are degraded (hydrolyzed) to smaller molecules (monomers), such as monosaccharides (simple sugars), amino acids, and long-chain fatty acids, respectively. Hydrolysis is performed by hydrolytic exoenzymes, such as cellulase, protease, and lipase, which are excreted by facultative and strict anaerobic fermentative microorganisms (Gerardi 2003). In the second process, the monomers derived from hydrolysis are fermented to volatile fatty acids (acetic acid, propionic, butyric, etc.), which then are used by microbes to produce methane at the third and fourth steps. Since during AD carbon is nearly exclusively removed as CH4 and CO2, the remaining inorganic elements in the feedstock are mineralized and almost fully preserved in the digestion liquor (digestate; Fig. 1). These elements are either as free ions (NH4+, PO43−, K+, SO42−, Fe2+, etc.) or as ion and/or surface bounded complexes. In general, the digestate is a very complex matrix where various free counterions and (bio-)solids interact with each other (Möller and Müller 2012). In the agro-industrial sector, AD is usually performed by mixing different substrates in order to obtain a balanced C/N ratio (around 20–30:1). Because most plant- and food-derived wastes and by-products are rich in C, thus having a high C/N ratio (>50:1), nitrogen-rich animal and poultry manures are typically used to adjust C/N to an optimum range (Mata-Alvarez et al. 2014). Because animal and poultry manures are also rich in other nutrients (P, K, S, etc.), digestates therefore are rich too in all of the essential nutrients required for microalgal growth (Xia and Murphy 2016).
2.1.1 Carbon
Carbon in digestates is either in inorganic (bicarbonate/carbonate) or in organic (volatile fatty acids, such as acetate, and undigested organic matter) forms. Regarding microalgae cultivation, bicarbonate and volatile fatty acids (mainly acetate) are the most significant C forms because they can be utilized by microalgae as C source for their growth. Bicarbonate/carbonate and volatile fatty acids (VFAs) are the end products of the acidogenic and acetogenetic stages of the digestion process, where organic polymers are biochemically broken down to monomers. The concentration of bicarbonate/carbonate and VFAs and composition of the latter can vary significantly depending on the substrate characteristics and AD parameters and on the overall process stability (Rincón et al. 2008). Bicarbonate/carbonate and VFAs have a significant role for the digestion process because, along with ammoniacal N, they are the main buffer systems for the pH in digestates (Georgacakis et al. 1982). Bicarbonate/carbonate concentration in digestates is typically in the range 500–1500 mg L−1. For the typical pH range of the digestates inorganic C is mainly (>95%) found as bicarbonate species (HCO3−).
Among the various VFAs contained in the digestates, the most abundant species is acetate (CHO2−). However, due to the inhibitory effect of VFAs on methanogenesis, their concentration should kept relative low (<500 mg/L) for a stable AD process. Recently, there is an increased interest in performing AD at low pH in order to favor the hydrolytic only phase and inhibiting methanogenesis to convert organic matter into VFAs, which are a potential C source for the production of different commodities such as biosurfactants, bioflocculants, and bioplastics (Wang et al. 2014). Regarding microalgae cultivation, these accumulated VFAs may serve as energy and/or C source for the mixotrophic or heterotrophic production of microalgal biomass (Venkata Mohan and Prathima Devi 2012; Chiranjeevi and Venkata Mohan 2017).
2.1.2 Nitrogen
Total N , which is the sum of organic and inorganic N, of the digestate can range between 1.2 and 9.1 g Kg−1 FM, while inorganic N can be 44–81% of total N (Table 1). N is mostly contained in the ammoniacal form (NH4+/NH3), while NO3− and NO2− are in trace concentrations. A fraction, however, of N remains in organic form, and its concentration depends mostly on the degree of biodegradation and mineralization of the organic matter. Ammoniacal N mainly derives from the mineralization of proteins and amino acids (contained in plant-derived substrates or from undigested feed proteins/amino acids excreted in manures), urea, or uric acid (main nitrogen form in animal and poultry manures, respectively), with a lower proportion originating from other nitrogenous compounds. A small fraction of ammoniacal N is utilized by anaerobes for their metabolic needs and their cell multiplication (Rajagopal et al. 2013). The ratio of NH4+ to NH3 (free ammonia) is mainly dictated by the pH and temperature. NH3 can be calculated from the total ammoniacal N by the following equation (Hansen et al. 1998):
where pH is the actual pH value of the solution and T is the temperature (in K). For the typical pH range of digestates, ammoniacal N is mainly (>95%) in the form of ammonium (NH4+).
The ionic status of ammonium allows it to interact with other ions in the digestate to form various complexes, with the most significant one to be the struvite (NH4MgPO4·6H2O) which precipitates in the solid state. Struvite formation occurs in two stages, (i) nucleation and (ii) crystal growth, and arises when the concentrations of Mg2+, NH4+, and PO43− surpass the struvite solubility product, which is also a function of pH. As pH increases, struvite solubility decreases and its formation is favored (Marti et al. 2008; Pastor et al. 2010).
2.1.3 Phosphorus
Total inorganic and organic P content in the digestates can range between 0.4 and 2.6 g kg−1 FM of which around 25–45% is in water-soluble forms (Table 1). After mineralization P is contained mainly in orthophosphates with HPO42− and PO43− (pKa2 ≈ 7.21) dominating speciation at the pH range typical for digestates. At higher pH the chemical equilibrium shifts toward favoring the formation of phosphate (PO43−), which tends to complex with cations (Ca2+, Mg2+, etc.) and subsequently precipitate as phosphate salts, such as Ca3(PO4)2 or MgHPO4. As mentioned before, at adequate concentration of NH4+ and Mg2+ struvite is formed; this is a very interesting form of fertilizer P. Marti et al. (2008) have shown that most P, 58% of the fixed P, precipitated as struvite, 15% as calcium phosphates in the form of hydroxyapatite (Ca5(PO4)3(OH)), and the other 27% was adsorbed on surfaces of the solids.
Digestates from the agro-industrial sector that contain animal manures are rich in P because typically the feeds of monogastric (nonruminants) animals such as swine and poultry are excessively supplemented with inorganic phosphate salts to provide the required P for their growth. This is because monogastric animals and poultry lack the enzyme phytase and cannot metabolize phytic acid, the principal P form in plant matter that comprises animal and poultry feed, typically a mixture of various crops such as corn, wheat, oat, and soya. Moreover, monogastric animals and poultry have a low uptake efficiency for P from phosphate forms, which results that almost 70% of the P contained in the feed is excreted unmetabolized in manures (Gupta et al. 2015; Jorquera et al. 2008). Consequently, cattle manure contains in general lower P amounts compared to swine and poultry (Kleinman et al. 2005).
2.1.4 Other Nutrients
Agro-industrial digestates, especially derived by the AD of animal manures , besides N and P, are also rich in other nutrients including K, S, Mg, Cu, Zn, and Fe. Some of these nutrients originate in the feed, while other nutrients (especially trace metals, such as Cu, Fe, Zn) originate mostly from the various feed supplements used to formulate feed rations. These elements are usually added in excess, as highly soluble metal salts, and therefore excess amounts are excreted in manures (Zhang et al. 2012). Regarding energy crops or crop residues, they contain very low amounts of trace metals, and therefore, when digestates as sole substrate (without manures), they have to be supplemented in the digesters (Brulé et al. 2013). In any case, regardless of the substrate type, it is very probable that digestate will contain all essential nutrients to support microalgal growth. However, due to the slight alkaline pH of the digestates, most of the cation nutrients might form several complexes and precipitate as carbonates and phosphates or attach to solid surface (Möller and Müller 2012). In this case their bioavailability for microalgal growth could be limited and might be necessary to be externally added to the cultivation medium.
2.2 Inhibitory Compounds and Contaminants Present in Digestates
Digestates, due to their complex nature, besides the valuable and reusable nutrients, contain also various organic, inorganic, or biological compounds that could potentially inhibit microalgal growth or affect the overall quality and safety of microalgal biomass. Besides the suspended solids and the colored dissolved compounds of the digestates that limits light penetration reducing growth rates, the most significant potential inhibitory compounds in digestates that might affect microalgal growth include ammonia, various organic acids, and heavy metals. On the other hand, the contamination of biomass with pathogens or chemical contaminants originated in digestates could restrict biomass utility for the production of various commodities.
2.2.1 Ammonia
One of the most significant inhibitory compound of digestates is ammoniacal N. In particular the free ammonia (NH3) form is highly toxic to microalgae. While ammonium is actively taken up by cells and therefore its intracellular concentration is metabolically regulated, NH3 diffuses passively and uncontrolled into the cells and at elevated concentrations act toxic. Ammonia acts by damaging the photosynthetic machinery and other cellular components, resulting in reduced photosynthesis activity and lower growth rates or even in cell death (Markou et al. 2016; Azov and Goldman 1982; Drath et al. 2008). Free ammonia has inhibitory effects on microalgae in relatively low concentrations (>25 mg-N/L) (Abeliovich and Azov 1976; Azov and Goldman 1982; Markou and Muylaert 2016); however, ammonia toxicity is microalgal species dependent, i.e., some species, such as the cyanobacterium Arthrospira platensis, display higher ammonia tolerance than other species (Markou et al. 2016; Markou and Muylaert 2016).
As mentioned before, in the NH4+/NH3 ionization system, the formation of NH3 is favored by alkaline pH (pKa ≈ 9.25). Microalgae are therefore more susceptible to ammonia toxicity when pH of the cultivation medium is high. This should be taken into consideration especially for microalgal cultures without a pH control, where pH may reach values higher than 10 due to the liberation of hydroxides (OH−) during CO2 uptake. At such pH values, ammoniacal N will be mostly found as free ammonia (>50% of the total ammoniacal N at pH > 9.25) increasing the digestate toxicity potential .
2.2.2 Organic Acids
Even as some microalgal species have the ability to utilize, mixotrophically or heterotrophically, organic acids such as fermentative acetate or butyrate as C and/or energy source (Turon et al. 2015), these could be inhibitory when their concentration is high. For example, butyrate exhibited inhibitory effects on Chlorella sorokiniana and Auxenochlorella protothecoides at concentrations higher than 0.1 gC L−1 and 0.25 gC L−1, respectively (Turon et al. 2015), or acetate on Chlamydomonas reinhardtii at concentrations higher than 0.4 g L−1 (Chen and Johns 1996b). However, significant higher acetate concentrations (> 3 g L−1) have been used for the cultivation of some microalgae without showing any inhibition, reflecting that the inhibition of organic acids is microalgal species dependent (Perez-Garcia et al. 2011; Chen and Johns 1996a), while cultivation mode (fed-batch, perfusion, chemostat) could improve the overall cultivation efficiency (Chen and Johns 1995, 1996a).
2.2.3 Heavy Metals
Heavy metals are generally defined as the metals or metalloids with a specific density >5 g mL−1, including Cu, Co, Cr, Cd, Fe, Zn, Pb, Hg, Mn, Ni, As, Mo, and V. Some of them, such as Cu, Co, Fe, Mn, and Mo, are essential for microalgal growth and are supplemented in various synthetic cultivation media as trace metals. These elements are contained in various enzymes or biomolecules and play a significant biological role. Depletion of these essential elements could have a negative effect on microalgal growth.
Digestates frequently contain heavy metals. These can originate in agro-industrial wastes and wastewaters that are contaminated by fertilizers, plant protection chemicals, or processing agents that contain heavy metals. Most manures contain relative high amounts of heavy metals especially Zn, Cu, and As as these are supplemented in animal feed as growth promoters or for the treatment of various diseases. They are usually added as soluble metal salts, commonly in excess amounts of the physiological requirements and therefore are excreted along with feces and urine (Zhang et al. 2012). Heavy metal species and concentrations in digestates are generally depended on the feedstock and in particular the ratio of the different wastes/wastewater used (Table 2). Toxicity of heavy on photosynthetic organisms, including microalgae, has been widely studied, and it is well known that at high concentrations, they damage the photosynthetic machinery affecting negatively cell growth. However, the level of toxicity depends on the heavy metal species, its concentration, and cultivation parameters, such as light intensity, pH, and vary with microalgal species (Švec et al. 2016; Napan et al. 2015; Torres et al. 2017). However, since digestates usually require dilution before, it is used for microalgal cultivation, their toxicity potential is rather low. No inhibition of microalgae cultivated in digestates is reported to be attributed to heavy metal toxicity. Contamination of biomass with heavy metals would though limit the potential of biomass to be used to produce several commodities (e.g., food, feed).
2.2.4 Biological Contamination
Digestates contain a plethora of microbes, the majority of which are strict anaerobes, but also include some facultative anaerobic acidogenic bacteria (Gerardi 2003). During microalgal cultivation facultative anaerobes as well microbes that externally contaminate the cultures could grow in a synergistic or competitive relationship with microalgae. The synergistic relationships are based on the fact that microbes degrade organic matter into CO2 which is then taken up by microalgae, while microalgae produce oxygen that is used by the microbes. Antagonism between microalgal and bacteria may occur when they compete for nutrients (Munoz and Guieysse 2006). On the other hand, some bacteria present in digestates produce phytohormones that are growth-promoting agents (Qi et al. 2017). Phytohormones play a regulatory role in microalgae cell division and elongation and in chlorophyll and protein metabolism and enhance tolerance to several stresses such as heavy metal toxicity, osmotic, and salt stresses (Pei et al. 2017; Salama et al. 2014). The addition of phytohormones to cultures promotes growth and increases biomass density (Pei et al. 2017). Presence of pathogens creates a significant disadvantage for the use of digestates for the cultivation of microalgae. Contamination of microalgal biomass with pathogens would definitely limit biomass utility in various applications (food, feed, high-value products). The term pathogens include all those agents, such as bacteria (e.g., Campylobacter spp., Clostridium sp., Escherichia coli, Listeria monocytogenes, Salmonella sp., or Yersinia enterocolitica), fungi (e.g., Aspergillus sp., Penicillium sp., Rhizomucor), protozoa, worms, viruses (e.g. enteroviruses, rotaviruses, adenoviruses, hepatitis E viruses, caliciviruses, reoviruses, parvoviruses), and prions that can cause diseases (Bicudo and Goyal 2003; Ray et al. 2013). The main source of pathogens in the digestates originates from animal and poultry manure used in the mixtures. During AD several pathogens are fully inactivated; however some are resistant and can survive. The major AD parameters that play a role in the inactivation of pathogens are time and temperature of treatment, the latter being the most significant one. Thermophilic (>50 °C) AD is generally more effective than mesophilic (>30–38 °C) for pathogen reduction; however some pathogens such as some spore-forming Clostridium or Bacillus can survive thermophilic AD (Sahlström 2003; Bagge et al. 2010). In several countries, a pasteurization stage (70 °C for 1 h) either before or after the AD digestion is integrated in the process to warrant the hygiene of the digestates ; however spore-forming pathogens are not always fully inactivated (Schnürer and Schnürer 2006; Sahlström 2003; Bagge et al. 2010).
2.2.5 Chemical Contaminants
Agro-industrial wastes/wastewater and hence their digestates can contain several other xenobiotic compounds that could pose a risk of contamination of microalgal biomass. The most important xenobiotics include pharmaceuticals (e.g., steroidal hormones, antibiotics, and parasiticides), mycotoxins, and dioxins (Ray et al. 2013; Van Boeckel et al. 2017) (Khan et al. 2008; Bártíková et al. 2016). Even as most of these xenobiotics are degraded during AD, there are some categories, such as steroidal hormones, or some antibiotics that are not extensively degraded. The efficiency of AD for degradation of xenobiotics varies widely and is a function of the physicochemical characteristics of the compound in question and some AD parameters, such as retention time and temperature (Stasinakis 2012).
3 Cultivation of Microalgae Applying Digestates
3.1 Removal of Nutrients from Digestates
Microalgae require a range of nutrients to synthesize the biomolecules that consist their biomass; C, N, P, K, Mg, S, Cl, Fe, Ca, Mn, Co, Cu, B, and Zn are essential for an unhindered cell growth. Lack of one or more of these essential nutrients will cause a cessation of cell growth resulting in biomass production reduction. Moreover, lack of nutrients will lead to alteration of the biochemical composition of biomass, typically triggering the accumulation of carbonaceous compounds (lipids or carbohydrates) and downregulation of protein production (Pancha et al. 2014; Kamalanathan et al. 2015). However, this biochemical composition alteration due to nutrient starvation/limitation in favor of carbonaceous compounds has been suggested as a strategy for the accumulation of lipids or carbohydrates as feedstock for various applications (see Sect. 4). Due to the fact that microalgae can remove nutrients from their surroundings, they have been proposed as a biological mean for the treatment of waste/wastewater for the removal of inorganic pollutants, such as N and P (Olguín 2012) which are among the main targets in the conventional wastewater treatment plants.
During microalgal cultivation on digestates, nitrogen is removed through three main mechanisms, i.e., biomass uptake, volatilization, and denitrification. Biomass uptake refers to the active or passive transport of the nutrients into the interior of the cells. Ν can be taken up being in various forms, such as ammoniacal, nitrite, nitrate, or organic form (N2 can be utilized only by a limited number of N-fixing cyanobacterial species). In general, ammoniacal form is the most preferable N form because it is already in a reduced status and can be utilized immediately by the metabolic pathway for protein synthesis. In contrast, nitrogen oxides have first to be reduced intracellularly, therefore consuming energy (Perez-Garcia et al. 2011). If different N forms are present in the cultivation medium, ammoniacal N is preferentially taken up, and only after it is exhausted other N forms are taken up (Fernandez and Galvan 2007; Vílchez and Vega 1994). Besides inorganic N uptake, microalgae can also grow on organic N, such as urea or amino acids (glycine, glutamate, glutamine). Urea is taken up indirectly, because it is first hydrolyzed to ammonia and carbonic acid which can then be taken up by cells. However, amino acid uptake is species dependent, and growth rates can vary between microalgal species (Neilson and Larsson 1980; Flores and Herrero 2005). Removal of N through volatilization occurs only when N is in the free ammonia form. As was mentioned before (Sect. 2.2), free ammonia dominates as pH increases, and therefore the potential of ammonia removal through volatilization increases at high pH. Depending on the cultivation conditions, ammonia volatilization losses can be significant (González-Fernández et al. 2011; Markou et al. 2014a). The pH is the most important factor for the volatilization of ammonia, whereas other various physicochemical characteristics (e.g., solid content, electrical conductivity) of the digestates do not have any significant influence on the volatilization potential (Markou et al. 2017). Removal of N through denitrification refers to the transformation of ammoniacal N into nitrite/nitrate and finally into molecular nitrogen (N2). Denitrification is a process that could take place when dissolved oxygen is not easily available and nitrifying microbes are present in an aqueous body, such as is case for microalgal-bacterial cultivation systems. Denitrification could account for 20–25% of the N removal (González-Fernández et al. 2011).
P is removed mainly through two mechanisms : biomass uptake and precipitation. P is taken up by cells mainly in the phosphate form (PO43−) by metabolically driven processes. However, microalgae can take up P from other inorganic or organic forms as well (Huang and Hong 1999; Whitton 1991). Organic P can be taken up after mineralization of the organic matter to produce as PO43−, or hydrolysis of other inorganic forms than PO43−, by extracellular phosphatase enzymes, the main mechanism of organic P uptake (Hua-sheng et al. 1995; Dyhrman and Ruttenberg 2006). The ability of microalgae to take up organic P depends on the chemical composition of the organic molecules (Dyhrman and Ruttenberg 2006). Precipitation of P complexes with polyvalent cations (Ca, Mg, etc.) might occur, especially in cultures where pH is increased due to photosynthesis. The presence of organic substances, such as humic acids, could favor the formation of phosphate-metal-humic complexes that are also of low bioavailability for microalgal uptake (Hartley et al. 1997; Hoffmann 1998; Li and Brett 2013). P removal from the solution is frequently reported to reach very high rates (>95%), attributed either to biomass uptake or precipitation (Yang et al. 2017; Franchino et al. 2013).
Although inorganic carbon (IC) is not a target for conventional wastewater treatment, microalgae require adequate amounts of CO2. Most microalgae can take up IC only from CO2 and/or HCO3−, while CO32− is extreme rarely taken up (Camiro-Vargas et al. 2005). Bicarbonate concentration in digestates, especially when they are used in a diluted form, seems not to be adequate for a satisfactory biomass production, and therefore it has to be externally provided to the cultures (Bjornsson et al. 2013; Park et al. 2010). As a source of CO2, either biogas (that contains about 35–45% CO2) or flue gases obtained after biogas combustion could be used (Kao et al. 2012; Salafudin et al. 2015).
For an unhindered cell growth and biomass production, digestates should contain the following nutrients in adequate amounts to cover metabolic needs: C, N, P, K, Mg, S, Cl, Fe, Ca, Mn, Co, Cu, B, and Zn. All these nutrients will be taken up during microalgal growth, and the degree of their removal from the medium depends on the microalgal species and the cultivation conditions (Markou 2015). As was mentioned before, digestates are a multipart medium, and the various ions interact with each other forming complexes that lead to some nutrients (e.g., P, Mg) to become unavailable to microalgae (Möller and Müller 2012). The unavailability of nutrients or an unbalanced C/N/P nutrient ratio could result in nutrient limitation with a negative effect on cell growth and biomass productivity (Beuckels et al. 2015). However, the level of nutrients availability will have an effect on the differential accumulation of target compounds like carbohydrates, lipids, and proteins. As nutrient availability decreases the accumulation of carbohydrates or lipids is triggered, while protein productivity increases as long bioavailability of nutrients increases (Dickinson et al. 2015).
3.2 Removal of Organic Matter
Organic matter removal during microalgae cultivation may occur through two main mechanisms: biomass uptake and degradation. As was mentioned before, microalgae can utilize some organic molecules as source of C and/or energy. Under mixotrophic conditions, i.e., in the presence of light, microalgae can take up organic molecules and utilize them as C and/or energy source, while under heterotrophic conditions where no light energy is available, microalgae take up organic molecules as C and energy source (Chojnacka and Marquez-Rocha 2004). Organic molecules that can be directly taken up by microalgae include several sugars (e.g., glucose, fructose), amino acids, organic acids (e.g., acetate, butyrate), and glycerol (Perez-Garcia et al. 2011). Removal of organic matter through degradation occurs either through their degradation by microalgae themselves or by various bacteria that are typically present in microalgal cultures. In both cases the degradation is carried out by various lytic enzymes that break organic matter down either into simpler molecules or into CO2 that is then taken up by microalgae. Both mechanisms contribute in a significant reduction of organic matter (measured as chemical oxygen demand – COD or biological chemical demand – BOD), reaching values as high as >90%.
However, organic matter cannot be removed completely because during microalgal growth, cells excrete extracellular organic matter (EOM) comprising of various compounds such as proteins, nucleic acids, lipids, and polysaccharides, with the latter a main portion of EOM (Myklestad 1995). However, depending on the cultivation mode (mixotrophic or heterotrophic), the EOM can be dominated by other compounds such as proteins (Wang et al. 2015). Among the main digestate constituents, it was reported that in cultures of Arthrospira platensis and Chlorella vulgaris, volatile fatty acids were removed at rates >90%, while proteins were removed only in cultures of C. vulgaris and not of A. platensis, whereas carbohydrates were accumulated in the medium of both cultures (Markou 2015). Hence, the total removal of organic matter from the cultivation medium supplemented with digestates is the difference between the organic matter removed and organic matter excreted by microalgae .
3.3 Cultivation Operational Parameters
Light penetration, mixing, and hydraulic retention time (HRT) are significant cultivation parameters that influence microalgal growth, especially when using digestates as the nutrient source, which are rich in suspended solids and dissolved colored compounds. The suspended solids contribute to turbidity, which along with the dissolved colored compounds absorb the incident light and reduce its penetration into the cultures, resulting in general in lower photosynthesis and biomass production (Wang et al. 2012; Depraetere et al. 2013; Curtis et al. 1994). Therefore, adequate mixing is important because it generates turbulence moving cells from dark zones to the light zones of the culture subjecting them to more light to conduct photosynthesis. The fluctuation in light intensity caused by mixing should be short enough (10 ms) for best light harvest (Eriksen 2008); however, strong mixing could cause shear stress reducing biomass production (Eriksen 2008; Marshall and Huang 2010). Turbulence caused by mixing is important because it also avoids cell sinking and the formation of nutrient or thermal gradients and increases the mass transfer between the liquid medium and the atmospheric CO2 and removes excess dissolved oxygen (Grobbelaar 2000).
HRT is a significant parameter because, for a given digestate type, it defines the load of the influents, the degree of nutrient removal, and the biomass concentration in the effluents. HRT depends on various parameters, such as light intensity, temperature, nutrient availability, microalgal strain(s), photobioreactor configuration, etc. (Whitton et al. 2015; Munoz and Guieysse 2006) and should be as high as it is needed for best nutrient removal and higher biomass concentrations and productivity. Depending on the cultivation conditions, for sufficient removal of inorganic and organic loads, HRT needs to be 2–10 days (Whitton et al. 2015; Munoz and Guieysse 2006). HRT length should be set to allow cells to reach their logarithmic growth phase to favor biomass productivity and wastewater treatment efficiency (Kim et al. 2014a; Medina and Neis 2007).
3.4 Pretreatment of Digestates to Facilitate Microalgal Growth
A pretreatment of digestates might be required before using them as a nutrient source for the cultivation of microalgae. The pretreatment targets the removal of growth-limiting or growth-inhibitory compounds, such as suspended solids, dissolved colored compounds, and ammonia, in order to render digestates more appropriate for microalgal growth. The simplest pretreatment is dilution by which the concentration of limiting/inhibitory compounds will be decreased at appropriate levels. Depending on the physicochemical characteristics of the digestates, they need a significant dilution, probably more than five times dilution (Xia and Murphy 2016). To address the often limited availability of operational water for the dilution, brackish water, seawater, or low-strength wastewater may be used, including the recycling of the medium after cell harvest (Farooq et al. 2015; Delrue et al. 2015; González-López et al. 2013; Deng et al. 2018).
A common pretreatment method of digestates is solid/liquid separation which generates a solid fraction rich in fibers and compounds attached to them and a liquid fraction rich in soluble compounds (Möller and Müller 2012; Hjorth et al. 2010) (Drosg et al. 2015). Solid/liquid separation, especially of digestates with high solid content, followed by filtration should be considered as a necessary step, as any suspended solid would decrease light penetration or may lead to cells clumping (Uggetti et al. 2014; Xia and Murphy 2016). Solid/liquid pretreatment will have a positive effect on HMs by reducing their concentration, since a great fraction of HMs will be attached onto the solids and removed from the liquid fraction. However, a fraction of P will as well be removed with the solid faction (Table 1)(Möller and Müller 2012), decreasing the available P for microalgal growth. Solid-liquid separation efficiency can be improved by the addition of precipitating agents; however, it should be taken into consideration that various essential nutrients could be removed as well. Laboratory studies have investigated other pretreatment methods, such as ammonia removal through stripping and decolorization by activated carbon (Marazzi et al. 2017), or decolorization using precipitation agents (Depraetere et al. 2013) or by nutrient recovery through adsorption onto geo-minerals (Markou et al. 2015; Markou et al. 2014b) prior microalgal cultivation, showing that generally the pretreatment improves microalgal growth and biomass production. Other pretreatment methods, such as coagulation and flocculation, flotation, and electrochemical treatment (Fu and Wang 2011; Gupta et al. 2012; Marazzi et al. 2017; Kim et al. 2014b; Depraetere et al. 2013), might also be employed; however, there is lack of studies for evaluating their impact on microalgal growth .
4 Potentials for Microalgal Biomass Uses
4.1 Biofuels
Microalgal biomass has been recently considered as a highly potential feedstock for the production of biofuels , such as biodiesel, bioalcohols, biogas, or bio-oil. Microalgal biomass could be used by any biomass-to-energy conversion technology available. Microalgae typically contain about 20–30% lipids; therefore they are of strong interest for biodiesel production (Lam and Lee 2012; Chisti 2007). For the production of biodiesel, lipids are transesterified, i.e., the triglycerides react with short-chain alcohol (i.e., methanol or ethanol) in the presence of catalyst converting them to fatty acid esters. However, one of the major challenges for an economically feasible and sustainable microalgal biodiesel production is to avoid drying of biomass, which after harvesting contains high moisture contents (90–95%) before biofuel production. Presence of water will inhibit several downstream processes, such as lipid extraction and transesterification (Lam and Lee 2012; Chisti 2007; Taher et al. 2014; Macías-Sánchez et al. 2015).
To avoid biomass drying, which is an energy consuming process, for the production of microalgal biofuels other biomass-to-energy conversion technologies that utilize wet biomass could be used; the ones with the greatest potential are AD, alcoholic fermentation, and hydrothermal liquefaction. The most challenging issue with AD is the recalcitrant nature of cell wall that hinders digestion. Most microalgal cell wall is composed of organic compounds with slow biodegradability, and therefore a disruption step is required to facilitate the release of the intracellular biomass compounds in order to be available for digestion (Gonzalez-Fernandez et al. 2015). Bioalcohol production is also a promising route for the production of microalgal biofuels (de Farias Silva and Bertucco 2016). Bioalcohol fermentation is commonly performed in two steps, hydrolysis and fermentation, which steps can be carried out separately or simultaneously. The main drawback of bioalcohol production is the complex, multistep, energy consuming and costly processes required (de Farias Silva and Bertucco 2016).
During hydrothermal liquefaction (HTL), i.e., under relative high temperature (200–350 °C) and relative high pressures (15–20 MPa), biomass is converted to a crude oily liquid (Guo et al. 2015). Compared to other technologies, such as biodiesel and bioalcohols, HTL is considered to be more efficient because lipids, proteins, and carbohydrates are converted into bio-oil with a final high energy density (Tian et al. 2014; Guo et al. 2015). However, since microalgae are relative rich in proteins, the content of N in the bio-oil is high, which lowers its quality and restricts its use (Biller et al. 2012).
4.2 Animal Feed
Because microalgae are rich in proteins, carbohydrates, lipids, and micronutrients, recent research on the use of microalgae for animal feed supplements received widespread attention (Chew et al. 2017). Adding microalgae biomass to animal feed provides vitamins, essential amino acids, polysaccharides, and n-3, n-6 polyunsaturated fatty acids, as well as minerals and pigments, such as carotenoids and chlorophyll (Priyadarshani and Rath 2012). Feeding livestock with microalgae at a replacement rate of 5–10% of the conventional feed can improve body weight gain, feed intake and feed conversion ratio, immune response, weight control, antioxidant status, fertility, and external appearance, such as healthy skin and fur (Certik and Shimizu 1999; Holman et al. 2012; Tsiplakou et al. 2017; Kulpys et al. 2009; Milledge 2011). Microalgae are able to synthesize all amino acids and become a source of essential amino acids. Allegedly, the average mass of most microalgal proteins is higher than traditional plant proteins and is similar to that of yeast, soy flour, and milk, with a well-balanced amino acids profile (Becker 2007). Adding microalgae to dietary supplements can improve the nutritional quality of meat, increase the ratio of PUFA/SFA (polyunsaturated fatty acids/total saturated fatty acids), and increase the DHA (docosahexaenoic acid) and total amount of n-3 fatty acids (Díaz et al. 2017). Microalgal carbohydrates are also an important nutrient component. Studies have found that Arthrospira carbohydrate is beneficial to animal internal organs (Kovač et al. 2013). Microalgae are rich sources of almost all important minerals and are therefore suitable to be used as animal feedstuffs for mineral supplementation (Christaki et al. 2011). The presence of copper, iodine, iron, potassium, zinc, and other elements in microalgae is abundant. At the same time, vitamins such as A, B1, B2, B6, B12, C, and E and nicotinic acid, anonicotin, biotin, and folic acid are also present in microalgae (Christaki et al. 2011; Priyadarshani and Rath 2012). However, long-term high-concentration microalgae in feed can lead to a decrease in the palatability of the feed and thus reduce the feed intake (Spolaore et al. 2006; Lamminen et al. 2017).
4.3 High-Value Products
A very interesting property of microalgae is their potential to produce a wide range of high-value products for various applications, such as pharmaceuticals, nutraceuticals, cosmeceuticals, and food additives. Microalgae could be an excellent source of carotenoids, such as astaxanthin (Aki et al. 2003), lutein (Fernández-Sevilla et al. 2010), phycocyanin, and phycoerythrin (Khajepour et al. 2015), or polyphenols, a source of mycosporine-like amino acids, or various secondary metabolites that have antioxidant, antibacterial, antitumor, or antidiabetic activities, improve immune system, etc. (Chew et al. 2017). Several of these high-value microalgal products are already commercialized, while there is a clear trend and high opportunities for new products to be placed in the market (Borowitzka 2013). However, these high-value products are very expensive to be produced, mainly due to their very low concentration in the microalgal biomass or due to the complex and costly extraction processes used. Therefore, the use of digestates for the production of high-value products is unlikely to lead to any significant improvement in the economics of the production of such commodities. However, the use of digestates could be an approach to support the sustainability of such production systems and especially when digestates could be derived by the leftover of microalgal biomass after the extraction of the target compounds. Such a scheme will be based on a closed-loop production system, where the nutrients contained in the biomass will be mineralized during AD and recycled back to the cultures for further biomass production (Prajapati et al. 2014).
4.4 Contamination Potentials
A great concern about using digestates for the production of microalgal feed or high-value products is the content of various hazardous pollutants, such as HMs, pathogens, and xenobiotics, which have the potential to contaminate the produced biomass, lowering its quality and rising safety issues upon its use and consumption. Contamination of microalgal biomass with hazardous pollutants can occur by cell surface sorption and/or intracellular accumulation. Surface sorption denotes the adhesion of the compounds in question onto the cell surface, while intracellular accumulation denotes the passive diffusion or active transportation of the compounds across cellular membranes into the interior of cells (Perales-Vela et al. 2006; Suresh Kumar et al. 2015; Basile et al. 2012). Microalgae display a very high sorption capacity for HMs (up to 100 mg g−1) and therefore have been considered as means for HMs removal from wastewater (Anastopoulos and Kyzas 2015; Suresh Kumar et al. 2015). However, this capability to take up high amounts of HMs could restrict the application potential of the production of microalgal commodities for human and animal consumption, since some HMs, such as Ni, As, Hg, and Cd, which are frequently contained in digestates, are highly toxic and their content limits on feed/food and products is strictly regulated worldwide.
It has been frequently reported that microalgae generate appropriate conditions, e.g., high pH and high concentration of dissolved oxygen, that result in a significant reduction (3–4 log) of the population of some indicator bacteria (Posadas et al. 2015; Heubeck et al. 2007; Schumacher et al. 2003; Al-Gheethi et al. 2017). However, the mechanisms underlying in the pathogen reduction during microalgal growth are still unclear, and there is lack of knowledge whether pathogens can be hosted by microalgal cells resulting in the contamination of the produced biomass. It is very probable that pathogens will be adsorbed in the microalgal cells because microalgal cell walls consist of several compounds, such as polysaccharides and proteins (e.g., adhesins), which have charged functional groups and favor the attachment of pathogens, such as viruses and bacteria (Verbyla and Mihelcic 2015; Marshall 1985).
Microalgal cultivation systems are able to remove xenobiotics by the following mechanisms: adsorption onto cell wall, cell uptake, volatilization, photodegradation and biological degradation, and transformation (Zhang et al. 2014; Wang et al. 2017). The extent of xenobiotics removal depends on the chemical compound and its physicochemical characteristics and the cultivation environmental conditions (light penetration, pH, retention time) (Zhang et al. 2014; Wang et al. 2017; Matamoros et al. 2015). Studies on the contamination of microalgal biomass with xenobiotics are scarce, but the available data show that there is a potential of biomass contamination with xenobiotics; for example Desmodesmus subspicatus was found to adsorb around 30% of the estrogens contained in the cultivation medium (Maes et al. 2014), while Phaeodactylum tricornutum displayed a sorption capacity for oxytetracycline of about 29 mg g−1 (Santaeufemia et al. 2016).
Given that contaminants have a variety of physicochemical characteristics (i.e., hydrophilic or hydrophobic properties, etc.), there is a high potential that using one of the available extraction methods (Cuellar-Bermudez et al. 2015; Günerken et al. 2015; Gerardo et al. 2014), they could be extracted as well along with the target compound. However, there is lack of related studies, and more research is needed to identify and develop methods for avoiding transferring contaminants along with the extracted target compounds.
5 Conclusions
Digestates contain all necessary macro- and micronutrients and can be utilized as cultivation medium (or supplement) for microalgal biomass production. However, some of the main physicochemical characteristics of the digestates, such as high content of inhibitory compounds, turbidity, and colored dissolved compounds might negatively influence microalgal growth, and therefore they need to be adjusted by using one or a combination of pretreating methods (e.g., dilution, solid/liquid separation, filtration, etc.) in order to render them appropriate for cell growth. So far, the majority of the published work regards laboratory-scale investigations, in which however it is demonstrated that microalgae can be successfully cultivated on media consisted from digestates. Low-value microalgal products, such as biofuels, seem not to be economical feasible yet, and therefore the best route of valorizing digestates with microalgae is the production of high-value products (feed, food supplemented, pigments, etc.). However, there are some concerns about the potential contamination of microalgal biomass with unwanted hazardous pollutants, such as heavy metals and pathogens originating in digestates. More research is needed toward this route in order to optimize the production of a safe and valuable microalgal biomass.
References
Abeliovich A, Azov Y (1976) Toxicity of ammonia to algae in seawage oxidation ponds. Appl Environ Microbiol 31:801–806
Akhiar A (2017) Characterization of liquid fraction of digestates after solid-liquid separation from anaerobic co-digestion plants. PhD Thesis, Université Montpellier, Montpellier
Akhiar A, Battimelli A, Torrijos M, Carrere H (2017) Comprehensive characterization of the liquid fraction of digestates from full-scale anaerobic co-digestion. Waste Manag 59:118–128. https://doi.org/10.1016/j.wasman.2016.11.005
Aki T, Hachida K, Yoshinaga M, Katai Y, Yamasaki T, Kawamoto S, Kakizono T, Maoka T, Shigeta S, Suzuki O (2003) Thraustochytrid as a potential source of carotenoids. J Am Oil Chem Soc 80(8):789
Al-Gheethi A, Mohamed R, Jais N, Efaq A, Halid AA, Wurochekke A, Amir-Hashim M (2017) Influence of pathogenic bacterial activity on growth of Scenedesmus sp. and removal of nutrients from public market wastewater. J Water Health 15:741. wh2017080
Anastopoulos I, Kyzas GZ (2015) Progress in batch biosorption of heavy metals onto algae. J Mol Liq 209:77–86. https://doi.org/10.1016/j.molliq.2015.05.023
Azov Y, Goldman JC (1982) Free ammonia inhibition of algal photosynthesis in intensive cultures. Appl Environ Microbiol 43(4):735–739
Bachmann S, Uptmoor R, Eichler-Löbermann B (2016) Phosphorus distribution and availability in untreated and mechanically separated biogas digestates. Sci Agric 73:9–17
Bagge E, Persson M, Johansson KE (2010) Diversity of spore-forming bacteria in cattle manure, slaughterhouse waste and samples from biogas plants. J Appl Microbiol 109(5):1549–1565
Bártíková H, Podlipná R, Skálová L (2016) Veterinary drugs in the environment and their toxicity to plants. Chemosphere 144:2290–2301
Basile A, Sorbo S, Conte B, Cobianchi RC, Trinchella F, Capasso C, Carginale V (2012) Toxicity, accumulation, and removal of heavy metals by three aquatic macrophytes. Int J Phytoremediation 14(4):374–387
Becker EW (2007) Micro-algae as a source of protein. Biotechnol Adv 25(2):207–210. https://doi.org/10.1016/j.biotechadv.2006.11.002
Beuckels A, Smolders E, Muylaert K (2015) Nitrogen availability influences phosphorus removal in microalgae-based wastewater treatment. Water Res 77 (0):98–106. https://doi.org/10.1016/j.watres.2015.03.018
Bicudo J, Goyal S (2003) Pathogens and manure management systems: a review. Environ Technol 24(1):115–130
Biller P, Ross AB, Skill S, Lea-Langton A, Balasundaram B, Hall C, Riley R, Llewellyn C (2012) Nutrient recycling of aqueous phase for microalgae cultivation from the hydrothermal liquefaction process. Algal Res 1(1):70–76
Bjornsson WJ, Nicol RW, Dickinson KE, McGinn PJ (2013) Anaerobic digestates are useful nutrient sources for microalgae cultivation: functional coupling of energy and biomass production. J Appl Phycol 25(5):1523–1528. https://doi.org/10.1007/s10811-012-9968-0
Borowitzka MA (2013) High-value products from microalgae-their development and commercialisation. J Appl Phycol 25(3):743–756. https://doi.org/10.1007/s10811-013-9983-9
Brulé M, Bolduan R, Seidelt S, Schlagermann P, Bott A (2013) Modified batch anaerobic digestion assay for testing efficiencies of trace metal additives to enhance methane production of energy crops. Environ Technol 34(13):1–12
Camiro-Vargas TK, Hernández-Ayón JM, Valenzuela-Espinoza E, Delgadillo-Hinojosa F, Cajal-Medrano R (2005) Dissolved inorganic carbon uptake by Rhodomonas sp. and Isochrysis aff. galbana determined by a potentiometric technique. Aquac Eng 33(2):83–95. https://doi.org/10.1016/j.aquaeng.2004.10.001
Certik M, Shimizu S (1999) Biosynthesis and regulation of microbial polyunsaturated fatty acid production. J Biosci Bioeng 87(1):1–14
Chen F, Johns MR (1995) A strategy for high cell density culture of heterotrophic microalgae with inhibitory substrates. J Appl Phycol 7(1):43–46. https://doi.org/10.1007/BF00003548
Chen F, Johns MR (1996a) Heterotrophic growth of Chlamydomonas reinhardtii on acetate in chemostat culture. Process Biochem 31(6):601–604. https://doi.org/10.1016/S0032-9592(96)00006-4
Chen F, Johns MR (1996b) Relationship between substrate inhibition and maintenance energy ofChlamydomonas reinhardtii in heterotrophic culture. J Appl Phycol 8(1):15–19. https://doi.org/10.1007/BF02186216
Chew KW, Yap JY, Show PL, Suan NH, Juan JC, Ling TC, Lee D-J, Chang J-S (2017) Microalgae biorefinery: high value products perspectives. Bioresour Technol 229:53–62
Chiranjeevi P, Venkata Mohan S (2017) Diverse acidogenic effluents as feedstock for microalgae cultivation: dual phase metabolic transition on biomass growth and lipid synthesis. Bioresour Technol 242(Supplement C):191–196. https://doi.org/10.1016/j.biortech.2017.04.059
Chisti Y (2007) Biodiesel from microalgae. Biotechnol Adv 25(3):294–306. https://doi.org/10.1016/j.biotechadv.2007.02.001
Chojnacka K, Marquez-Rocha FJ (2004) Kinetic and stoichiometric relationships of the energy and carbon metabolism in the culture of microalgae. Biotechnology 3:21–34
Christaki E, Florou-Paneri P, Bonos E (2011) Microalgae: a novel ingredient in nutrition. Int J Food Sci Nutr 62(8):794–799
Cuellar-Bermudez SP, Aleman-Nava GS, Chandra R, Garcia-Perez JS, Contreras-Angulo JR, Markou G, Muylaert K, Rittmann BE, Parra-Saldivar R (2017) Nutrients utilization and contaminants removal. A review of two approaches of algae and cyanobacteria in wastewater. Algal Res 24:438–449. https://doi.org/10.1016/j.algal.2016.08.018
Cuellar-Bermudez SP, Aguilar-Hernandez I, Cardenas-Chavez DL, Ornelas-Soto N, Romero-Ogawa MA, Parra-Saldivar R (2015) Extraction and purification of high-value metabolites from microalgae: essential lipids, astaxanthin and phycobiliproteins. Microb Biotechnol 8(2):190–209
Curtis T, Mara DD, Dixo N, Silva SA (1994) Light penetration in waste stabilization ponds. Water Res 28(5):1031–1038
de Farias Silva CE, Bertucco A (2016) Bioethanol from microalgae and cyanobacteria: a review and technological outlook. Process Biochem 51(11):1833–1842. https://doi.org/10.1016/j.procbio.2016.02.016
Delrue F, Imbert Y, Fleury G, Peltier G, Sassi J-F (2015) Using coagulation–flocculation to harvest Chlamydomonas reinhardtii: coagulant and flocculant efficiencies, and reuse of the liquid phase as growth medium. Algal Res 9(Supplement C):283–290. https://doi.org/10.1016/j.algal.2015.04.004
Deng X-Y, Gao K, Addy M, Li D, Zhang R-C, Lu Q, Ma Y-W, Cheng Y-L, Chen P, Liu Y-H, Ruan R (2018) Cultivation of Chlorella vulgaris on anaerobically digested swine manure with daily recycling of the post-harvest culture broth. Bioresour Technol 247(Supplement C):716–723. https://doi.org/10.1016/j.biortech.2017.09.171
Depraetere O, Foubert I, Muylaert K (2013) Decolorisation of piggery wastewater to stimulate the production of Arthrospira platensis. Bioresour Technol 148:366–372. https://doi.org/10.1016/j.biortech.2013.08.165
Deublein D, Steinhauser A (2008) Biogas from waste and renewable resources: an introduction. Willey-VCH, Weinheim
Díaz M, Pérez C, Sánchez C, Lauzurica S, Cañeque V, González C, De La Fuente J (2017) Feeding microalgae increases omega 3 fatty acids of fat deposits and muscles in light lambs. J Food Compos Anal 56:115–123
Dickinson KE, Bjornsson WJ, Garrison LL, Whitney CG, Park KC, Banskota AH, McGinn PJ (2015) Simultaneous remediation of nutrients from liquid anaerobic digestate and municipal wastewater by the microalga Scenedesmus sp. AMDD grown in continuous chemostats. J Appl Microbiol 118(1):75–83
Drath M, Kloft N, Batschauer A, Marin K, Novak J, Forchhammer K (2008) Ammonia triggers photodamage of photosystem II in the cyanobacterium Synechocystis sp. strain PCC 6803. Plant Physiol 147(1):206–215
Drosg B, Fuchs W, Al Seadi T, Madsen M, Linke B (2015) Nutrient recovery by biogas digestate processing. IEA Bioenergy, pp 7–11
Dyhrman ST, Ruttenberg KC (2006) Presence and regulation of alkaline phosphatase activity in eukaryotic phytoplankton from the coastal ocean: implications for dissolved organic phosphorus remineralization. Limnol Oceanogr 51(3):1381–1390
Eich-Greatorex S, Vivekanand V, Estevez MM, Schnürer A, Børresen T, Sogn TA (2018) Biogas digestates based on lignin-rich feedstock – potential as fertilizer and soil amendment. Arch Agron Soil Sci 64(3):347–359. https://doi.org/10.1080/03650340.2017.1352086
Eriksen NT (2008) The technology of microalgal culturing. Biotechnol Lett 30(9):1525–1536
Farno E, Baudez JC, Parthasarathy R, Eshtiaghi N (2014) Rheological characterisation of thermally-treated anaerobic digested sludge: impact of temperature and thermal history. Water Res 56:156–161. https://doi.org/10.1016/j.watres.2014.02.048
Farooq W, Moon M, Ryu B-G, Suh WI, Shrivastav A, Park MS, Mishra SK, Yang J-W (2015) Effect of harvesting methods on the reusability of water for cultivation of Chlorella vulgaris, its lipid productivity and biodiesel quality. Algal Res 8(Supplement C):1–7. https://doi.org/10.1016/j.algal.2014.12.007
Fernandez E, Galvan A (2007) Inorganic nitrogen assimilation in Chlamydomonas. J Exp Bot 58(9):2279–2287. https://doi.org/10.1093/jxb/erm106
Fernández-Sevilla J, Acién Fernández F, Molina Grima E (2010) Biotechnological production of lutein and its applications. Appl Microbiol Biotechnol 86(1):27–40
Flores E, Herrero A (2005) Nitrogen assimilation and nitrogen control in cyanobacteria. Biochem Soc Trans 33(1):164–167
Franchino M, Comino E, Bona F, Riggio VA (2013) Growth of three microalgae strains and nutrient removal from an agro-zootechnical digestate. Chemosphere 92(6):738–744
Fu F, Wang Q (2011) Removal of heavy metal ions from wastewaters: a review. J Environ Manag 92(3):407–418. https://doi.org/10.1016/j.jenvman.2010.11.011
Günerken E, D’Hondt E, Eppink MHM, Garcia-Gonzalez L, Elst K, Wijffels RH (2015) Cell disruption for microalgae biorefineries. Biotechnol Adv 33(2):243–260. https://doi.org/10.1016/j.biotechadv.2015.01.008
Georgacakis D, Sievers DM, Iannotti EL (1982) Buffer stability in manure digesters. Agric Wastes 4(6):427–441. https://doi.org/10.1016/0141-4607(82)90038-5
Gerardi MH (2003) The microbiology of anaerobic digesters, Wastwater microbiology series. Willey, Hoboken, New Jersey
Gerardo ML, Oatley-Radcliffe DL, Lovitt RW (2014) Integration of membrane technology in microalgae biorefineries. J Membr Sci 464:86–99
González-Fernández C, Molinuevo-Salces B, García-González MC (2011) Nitrogen transformations under different conditions in open ponds by means of microalgae–bacteria consortium treating pig slurry. Bioresour Technol 102(2):960–966
Gonzalez-Fernandez C, Sialve B, Molinuevo-Salces B (2015) Anaerobic digestion of microalgal biomass: challenges, opportunities and research needs. Bioresour Technol 198:896–906
González-López CV, Cerón-García MC, Fernández-Sevilla JM, González-Céspedes AM, Camacho-Rodríguez J, Molina-Grima E (2013) Medium recycling for Nannochloropsis gaditana cultures for aquaculture. Bioresour Technol 129(Supplement C):430–438. https://doi.org/10.1016/j.biortech.2012.11.061
Grobbelaar JU (2000) Physiological and technological considerations for optimising mass algal cultures. J Appl Phycol 12(3–5):201–206
Guo Y, Yeh T, Song W, Xu D, Wang S (2015) A review of bio-oil production from hydrothermal liquefaction of algae. Renew Sust Energ Rev 48:776–790
Gupta RK, Gangoliya SS, Singh NK (2015) Reduction of phytic acid and enhancement of bioavailable micronutrients in food grains. J Food Sci Technol 52(2):676–684
Gupta VK, Ali I, Saleh TA, Nayak A, Agarwal S (2012) Chemical treatment technologies for waste-water recycling—an overview. RSC Adv 2(16):6380–6388
Hansen KH, Angelidaki I, Ahring BK (1998) Anaerobic digestion of swine manure: inhibition by ammonia. Water Res 32(1):5–12. https://doi.org/10.1016/s0043-1354(97)00201-7
Hartley AM, House WA, Callow ME, Leadbeater BSC (1997) Coprecipitation of phosphate with calcite in the presence of photosynthesizing green algae. Water Res 31(9):2261–2268. https://doi.org/10.1016/S0043-1354(97)00103-6
Heubeck S, Craggs R, Shilton A (2007) Influence of CO2 scrubbing from biogas on the treatment performance of a high rate algal pond. Water Sci Technol 55(11):193–200
Hjorth M, Christensen KV, Christensen ML, Sommer SG (2010) Solid–liquid separation of animal slurry in theory and practice. A review. Agron Sustain Dev 30(1):153–180
Hoffmann JP (1998) Wastewater treatment with suspended and nonsuspended algae. J Phycol 34(5):757–763. https://doi.org/10.1046/j.1529-8817.1998.340757.x
Holman B, Kashani A, Malau-Aduli A (2012) Growth and body conformation responses of genetically divergent Australian sheep to Spirulina (Arthrospira platensis) supplementation. Am J Exp Agric 2:160–173
Hua-sheng H, Hai-li W, Bang-qin H (1995) The availability of dissolved organic phosphorus compounds to marine phytoplankton. Chin J Oceanol Limnol 13(2):169–176. https://doi.org/10.1007/bf02846823
Huang B, Hong H (1999) Alkaline phosphatase activity and utilization of dissolved organic phosphorus by algae in subtropical coastal waters. Mar Pollut Bull 39(1–12):205–211. https://doi.org/10.1016/S0025-326X(99)00006-5
Jorquera M, Martínez O, Maruyama F, Marschner P, de la Luz Mora M (2008) Current and future biotechnological applications of bacterial Phytases and Phytase-producing bacteria. Microbes Environ 23(3):182–191. https://doi.org/10.1264/jsme2.23.182
Kamalanathan M, Gleadow R, Beardall J (2015) Impacts of phosphorus availability on lipid production by Chlamydomonas reinhardtii. Algal Res 12:191–196. https://doi.org/10.1016/j.algal.2015.08.021
Kao C-Y, Chiu S-Y, Huang T-T, Dai L, Hsu L-K, Lin C-S (2012) Ability of a mutant strain of the microalga chlorella sp. to capture carbon dioxide for biogas upgrading. Appl Energy 93:176–183
Khajepour F, Hosseini SA, Ghorbani Nasrabadi R, Markou G (2015) Effect of light intensity and photoperiod on growth and biochemical composition of a local isolate of Nostoc calcicola. Appl Biochem Biotechnol 176(8):2279–2289. https://doi.org/10.1007/s12010-015-1717-9
Khan S, Roser D, Davies C, Peters G, Stuetz R, Tucker R, Ashbolt N (2008) Chemical contaminants in feedlot wastes: concentrations, effects and attenuation. Environ Int 34(6):839–859
Kim B-H, Kang Z, Ramanan R, Choi J-E, Cho D-H, Oh H-M, Kim H-S (2014a) Nutrient removal and biofuel production in high rate algal pond using real municipal wastewater. J Microbiol Biotechnol 24(8):1123–1132
Kim H-C, Choi WJ, Maeng SK, Kim HJ, Kim HS, Song KG (2014b) Ozonation of piggery wastewater for enhanced removal of contaminants by S. Quadricauda and the impact on organic characteristics. Bioresour Technol 159(Supplement C):128–135. https://doi.org/10.1016/j.biortech.2014.02.061
Kleinman PJ, Wolf AM, Sharpley AN, Beegle DB, Saporito LS (2005) Survey of water-extractable phosphorus in livestock manures. Soil Sci Soc Am J 69(3):701–708
Kovač DJ, Simeunović JB, Babić OB, Mišan AČ, Milovanović IL (2013) Algae in food and feed. Food Feed Res 40(1):21–31
Kulpys J, Paulauskas E, Pilipavicius V, Stankevicius R (2009) Influence of cyanobacteria Arthrospira (Spirulina) platensis biomass additive towards the body condition of lactation cows and biochemical milk indexes. Agron Res 7:823–835
Lam MK, Lee KT (2012) Microalgae biofuels: a critical review of issues, problems and the way forward. Biotechnol Adv 30(3):673–690. https://doi.org/10.1016/j.biotechadv.2011.11.008
Lamminen M, Halmemies-Beauchet-Filleau A, Kokkonen T, Simpura I, Jaakkola S, Vanhatalo A (2017) Comparison of microalgae and rapeseed meal as supplementary protein in the grass silage based nutrition of dairy cows. Anim Feed Sci Technol 234:295–311
Li B, Brett MT (2013) The influence of dissolved phosphorus molecular form on recalcitrance and bioavailability. Environ Pollut 182 (0):37–44. https://doi.org/10.1016/j.envpol.2013.06.024
Lin H, Gan J, Rajendran A, Reis CER, Hu B (2015) Phosphorus removal and recovery from Digestate after biogas production, Ch. 24. In: Biernat K (ed) Biofuels - status and perspective. InTech, Rijeka. https://doi.org/10.5772/60474
Lora Grando R, de Souza Antune AM, da Fonseca FV, Sánchez A, Barrena R, Font X (2017) Technology overview of biogas production in anaerobic digestion plants: a European evaluation of research and development. Renew Sust Energ Rev 80(Supplement C):44–53. https://doi.org/10.1016/j.rser.2017.05.079
Lukehurst C, Frost P, Al Seadi T (2010) Utilisation of digestate from biogas plants as biofertiliser. IEA Bioenergy, Hoboken, New Jersey
Macías-Sánchez MD, Robles-Medina A, Hita-Peña E, Jiménez-Callejón MJ, Estéban-Cerdán L, González-Moreno PA, Molina-Grima E (2015) Biodiesel production from wet microalgal biomass by direct transesterification. Fuel 150:14–20. https://doi.org/10.1016/j.fuel.2015.01.106
Maes HM, Maletz SX, Ratte HT, Hollender J, Schaeffer A (2014) Uptake, elimination, and biotransformation of 17α-ethinylestradiol by the freshwater alga Desmodesmus subspicatus. Environ Sci Technol 48(20):12354–12361
Marazzi F, Sambusiti C, Monlau F, Cecere SE, Scaglione D, Barakat A, Mezzanotte V, Ficara E (2017) A novel option for reducing the optical density of liquid digestate to achieve a more productive microalgal culturing. Algal Res 24(Part A):19–28. https://doi.org/10.1016/j.algal.2017.03.014
Marcilhac C, Sialve B, Pourcher A-M, Ziebal C, Bernet N, Béline F (2014) Digestate color and light intensity affect nutrient removal and competition phenomena in a microalgal-bacterial ecosystem. Water Res 64:278–287
Markou G (2015) Fed-batch cultivation of Arthrospira and Chlorella in ammonia-rich wastewater: optimization of nutrient removal and biomass production. Bioresour Technol 193 (0):35–41. https://doi.org/10.1016/j.biortech.2015.06.071
Markou G, Agriomallou M, Georgakakis D (2017) Forced ammonia stripping from livestock wastewater: the influence of some physico-chemical parameters of the wastewater. Water Sci Technol 75(3):686–692
Markou G, Depraetere O, Muylaert K (2016) Effect of ammonia on the photosynthetic activity of Arthrospira and Chlorella: a study on chlorophyll fluorescence and electron transport. Algal Res 16:449–457. https://doi.org/10.1016/j.algal.2016.03.039
Markou G, Depraetere O, Vandamme D, Muylaert K (2015) Cultivation of Chlorella vulgaris and Arthrospira platensis with recovered phosphorus from wastewater by means of zeolite sorption. Int J Mol Sci 16(2):4250–4264. https://doi.org/10.3390/ijms16024250
Markou G, Muylaert K (2016) Effect of light intensity on the degree of ammonia toxicity on PSII activity of Arthrospira platensis and Chlorella vulgaris. Bioresour Technol 216:453–461. https://doi.org/10.1016/j.biortech.2016.05.094
Markou G, Vandamme D, Muylaert K (2014a) Ammonia inhibition on Arthrospira platensis in relation to the initial biomass density and pH. Bioresour Technol 166:259–265. https://doi.org/10.1016/j.biortech.2014.05.040
Markou G, Vandamme D, Muylaert K (2014b) Using natural zeolite for ammonia sorption from wastewater and as nitrogen releaser for the cultivation of Arthrospira platensis. Bioresour Technol 155 (0):373–378. https://doi.org/10.1016/j.biortech.2013.12.122
Marshall JS, Huang Y (2010) Simulation of light-limited algae growth in homogeneous turbulence. Chem Eng Sci 65(12):3865–3875. https://doi.org/10.1016/j.ces.2010.03.036
Marshall KC (1985) Mechanisms of bacterial adhesion at solid-water interfaces. In: Savage DC, Fletcher M (eds) Bacterial adhesion: mechanisms and physiological significance. Springer US, Boston, pp 133–161. https://doi.org/10.1007/978-1-4615-6514-7_6
Marti N, Bouzas A, Seco A, Ferrer J (2008) Struvite precipitation assessment in anaerobic digestion processes. Chem Eng J 141(1):67–74. https://doi.org/10.1016/j.cej.2007.10.023
Mata-Alvarez J, Dosta J, Romero-Güiza MS, Fonoll X, Peces M, Astals S (2014) A critical review on anaerobic co-digestion achievements between 2010 and 2013. Renew Sust Energ Rev 36(Supplement C):412–427. https://doi.org/10.1016/j.rser.2014.04.039
Matamoros V, Gutiérrez R, Ferrer I, García J, Bayona JM (2015) Capability of microalgae-based wastewater treatment systems to remove emerging organic contaminants: a pilot-scale study. J Hazard Mater 288:34–42
Medina M, Neis U (2007) Symbiotic algal bacterial wastewater treatment: effect of food to microorganism ratio and hydraulic retention time on the process performance. Water Sci Technol 55(11):165–171
Milledge JJ (2011) Commercial application of microalgae other than as biofuels: a brief review. Rev Environ Sci Biotechnol 10(1):31–41
Möller K, Müller T (2012) Effects of anaerobic digestion on digestate nutrient availability and crop growth: a review. Eng Life Sci 12(3):242–257
Monlau F, Sambusiti C, Ficara E, Aboulkas A, Barakat A, Carrere H (2015) New opportunities for agricultural digestate valorization: current situation and perspectives. Energy Environ Sci 8(9):2600–2621
Mudryk K, Frączek J, Jewiarz M, Wróbel M, Dziedzic K (2016) Analysis of mechanical dewatering of Digestate. Agric Eng 20. https://doi.org/10.1515/agriceng-2016-0073
Munoz R, Guieysse B (2006) Algal-bacterial processes for the treatment of hazardous contaminants: a review. Water Res 40(15):2799–2815. https://doi.org/10.1016/j.watres.2006.06.011
Myklestad SM (1995) Release of extracellular products by phytoplankton with special emphasis on polysaccharides. Sci Total Environ 165(1):155–164
Napan K, Teng L, Quinn JC, Wood BD (2015) Impact of heavy metals from flue gas integration with microalgae production. Algal Res 8:83–88. https://doi.org/10.1016/j.algal.2015.01.003
Neilson AH, Larsson T (1980) The utilization of organic nitrogen for growth of algae: physiological aspects. Physiol Plant 48(4):542–553. https://doi.org/10.1111/j.1399-3054.1980.tb03302.x
Olguín EJ (2012) Dual purpose microalgae–bacteria-based systems that treat wastewater and produce biodiesel and chemical products within a Biorefinery. Biotechnol Adv 30(5):1031–1046. https://doi.org/10.1016/j.biotechadv.2012.05.001
Oliveira I, Reed J, Abu-Orf M, Wilson V, Jones D, Esteves S (2015) Impact of digestate storage conditions and rheological properties: preliminary investigations on conditioning and dewatering. Paper presented at the IWA Specialist conference on sludge management: SludgeTech 2015, United Kingdom, 29/06/15
Pancha I, Chokshi K, George B, Ghosh T, Paliwal C, Maurya R, Mishra S (2014) Nitrogen stress triggered biochemical and morphological changes in the microalgae Scenedesmus sp. CCNM 1077. Bioresour Technol 156:146–154. https://doi.org/10.1016/j.biortech.2014.01.025
Park J, Jin H-F, Lim B-R, Park K-Y, Lee K (2010) Ammonia removal from anaerobic digestion effluent of livestock waste using green alga Scenedesmus sp. Bioresour Technol 101(22):8649–8657. https://doi.org/10.1016/j.biortech.2010.06.142
Pastor L, Mangin D, Ferrer J, Seco A (2010) Struvite formation from the supernatants of an anaerobic digestion pilot plant. Bioresour Technol 101(1):118–125. https://doi.org/10.1016/j.biortech.2009.08.002
Pei H, Jiang L, Hou Q, Yu Z (2017) Toward facilitating microalgae cope with effluent from anaerobic digestion of kitchen waste: the art of agricultural phytohormones. Biotechnol Biofuels 10(1):76
Perales-Vela HV, Peña-Castro JM, Canizares-Villanueva RO (2006) Heavy metal detoxification in eukaryotic microalgae. Chemosphere 64(1):1–10
Perez-Garcia O, Escalante FME, de Bashan LE, Bashan Y (2011) Heterotrophic cultures of microalgae: metabolism and potential products. Water Res 45(1):11–36. https://doi.org/10.1016/j.watres.2010.08.037
Posadas E, del Mar MM, Gomez C, Acién FG, Muñoz R (2015) Influence of pH and CO 2 source on the performance of microalgae-based secondary domestic wastewater treatment in outdoors pilot raceways. Chem Eng J 265:239–248
Prajapati SK, Kumar P, Malik A, Vijay VK (2014) Bioconversion of algae to methane and subsequent utilization of digestate for algae cultivation: a closed loop bioenergy generation process. Bioresour Technol 158:174–180
Priyadarshani I, Rath B (2012) Commercial and industrial applications of micro algae–a review. J Algal Biomass Utln 3(4):89–100
Qi G, Pan Z, Andriamanohiarisoamanana FJ, Yamashiro T, Iwasaki M, Kawamoto K, Umetsu K (2017) Isolation and characterization of plant growth promoting bacteria (PGPB) from anaerobic digestate and their effect on common wheat (Triticum aestivum) seedling growth. Int J Environ Agric Res 3(11):46–52
Rajagopal R, Massé DI, Singh G (2013) A critical review on inhibition of anaerobic digestion process by excess ammonia. Bioresour Technol 143:632–641
Ray P, Zhao Z, Knowlton K (2013) Emerging contaminants in livestock manure: hormones, antibiotics and antibiotic resistance genes. Sustainable Animal Agriculture, Wallingford, pp 268–283
Rincón B, Borja R, González JM, Portillo MC, Sáiz-Jiménez C (2008) Influence of organic loading rate and hydraulic retention time on the performance, stability and microbial communities of one-stage anaerobic digestion of two-phase olive mill solid residue. Biochem Eng J 40(2):253–261. https://doi.org/10.1016/j.bej.2007.12.019
Risberg K, Cederlund H, Pell M, Arthurson V, Schnürer A (2017) Comparative characterization of digestate versus pig slurry and cow manure – chemical composition and effects on soil microbial activity. Waste Manag 61:529–538. https://doi.org/10.1016/j.wasman.2016.12.016
Sahlström L (2003) A review of survival of pathogenic bacteria in organic waste used in biogas plants. Bioresour Technol 87(2):161–166
Salafudin, Setyobudi RH, Wahono SK, Nindita A, Adinurani PG, Nugroho YA, Sasmito A, Liwang T (2015) Biological purification system: integrated biogas from small anaerobic digestion and natural microalgae. Procedia Chem 14:387–393. https://doi.org/10.1016/j.proche.2015.03.069
Salama E-S, Kabra AN, Ji M-K, Kim JR, Min B, Jeon B-H (2014) Enhancement of microalgae growth and fatty acid content under the influence of phytohormones. Bioresour Technol 172:97–103
Salama E-S, Kurade MB, Abou-Shanab RA, El-Dalatony MM, Yang I-S, Min B, Jeon B-H (2017) Recent progress in microalgal biomass production coupled with wastewater treatment for biofuel generation. Renew Sust Energ Rev 79:1189–1211
Santaeufemia S, Torres E, Mera R, Abalde J (2016) Bioremediation of oxytetracycline in seawater by living and dead biomass of the microalga Phaeodactylum tricornutum. J Hazard Mater 320:315–325
Schnürer A, Schnürer J (2006) Fungal survival during anaerobic digestion of organic household waste. Waste Manag 26(11):1205–1211
Schumacher G, Blume T, Sekoulov I (2003) Bacteria reduction and nutrient removal in small wastewater treatment plants by an algal biofilm. Water Sci Technol 47(11):195–202
Spolaore P, Joannis-Cassan C, Duran E, Isambert A (2006) Commercial applications of microalgae. J Biosci Bioeng 101(2):87–96. https://doi.org/10.1263/jbb.101.87
Stasinakis AS (2012) Review on the fate of emerging contaminants during sludge anaerobic digestion. Bioresour Technol 121:432–440
Suresh Kumar K, Dahms H-U, Won E-J, Lee J-S, Shin K-H (2015) Microalgae – a promising tool for heavy metal remediation. Ecotoxicol Environ Saf 113:329–352. https://doi.org/10.1016/j.ecoenv.2014.12.019
Švec P, Kováčik J, Hedbavný J, Babula P, Rotková G, Klejdus B (2016) Impact of anions, cations, and pH on manganese accumulation and toxicity in the green alga Scenedesmus quadricauda. Water Air Soil Pollut 227(5):161
Taher H, Al-Zuhair S, Al-Marzouqi AH, Haik Y, Farid M (2014) Effective extraction of microalgae lipids from wet biomass for biodiesel production. Biomass Bioenergy 66:159–167. j.biombioe.2014.02.034
Tambone F, Genevini P, D’Imporzano G, Adani F (2009) Assessing amendment properties of digestate by studying the organic matter composition and the degree of biological stability during the anaerobic digestion of the organic fraction of MSW. Bioresour Technol 100(12):3140–3142. https://doi.org/10.1016/j.biortech.2009.02.012
Tian C, Li B, Liu Z, Zhang Y, Lu H (2014) Hydrothermal liquefaction for algal biorefinery: a critical review. Renew Sust Energ Rev 38:933–950
Torres EM, Hess D, McNeil BT, Guy T, Quinn JC (2017) Impact of inorganic contaminants on microalgae productivity and bioremediation potential. Ecotoxicol Environ Saf 139:367–376. https://doi.org/10.1016/j.ecoenv.2017.01.034
Tsiplakou E, Abdullah M, Mavrommatis A, Chatzikonstantinou M, Skliros D, Sotirakoglou K, Flemetakis E, Labrou N, Zervas G (2017) The effect of dietary Chlorella vulgaris inclusion on goat’s milk chemical composition, fatty acids profile and enzymes activities related to oxidation. J Anim Physiol Anim Nutr 102(1):142–151
Turon V, Baroukh C, Trably E, Latrille E, Fouilland E, Steyer J-P (2015) Use of fermentative metabolites for heterotrophic microalgae growth: yields and kinetics. Bioresour Technol 175:342–349
Uggetti E, Sialve B, Latrille E, Steyer J-P (2014) Anaerobic digestate as substrate for microalgae culture: the role of ammonium concentration on the microalgae productivity. Bioresour Technol 152:437–443. https://doi.org/10.1016/j.biortech.2013.11.036
Van Boeckel TP, Glennon EE, Chen D, Gilbert M, Robinson TP, Grenfell BT, Levin SA, Bonhoeffer S, Laxminarayan R (2017) Reducing antimicrobial use in food animals. Science 357(6358):1350–1352
Venkata Mohan S, Prathima Devi M (2012) Fatty acid rich effluent from acidogenic biohydrogen reactor as substrate for lipid accumulation in heterotrophic microalgae with simultaneous treatment. Bioresour Technol 123(Supplement C):627–635. https://doi.org/10.1016/j.biortech.2012.07.004
Verbyla ME, Mihelcic JR (2015) A review of virus removal in wastewater treatment pond systems. Water Res 71:107–124. https://doi.org/10.1016/j.watres.2014.12.031
Vílchez C, Vega JM (1994) Nitrite uptake by Chlamydomonas reinhardtii cells immobilized in calcium alginate. Appl Microbiol Biotechnol 41(1):137–141
Wang H, Xiong H, Hui Z, Zeng X (2012) Mixotrophic cultivation of Chlorella pyrenoidosa with diluted primary piggery wastewater to produce lipids. Bioresour Technol 104:215–220
Wang K, Yin J, Shen D, Li N (2014) Anaerobic digestion of food waste for volatile fatty acids (VFAs) production with different types of inoculum: effect of pH. Bioresour Technol 161(Supplement C):395–401. https://doi.org/10.1016/j.biortech.2014.03.088
Wang Y, Guo W, Yen H-W, Ho S-H, Lo Y-C, Cheng C-L, Ren N, Chang J-S (2015) Cultivation of Chlorella vulgaris JSC-6 with swine wastewater for simultaneous nutrient/COD removal and carbohydrate production. Bioresour Technol 198:619–625
Wang Y, Liu J, Kang D, Wu C, Wu Y (2017) Removal of pharmaceuticals and personal care products from wastewater using algae-based technologies: a review. Rev Environ Sci Biotechnol. https://doi.org/10.1007/s11157-017-9446-x
Weiland P (2010) Biogas production: current state and perspectives. Appl Microbiol Biotechnol 85(4):849–860
Whitton B (1991) Use of phosphatase assays with algae to assess phosphorus status of aquatic environments. Bioindicators and environmental management Academic Press, London, pp 295–310
Whitton R, Ometto F, Pidou M, Jarvis P, Villa R, Jefferson B (2015) Microalgae for municipal wastewater nutrient remediation: mechanisms, reactors and outlook for tertiary treatment. Environ Technolo Rev 4(1):133–148
Xia A, Murphy JD (2016) Microalgal cultivation in treating liquid digestate from biogas systems. Trends Biotechnol 34(4):264–275
Yang S, Xu J, Wang Z-M, Bao L-J, Zeng EY (2017) Cultivation of oleaginous microalgae for removal of nutrients and heavy metals from biogas digestates. J Clean Prod 164:793–803. https://doi.org/10.1016/j.jclepro.2017.06.221
Zhang D, Gersberg RM, Ng WJ, Tan SK (2014) Removal of pharmaceuticals and personal care products in aquatic plant-based systems: a review. Environ Pollut 184:620–639
Zhang F, Li Y, Yang M, Li W (2012) Content of heavy metals in animal feeds and manures from farms of different scales in Northeast China. Int J Environ Res Public Health 9(8):2658–2668. https://doi.org/10.3390/ijerph9082658
Zuliani L, Frison N, Jelic A, Fatone F, Bolzonella D, Ballottari M (2016) Microalgae cultivation on anaerobic Digestate of municipal wastewater, sewage sludge and agro-waste. Int J Mol Sci 17(10):1692. https://doi.org/10.3390/ijms17101692
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Markou, G., Wang, L., Ye, J., Unc, A. (2019). Cultivation of Microalgae on Anaerobically Digested Agro-industrial Wastes and By-Products. In: Gupta, S., Bux, F. (eds) Application of Microalgae in Wastewater Treatment. Springer, Cham. https://doi.org/10.1007/978-3-030-13909-4_7
Download citation
DOI: https://doi.org/10.1007/978-3-030-13909-4_7
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-13908-7
Online ISBN: 978-3-030-13909-4
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)