Abstract
As food wastage becomes an increasingly dire problem, research has been conducted on food waste management. Anaerobic digestion has been presented as an alternative to traditional methods of food waste disposal, as it is able to produce biogas as a renewable energy source. However, its byproduct, liquid food waste digestate, needs to be treated appropriately before disposal. Food waste digestate usually contains a high percentage of ammonia, phosphorus, and other organic compounds. Hence, microalgae cultivation in food waste digestate has been suggested as a treatment method, as various strains of microalgae are effective in removal of nitrogen, phosphorus, heavy metals, and toxins from wastewater, while using compounds present to synthesize valuable biomass. Thus, microalgae as a treatment for food waste digestate is promising. In this study, food waste was taken from a local anaerobic digester, filtered and used for the heterotrophic cultivation of three different strains of microalgae. Hetero-trophic cultivation was carried out as it does not require light and is easier to incorporate into biorefineries. From the three strains, Chlorella sorokiniana has been found to have the best growth rate, reaching a final dry cell weight of 0.144 g/L, and was thus used for subsequent experiments. C. sorokiniana was then cultivated in different glucose and food waste digestate concentrations to investigate microalgal performance and potential products. Results found that C. sorokiniana was effective in ammonia removal, with the highest removal at 250.8 ppm and had the highest protein and carbohydrate percentage contents at 47% and 57.1% respectively.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
- Microalgae
- Wastewater treatment
- Heterotrophic cultivation
- Protein production
- Carbohydrate production
- Ammonium ion removal
- Food waste digestate
1 Introduction
In 2021, about 17% of global food production was wasted, according to a report by the United Nations [1]. Food waste (FW) refers to materials intended for human consumption which are discarded, lost, degraded, or contaminated [2]. In America alone, avoidable FW exceeds 55,000,000 tonnes per year, producing greenhouse gas emissions of CO2equivalent to 2% of the nation’s total annual emission [3]. China generates around 90,000,000 tonnes of FW yearly [4], and locally in Singapore, 665,000 tonnes of FW was generated just in the year of 2020 itself [5]. With the increasing amount of FW generated globally, the need for proper FW treatment and disposal is on the rise.
FW, if disposed untreated, can cause various environmental problems of detrimental consequences. The traditional method of disposing of FW in landfills results in the release of methane into the atmosphere during organic decomposition, which is 80 times more powerful than CO2 as a greenhouse gas [6]. Another conventional method of food waste management is incineration at Waste-To-Energy (WTE) plants. It makes use of burning of waste to generate electricity, causing airborne pollution [7]. WTE plants also do not fully utilize the nutrients still present in the FW such as nitrogen, potassium, and phosphorus [8]. Hence currently, new and innovative ways of FW treatment are being investigated, one of which is anaerobic digestion of FW, using bacterial breakdown [9]. Compared to traditional methods of FW disposal, anaerobic digestion is seen as a promising method of generating methane, which is captured as biofuel as a form of renewable energy. Food waste as a substrate for anaerobic digestion may also have the potential to provide a higher biogas yield compared to other forms of widely used substrate, such as manure and corn silage [10].
However, anaerobic digestion also produces a byproduct, a sludge digestate consisting of the end products of anaerobic digestion. FW digestate often contains macronutrients and micronutrients, and is generally rich in nitrogen, potassium and sodium [11]. Due to its high organic ion content, direct disposal of it without treatment can cause environmental pollution. The most common treatment method is using it as agricultural fertiliser [12], but liquid digestate fertilizer might cause run-off and eventual eutrophication of water systems [13]. Using liquid FW digestate for land-based agriculture also requires a large land size and its suitability depends on the type of crops and time of the year [14]. For countries which do not have large land spaces dedicated to agricultural use, using FW digestate as fertilizer may not be as effective. However, it is a highly viable medium for the cultivation of different strains of microalgae simultaneously with the treatment of digestate, a method which requires only a small land size [15].
Microalgae cultivation is a novel and promising way of utilizing FW digestate [16, 17]. Various strains of microalgae have been found to be effective in removing nitrogen, phosphorus and other compounds in waste, utilizing these compounds for growth and producing high yield of valuable products such as carbohydrates which can be converted into bioethanol, lipids which can be converted into biofuel, proteins as animal feed, and various vitamins [18]. Generally, the large-scale commercialisation of microalgae production has been hindered by the expensive biomass production procedure and product extraction steps [19]. However, using FW digestate can reduce the cost price of producing a viable medium for microalgae cultivation. As such, cultivation of microalgae using FW digestate could be beneficial, since high value products can be obtained with the concurrent removal of compounds in food waste.
Several studies have investigated the possibility of using FW digestate as a medium to cultivate microalgae, but most of them researched on the autotrophic or mixotrophic method of cultivation [15, 20, 21], which requires the presence of light. Despite various added benefits of heterotrophic cultivation method, it is rarely studied in FW digestate treatment. Studies which investigated the heterotrophic cultivation method [22, 23] only took into consideration one specific strain of microalgae. Some benefits of heterotrophic methods include its ability to be integrated into current biorefinery systems due to it occuring in the absence of light, as large-scale operations in fermenters are carried out in the dark. Heterotrophic cultivation also requires less manpower in terms of operation and daily maintenance, and has been reported to be able to enhance the algal biomass yield up to 25 fold compared to the autotrophic method [24]. Hence, heterotrophic cultivation was chosen in this study to investigate its suitability as a cultivation method for microalgae in FW digestate.
This study aims to investigate the suitability of heterotrophic cultivation of microalgae in FW digestate for nutrient removal in digestate and nutrient remediation by microalgae [15, 21, 25, 26]. Three different strains of freshwater microalgae strains were cultivated and their ability to grow heterotrophically in a medium of local FW digestate was compared. Chlorella sorokiniana was found to have the highest growth rate out of the three strains and used for the subsequent experiments, where mediums of different digestate and glucose concentrations were used to cultivate C. sorokiniana in order to investigate microalgal performance in both ammonium ion removal and biomass production. The hypothesis is that with an increasing percentage of FW digestate in the medium, the growth rate of microalgae will increase initially, but when the percentage concentration of digestate increases beyond the tolerable ammonium ion concentration of the microalgae, the cells will stop growing. With an increasing concentration of glucose, cell growth is also expected to increase, as carbon will no longer be a limiting factor. Biomass production and nutrient remediation is also expected to increase with increasing digestate and glucose, as the cell growth increases.
2 Materials and Methods
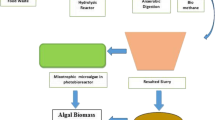
2.1 Filtration of Food Waste Digestate
Food waste digestate was collected from a local anaerobic digester. Digestate was then centrifuged at 4 ℃ and 10,000 rpm for 10 min, then the supernatant was recovered and vacuum filtered using 90, 45, and 25 mm filter papers in order to remove solids. A sample was taken from the resultant liquid food waste digestate to determine its cation and anion concentration by ionic chromatography. By composition, the digestate contains Na+ (1627 ppm), K+ (1604 ppm), Cl− (1709 ppm), \({\text{SO}}_4^{2 - }\) (184 ppm), PO4 (70 ppm), and \({\text{NH}}_4^+\) (2509 ppm) Subsequently, the FW digestate was refrigerated until the commencement of the experiment.
2.2 Pre-cultivation of Microalgal Strains
Three strains of freshwater microalgae Chlorella vulgaris (UTEX259), Chlorella sorokiniana (UTEX1230) and Scenedesmus obliquus (UTEX393) were purchased from the University of Texas, Austin. For inoculation, each of the 3 strains were pre-cultivated in Bold’s Basal Medium (BBM) prepared from 0.01 g/L (v/v) NaCl, NaNO3, CaCl2⋅2H2O, MgSO4⋅7H2O, K2HPO4, KH2PO4, and 0.01 g/L (v/v)
Na2EDTA⋅2H2O, FeSO4⋅7H2O, H3BO3, and trace metal solution. A 2.5 g/L glucose was added as an organic carbon source. The medium was inoculated using 40 ml cell culture flasks wrapped in aluminium foil, shaken at 115–120 rpm and maintained at 24–25 ℃ for the next 4 days.
2.3 Cultivation of Different Microalgal Strains in Filtered Food Waste Digestate
From the pre-cultivated strains, the optical density (OD) was measured to determine the cell concentration (see Sect. 2.5), and 0.05 g/L of cells were collected and added to 150 ml baffled flasks in duplicates. A 15% of digestate (v/v) was added to each flask. BBM used is similar to that in Sect. 2.2 but omits NaNO3 and NaCl as the ions in these solutions are already present in the digestate. The baffled flasks were wrapped in aluminium foil, shaken at 115–120 rpm and maintained at room temperature. Medium was inoculated for the next 8 days.
2.4 Cultivation of Chlorella sorokiniana in Different Concentrations of Glucose and Digestate
Chlorella sorokiniana cultivated in Sect. 2.3 had the highest growth rate out of the three strains, and was thus used for this experiment. Initial cultivation process is similar to that in Sect. 2.3, but for three flasks, filtered digestate was added in 2.5%, 5% and 10% (v/v) respectively with 2.5 g/L glucose. For the other three flasks, filtered digestate was added in 2.5%, 5%, and 10%, respectively, with 10 g/L glucose. The pH of the culture medium was kept at 7–7.5 through 1M NaOH addition. The medium was inoculated in the same conditions as in Sect. 2.3 for the next 8 days.
2.5 Analytical Methods
For all above experiments, 1ml of samples were taken from each flask everyday. Microalgal growth was determined by diluting samples with distilled water to an optical density (OD) below 0.8, and the OD was measured at wavelength of 540 nm (OD540).
Dry cell weight (DCW) was calculated using the correlations developed in previous studies.
pH of the medium was measured using a pH metre (Orion 4-Star, Thermo Scientific, USA).
At the end of the experiment in Sect. 2.4, C. sorokiniana was harvested through centrifugation, washed and freeze dried at − 80 ℃ for determination of biomass composition. The cation concentration in the medium was determined using ionic chromatography. Protein was extracted in duplicates from all the dried cell samples by the hot alkali method. The protein content was determined using the Lowry’s Method of Protein Analysis. Carbohydrate was extracted via acid hydrolysis, and the carbohydrate content was determined using the Calorimetric Dubois Method for determination of sugar and related substances.
3 Results and Discussions
3.1 Growth of Different Microalgal Strains in FW Digestate
Chlorella vulgaris, S. obliquus and C. sorokiniana were cultivated in 15% (v/v) FW digestate for 8 days. As presented in Fig. 1, C. sorokiniana reached the highest DCW concentration, with its DCW reaching 0.144 g/L. For C. sorokiniana, the DCW increased by almost 3 fold from its initial cell concentration on Day 0. Both C. vulgaris and S. obliquus had a peak in growth on Day 2, where their DCW reached 0.112 g/L and 0.0702 g/L. However, both the growth of C. vulgaris and S. obliquus experienced a decline in DCW from Day 2 to Day 7, with final DCW at 0.07546 g/L and 0.05406 g/L respectively. In the three strains of microalgae cultivated, only C. sorokiniana had an increasing trend of DCW all throughout the experiment duration, with C. vulgaris and S. obliquus both experiencing a decrease in DCW from Day 2 onwards. This might be due to the high ammonium ion content in the FW digestate, which might have exceeded the ammonia tolerance limit in C. vulgaris and S. obliquus [27], inhibiting further growth, as ammonia is toxic to microalgae in high concentrations. Cultivation with digestate also might not be the most optimal method of cultivation of those 2 strains [28, 29], although the ammonium ion concentration was suitable for C. sorokiniana [30]. This corroborates our results and thus, C. sorokiniana was determined to have the most optimal growth rate.
3.2 Growth of Chlorella sorokiniana in Different Glucose Concentrations
Since the growth rates of microalgae in 15% digestate and 2.5 g/L glucose were not significant, for subsequent investigations, this study varied the glucose and digestate concentration to investigate their effects on cell growth. Three percentages of digestate (v/v) 2.5%, 5%, and 10% were chosen. Two concentrations of glucose, 2.5 and 10g/L were used. Comparing Fig. 2 (left) and Fig. 2 (right), C. sorokiniana reached a generally higher DCW in 10 g/L glucose than 2.5 g/L glucose. In 2.5 g/L glucose concentration, culture with 2.5% FW digestate reached a final DCW of 0.961 g/L, but with 10 g/L glucose concentration, culture with 2.5% FW digestate reached a final DCW of 1.22 g/L. For cultures with 5% digestate, final DCW is 0.523 g/L in 2.5 g/L glucose concentration, compared to 2.00 g/L in 10 g/L glucose concentration. For cultures with 10% digestate, the final DCW is 1.24 g/L in 2.5 g/L glucose concentration and 3.05 g/L in 10 g/L glucose, with an almost 3 times difference between them.
Glucose was utilized for this experiment as it has been found to be amongst the organic carbon sources to support growth of microalgal strains under heterotrophic conditions [31, 32]. It is also easier to obtain than other organic carbon sources as it is produced on a large scale and yields a high level of substrate [33]. Increasing the glucose concentration of the culture increases the growth rate of C. sorokiniana significantly under heterotrophic conditions [34], as organic carbon will no longer be a limiting factor in glucose catabolic pathways, which are crucial for microalgal growth. This corroborates with the results obtained above.
3.3 Growth of Chlorella sorokiniana in Different Digestate Concentrations
Growth of C. sorokiniana in different FW digestate percentages was also compared. In Fig. 2 (left), it was observed that the culture with 10% digestate had a higher trend of DCW increase compared to the culture with 5% digestate and 2.5% digestate, reaching a DCW of 1.24 g/L at the end of 8 days. Culture with 2.5% digestate and 5% digestate reached a final DCW of 0.523 g/L and 0.961 g/L, respectively. In Fig. 2 (right), a similar trend is observed. Culture with 2.5% digestate reached 1.23 g/L of final DCW, culture with 5% digestate reached 2.00 g/L of final DCW, and culture with 10% digestate reached a final DCW of 3.05 g/L. An increase in the percentage of digestate is found to have generally increased the growth rate of C. sorokiniana, with culture of 10% digestate having the most DCW in both 2.5 and 10 g/L glucose environments. This is likely because the digestate consists of ammonium ion, the sole nitrogen-containing nutrient in the medium. Nitrogen is required by microalgae as a vital macronutrient which regulates the metabolism of microalgae [35], thus controlling its growth. With an optimum concentration of ammonium ion, cell growth can be enhanced.
3.4 Biomass Production and Nutrient Remediation of Chlorella sorokiniana in Different Glucose and Digestate Concentrations
Table 1 shows the comparison of protein and carbohydrate content in each of the cultures after the experiment, and the change in ammonium ion content in the FW digestate medium before and after the experiment for each cell culture. The carbohydrate content for the culture with 10% digestate and 2.5 g/L glucose is vastly different from other carbohydrate content. It is considered as an experimental error and not taken into account for subsequent data analysis. The protein and carbohydrate production of each flask is more clearly represented in Fig. 3 (left) and Fig. 3 (right).
Comparing different digestate concentrations, it was observed that as the digestate percentage supplied was increased, the protein content in the microalgal cells increases. The highest final protein content is found in cells cultivated in 10% digestate and 2.5 g/L glucose, of 47.3%. As the digestate percentage increases, the carbohydrate percentage decreases, with the highest carbohydrate percentage and the lowest carbohydrate percentage present in culture with 2.5% digestate, and culture with 5% digestate, respectively. The increase in protein and decrease in carbohydrate percentages may be due to the decreasing Carbon/Nitrogen (C/N) ratio as the digestate increases. It has been reported that microalgae utilize carbon in medium 25–30 times faster than nitrogen, hence a low C/N ratio may result in more protein being produced [36, 37].Studies have also observed that the highest rate of carbohydrate synthesis in microalgae was present in the culture cultivated under nutrient limitation [38], where the highest carbohydrate concentrations were also found in cultures with 2.5% of digestate, limiting the nitrogen content. For nutrient remediation, an increase in digestate increases the ammonium ion absorbed by microalgal cells, with the greatest change being 250.8 ppm of ammonium ion absorbed, which is comparable to other methods of nutrient remediation.
Comparing the cultures with the same digestate percentage but different glucose concentrations, an increase in glucose caused a decrease in protein percentage, with the biggest difference being 47.3 and 24.2%. However, an increase in glucose was observed to have increased the carbohydrate percentage with the highest found from the culture with 10 g/L glucose, at 57.1%. An increase in glucose concentration was not found to have a major impact on the ammonium ion taken in by the cells, although a difference of 32.8 ppm was observed at 10% digestate content. A higher glucose concentration increases the C/N ratio in the cultivation medium, and thus results in a lower protein percentage but a higher carbohydrate percentage. The high C/N ratio causes more carbon nutrient uptake, and this may induce the storage of carbohydrates and lipids in some microalgal species [39], thus decreasing protein concentration overall.
4 Conclusion
This study found that using FW digestate for microalgal cultivation holds potential in terms of ammonium ion removal and production of protein and carbohydrates. Depending on the method of utilization of microalgae after cultivation, different C/N ratios should be chosen to optimize protein or carbohydrate content. Growth was not found to be limited at high FW digestate percentages, suggesting that C. sorokiniana has not yet reached its ammonia tolerance capacity. However, this method requires more research before it can be applied large scale, as dilution of FW digestate is not cost effective [26]. Another cost factor of heterotrophic cultivation is that it requires an organic carbon source, as of now, the most commonly used is glucose. However, further research can be conducted to obtain carbon sources suitable for microalgae cultivation from other waste sources.
References
UNEP food waste index report 2021 [Internet]. UNEP—UN Environment Programme [cited 21 January 2022]. https://www.unep.org/resources/report/unep-food-waste-index-report-2021
Girotto, F., Alibardi, L., & Cossu, R. (2015). Food waste generation and industrial uses: A review. Waste Management, 45, 32–41.
Venkat, K. (2022). The climate change and economic impacts of food waste in the United States [Internet]. Centmapress.ilb.uni-bonn.de [cited 21 January 2022]. http://centmapress.ilb.uni-bonn.de/ojs/index.php/fsd/article/view/198
Zhang, C., Su, H., Baeyens, J., & Tan, T. (2014). Reviewing the anaerobic digestion of food waste for biogas production. Renewable and Sustainable Energy Reviews, 38, 383–392.
(2022). Commentary: Singapore’s festive indulgence creates enormous food waste [Internet]. CAN [cited 21 January 2022]. https://www.chan-nelnewsasia.com/commentary/food-waste-christmas-festive-indulgence-2397511
(2022). [Internet]. Environmental defence fund [cited 21 January 2022]. https://www.edf.org/climate/methane-crucial-opportunity-climate-fight
Bosello, F. Energy from waste
Kuppusamy, S., Venkateswarlu, K., & Megharaj, M. (2017). Evaluation of nineteen food wastes for essential and toxic elements. International Journal of Recycling of Organic Waste in Agriculture [Internet]. SpringerLink. Springer [cited 2022 Jan 21]. https://doi.org/10.1007/s40093-017-0178-2
Premo, B. (2022). Anaerobic digestion: A solution to diverting wasted food [Internet]. Center for EcoTechnology [cited 21 January 2022]. https://www.centerfore-cotechnology.org/anaerobic-digestion-a-solution-to-diverting-food-waste/#:~:text=Anaero-bic%20Digestion%20is%20a%20process,biogas%20composed%20mostly%20of%20me-thane
Curry, N., & Pillay, P. (2012). Biogas prediction and design of a food waste to energy system for the urban environment. Renewable Energy, 41, 200–209.
Logan, M., & Visvanathan, C. (2019). Management strategies for anaerobic digestate of organic fraction of municipal solid waste: Current status and future prospects. Waste Management & Research: The Journal for a Sustainable Circular Economy, 37(1_suppl), 27–39.
Sayedin, F., Kermanshahi-pour, A., He, Q., Tibbetts, S., Lalonde, C., & Brar, S. (2020). Microalgae cultivation in thin stillage anaerobic digestate for nutrient recovery and bioproduct production. Algal Research, 47, 101867.
Xia, A., & Murphy, J. (2016). Microalgal cultivation in treating liquid digestate from biogas systems. Trends in Biotechnology, 34(4), 264–275.
Praveen, P., Guo, Y., Kang, H., Lefebvre, C., & Loh, K. (2018). Enhancing microalgae cultivation in anaerobic digestate through nitrification. Chemical Engineering Journal, 354, 905–912.
Chuka-ogwude, D., Ogbonna, J., Borowitzka, M., & Moheimani, N. (2020). Screening, acclimation and ammonia tolerance of microalgae grown in food waste digestate. Journal of Applied Phycology, 32(6), 3775–3785.
Prajapati, S., Kumar, P., Malik, A., & Vijay, V. (2014). Bioconversion of algae to methane and subsequent utilization of digestate for algae cultivation: A closed loop bioenergy generation process. Bioresource Technology, 158, 174–180.
Bjornsson, W., Nicol, R., Dickinson, K., & McGinn, P. (2013). Anaerobic digestates are useful nutrient sources for microalgae cultivation: Functional coupling of energy and biomass production. Journal of Applied Phycology, 25(5), 1523–1528.
Dourou, M., Dritsas, P., Baeshen, M., Elazzazy, A., Al-Farga, A., & Aggelis, G. (2020). High-added value products from microalgae and prospects of aquaculture wastewaters as microalgae growth media. FEMS Microbiology Letters, 367(12).
Tan, J., Lee, S., Chew, K., Lam, M., Lim, J., Ho, S., et al. (2020). A review on microalgae cultivation and harvesting, and their biomass extraction processing using ionic liquids. Bioengineered, 11(1), 116–129.
Stiles, W., Styles, D., Chapman, S., Esteves, S., Bywater, A., Melville, L., et al. (2018). Using microalgae in the circular economy to valorise anaerobic digestate: Challenges and opportunities. Bio-resource Technology., 267, 732–742.
Koutra, E., Economou, C., Tsafrakidou, P., & Kornaros, M. (2018). Bio-based products from micro-algae cultivated in digestates. Trends in Biotechnology, 36(8), 819–833.
Sobhi, M., Guo, J., Cui, X., Sun, H., Li, B., Aboagye, D., et al. (2019). A promising strategy for nutrient recovery using heterotrophic indigenous microflora from liquid biogas digestate. Science of the Total Environment, 690, 492–501.
Pleissner, D., Lindner, A., & Händel, N. (2021). Heterotrophic cultivation of Galdieria sulphuraria under non-sterile conditions in digestate and hydrolyzed straw. Bioresource Technology., 337, 125477.
Morales-Sánchez, D., Martinez-Rodriguez, O., & Martinez, A. (2016). Heterotrophic cultivation of microalgae: Production of metabolites of commercial interest. Journal of Chemical Technology and Biotechnology, 92(5), 925–936.
Nwoba, E., Mickan, B., & Moheimani, N. (2019). Chlorella sp. growth under batch and fed-batch conditions with effluent recycling when treating the effluent of food waste anaerobic digestate. Journal of Applied Phycology, 31(6), 3545–3556.
Torres Franco, A., da Encarnação, A. S., Passos, F., de Lemos, C. C., Mota Filho, C., & Cunha, F. C. (2018). Treatment of food waste digestate using microalgae-based systems with low-intensity light-emitting diodes. Water Science and Technology., 78(1), 225–234.
Cai, T., Park, S., & Li, Y. (2013). Nutrient recovery from wastewater streams by microalgae: Status and prospects. Renewable and Sustainable Energy Reviews., 19, 360–369.
Safi, C., Zebib, B., Merah, O., Pontalier, P., & Vaca-Garcia, C. (2014). Morphology, composition, production, processing and applications of Chlorella vulgaris: A review. Renewable and Sustainable Energy Reviews, 35, 265–278.
Mandal, S., & Mallick, N. (2009). Microalga Scenedesmus obliquus as a potential source for bio-diesel production. Applied Microbiology and Biotechnology., 84(2), 281–291.
Kim, S., Park, J., Cho, Y., & Hwang, S. (2013). Growth rate, organic carbon and nutrient removal rates of Chlorella sorokiniana in autotrophic, heterotrophic and mixotrophic conditions. Bioresource Technology., 144, 8–13.
Theriault, R. (1965). Heterotrophic growth and production of Xanthophylls by Chlorella pyrenoidosa. Applied Microbiology., 13(3), 402–416.
Samejima, H., & Myers, J. (1958). On the heterotrophic growth of Chlorella pyrenoidosa. Journal of General Microbiology., 18(1), 107–117.
Cheirsilp, B., & Torpee, S. (2012). Enhanced growth and lipid production of microalgae under mixotrophic culture condition: Effect of light intensity, glucose concentration and fed-batch cultivation. Bioresource Technology., 110, 510–516.
Kim, H., Park, W., Lee, B., Seon, G., Suh, W., & Moon, M., et al. (2019). Optimization of heterotrophic cultivation of Chlorella sp. HS2 using screening, statistical assessment, and validation. Scientific Reports, 9(1).
Zarrinmehr, M., Farhadian, O., Heyrati, F., Keramat, J., Koutra, E., Kornaros, M., et al. (2020). Effect of nitrogen concentration on the growth rate and biochemical composition of the microalga, Isochrysis galbana. The Egyptian Journal of Aquatic Research., 46(2), 153–158.
Barik, D. (2019). Energy from toxic organic waste for heat and power generation. Woodhead Publishing.
(2004). Handbook of water and wastewater microbiology. Choice Reviews Online, 41(07):41-4037–41-4037.
Recht, L., Zarka, A., & Boussiba, S. (2012). Patterns of carbohydrate and fatty acid changes under nitrogen starvation in the microalgae Haematococcus pluvialis and Nannochloropsis sp. Applied Microbiology and Biotechnology., 94(6), 1495–1503.
Hannon, M., Gimpel, J., Tran, M., Rasala, B., & Mayfield, S. (2010). Biofuels from algae: Challenges and potential. Biofuels, 1(5), 763–784.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this paper
Cite this paper
Jiayue, Z., Parakh, S., Tong, Y. (2023). Heterotrophic Cultivation of Microalgae in Food Waste Digestate for Simultaneous Biomass Production and Nutrient Remediation. In: Guo, H., et al. IRC-SET 2022. Springer, Singapore. https://doi.org/10.1007/978-981-19-7222-5_40
Download citation
DOI: https://doi.org/10.1007/978-981-19-7222-5_40
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-19-7221-8
Online ISBN: 978-981-19-7222-5
eBook Packages: Physics and AstronomyPhysics and Astronomy (R0)