Abstract
Seeds of date palm trees are becoming one of the nature solutions for pollution as their interesting adsorption properties can compete with other conventional adsorbents such as activated carbons. In the Middle East, where massive amounts of palm pits are being disposed daily, researchers are interested in exploit these waste in beneficial applications such as water treatment from toxins. This chapter is reviewing the latest research that investigates the possible routes of extracting useful substances from palm dates including oil, cellulose and phenol. It also focuses on the chemical composition, surface morphology and microstructure of the treated palm seeds. Such remarkable properties reflect the outstanding behavior of the extracts during adsorption experiments. It is discussed here, how palm seeds can be effectively applied in heavy metal (Cu, Pb, Zn, Cr, Au, Br and Ni) removal from aqueous solution. Degradation of dyes (Rhodamine B (RhB), Methylene blue (MB), Congo red (CR) and Crystal violet (CV)) and other pollutants are also considered under different experimental conditions.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
11.1 Introduction
Clean water scarcity has been a global environmental issue due to the different and massive amounts of pollutants that residue daily as a product of industrial activities (Natarajan et al. 2018; Naushad et al. 2015a). Such pollutants include heavy metals (Kobielska et al. 2018; Shahat et al. 2015), dyes (Abbas and Ahmed 2016; Javadian et al. 2014), acids (Salman and Al-saad 2012) and also microbes (Li et al. 2018). Research studies have focused on the removal techniques of these un-degradable pollutants through several chemical and physical methods such as adsorption (Danish et al. 2012; Rajamohan et al. 2014), catalysis (Zhou et al. 2018) and filtration (Barnaby et al. 2017). Adsorption has been one of the effective routes to get rid of these pollutants and it depends mainly of the applied adsorbent which has to own certain chemical and physical properties (Ahsaine et al. 2018; Mittal et al. 2016) as well as being safe to environment (Banerjee et al. 2018). Activated carbon (Ac) (Farooq et al. 2017) nanoparticles (Khosravi et al. 2018) and biosorbents (Wen et al. 2018) are well known adsorbents for the removal of pollutants from aqueous solutions. Biological, green or echo-friendly materials has emerged to be efficient adsorbers to pollutants beside their low cost, easy preparation and environmental friendly (Amiri et al. 2017; Naushad et al. 2015b) such as walnut shell (Banerjee et al. 2018) coconut (Johari et al. 2016; Etim et al. 2016) and tea (Shen et al. 2017). Among these bio-adsorbents and in particular regions of the word (Middle East and North Africa) (Al-Mutairi 2010), the seeds of palm dates fruit have shown a great potential to be an excellent catalyst for adsorption procedures (Ahmed 2016). Due to the high consumption of dates in various regions, millions of tons of date pits are produced daily. Aside with their applications in biodiesel production (Al-Muhtaseb et al. 2016), in batteries (Izanzar et al. 2018) and other environmental applications (Jamil et al. 2016; Naushad et al. 2015c), it has been reported that date pits can be an excellent alternative to activated carbon which is used excessively for pollutants removal procedure (Al-Mutairi 2010). Palm dates have shown to possesses interesting properties such as high porosity and high surface area that makes it low cost and clean adsorber after undergoing extraction and pre-treatment stage (Ahmed 2016). Several research works focused on the applications of palm dates for water treatment and in particular for the removal of metals (Mahdi et al. 2018), dyes (Daoud et al. 2017) and others through adsorption. Physical and chemical treatment is very essential as it could dramatically affect the adsorption process as well as it alters the morphology of the adsorbent and the grain size and hence the surface area which is the key factor of the adsorption process (Gueu et al. 2006). This chapter provides an over view of the extraction characteristics of palm dates as well as a particular focus on its applications in the removal of metals, dyes and other from water.
11.2 Extraction Procedures and Synthesis Methods of Date Palm Seeds
Dates are the fruit of the palm tree. It has a very important nutritional value. It has been considered an important food in the past. Dates contain a large proportion of important minerals for the human body in its construction and protect it from many of the disease (Farsi et al. 2007). Date seed has also many benefits for human and animals and it is an important part of date represented the steel body, and is rectangular in shape, and pointed at both ends, and occupies the center of the fruit, and weighing between 0.5 and 4 g with length of 12–20 mm and usually the length of the seed is equal to three times the width. Date seeds represent 10–20% of the total weight of the fruit and contain protein, carbon hydrate, fiber, foodstuff, ash, fat water and oil (Barreveld 1993). It also contains many of important elements such as sodium, Potassium, Calcium, Iron, Copper, Magnesium, Manganese, Zinc and phosphorus. The common names are, date seeds, dates pits, date kernels, date stones, date pips (Ali et al. 2015). The different ripening stages of date palm according to days post pollination (DPP) shown in Fig. 11.1 (Ghnimi et al. 2017).
Schematic diagram of different ripening stages of date palm according to days post pollination (DPP). (Ghnimi et al. 2017)
Bio-fuel production from date palm seed oil (Pheonix Canariensis) is a new trend, and solvent extraction is one of the traditional technique used for this purpose. This is a cheapest method and it is applied to produce oil such as Jojoba oil, soybean oil, palm oil and jatropha oil from oil seeds (Devshony et al. 1992). Many factors which affect the solid liquid extraction, like particle size, physical and chemical properties of the solvent solubility, temperature and solvent agitation. The extraction equipment also requires safety considerations such as, environmental factors, solvent and dust loadings and working environment. The main application of this procedure includes the extraction of oil from oil seeds and metal salts fro their ores (Rahman et al. 2007). Rate of extraction of oil from date seed depends on type of solvent, partial size of date seed, time of extraction and temperature (Mohsen et al. 1995).
The aim of this work is to produce bio-oil from date palm seeds by conventional Soxhlet apparatus method. The yield of this production is optimized with respect to grain size of the crushed seeds, extraction time and choice of the solvents used (Abd-Alla and El-Enany 2012). The obtained bio-oil were charcterized using Fourier Transform Infrared Spectroscopy (FT-IR) for the identification of functional groups and Gas Chromatography – Mass Spectrum for the identification of fatty acids composition, iodine value, saponification value and kinetics value are determined by this method (Abd-Alla and El-Enany 2012).
Phoenix canariensis (Date palm) is mainly found in the Canary Islands and in countries like Australia and California. The Canary Island Date Palm, a very large palm grown for its rounded crown and its massive trunk, is mainly used in parks and gardens for avenue planting. The norm of chemical parameters of Phoenix canariensis are shown in Table 11.1 (Nehdi et al. 2010). For its growth, it requires a well-drained fertile moist, organic rich and loamy soil. It has a longer life span up to 150 years with less water requirement. The different methods used, for the extraction and synthesis are discussed below. The date palm seeds vary in amount of their oil, starch and other components. The seed is usually obtained from a small embryo and hard endosperm made up of cellulose.
Date palm seed weigh approximately 5.6–14.2%, in that 6.46% I moisture, 5.22% protein, 16.2% fiber, 8.49% fat, 62.51% carbohydrate and 1.12% ash. It contain several minerals like potassium, magnesium, calcium, phosphorous, sodium, iron and several acids like, fatty acids, oleic acid and lauric acid. The chemical composition, physical and chemical properties of Phoenix canariensis shown in Table 11.2 (Nehdi et al. 2010).
11.2.1 Solvent Extraction Using Soxhlet Apparatus for Protein Fraction from Date Palm Seeds
The seeds are manually isolated, soaked in water and washed to remove any remaining dirt. The flesh of the date is air dried for a week, and further dried overnight at 40 °C. The seeds are ground into a fine powder and defatted by extraction with hexane using a Soxhlet apparatus. The defatted powder is then dried to form a date seed powder (DSP). The Fig. 11.2 shows schematic diagram of conventional Soxhlet apparatus used in the laboratory and the outline diagram of the apparatus (Luque de Castro and Priego-Capote 2010).
Schematic diagram of conventional Soxhlet apparatus. (Luque de Castro and Priego-Capote 2010)
The preparation of oil from date palm seed is carried out by placing 15 g of dried DPSP into an extraction thimble and sealed with a piece of cotton wool. The thimble is then inserted in a Soxhlet extraction flask. Anti-bumping agent granules are added before adding 300 ml of hexane to a cleaned dried and weighed round bottom flask. The extraction unit is then placed over an electric heating mantle. The temperature of the heater is adjusted to boil the hexane.
When the solvent starts to boil, some amount of hexane drips out from the condenser into the sample chamber where it extracts the oil from the DPSP. The extraction is continued for 10 h or until the solvent in the sample chamber becomes colorless which indicates that it was free from oil and that all the oil had been extracted (Ibrahim et al. 2012). At this point, the heat source is switched off. The sample is removed from the thimble and left overnight to dry at room temperature. The defatted date palm seed powder (DDPSP) is kept in plastic container at −20 °C until further use.
11.2.2 Extraction of Cellulose from Date Palm Seeds by Van Soest Analysis Method
The seeds of date palm are collected and directly isolated from date fruit, which are fully ripped. The obtained sample is washed with distilled water to remove any adhering date flesh, then air-dried and preserved at −20 °C. The as-dried date seeds are ground into powder with a microphyte disintegrator FZ10 in order to pass a 495 μm sieve. The size of the samples must be small to make sure the reaction reagent and fibers are optimum during the extraction process. Powdered date seeds (20 g) are sequentially subjected to Soxhlet extraction with water and petroleum for 8 h. each and followed by alkaline extraction: consistency (5%); NaOH (2%); temperature (70 °C) and treatment time (160 min) (Al-Hooti et al. 1998). The bleaching step is carried out with acidified sodium chlorite (1.7%). The cellulose is extracted from holo cellulose with 10% KOH, containing 1% H3BO3 for 10 h at room temperature with a solid-to-liquor ratio of 1/25.
11.2.3 Extraction of Date Palm Seed Oil by Supercritical Carbon Dioxide Method
The collected date palm is usually preserved in a refrigerator. The flesh is separated from the seed manually and washed to eliminate any traces of impurities. Pure carbon dioxide (99.99%) which is equipped with supercritical fluid extractor is used throughout the experiment. 10 g of sample and size 2 mm of maximum dimension is weighed accurately in a glass dish that has been dried previously in an oven and set to operate at 103 ± 2 °C for 4 h (Hosam and Wissam 2011). The dish is weighed after cooling in the desiccators. The ground date seeds are sieved using plates of 0.5, 0.4, 0.3 and 0.2 mm pore size. Supercritical Carbon Dioxide (SC-CO2) extraction is conducted at constant temperature and pressure, 40 °C and 30 MPa for different particle size. The schematic diagram of supercritical carbondioxide (SC-CO2) apparatus shown in Fig. 11.3 (Pradhan et al. 2009).
Schematic diagram of supercritical CO2 apparatus. (Pradhan et al. 2009)
SC-CO2 extraction of date’s fruit seed is carried out using laboratory-scale supercritical fluid extraction system. In this method a continuous flow of CO2 gas with 99.9% purity is passed into the extractor with a flow rate of 24 mL/min and a temperature range of 40, 50, 60, 70 and 80 °C and a pressure range of 27.6, 34.5 and 41.4 MPa were used. An approximate amount of fine date palm powder were placed in an extraction vessel and extraction is set to be 40 min. The extracted oil is collected by placing a vial at the outlet of the restrictor (Crabbe et al. 2001).
11.2.4 Extraction Method for the Identification of Biomedical Protein from Date Palm Seed and Flesh
The seeds are manually removed from the fruits and washed to remove any remained date flesh. The seeds are air dried at room temperature and the flesh is cut into small pieces. Date seed and flesh are ground into powder using a precooled mortar in the presence of liquid nitrogen. The powder is further ground into fine powder by using a blender. All date samples are then kept at −80 °C until further use (Ambigaipalan and Shahidi 2015).
11.2.4.1 TCA-Acetone (TCA-A) Extraction Method
1.5 g of seed (2.0 g of flesh) powder issuspended in 12 mL of ice-cold extraction buffer [50 mMTris-HCl (pH 8.5), 5 mM EDTA (pH 8.5), 100 mMKCl, 2% v/v 2-mercaptoethanol] and vortexed for 15 min at 4 °C. After centrifugation (15,000 g, 10 min, 4 °C), the supernatant is collected and precipitated overnight with four to five volumes of acetone containing 10% w/v TCA at – 20 °C.
The supernatant is removed after centrifugation (15,000 g, 30 min, 4 °C) and the pellet is washed twice in ice-cold acetone/0.2% DTT. The pellet is incubated for 1 h between the two rinsing steps at −20 °C. After centrifugation at 10,000 g for 5 min at 4 °C, the supernatant is removed and the pellet is air-dried. The pellet is then resuspended in 400-μL resolubilisation buffer (7 M urea, 2 M thiourea, 4% w/v CHAPS) and vortexed for 1 hr. at room temperature. After centrifugation (10,000 g, 10 min, 4 °C), the supernatant is collected (Carpentier et al. 2005).
11.2.4.2 (Phe) Extraction Method
In Phe extraction method, 1.5 g of seed (2.0 g for flesh) powder is suspended in 10 mL of ice-cold extraction buffer [50 mMTris-HCl (pH 8.5), 5 mM EDTA (pH 8.5), 100 mMKCl, 2% v/v 2-mercaptoethanol, 30% w/v sucrose] and vortexed for 30 s Ice-cold Tris-buffered phenol (pH 8.0, 10 mL) is then added to the sample and vortexed for 15 min at 4 °C. After centrifugation (15,000 g, 10 min, 4 °C) the phenolic phase is collected and re-extracted with the same volume of extraction buffer and vortexed for 30 s. After centrifugation (15,000 g, 10 min, 4 °C) the phenolic phase is collected and precipitated overnight with 5 volumes of 100 mM ammonium acetate in methanol at −20 °C (Saravanan and Rose 2004). The supernatant is then removed after centrifugation (15,000 g, 30 min, 4 °C) and the pellet is washed twice in ice-cold acetone/0.2% DTT. It is then centrifuged and suspended in buffer.
11.2.4.3 TCA-Acetone-Phenol (TCA-A-Phe) Extraction Method
In this TCA-A-Phe extraction method, 1.5 g of seed (2.0 g for flesh) powder of is suspended in 10 mL of 10% w/v TCA in acetone and vortexed for 30 s. After centrifugation (15,000 g, 10 min, 4 °C) the supernatant is removed and then 10 mL of 100 mM ammonium acetate in methanol at −20 °C was added, vortexed for 30 s and centrifuged (15,000 g, 10 min, 4 °C). The supernatant is removed after centrifugation and washing steps were repeated twice with 10 mL of ice cold acetone at −20 °C. After centrifugation (15,000 g, 10 min, 4 °C), the supernatant is removed and the resultant pellet is air dried at room temperature to remove residual acetone (Wang et al. 2006). The following steps (Phe extraction, methanol/ammonium acetate precipitation, and pellet resolubilisation) are performed in the same way as in Phe extraction method.
11.2.5 Preparation and Extraction of Date Palm Nanoparticle by Agar Well Diffusion Method
Date seeds are soaked in water to remove any adhering date flesh, air-dried, then further dried in an air oven at 60 °C. The dried date palm seeds are ground and passed through 1–2 mm screens to produce date palm seeds flour or date-pits powder. The powder is then kept in the freezer at −20 °C (Saleh and Otaibi 2013). About 5 g of date palm seed powder is weighed and poured into a flask including 20–50 mL of 38% hydrochloric acid solution. The flask is then kept under stirring at a speed of 1000 rpm at a temperature of 30 + 2 °C. The date palm seed nanoparticles were filtered through a Millipore filter having a pore size of 220 nm. Alternately, the solution was centrifuged at about 9000 rpm for about 15 min to isolate the date palm seed nanoparticles.
11.2.6 Preparation and Extraction of Date Palm by Acid Hydrolysis
Date palm seeds are extracted with ether for 11 h and with aqueous methanol (70%) for 18 h in a continuous extraction apparatus (Soxhlet) until exhaustion. The aqueous extract is concentrated to a small volume and partitioned successively with chloroform and n-butanol. The n-butanol extract is then fractionated on a silica gel 60 (63–200 mesh) column chromatography and eluted with chloroform followed by step-wise addition of methanol to afford four fractions (Nagwa et al. 2009). Each fraction is subjected to sephadex and eluted with a mixture of methanol and water. All separation processes were performed using Whatman No. 1 paper with (S1) n-butanol-HOAc-H2O (4:1:5, top layer) and 15% aqueous HOAc. The solution of each compound in 6% aqueous HCl is refluxed for 2 h. The reaction mixture is diluted with water and extracted with ether.
11.2.7 Preparation and Extraction of Date Palm by FRAP (Ferric Reducing Antioxidant Power Assay) Method
The date palm samples were packed in polyethylene bags, sealed and stored at 2–8 °C. The pits were removed from the flesh, washed to get rid of any adhering date flesh, and air-dried. Then, they were further dried at about 50 °C for 4 h. Date pits of each variety were separately milled up to 1–2 mm and then preserved at 2–8 °C.
About 0.02 g of powdered date seeds was shaked with 5 mL of solvent in a glass tube at room temperature, two times for 30 min and then centrifuged. Water, methanol:water (50:50, v/v), methanol, DMSO and water:methanol:acetone: formic acid (20:40:40:0.1) (3) were used as solvents. The extraction was carried out using these solvents to compare the antioxidant activities and the total phenolic contents of each extract.
In ferric reducing antioxidant power assay method, reduction of a ferric-tripyridyltriazine complex to its colored ferrous form in the presence of antioxidants. The FRAP reagent contained 5 mL of 10 mmol/L solution of TPTZ (2,4,6-tripyridyl-s-triazine) in 40 mmol/L HCl, 5 mL of a 20 mmol/L solution of FeCl3and 50 mL of a 0.3 mol/L acetate buffer solution, pH 3.6 was maintained and warmed at 37 °C. The date palm seed extract of 50 μL was mixed with 1.5 mL FRAP reagent and after incubation at 37 °C for 10 min, the absorbance of the reaction mixture was measured at 593 nm (Benzie and Strain 1996).
11.2.8 Preparation and Extraction Date Palm Seed by Aqueous and Ethanolic Method
Palm date was collected at the initial growth stage and stored in a refrigerator at 4 °C, then removed the flesh of date from the pits. The pits were dried and crushed to fine powder. Fruits and seeds were defatted by using n-hexane then extracted three times with 500 ml methanol at room temperature for 24 h by a magnetic stirrer. To obtain crude methanol, the extracts were filtered and centrifuged at 6000 radius centrifugation force for 30 min at 3 °C (Pujari et al. 2011), then the supernatant was concentrated under low pressure at 40 °C for 1 to 2 h using a rotary evaporator. These extracts were stored in dark glass bottles at −4 °C. Also, the dry date palm seeds were soaked in water (1:5 w/v) at 40 °C then stirred for 24 h at a temperature of 4 °C. This mixture was centrifuged at 6000 RCF for 20 min and the precipitate was discarded and the supernatant liquid was stored at 4 °C. The flowchart for the procedure of Ethanolic and aqueous extraction was shown in Fig. 11.4 (Al-Farsi and Lee 2008).
Flowchart for the extraction procedure for Ethanolic and aqueous method. (Al-Farsi and Lee 2008)
11.2.8.1 Ethanolic Extract
100 g of the fresh blended date palm seed were extracted exhaustively with 1 L of ethanol and the mixture sieved, then the remaining ethanol in the extract was evaporated to obtain the concentrated extract, which was reconstituted in distilled water at a concentration of 1 mg/ ml and stored in the refrigerator (Amani et al. 2016).
11.2.8.2 Aqueous Extract
100 g of the fresh blended date palm seed were extracted exhaustively with 1 L of water and the mixture sieved, then the remaining methanol in the extract was evaporated to obtain the concentrated extract, which was reconstituted in distilled water at a concentration of 1 mg/ ml and stored in the refrigerator.
11.2.9 Preparation and Extraction of Date Palm Seed by Isoelectric Precipitation Method
The date palm seeds were soaked in water, washed to remove any adhering date flesh, and then air-dried. The fine powdered samples were collected, stored and then further dried at 50 °C. Date pits, of each variety, were separately milled up to 1–2 mm and then preserved at −20 °C. Lipid extraction was carried out with a SER 148 solvent extractor equipped with six Soxhlet posts. The extraction was carried out for 30 min, with thimbles immersed in boiling petroleum ether, and 60 min of reflux washing (Besbes et al. 2009). After removing solvent, using a rotavapour apparatus, the obtained defatted date seeds were used for preparation of fibro-protein extract.
The fibro-protein extract from defatted date seed was prepared according to the isoelectric precipitation method. The defatted date seed flower was mixed with distilled water (1:10 w/v), adjusted to pH 10 with NaOH and after stirring for at least 40 min, was centrifuged at 6500 g for 20 min at 4 °C. Then, the residue was mixed with distilled water (1:5 w/v), readjusted to pH 10 and centrifuged following the same process. The supernatant liquid of both centrifugations were blended and used as mother solution for DSFPE production (Tsaliki et al. 2002). This solution was adjusted at pH 4.5 with 0.1 HCl, centrifuged, freeze and lyophilized to obtain the DSFPE.
11.2.10 Preparation and Extraction of Date Palm Seed by Aqueous Ethanolic Method
In this method, date palms and flesh and pits were separated. Palm seeds were washed to remove adherent fruit material. After washing and drying, seeds were roasted and grounded to obtain fine date seeds powder, which was stored in plastic jars at ambient temperature. The solvent extraction was carried out using aqueous ethanol followed by extraction. In this method, 50 g of date seeds powder was added in 149 mL of aqueous ethanol in 250 mL conical flask, 1 mL of acetic acid and 40 mL of distilled water were added into flask. The mixture of solvent and date seeds powder was placed in orbital shaker for 3–4 h at 280 rpm with controlled temperature of 20 °C. The mixture was filtered through Whatmann filter paper No. 2. The solvent was evaporated using rotary evaporator under reduced pressure at 40 °C. The final extract was stored at – 40 °C (Farsi et al. 2005).
11.2.11 Different Method for the Preparation and Extraction of Anti – oxidant from Date Palm Seeds
11.2.11.1 Decoction Method
The date palm seeds powder is boiled in a process called decoction method in order to obtain a thick liquid solution which is rich in antioxidants. 5 g of sample is mixed with 100 ml deionized water and boiled at 100 °C for 30 min in water bath. Then, the mixture is centrifuged at 10000 rpm for 10 min. The liquid portion is filtered using muslin cloth, and the filtrate is used to determine the antioxidants (Lee et al. 2008).
11.2.11.2 Hydro-alcoholic Method
Antioxidants can be extracted from date palm seeds by the hydro-alcoholic cold separation method. In this method, 10 g sample is mixed with petroleum ether ethanol 90% at a ratio of 1:1 (v/v) in flask capped by cotton wool and shaken on a rotary shaker for 24 h. The solution is centrifuged at 10000 rpm for 10 min and filtered using eight layers of muslin cloth. The combined filtrate is concentrated in a rotary evaporator at 60 °C under reduced pressure to evaporate the petroleum ether and yield a thick solution. The hydro-alcoholic method achieved higher amount of total phenols and flavanones than methanolic and aqueous method. It is found that higher yield of antioxidants was achieved by the decoction method compared to the hydro-alcoholic method (Milovanovic et al. 2007).
11.2.11.3 Ultrasound Method
In this method, in a 25 ml flask, 2.5 g powdered date palm seeds were mixed with 20 ml methanol and the mixture is sonicated at 20 khz for 30 min at a temperature of 23 °C filtered using eight layers of muslin cloth. The combined filtrate is concentrated in a rotary evaporator at 60 °C under reduced pressure to evaporate the methanol. This method shows better yield and reduced time, and the effect of ultrasound mainly depends on the nature of the solvent used for extraction. The solvent in the ratio of 3:1 i.e. acetone: water with chloroform yields a high amount of phenol from date palm seeds (Pan et al. 2011).
11.2.11.4 Solvents Method
In this 1 g of fine form of date palm seeds were mixed with suitable solvents such as acetone, DMF, methanol, ethyl acetate and ethanol. Then the solution kept in a water bath for 60 °C for 4 h and then centrifuged at 10000 rpm for 10 min and filtered using muslin cloths. Then the obtained filtrate was concentrated using a rotary evaporator at 60 °C under reduced pressure. It was found that, the antioxidant extraction yield is influenced by the nature of the different solvents used and its concentration. For the extraction of anthocyanins, acid such as methanol or ethanol is used, DMF used as a solvent to extract polyphenol from tea than acetone (Paniwnyk et al. 2001).
11.2.12 Determination of Phenolic Content from Date Palm Seed by Folin-Ciocalteu Method
The date palms were purchased; pits removed and were stored in a refrigerator at 4 °C until used. The extraction procedure was carried out on the date seed powder. In a 125 mL Erlenmeyer flask, 1 g of date palm seed fine powder were mixed with 20 ml de-ionised water (20:1 ratio). The flask then placed in a water bath for 25 °C for 1 h, then the obtained solution was centrifuged at 1000 rpm for 10 min and then filtered. The solvent was evaporated by using a rotary evaporator at 60 °C (Hong et al. 2006).
Finally, the sample was analyzed for total antioxidants (TA), total phenolic (TP) and total flavonoid (TF) using a spectrophotometer. The same procedure was followed with all the solvents.
11.2.13 Extraction and Preparation of Silver Nanoparticle from Date Palm Seeds by Microwave Assisted Green Synthesis
In this extraction method, date palm seeds were dried, powdered, sieved and stored in the form of fine powder. From this, 0.3 g of date palm fine powder was mixed with 25 ml of double distilled water and exposed to microwave at 300 W. After cooling, the obtained reddish brown extract was filtered and stored at 5 °C. Similar procedure was followed for the synthesis of silver nanoparticle, 1 ml date palm extract mixed with 10 ml silver nitate solution and then irradiation at 300 W for 30 s in microwave. After cooling, this mixture was mixed with 0.1 ml 4-nitrophenol solution and 1.85 ml sodium brohydride solution (Aitenneite et al. 2016).
11.2.14 Extraction Method for the Identification Protein from Date Palm Seed by HPLC – PDA Analysis
The pits separated from the flesh of date palm, crushed into fine powder and around 100 g are taken in a flask. The fine powder was equally separated in individual flasks, immersed and extracted three times with 500 ml of n-hexane. The flask was shaken for 24 h with 3 h interval using a magnetic stirrer, and then filtered with Whatman filter paper. The solvent was removed under reduced pressure at 40 °C using a rotary evaporator to obtain hexane crude extracts.
The immersed seeds were separately extracted three times with methanol (500 ml) at room temperature for 24 h using a magnetic stirrer. The methanolic extracts were filtered and then centrifuges at 6000 for 30 min at 3 °C. The supernatant liquid was concentrated under reduced pressure at 40 °C for 1–2 h using a rotary evaporator to obtain methanol crude extracts. The n-hexane and methanol extracts were kept in dark glass bottles at −4 °C. Also, the dry date palm fruits were soaked in water (1:5 w/v) at 40 °C then stirred for 24 h at 4 °C (Wang et al. 2004). The mixture was centrifuged at 6000 RCF for 20 min to discard the precipitate and the supernatant was stored at 4 °C.
11.2.15 Extraction of Date Palm Seed by Ultrasonication Assisted Extraction
Dried date palm seeds were powdered to a uniform particle size. Sample-to-solvent ratio was kept constant for all the procedure. Extraction carried out in ultrasonic bathat 35KHz. Samples (2 g) were powdered and placed into Erlenmeyer flasks (250 mL) Samples were exhaustively extracted with different proportions of ethanol-water (from 0% to 100%) at different extraction time (from 5 to 45 min), in different ratios of aqueous ethanol to raw material (from 10 to 70 mL/g) and at extraction temperature varying from ambient temperature to 65 °C. Absolute methanol and 50% ethanol were used for extraction. Mostly, ethanol was selected as extraction solvent due to its low toxicity (Vijay et al. 2012). The mixtures were centrifuged at 2000 × g for 20 min at 4 °C and the supernatants were collected for the antioxidant activity determination. Figure 11.5 shows the experimental set up design for the ultrasonication assisted extraction method (Luque de Castro and Priego-Capote 2010).
Schematic diagram for the experimental set up for ultrasonication assisted extraction method. (Luque de Castro and Priego-Capote 2010)
11.2.16 Estimation of Flavonoid Content in Date Palm Seed by Aluminum Chloride Colorimetric Method
Date palm seeds were collected and dried into powder with uniform particle size. In this method, sample-to-solvent ratio was kept constant for all the procedure, i.e. 1 g seed powder in 50 ml solvent. Absolute methanol (Merck, Mumbai) and 50% ethanol were used for the extraction.
Aluminum chloride (AlCl3) colorimetric method was used for flavonoids determination. 0.5 mL of each plant extract was separately mixed with 1.5 mL of methanol, 0.1 mL of 10% aluminum chloride, 0.1 mL of 1 M potassium acetate and 2.8 mL of distilled water. The reaction mixture was allowed to stand at room temperature for 30 min and then further analyses were conducted (Dobias et al. 2010).
11.3 Physico–Chemical Characteristics of Date Palm Seeds
11.3.1 Date Palm Seeds as a Precursor for Porous Carbon
There are many reports about using of date palm seeds as low-cost sorbent in the adsorption process for the purification of removal wastewater from pollutants. This approach has few options: the use of non-activated date pits as solid adsorbent and the use of activated date palm seeds carbons (biochar) obtained from date palm seeds as adsorbent for hazardous dyes and heavy metals from wastewater (Fig. 11.6).
Activated carbons can be prepared from date palm seeds by using two steps:
-
Step 1 – carbonization: means thermal treatment (pyrolysis) of the carbon precursors under inert gas atmosphere and high temperatures (600–700 °C) to obtain a biochar – a stable solid rich in carbon; but very often the obtained biochar shows low adsorption capacity and needs an activation step to increase its pore volume, porosity and surface area;
-
Step 2 – activation:
-
physical activation: using of CO2 atmosphere or steam at temperatures above 700 °C; the high level of internal carbon mass is undesirable so it must to be removed to obtain a carbon with well-developed structure (Ahmadpour and Do 1996; Belhachemi et al. 2009);
-
chemical activation: the carbonization of the palm date pits precursor in the presence of chemical activators such as KOH, K2CO3, NaOH, Na2CO3, AlCl3, FeCl3, ZnCl2, H3PO4 H2SO4 at temperatures lower compared to the physical activation (400–800 °C) and for shorter periods of time; these conditions help to get activated carbons with high surface area and porosity (Ao et al. 2018);
-
physico-chemical activation: simultaneous combination of physical and chemical activations after carbonization process in the temperature range 600–850 °C with using the potassium hydroxide, zinc chloride or orthophosphoric acid (as dehydrating agents) and CO2 or steam (as oxidizing agents) leading to obtain a high porous structure (Ahmed 2016).
-
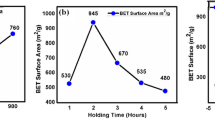
A wide number of studies have reported the preparation of activated carbon from date palm seeds by application physical or chemical activation. Ogungbenro et al. (2017) reported about activated carbon prepared from palm date seeds by physical activation for CO2 capture applications. It was shown, that the optimal temperature for thermal treatment is 800 °C, while activated carbon obtained at 900 °C had the highest CO2 adsorption capacity equal 141.14 mg/g. Md. A. Islam et al. (2015) prepared mesoporous activated carbon from palm date seed by alkaline activation of hydrochar. The activated carbon had high BET surface area equal 1282.49 m2/g and the maximum adsorption capacity in regarding to methylene blue dye 612.1 mg/g. A. El Nemr et al. (2008) prepared activated carbon from date palm seed by dehydrating methods using boiling for 20 h with concentrated sulfuric acid (98%, 2.0 l). Its capacity to remove toxic chromium from different aqueous solutions was evaluated, reaching a maximum adsorption capacity 120.48 mg.g−1 (for Cr(VI) concentration is 75 mg.l−1 and carbon adsorbent concentration is 4 g.l−1).
Magnetic biocomposite was fabricated by (M. Gazi et al. 2017) through the co-precipitation of palm seed-based biochar in the presence of magnetite particles (Fig. 11.7). Such magnetically separable palm seed-based biochar was investigated for the removal of Ni2+ ions. The as-prepared biocomposite has a surface area of 135 m2.g−1 and a saturation magnetization of 65.8 Am2.kg−1, with the Ni(II) removal ability reaching 87% (28 mg.g−1) at pH 3. It was shown that the magnetic biocomposite can be regenerated without loss of its activity.
Magnetic palm seed-based biochar and it’s activity in nickel removal (a) magnetically separable palm seed-based biochar; (b) adsorption isotherms for magnetic biochar at different temperatures; (c, d) adsorption-desorption cycles using HCl (c) and H2O (d) regenerants). (Reprinted from M. Gazi et al. (2017), Copyright (2017), with permission from Elsevier)
11.3.2 Chemical Composition, Surface Morphology and Microstructure
The different techniques used for physico-chemical characterization includes surface morphology, microtexture and element composition of date palm seeds biomass, such as FTIR, SEM, BET, TG-DTA-DTG etc. Approximate analysis have shown that the chemical composition of palm date seeds is follow: 4.9% moisture, 76.6% volatile matter, 10.8% ash, 7.7% fixed carbon (Hani H. Sait et al. 2012).
Scanning electron microscopy (SEM) observations usually reveal a porous microstructure of activated carbon. From Fig. 11.8a it is clearly seen that crushed raw date seeds have non-porous microstructure. After thermal treatment (for example, at 900 °C) the porosity increases and large pores clearly seen on the activated carbon texture (Fig. 11.8b), which leads for increasing of adsorption capacity in regarding to CO2 (A. E. Ogungbenro et al. 2018).
SEM of crushed raw date seeds (a) and carbon samples after physical activation (b) with enhance adsorption capacity for CO2 capture. (Reprinted from A. E. Ogungbenro et al. (2018), Copyright (2018), with permission from Elsevier)
Fourier-transform infrared spectroscopy (FTIR) helps to obtain information about the lignocellulosic composition of palm date seeds and the presence of different surface functional groups (alkene, ester, aromatic, alkanone, alcohol, hydroxyl, ether and carboxyl) (A. E. Ogungbenro et al. 2018). Usually the FTIR spectra of palm date seeds and activated carbon consists of several main adsorption peaks (Table 11.3). The broad adsorption peak at 3500–3500 cm−1 corresponds to free hydroxyl groups on the palm date seeds surface. The peak around ~2900 cm−1 related to asymmetric and symmetric stretching modes of the C–H bond of –CH2 group. The peak around 1700 cm−1 corresponds to the stretching vibration of carboxyl group (–COOH, –COOCH3) and carboxylic acids or their esters. The peaks at 1500, 1380, and 1100–1150 cm−1 are related C–C and C–O bonds (Table 11.3) (D. Pathania et al. 2016). As pyrolysis occurs, infrared peaks become less intense due to fragmentation of correspondent functional groups. The bands around 3500 and 2900 cm−1 disappear due to decrease in moisture amount and aliphatic compounds in the seeds. The physical and chemical activation leads to stretching of the carbonyl group C=O (observed in the peak at ~1700–1600 cm−1) (Ogungbenro et al. 2018).
M.A. Al-Ghouti et al. (2010) investigated the adsorption mechanisms of methylene blue dye onto date palm seeds through FTIR spectroscopy (Fig. 11.4). The FTIR data indicated interactions of the methylene blue molecules with functional groups on the data palm seeds surface (Fig. 11.9a). The hydrogen bonding between the seed’s surface hydroxyl groups and the nitrogen atoms of dye molecules and electrostatic attraction as two mechanisms of adsorption on the palm pits surface were observed (Fig. 11.9b and c).
Adsorption mechanisms of removing MB dye using date pits adsorbent: (a) FTIR spectra of the raw date pits and raw date pits-MB loaded samples respectively; (b) hydrogen bonding between methylene blue dye molecule and OH-groups on the seeds surface; (c) electrostatic attraction between dye molecule and the cellulose unit of the seeds surfaces; (d) SEM micrographs for the raw date pits and (e) raw date pits-MB loaded samples respectively. (Reprinted from M.A. Al-Ghouti et al. (2010), Copyright (2010), with permission from Elsevier)
11.4 Palm Seeds Applications in The Removal of Pollutants from Water
Treated palm dates stones is a potential bio-adsorber that has been used effectively to eliminate residuals and un-degradable materials such as metals (Danish et al. 2012), dyes (Daoud et al. 2017) and acids (Salman and Al-saad 2012) from aqueous solutions. Adsorption is well known as an efficient chemical approach that depends mainly in chemical bonding between adsorber and adsorbents (Ahmed 2016) and hence it is highly affected by the chemical and physical conditions of the medium such as pH (Mahdi et al. 2018), temperature (Danish et al. 2017), time (Al-Saidi 2016), string speed (Islam et al. 2015) and initial concentration of the adsorber and pollutants (Salman and Al-saad 2012). Table 11.4 illustrates the different pollutants used for adsorption procedures applying palm seeds stones.
Adsorption process would reach an equilibrium stage where no more pollutant can be adsorbed. The amount of the adsorbed pollutants at equilibrium and at any time can be calculated applying the following Eqs. (11.1–11.2)
where Ci, Ce, and Ct (mg/L) are the liquid phase concentrations at initial, equilibrium, and time t (min), respectively, V is the solution volume (mL) and W is the adsorbent mass used (g) (Pathania et al. 2016). Hence the removal efficiency can be also determined from the equation:
Different fitting models can be chosen to explain the behavior and mechanism of a certain removal procedure with consideration to the different parameters such as equilibrium, adsorption constants, adsorption energy, adsorbed solute and concentration solute. These models include Pseudo (Islam et al. 2015) Langmuir, Freundlich, Koble–Corrigan, Redlich–Peterson, Tempkin, Dubinin–Radushkevich and Generalized isotherm equations (El Nemr et al. 2008).
11.4.1 Heavy Metal Removal
The idea of heavy metal removal by applying extracts from palm seeds has been proposed since many years because of it low cost and availability (Gueu et al. 2006). Activated carbon was extracted from the seed shell of palm trees and used to prepare an adsorbent (GA) for heavy metals (Cu, Pb and Zn). The catalyst was characterized in term of Ash content, acidity group and surface area (95 m2 g−1), which plays the major role in the adsorption process. Adsorption test was carried out with a metal solution of 300 mg/L for each Cu, Pb and Zn with three different adsorbent mass (2, 4 and 6 g). The results were compared with other adsorbent extracted from coconut shell. It was found that a considerable percentage of metal was adsorbed due to the precedence of GA adsorbent as the removal increases with GA amount. The process was shown to be more efficient with Pb metal as it reached 50.67% in 30 min. It was also seen that the adsorption percentage raised dramatically by changing the pH from 2 to 4 and then remains almost steadily from pH 4 to 10 (Gueu et al. 2006).
Activated Carbon from date palm seeds were also used for the adsorption of chromium Cr+6 from wastewater. Full adsorption kinetics were applied using different amount of Cr+6 (25, 50, 75, 125 mg−1) in 100 mL of water. During a period of 180 min, a 100% of removal efficiency was achieved at 25 mg/L of Cr+6. This efficiency decreased gradually with increasing the initial concentration until reaching 70% at 125 mg/L and pH 1 which was found to be the optimum acidity for the reaction. The removal efficiency was affected negatively by raising the pH level of the medium (from 1 to 8) (El Nemr et al. 2008).
Date pits have been involved in the removal of Nobel metals (Au) pollutants from water. Dried and crushed date pits of palm trees in KSA have been through several chemical treatments before applying it as an adsorbent. The characterization of the adsorber was investigated in term of morphology and chemical properties. Scanning electron microscopy observations showed a smooth surface that become rougher by the adsorption of Au particles (Fig. 11.10a–b). Batch adsorption procedure with 100 mL of aqueous solution and 10 mM of Au ions with presence of 0.5 molL-1 of HCl showed a remarkable percentage of removal efficiency. Figure 11.10c shows a maximum of 90% in 120 min where the reaction reached its equilibrium. It is shown that the first stage (40 min) experienced higher rate of adsorption due to the availability of adsorption sites that eventually will be filled with the Au particles which prevents further adsorption and the reaction reaches equilibrium. For further explanation of the Au attachment to the to the palm seed sdsorber, FTIR was applied before and after the adsorption process to investigate the difference in chemical bonding (Fig. 11.10d). A shift in the hydroxyal group was noticed after adsorption which indicates the formation of new hydrogen bond which cause the Au particles to interact with the adsorber. Also, several shifts to lower wavelengths were seen in other existing bonds. XRD analysis was also carried out to confirm the pure metallic Au formation on the surface of the adsorber (Al-Saidi 2016).
SEM images of RDPs taken (a) before and (b) after adsorption of Au(III); (c) FT-IR spectra of RDPs (A) before and (B) after adsorption of Au(III); (d) the effect of time on removal percentage of Au(III) by RDPs. Conditions: test solution (100 mL), HCl (0.5 mol L−1), RDP doze (0.1 ± 0.001 g), shaking speed (150 rpm), and temperature (25 ± 0.1 °C). (Al-Saidi 2016)
Date pits were applied to desalinated water to clear Bromide ions. It went through the conventional physical and chemical treatments to prepare two adsorbents (roasted and unroasted) that were compared with commercial activated carbon (Al-Ghouti et al. 2017). SEM images showed a narrow pore microstructure associated with both roasted and unroasted. However, the roasted adsorbent showed more pure surface with less contaminations. The adsorption process was shown to be affected by the pH of the medium as pH 4 (weak acidic medium) showed the maximum adsorption of 57% of Br. This was explained as the higher values of pH (pH 8 and 11) might change the ionization and the charge on the adsorbent surface and hence affect the uptake of the negative Br- ions. In the other hand, the excess of the H+ in the weak acidic medium (pH = 4) changes the anion groups on the surface to cationic groups that assess the adsorption process. With further increase in acidity (pH = 2), the adsorption was found to be decreased because of the creation of chlorine ions that negatively affect the process (Al-Ghouti et al. 2017).
Two adsorption isotherm fittings (Freundlich and Langmuir) were applied to explain the mechanism of the process. It was indicated clearly that the non-linear model (Freundlich) fits well with the adsorption behavior (Fig. 11.11a). Kinetic models showed that the surface functional groups as well as the pore microstructure of the date pits adsorber highly influenced the efficiency of the adsorption. It was also shown that the grain size affects the kinetics of the removal as three different ranges of grain size were investigated (0.5–1, 0.25–0.5 and 0.125–0.25 mm). With larger range of grain size, more fluctuation was observed in the first 60 min of the adsorption process where all the sizes have the same behavior after reaching the saturation level (Fig. 11.11b). The effect of the initial concentration and the adsorbant mass were also reported (Al-Ghouti et al. 2017).
(a) Adsorption isotherm fitting to experimental data using Freundlich and Langmuir isotherms for roasted date pits adsrorber; (b) effect of particle size on the removal efficiency of Br- with roasted palm date pits: mass 1.0 g, KBr initial concentration 200 mg L−1, and pH 4, at particle size 0.5–1.0 mm, 0.25–0.5 mm, and 0.125–0.25 mm. (Al-Ghouti et al. 2017)
Moreover, Ni2+ and Cu2+ ions were shown to be effectively removed from aqueous solution by applying biochar samples prepared from palm dates pits using different calcinations temperatures (350, 450 and 550) and time periods (1, 2 and 3 h). adsorption procedures were carried out through Batch experiment (Fig. 11.12a) and all samples showed a considerable efficiency in the removal of Ni and Cu ions with the maximum rate for the sample with highest calcination temperature and time (550 °C at 3 h). Therefore, the latter sample was chosen for further experiments to investigate the effect of pH, particle size and contact time. It was shown that as pH increases from 2 to 6, the adsorption capacity increases too by 30% (Fig. 11.12b). The lower adsorption in lower pH medium referred to the creation of H+ ions that decrease the active adsorption sites in the adsorbent. It is well-known that the pH of a medium would highly affect the surface functional groups such as carboxyl (COOH), Amino (NH) and hydroxyal (OH hence strongly affect the adsorption process. Experiments also revealed that at a certain particle size range (0.6–1.4 mm) of the prepared adsorber, the removal efficiency reached its maximum. Lower and higher ranges of sizes caused lower rates of removal efficiencies. This was more noticeable in the case of Ni adsorption (Fig. 11.12c) (Mahdi et al. 2018).
(a) Fixed bed adsorption arrangement; (b) effect of pH on the adsorption of Cu2+ and Ni2+ onto biochar (room temperature; biochar: solution = 10 g L−1; Co = 1.0 mM); (c) effect of biochar particle size on the adsorption of Cu2+ and Ni2+ (room temperature; biochar: solution = 10 g L−1; Co = 0.5 mM). (Mahdi et al. 2018)
11.4.2 Removal of Dyes
Dyes are one of the most found pollutants in water and most of them are organic and non-biodegradable, thereby they accumulate in water causing serious problems to environment habitat and human health (Ahsaine et al. 2018; Pathania et al. 2015). Date seeds were shown to have a considerable effect during the removal process of these dyes from water. Table 11.5 shows some of the dyes that can be adsorbed by treated date seeds adsorber.
Mesoporous activated carbon was produced from palm tree seeds and applied for the degradation of MB dye that reached its maximum (450 mg.g−1)) around 700 min (Islam et al. 2015). It was also shown that the amount of the dye removal depends on the amount of the palm seeds adsorbent (Fig. 11.13a). At a very low concentration (50 mg.L−1), almost no removal achieved whereas by increasing the amount gradually to 100, 200, 300, 400 and 500 mg.L−1, the removal efficiency started to increase too until it reached its maximum at the highest amount of adsorbent. Performing the adsorption experiment with different pH showed that the removal efficiency is better at higher pH (10 and 12). Adsorption isotherm studies showed that the Pseudo second order model has better fitting to the adsorption behavior (Fig. 11.13b) (Islam et al. 2015).
(a) Effect of contact time and initial concentration on removal of MB onto HTC–PDS3 at 30 °C (b) Adsorption isotherm fitting to experimental data using Pseudo first and second order isotherms. (Islam et al. 2015)
Also, activated carbon from date seeds has been applied efficiently for the removal of RhB dye (Danish et al. 2017). FESEM images taken before and after the adsorption process revealed the difference in the morphology of the adsorbent after attachment with the dye. Figure 11.14a showed lower porosity for the adsorbent after dye adsorption confirming that the dye molecules are attached to the adsorbent and significantly blocking the pores. EDS analysis showed changes in elemental composition of the adsorbent as a result of the dye attachment as the carbon weigh percent increased slightly due to the attachment with the organic dye that contains carbons in its chemical chain. The effect of pH and initial concentration were confirmed too showing the same trend of the previous studies. Intensive analysis was carried out to explain the behavior of the adsorption by applying various fitting models and Box-Behnken design experiment Fig. 11.14b where the number of parameters such as dosage, concentration, time, temperature and pH were taken into account simultaneously. Moreover, the process maintained its efficiency over four adsorption cycles (Fig. 11.14c) (Danish et al. 2017).
FESEM images of activated carbon extracted from date seeds (A) before and (B) after RhB adsorption; (b) 3D response surface graph for adsorption capacity versus (a) time and adsorbent dose (b) temperature and adsorbent dose (c) temperature and time; (c) Adsorption efficiency and reusability of activated carbon from dates pits for RhB in various recycles. (Danish et al. 2017)
Furthermore, Congo Red (CR) dye was shown to be efficiently removed from aqueous solution by applying the palm seeds adsorber (Pathania et al. 2016). FTIR used to study the effect of the adsorption and to have more understanding to its mechanism, revealed several peaks shift into higher/lower wavelengths which indicates weakness/strength of the corresponding bonds. New peaks immerged as a result of the attachment between the dye and the adsorbent as well as the creation of new chemical bonds (Fig. 11.15a). Similar to other studies, the adsorption was influenced by pH and initial concentration. Moreover, the effect of temperature was examined too and higher temperature medium (55 °C) showed higher adsorption capacity, due to the higher diffusion of the dye molecules into the adsorbent pores. The effect of the ionic strength of the medium (Fig. 11.15b) by adding different amount of NaCl salt from 0 to 1.5 M, indicated that the removal efficiency was positively enhancing from 83.45 to 89.83%, respectively. The presence of NaCl increases the dye agglomerates and hence causes better attachment to the adsorber (Pathania et al. 2016).
(a) FTIR spectrum of the date pits adsorber before (a) and (b) after adsorption; (b) effect of ionic strength for CR dye adsorption onto date pits [dye concentration 20 mg/L, sorbent dosage 0.60 mg, pH 2.0, contact time 120 min, temperature 30 °C]. (Pathania et al. 2016)
It is very essential to mention here that the influence of pH, temperature and initial concentration is different and the trend is changing form a study to another based on many reasons including the chemical reaction between the dye and the adsorbent as different dyes and different absorbents combination is not necessarily affected by experimental conditions in the same manner. Table 11.2 illustrates the chemical composition of different dyes.
Another study, investigated the efficiency of the treated palm seeds on the adsorption of the reactive dye (BEZAKTIV Red S-MAX) and compared with the commercial activated carbon. Three temperatures where applied for the adsorption experiment (298, 308 and 318 K) and the results was similar to previous studies as the maximum adsorption occurred at the highest temperature (Fig. 11.16a). However, for pH effect, the result was different than other reported studies. It was found that the optimum pH for the reaction is 8 while at pH 10 the adsorption decreased (Fig. 11.16b). This was related to the surface properties of the adsorbent and the degree of ionization of the dye (Daoud et al. 2017).
(a) Effect of solution temperature on dye uptake on RPK (date pits), NJK (other adsorbers) and CAC (activated carbon); (b) The variation of the pH Effect on the adsorption of dye uptake on RPK (date pits), NJK (other adsorbers) and CAC (activated carbon). (Daoud et al. 2017)
On the other hand, another study showed different effect of the initial concentration when Crystal violet (CV) dye was adsorbed by treated palm seeds (El Messaoudi et al. 2016) as the adsorption was found to increase with increasing the dye concentration (in other words with decreasing the actual amount relative amount of adsorbent to dye) (Fig. 11.17a). This was explained as the enhancement of mass transfer rate that increases the effective mass ratio of the dye/adsorbent. The same behavior was also seen with MB dye using the same adsorbent. The combined effect of temperature and dye concentration showed that the influence of temperature is almost unnoticeable until reaching a certain dye concentration of 480 mg.L−1 where higher temperature causes a higher adsorption rate (Fig. 11.17b) (El Messaoudi et al. 2016).
Effect of (a) initial concentration of and (b) temperature in the adsorption of CV dye. (El Messaoudi et al. 2016)
11.4.3 Other Pollutants
Palm dates seeds have also shown to be efficient in the removal of other pollutants such as Nitrophenols which is a highly toxic acid that comes from pesticides applications, refineries and manufacturing. A statistical analysis design was applied for the adsorption batch experiment depending on pH, time, concentration, temperature and speed of shaking with a comparison of activated carbon. It was found that the efficiency of the removal of the date pits is 15% higher than the activated carbon. Toxicity analysis were performed too (Al-Mutairi 2010).
Chemically and thermally treated date stone were applied to remove three types of Drin pesticides (aldrin, dieldrin and endrin) from aqueous solutions. Three particle size ranges of the adsorbent were prepared and the smaller sizes were associated with the highest removal efficiency due to their larger surface area and hence this size range was applied for further investigations. It was shown that each type of acids has different response to the palm date adsorber and the Aldrin showed the highest amount of adsorption with time before it reaches equilibrium (Fig. 11.18a). It is very essential to mention here that the treatment of the date stone highly influenced the adsorption process (Fig. 11.18b). The effect of temperature was examined too as the temperature of the medium was raised gradually from 10 to 40 °C (by 5 °C in each interval). For the three acids, it was found that the removal percentage was decreased with increasing temperature. This result was different than results reported in previous mentioned studies and it was related to the solubility of the pesticides as increasing temperature will make them more soluble and hence affect negatively their bonding with the palm seeds adsorber. (El Bakouri et al. 2009).
(a) Adsorption kinetics of pesticides on acid-treated date stones (b) Adsorption isotherms of endrin on treated and untreated date stones determined at 25 °C. (El Bakouri et al. 2009)
Palm seeds were found to be effective for the adsorption of other pollutants too. For examples acids, that undergo the same removal mechanisms of dyes and metals and the same fitting models can be applied (Salman and Al-saad 2012). Moreover, activated carbon was extracted from palm dates and used to remove 2,4-Dichlorophenoxyacetic acid. Three isotherms models were applied to fit the behavior of the removal (Lamgmuir, Freundlicn and Temkin) at three different temperatures 30, 40 and 50 °C. Here the effect of temperature is related to the chemical nature of the adsorbent. Endothermic nature materials tend to attach more with the adsorber in high temperatures whereas exothermic behaves in opposite way and its bonding with adsorber become weaker at higher temperatures (Salman and Al-saad 2012).
11.5 Conclusion
This chapter focused on the applications of date seeds in the removal of different pollutants from wastewater. Most of these pollutants are metals, dyes and acids which are very difficult to be biologically degraded in nature causing major hazard to the environment. It was shown that the stones of the palm dates represent a great potential adsorber for these different pollutants with a very high rate of removal that can be compared with other well-known adsobers such as activated carbon. Studies revealed that the extraction and treatment procedure of the date stones adsorber affect greatly the removal efficiency. This comes along with the adsorption experimental parameters mainly pH, temperature and initial concentration. Understanding the adsorption kinetics needs fitting with standard adsorption models that take into account all of these different factors. Therefore, it can be concluded that the palm date stones which is mostly considered as a food waste can be turned to an efficient adsorber of highly toxic pollutants. Such treated water can be reused in other domestic applications heading in the way of sustainable water resources and cleaner environment.
References
Abbas AF, Ahmed MJ (2016) Mesoporous activated carbon from date stones (Phoenix dactylifera L.) by one-step microwave assisted K2CO3 pyrolysis. J Water Process Eng 9:201–207. https://doi.org/10.1016/j.jwpe.2016.01.004
Abd-Alla MH, El-Enany AWE (2012) Production of acetone– butanol–ethanol from spoilage date palm (Phoenix dactylifera L.) fruits by mixed culture of Clostridium acetobutylicum and Bacillus subtilis. Biomass Bioenergy 42:172–178. https://doi.org/10.1016/j.biombioe.2012.03.006
Ahmadpour A, Do DD (1996) The preparation of active carbons from coal by chemical and physical activation. Carbon 34(4):471–479. https://doi.org/10.1016/0008-6223(95)00204-9
Ahmed MJ (2016) Preparation of activated carbons from date (Phoenix dactylifera L.) palm stones and application for wastewater treatments: review. Process Saf Environ Prot 102:168–182. https://doi.org/10.1016/j.psep.2016.03.010
Ahsaine HA, Zbair M, Anfar Z, Naciri Y, El Alem N (2018) Cationic dyes adsorption onto high surface area ‘almond shell’ activated carbon: kinetics, equilibrium isotherms and surface statistical modeling. Mater Today Chem 8:121–132. https://doi.org/10.1016/j.mtchem.2018.03.004
Aitenneite H, Abboud Y, Tanane O, Solhy A, Sebti S, Bouari A (2016) Rapid and green microwave-assisted synthesis of silver nanoparticles using aqueous phoenix dactylifera L. (date palm) leaf extract and their catalytic activity for 4-Nitrophenol reduction. J Mater Environ Sci 7:2335–2339. http://Document/vol7/vol7_N7/251-JMES
Al-Farsi MA, Lee CY (2008) Optimization of phenolics and dietary fibre extraction from date seeds. Food Chem 108:977–985. https://doi.org/10.1016/j.foodchem.2007.12.009
Al-Ghouti MA, Li J, Salamh Y, Al-Laqtah N, Walker G, Ahmad MNM (2010) Adsorption mechanisms of removing heavy metals and dyes from aqueous solution using date pits solid adsorbent. J Hazard Mater 176(1–3):510–520. https://doi.org/10.1016/j.jhazmat.2009.11.059
Al-Ghouti MA, Al Disi ZA, Nasser Al-Kaabi N, Khraisheh M (2017) Mechanistic insights into the remediation of bromide ions from desalinated water using roasted date pits. Chem Eng J 308:463–475. https://doi.org/10.1016/j.cej.2016.09.091
Al-Hooti S, Sidhu J, Qabazard H (1998) Chemical composition of date seed fruit cultivars of United Arab Emirates. J Food Sci Technol 35:44–46. https://doi.org/10.1016/0308-8146(95)00051-J
Ali MA, Al-Hattab TA, Al-Hydary IA (2015) Extraction of date palm seed oil (Phoenix Dactylifera) by Soxhlet apparatus. Int J Adv Eng Technol 8:261–271. http://ijaet.org/media/3I27-IJAET0827749-v8-iss3-261-271
Al-Muhtaseb AH, Jamil F, Al-Haj L, Al-Hinai MA, Baawain M, Myint MTZ, Rooney D (2016) Efficient utilization of waste date pits for the synthesis of green diesel and jet fuel fractions. Energy Convers Manag 127:226–232. https://doi.org/10.1016/j.enconman.2016.09.004
Al-Mutairi NZ (2010) 2,4-Dinitrophenol adsorption by date seeds: effect of physico-chemical environment and regeneration study. Desalination 250:892–901. https://doi.org/10.1016/j.desal.2008.10.035
Al-Saidi HM (2016) The fast recovery of gold(III) ions from aqueous solutions using raw date pits: kinetic, thermodynamic and equilibrium studies. J Saudi Chem Soc 20:615–624. https://doi.org/10.1016/j.jscs.2013.06.002
Amani MM, Abdel AH, Seham AM, Azza A, Gehan S (2016) Aqueous and methanolic extracts of palm date seeds and fruits (phoenix dactylifera) protects against diabetic nephropathy in type II diabetic rats. Biochem Physiol 5:2168–2177. https://doi.org/10.1080/10408398.2010.499824
Ambigaipalan P, Shahidi F (2015) Antioxidant potential of date (Phoenix dactylifera L.) seed protein hydrolysates and carnosine in food and biological systems. J Agric Food Chem 63:864–871. https://doi.org/10.1021/jf505327b
Amiri M, Salavati-Niasari M, Akbari A, Gholami T (2017) Removal of malachite green (a toxic dye) from water by cobalt ferrite silica magnetic nanocomposite: herbal and green sol-gel autocombustion synthesis. Int J Hydrog Energy 4:24846–24860. https://doi.org/10.1016/j.ijhydene.2017.08.077
Ao W, Fu J, Mao X, Kang Q, Ran C, Liu Y, Zhang H, Gao Z, Li J, Liu G, Dai J (2018) Microwave assisted preparation of activated carbon from biomass: a review. Renew Sust Energ Rev 92:958–979. https://doi.org/10.1016/j.rser.2018.04.051
Banerjee M, Basu RK, Das SK (2018) Cr(VI) adsorption by a green adsorbent walnut shell: adsorption studies, regeneration studies, scale-up design and economic feasibility. Process Saf Environ Prot 116:693–702. https://doi.org/10.1016/j.psep.2018.03.037
Barnaby R, Liefeld A, Jackson BP, Hampton TH, Stanton BA (2017) Effectiveness of table top water pitcher filters to remove arsenic from drinking water. Environ Res 158:610–615. https://doi.org/10.1016/j.envres.2017.07.018
Barreveld WH (1993) Date palm products: FAO agricultural services bulletin no. 101. Food and Agriculture Organization of the United Nations Rome. http://www.fao.org/docrep/t0681e/t0681e00.htm
Belhachemi M, Rios RVRA, Addoun F, Silvestre-Albero J, Sepúlveda-Escribano A, Rodríguez-Reinoso F (2009) Preparation of activated carbon from date pits: effect of the activation agent and liquid phase oxidation. J Anal Appl Pyrolysis 86(1):168–172. https://doi.org/10.1016/j.jaap.2009.05.004
Benzie IFF, Strain JJ (1996) The reducing ability of plasma as a measure of ‘antioxidant power’- the FRAP assay. Anal Biochem 239:70–76. https://doi.org/10.1006/abio.1996.0292
Besbes S, Drira L, Blecker C, Deroanne C, Attia H (2009) Adding value to hard date (Phoenix dactylifera L.): compositional, functional and sensory characteristics of date jam. Food Chem 112:406–411. https://doi.org/10.1016/j.foodchem.2008.05.093
Carpentier SC, Witters E, Laukens K, Deckers P, Swennen R, Panis B (2005) Preparation of protein extracts from recalcitrant plant tissues: an evaluation of different methods for two-dimensional gel electrophoresis analysis. Proteomics 5:2497–2507. https://doi.org/10.1002/pmic.200401222
Crabbe E, Hipolito CN, Kobayashi G, Sonomoto K, Ishizaki A (2001) Biodiesel production from crude palm oil and evolution of butanol extraction and fuel properties. Process Biochem 37:65–71. https://doi.org/10.1016/S0032-9592(01)00178-9
Danish M, Hashim R, Ibrahim MN, Rafatullah M, Sulaiman O (2012) Surface characterization and comparative adsorption properties of Cr(VI) on pyrolysed adsorbents of Acacia Mangium wood and Phoenix dactylifera L. stone carbon. J Anal Appl Pyrolysis 97:19–28. https://doi.org/10.1016/j.jaap.2012.06.001
Danish M, Khanday WA, Hashim R, Binti Sulaiman NS, Akhtar MN, Nizami M (2017) Application of optimized large surface area date stone (Phoenix dactylifera) activated carbon for Rhodamin B removal from aqueous solution: box-Behnken design approach. Ecotoxicol Environ Saf 139:280–290. https://doi.org/10.1016/j.ecoenv.2017.02.001
Daoud M, Benturki O, Kecira Z, Girods P, Donnot A (2017) Removal of reactive dye (BEZAKTIV red S-MAX) from aqueous solution by adsorption onto activated carbons prepared from date palm rachis and jujube stones. J Mol Liq 243:799–809. https://doi.org/10.1016/j.molliq.2017.08.093
Devshony S, Ethesola A, Shani A (1992) Characterization and some potential application of date palm (Pheonix Dactylifera L) seeds and seed oil. J Am Oil Chem Soc 69:595–597. https://doi.org/10.1007/BF02636115
Dobias P, Pavlikova P, Adam M, Eisner A, Benova B, Venture K (2010) Comparison of pressurised fluid and ultrasonic extraction methods for analysis of plant antioxidants and their antioxidant capacity. Cent Eur J Chem 8:87–95. https://doi.org/10.2478/s11532-009-0125-9
El Bakouri H, Usero J, Morillo J, Rojas R, Ouassini A (2009) Drin pesticides removal from aqueous solutions using acid-treated date stones. Bioresour Technol 100:2676–2684. https://doi.org/10.1016/j.biortech.2008.12.051
El Messaoudi N, El Khomri M, Bentahar S, Dbik A, Lacherai A, Bakiz B (2016) Evaluation of performance of chemically treated date stones: application for the removal of cationic dyes from aqueous solutions. J Taiwan Inst Chem Eng 67:244–253. https://doi.org/10.1016/j.jtice.2016.07.024
El Nemr A, Khaled A, Abdelwahab O, El-Sikaily A (2008) Treatment of wastewater containing toxic chromium using new activated carbon developed from date palm seed. J Hazard Mater 152(1):263–275. https://doi.org/10.1016/j.jhazmat.2007.06.091
Etim UJ, Umoren SA, Eduok UM (2016) Coconut coir dust as a low cost adsorbent for the removal of cationic dye from aqueous solution. J Saud Chem Soc 20:S67–S76. https://doi.org/10.1016/j.jscs.2012.09.014
Farooq M, Bell AH, Almustapha MN, Andresen JM (2017) Bio-methane from an-aerobic digestion using activated carbon adsorption. Anaerobe 46:33–40. https://doi.org/10.1016/j.anaerobe.2017.05.003
Farsi MA, Alasalvar C, Morris A, Baron M, Shahidi F (2005) Comparison of antioxidant activity, anthocyanins, carotenoids, and phenolics of three native fresh and sun-dried date (Phoenix dactylifera L.) varieties grown in Oman. J Agric Food Chem 53:7592–7599. https://doi.org/10.1021/jf050579q
Farsi MA, Alasalvar C, Alabid M, Shoaily KA, Amry MA, AlRawah M (2007) Compositional and function characteristics of dates, syrups, and their by-products. Food Chem 104:943–947. https://doi.org/10.16966/2470-6086.108
Gazi M, Oladipo AA, Azalok KA (2017) Highly efficient and magnetically separable palm seed-based biochar for the removal of nickel. Sep Sci Technol 53(7):1124–1131. https://doi.org/10.1080/01496395.2017.1340955
Ghnimi S, Umer S, Karim A, Kamal-Eldin A (2017) Date fruit (Pheonix dactylifera L): an underutilized food seeking industrial valorization. NFS J 6:1–10. https://doi.org/10.1016/j.nfs.2016.12.001
Gueu S, Yao B, Adouby K, Ado G (2006) Heavy metals removal in aqueos solution by activated carbon prepared by coconut shell and seed shell of the palm tree. J Appl Sci 6(13):2789–2793. http://docsdrive.com/pdfs/ansinet/jas/2006/2789-2793.pdf
Hong Y, Tomas BF, Kader A, Mitchell A (2006) The flavonoid glycosides and procyanidin composition of Deglet Noor dates. J Agric Food Chem 54:2405–2411. https://doi.org/10.1021/jf0581776
Hosam MH, Wissam HI (2011) Nutritional quality of 18 date fruit varieties. Int J Food Sci Nutr 62:544–551. https://doi.org/10.3109/09637486.2011.558073
Ibrahim A, Akasha LC, Stephen RE (2012) Extraction and characterisation of protein fraction from date palm fruit seeds. Int J Food Eng 6:875–877. http://scholar.waset.org/1307-6892/3421
Islam MA, Tan IAW, Benhouria A, Asif M, Hameed BH (2015) Mesoporous and adsorptive properties of palm date seed activated carbon prepared via sequential hydrothermal carbonization and sodium hydroxide activation. Chem Eng J 270:187–195. https://doi.org/10.1016/j.cej.2015.01.058
Izanzar I, Dahbi M, Kiso M, Doubaji S, Komaba S (2018) Hard carbons issued from date palm as efficient anode materials for sodium-ion batteries. Carbon 137:165–173. https://doi.org/10.1016/j.carbon.2018.05.032
Jamil F, Al-Muhtaseb AH, Al-Haj L, Al-Hinai MA, Hellier P, Rashid U (2016) Optimization of oil extraction from waste “date pits” for biodiesel production. Energy Convers Manag 117:264–272. https://doi.org/10.1016/j.enconman.2016.03.025
Javadian H, Angaji MT, Naushad M (2014) Synthesis and characterization of polyaniline/γ-alumina nanocomposite: a comparative study for the adsorption of three different anionic dyes. J Ind Eng Chem 20:3890–3900. https://doi.org/10.1016/j.jiec.2013.12.095
Johari K, Saman N, Song ST (2016) Adsorption enhancement of elemental mercury by various surface modified coconut husk as eco-friendly low-cost adsorbents. Int Biodeter Biodegr 109:45–52. https://doi.org/10.1016/j.ibiod.2016.01.004
Khosravi R, Moussavi G, Ghaneian MT (2018) Chromium adsorption from aqueous solution using novel green nanocomposite: adsorbent characterization, isotherm, kinetic and thermodynamic investigation. J Mol Liq 256:163–174. https://doi.org/10.1016/j.molliq.2018.02.033
Kobielska PA, Howarth AJ, Farha OK, Nayak S (2018) Metal–organic frameworks for heavy metal removal from water. Coord Chem Rev 358:92–107. https://doi.org/10.1016/j.ccr.2017.12.010
Lee SU, Lee JH, Choi SH, Lee JS, Kameyama MO, Kozukue N, Levin CE, Friedman M (2008) Flavonoid content in fresh, home-processed, and light-exposed onions and in dehydrated commercial onion products. J Agric Food Chem 56:8541–8548. https://doi.org/10.1021/jf801009p
Li Q, Xinru C, Yuwen C, Yang X, Qiao W, YouLi Y, Wang X (2018) Strategies for the stable performance and rapid inhibition recovery of a thermophilic digester treating coffee wastes and the synergistic effects of microbes. Int Biodeter Biodegr. https://doi.org/10.1016/j.ibiod.2018.02.014
Luque de Castro MD, Priego-Capote F (2010) Soxhlet extraction: past and present panacea. J Chromatogr A 1217:2383–2389. https://doi.org/10.1016/j.chroma.2009.11.027
Mahdi Z, Yu QJ, El Hanandeh A (2018) Investigation of the kinetics and mechanisms of nickel and copper ions adsorption from aqueous solutions by date seed derived biochar. J Environ Chem Eng 6:1171–1181. https://doi.org/10.1016/j.jece.2018.01.021
Milovanovic V, Radulovic N, Todorovic Z, Stankovic M, Stojanovic G (2007) Antioxidant, antimicrobial and genotoxicity screening of hydro-alcoholic extracts of five Serbian Equisetum species. Plant Foods Hum Nutr 62:113–119. https://doi.org/10.1007/s11130-007-0050-z
Mittal A, Naushad M, Sharma G et al (2016) Fabrication of MWCNTs/ThO2 nanocomposite and its adsorption behavior for the removal of Pb(II) metal from aqueous medium. Desalin Water Treat 57:21863–21869. https://doi.org/10.1080/19443994.2015.1125805
Mohsen AE, Nezam M, Din EM (1995) Technological study on Dibis production from the Siwi date. Egypt J Food Sci 23:229–239. https://doi.org/10.1016/j.sjbs.2012.12.004
Nagwa MA, Lamia TA, Abou EK, Nabil ES, Lalita MC, Tom JM (2009) Flavonoid constituents and antimicrobial activity of date (phoenix dactylifera L.) seeds growing in Egypt. Med Aromat Plant Sci Biotechnol 49:719–721. https://doi.0906/MAPSB_3(SI1)/MAPSB_3(SI1)1-5o
Natarajan S, Hari CB, Tayade RJ (2018) Recent advances based on the synergetic effect of adsorption for removal of dyes from waste water using photocatalytic process. J Environ Sci 65:201–222. https://doi.org/10.1016/j.jes.2017.03.011
Naushad M, ALOthman ZA, Awual MR et al (2015a) Adsorption kinetics, isotherms, and thermodynamic studies for the adsorption of Pb2+ and Hg2+ metal ions from aqueous medium using Ti(IV) iodovanadate cation exchanger. Ionics (Kiel) 21:2237–2245. https://doi.org/10.1007/s11581-015-1401-7
Naushad M, Ahamad T, Alothman ZA et al (2015b) Synthesis, characterization and application of curcumin formaldehyde resin for the removal of Cd2+ from wastewater: kinetics, isotherms and thermodynamic studies. J Ind Eng Chem 29:78–86. https://doi.org/10.1016/j.jiec.2015.03.019
Naushad M, Khan MR, ALOthman ZA et al (2015c) Removal of BrO3− from drinking water samples using newly developed agricultural waste-based activated carbon and its determination by ultra-performance liquid chromatography-mass spectrometry. Environ Sci Pollut Res 22:15853–15865. https://doi.org/10.1007/s11356-015-4786-y
Nehdi I, Omri S, Khalil MI, Al-Resayes SI (2010) Characteristics and chemical composition of date palm (Pheonix canariensis) seeds and seed oil. Ind Crop Prod 32:360–365. https://doi.org/10.1016/j.indcrop.2010.05.016
Ogungbenro AE, Quang DV, Al-Ali K, Abu-Zahra MRM (2017) Activated carbon from date seeds for CO2 capture applications. Energy Procedia 114:2313–2321. https://doi.org/10.1016/j.egypro.2017.03.1370
Ogungbenro AE, Quang DV, Al-Ali KA, Vega LF, Abu-Zahra MRM (2018) Physical synthesis and characterization of activated carbon from date seeds for CO2 capture. J Environ Chem Eng 6(4):4245–4252. https://doi.org/10.1016/j.jece.2018.06.030
Pan Z, Qu W, Ma H, Atungulu GG, McHugh TH (2011) Continuous and pulsed ultrasound-assisted extractions of antioxidants from pomegranate Peel. Ultrason Sonochem 18:1249–1257. https://doi.org/10.1016/j.ultsonch.2011.01.005
Paniwnyk L, Beaufoy E, Lorimer JP, Mason TJ (2001) The extraction of Rutin from flower buds of Sophora Japonica. Ultrason Sonochem 8:299–301. https://doi.org/10.1016/S1350-4177(00)00075-4
Pathania D, Sharma G, Kumar A et al (2015) Combined sorptional–photocatalytic remediation of dyes by polyaniline Zr(IV) selenotungstophosphate nanocomposite. Toxicol Environ Chem 97:526–537. https://doi.org/10.1080/02772248.2015.1050024
Pathania D, Sharma A, Siddiqi ZM (2016) Removal of Congo red dye from aqueous system using Phoenix dactylifera seeds. J Mol Liq 219:359–367. https://doi.org/10.1016/j.molliq.2016.03.020
Pradhan RC, Venkatesh M, Rout PK, Naik S, Dalai AK (2009) Supercritical CO2 extraction of fatty oil from flaxseed and comparison with screw press expression and solvent extraction processes. J Food Eng 98:393–397. https://doi.org/10.1016/j.jfoodeng.2009.11.021
Pujari RR, Vyawahare NS, Kagathara VG (2011) Evaluation of antioxidant and neuroprotective effect of date palm (Phoenix dactylifera L.) against bilateral common carotid artery occlusion in rats. Ind J Exp Biol 49:627–633.http://nopr.niscair.res.in/handle/123456789/12528
Rahman MS, Kasapis S, Al-Kharusi NSZ, Al-Marhubi IM, Khan AJ (2007) Composition characterization and thermal transition of date pits powders. J Food Eng 80:1–10. https://doi.org/10.1016/j.jfoodeng.2006.04.030
Rajamohan N, Rajasimman M, Dilipkumar M (2014) Parametric and kinetic studies on biosorption of mercury using modified Phoenix dactylifera biomass. J Taiwan Inst Chem Eng 45:2622–2627. https://doi.org/10.1016/j.jtice.2014.07.004
Sait HH, Hussain A, Salema AA, Ani FN (2012) Pyrolysis and combustion kinetics of date palm biomass using thermogravimetric analysis. Bioresour Technol 118:382–389. https://doi.org/10.1016/j.biortech.2012.04.081
Saleh FA, Otaibi MM (2013) Antibacterial activity of date palm (PheonixDectyliferaL) fruit at different ripening stages. J Food Process Technol 4:285–290. https://doi.org/10.4172/2157-7110.1000285
Salman JM, Al-saad KA (2012) Adsorption of 2, 4-dichlorophenoxyacetic acid onto date seeds activated carbon: equilibrium, kinetic and thermodynamic studies. Int J Chem Sci 10(2):677–690. http://www.tsijournals.com/abstract/adsorption-of-2-4dichlorophenoxyacetic-acid-onto-date-seeds-activated-carbon-equilibrium-kinetic-and-thermodynamic-studi-10635.html
Saravanan RS, Rose JKC (2004) A critical evaluation of sample extraction techniques for enhanced proteomic analysis of recalcitrant plant tissues. Proteomics 4:2522–2532. https://doi.org/10.1002/pmic.200300789
Shahat A, Awual MR, Khaleque MA et al (2015) Large-pore diameter nano-adsorbent and its application for rapid lead (II) detection and removal from aqueous media. Chem Eng J 273:286–295. https://doi.org/10.1016/j.cej.2015.03.073
Shen B, Tian L, Li F et al (2017) Elemental mercury removal by the modified bio-lchar from waste tea. Fuel 187:189–196. https://doi.org/10.1016/j.fuel.2016.09.059
Tsaliki E, Kechagia U, Doxastakis G (2002) Evaluation of the properties of cottonseed protein isolates. Food Hydrocoll 16:645–652. https://doi.org/10.1016/S0268-005X(02)00030-9
Vijay K, Ankit G, Madhu N (2012) Comparative study of various methods for extraction of antioxidant and antibacterial compounds from plant seeds. J Nat Remedies 12:162–173. https://Ankit_Gupta6/publication/235437071
Wang H, Johnson LA, Wang T (2004) Preparation of soy protein concentration and isolate from extruded-expel led soybean meals. J Am Oil Chem Soc 81:713–717. https://doi.org/10.1007/s11746-004-966-8
Wang W, Vignani R, Scali M, Cresti M (2006) A universal and rapid protocol for protein extraction from recalcitrant plant tissues for proteomic analysis. Electrophoresis 27:2782–2786. https://doi.org/10.1002/elps.200500722
Wen X, Du C, Zeng G, Huang D, Zhang J, Yin L, Tan S (2018) A novel biosorbent prepared by immobilized Bacillus licheniformis for lead removal from wastewater. Chemosphere 200:173–179. https://doi.org/10.1016/j.chemosphere.2018.02.078
Zhou Y, Jin C, Li Y, Shen W (2018) Dynamic behavior of metal nanoparticles for catalysis. NanoToday 20:101–120. https://doi.org/10.1016/j.nantod.2018.04.005
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Al-Najar, B., Bououdina, M., Judith Vijaya, J., Nair, R.R., Tatarchuk, T. (2019). Removal of Toxins from the Environment Using Date Palm Seeds. In: Naushad, M., Lichtfouse, E. (eds) Sustainable Agriculture Reviews 34. Sustainable Agriculture Reviews, vol 34. Springer, Cham. https://doi.org/10.1007/978-3-030-11345-2_11
Download citation
DOI: https://doi.org/10.1007/978-3-030-11345-2_11
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-11344-5
Online ISBN: 978-3-030-11345-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)