Abstract
Plant oils have many additional applications other than biofuel. Potential industrial applications of plant lipids include the production of lubricants, solvents, surfactants, bioplastics, and rubber. There has also been a surge of interest in the utilization of plants as “biofactories” for the production of bioactive oils for human nutrition. Several types of plant-derived lipids are used for such purposes, including fatty acids within triacylglycerol, wax esters, and lipid-based polymers. Attempts to engineer plants that synthesize high levels of useful lipids are ongoing; however, this approach has proven challenging.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
-
Plant oils have many additional applications other than biofuel.
-
Potential industrial applications of plant lipids include the production of lubricants, solvents, surfactants, bioplastics, and rubber.
-
There has also been a surge of interest in the utilization of plants as “biofactories” for the production of bioactive oils for human nutrition.
-
There are several types of plant-derived lipids used for such purposes, including fatty acids within triacylglycerol , wax esters, and lipid-based polymers.
-
Attempts to engineer plants that synthesize high levels of useful lipids are ongoing; however, this approach has proven challenging.
5.1 Introduction
Although the production of plant-derived biodiesel has seemingly been at the forefront of nontraditional uses of oilseed crops, there are several additional applications for plant lipids that have been gaining momentum in recent years. As is the case for fuel, our society relies heavily on petroleum as a feedstock for numerous industrial products, such as lubricants, solvents, agricultural chemicals , surfactants, polymers, and food processing compounds (Fig. 5.1). As petrochemical stocks dissipate and concern for the environment grows, however, the manufacture of industrial petrochemicals is sure to be hard hit. The fact that many of these industrial products have an even greater value per unit than hydrocarbon fuels means that the development of sustainable bio-based raw materials for industrial use is becoming increasingly important (Vanhercke et al. 2013). Only about 10% of the global petrochemical feedstock is used for production of these value-added industrial products (Carlsson 2009). Therefore, unlike the development of biofuel for plant oils, the use of plant oils for production of value-added materials will have less of an effect on the global supply of edible oils.
While the majority of plant lipids currently valued for these purposes are derived from storage triacylglycerol (TAG), they are by no means the only lipids of importance that can be obtained from plant sources. In this chapter we will be discussing various types of industrially important lipid-related molecules, including fatty acids, wax esters, and lipid-based polymers. Although not bioproducts in the strictest sense, we will also consider the production of bioactive oils in plants for human and animal nutrition. Finally we will touch upon the challenges that have been encountered in attempts to use biotechnology as a means to harness the lipid biosynthetic pathways of plants for the production of molecules of interest.
5.2 Industrial Feedstocks
While there are a wide range of bio-based molecules that could be used for industrial purposes, plant lipids are particularly compatible for replacing petrochemical -based feedstocks due to their similar linear carbon chain structures. In fact, many plant-derived industrial feedstocks offer a variety of advantages over fossil fuel -based molecules. For example, while petroleum must be broken down and rebuilt into specialty chemicals, plants begin the process from simple precursors and build the complex molecules themselves, which has the potential to be very beneficial in terms of time, energy, and processing. Furthermore, a number of plant-based chemicals are actually known to perform better than their synthetic counterparts, as is the case for natural rubber.
Unfortunately, there are presently also several drawbacks to the use of plant oils for industrial purposes. The first of these is price – plant oils are currently more expensive than petroleum; however, they are becoming more competitive as petroleum prices rise. The second is availability – the majority of plant oils of industrial interest are often not present in domesticated crops and therefore tend to have limited distribution and variable supply.
5.2.1 Fatty Acids
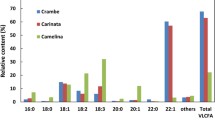
Currently, the major food oil crops dominate in terms of agricultural production and include canola-type Brassica napus , oil palm (mainly Elaeis guineensis ), soybean ( Glycine max ), cotton (Gossypium spp.) seed, peanut ( Arachis hypogea ), corn ( Zea mays ), sunflower ( Helianthus annuus ), and olive ( Olea europaea ), along with several less significant crops such as coconut ( Cocos nucifera ), flax ( Linum usitatissimum ) seed, sesame ( Sesamum indicum ), and safflower ( Carthamus tinctorius ) (Weselake et al. 2017). Industrial oils for various applications make use of the natural diversity of fatty acid structures found within plant storage TAG (Fig. 5.2); however, the majority of the food-use oils derived from the crops mentioned above consist of only five main fatty acids, including palmitic , stearic , oleic, linoleic, and α-linolenic acids (Fig. 4.2). Indeed, most fatty acids of interest for industrial use occur in a limited range of species (Table 5.1) or only in very minor quantities in domesticated crop species. As such, only a small number of fatty acids are actually used for industrial purposes at present compared with the large number produced in nature (over 300 types) (Badami and Patil 1980).
5.2.1.1 Medium-Chain Saturated Fatty Acids
One major use of plant oils for industrial purposes relies on the presence of a high proportion of saturated fatty acids of medium chain length, which are used for the production of surfactants such as detergents, soaps, and other personal care products. As mentioned in the previous chapter, the synthesis of fatty acids in plants normally occurs through the sequential addition of 2 carbon units at a time and terminates when the chain reaches 16 or 18 carbons. This occurs through the action of an acyl-acyl carrier protein (ACP) thioesterase (TE), which catalyzes the release of the new fatty acid chain for subsequent export from the plastid to the cytosol and incorporation into TAG (see Fig. 4.2). In certain tropical plants, specialized chain-length-specific TEs catalyze the release of fatty acids at an earlier stage in their synthesis, when the chain is 10, 12, or 14 carbons in length (Voelker et al. 1997).
The main fatty acid of this type currently in use industrially is lauric acid (12:0), which is found naturally in plants such as oil palm and coconut (Fig. 5.2, Table 5.1). Caprylic (8:0) and capric (10:0) acids are also useful in a range of industrial applications. Unfortunately, there are currently few commercially viable plants that are rich in any of these fatty acids, and they are therefore either fractionated out as very minor components of palm kernel or coconut oils or produced from petroleum. To make matters worse, the expanding production of oil palm is causing a considerable amount of habitat destruction in tropical regions, which makes finding an alternative source of these fatty acids of the utmost importance. Interestingly, members of the genus Cuphea , which produce extremely high levels of these three fatty acids (Badami and Patil 1980; Kim et al. 2015), may offer a potential source in temperate regions. Initial attempts to genetically engineer oilseed crops to produce these valuable fatty acids have also met with some success in certain cases, which bodes well for their future production in agronomically amenable plant species.
The production of medium-chain saturated fatty acids in transgenic plants represents an early success story. Lauric acid is typically found in tropical oils such as coconut and palm kernel oil and is widely used in food, pharmaceutical, and cosmetic industries. One of the earliest examples of metabolically engineering plants for oil quality was achieved through the expression of a 12:0-ACP -specific TE from the seeds of California bay laurel ( Umbellularia californica ) in canola, whereby they achieved up to 60% lauric acid in the second generation. However, the vast majority of this fatty acid was only present in the sn-1 and sn-3 positions (the two outer positions on the glycerol backbone) of TAG, suggesting that these plants were unable to incorporate 12:0 at the middle sn-2 position, which limited further increases in yield (Voelker et al. 1996; Wiberg et al. 2000).
In nature, only select plants are capable of introducing saturated fatty acids into the sn-2 position of TAG, which greatly influences the levels at which the plant can accumulate this type of fatty acid. Members of Lauraceae, Myristicaceae, and Lythraceae, as well as coconut, are known to produce seed TAG with a predominance of medium-chain saturated acyl groups at all three sn positions and are thus able to amass up to 90% of these fatty acids. This is very likely a direct result of their unusual lysophosphatidic acid acyltransferase (LPAAT) enzymes, which show a selective preference for this type of acyl substrate . LPAAT catalyzes the acyl-CoA-dependent acylation of lysophosphatidic acid (LPA) to produce phosphatidic acid (PA) in the Kennedy pathway (Fig. 5.3). While enzyme specificity refers to the ability of an enzyme to use a particular substrate when presented in isolation from others, and is thus not all that reflective of what occurs in a living plant cell , enzyme selectivity refers to the preference of an enzyme to use a particular substrate when presented with a mix of possible substrates. As one may expect, when transgenic plants were generated that produced both the specialized California bay laurel TE and an LPAAT from coconut, lauric acid was also observed at the sn-2 position of the glycerol backbone of TAG, and total levels of this fatty acid were increased (Knutzon et al. 1999; Wiberg et al. 2000). This approach involving the introduction of an LPAAT with a preference for particular fatty acids may also prove useful for overcoming low yields when metabolically engineering plants to produce other fatty acids that are naturally excluded from the sn-2 position, such as erucic acid (Lassner et al. 1995).
The role of lysophosphatidic acyltransferase (LPAAT) in the Kennedy pathway leading to triacylglycerol (TAG). Other abbreviations: CoA coenzyme A, DAG diacylglycerol, FA-CoA fatty acyl-coenzyme A, G3P sn-glycerol-3-phosphate, LPA lysophosphatidic acid . Information on this pathway can be found in numerous reviews on plant lipid biosynthesis (e.g., Chen et al. 2015)
5.2.1.2 Monounsaturated Fatty Acids
Monounsaturated fatty acids are also incredibly useful for industrial purposes (Harwood et al. 2017). This is due, at least in part, to the fact that when compared to oils rich in polyunsaturated fatty acids (PUFAs) , those containing high levels of monounsaturated fatty acids are far more resistant to oxidation. This makes for more stable oil and allows its direct use in such industrial products as biolubricants . In addition, monounsaturated fatty acids can be easily cleaved at their double-bond sites through chemical processing to yield many highly desirable feedstocks for industrial use, such as monomers that can be used to produce various types of nylon (polyamides).
Oleic acid (18:1Δ9cis; hereafter referred to as 18:1) is probably the most common of the monounsaturated fatty acids and typically makes up approximately 25–55% of the oil from major oilseed crops (Table 4.2). It is generated via the action of a stearoyl-ACP desaturase (SAD) enzyme , which catalyzes the addition of a double bond at a specific position within a fatty acid chain (see Fig. 4.2). In this case, the enzyme is termed as Δ9 SAD , which catalyzes the insertion of a double bond at the Δ9 position of the stearoyl moiety (18:0) of stearoyl-ACP. Although high oleic acid cultivars (75–85%) of several oilseed crops have been bred for food use (Takagi and Rahman 1996; Schierholt et al. 2000), the significant residual levels of PUFAs that remain in these oils cause issues for industrial applications.
There are also a number of naturally occurring plant oils that possess monounsaturated fatty acids with double bonds at alternative positions. For example, seeds from Umbelliferae species, such as Daucus carota (carrot) and Coriandrum sativum , produce seed oil with the major constituent being petroselinic acid (18:1Δ6cis). This fatty acid has the potential to be very significant for industrial use as it can be split using ozonolysis to yield lauric acid and adipic acid (6:0), which is a building block of 6,6 nylon, a product that is currently generated from petroleum and has a very large annual production . The synthesis of this unusual monounsaturated fatty acid results, at least in part, from the activity of a plastidial Δ4 16:0-ACP desaturase that catalyzes the addition of a double bond to palmitoyl-ACP, with the resulting molecule then being elongated to petroselinic acid (Cahoon and Ohlrogge 1994). This desaturase has distinct substrate specificity in terms of acyl chain length compared to other acyl-ACP desaturases.
Additional examples of acyl-ACP desaturases with unique substrate specificities also exist, including the Δ9 18:0-ACP desaturase from Thunbergia alata (Cahoon et al. 1994) and Δ9 16:0-ACP desaturases from Doxantha spp. and Asclepia syriaca (Cahoon et al. 1997a, 1998), which in the latter case allows the production of palmitoleic acid (16:1Δ9cis). This fatty acid and its elongation product cis-vaccenic acid (18:1Δ11cis) are two additional monounsaturated fatty acids that have garnered industrial interest due to the recent development of olefin metathesis. This technology allows the production of 1-octene from either of these fatty acids, which is a very valuable industrial feedstock with particular use in the generation of polyethylene and plasticizers. Both palmitoleic acid and cis-vaccenic acid are normally present in only very small amounts (<2%) in the majority of plant oils, with the exception of such species as Hippophae rhamnoides (sea buckthorn) and Doxantha unguis-cati (cat’s claw), which can accumulate up to 80% of these two fatty acids in its seed oil (Chisholm and Hopkins 1965). As has been the case for the aforementioned monounsaturated fatty acids, the major determinant of whether these fatty acids are present at high levels in a particular species appears to be the presence of a specific acyl-ACP desaturase. Interestingly, very few amino acid differences need to exist in these various desaturase enzymes for distinct changes in substrate specificities to occur; in fact, it has been found that the alteration of as few as five amino acid residues can result in the conversion of a Δ6 16:0-ACP desaturase into a Δ9 18:0-ACP desaturase (Cahoon et al. 1997b).
Another relatively uncommon monounsaturated fatty acid is the very long-chain erucic acid (22:1Δ13cis), which is used to produce erucamide (a slipping agent used in the production of polyethylene and propylene films) (McVetty et al. 2016). This fatty acid is generated through the sequential elongation of oleoyl-CoA by an extraplastidial elongase system (see Fig. 4.3). Modern-day canola, in which erucic acid is essentially absent, is the result of a nonfunctional fatty acid elongase 1 (Katavic et al. 2002). Erucic acid is produced by such plants as Limnanthes douglasii and members of the Brassicaceae. Currently, it is derived mainly from the oil of high-erucic rapeseed (HEAR), which contains 45–55% of this fatty acid. Unfortunately, the continued production of HEAR is set to become increasingly difficult due to the ever-expanding growth of food-use canola (low-erucic acid rapeseed), as the two crops are cross-fertile. This means that HEAR must be grown in complete isolation from canola crops to preclude contamination with erucic acid, which is thought to be detrimental to our health when ingested (Zhang et al. 1991), a feat that will be highly challenging. As a result of this, biotechnological approaches to produce high levels of erucic acid in alternative oilseed crops are underway (Zhu et al. 2016). Indeed, the production of up to almost 80% erucic acid in transgenic Crambe abyssinica was achieved using a gene stacking strategy involving multiple expression cassettes (Li et al. 2012).
5.2.1.3 Unusual Fatty Acids
In addition to the fatty acids described above, there are several specialty industrial oils that are derived from fatty acids found only in very specific plants that contain unusual functional groups, such as hydroxyl groups or epoxy bridges, unusual double-bond structures, or cyclic structures (Fig. 5.2). Many of these fatty acids result from the activity of divergent members of the fatty acid desaturase (FAD) 2 family, which is typically an endoplasmic reticulum (ER)-bound enzyme that catalyzes the addition of the second double bond at the Δ12 position of oleic acid to produce linoleic acid (18:2Δ9cis,12cis; hereafter referred to as 18:2) (Okuley et al. 1994). As indicated previously, FAD2 and FAD3 catalyze PUFA formation at the level of phosphatidylcholine (PC) (see Fig. 4.3). While functional variants of this enzyme are rare in nature, there are several plant species that accumulate storage TAG with extremely elevated amounts of these unusual fatty acids.
One fairly well-known example is castor oil, which is obtained from the castor bean plant ( Ricinus communis ) and contains very high levels (90%) of the hydroxy fatty acid ricinoleic acid (12-hydroxy-18:1Δ9cis) (Fig. 5.2, Table 5.1) (Badami and Patil 1980), which is synthesized from oleic acid through the action of a FAD2 -related hydroxylase (Broun and Somerville 1997). This rare fatty acid is becoming an increasingly important industrial feedstock, as it has the ability to undergo pyrolytic cleavage at the Δ12 position, resulting in the production of undecylenic acid (11:1Δ10cis), which is used in the production of various cosmetics, pharmaceuticals, and nylon (McKeon 2016). Castor oil can also be used directly in several applications, including the production of polyurethane and high-performance lubricants (McKeon 2016; Vanhercke et al. 2013). Unfortunately, the potential for castor as a future industrial oil crop is very limited due to the presence of a highly toxic protein (ricin) in its seeds, along with many allergens; as a result, attempts to produce alternative sources of this fatty acid through genetic engineering are currently under way.
When the castor fatty acid hydroxylase, which catalyzes the hydroxylation of oleic acid to ricinoleic acid, was introduced during seed development in the model plant Arabidopsis thaliana , only 18% ricinoleic acid was produced (Broun and Somerville 1997). While this result was promising conceptually, much higher levels are required for the plant to be useful in an industrial sense. Interestingly, both castor diacylglycerol acyltransferase (DGAT) 2 and phospholipid:diacylglycerol acyltransferase (PDAT) were subsequently shown to prefer substrates containing ricinoleic acid and have thus been implicated in the accumulation of high amounts of this fatty acid in this species. The DGAT -catalyzed reaction was discussed in Chapter 4 and is shown again in Fig. 5.4 in the context of a plant cell that has been engineered to produce TAG containing ricinoleic acid. In contrast, PDAT catalyzes the acyl-CoA -independent formation of TAG using PC as a fatty acyl donor (Fig. 5.4) (Ståhl et al. 2004). Co-introduction of castor DGAT2 and PDAT, along with the castor fatty acid hydroxylase, resulted in a significant boost in ricinoleic acid levels in transgenic A. thaliana seeds (Burgal et al. 2008; Kim et al. 2011; Van Erp et al. 2011). Similarly, when castor phosphatidylcholine :diacylglycerol cholinephosphotransferase (PDCT) was co-introduced along with the castor fatty acid hydroxylase in A. thaliana, ricinoleic acid levels in the seed oil were also increased compared to when the hydroxylase was introduced by itself (Hu et al. 2012). PDCT catalyzes the transfer of the phosphocholine head group of PC-modified fatty acids, such as ricinoleic acid, to diacylglycerol (DAG) produced in the Kennedy pathway (Fig. 5.4) (Lu et al. 2009). As a result, hydroxy fatty acid -enriched DAG is now available for the Kennedy pathway leading to TAG, and DAG enriched in oleic acid originally produced in the Kennedy pathway is now available for further hydroxylation at the level of PC. Even with these further modifications, the ricinoleic acid content still only reached about 27%, indicating that additional engineering work was required to achieve higher levels. In this regard, additional enzyme-catalyzed reactions are being targeted for modification. For example, phospholipase A2 (PLA2) catalyzes the release of fatty acyl chains from the middle position (sn-2) of PC . Interestingly, a low molecular mass PLA2 from castor has been shown to exhibit preference for hydroxy fatty acyl chains (Bayon et al. 2015). Introduction of this castor PLA2 into transgenic A. thaliana expressing a gene encoding castor oleic acid hydroxylase resulted in a significant decrease in the ricinoleic acid content of PC .
The interplay of triacylglycerol (TAG) biosynthesis with membrane metabolism in a hypothetical plant system producing TAG enriched in hydroxy fatty acids. These pathways may also apply to other fatty acids produced on phosphatidylcholine (PC) such as α-linolenic acid. Diacylglycerol (DAG) produced in the Kennedy pathway can be incorporated into PC where hydroxylation of oleic acid (18:1) to form ricinoleic acid (12-OH 18:1Δ9cis) occurs at the middle position of the glycerol backbone. Various acyl-trafficking processes can lead to the formation of TAG enriched in ricinoleic acid . Other abbreviations: CoA coenzyme A, DAG diacylglycerol, DGAT diacylglycerol acyltransferase, FA-CoA fatty acyl-coenzyme A, G3P sn-glycerol-3-phosphate, LPA lysophosphatidic acid, LPAAT lysophosphatidic acid acyltransferase, LPC lysophosphatidylcholine , PA phosphatidic acid , Pi inorganic phosphate, PAP phosphatidic acid phosphatase , PDAT phospholipid:diacylglycerol acyltransferase , PDCT phosphatidylcholine :diacylglycerol cholinephosphotransferase, phocho phosphocholine head group, PLA2 phospholipase A2, PLD phospholipase D . This figure is based on information from the following sources: Vanhercke et al. (2013), Bayon et al. (2015), Chen et al. (2015), Yang et al. (2017)
The long-chain monounsaturated epoxy fatty acid vernolic acid (12,13-epoxy-18:1Δ9cis) is produced in abundance in the seed oil of Asteraceae , Vernonia , and Euphorbia spp. (Fig. 5.2, Table 5.1). This unusual fatty acid , which contains an epoxy group, can be used as a feedstock for the production of adhesives, varnishes, paints, and industrial coatings. The epoxy group of vernolic acid is added through the catalytic action of either a FAD2 -related epoxygenase or a cytochrome P450 , depending on the species (Cahoon et al. 2002). Yet another example of FAD2-like enzymes with a divergent function are the FAD2-related conjugases commonly found in Momordica charantia , Impatiens balsamina , and Vernicia fordii (tung tree) (Dyer et al. 2002). These enzymes catalyze the formation of conjugated fatty acids, which contain non-methylene-interrupted double bonds within their structure , from either linoleic acid or α-linolenic acid (18:3Δ9cis,12cis,15cis; hereafter referred to as 18:3) acid. M. charantia and tung tree seed in particular contain very high levels of the conjugated α-eleostearic acid (18:3Δ9cis,11trans,13trans), which accumulates to 60% and 82% of their total seed oil, respectively. Tung tree oil possesses very unique drying properties and is highly valued for the protection of furniture. As is the case for castor , the potential for the future expansion of the production of the majority of plants that synthesize these unusual fatty acids is very limited due to their unsuitability as commercial crops. Unfortunately, transgenic approaches in which these genes have been introduced into non-native plant species have shown only minimal success as of yet. For example, introduction of a Vernonia galamensis Δ12-epoxygenase, along with a DGAT2 from the same species, in transgenic plants has resulted in the production of seeds containing up to only 26% vernolic acid (Li et al. 2010).
5.2.2 Plant-Derived Wax Esters
TAGs and their associated fatty acids certainly dominate in terms of the plant lipids most commonly used for industrial applications at present. However, wax esters, which are made up of a long-chain fatty acid esterified to a long-chain fatty alcohol , are another class of plant lipid that is used in the production of specialized industrial products. Plant waxes are present at high levels in their cell walls, where they function in light absorption and reflection, and also provide protection from desiccation and resistance to pathogens (Kolattukudy 1970). These functional properties are a result of their solid state, high hydrophobicity, and excellent resistance to hydrolytic degradation, all of which are also incredibly useful for potential industrial applications, especially in the form of lubricants (Heilmann et al. 2012).
The plant wax that is most commonly used for industrial purposes at present is obtained from the leaves of the carnauba palm tree ( Copernicia prunifera ), and is highly valued as a surface polish and protectant, with many additional industrial uses (Vanhercke et al. 2013). Wax esters derived from the seeds of the desert shrub jojoba ( Simmondsia chinensis ), however, are also gaining interest. The seed oil produced by this species is unique, as straight-chain wax esters that are in liquid form at room temperature serve as the predominant storage lipid in their seeds in place of TAG, accumulating to levels of up to 50% of their weight (Ohlrogge et al. 1978). Currently, they are used as ingredients in cosmetics and personal care products such as moisturizers, shampoos, and conditioners, as they are quite similar to human sebum. They are also used as an alternative of sperm whale oil, which is likewise made up mainly of wax esters and was widely used in high-pressure and high-temperature lubricants prior to the ban on importing whale oil to the USA in 1971. Unfortunately, since jojoba wax esters are made up mainly of relatively short monounsaturated C20 and C22 fatty acids and fatty alcohols, they have too high of a melting point (approximately 9 °C) for their widespread application as lubricants, particularly in colder climates. While this plant is grown commercially for its seed wax in certain parts of the world, its agricultural expansion is limited by its low yield and requirement for a warm climate. In addition, harvest of the beans is typically carried out by hand as maturation occurs sporadically, which makes it a very labor-intensive crop. Therefore, its wax esters are prohibitively expensive and are not able to compete with cheaper petroleum-based lubricants (Vanhercke et al. 2013).
Similarly to TAGs, the specific properties of wax esters result from their carbon chain length and in some cases from the occurrence of functional groups such as methyl branches or diesters (Biester et al. 2012). However, it appears that wax ester synthesis may be a more straightforward process than TAG biosynthesis, and in principle only three additional enzymes would be required for their production in seeds: a fatty acid elongase to yield high levels of fatty acids with a chain of at least 20 carbons in length, a fatty acid reductase (FAR) to convert fatty acids to fatty alcohols, and a wax synthase (WS) to esterify a fatty alcohol to a fatty acid. This final step in the biosynthesis of wax esters is somewhat analogous to the final DGAT-catalyzed acylation of DAG to produce TAG in the Kennedy pathway, and in some cases, DGAT enzymes possess dual WS/DGAT activity (Kalscheuer and Stienbüchel 2003; Li et al. 2008). As expected, the transgenic production of wax esters in plant seeds has proven to be very promising.
In theory, the transgenic production of wax esters would require the activity of only a fatty acid elongase to generate high levels of fatty acids with chain lengths of C20 or longer, as well as those of a FAR and WS. This was indeed confirmed in transgenic A. thaliana through the introduction of a fatty acid elongase, along with a jojoba FAR and WS (Lardizabal et al. 2000), whereby a major proportion of seed TAG was replaced by wax esters (up to 70% of the oil by weight). Since the introduced wax ester biosynthesis pathway was running alongside the naturally occurring (endogenous) TAG biosynthetic pathway in these plants, it is reasonable to assume that suppression of TAG assembly could result in even better results.
In addition to their use as biolubricants , wax esters can also be hydrolyzed to yield fatty acids and fatty alcohols. Indeed, wax ester biosynthesis may provide a more amenable system for engineering the accumulation of useful fatty acids. Therefore, other fatty acid-modifying enzymes, such as hydroxylases or TEs, could be incorporated into the mix to provide substrates for the synthesis of various wax esters. By combining various permutations of genes encoding these enzymes, a very large number of wax esters with different compositions and functionalities could thus be achieved. In line with this, the worldwide EU-FP7 ICON (Industrial Oil crops producing added value Oils for Novel chemicals) project was established in an attempt to further research in this area (http://icon.slu.se).
5.2.3 Lipid-Derived Polymers
Polymers are large molecules that are made up of a number of repeated monomer subunits covalently linked through the process of polymerization. Examples of lipid-derived polymers include many plastics, Styrofoam, and rubber. The properties of a particular polymer are influenced by several factors including structure and architecture, as well as chain length.
5.2.3.1 Bioplastics
In the USA alone, two million plastic bottles are used every 5 min, and 60,000 plastic bags are used every 5 s (Rauber 2011). The majority of this plastic is petroleum-based and is not recycled nor is it biodegradable. Therefore, the bulk of it ends up either in landfills or the ocean. Plastics are composed of high molecular weight polymers of simple monomers, such as ethylene and styrene. While most are hydrocarbon-based with N, O, S, or halogen (chlorine or fluorine) substitutions, a minority are based on silicone. There are many different types of plastics, including polyethylene, polypropylene, polyvinylchloride, polytetrafluoroethylene, nylon, and polystyrene, which are classified according to their composition or functional properties.
One class of plastic that is generated in nature are the polyhydroxyalkanoates (PHAs) , which, unlike starch-based plastics such as polylactic acid , are biodegradable and UV-stable. PHAs are linear polymers of 3-hydroxy fatty acids with various side chains that give them different properties. They are a storage compound produced in response to nutrient deficiency or excess carbon supply by certain soil bacteria through the fermentation of either lipids or sugars. The particular polymer generated depends upon both the bacterial species and growth conditions, with the resulting molecules accumulating as intracellular granules (Fig. 5.5) (Suriyamongkoi et al. 2007).
Polyhydroxyalkanoates (top), which accumulate as granules within the bacteria that produce them (bottom) (Image source: AOCS Lipid Library http://lipidlibrary.aocs.org/Biochemistry/content.cfm?ItemNumber=41298)
There are two main classes of PHAs, which are based upon the chain length of their hydroxy fatty acids. Short-chain PHAs comprise hydroxy fatty acids with three to five carbon atoms and have properties that are similar to polypropylene. Medium-chain PHAs comprise hydroxy fatty acids with 6–14 carbon atoms and resemble elastomers and rubbers. The simplest and most commonly occurring form of PHA is poly-β-hydroxybutyrate (PHB), which is made up of 1000–3000 short-chain hydroxy fatty acid monomers. While PHB on its own is rather brittle and stiff, copolymerization with other fatty acids, such as β-hydroxyvalerate, results in increased flexibility (Suriyamongkoi et al. 2007). PHA containers undergoing aerobic biodegradation (Madison and Huisman 1999) are shown in Fig. 5.6.
Degradation of polyhydroxyalkanoate containers in aerobic sewage sludge after treatment for 0, 2, 4, 6, 8, and 10 weeks (left to right) (Reproduced from Madison and Huisman (1999) with permission from the American Society for Microbiology)
Although plants are not natural producers of PHAs, oilseed crops have been targeted as potential “factories” for this polymer as the products of fatty acid breakdown can provide abundant substrate for its synthesis. In bacteria, there are three enzymes required for the conversion of propionyl-CoA and/or acetyl -CoA to PHA : β-ketothiolase , acetoacetyl-CoA reductase , and PHA synthase . Plants already possess their own ketothiolase, which is involved in isoprenoid metabolism, and would therefore theoretically only require the introduction of bacterial acetoacetyl-CoA reductase and PHA synthase. To date, several approaches have been used to successfully produce PHAs in plants at moderate levels (Table 5.2) (Bohmert-Tatarev et al. 2011); however, further research will likely be necessary to increase the yield of this product in order for these plants to be of industrial use.
5.2.3.2 Natural Rubber
Natural rubber consists of a high molecular weight polymer of isoprene (most often cis-1,4-polyisoprene), with other ill-defined minor components that contribute to its functionality, and is used extensively in many products with a global consumption of about 11 million metric tons per year. Natural rubber is synthesized within microscopic particles produced in the cytosol of certain plants and fungi, although the precise genetic mechanism of its biosynthesis has yet to be fully elucidated. What is known is that it comprises a rather complicated side branch of the isoprenoid pathway involving a rubber transferase (or rubber transferase complex) that is embedded within a membrane surrounding the rubber particle core (Cornish and Xie 2012). To confuse matters further, it appears that different species tend to exhibit variations in their exact mode of rubber biosynthesis, accumulation, and compartmentalization.
Currently, the main commercial source of natural rubber is the latex of the rubber tree ( Hevea brasiliensis ). Unfortunately, rubber tree plantations are very labor-intensive due to the fact that the latex cannot be harvested mechanically, and they are limited to warm, humid, and sunny climates such as Thailand, Indonesia, and Malaysia, which together account for the majority of its production. While H. brasiliensis is native to South America, it is not cultivated widely there due to the presence of South American leaf blight. While this fungal pathogen appears to remain limited to South America at this time, the fact that the genetic base of the various plantations worldwide is extremely narrow makes the commercial production of H. brasiliensis incredibly susceptible to widespread failure if/when this disease were to spread (Onokpise and Louime 2012). To make matters worse, rubber tree plantations are quickly being replaced with palm oil plantations to meet biofuel demands and increase profit to the farmers, and allergies to H. brasiliensis latex are very common and on the rise (Bousquet et al. 2006). As a result of all these factors, the bulk of rubber produced today is generated synthetically from petroleum. Natural rubber, however, is known to have superior performance to synthetic rubber in some applications, such as aircraft tires and medical devices (Imle 1978), making synthetic rubber not only an unsustainable and environmentally unfriendly source of this product but also an inadequate one.
Rubber-containing latex is produced in varying amounts by approximately 2500 plant species and is generally exuded following injury where it mainly plays a role in disease resistance, tolerance to environmental stress, and wound healing. However, in the vast majority of these species, the plant is difficult to cultivate and/or tap, or the rubber is not suited for industrial purposes (Belcher et al. 2004). Unfortunately, due to the complexity of the rubber biosynthetic pathway, little progress has been made as of yet in the metabolic engineering of plants capable of producing this product.
There are two major candidate species that are currently being studied for their potential as alternative sources of natural rubber: guayule ( Parthenium argentatum ) and Russian dandelion (Taraxacum kok-saghyz) (Table 5.3) (Van Beilen and Poirier 2007). Guayule is a flowering shrub in the Asteraceae family that is native to the southwestern USA and northern Mexico. It has been explored as an alternative source of rubber several times throughout history, but following World War II, its expense compared to H. brasiliensis-derived rubber was considered to be prohibitive. Despite its cost, as well as issues with its ability to only produce rubber seasonally and technical challenges with extraction and processing, it has seen a growing resurgence in interest due to the fact that unlike rubber extracted from H. brasiliensis, it is hypoallergenic, which makes it an ideal candidate for various medical applications (Van Beilen and Poirier 2007). Another attraction of this species is its potential as a biofuel crop, as it is a nonfood species and can be grown in arid regions where food crops would fail, thus limiting the food versus fuel conundrum.
Russian dandelion was grown on a rather large scale in the Soviet Union in the 1930s and 1940s, as well as in other parts of the world during World War II when supplies of H. brasiliensis-derived rubber were threatened. During this time, it was found that tires produced using rubber derived from Russian dandelion were as resilient as those from H. brasiliensis rubber and better than those generated from guayule. As is the case for guayule-derived rubber, its expense compared to that of H. brasiliensis has prohibited its further development, although there has been a resurgence of interest in this species. While it is potentially even more allergenic than rubber obtained from H. brasiliensis and would require domestication, its short life span and amenability to tissue culture and transformation would facilitate agronomic improvement (Van Beilen and Poirier 2007).
5.3 Production of Bioactive Oils in Plants
Over the past few decades, a great number of studies have shown a link between the consumption of certain lipids, such as fat-soluble vitamins, phytosterols, carotenoids, and particular polyunsaturated fatty acids , and improved human health. Indeed, many of these compounds have been associated with the prevention, delay, or treatment of such serious diseases as cancer, osteoporosis, cardiovascular disease, and a number of immune disorders, to name a few (Chen et al. 2013). These oils occur naturally in certain foods and are termed bioactive , which refers to their potential ability to promote human health. Bioactive oils are often categorized as nutraceuticals since they have the potential to offer health benefits beyond normal nutrition. While milk products, fish, and particular vegetables and seeds can often be a rich source of these healthy molecules, it has been widely reported that the general public is in fact not consuming enough of many of them. Therefore, it is now becoming the norm within the food industry to fortify foods, either for human consumption or animal feed, with bioactive lipids. In an attempt to produce some of these compounds at higher levels in a more sustainable manner, metabolic engineering of plants is being explored (Weselake et al. 2017). Although bioactive oils are not bioproducts in the strictest sense, it is useful to discuss the molecular strategies leading to their production in transgenic plants.
5.3.1 Very Long-Chain Polyunsaturated Fatty Acids
One of the most beneficial types of bioactive lipids are believed to be the omega-3 very long-chain polyunsaturated fatty acids (VLC-PUFAs) , which are 20 carbons or more in length with at least three methylene-interrupted double bonds in the cis-position , the first of which is located three carbons from the methyl end of the chain (Venegas-Calerón et al. 2010). In particular, eicosapentaenoic acid (EPA; 20:5Δ8cis,11cis,14cis,17cis) and docosahexaenoic acid (DHA; 22:6Δ4cis,7cis,10cis,13cis,16cis,19cis) (Fig. 5.2) have been shown to have a myriad of health benefits. For example, clinical trials have demonstrated protective roles for these fatty acids in the prevention of cardiovascular disease, and they are also linked to the prevention of obesity and type 2 diabetes, as well as having a role in neonatal development (Horrocks and Yeo 1999; Innis 2000; Das 2002). Furthermore, they are currently being studied for a vast number of additional health-promoting properties , such as their ability to protect against certain types of cancer (Rose and Connolly 1999), attention-deficit disorder (Richardson and Puri 2002), and dementia (Morris et al. 2003). Interestingly, stearidonic acid (18:4Δ6cis,9cis,12cis,15cis), which is an intermediate in the pathway leading to EPA and DHA , and flax oil containing this PUFA have also been shown to have anticancer properties (Subedi et al. 2015; Yu et al. 2015). While EPA and DHA are vital components of human metabolism, most animals (including humans) have a very limited ability to produce them from their α-linolenic acid (18:3) precursor, and therefore, their intake via food is a necessity (Riediger et al. 2009). The principal source of EPA and DHA for human nutrition is cold-water marine fish such as salmon, mackerel, and tuna, which are used for both direct consumption and the isolation of nutritional additives. Like the majority of other animals, these fish do not synthesize these fatty acids themselves but accumulate them from their dietary intake of marine microbes such as algae, which are able to generate VLC-PUFAs de novo (Williams and Burdge 2006).
Unfortunately, due to decades of overfishing (Pauly et al. 2005), as well as concerns relating to marine pollution and the resulting accumulation of dioxins, heavy metals, and polychlorinated biphenyls in fish (Yokoo et al. 2003), there is a dire need to develop alternative and sustainable sources of these VLC-PUFAs . Indeed, even the use of farmed fish for this purpose has proven problematic in terms of sustainability, since farmed fish require greater input amounts of EPA - and DHA -containing feed than what can be harvested from the finished product. Several strategies are currently under study in an attempt to overcome these challenges. For example, approaches in which algal sources themselves are utilized as a production platform for the synthesis of these fatty acids are currently used for certain high-value applications such as infant formula. Such cultures, however, are expensive to maintain, necessitate specialized facilities, have a significant environmental footprint, and are limited in terms of scalability (Lee 2001). In contrast, the metabolic engineering of plants to produce relatively high levels of EPA and/or DHA is proving to be achievable, which holds out promise for a sustainable means of generating these bioactive fatty acids.
While certain lower plants (such as mosses) have the capacity to synthesize significant amounts of VLC-PUFAs , these fatty acids are virtually absent from higher plants, although they can provide a rich source of their precursor fatty acid, α-linolenic acid (Venegas-Calerón et al. 2010). In theory, the conversion of native plant fatty acids to VLC-PUFAs requires a minimum of three additional enzymes, including two ER -bound FADs and an elongase. To date, genes encoding these enzymes have been successfully isolated from a range of VLC-PUFA-synthesizing organisms, and several have been expressed in oilseed crops, providing proof of concept that the VLC-PUFA pathway could operate in a higher plant system (Abbadi et al. 2004; Qi et al. 2004); however, only very low levels of EPA and in some cases DHA were obtained. Additionally, the majority of these transgenic plants also contained high levels of undesirable omega-6 metabolic intermediates, which are completely lacking in marine oils. Very recently, a new study has been published in which researchers successfully generated transgenic Camelina sativa that produced levels of both EPA and DHA that were equivalent to those seen in fish oils through the introduction of multigene constructs expressing five to seven cassettes encoding various desaturases and elongases (Ruiz-Lopez et al. 2014). This study provides promise that generating a sustainable, terrestrial, source of EPA and DHA will indeed be feasible in the future.
5.3.2 Conjugated Linolenic Acids
Although PUFAs typically possess double bonds that are separated by at least one methylene group, the seeds of certain plant species produce oils that are rich in PUFAs bearing conjugated double bonds. Conjugated linolenic acids (CLNAs) bear three conjugated double bonds and can be found in the seed oils of plants from several families, including Rosaceae, Punicaceae, Chrysobalanaceae, Lythraceae, Cucurbitaceae, and Euphorbiaceae (Badami and Patil 1980). Many of these oils are currently under intense study for their nutraceutic applications , especially due to growing evidence of their cytotoxic and antiproliferative effects on tumor cells (Igarashi and Miyazawa 2000; Tsuzuki et al. 2004; Shinohara et al. 2012), as well as their alteration of lipid metabolism in animals (Koba et al. 2002).
Punicic acid (18:3Δ9cis,11trans,13cis) is one such fatty acid and is found predominantly in pomegranate ( Punica granatum ) seeds where it constitutes approximately 65% of the oil. Pomegranate oil/punicic acid has demonstrated anticancer activity against prostrate (Gasmi and Sanderson 2010) and breast cancer cells (Sturgeon and Ronnenberg 2010) and has also been linked to the prevention of obesity (Arao et al. 2004), type 2 diabetes (Vroegrijk et al. 2011), osteoporosis (Spilmont et al. 2013), and inflammatory disease (Bassaganya-Riera et al. 2011). Furthermore, it has also been established in human clinical trials that the consumption of pomegranate seed oil has a positive effect on cardiovascular health (Mirmiran et al. 2010). Tropical and subtropical pomegranate crops are very limited in their agronomic range, require warm climates, exhibit susceptibility to various insect pests and fungal pathogens, and currently require hand-harvesting. Therefore, the transfer of genes responsible for the biosynthesis of punicic acid into other crop plants would be advantageous.
Like other conjugated fatty acids, punicic acid is synthesized by a divergent FAD2 enzyme termed a conjugase (FADX). This enzyme acts to convert the Δ12 double bond of linoleic acid (18:2) into two conjugated double bonds (Δ11trans and Δ13cis). As has been the situation in many other attempts to produce non-native fatty acids in transgenic plants, only very low levels of punicic acid were obtained in initial studies whereby a FADX gene was expressed alone in transgenic plants (Koba et al. 2007), which is almost surely the result of a requirement for a network of additional enzymes exhibiting a preference for this fatty acid. Indeed, the production of up to 21% punicic acid in the seed oil of A. thaliana was demonstrated when the P. granatum FADX gene was expressed along with P. granatum FAD2 in a background where the elongation of C18 fatty acids was suppressed (Mietkiewska et al. 2014). Once again, these results suggest that even higher amounts of punicic acid could be generated in desired crop species through the incorporation of further genes within the network.
5.4 Challenges Associated with the Metabolic Engineering of Lipid Composition in Plants
The specialty lipids described above each yield tremendous potential for an expansion of use as industries as a whole make a shift toward the production of value-added compounds. Unfortunately, in most cases the species that synthesize these molecules tend to suffer from various limitations in terms of further agronomic expansion, including a requirement for narrow growth conditions, labor-intensiveness, and lack of domestication. Alternatively, as is the case for PHAs and VLC -PUFAs, the native producer is not a land plant species. As a result, a substantial amount of research is now being conducted in an attempt to engineer the production of various molecules with commercial applications in plant species with superior agronomic traits. Several target oilseed species have been recognized for this purpose, such as C. sativa, C. abyssinica, and B. carinata, which tend to have many of the same attributes as alternative biodiesel crops (Zhu et al. 2016). Ideally, these target species are nonfood crops that would not outcross with any food crop, are amenable to genetic transformation and tissue culture, and would allow large-scale production and incorporation into existing agricultural practices. Furthermore, an ability to grow on marginal land that is not suitable for other agricultural purposes would be an incredible advantage, as it would provide the added benefit of reducing competition with current food crops.
In the case of industrial oils, the hope is that plant oils, waxes, and lipid-derived polymers could 1 day be produced on a scale that would significantly reduce the use of petrochemicals for these purposes. As discussed above, many genes involved in the biosynthesis of industrially useful fatty acids and lipids have been cloned, and in certain instances (e.g., the production of unusual fatty acids and wax esters) their synthesis is under relatively simple genetic control. Therefore, it would seem that expressing these genes in transgenic plants would be fairly straightforward, and in certain cases, as discussed, results have been promising. However, as a whole, the results of these experiments have been fairly unpredictable, and the amounts of value-added product generated have virtually always fallen short of levels present in the species from which the transgenes were derived (Dyer et al. 2008). There are likely many reasons for these disappointing results but most almost certainly stem from the complexity of lipid biosynthetic pathways. Since it is necessary to have a relatively high content of the desired lipid molecule within the plant in order to maximize yield for most applications, the development of these transgenic plants will not only involve achieving the synthesis of the target molecule but will also necessitate genetic modification to enable very high levels of accumulation (see Sect. 4.7).
5.4.1 Preference of Kennedy Pathway Acyltransferases for Particular Substrates
In many cases where metabolic engineering of TAG composition has not yielded impressive gains in the fatty acid of choice, it has been suggested that the transgenic seeds lacked the enzymes required to efficiently acylate the novel fatty acids onto the glycerol backbone of TAG, resulting in an accumulation of these fatty acids in the acyl -CoA pool and their subsequent degradation via beta-oxidation . Conversely, in the native plant species that naturally produce these fatty acids, their Kennedy pathway acyltransferases are often specialized to preferentially utilize these fatty acids as substrates (Davies et al. 1995; Kroon et al. 2006; Yu et al. 2006). In particular, LPAAT and DGAT2 enzymes, which catalyze the acylation of the sn-2 and sn-3 positions of TAG (see Figs. 5.3 and 5.4), respectively, from species that produce unusual fatty acids, often appear to exhibit clear substrate preferences. As such, the co-introduction of these enzymes along with the target catalytic enzyme will be of the utmost importance in the production of unusual fatty acids at high levels (Burgal et al. 2008; Nath et al. 2009; Li et al. 2010; Kim et al. 2011; Van Erp et al. 2011).
5.4.2 The Importance of Acyl Transfer from Phosphatidylcholine to Triacylglycerol in Fatty Acid Composition
Until fairly recently, it was believed that the biosynthesis of fatty acids and their incorporation into TAG was an essentially linear process that included the generation of saturated or monounsaturated acyl chains of various lengths in the plastid via the fatty acid synthesis pathway, followed by their export to the cytosol, modification of the acyl groups (mainly while bound to phosphatidylcholine [PC] ), return of acyl groups to the acyl -CoA pool, and subsequent assembly onto the sn-1, sn-2, and sn-3 positions of a glycerol backbone through the activity of ER -bound acyl-CoA-dependent Kennedy pathway enzymes to form TAG (Vanhercke et al. 2013). This process is now understood to be far more complex than originally thought, with complicated networks mediating the movement of acyl groups between pools of acyl-PC, acyl-CoA, and TAG precursors. The pathways outlined in Fig. 5.4 give us a glimpse of this complexity. Indeed, several enzymes have been identified that are involved in acyl trafficking between PC , which is the site of many fatty acid modification reactions, and DAG, TAG, or the acyl-CoA pool (Chen et al. 2015).
The role of PDAT in channelling hydroxy fatty acids into TAG, along with PDCT , was discussed earlier in this chapter (Fig. 5.4). Yet another acyl -CoA-independent route for DAG synthesis involves the enzyme phospholipase D (PLD) which catalyzes the removal of the choline group from PC to form PA (Yang et al. 2017) (Fig. 5.4). PA is a substrate for the Kennedy pathway enzyme phosphatidic acid phosphatase (PAP), which catalyzes the removal of phosphate (Pi) from PA to generate DAG.
In terms of acyl movement between PC and acyl-CoA , lysophosphatidylcholine acyltransferase (LPCAT) appears to catalyze acyl exchange between the sn-2 position of PC and the acyl-CoA pool (Yurchenko et al. 2009). There is also evidence that the DGAT-catalyzed reaction may encourage the removal of modified fatty acids from the sn-2 position of PC through biochemical coupling of the reverse reaction catalyzed by LPCAT (i.e., formation of acyl-CoA and lysophosphatidylcholine [LPC]) and the forward reaction catalyzed by DGAT (i.e., formation of TAG and CoA) (Pan et al. 2015).
The extent of acyl movement through PC probably differs markedly between plant species and could play a key role in the ability of a plant to incorporate unusual fatty acids into TAG. Perhaps even more importantly, particular routes through this complicated network have likely become specialized for unusual acyl chains (such as hydroxyl fatty acyl chains) in different plants, possibly in an effort to channel potentially damaging acyl groups away from membranes and into storage lipids. While the precise mechanisms of these adaptations remain to be fully elucidated, they almost certainly involve the concerted action and specialization of enzymes involved in PC editing and PC to DAG conversion (Napier and Graham 2010).
The lack of efficient removal of unusual fatty acids from the site of their synthesis on PC has also been identified as a bottleneck in many transgenic plants (Bates and Browse 2011). As a result, it is not at all surprising that transgenic oil species that do not normally synthesize these unusual fatty acids are often suboptimal in attempts at their biosynthesis. As a result, it is becoming clear that in order to produce these target fatty acids at useful levels in transgenic plants, several enzymes in addition to the Kennedy pathway acyltransferases will require modification, including PDAT , PDCT , PLA2 , PLD , and LPCAT (Van Erp et al. 2011; Hu et al. 2012; Bayon et al. 2015; Chen et al. 2015; Yang et al. 2017).
5.5 Closing Comments
Plant oils have the potential to be used for a large number of industrial and nutraceutic applications and could provide a sustainable replacement for current sources of these molecules. However, it will be necessary to produce these lipids on a much larger scale than they are presently in their native species, and metabolic engineering will likely play a strong role in this expansion. The engineering of oils and waxes, as well as other plant lipids , is currently set to flourish due to a number of major advances in this field. These advances have taken into account the complex nature of lipid biosynthesis in plants and have resulted in the combination of various engineering strategies to enable maximization of both the efficiency and specificity of the introduced pathway . Indeed, the generation of plants producing the VLC-PUFAs EPA and DHA (Ruiz-Lopez et al. 2014) may be the most complex genetic engineering goal achieved to date in plants.
Further advances in this field are also likely on the horizon, as additional genes necessary for the high-level production of these compounds are uncovered and put to use. Furthermore, the majority of transgenic approaches utilized to date have been carried out through the addition of a pathway, whereby it must compete with existing pathways in the target plant. This means that the introduced pathways are not likely operating at their full potential; as such, inactivating the function of naturally occurring networks and including novel pathways as substitutions rather than additions could provide an enhancement in the accumulation of useful lipids (Chapman and Ohlrogge 2012). Future studies will almost certainly take this into consideration, and it is anticipated that 1 day soon, the engineering of plants to produce non-native lipids at levels that are equivalent to or better than those present in their native host species will be a reality.
References
Abbadi A, Domergue F, Bauer J, Napier JA, Welti R, Zähringer U et al (2004) Biosynthesis of very-long-chain polyunsaturated fatty acids in transgenic oilseeds: constraints on their accumulation. Plant Cell 16:2734–2748
Arao K, Yotsumoto H, Han S-Y, Nagao K, Yanagita T (2004) The 9cis, 11trans, 13cis isomer of conjugated linolenic acid reduces apolipoprotein B100 secretion and triacylglycerol synthesis in HepG2 cells. Biosci Biotech Bioch 68:2643–2645
Badami RC, Patil KB (1980) Structure and occurrence of unusual fatty acids in minor seed oils. Prog Lipid Res 19:119–153
Bassaganya-Riera J, DiGuardo M, Climent M, Vives C, Carbo A, Jouni ZE, Einerhand AWC, O’Shea M, Hontecillas R (2011) Activation of PPARϒ and δ by dietary punicic acid ameliorates intestinal inflammation in mice. Br J Nutr 106:878–886
Bates PD, Browse J (2011) The pathway of triacylglycerol synthesis through phosphatidylcholine in Arabidopsis produces a bottleneck for the accumulation of unusual fatty acids in transgenic seeds. Plant J 68:387–399
Bayon S, Chen G, Weselake RJ, Browse J (2015) A small phospholipase A2-α from castor catalyzes removal of hydroxy fatty acids from phosphatidylcholine in transgenic Arabidopsis seeds. Plant Physiol 167:1259–1270
Belcher B, Rujehan IN, Achdiawan R (2004) Rattan, rubber, or oil palm: cultural and financial considerations for farmers in Kalimantan. Econ Bot 58:S77–S87
Biester EM, Hellenbrand J, Gruber J, Hamberg M, Frentzen M (2012) Identification of avian wax synthases. BMC Biochem 13:4
Bohmert-Tatarev K, McAvoy S, Daughtry S, Peoples OP, Snell KD (2011) High levels of bioplastic are produced in fertile transplastomic tobacco plants engineered with a synthetic operon for the production of polyhydroxybutyrate. Plant Physiol 155:1690–1708
Bousquet J, Flahault A, Vandenplas O, Ameille J, Duron J-J, Pecquet C, Chevrie K, Annesi-Maesano I (2006) Natural rubber latex allergy among health care workers: a systematic review of the evidence. J Allergy Clin Immunol 118:447–454
Broun P, Somerville C (1997) Accumulation of ricinoleic, lesquerolic, and densipolic acids in seeds of transgenic Arabidopsis plants that express a fatty acyl hydroxylase cDNA from castor bean. Plant Physiol 113:933–942
Burgal J, Shockey J, Lu C, Dyer J, Larson T, Graham I, Browse J (2008) Metabolic engineering of hydroxy fatty acid production in plants: RcDGAT2 drives dramatic increases in ricinoleate levels in seed oil. Plant Biotechnol J 6:819–831
Cahoon EB, Ohlrogge JB (1994) Metabolic evidence for the involvement of a Δ4-palmitoyl-acyl carrier protein desaturase in petroselinic acid synthesis in coriander endosperm and transgenic tobacco cells. Plant Physiol 104:827–837
Cahoon EB, Becker CK, Shanklin J, Ohlrogge JB (1994) cDNAs for isoforms of the Δ9-stearoyl-acyl carrier protein desaturase from Thunbergia alata endosperm. Plant Physiol 106:807–808
Cahoon EB, Coughlan SJ, Shanklin J (1997a) Characterization of a structurally and functionally diverged acyl-acyl carrier protein desaturase from milkweed seed. Plant Mol Biol 33:1105–1110
Cahoon EB, Lindqvist Y, Schneider G, Shanklin J (1997b) Redesign of soluble fatty acid desaturases from plants for altered substrate specificity and double bond position. Proc Natl Acad Sci U S A 94:4872–4877
Cahoon EB, Shah S, Shanklin J, Browse J (1998) A determinant of substrate specificity predicted from the acyl-acyl carrier protein desaturase of developing cat’s claw seed. Plant Physiol 117:593–598
Cahoon EB, Ripp KG, Hall SE, McGonigle B (2002) Transgenic production of epoxy fatty acids by expression of a cytochrome P450 enzyme from Euphorbia lagascae seed. Plant Physiol 128:615–624
Carlsson A (2009) Plant oils as feedstock alternatives to petroleum – a short survey of potential oil crop platforms. Biochimie 91:665–670
Chapman KD, Ohlrogge JB (2012) Compartmentation of triacylglycerol accumulation in plants. J Biol Chem 287:2288–2294
Chen B, McClements DJ, Decker EA (2013) Design of foods with bioactive lipids for improved health. Annu Rev Food Sci Technol 4:35–56
Chen G, Woodfield HK, Pan X, Harwood JL, Weselake RJ (2015) Acyl-trafficking during plant oil accumulation. Lipids 50:1057–1068
Chisholm MJ, Hopkins CY (1965) Fatty acids of doxantha seed oil. J Am Oil Chem Soc 42:49–50
Cornish K, Xie W (2012) Natural rubber biosynthesis in plants: rubber transferase. Methods Enzymol 515:63–82
Das UN (2002) The lipids that matter from infant nutrition to insulin resistance. Prostag Leukotr Essent Fatty Acids 67:1–12
Davies HM, Hawkins DJ, Nelsen JS (1995) Lysophosphatidic acid acyltransferase from immature coconut endosperm having medium chain length substrate specificity. Phytochemistry 39:989–996
Dyer JM, Chapital DC, Kuan JC, Mullen RT, Turner C, McKeon TA, Pepperman AB (2002) Molecular analysis of a bifunctional fatty acid conjugase/desaturase from tung: implications for the evolution of plant fatty acid diversity. Plant Physiol 130:2027–2038
Dyer JM, Stymne S, Green AG, Carlsson AS (2008) High-value oils from plants. Plant J 54:640–655
Gasmi J, Sanderson JT (2010) Growth inhibitory, antiandrogenic, and pro-apoptotic effects of punicic acid in LNCaP human prostate cancer cells. J Agric Food Chem 58:12149–12156
Harwood JL, Woodfield HK, Chen G, Weselake RJ (2017) Modification of oil crops to produce fatty acids for industrial applications. In: Ahmad M (ed) Fatty acids: chemistry, synthesis, and applications. Academic Press and AOCS Press, Urbana, pp 188–235
Heilmann M, Iven T, Ahmann K, Hornung E, Stymne S, Feussner I (2012) Production of wax esters in plant seed oils by oleosomal co-targeting of biosynthetic enzymes. J Lipid Res 53:2153–2161
Horrocks LA, Yeo YK (1999) Health benefits of docosahexaenoic acid (DHA). Pharmacol Res 40:211–225
Hu Z, Ren Z, Lu C (2012) The phosphatidylcholine diacylglycerol cholinephosphotransferase is required for efficient hydroxy fatty acid accumulation in transgenic Arabidopsis. Plant Physiol 158:1944–1954
Igarashi M, Miyazawa T (2000) Newly recognized cytotoxic effect of conjugated trienoic fatty acids on cultured human tumor cells. Cancer Lett 148:173–179
Imle EP (1978) Hevea rubber – past and future. Econ Bot 32:264–277
Innis SM (2000) The role of dietary n-6 and n-3 fatty acids in the developing brain. Dev Neurosci-Basel 22:474–480
Kalscheuer R, Stienbüchel A (2003) A novel bifunctional wax ester synthase/acyl-CoA:diacylglycerol acyltransferase mediates wax ester and triacylglycerol biosynthesis in Acinetobacter calcoaceticus ADP1. J Biol Chem 278:8075–8082
Katavic V, Mietkiewska E, Barton DL, Gibilin EM, Reed DW, Taylor DC (2002) Restoring enzyme activity in non-functional low erucic acid Brassica napus fatty acid elongase 1 by a single amino acid substitution. Eur J Biochem 269:56255631
Kim HU, Lee K-R, Go YS, Jung JH, Suh M-C, Kim JB (2011) Endoplasmic reticulum-located PDAT1-2 from castor bean enhances hydroxy fatty acid accumulation in transgenic plants. Plant Cell Physiol 52:983–993
Kim HJ, Silva JE, Vu HS, Mockaitis K, Nam J-W, Cahoon EB (2015) Toward production of jet fuel functionality in oilseeds: identification of FatB acyl-acyl carrier protein thioesterases and evaluation of combinatorial expression strategies in Camelina seeds. J Exp Bot 66:4251–4265
Knutzon DS, Hayes TR, Wyrick A, Xiong H, Davies M, Voelker TA (1999) Lysophosphatidic acid acyltransferase from coconut endosperm mediates the insertion of laurate at the sn-2 position of triacylglycerols in lauric rapeseed oil and can increase total laurate levels. Plant Physiol 120:739–746
Koba K, Akahoshi A, Yamasaki M, Tanaka K, Yamada K, Iwata T, Kamegai T, Tsutsumi K, Sugano M (2002) Dietary conjugated linolenic acid in relation to CLA differently modifies body fat mass and serum and liver lipid levels in rats. Lipids 37:343–350
Koba K, Imamura J, Akashoshi A, Murase-Kohno J, Nishizono S et al (2007) Genetically modified rapeseed oil containing cis-9, trans-11, cis-13 octadecatrienoic acid affects body fat mass and lipid metabolism in mice. J Agric Food Chem 55:3781–3748
Kolattukudy PE (1970) Plant waxes. Lipids 5:259–275
Kroon JTM, Wei W, Simon WJ, Slabas AR (2006) Identification and functional expression of a type 2 acyl-CoA:diacylglycerol acyltransferase (DGAT2) in developing castor bean seeds which has high homology to the major triglyceride biosynthetic enzyme of fungi and animals. Phytochemistry 67:2541–2549
Lardizabal KD, Metz JG, Ssakamoto T, Hutton WC, Pollard MR, Lassner MW (2000) Purification of a jojoba embryo wax synthase, cloning of its cDNA, and production of high levels of wax in seeds of transgenic Arabidopsis. Plant Physiol 122:645–656
Lassner MW, Levering CK, Davies HM, Knutzon DS (1995) Lysophosphatidic acid acyltransferase from meadowfoam mediates insertion of erucic acid at the sn-2 position of triacylglycerol in transgenic rapeseed oil. Plant Physiol 109:1389–1394
Lee YK (2001) Microalgal mass culture systems and methods: their limitation and potential. J Appl Phycol 13:307–315
Li F, Wu X, Lam P, Bird D, Zheng H, Samuels L, Reinhard Jetter R, Kunst L (2008) Identification of the wax ester synthase/acyl-coenzyme A:diacylglycerol acyltransferase WSD1 required for stem wax ester biosynthesis in Arabidopsis. Plant Physiol 148:97–107
Li R, Yu K, Hatanaka T, Hildebrand DF (2010) Vernonia DGATs increase accumulation of epoxy fatty acids in oil. Plant Biotechnol J 8:184–195
Li X, van Loo EN, Gruber J, Fan J, Guan R, Frentzen M, Stymne S, Zhu L-H (2012) Development of ultra-high erucic acid oil in the industrial oil crop Crambe abyssinica. Plant Biotechnol J 10:862–870
Lu C, Xin Z, Ren Z, Miquel M, Browse J (2009) An enzyme regulating triacylglycerol composition is encoded by the ROD1 gene of Arabidopsis. Proc Natl Acad Sci USA 106:18837–18842
Madison LL, Huisman GW (1999) Metabolic engineering of poly(3-hydroxyalkanoates): from DNA to plastic. Microbiol Mol Biol Rev 63:21–53
McKeon TA (2016) Brassica spp. oils. In: McKeon TA, Hildebrand DF, Hayes DG, Weselake RJ (eds) Industrial oil crops. Elsevier/AOCS Press, New York/Urbana, pp 75–112
McVetty PBE, Mietkiewska E, Omonov T, Curtis J, Taylor DC, Weselake RJ (2016) Brassica spp. oils. In: McKeon TA, Hildebrand DF, Hayes DG, Weselake RJ (eds) Industrial oil crops. Elsevier/AOCS Press, New York/Urbana, pp 113–156
Mietkiewska E, Miles R, Wickramarathna A, Sahibollah AF, Greer MS, Chen G, Weselake RJ (2014) Combined transgenic expression of Punica granatum conjugase (FADX) and FAD2 desaturase in high linoleic acid Arabidopsis thaliana mutant leads to increased accumulation of punicic acid. Planta 240:575–583
Mirmiran P, Fazeli MR, Asghari G, Shafiee A, Azizi F (2010) Effect of pomegranate seed oil on hyperlipidaemic subjects: a double-blind placebo-controlled clinical trial. Br J Nutr 104:402–406
Morris MC, Evans DA, Bienias JL, Tangney CC, Bennett DA, Wilson RS et al (2003) Consumption of fish and n-3 fatty acids and the risk incident of Alzheimer disease. Arch Neurol-Chicago 60:940–946
Napier JA, Graham IA (2010) Tailoring plant lipid composition: designer oilseeds come of age. Curr Opin Plant Biol 13:329–336
Nath UK, Wilmer JA, Wallington EJ, Becker HC, Mollers C (2009) Increasing erucic acid content through combination of endogenous low polyunsaturated fatty acids alleles with Ld-LPAAT + Bn-fae1 transgenes in rapeseed (Brassica napus L.). Theor Appl Genet 118:765–773
Ohlrogge JB, Pollard MR, Stumpf PK (1978) Studies on the biosynthesis of waxes by developing jojoba seed tissue. Lipids 13:203–210
Okuley J, Lightner J, Feldmann K, Yadav N, Lark E, Browse J (1994) Arabidopsis FAD2 gene encodes the enzyme that is essential for polyunsaturated lipid synthesis. Plant Cell 6:147–158
Onokpise O, Louime C (2012) The potential of the South American leaf blight as a biological agent. Sustainability 4:3151–3157
Pan X, Chen G, Kazachkov M, Greer MS, Caldo KM, Zou J, Weselake RJ (2015) In vivo and in vitro evidence for biochemical coupling of reactions catalyzed by lysophosphatidylcholine acyltransferase and diacyglycerol acyltransferase. J Biol Chem 290:18068–18078
Pauly D, Watson R, Alder J (2005) Global trends in world fisheries: impacts on marine ecosystems and food security. Philos Trans R Soc London B 360:5–12
Philip S, Keshavarz T, Roy I (2007) Polyhydroxyalkanoates: biodegradable polymers with a range of applications. J Chem Technol Biotechnol 82:233–247
Qi B, Fraser T, Mugford S, Dobson G, Sayanova O, Butler J et al (2004) Production of very long chain polyunsaturated omega-3 and omega-6 fatty acids in plants. Nat Biotechnol 22:739–745
Rauber P (2011) Beyond oil in 20 years. Sierra Magazine. Available online at: http://vault.sierraclub.org/sierra/201101/beyondoil.aspx
Richardson AJ, Puri BK (2002) A randomized double-blind, placebo-controlled study of the effects of supplementation with highly unsaturated fatty acids on ADHD-related symptoms in children with specific learning difficulties. Prog Neuro-Psychopharmacol Biol Psychiatry 26:233–239
Riediger ND, Othman RA, Suh M, Moghadasian MH (2009) A systemic review of the roles of n-3 fatty acids in health and disease. J Am Diet Assoc 109:668–679
Rose DP, Connolly JM (1999) Omega-3-fatty acids as cancer chemopreventive agents. Pharmacol Ther 83:217–224
Ruiz-Lopez N, Haslam RP, Napier JA, Sayanova O (2014) Successful high-level accumulation of fish oil omega-3 long-chain polyunsaturated fatty acids in a transgenic oilseed crop. Plant J 77:198–208
Schierholt A, Becker HC, Ecke W (2000) Mapping a high oleic acid mutation in winter oilseed rape (Brassica napus L.). Theor Appl Genet 101:897–901
Shinohara N, Tsuduki T, Ito J, Honma T, Kijima R, Sugawara S, Arai T, Yamasaki M, Ikezaki A, Yokoyama M, Nishiyama K, Nakagawa K, Miyazawa T, Ikeda I (2012) Jacaric acid, a linolenic acid isomer with a conjugated triene system, has a strong antitumor effect in vitro and in vivo. BBA-Mol Cell Biol L 1821:980–988
Spilmont M, Léotoing L, Davicco M-J, Lebecque P, Mercier S, Miot-Noirault E, Pilet P, Rios L, Wittrant Y, Coxam V (2013) Pomegranate seed oil prevents bone loss in a mice model of osteoporosis, through osteoblastic stimulation, osteoclastic inhibition and decreased inflammatory status. J Nutr Biochem 24:1840–1848
Ståhl U, Carlsson AS, Lenman M, Dahlqvist A, Huang B, Banaś W, Banaś A, Stymne S (2004) Cloning and functional characterization of a phospholipid:diacylglycerol acyltransferase from Arabidopsis. Plant Physiol 135:1324–1335
Sturgeon SR, Ronnenberg AG (2010) Pomegranate and breast cancer: possible mechanisms of prevention. Nutr Rev 68:122–128
Subedi K, Yu H-M, Weselake RJ, Meesapyodsuk D, Qiu X, Shah S, Field CJ (2015) Stearidonic acid- enriched flax oil reduces the growth of human breast cancer in vitro and in vivo. Breast Cancer Res Treat 149:17–29
Suriyamongkoi P, Weselake R, Narine S, Moloney M, Shah S (2007) Biotechnological approaches for the production of polyhydroxyalkanoates in microorganisms and plants – a review. Biotechnol Adv 25:148–175
Takagi Y, Rahman SM (1996) Inheritance of high oleic acid content in the seed oil of soybean mutant M23. Theor Appl Genet 92:179–182
Taylor DC, Smith MA, Fobert P, Mietkiewska E, Weselake RJ (2011) Plant systems – metabolic engineering of higher plants to produce bio-industrial oils. In: Moo-Young M (ed) Comprehensive biotechnology, vol 4, 2nd edn. Elsevier, Amsterdam, pp 67–85
Tsuzuki T, Tokuyama Y, Igarashi M, Miyazawa T (2004) Tumor growth suppression by α-eleostearic acid, a linolenic acid isomer with a conjugated triene system, via lipid peroxidation. Carcinogenesis 25:1417–1425
Van Beilen JB, Poirier Y (2007) Establishment of new crops for the production of natural rubber. Trends Biotechnol 25:522–529
Van Erp H, Bates PD, Burgal J, Shockey J, Browse J (2011) Castor phospholipid:diacylglycerol acyltransferase facilitates efficient metabolism of hydroxy fatty acids in transgenic Arabidopsis. Plant Physiol 155:683–693
Vanhercke T, Wood CC, Stymne S, Singh SP, Green AG (2013) Metabolic engineering of plant oils and waxes for industrial feedstocks. Plant Biotechnol J 11:197–210
Venegas-Calerón M, Sayanova O, Napier JA (2010) An alternative to fish oils: metabolic engineering of oil-seed crops to produce omega-3 long chain polyunsaturated fatty acids. Prog Lipid Res 49:108–119
Voelker TA, Hayes TR, Cranmer AM, Turner JC, Davies HM (1996) Genetic engineering of a quantitative trait: metabolic and genetic parameters influencing the accumulation of laurate in rapeseed. Plant J 9:229–241
Voelker TA, Jones A, Cranmer AM, Davies HM, Knutzon DS (1997) Broad-range and binary-range acyl-acyl-carrier-protein thioesterases suggest an alternative mechanism for medium-chain production in seeds. Plant Physiol 114:669–677
Vroegrijk IOCM, van Diepen JA, van den Berg S, Westbroek I, Keizer H, Gambelli L, Hontecillas R, Bassanganya-Riera J, Zondag GCM, Romijn JA, Havekes LM, Voshol PJ (2011) Pomegranate seed oil, a rich source of punicic acid, prevents diet-induced obesity and insulin resistance in mice. Food Chem Toxicol 49:1426–1430
Weselake RJ, Woodfield HK, Field CJ, Harwood JL (2017) Production of edible oils through metabolic engineering. In: Akoh C (ed) Food lipids –chemistry, nutrition, and biotechnology, 4th edn. CRC Press/Taylor & Francis Group, Boca Raton, pp 973–995
Wiberg E, Edwards P, Byrne J, Stymne S, Dehesh K (2000) The distribution of caprylate, caprate and laurate in lipids from developing and mature seeds of transgenic Brassica napus L. Planta 212:33–40
Williams CM, Burdge G (2006) Long-chain n-3 PUFA: plant v marine sources. Proc Nutr Soc 65:42–50
Yang W, Wang G, Li J, Bates PD, Wang X, Allen DK (2017) Phospholipase D enhances diacylglycerol flux into triacylglycerol. Plant Physiol 174:110–123
Yokoo EM, Valente JG, Grattan I, Schmidt SL, Platt I, Silbergeld EK (2003) Low level methylmerucury exposure affects neurophysiological function in adults. Environ Health 2:8
Yu K, McCracken CT, Li R, Hildebrand DF (2006) Diacylglycerol acyltransferases from Vernonia and Stokesia prefer substrates with vernolic acid. Lipids 41:557–566
Yu H-M, Newell M, Subedi K, Weselake RJ, Mazurak V, Field CJ (2015) Bypassing the Δ6-desaturase enzyme and directly providing n-3 and n-6 PUFA pathway intermediates reduces the survival of two human breast cancer cell lines. Eur J Lipid Sci Technol 117: 1378–1390
Yurchenko OP, Nykiforuk CL, Moloney MM, Stahl U, Banas A, Stymne S, Weselake RJ (2009) A 10 kDa acyl-CoA binding protein (ACBP) from Brassica napus enhances acyl exchange between acyl-CoA and phosphatidylcholine. Plant Biotechnol J 7:602–610
Zhang LS, Tan Y, Ouyang YL, Wang RS (1991) Effects of high erucic acid rapeseed oil on fatty acid oxidation in rat liver. Biomed Environ Sci 4:262–267
Zhu L-H, Krens F, Smith MA, Li X, Qi W, van Loo EN, Iven T, Feussner I, Nazarenus TJ, Huai D, Taylor DC, Zhour X-R, Green AG, Shockey J, Klasson KT, Mullen RT, Huang B, Dyer JM, Cahoon E (2016) Dedicated industrial oilseed crops as metabolic engineering platforms for sustainable industrial feedstock production. Sci Rep 6:22181
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer Science+Business Media, LLC, part of Springer Nature
About this chapter
Cite this chapter
Singer, S.D., Weselake, R.J. (2018). Production of Other Bioproducts from Plant Oils. In: Chen, G., Weselake, R., Singer, S. (eds) Plant Bioproducts. Springer, New York, NY. https://doi.org/10.1007/978-1-4939-8616-3_5
Download citation
DOI: https://doi.org/10.1007/978-1-4939-8616-3_5
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4939-8614-9
Online ISBN: 978-1-4939-8616-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)