Abstract
The 20 and 22 carbon n-3 long-chain polyunsaturated fatty acids (LCPUFA) inhibit the growth of tumors in vitro and in animal models, but less is known about the 18 carbon n-3, stearidonic acid (SDA). This study aimed to establish and determine a mechanism for the anti-cancer activity of SDA-enriched oil (SO). SO (26 % of lipid) was produced by genetically engineering flax and used to treat human tumorigenic (MDA-MB-231, MCF-7) and non-tumorigenic (MCF-12A) breast cells. Nu/nu mice bearing MDA-MB-231 tumor were fed SO (SDA, 4 % of fat). Cell/tumor growth, phospholipid (PL) composition, apoptosis, CD95, and pro-apoptotic molecules were determined in SO-treated cells/tumors. Compared to a control lipid mixture, SO reduced (p < 0.05) the number of tumorigenic, but not MCF-12A cells, and resulted in higher concentration of most of the n-3 fatty acids in PL of all cells (p < 0.05). However, docosapentaenoic acid increased only in tumorigenic cells (p < 0.05). SO diet decreased tumor growth and resulted in more n-3 LCPUFA, including DPA and less arachidonic acid (AA) levels in major tumor PL (p < 0.05). Treatment of MDA-MB-231 cells/tumors with SO resulted in more apoptotic cells (in tumors) and in vivo and in vitro, more CD95+ positive cells and a higher expression of apoptotic molecules caspase-10, Bad, or Bid (p < 0.05). Supplementing SO alters total PL and PL classes by increasing membrane content of n-3 LCPUFA and lowering AA (in vivo), which is associated with increased CD95-mediated apoptosis, thereby suggesting a possible mechanism for reduce tumor survival.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Despite treatment improvements, further advancement is needed to improve treatment and extend the lives of women affected with breast cancer. There are promising results showing that long-chain n3 polyunsaturated fatty acids (LCPUFA), primarily eicosapentaenoic acid (EPA; C20:5n-3) and docosahexaenoic acid (DHA; C22:6n-3), can reduce the growth of human breast tumor cells in vitro [1–4] and in experimental models of breast cancer [5–9]. A key mechanism for anti-cancer effects of n-3 LCPUFA is via incorporation into membrane phospholipid (PL) and their subsequent effect on membrane-mediated functions and signals (reviewed by [10]). Changes in n-3 LCPUFA content in membrane lipids have been associated with changes in membrane content of the CD95 death receptor [2], raft-associated epidermal growth factor receptor and phosphorylation [2, 4, 11], and changes in caspases, pro-apoptotic proteins, and NF-kB, all which ultimately reduce cell survival [10, 12–14]. N-3 LCPUFA are preferentially enriched in the membrane PL, primarily phosphatidylethanolamine (PE), phosphatidylcholine (PC), and phosphatidylinositol (PI) [15]. These PLs are involved in many functions that could alter cell growth or death. For example, PE serves as chaperone for proteins moving from cytoplasm to membrane [16], and PC and PI are sources of diacylglycerol for signaling function [17, 18]. Changes in the n-3 fatty acid composition of PC have been demonstrated to reduce tumor cell growth, and alternations in PE composition lead to mitochondrial-mediated apoptosis [16, 18, 19]. PI is a major source of arachidonic acid (AA; 20:4n-6), important for eicosanoid synthesis [17], compounds which promote tumor progression and metastasis [20].
α-Linolenic acid (ALA; C18:3n-3) is the main dietary source of n-3 fatty acids, but most studies do not find the same anti-cancer activity [1, 21, 22] as EPA and DHA. This might be due to the limited conversion in vivo [23, 24] to EPA and DHA. Feeding stearidonic acid (SDA; C18:4n-3), however, has been demonstrated to increase tissue EPA content [22, 25], overcoming to some extent the requirement for the ∆6-desaturase-catalyzed reaction (Fig. 1). SDA has also been shown to have inhibitory effect on cell growth in colorectal cancer and fibroblast cells [21, 26], but its effect has not been studied in breast cancer cells. SDA exists in low amounts in the diet but SDA-enriched seed oils have been produced by genetic engineering, the introduction of a ∆6-desaturase in both flax (Linum usitatissimum) [27, 28] and soybean (Glycine max) [29, 30]. Although these enriched seed oils contain moderate concentrations of SDA (10–15 %) [27, 28], they also contain appreciable amounts of n-6 fatty acids [3, 31–33], which may negate the potential n-3 anti-cancer activity. The objective of this study was to determine the anti-cancer effect and incorporation in breast cancer cell PL in vitro and in vivo of the fatty acids in a genetically modified flax containing SDA.
Materials and methods
Production and identification of stearidonic acid-enriched flax oil (SO)
The Δ6-DESATURASE gene isolated from Pythium irregular [34] was amplified using two primers with BglII and NcoI restriction sites. The Δ6-ELONGASE gene from Thraustochytrium sp was re-amplified from BJ5 [35] using the primers with NotI and XbaI. Genes were cloned separately into pCR4-TOPO TA cloning vector, and subsequently released from the intermediate vector and cloned, respectively, into the cloning sites of pUC19 under control of the Cnl1 flax seed-specific promoter and OCS terminator. The two-gene cassette was released from the pUC19, blunt ended with Pfu end filling, and inserted into a plant expression vector pX1. Flax line F06396B was transformed by Agrobacterium-mediated plant transformation technique [36], with some modifications. Surface-sterilized seeds were grown in Murashige and Skoog salt medium supplemented with sucrose (15 g L−1). Hypocotyl explant segments were then co-cultivated with Agrobacterium tumefaciens cell culture (containing pX1 vector) of OD600 = 0.5 for 2 h. The explants were then cultured on F2 medium (Murashige and Skoog salts with 1.20 mg L−1 benzyl adenine and 0.08 mg L−1 1-naphthaleneacetic acid) for 2 days. The callus and shoots were induced by repeated sub-culturing of the explants, and the green shoots were placed in shoot development medium containing 2.0 mg L−1 indole-3-butyric acid and 0.1 mg L−1 1-naphthaleneacetic acid for few weeks. Shoots were transferred to root-inducing medium, and plants were moved into soil once good root was developed.
Developing embryos from transformed plants were cultured on selection plates containing kanamycin to determine their segregation ratio. Transgenic plants were grown to subsequent generations until they became homozygous and increased through propagation of plants in controlled growth chambers (16 h light at 21 °C and 600 ppm CO2/8 h dark at 16 °C and 400 ppm CO2; approximately 1,300 μ mol m−2 s−1 light intensity) using ProMix BX potting soil (J.R. Johnson Supply, Minneapolis, MN, USA). The fatty acid composition of the seed oil from a regular flax line, breeding line F06396B (n = 4), and SDA flax (n = 5) was determined by gas chromatography–mass spectrometry.
Cell culture experiments
MDA-MB-231 (ER-/PR-) cells were maintained in Iscove’s Modified Dulbecco’s Medium; MCF-7 (ER+/PR+) cells in Minimal Essential Medium with 10 µg/ml insulin; and 1 % sodium pyruvate and MCF-12A (non-tumorigenic) cells in Dulbecco’s Modified Eagle Medium with 20 ng/mL epidermal growth factor, 100 ng/ml cholera toxin, 500 ng/ml hydrocortisone, and 10 µg/mL insulin. All media were supplemented with 5 % v/v fetal calf serum (FCS) and 1 % v/v penicillin and streptomycin (all media components from Life Technologies, Burlington, ON, Canada, cell lines from American Type Culture Collection, Manassas, VA, USA) and maintained in 75 cm2 tissue culture flasks (Fisher Scientific, Ottawa, ON, Canada) at 37 °C in 5 % CO2 at 98 % relative humidity.
Palmitic acid (PA; C16:0), stearic acid (SA; C18:0), γ-linolenic acid (GLA; C18:3n-6), ALA, and eicosadienoic acid (EDA; C20:2n-6) from NuChek Prep Inc (Elysian, MN, USA), and oleic acid (OA; C18:1n-9), linoleic acid (LA; C18:2n-6), and SDA from Matreya (Pleasant Gap, PA, USA) were conjugated to bovine serum albumin (Fisher Scientific, Edmonton, AB, Canada) as previously described [2]. Cells were plated in triplicate at a density of 2.6 × 104 cells per well for MDA-MB-231 and MCF-12A cells, and 5.2 × 104 cells per well for MCF-7 cells in a 24-well plate (Corning Inc., Tewksbury, MA, USA) for 72 h. Cells were then treated in triplicate with a mixture of fatty acids (PA (6.0 %), SA (3.1 %), OA (13.4 %), LA (3.4 %), GLA (15.0 %), ALA (31.2 %), SDA (26.1 %), and EDA (0.7 %)) that represented the major fatty acids in the SO lipid. The treatment concentration of 150 µM was determined in a pilot dose study with MDA-MB-231 (Fig. 2a) and the growth inhibitor effects confirmed in the MCF-7 cells (Fig. 2b). Treatment media were replaced daily. All conditions were supplemented with 40 μM OA and LA. This was previously found to provide sufficient fatty acids to maintain growth and ensure sufficient n-6 fatty acids to maintain AA concentrations in the tumor [2]. After 48 h, media were removed, cells detached (0.25 % trypsin, Fisher Scientific, Ottawa, ON, Canada), and the number of viable (trypan blue excluded) cells counted under a microscope.
Effect of SO fatty acid mixture on cell viability of MDA-MB-231, MCF-7, and MCF-12A cells. a Effect of different concentrations of SO fatty acid mixture (25–300 μM) on MDA-MB-231 cells, b effect of treating MDA-MB-231, MCF-7, and MCF-12A cells with 150 μM SO fatty acid mixture. Values are mean ± SEM (n = 3 per cell line per treatment), expressed as percentage of the control, a mixture of oleic acid (OA, C18:1n-9) and linoleic acid (LA, C18:2n-6). Asterisk indicates a significant difference compared to control (p < 0.05), hash indicates a significant difference to MCF-12A. The control is set at 100 % for all cell lines so only one bar is shown
Experimental animals and diet
Animal experiments were reviewed and approved by the University of Alberta Animal Policy and Welfare Committee and were in accordance with the Canadian Council on Animal Care guidelines. Diets were nutritionally complete and contained 20 % w/w fat [6, 37]. The fatty acid composition of the diets (Table 1) was achieved by blending oils (Supplementary Table 1) so as to obtain a polyunsaturated to saturated ratio of 0.5 and a SDA content in the SO diet of 4 % w/w fat. Diets were irradiated for 72 h at 8 kGy and stored at −20 °C until used. Fatty acid analysis by GC pre- and post-irradiation confirmed that the fat composition was not altered by irradiation (data not shown).
Immunodeficient 6-week-old female nu/nu mice (Charles River Laboratories International, Inc) were housed in bio-containment under aseptic conditions with autoclaved bedding and water. After 3 days of feeding the control diet (Table 1), MDA-MB-231 cells (2 × 106 cells/100 μL in 5 % FCS Iscove’s media) were injected subcutaneously below the upper right scapula [38]. Mice were fed control diet ad libitum until the tumor reached approximately 50 mm3 [39, 40], and then randomized (n = 5 each group) to a diet (Table 1) for 4 weeks. Mice were euthanized and tumors were carefully removed, weighed, flash frozen for lipid extraction, fixed with formalin for immunohistochemistry, or homogenized for protein analysis. The individuals performing the excision and weighing of the tumor and all subsequent assays were blinded to the treatments.
Analysis of cells and tumors
Lipids from cultured cells or tumors were extracted using a modified Folch procedure [41, 42]. PL fatty acid composition of total PL or the major PL classes (separated by thin layer chromatography) was determined by gas–liquid chromatography as previously reported [43]. Following treatment of MDA-MB-231 with SO, cells were prepared for flow cytometry as described [2]. Cells incubated with anti-CD95 or isotope control (BD Biosciences, Mississauga, ON, Canada) were analyzed on a FACSCanto™ II (BD Biosciences, Mississauga, ON, Canada) and quantified using Kaluza software.
Homogenized tumor tissue and MDA-MB-231 whole cell lysates were prepared for Western blot analysis as described [4]. Primary antibodies to CD95, Bid, Bad, GAPDH (loading control) (Cell Signaling Technology, Whitby, ON, Canada), and caspase-10 (Abcam, Toronto, ON, Canada) were used. Membranes were developed using Pierce ECL 2 Western Blotting Substrate (Fisher Scientific, Edmonton, AB, Canada), visualized on a Typhoon™ Trio+ variable mode imager (GE Life Sciences, Baie d’Urfe, PQ, Canada) and relative intensities of band signals quantified using ImageQuant TL software.
Tumor sections were stained with anti-CD95, and the reactive cells were visualized using ImmunoDetector liquid DAB (Bio SB Inc., Santa Barbara, CA, USA). Apoptotic cells were identified using ApopTag plus peroxidase in situ apoptosis detection kit (EMD Millipore Corporation, Etobicoke, ON, Canada), according to manufacturer’s recommendation. Sections were imaged using AxioCam (Carl Zeiss microscopy), and the proportion of positive cells was determined.
Statistical analysis
Statistical analyses were carried out using SPSS, version 21. Data were tested for normal distribution and once confirmed analyzed by one-way analysis of variance ANOVA, followed by post hoc analysis using Tukey’s test to identify significant (p < 0.05) difference among fatty acids and treatments. To compare differences between diet treatments in the tumor study or SO treatment in cell culture, unpaired t-tests were performed.
Results
Production of the SDA-enriched flax seed oil
FA analysis of transgenic flax seeds indicated that several new FAs were produced compared with the non-transformed flax (Supplementary Table 1). Among them, SDA was the most abundant, approximately 26 % of total FA, followed by GLA of approximately 15 %.
In vitro cell growth
The number of viable MDA-MB-231 and MCF-7 cells was lower (p < 0.05), 84.8 and 81.4 %, respectively, in response to the mixture of fatty acids representing SO oil, compared to the control (Fig. 2b). Cell viability of the non-malignant MCF-12A cells did not differ significantly compared to control and was greater (p < 0.05) than MCF-7 or MDA-MB-231 cells (Fig. 2b).
Effect of feeding the SO diet on food intake, body, and tumor weight
There was no difference in body weight (Fig. 3a) and average daily food intake (Fig. 3b) of MDA-MB-231 tumor-bearing nu/nu mice fed the control or the SO diet, respectively. The final tumor weight was significantly lower with SO diet compared to the control (Fig. 3c).
Effect of control diet or stearidonic acid-enriched (SO) diet on body weight, food intake, and the growth of MDA-MB-231 tumors in nu/nu mice. After injection of cancer cells, animals were maintained on a control diet for 4 weeks, and then either a control diet or SO diet for an additional 4 weeks. a Changes in body weight, b average daily food intake, and c at the end of the experiment, each tumor was removed and weighed. Values are mean ± SEM (n = 5 per treatment). Asterisks indicate a significant difference between control and SO diet (p < 0.05)
Effect of SO treatment on total PL fatty acid composition of cultured cells
After SO treatment, the major components of SDA-enriched oil, SDA (Fig. 4a), and GLA were higher in the PL of MDA-MB-231, MCF-7, and MCF-12A compared to control treatment (Supplementary Table 2). In response to SO treatment, the levels of fatty acids intermediate eicosatrienoic acid (ETE, 20:3n-3) were higher in MCF-12A and eicosatetraenoic acid (ETA, C20:4n-3) higher in all cell lines; however, DHA levels were similar to those in control-treated cells (Supplementary Table 2). The concentration of EPA (Fig. 4b) was higher in the PL of all cell lines, and the levels of docosapentaenoic acid (DPA) (Fig. 4c) and dihomo γ-linoleic acid (DGLA, C20:3n-6) (Supplementary Table 2) were higher only in the PL of tumorigenic cells. The relative concentration of AA increased significantly in MDA-MB-231 and 2-fold in MCF-7 after SO treatment but did not change in MCF-12A cells (Fig. 4d).
Whole cell phospholipid composition in MDA-MB-231, MCF-7, and MCF-12A in response to SO mixture of fatty acids compared to mixture of oleic acid and linoleic acid (OA, C18:1n-9/LA, C18:2n-6) as a control. a Stearidonic acid (SDA, C18:4n-3), b eicosapentaenoic acid (EPA, C20:5n-3), c docosapentaenoic acid (DPA, 22:5n-3), and d Arachidonic acid (AA, C20:4n-6). Values are mean percent composition ± SEM (n = 3–5 per cell line treatment) corresponding to (OA/LA) as control. Asterisks indicate a significant difference compared to respective control treatment (p < 0.05)
Fatty acid composition of PL classes in vivo and in vitro
SDA concentration was increased in PC, PE, and PI of SO tumors (Fig. 5a). EPA increased in PC and PE (Figs. 5b, 6a), and DPA increased in PC, PE, and PI (Figs. 5c, 6b) both in vivo and in vitro. Tumors had lower AA in PE and PI in the SO diet group, whereas cultured cells had an increase of AA in PC and PE (Figs. 5d, 6c). The relative proportion of DGLA in PC and PE, and ETA in PC, PE, PI, and PS was increased with SO diet, however, that of DHA did not differ in PL classes (Supplementary Table 3).
Fatty acid composition of major phospholipid classes in MDA-MB-231 tumors implanted into nu/nu mice fed either a control or stearidonic acid-enriched (SO) diet. a Stearidonic acid (SDA, C18:4n-3), b eicosapentaenoic acid (EPA, C20:5n-3), c docosapentaenoic acid (DPA, C22:5n-3), and d arachidonic acid (AA, C20:4n-6). Values are shown as mean percentage ± SEM (n = 5 per treatment) corresponding to control. Asterisks indicate a significant difference compared to respective control (p < 0.05). PC phosphatidylcholine, PE phosphatidylethanolamine, PI phosphatidylinositol
Composition of fatty acids in major phospholipid classes in MDA-MB-231 cells treated with the SO mixture of fatty acids. a eicosapentaenoic acid (EPA, C20:5n-3), b docosapentaenoic acid (DPA, 22:5n-3), and c arachidonic acid (AA, C20:4n-6). Values are mean percent composition ± SEM (n = 3). Asterisks indicate a significant differences between control and SO treatments (p < 0.05). PC phosphatidylcholine, PE phosphatidylethanolamine, PI phosphatidylinositol
CD95 expression and pro-apoptotic molecules in vitro and in vivo
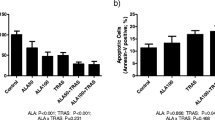
Total surface expression of CD95 and downstream proteins caspase-10 and Bad were significantly higher in SO-treated MDA-MB-231 cells (Fig. 7a, b). SO tumors also had a higher proportion of CD95-positive cells (Fig. 8a, b, c), higher protein levels of CD95, caspase-10 and Bid (Fig. 8d), and more apoptotic cells (Fig. 9a, b, c) compared to control tumors.
Effect of SO fatty acid mixture on the proportion of Fas ligand receptor (CD95) and pro-apoptotic molecules in MDA-MB-231 cell. a Flow cytometry analysis of CD95+ cells, b Western blot analysis of caspase-10 and Bad. Values are shown as mean ± SEM (n = 3–4 per treatment). Expression level of proteins is shown by relative fold change to control treatment (OA, C18:1n-9//LA, C18:2n-6) which is indicated by dotted line. Asterisks indicate a significant difference compared to control treatment (p < 0.05)
Effect of control diet or stearidonic acid-enriched (SO) diet on the expression of Fas ligand receptor (CD95) and pro-apoptotic molecules in MDA-MB-231 tumors from nu/nu mice. Immunohistochemical analysis of CD95 in tumors from nu/nu mice treated with a control diet and b SO diet. Positive staining is dark brown color outside of nuclei. Nuclei are green, stained with methyl green. Arrows indicate CD95+ cells. Scale bar represents 50 µM. c Relative quantitation of CD95+ cells. d Western blot analysis of CD95, caspase-10, and Bid. Expression level of proteins is shown by relative fold change to control which is indicated by dotted line. Values are shown as mean ± SEM (n = 3–4 per treatment). Asterisks indicate a significant difference compared to control (p < 0.05)
Immunohistochemical study of apoptotic cell in tumor tissues by TUNEL staining in response to control diet or stearidonic acid-enriched (SO) diet. Representative images show apoptotic positive cells on tumors with a control diet and b SO diet. Arrows indicate cells undergoing apoptosis which are dark brown color specific to nucleus. Non-apoptotic nuclei are shown by green color after counterstain with methyl green. Scale bar indicates 50 µM. c Relative quantitation of TUNEL-positive cells, determined by the average percent of positive (apoptotic cells)/total number of cells counted from representative sections. Values indicate mean (%) ± SEM (n = 3 per treatment). Asterisk indicates a significant difference compared to control (p < 0.05)
Discussion
Treating MDA-MB-231 and MCF-7 cells with SO fatty acids significantly reduced their growth. Feeding a diet containing SO, compared to a diet with a fat composition more similar to the North American population [44], reduced tumor growth of MDA-MB-231 cells in nu/nu mice. The SDA content of the SO produced from the current study was 26 % which was considerably higher than the 10–15 % reported in previous studies [27, 28]. This higher level of SDA is attributable to the use of high ALA (approximately 75 %) flax breeding line (F06396B). SO contained a mixture of fatty acids (ALA, SDA, and GLA in the highest concentration). Whether it was individual fatty acids in the SO or the combination of fatty acids that reduced tumor growth was not determined in the current study. The SDA content likely contributes to the anti-cancer effects as it has been demonstrated that feeding SDA, but not ALA, reduces tumor growth in an animal model of colorectal cancer [22]. Previous studies have reported tumor cell growth-promoting effects of LA and AA [31, 33], while GLA had variable effects depending on cell lines and doses [1, 45]. Our data suggest that GLA, at least in the concentrations used in the current study, does not promote tumor growth but may have contributed to the higher AA concentration, we observed in MDA-MB-231 cells in vitro. Interestingly, SO fatty acid mixture did not have inhibitory effect in non-tumorigenic cell line MCF-12A, suggesting that SO at the concentrations studied may have anti-growth effects only in human breast cancer MDA-MB-231 and MCF-7 cells.
The anti-cancer effects of SO seen in this study were accompanied by evidence of tumor apoptosis (twofold) in mice bearing the MDA-MB-231 tumor, and an increased expression of CD95, caspase-10, and other pro-apoptotic molecules (Bid/Bad) in both tumors and cultured cells. Our earlier work in the same breast cancer cells reported that EPA and DHA increased apoptosis [4]. The results of the current study suggest that this was mediated by increased surface expression of CD95 that would subsequently activate caspase-8 or 10 [46, 47]. Several cancer drugs work via the activation of CD95 but cancer cells have shown to have a lower expression of CD95 [47, 48]. Feeding SO might offer a way to facilitate CD95-induced apoptosis.
Increases in EPA and DHA concentration of PL have been demonstrated to decrease cell proliferation and increase apoptosis, by changes in EGFR/AKT/NFkappaB cell survival pathway or by inducing CD95-mediated apoptosis [2–4, 15]. EPA, but not DHA, increased with SO treatment in the current study, and this fatty acid has been demonstrated to inhibit human breast cancers both in vitro [1–4, 49–52] and in vivo [7, 50] and induce apoptosis [2, 4, 53]. DPA was increased with SO treatment by more than threefold in tumorigenic cells. Although proliferation was not measured specifically in the current study, DPA has been reported to decrease fatty acid synthase activity [54] which inhibits breast cancer cell growth [55–57] and has inhibitory effects on angiogenesis (via VEGFR2 signaling) [58, 59]. Increased ETA in PL may also have contributed to the apoptotic activity as this fatty acid was reported to inhibit cyclooxygenase-2 [60, 61], which could increase apoptosis and decrease proliferation [62] and reduce the growth of breast tumors in mice [63, 64].
Feeding SDA has been shown to increase EPA and DPA in plasma, heart, and liver of healthy rodents, humans, and dogs [25, 65, 66]. A previous study in MDA-MB-231 cells reported that SDA treatment increased the concentrations of ETA, EPA, and DPA, the n-3 LCPUFA downstream from SDA [67]. Our study extended these findings by determining the changes in specific PL classes in MDA-MB-231 cells and tumors. Although SDA was incorporated into cells and tumor PL, the resulting proportion was very small. Both in vivo and in vitro EPA and DPA were incorporated to a greater extent into PE. PE is the major mediator of protein translocation [16], and our group has demonstrated that changes in the n-3 LCPUFA composition are associated with movement of CD95 into lipid rafts in the membrane of MDA-MB-231 cells [2]. A higher concentration of EPA and DPA occurred in the PC moiety with SO treatment/diet. The changes in those fatty acids in PC were associated with decreased cell proliferation and increased apoptosis in tumor cells [15]. The n-3 LC PUFA concentration of PI was also increased in vitro and in vivo by SO treatment. PI is the main source of diacylglycerol in the plasma membrane which via protein kinase C (PKC) hydrolysis produces signaling molecules [17] involved in apoptosis [68, 69]. PI is also very high in AA, and a significant decrease in the AA content of PI (also PE) was observed in tumors from mice fed the SO diet. It is possible that the anti-carcinogenic effect of SO on tumors could be via the reduction in the availability of precursor fatty acid AA in PL, particularly PI, for the biosynthesis of eicosanoids and other membrane-generated signals involved in tumor cell growth (reviewed by [70]). However, it is unlikely that this decreased availability of AA in PL is the primary mechanism for the anti-apoptotic effects in this tumor type as in vitro incubation with SO resulted in a higher concentration of AA in MDA-MB-231 (in PC and PE) and MCF-7 cells. The higher AA concentration in PL after in vitro treatment is most likely due to the presence of higher concentration of AA precursor GLA (15.0 %) in our SO fatty acid mixture (whereas in vivo it only contributed 2.5 % of total fat).
Interestingly, we observed the relative membrane change of major fatty acids DGLA, EPA, and DPA after SO treatment, which were higher in MDA-MB-231 followed by MCF-7 and MCF-12A, which could in part be due to the faster growth of the MDA-MB-231 cells [71]. In MCF-7, compared to MDA-MB-231 and MCF-12A cells, the lower concentration of many fatty acids in the elongation and saturation pathway beyond ALA and LA was likely due to limited ∆5- and ∆6-activity in MCF-7 [3] which is the major, albeit not the only method of two-carbon elongation in MCF-7 [3, 15, 72].
In conclusion, we demonstrated anti-cancer activity of SO on breast cancer cells and tumors. We are proposing that the change in membrane n-3 LCPUFA (ETA, EPA, and DPA) composition similar to what we previously reported for DHA [2] facilitated CD95-mediated tumor death. In addition, this study provided evidence of an alternative dietary source of n-3 LCPUFA that could be used in the future in clinical trials aimed at treating breast cancer.
Abbreviations
- AA:
-
Arachidonic acid
- ALA:
-
α-linolenic acid
- CD95:
-
Fas ligand death receptor
- DGLA:
-
Dihomo γ-linolenic acid
- DHA:
-
Docosahexaenoic acid
- DPA:
-
Docosapentaenoic acid
- EPA:
-
Eicosapentaenoic acid
- ETA:
-
Eicosatetraenoic acid
- GC:
-
Gas chromatography
- GLA:
-
γ-linolenic acid
- LA:
-
Linoleic acid
- LCPUFA:
-
Long-chain polyunsaturated fatty acid
- OA:
-
Oleic acid
- PC:
-
Phosphatidylcholine
- PE:
-
Phosphatidylethanolamine
- PI:
-
Phosphatidylinositol
- PL:
-
Phospholipid
- PS:
-
Phosphatidylserine
- SDA:
-
Stearidonic acid
- SO:
-
SDA-enriched oil
References
Chajes V, Sattler W, Stranzl A, Kostner GM (1995) Influence of n-3 fatty acids on the growth of human breast cancer cells in vitro: relationship to peroxides and vitamin-E. Breast Cancer Res Treat 34(3):199–212
Ewaschuk JB, Newell M, Field CJ (2012) Docosahexanoic acid improves chemotherapy efficacy by inducing CD95 translocation to lipid rafts in ER(-) breast cancer cells. Lipids 47(11):1019–1030. doi:10.1007/s11745-012-3717-7
Grammatikos SI, Subbaiah PV, Victor TA, Miller WM (1994) n-3 and n-6 fatty acid processing and growth effects in neoplastic and non-cancerous human mammary epithelial cell lines. Br J Cancer 70(2):219–227
Schley PD, Jijon HB, Robinson LE, Field CJ (2005) Mechanisms of omega-3 fatty acid-induced growth inhibition in MDA-MB-231 human breast cancer cells. Breast Cancer Res Treat 92(2):187–195. doi:10.1007/s10549-005-2415-z
Kang KS, Wang P, Yamabe N, Fukui M, Jay T, Zhu BT (2010) Docosahexaenoic acid induces apoptosis in MCF-7 cells in vitro and in vivo via reactive oxygen species formation and caspase 8 activation. PLoS ONE 5(4):e10296. doi:10.1371/journal.pone.0010296
Robinson LE, Clandinin MT, Field CJ (2001) R3230AC rat mammary tumor and dietary long-chain (n-3) fatty acids change immune cell composition and function during mitogen activation. J Nutr 131(7):2021–2027
Rose DP, Connolly JM (1999) Omega-3 fatty acids as cancer chemopreventive agents. Pharmacol Ther 83(3):217–244
Rose DP, Connolly JM, Rayburn J, Coleman M (1995) Influence of diets containing eicosapentaenoic or docosahexaenoic acid on growth and metastasis of breast cancer cells in nude mice. J Natl Cancer Inst 87(8):587–592
Wu M, Harvey KA, Ruzmetov N, Welch ZR, Sech L, Jackson K, Stillwell W, Zaloga GP, Siddiqui RA (2005) Omega-3 polyunsaturated fatty acids attenuate breast cancer growth through activation of a neutral sphingomyelinase-mediated pathway. Int J Cancer 117(3):340–348. doi:10.1002/ijc.21238
Field CJ, Schley PD (2004) Evidence for potential mechanisms for the effect of conjugated linoleic acid on tumor metabolism and immune function: lessons from n-3 fatty acids. Am J Clin Nutr 79(6 Suppl):1190S–1198S
Sawyer MB, Field CJ (2010) Possible Mechanism of n-3 PUFA anti-tumor action. In: Calviello G, Serini S (eds) Dietary omega-3 polyunsaturated fatty acids and cancer, Diet and Cancer, vol 1. Springer Science, New York, pp 3–38. doi:10.1007/978-90-481-3579-0_1
Gu Z, Suburu J, Chen H, Chen YQ (2013) Mechanisms of omega-3 polyunsaturated fatty acids in prostate cancer prevention. Biomed Res Int 2013:824563. doi:10.1155/2013/824563
Hardman WE (2004) (n-3) Fatty acids and cancer therapy. J Nutr 134(12 Suppl):3427S–3430S
Deshpande R, Mansara P, Suryavanshi S, Kaul-Ghanekar R (2013) Alpha-linolenic acid regulates the growth of breast and cervical cancer cell lines through regulation of NO release and induction of lipid peroxidation. J Mol Biol 2:6–17
Corsetto PA, Montorfano G, Zava S, Jovenitti IE, Cremona A, Berra B, Rizzo AM (2011) Effects of n-3 PUFAs on breast cancer cells through their incorporation in plasma membrane. Lipids Health Dis 10:73. doi:10.1186/1476-511X-10-73
Baumgartner P, Geiger M, Zieseniss S, Malleier J, Huntington JA, Hochrainer K, Bielek E, Stoeckelhuber M, Lauber K, Scherfeld D, Schwille P, Waldele K, Beyer K, Engelmann B (2007) Phosphatidylethanolamine critically supports internalization of cell-penetrating protein C inhibitor. J Cell Biol 179(4):793–804. doi:10.1083/jcb.200707165
Neri LM, Bortul R, Borgatti P, Tabellini G, Baldini G, Capitani S, Martelli AM (2002) Proliferating or differentiating stimuli act on different lipid-dependent signaling pathways in nuclei of human leukemia cells. Mol Biol Cell 13(3):947–964. doi:10.1091/mbc.01-02-0086
Wright MM, Howe AG, Zaremberg V (2004) Cell membranes and apoptosis: role of cardiolipin, phosphatidylcholine, and anticancer lipid analogues. Biochem Cell Biol 82(1):18–26. doi:10.1139/o03-092
Yao Y, Huang C, Li ZF, Wang AY, Liu LY, Zhao XG, Luo Y, Ni L, Zhang WG, Song TS (2009) Exogenous phosphatidylethanolamine induces apoptosis of human hepatoma HepG2 cells via the bcl-2/Bax pathway. World J Gastroenterol WJG 15(14):1751–1758
Wang D, Dubois RN (2010) Eicosanoids and cancer. Nature reviews. Cancer 10(3):181–193. doi:10.1038/nrc2809
Leitzmann MF, Stampfer MJ, Michaud DS, Augustsson K, Colditz GC, Willett WC, Giovannucci EL (2004) Dietary intake of n-3 and n-6 fatty acids and the risk of prostate cancer. Am J Clin Nutr 80(1):204–216
Petrik MB, McEntee MF, Johnson BT, Obukowicz MG, Whelan J (2000) Highly unsaturated (n-3) fatty acids, but not alpha-linolenic, conjugated linoleic or gamma-linolenic acids, reduce tumorigenesis in Apc(Min/+) mice. J Nutr 130(10):2434–2443
Burdge GC, Wootton SA (2002) Conversion of alpha-linolenic acid to eicosapentaenoic, docosapentaenoic and docosahexaenoic acids in young women. Br J Nutr 88(4):411–420. doi:10.1079/BJN2002689
Emken EA, Adlof RO, Gulley RM (1994) Dietary linoleic acid influences desaturation and acylation of deuterium-labeled linoleic and linolenic acids in young adult males. Biochim Biophys Acta 1213(3):277–288
Yamazaki K, Fujikawa M, Hamazaki T, Yano S, Shono T (1992) Comparison of the conversion rates of alpha-linolenic acid (18:3(n-3)) and stearidonic acid (18:4(n-3)) to longer polyunsaturated fatty acids in rats. Biochim Biophys Acta 1123(1):18–26
Cantrill RC, Huang YS, Ells GW, Horrobin DF (1993) Comparison of the metabolism of alpha-linolenic acid and its delta 6 desaturation product, stearidonic acid, in cultured NIH-3T3 cells. Lipids 28(3):163–166
Abbadi A, Domergue F, Bauer J, Napier JA, Welti R, Zahringer U, Cirpus P, Heinz E (2004) Biosynthesis of very-long-chain polyunsaturated fatty acids in transgenic oilseeds: constraints on their accumulation. Plant Cell 16(10):2734–2748. doi:10.1105/tpc.104.026070
Ruiz-Lopez N, Haslam RP, Venegas-Caleron M, Larson TR, Graham IA, Napier JA, Sayanova O (2009) The synthesis and accumulation of stearidonic acid in transgenic plants: a novel source of ‘heart-healthy’ omega-3 fatty acids. Plant Biotechnol J 7(7):704–716. doi:10.1111/j.1467-7652.2009.00436.x
Eckert H, La Vallee B, Schweiger BJ, Kinney AJ, Cahoon EB, Clemente T (2006) Co-expression of the borage Delta 6 desaturase and the Arabidopsis Delta 15 desaturase results in high accumulation of stearidonic acid in the seeds of transgenic soybean. Planta 224(5):1050–1057. doi:10.1007/s00425-006-0291-3
Harris WS (2012) Stearidonic acid-enhanced soybean oil: a plant-based source of (n-3) fatty acids for foods. J Nutr 142(3):600S–604S. doi:10.3945/jn.111.146613
Chang NW, Wu CT, Chen DR, Yeh CY, Lin C (2013) High levels of arachidonic acid and peroxisome proliferator-activated receptor-alpha in breast cancer tissues are associated with promoting cancer cell proliferation. J Nutr Biochem 24(1):274–281. doi:10.1016/j.jnutbio.2012.06.005
Maillard V, Hoinard C, Steghens JP, Jourdan ML, Pinault M, Bougnoux P, Chajes V (2002) Interaction of dietary beta-carotene and alpha-linolenic acid: effect on promotion of experimental mammary tumours. IARC Sci Publ 156:403–404
Reyes N, Reyes I, Tiwari R, Geliebter J (2004) Effect of linoleic acid on proliferation and gene expression in the breast cancer cell line T47D. Cancer Lett 209(1):25–35. doi:10.1016/j.canlet.2003.12.010
Hong H, Datla N, Reed DW, Covello PS, MacKenzie SL, Qiu X (2002) High-level production of gamma-linolenic acid in Brassica juncea using a delta6 desaturase from Pythium irregulare. Plant Physiol 129(1):354–362. doi:10.1104/pp.001495
Wu G, Truksa M, Datla N, Vrinten P, Bauer J, Zank T, Cirpus P, Heinz E, Qiu X (2005) Stepwise engineering to produce high yields of very long-chain polyunsaturated fatty acids in plants. Nat Biotechnol 23(8):1013–1017. doi:10.1038/nbt1107
Mlynarova L, Bauer M, Nap JP, Pretova A (1994) High efficiency Agrobacterium-mediated gene transfer to flax. Plant Cell Rep 13(5):282–285. doi:10.1007/BF00233320
Robinson LE, Field CJ (1998) Dietary long-chain (n-3) fatty acids facilitate immune cell activation in sedentary, but not exercise-trained rats. J Nutr 128(3):498–504
Hoke EM, Maylock CA, Shacter E (2005) Desferal inhibits breast tumor growth and does not interfere with the tumoricidal activity of doxorubicin. Free Radic Biol Med 39(3):403–411. doi:10.1016/j.freeradbiomed.2005.03.029
Marlind J, Kaspar M, Trachsel E, Sommavilla R, Hindle S, Bacci C, Giovannoni L, Neri D (2008) Antibody-mediated delivery of interleukin-2 to the stroma of breast cancer strongly enhances the potency of chemotherapy. Clin Cancer Res 14(20):6515–6524. doi:10.1158/1078-0432.CCR-07-5041
North WG, Pang RH, Gao G, Memoli VA, Cole BF (2011) Native MAG-1 antibody almost destroys human breast cancer xenografts. Breast Cancer Res Treat 127(3):631–637. doi:10.1007/s10549-010-1009-6
Field CJ, Ryan EA, Thomson AB, Clandinin MT (1988) Dietary fat and the diabetic state alter insulin binding and the fatty acyl composition of the adipocyte plasma membrane. Biochem J 253(2):417–424
Folch J, Lees M, Sloane Stanley GH (1957) A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem 226(1):497–509
Schonberg S, Krokan HE (1995) The inhibitory effect of conjugated dienoic derivatives (CLA) of linoleic acid on the growth of human tumor cell lines is in part due to increased lipid peroxidation. Anticancer Res 15(4):1241–1246
Field CJ, Angel A, Clandinin MT (1985) Relationship of diet to the fatty acid composition of human adipose tissue structural and stored lipids. Am J Clin Nutr 42(6):1206–1220
Menendez JA, Ropero S, del Barbacid MM, Montero S, Solanas M, Escrich E, Cortes-Funes H, Colomer R (2002) Synergistic interaction between vinorelbine and gamma-linolenic acid in breast cancer cells. Breast Cancer Res Treat 72(3):203–219
Barnhart BC, Alappat EC, Peter ME (2003) The CD95 type I/type II model. Semin Immunol 15(3):185–193
Fulda S, Strauss G, Meyer E, Debatin KM (2000) Functional CD95 ligand and CD95 death-inducing signaling complex in activation-induced cell death and doxorubicin-induced apoptosis in leukemic T cells. Blood 95(1):301–308
Kim HS, Lee YS, Kim DK (2009) Doxorubicin exerts cytotoxic effects through cell cycle arrest and Fas-mediated cell death. Pharmacology 84(5):300–309. doi:10.1159/000245937
Bernard-Gallon DJ, Vissac-Sabatier C, Antoine-Vincent D, Rio PG, Maurizis JC, Fustier P, Bignon YJ (2002) Differential effects of n-3 and n-6 polyunsaturated fatty acids on BRCA1 and BRCA2 gene expression in breast cell lines. Br J Nutr 87(4):281–289. doi:10.1079/BJNBJN2002522
Biondo PD, Brindley DN, Sawyer MB, Field CJ (2008) The potential for treatment with dietary long-chain polyunsaturated n-3 fatty acids during chemotherapy. J Nutr Biochem 19(12):787–796. doi:10.1016/j.jnutbio.2008.02.003
Schley PD, Brindley DN, Field CJ (2007) (n-3) PUFA alter raft lipid composition and decrease epidermal growth factor receptor levels in lipid rafts of human breast cancer cells. J Nutr 137(3):548–553
Yamamoto D, Kiyozuka Y, Adachi Y, Takada H, Hioki K, Tsubura A (1999) Synergistic action of apoptosis induced by eicosapentaenoic acid and TNP-470 on human breast cancer cells. Breast Cancer Res Treat 55(2):149–160
Corsetto PA, Cremona A, Montorfano G, Jovenitti IE, Orsini F, Arosio P, Rizzo AM (2012) Chemical-physical changes in cell membrane microdomains of breast cancer cells after omega-3 PUFA incorporation. Cell Biochem Biophys 64(1):45–59. doi:10.1007/s12013-012-9365-y
Kaur G, Cameron-Smith D, Garg M, Sinclair AJ (2011) Docosapentaenoic acid (22:5n-3): a review of its biological effects. Prog Lipid Res 50(1):28–34. doi:10.1016/j.plipres.2010.07.004
Hunt DA, Lane HM, Zygmont ME, Dervan PA, Hennigar RA (2007) MRNA stability and overexpression of fatty acid synthase in human breast cancer cell lines. Anticancer Res 27(1A):27–34
Jin Q, Yuan LX, Boulbes D, Baek JM, Wang YN, Gomez-Cabello D, Hawke DH, Yeung SC, Lee MH, Hortobagyi GN, Hung MC, Esteva FJ (2010) Fatty acid synthase phosphorylation: a novel therapeutic target in HER2-overexpressing breast cancer cells. Breast Cancer Res 12(6):R96. doi:10.1186/bcr2777
Menendez JA, Vellon L, Mehmi I, Oza BP, Ropero S, Colomer R, Lupu R (2004) Inhibition of fatty acid synthase (FAS) suppresses HER2/neu (erbB-2) oncogene overexpression in cancer cells. Proc Natl Acad Sci USA 101(29):10715–10720. doi:10.1073/pnas.0403390101
Kunkel P, Ulbricht U, Bohlen P, Brockmann MA, Fillbrandt R, Stavrou D, Westphal M, Lamszus K (2001) Inhibition of glioma angiogenesis and growth in vivo by systemic treatment with a monoclonal antibody against vascular endothelial growth factor receptor-2. Cancer Res 61(18):6624–6628
Tsuji M, Murota SI, Morita I (2003) Docosapentaenoic acid (22:5, n-3) suppressed tube-forming activity in endothelial cells induced by vascular endothelial growth factor. Prostaglandins Leukot Essent Fatty Acids 68(5):337–342
Bierer TL, Bui LM (2002) Improvement of arthritic signs in dogs fed green-lipped mussel (Perna canaliculus). J Nutr 132(6 Suppl 2):1634S–1636S
Rainsford KD, Whitehouse MW (1980) Gastroprotective and anti-inflammatory properties of green lipped mussel (Perna canaliculus) preparation. Arzneimittelforschung 30(12):2128–2132
Basu GD, Pathangey LB, Tinder TL, Lagioia M, Gendler SJ, Mukherjee P (2004) Cyclooxygenase-2 inhibitor induces apoptosis in breast cancer cells in an in vivo model of spontaneous metastatic breast cancer. Mol Cancer Res 2(11):632–642
Kundu N, Yang Q, Dorsey R, Fulton AM (2001) Increased cyclooxygenase-2 (cox-2) expression and activity in a murine model of metastatic breast cancer. Int J Cancer 93(5):681–686
Rozic JG, Chakraborty C, Lala PK (2001) Cyclooxygenase inhibitors retard murine mammary tumor progression by reducing tumor cell migration, invasiveness and angiogenesis. Int J Cancer 93(4):497–506
Harris WS, Lemke SL, Hansen SN, Goldstein DA, DiRienzo MA, Su H, Nemeth MA, Taylor ML, Ahmed G, George C (2008) Stearidonic acid-enriched soybean oil increased the omega-3 index, an emerging cardiovascular risk marker. Lipids 43(9):805–811. doi:10.1007/s11745-008-3215-0
James MJ, Ursin VM, Cleland LG (2003) Metabolism of stearidonic acid in human subjects: comparison with the metabolism of other n-3 fatty acids. Am J Clin Nutr 77(5):1140–1145
Horia E, Watkins BA (2005) Comparison of stearidonic acid and alpha-linolenic acid on PGE2 production and COX-2 protein levels in MDA-MB-231 breast cancer cell cultures. J Nutr Biochem 16(3):184–192. doi:10.1016/j.jnutbio.2004.11.001
Majumder PK, Pandey P, Sun X, Cheng K, Datta R, Saxena S, Kharbanda S, Kufe D (2000) Mitochondrial translocation of protein kinase C delta in phorbol ester-induced cytochrome c release and apoptosis. J Biol Chem 275(29):21793–21796. doi:10.1074/jbc.C000048200
Mandil R, Ashkenazi E, Blass M, Kronfeld I, Kazimirsky G, Rosenthal G, Umansky F, Lorenzo PS, Blumberg PM, Brodie C (2001) Protein kinase Calpha and protein kinase Cdelta play opposite roles in the proliferation and apoptosis of glioma cells. Cancer Res 61(11):4612–4619
Larsson SC, Kumlin M, Ingelman-Sundberg M, Wolk A (2004) Dietary long-chain n-3 fatty acids for the prevention of cancer: a review of potential mechanisms. Am J Clin Nutr 79(6):935–945
Bernhardt G, Reile H, Birnbock H, Spruss T, Schonenberger H (1992) Standardized kinetic microassay to quantify differential chemosensitivity on the basis of proliferative activity. J Cancer Res Clin Oncol 118(1):35–43
Hansen-Petrik MB, McEntee MF, Johnson BT, Obukowicz MG, Masferrer J, Zweifel B, Chiu CH, Whelan J (2002) Selective inhibition of Delta-6 desaturase impedes intestinal tumorigenesis. Cancer Lett 175(2):157–163
Acknowledgments
The authors acknowledge the technical support of Susan Goruk, Chris Kazala, and Robin Miles. Funding for this project came in part from the Canadian Institute of Health Sciences (CIHR) and in part from an investigator initiated grant to a provincial government agency Alberta Innovates Biosolutions (AIBS) that administered the funds to this project from their funding partner, the Alberta Canola Producers (ACP). RJW is grateful for the support provided by the Canada Research Chairs Program.
Conflicts of interest
The authors have no financial association with the owner of the patent or the funders of this project.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Subedi, K., Yu, HM., Newell, M. et al. Stearidonic acid-enriched flax oil reduces the growth of human breast cancer in vitro and in vivo. Breast Cancer Res Treat 149, 17–29 (2015). https://doi.org/10.1007/s10549-014-3212-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-014-3212-3