Abstract
The centrosome, known as the cell’s major microtubule organizing center, is a multifunctional organelle of ca. 1 μm in diameter without membrane boundaries; it relies on precise regulation to nucleate microtubules for specific functions throughout the cell cycle. The centrosome also serves as hub for signal transduction molecules and orchestrates signal transduction through its microtubule network. It holds key roles in cell cycle regulation and directly or indirectly affects cell cycle progression and cellular metabolism.
Centrosome dysfunctions have been implicated in numerous diseases and disorders including neurodegenerative diseases and cancer. Centrosome functions are also affected in aging cells in which aneuploidy is associated with loss of centrosome and microtubule integrity leading to chromosome mis-segregation, as seen in senescing somatic cells and in mammalian reproductive cells in which meiotic spindles become dysfunctional resulting in fertility problems and developmental disorders.
This chapter is centered on centrosome–microtubule interactions and their dysfunctions in disease and disorders with focus on (1) Centrosome–microtubule dynamics; (2) Centrosome dysfunctions in aging cells; (3) Centrosome dysfunctions in cancer cells. Several avenues are discussed to understand centrosome abnormalities and restore function in affected reproductive and somatic cells.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Cytoskeleton
- Microtubules
- Intermediate filaments
- Microfilaments
- Centrosomes
- Septins
- Cytoskeletal abnormalities
Introduction
The centrosome, known as the primary microtubule organizing center (MTOC) of eukaryotic cells, is a multifunctional organelle of ca. 1 μm in diameter without membrane boundaries. It plays essential roles in all cell cycle stages and undergoes remodeling during the transition from interphase to mitosis (G2/M) to become division-competent and assemble a functional mitotic apparatus that is able to separate chromosomes accurately to the dividing daughter cells (reviewed in [1]). Centrosome dysfunctions have been implicated in numerous diseases and disorders including Alstrom syndrome, Bardet–Biedl syndrome, retinitis pigmentosa, deafness, obesity, diabetes mellitus, lissencephaly, mental and behavioral disorders, malformations, juvenile autosomal recessive Parkinson disease, rheumatoid arthritis, polycystic kidney disease, ciliopathies, cancer and others (reviewed by Badano et al. [2], Nigg and Raff [3], Gerdes et al. [4], Bettencourt-Dias et al. [5], Schatten [6]). The centrosome is a versatile organelle that functions as major hub for signal transduction molecules and orchestrates signal transduction through its microtubule network. The centrosome further is involved in Golgi functions, in directing organelle movement such as mitochondria and cargo such as enzyme-containing vesicles and therefore, centrosome dysfunctions have wide-ranging impacts on cellular metabolism and cellular health. As detailed in Chap. 10 by Li and Hu, the centrosome also plays a role in primary cilia formation, thereby interacting with the extra- and intracellular microenvironment. Detailed data are available for primary cilia dysfunctions in polycystic kidney disease in which the proteins polycystin-1, polycystin-2, polaris, and cystin are affected [7].
The structure and functional components of the centrosome and the centrosome duplication cycle have been reviewed previously [1, 8, 9–12, 187] and will only be briefly addressed here while this chapter is focused on centrosome–microtubule interactions and their dysfunctions in disease and disorders. As indicated above, numerous diseases have directly been linked to centrosome dysfunctions (reviewed by Badano et al. [2], Nigg and Raff [3], Gerdes et al. [4], Bettencourt-Dias et al. [5], Schatten [6]) and others have been linked to centrosome-related signaling dysfunctions. Some of the major diseases and disorders linked to centrosomes will be addressed here while others will be mentioned and references will be provided. In this chapter we will primarily focus on (1) Centrosome–microtubule dynamics; (2) Centrosome dysfunctions in aging cells; (3) Centrosome dysfunctions in cancer cells.
Section 1: Centrosome–Microtubule Dynamics
As indicated above, the centrosome is a multifunctional cellular organelle without membrane boundaries that relies on precise regulation to nucleate microtubules for specific and varying functions throughout the cell cycle. Several regulators of centrosome functions have been determined in which kinases and phosphatases as well as post-translational modifications play major roles. Such modifications allow centrosomes to nucleate and organize different microtubule formations. Whereas the interphase centrosome nucleates the large interphase aster that radiates from the nucleus-associated interphase centrosome the mitotic centrosomes organize polar asters and the central mitotic spindle that is critical for chromosome alignment and separation. As will be detailed below significant remodeling of the centrosome takes place at the transition from G2/M to modify the interphase centrosome into division-competent mitotic centrosomes, a process termed centrosome maturation. During this process centrosomes become enabled to nucleate an increased number of mitotic microtubules as a result of increased amounts of γ-tubulin that associate with the mitotic centrosome.
As mentioned above, the centrosome holds key roles in cell cycle regulation and in several other complex cellular functions that directly or indirectly affect cell cycle progression and cellular metabolism. Numerous kinases are involved in the transition from G2 to mitosis [9–12]. These kinases primarily play a role in centrosome protein phosphorylation while dephosphorylation takes place when cells exit mitosis.
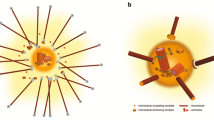
In a typical mammalian somatic cell, a perpendicularly oriented centriole pair is embedded in a centrosomal matrix (Fig. 5.1) that oftentimes is also referred to as pericentriolar material (PCM). The centrosomal matrix (PCM) is composed of a lattice of coiled-coil proteins and contains specific centrosomal proteins including the γ-tubulin ring complexes (γ-TuRCs), pericentrin, centrin, and calcium-sensitive components ([14]; reviewed in [1]). The composition of proteins embedded within the centrosomal matrix varies in different cell cycle stages while centrioles, on the other hand, do not significantly change in their molecular composition throughout the cell cycle. However, centriole duplication takes place in a precisely regulated process to assure that centrioles are duplicated only once during the cell cycle. In mammalian cells, centrioles display a typical composition of nine outer triplet microtubules forming a barrel-shaped small tube without containing central microtubules. Centrioles duplicate through a semiconservative duplication process during which a younger (daughter) centriole forms perpendicular to the older (mother) centriole. The mother centriole is distinguished from the daughter centriole by appendages as a characteristic feature which indicates structural and functional differences. In mammalian cells, centrioles are involved in the assembly of specific centrosome proteins and in the duplication of centrosomal material [15]. The exact composition of centrosomal material is not yet clear, as it is difficult to generate precise data with our currently available methods but it has been reported that as many as 500 proteins may be associated with the interphase centrosome structure [16] although it is likely that a large number of these proteins may be centrosome-associated proteins or proteins that are temporarily localized to centrosomes during specific cell cycle stages. A more conservative estimate may include about 60–100 centrosomal proteins to be present in a typical somatic cell interphase centrosome (reviewed in [17]). Of these, centrosome core proteins are tightly associated with the centrosome matrix while others are part of the cell cycle-dependent structural centrosomal changes in most cell systems.
A typical centrosome in somatic cells is composed of centrosomal material, also referred to as pericentriolar material (PCM), surrounding two perpendicularly oriented centrioles. Embedded in this matrix are centrosomal proteins such as γ-tubulin and the γ-tubulin ring complexes that nucleate microtubules along with numerous associated proteins as described in the text. Other components within the centrosomal matrix include the microtubule anchoring complexes. Modified from Schatten and Sun [13]
So far, purified centrosomes have been analyzed by mass spectrometry, revealing several classes of proteins that include structural proteins (alpha-tubulin, beta-tubulin, γ-tubulin, γ-tubulin complex components 1–6, centrin 2 and 3, AKAP450, pericentrin/kendrin, ninein, pericentriolar material 1 (PCM1), ch-TOG protein, C-Nap1, Cep250, Cep2, centriole-associated protein CEP110, Cep1, centriolin, centrosomal P4.1-associated protein (CPAP), CLIP-associating proteins CLASP1 and CLASP 2, ODF2, cenexin, Lis1, Nudel, EB1, centractin, myomegalin); regulatory molecules (cell division protein 2 (Cdc2), Cdk1, cAMP-dependent protein kinase type II-alpha regulatory chain, cAMP-dependent protein kinase-alpha catalytic subunit, serine/threonine protein kinase Plk1, serine/threonine protein kinase Nek2, serine/threonine protein kinase Sak, Casein kinase I, delta and epsilon isoforms, protein phosphatase 2A, protein phosphatase 1 alpha isoform, 14-3-3 proteins, epsilon and gamma isoforms); motor and motor-related proteins (dynein heavy chain, dynein intermediate chain, dynein light chain, dynactin 1, p150 Glued, dynactin 2, p50, dynactin 3); and heat shock proteins (heat shock protein Hsp90, TCP subunits, and heat shock protein Hsp73).
Gamma-tubulin is an essential centrosomal protein that is primarily found in the centrosome matrix structure, but it can also serve as nucleating sites in areas other than the centrosome and it can be associated with microtubule walls. It also is important for microtubule nucleation from the Golgi and it associates with the plasma membrane during cellular polarization. The major nucleating complex for microtubules from centrosomes is the ca. 2.2-MDa γ-TuRC that is present in all cells studied so far [18] and consists of 12 or 14 γ-tubulin molecules. Hundreds of γ-TuRCs may be embedded in the centrosome matrix, dependent on the requirements for microtubule nucleation which differs in different cell cycle stages. The large γ-TuRC contains 5-7 small complexes, the γTuSCs (around 280 kDa) that each comprises two molecules of γ-tubulin and one molecule each of GCP (γ-tubulin complex protein) 2 and 3 [19]. The γ-TuSCs associate with the γ-TuRC by condensation and association with proteins GCP4, GCP5, GCP6, and GCP-WD/NEDD1. Two functional genes for γ-tubulin have been identified for mammalian cells (TUBG1, TUBG2). Posttranslational modifications of γ-tubulin have been reported which includes phosphorylation and mono-ubiquitination. Complexes of γ-tubulin with protein tyrosine kinases of the Src family, polo-like kinase, microtubule affinity-regulating kinase 4 (MARK 4) or phosphoinositide 3-kinase have also been documented. Various proteins and protein complexes are needed to anchor the γ-TuRC to the centrosome matrix including the large coiled-coil A-kinase anchoring proteins [19–22, 23–26], Cep135 [27], ninein, augmin, Cep192/SPD2, AKAP450/CG-NAP, pericentrin/kendrin, and CDK5RAP2/centrosomin. Dynactin plays a major role in microtubule anchorage at centrosomes as well as at non-centrosomal anchorage sites. It is preferentially localized to the mother centriole [28–30]. Several of the microtubule minus-end binding proteins including those of the γ-TuRC are accumulated at the proximal ends of centrioles. Tubulin polyglutamylation of the centriole walls modulates interaction between tubulin- and microtubule-associated proteins. Much interest has been focused on how the activity of the γ-TuRC is regulated and significant new data have been produced that identified new components that interact with or regulate the γ-TuRC such as NME7 [31] and TACC3 Protein [32]. More specific information on γ-TuRC regulation is available in several recent original and review papers [31–34]. New methods including subdiffraction-resolution fluorescence microscopy combined with site-specific antibody analyses have generated new insights into high-order spatial organization of the centrosome structure [35–38].
As mentioned above, the number of microtubules nucleated by the γ-TuRC varies in different cell cycle stages. In interphase, fewer but longer microtubules are nucleated, while in mitosis, γ-TuRCs become increased to nucleate more microtubules. Mitotic microtubules are shorter, larger in number, and highly dynamic. The regulation of microtubule nucleation includes cell cycle-specific proteins that participate in the centrosome maturation process and include the small GTPase Ran, Aurora A kinase, polo-like kinases, and others (reviewed in [6]).
Pericentrin forms a ca. 3-MDa complex with γ-tubulin and depends on dynein for assembly onto centrosomes [39]. Pericentrin is part of the pericentrin/AKAP450 centrosomal targeting (PACT) domain [40] involved in recruiting γ-tubulin to centrosomes [20, 21]. Mutation of the pericentrin gene results in loss of recruitment of several other centrosomal proteins which becomes manifested in diseases or disorders (reviewed in [2]). Centrins are primarily associated with centrioles, but are also components of centrosomes with an essential role in centrosome duplication ([15, 41–43]; reviewed in [44]).
NuMA (Nuclear Mitotic Apparatus protein): One of the critical proteins enabling mitotic and meiotic centrosome functions is NuMA. NuMA becomes a significant centrosome-associated protein during mitosis as well as meiosis when it forms an insoluble crescent around the centrosome area facing toward the central mitotic or meiotic spindle. NuMA is important for cross-linking spindle microtubules and for tethering microtubules precisely into the bipolar mitotic or meiotic apparatus [45]. NuMA is a multifunctional protein (reviewed in [46, 47] that serves as nuclear matrix protein in the nucleus during interphase but it is not associated with interphase centrosomes. NuMA becomes dispersed into the cytoplasm during nuclear envelope breakdown and associates with microtubules for translocation to the centrosomal area. Cdk1/cyclin B-dependent phosphorylation is important for this process to take place [48]; the association with microtubules and translocation to centrosomes requires dynein–dynactin-mediation; failure of this mediation will result in meiotic and mitotic dysfunctions [185].
Regulation of the Centrosome Complex
The regulation of the centrosome complex is critically important for accurate functions throughout the cell cycle and for coordination with several cell cycle events. It includes accurate duplication of the centrioles as well as the centrosomal material for precise coordination of centrosome and chromosome dynamics. In mitosis as well as meiosis, centrosomes organize microtubules that attach to kinetochores as part of a complex molecular machinery that assures accurate separation and equal distribution of chromosomes to the dividing cells. Centrosomes and chromosomes undergo coordinated duplication cycles in parallel pathways to assure accurate cell divisions. Cell cycle abnormalities occur when these events become misregulated and desynchronized, as will be addressed in sections 2 and 3.
Centrosome Duplication
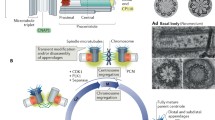
It is important that centrosomes are duplicated only once during the cell cycle in a process that is well synchronized with the DNA cycle to assure precise chromosome partitioning to the dividing daughter cells (Fig. 5.2). Centrosome duplication and DNA replication both require hyperphosphorylation of the retinoblastoma (RB) protein and activation of Cdk2. The program of duplication and block for reduplication has been reviewed (reviewed in [1, 9–12]) and it has been shown that centrosome duplication starts to take place shortly before the G2 cell cycle stage in a precisely orchestrated duplication program. The process begins with disorientation of the pair of centrioles, centriole duplication, centriole disjunction, and separation of sister centrioles (reviewed in [49, 50]). While this process has been well studied in somatic cells and quite detailed knowledge has been accumulated on centriole duplication, we still do not yet fully understand duplication of centrosomal material and centrosome dynamics throughout the cell cycle. Centrosome duplication and separation is frequently correlated to the better understood centriole dynamics and centriole duplication cycle. Our knowledge about centrosome duplication primarily relates to phosphorylation (reviewed in [9–12]). We know that the initiation of centrosome duplication is under cytoplasmic control and driven by cyclin-dependent kinase 2 (Cdk2) complexed with cyclin E or cyclin A that rises during the late G1 stage (reviewed in [51]). The initiation of centrosome duplication further requires calcium/calmodulin-dependent kinase II (CaMKII) [52] that localizes to spindle poles [53] and phosphorylates centrosome proteins in vitro [54]. Specific centrosome proteins depend on multiple signaling to allow the transition from G2 to mitosis; the G2/M cell cycle transition is critical for centrosome phosphorylation to become division competent and allow cell proliferation (reviewed in [1]). Polo-like kinases are required for multiple stages of mitotic progression and they are further involved in centrosome separation [55–57].
Centrosome cycle in somatic cells. (A) The interphase centrosome is located next to the nucleus and organizes the interphase microtubule array. (B) Centriole and centrosome duplication is synchronized with DNA duplication during the S phase. (C) Separation of the duplicated centrosome complex to the forming mitotic poles takes place during early prophase. (D) Establishment of the bipolar mitotic apparatus with centrosomes localized at the center of the mitotic poles. (E) Compacted metaphase centrosomes organize a well-focused metaphase spindle. (F) Reorganized centrosomal material closely associated with the reforming daughter nuclei of dividing cells. (G–I) Centrosomal abnormalities resulting in tripolar (G) or multipolar (H–I) spindle formations. Modified from Schatten [6]
The block to centriole reduplication may involve ubiquitin-mediated proteolysis of centrosomal proteins, as several components of the SCF (Skp1/cullin/Fbox; ubiquitin ligase complex) proteolysis pathway as well as the 26S proteasome are localized to centrosomes in human cells [9–11, 58–61]. The centrosomal protein centrin plays a role in centrosome disjunction at the G2/prophase transition, and it has also been shown that the Nek2 kinase is involved in this process ([62]; reviewed in [63]). Centrosome separation is in part driven by plus- and minus end-directed microtubule motor proteins which takes place in interphase around the nucleus and during mitosis at the mitotic poles.
As indicated above, the G2/M transition represents a critical phase during which centrosomes mature to become division-competent in a process requiring Cdk1/cyclin B as well as Cdk1/cyclin A (reviewed in [64]). Cdk1/cyclin B activation is detected in centrosomes during prophase [65]. During the G2/M phase several important centrosomal proteins are acquired including polo-like kinase 1 (Plk1) [66], and NuMA [67]. On the other hand, interphase centrosome proteins such as C-Nap1 [68] or Nlp [69] are removed. γ-TuRC recruitment to the centrosome increases prior to mitosis to nucleate increased numbers of microtubules for spindle formation. Polo and Aurora A kinases [70] and cdc2/cyclin B kinase [65] are precisely regulated during G2/M and during mitosis. Misregulation is associated with diseases and disorders as will be addressed in sections 2 and 3. Exit from mitosis requires degradation of cyclin B which is achieved by proteins binding to the anaphase-promoting complex/cyclosome (APC/C); the activated APC/CCdc20 degrades cyclin B and securin to allow cell cycle exit from mitosis [71–74]. Microtubule motor proteins are important for the assembly of a functional mitotic centrosome which includes shuttling of the proteins pericentrin, centrin, ninein, and NuMA along microtubules toward the centrosomal area.
As of now we do not yet fully understand how centrosome proteins are associated with the centrosomal matrix but it is possible that the centrosomal matrix may play a role in clustering of centrosome proteins during mitosis ([75]; reviewed in [6]). Invertebrate models have provided some information on the centrosomal matrix structure which revealed that this material may contain fibrous cytoskeleton-like material. In the Spisula model, material left after high-salt extraction of centrosomal proteins contained a fibrous component [76, 77], perhaps composed of filament-like material that may have intermediate filament-like characteristics. Our own studies on sea urchin centrosomes revealed filament-like proteins that could be detected with Ah6, a monoclonal antibody to intermediate filament-like proteins [78]. In addition to these proteins microtubule-associated proteins (MAPs) in the centrosomal matrix may be involved in centrosome clustering. Other proteins that may play a role in functions of the centrosomal matrix includes HSET (kinesin-related protein), as HSET depletion blocks centrosome clustering and promotes multipolar divisions [79].
The interactions of microtubules with centrosomes and their interdependent regulation is complex and we do not yet fully understand the factors that affect centrosomes and microtubules and their influence on each other which includes signal transduction pathways that play a role in communication between microtubules and centrosomes. Signal transductions that have been explored allowed close insights into this important relationship in somatic as well as in reproductive cells and determined critically important molecular mechanisms that effectively regulate centrosome and microtubule dynamics, their interactions with each other, and communication with other cellular components. Many of the signaling molecules that have been identified to colocalize with centrosomes use centrosomes as their central docking station for cellular communications in which microtubules provide the distribution network. As centrosomes are able to modify the microtubule network they can facilitate changes to accommodate cell cycle-dependent signaling requirements. Key signaling molecules that associate with centrosomes include the mitogen activated protein kinase (MAPK) that plays a critical role in centrosome and microtubule regulation during meiosis, mitosis, and cell division ([80]; reviewed in [1]). Polo and Aurora A kinases [70], and cdc2/cyclin B kinase [65] are other important meiotic and mitotic cell cycle regulators that are concentrated at the centrosome (reviewed in [1]). These kinases are critical for centrosome regulation, as abnormalities have been linked to centrosome pathologies affecting cellular health. In mouse meiotic maturation p38α MAPK, a centrosome-associated protein, has been shown to regulate spindle assembly, spindle length and chromosome segregation [81]. Depletion of p38α affects other proteins and results in spindle pole defects and aneuploidy.
Posttranslational Tubulin Modifications
The effects of posttranslational modifications on microtubule dynamics and functions have been reviewed [82–84] and their importance for modulations of cytoskeletal and cellular functions have been highlighted. PTMs are chemical modifications that regulate microtubule activity and interactions with other cellular molecules and components by creating marks on microtubules for specialized interactions and function-specific activities [83]. PTM-related microtubule dysfunctions have been linked to diseases such as cancer, diabetes, heart diseases, neurodegenerative diseases, and various others (reviewed in [82, 84]).
Several tubulin PTMs have been studied in somatic and reproductive cells and include acetylation and detyrosination/tyrosination. These PTMs have been implicated in microtubule stability, in networking with other proteins, and in targeted associations with the microtubule motor proteins dynein and/or kinesin that accommodate transport of cargo molecules along microtubules. The association of dynein with microtubules is important for transport of centrosome proteins such as pericentrin and centrosome-associated proteins such as NuMA to remodel centrosomes throughout the cell cycle (reviewed in [1, 82]). These PTMs are also important for localized function-specific stabilization of microtubules [83, 85, 86] which is an important aspect for stabilizing labile microtubules at the minus ends facing the centrosomal area.
Acetylation of microtubules is a reversible PTM that is mediated by acetyltransferase [87] while tubulin deacetylation is mediated by two known enzymes, the histone deacetylase 6 (HDAC6) [88, 89] to reverse acetylation of Lys40, and sirtuin 2 (SIRT2) [90]. HDAC6 functions can be inhibited by trichostatin A (TSA) [91, 92] and tubastatin A [93]. The reversible detyrosination/tyrosination cycle plays a role in the recruitment of microtubule-binding proteins and specific molecular motors. Detyrosination is achieved by the removal of a Tyr functional group from tubulin, whereas tyrosination is achieved by re-addition of Tyr that returns tubulin to its nascent state [94].
The Primary Cilia-Centrosome Cycle
Primary cilia are tightly correlated with the centrosome cycle and the regulatory relationship between primary cilia functions and the cell cycle has clearly been established (reviewed in [95, 186]). The primary cilium–centriole–centrosome cycle starts during G1 when the distal end of the mother centriole becomes associated with a membrane vesicle (reviewed by Pan and Snell [95]) followed by growth into an axoneme that is surrounded by the enlarging ciliary vesicle that fuses with the plasma membrane. During the subsequent S phase centrioles duplicate and lengthen; the mature length of the primary cilium is achieved during G2. As detailed in Chap. 10 by Li and Hu, the primary cilium is a non-motile single cilium composed of 9 outer microtubule doublets with no central microtubule pair (“9 + 0”); it is covered by a specialized receptor-rich plasma membrane. The primary cilium protrudes from almost all cells in our body [96–98] and it communicates signals from the external cellular environment to the cell body. The molecular aspects of this cilium and functions are addressed in Chap. 10 by Li and Hu of this book. Numerous diseases have been associated with primary cilia dysfunctions that have been well elaborated for polycystic kidney syndrome and include many diseases and disorders grouped under the umbrella of ciliopathies. Several studies have revealed details of signal transduction cascades between primary cilia and the centrosome that are essential for accurate cell cycle progression [1, 97–103]. These are reviewed in Chap. 10 by Li and Hu.
Section 2: Centrosome Dysfunctions in Aging Cells
By now centrosome dysfunctions in diseases and disorders have been studied by a number of different investigators (reviewed by Badano et al. [2], Nigg and Raff [3], Gerdes et al. [4], Bettencourt-Dias et al. [5], Schatten [6]) but centrosome dysfunctions in aging cells are still largely unexplored although it has clearly been shown that centrosomes and the cytoskeleton are affected by aging in reproductive cells [13], in stem cells [104, 105], and in various cultured cells [106, 107]. The best examples of centrosome and cytoskeletal changes during aging come from oocyte cells in which aging occurs rapidly when fertilization does not take place within a certain time frame (reviewed in [108–110]). Furthermore, oocyte aging is well known to be associated with aneuploidy primarily in women past 35 years of age which results in low fertilization rates, affecting many women in advanced ages who desire to have children and seek treatment in in vitro fertilization (IVF) clinics.
Oocytes of most mammalian species are arrested at the meiosis II (MII) stage and remain arrested until fertilization takes place which typically occurs soon after ovulation. The window for optimal fertilization varies in different species. If fertilization does not take place within a certain time frame unfertilized oocytes remain in the oviduct or in culture and will undergo time-dependent quality changes in a process termed oocyte aging. In unfertilized MII stage oocytes several aging effects take place in humans and all mammalian animal models studied so far. In humans, oocyte aging occurs within a 24 h time frame and includes changes in calcium metabolism, decrease in enzyme activity, decrease in essential organelle functions such as mitochondria resulting in decrease in ATP production, destabilization of the microfilament and microtubule cytoskeleton, loss of centrosome integrity associated with loss of spindle integrity, and loss of cohesion between sister chromatids resulting in chromosome mis-segregation and aneuploidy. Loss of spindle integrity includes dispersion of centrosomal proteins including γ-tubulin and NuMA from the centrosome core structure. The mechanisms underlying these changes and consequences or causes are not well understood but several pieces of information have been accumulated in recent years. It has been shown in human oocytes that microtubules become destabilized in aging oocytes which prevents accurate motor-driven transport of centrosomal proteins along microtubules to form and maintain a functional centrosome [111, 185]. Because of the high demand to overcome the effects of oocyte aging to allow fertilization, embryo development and the birth of healthy babies this area of research has progressed more rapidly compared to research on aging in somatic cells and studies on oocyte aging have become important for procedures that can be applied in IVF clinics to overcome the aging effects. One of the important goals to overcome the effects of aging is to understand and target the mechanisms underlying loss of spindle integrity and prevent aneuploidy [13].
In fresh oocytes, centrosome dynamics are precisely regulated and include active maintenance of centrosomes until fertilization takes place. As mentioned above, the MII spindle in mammalian reproductive systems is highly dynamic and becomes unstable if regulation by a complex set of kinases and other regulatory proteins fails or if fertilization does not take place within a certain period of time (reviewed in [108, 110, 112, 113]). The kinases involved in the process of meiosis includes CDK1/cyclin B and other kinases such as PKA, AKT, MAPK, Aurora A, CaMKII, the phosphatases CDC5, CDK14s and others that participate in the meiotic process.
As mentioned above, aneuploidy resulting from oocyte aging is associated with disintegration of centrosomal proteins such as NuMA and γ-tubulin from the centrosomal core structure of the MII spindle which coincides with the formation of numerous small centrosomal aggregates in the ooplasm. It is not yet clear whether the centrosomal core structure itself is affected by oocyte aging or whether microtubule destabilization results in loss of microtubule motor activities in which transport of centrosomal proteins such as NuMA and pericentrin is impaired, thereby affecting proper maintenance of a functional centrosome. It is possible that loss of microtubule stability is the result of loss of microtubule acetylation [85] that prevents accurate association of the motor proteins dynein and kinesins with microtubules (reviewed in [82]). Other factors that affect microtubule and centrosome dynamics and stability include signal transductions that may be misguided in aging oocytes (reviewed in [1, 13]), resulting in an inability to maintain spindle integrity.
Dispersion of centrosomal components including NuMA and γ-tubulin from the meiotic spindle poles in aging oocytes has been reported for several non-rodent mammalian animal models as well as for humans ([114, 115]; reviewed in [108, 111, 116]). Oocyte aging affects meiotic regulation which not only can lead to aneuploidy affecting fertilization but also to subsequent cell and developmental abnormalities resulting in abortion, disease, or developmental defects (reviewed in [108, 110, 112, 113, 116]). Diseases that may be associated with meiotic aneuploidies to become manifested later in life include childhood cancer with characteristic centrosome dysfunctions that may originate from aberrant oocyte centrosomes.
Centrosomes are primarily located at the two meiotic spindle poles in mature MII oocytes. It is important to emphasize that, while these centrosomes serve functions as known for mitotic centrosomes, there are important differences between meiotic centrosomes and mitotic centrosomes. Whereas a typical somatic cell centrosome contains a pair of centrioles (reviewed in [1]) centrally located within the centrosome, centrosomes of the oocyte’s meiotic spindle are acentriolar. These acentriolar centrosomes contain centrosomal proteins that are known for mitotic centrosomes embedded in the centrosome matrix including γ-tubulin, pericentrin, centrin, and the nuclear mitotic apparatus protein, NuMA, but the central centriole pair is absent (reviewed in [1]). As the quantity and specific composition of centrosomal proteins may differ in different cell systems it can also be different in oocytes of different animal species.
As mentioned above, in aging oocytes one of the most noticeable features of aging is the deterioration of the meiotic spindle with disintegration of centrosomal proteins from the centrosomal core structure that is correlated with loss of microtubule stability (reviewed in [13]). As we do not yet fully understand the underlying reasons for centrosome and microtubule instability in aging MII spindles it is possible that the absence of centrioles plays a role in the rapid loss of spindle integrity. In somatic cells centrioles are intimately involved in centrosome dynamics, potentially contributing to stability that may be absent in acentriolar centrosomes.
In a variety of other cell systems centrosome dysfunctions have also been implicated in aging; for example, supernumerary centrosome abnormalities have been observed in senescing cells [106] which may be the result of cell cycle abnormalities in which signal transductions are altered. As indicated above, centrosomes undergo remodeling at the transition from G2/M which is the stage during which many of the cell cycle regulators are downregulated in aging cells and affect centrosome functions. We know that specific kinases and phosphatases are important for cytoskeletal regulation in meiotic and mitotic spindles (reviewed in [82, 117]); in aging cells, studies have shown that centrosomes have lower activity in centrosome-associated protein kinases [118, 119] which includes Plk that serves an important role in centrosome functions [107]. Furthermore, mis-orientation of centrosomes has been shown in aging stem cells which results in decrease in cell divisions [104], contributing to declines in spermatogenesis during aging.
Microtubule associated proteins (MAPs) and posttranslational modifications play important roles in the regulation of microtubule stability. Such stabilizing factors may be lost in aging cells contributing to microtubule instability. Other factors for microtubule stabilization include interactions with centrosomes and cell membranes (reviewed in [82]). Individual microtubules if not regulated by specific kinases and phosphatases undergo individual microtubule aging which becomes important when considering aging of microtubules in the meiotic spindle of aging oocytes (reviewed in [82, 117]). Changes in posttranslational tubulin modifications have been associated with loss of microtubule stability with strong effects on microtubule functions in several cell systems. PTM changes resulting in microtubule instability during aging have also been determined in neuronal cells in which microtubule PTM dysfunctions have been linked to diseases such as Alzheimer’s and Parkinson’s.
Our previous experiments have shown that MII spindle microtubules are acetylated at the microtubule-centrosome interface area [85] which was correlated with microtubule stabilization. In aging oocytes loss of spindle integrity is first seen in this specific area which suggests that loss of acetylation plays a role in spindle instability and is in part a causative factor for aneuploidy. Restoring stability of these microtubules may be possible by treatment of aging oocytes with deacetylation inhibitors such as inhibitors of HDA6 that have been used in cancer cell therapy [120, 121]. This treatment strategy may allow microtubule stabilization in the meiotic spindle to prevent de-acetylation-related microtubule instability.
Our previous studies have also shown that restoring signal transduction that may have been impacted during aging will halt or reverse the aging process (reviewed in [13]). For example, the use of caffeine to delay or prevent oocyte aging has been proposed by Kikuchi et al. [122, 123] who found that controlling the activity of MPF can reverse oocyte aging (reviewed in [108]). These investigators also showed that both MPF and MAPK are critical for maintaining oocyte spindle integrity, and that MPF and MAPK activities gradually decrease during oocyte aging [124–128]. Continuous treatment with 10 mM caffeine could prevent the decline in MPF and MAPK activity in aging bovine oocytes ([129]; reviewed in [108]) and continuous treatment with caffeine could restore spindle integrity in aging porcine oocytes [112] with chromosomes, microtubules and the centrosomal proteins γ-tubulin and NuMA displaying normal appearance as known for fresh oocytes (reviewed in [13]).
Section 3: Centrosome Dysfunctions in Cancer Cells
The incidence of cancer development and progression increases with aging and multiple factors play a role in changes that take place during this process. It is well known that the mutation rate increases in cells that have reached replicative senescence. Misguided signal transductions have been implicated in cancer development and progression which may in part be similar to those seen during physiological aging. One hallmark characteristic common to all cancer cells is abnormal cell division which is strongly related to abnormal centrosome functions. It has been recognized as early as 1914 [130] that centrosomes are affected in cancer cells which adversely affects chromosome segregation and cell division, two hallmark features that are clearly seen in cancer cells and tissue ([131–133]; reviewed in [1]).
Theodor Boveri’s classic remarkable discoveries and brilliant data interpretations ([130]; translated into English in [134]) ignited a new era in modern cancer research when the significance of centrosomes was again recognized and when it was possible to apply new technologies to explore changes in centrosomes as important aspect for abnormal cancer cell proliferation (reviewed in [1]). While well-regulated centrosomes form the bipolar mitotic spindle in mitosis cancer cell centrosomes frequently form multipolar spindles with consequences for aneuploidy and genomic instability. Abnormal multipolar mitoses resulting from supernumerary centrosomes have been well documented in numerous cancers and have been well analyzed in HPV-associated lesions in cervical cancers in which centrosome abnormalities are already detected in early stages of tumor development [135]. While environmental insult and in some cases viral infections are known to be cancer-inducing factors we do not yet fully understand the underlying mechanisms leading to centrosomal abnormalities which in many cases may have multifactorial components. As cause and effect studies are still being explored in attempts to determine when centrosomes become dysfunctional during the cascade of events leading to the observed abnormalities (reviewed in [136]), it has clearly been determined that cancer cell centrosomes are significantly different from noncancer cell centrosomes which includes their state of abnormal phosphorylation which had first been recognized when examining breast adenocarcinoma cells [137]. Increased phosphorylation of cancer cell centrosomes [137] is associated with increases in microtubule nucleation and abnormal organization leading to aberrant attachment of microtubules to chromosomes (reviewed in [6]). Increased γ-tubulin expression has been shown in breast carcinoma cells [138, 139] and gliomas.
Centrosomes have been recognized as major microtubule organizing centers (MTOCs) and their role as major hub for signal transduction molecules that participate in signal transduction cascades through the microtubule network. The effects of misguided signal transduction on the formation of abnormal centrosomes and in turn the effects of abnormal centrosomes on signal transduction has been recognized. This interdependence may be part of a vicious cycle in which regulation of centrosomes and regulation of signal transduction by centrosomes are both affected in cancer cells.
Several proteins in cancer cell centrosomes are overexpressed or display abnormalities and include the centrosome-associated protein NuMA. The NuMA region on chromosome 11q13 has been associated with breast cancer susceptibility [140]. NuMA misregulation may further contribute to abnormalities in cancer cells, as NuMA requires specific signaling for its centrosome-associated functions in which signaling of cyclin B is important (reviewed in [46]); cyclin B signals may be affected in cancer cells. For NuMA’s relocation into the nucleus following exit from mitosis it has to become dissociated from the mitotic spindle poles. This process requires cdc1/cyclin B activity [141]. Destruction of cyclin B allows exit from mitosis. If NuMA does not become relocated properly into the nucleus for its interphase functions NuMA can form cytoplasmic focal points in the cytoplasm that organize abnormal microtubule asters [141] that can contribute to mitotic abnormalities. Such abnormal mitotic formations in cancer cells have also been observed to originate from basal bodies of dislodged primary cilia that become located in the cancer cell cytoplasm and form supernumerary nucleation sites for microtubule-based asters that participate in aberrant chromosome segregation [131–133, 142].
Dysfunctions of structural, regulatory, and motor-related proteins may be other contributors to centrosome abnormalities. In cancer cells, the cell cycle coordination between chromosomes and centrosomes are lost, resulting in asynchronous misregulation and misguided duplication cycles. Multipolar centrosomes that separate chromosomes unequally to the dividing cells will contribute to imbalanced distribution of chromosomes resulting in cells that may lack tumor suppressor genes while others may have increases in tumor promoting genes.
Dissociation of centrosome cycles from DNA cycles have been reported after irradiation. Loss of proteins that are important for critical cell functions may lead to loss of cell polarity and increased cancer cell formations. For example, loss of Plk3 function will result in loss of cell shape [57], affecting microtubule functions underneath the plasma membrane resulting in loss of cellular polarity in cancer cells and tissue. Cascades of events may follow and may include loss of signal transduction processes, imbalanced or disrupted transport of centrosome proteins resulting in additional centrosomal pathologies related to centrosome and microtubule functions with consequences for failures in organelle and vesicle distribution. Secondary pathologies can develop as a result of disruption in transport leading to interconnected communication failures for which cause and effects are difficult to establish. Signal transduction events have been well studied in breast cancer in which numerous centrosomal abnormalities have been reported (reviewed by Kais and Parvin [58, 187], Fisk [9], Fukasawa [11], Korzeniewski and Duensing [143], Saladino et al. [144], Yan and Chng [145].
The causes for changes leading to aberrant centrosomes in cancer cells are known in some cases but not in others. We know that environmental stress can result in the formation of aggresomes, aggregates that are thought to be the result of misfolded proteins [146–150]; they are oftentimes located in close proximity to centrosomes and some of the aggresomes contain γ-tubulin. Aggresomes are associated with disease or disorders including Parkinson’s and dementia [151].
Other factors that play a role in cancer initiation and progression include epigenetic modifications which includes aberrant hypermethylation that has been implicated in inactivation of checkpoint genes that may influence cell cycle-dependent centrosome abnormalities. Such abnormalities have been reported for pancreatic cancer [152, 153].
Among the best-studied changes in cancer cell centrosomes is overexpression of specific centrosome proteins that results in abnormal centrosome configurations and aneuploidy [133, 154]. These studies strongly correlate abnormal centrosomes with cancer development and progression in which increased centrosome number and volume, supernumerary centrioles, accumulation of increased PCM, and abnormal phosphorylation of centrosomes are characteristic for cancer cells in which cell polarity is lost [2, 137]. Centrosome misregulation is associated with abnormal microtubule nucleation, abnormal spindle formation, and chromosomal mis-segregation. As mentioned above, loss of tumor suppressor genes are among the factors that affect accurate centrosome functions.
Changes in Aurora A have been implicated in centrosome amplification in breast cancer and other cancers. In animal models, overexpression of Aurora A kinase (AURKA), an important centrosome-associated serine/threonine kinase, was strongly associated with tumor development [155, 156]. These experiments showed that Aurora A localizes to centrosomes and overexpression of Aurora A causes multipolar mitotic spindles that play a role in early development of mammary tumors. Further studies [156] showed that the pro-survival AKT pathway is activated, preventing cell death while promoting abnormal cell proliferation in which tetraploid cells with accumulated centrosomes were generated.
Genes implicated in centrosome amplification are in part responsible for deregulation of centrosome duplication and subsequent reactions that lead to cascades of cell cycle-related abnormalities. Critical cell cycle regulators are lost in cancer cells which includes loss of the tumor suppressor p53, resulting in multiple cycles of centrosome duplication in one S phase in which centrosome numbers become increased [157]. Viral oncoproteins can inactivate p53 resulting in cells with supernumerary centrosomes which has clearly been shown for the papillomavirus (reviewed in [135]). Loss of p53 following genotoxic stress or mitogenic stimulation has been documented for breast cancer cells in which changes in the CDK2/cyclin-dependent pathway has been implicated [158, 159].
Other tumor suppressor genes have directly or indirectly been implicated in changes of centrosome functions and include the breast- and ovary-specific tumor suppressor gene BRCA1 that has been shown to play a role in deregulation of centrosome duplication. BRCA1 is involved in G2/M checkpoint functions and it plays a role in preventing centrosome overduplication. While we do not yet fully understand the precise mechanisms by which BRCA1 affects centrosome regulation a model for the regulation of the centrosome by BRCA1 has been presented by Kais and Parvin [58]. The model suggests that in regulated cell cycles BRCA1 ubiquitinates the already duplicated centrosomes to inhibit reduplication. Supernumerary centrosome are the result of loss of BRCA1 during the S phase. The model suggests that overexpression of AURKA mimics the effects of BRCA1 loss. Furthermore, overexpressed AURKA overrides the spindle checkpoint and may thereby contribute to abnormal mitosis.
In regulated cell cycles BRCA1 forms a complex with the BRCA1-associated RING domain 1 (BARD1) functioning as E3 ubiquitin ligase. The BRCA1–BARD1 complex plays a role in maintaining centrosome homeostasis by ubiquitinating γ-tubulin, preventing abnormal duplication and abnormal microtubule nucleation by γ-tubulin. Centrosome abnormalities have also been reported in transgenic mice in which the BRCA1-associated centrosomal ninein-like protein (Nlp) is overexpressed, causing spontaneous breast tumorigenesis perhaps as a result of Nlp mimicking BRCA1 loss [160]. BRCA2 also plays a role in centrosome functions, as in regulated cell cycles the BRCA2-associated protein NPM forms a complex with ROCK2 to maintain numerical centrosome integrity; centrosome overduplication and fragmentation may be the result of aberrant regulation of this protein [113].
The centrosomal kinase Nek2 is important for centrosome regulation and centrosome accumulation has been reported in breast epithelial cells in which Nek2 was misregulated [161]. Several other centrosomal components are involved in cancer initiation and progression [162–165] that have been detailed in recent reviews [6, 58, 9, 11, 143–145, 187]. Other factors also play a role in centrosome abnormalities and may include structural defects of the centrosome matrix (reviewed in [6]). One line of research has focused more recently on centrosome clustering as an important factor in centrosome abnormalities in cancer cells. Centrosome clustering is important for mitosis to accumulate centrosomal material equally at the two mitotic poles. Studies have shown that centrosome amplification, cell cycle control dysfunctions, and aggregation of centrosomal material at the mitotic spindle poles are associated with centrosome clustering abnormalities (reviewed in [75]). While multipolar mitotic cells are easy to identify as abnormalities, amplified cancer cell centrosomes can cluster into abnormal bipolar spindles. These abnormalities are not easily discernible from regular bipolar spindles with non-amplified centrosomes but they will nucleate and organize abnormal microtubule formations resulting in chromosome mis-segregations and aneuploidy as shown in Drosophila cells [166] as well as in cancer cells [75, 144]. We do not yet fully understand the mechanisms underlying centrosome clustering in normal cells and dysfunctions in cancer cells; more research is needed to analyze the regulation of centrosome clustering. Studies by Kwon et al. [79] have determined that the actin cytoskeleton plays a role in centrosome clustering. Our previous experiments using the invertebrate sea urchin as experimental system revealed that microtubules and microfilaments are required for centrosome dynamics (Schatten et al. [167]) which may also be important for centrosome clustering. We do not yet know whether or not the filamentous components identified in sea urchin centrosomes [78] and in Spisula extracts [76, 77] or perhaps other cytoskeletal elements (reviewed in [6]) play a role in the mechanisms underlying centrosome clustering. Microtubule motor proteins have also been implicated in the clustering process which has been discussed in detail by Krämer et al. [75]. It has been proposed that preventing centrosome clustering into an abnormal bipolar mitotic apparatus may provide new targets for cancer therapy. Centrosomes that are not able to cluster form multiple microtubule asters followed by fragmented cell divisions and cell death with fewer chances for cancer cell viability [168].
The Role of Primary Cilia in Cancer Development and Progression
Because of the close relationships between centrosomes and primary cilia centrosome abnormalities may also affect primary cilia formation and functions which may further contribute to cancer development and progression. During progressive stages of cancer development the basal body of the primary cilium becomes dislodged and locates in the cytoplasm [131, 132, 142] where it may form small asters and participate in the mitotic process during subsequent cell divisions. As the oncogenic Aurora A kinase (Aurora A) is localized to the basal body of primary cilia [169] it may further play a role in centrosome amplification and contribute to primary cilia-cell cycle dysfunctions.
Centrosomes as Target for Cancer Therapy and Prevention
Cancer is a complex heterogeneous disease that can have different causes and different centrosome abnormalities which presents complexities for the design of effective treatment strategies (reviewed in [6]) requiring multiple targeted treatment approaches to eradicate different subpopulations of cells in cancer tissue. Centrosomes are increasingly being discussed as new targets for cancer treatment, as centrosomes are central to cell division and may be a major driver for abnormal cell divisions.
Targeting cancer cell centrosomes may include targeting misguided signaling pathways, overexpressed centrosome proteins, abnormal centrosome clustering, abnormal primary cilia dynamics, overexpressed phosphorylation such as Aurora A that is implicated in centrosome hyperphosphorylation or other components in the phosphorylation cascade; it further includes different molecules that play a role in centrosome function such as the aryl hydrocarbon receptor (AhR) and cyclin E, as reported by Korzeniewski et al. [164], and several others (reviewed in [6]). Such approaches to target multiple centrosome abnormalities are possible either through the development of new pharmaceuticals or through plant derivatives or dietary ingredients that have been shown to affect centrosome–microtubule interactions during mitosis and cell division.
Plant derivatives that have been developed into a cancer-targeting pharmaceuticals includes paclitaxel (or taxol), originally isolated from the plant Taxus brevifolia. Taxol primarily inhibits microtubule depolymerization, thereby preventing progression of mitosis and cell division [170, 171]. It has been shown that taxol interacts with microtubules at the centrosome–microtubule nucleation sites [172, 173], and it had been proposed that centrosomes in taxol-treated cells may lose their capacity to nucleate microtubules [172]. Other drugs that had been explored as anti-cancer drugs include colcemid and nocodazole that prevent and disrupt microtubule polymerization.
Currently, curcumin, a natural polyphenol found in the rhizomes of Curcuma longa (turmeric) is being investigated for its anti-cancer activities which led to new promising strategies including the use of theragnostic curcumin-encapsulated nanoparticles that will increase bioavailability and allow more potent clinical applications [174]. Our recent preliminary experiments showed an effect of curcumin on the microtubule cytoskeleton in several cancer cell lines (Schatten et al. unpublished).
Other studies have focused on the antimitotic drug griseofulvin that arrests cells at the G2/M transition stage in a concentration-dependent manner [175–178]. Studies have shown that griseofulvin affects the NFκB pathway and that the NFκB pathway and centrosome dynamics are connected [179]. This finding is intriguing, as griseofulvin has recently been shown to specifically inhibit supernumerary centrosome clustering in cancer cells which indicates its potential as drug that interferes with centrosome dynamics (reviewed in [75]). This line of potential drug development has not yet been explored in detail but it is well worth pursuing, as prevention of centrosome clustering may preferentially affect cancer cell centrosomes leading to cellular fragmentation followed by cell death. Griseofulvin has already been approved as an effective orally administered antifungal drug that interferes with microtubule dynamics in vivo and in vitro [176, 180–184] and it induces multipolar mitoses in tumor cells [175, 177, 178, 181]. It may interfere with microtubule minus ends at the centrosome–microtubule interacting sites.
Other drugs that are currently considered to interfere with the microfilament system in cancer cells are discussed in Chap. 16 of this book by Brayford et al.
Conclusion and Future Directions
In recent years, significant progress has been made in our understanding of centrosome dynamics and centrosome interactions with microtubules in several cell systems. Centrosome dysfunctions have been identified and characterized in aging cells and in cancer cells which allowed targeting of centrosomes for therapeutic interventions. New imaging methods have been applied to analyze centrosome structure in more detail than previously possible and new technological advances have allowed close insights into the composition and regulation of the centrosome organelle and its interactions with other cellular components. Studies in reproductive and somatic cells have determined centrosome abnormalities in aging cells in which centrosomal proteins disperse from the centrosomal matrix leading to centrosome disintegration and microtubule instability. These studies have also determined that the aging process is reversible to a certain extent by experimentally manipulating specific signal transduction processes. New information on cancer cell centrosomes has allowed an analysis of detailed signal transductions that are misguided in cancer cells and lead to centrosome hyperphosphorylation with centrosomes being phosphorylated throughout the cell cycle without undergoing dephosphorylation which takes place during exit from mitosis in regulated cell cycles. Centrosome amplification and multipolar centrosomes have further been analyzed in cancer cells and these abnormalities suggested new target sites for cancer therapies which includes inhibiting misguided signaling pathways, or inhibiting centrosome clustering to induce excessive centrosome fragmentation resulting in cancer cell fragmentation followed by cell death.
Many questions remain to be answered and include questions on the nature of the centrosomal matrix and on how centrosomal proteins associate with matrix components. We also do not yet fully understand the mechanisms of centrosome duplication, especially in reproductive cell systems in which centrosome abnormalities have been implicated in developmental disorders and/or embryo loss. Understanding the mechanisms that play a role in a regulated centrosome cycle will allow to determine molecular abnormalities that may be corrected in centrosome-impaired diseases or disorders.
References
Schatten H (2008) The mammalian centrosome and its functional significance. Histochem Cell Biol 129:667–686
Badano JL, Teslovich TM, Katsanis N (2005) The centrosome in human genetic disease. Nat Rev Genet 6:194–205
Nigg EZ, Raff JW (2009) Centrioles, centrosomes, and cilia in health and disease. Cell 139:663–678
Gerdes JM, Davis EE, Katsanis N (2009) The vertebrate primary cilium in development, homeostasis, and disease. Cell 137:32–45
Bettencourt-Dias M, Hildebrandt F, Pellman D, Woods G, Godinho SA (2011) Centrosomes and cilia in human disease. Trends Genet 27(8):307–315
Schatten H (2013) The impact of centrosome abnormalities on breast cancer development and progression with a focus on targeting centrosomes for breast cancer therapy. In: Schatten H (ed) Cell and molecular biology of breast cancer. Springer Science and Business Media/LLC, New York
Yoder BK et al (2002) The polycystic kidney disease proteins, polycystin-1, polycystin-2, polaris, and cystin, are co-localized in renal cilia. J Am Soc Nephrol 13:2508–2516
Schatten H, Sun Q-Y (2010) The role of centrosomes in fertilization, cell division and establishment of asymmetry during embryo development. Semin Cell Dev Biol 21:174–184
Fisk HA (2012) Many pathways to destruction: the centrosome and its control by and role in regulated proteolysis (Chapter 8). In: Schatten H (ed) The centrosome. Springer Science and Business Media, New York
Prosser SL, Fry AM (2012) Regulation of the centrosome cycle by protein degradation (Chapter 9). In: Schatten H (ed) The centrosome. Springer Science and Business Media, New York
Fukasawa K (2012) Molecular links between centrosome duplication and other cell cycle associated events (Chapter 10). In: Schatten H (ed) The centrosome. Springer Science and Business Media, New York
Boutros R (2012) Regulation of centrosomes by cyclin-dependent kinases (Chapter 11). In: Schatten H (ed) The centrosome. Springer Science and Business Media, New York
Schatten H, Sun QY (2015) Centrosome and microtubule functions and dysfunctions in meiosis: implications for age-related infertility and developmental disorders. Reprod Fertil Dev. doi:10.1071/RD14493. [Epub ahead of print] PMID:25903261
Salisbury JL (2004) Centrosomes: Sfi1p and centrin unravel a structural riddle. Curr Biol 14:R27–R29
Salisbury JL, Suino KM, Busby R, Springett M (2002) Centrin-2 is required for centriole duplication in mammalian cells. Curr Biol 12:1287–1292
Andersen JS, Wilkinson CJ, Mayor T, Mortensen P, Nigg EA, Mann M (2003) Proteomic characterization of the human centrosome by protein correlation profiling. Nature 426:570–574
Wilkinson CJ, Andersen JS, Mann M, Nigg EA (2004) A proteomic approach to the inventory of the human centrosome. In: Nigg E (ed) Centrosomes in development and disease. Wiley-VCA, Weinheim, pp 125–142
Hannak E, Oegema K, Kirkham M, Gonczy P, Habermann B, Hyman AA (2002) The kinetically dominant assembly pathway for centrosomal asters in Caenorhabditis elegans is γ-tubulin dependent. J Cell Biol 157:591–602
Murphy S, Urbani L, Stearns T (1998) The mammalian gamma-tubulin complex contains homologues of yeast spindle pole body component sspc97p and spc98p. J Cell Biol 141:663–674
Dictenberg J, Zimmerman W, Sparks C, Young A, Vidair C, Zheng Y, Carrington W, Fay F, Doxsey SJ (1998) Pericentrin and gamma tubulin form a protein complex and are organized into a novel lattice at the centrosome. J Cell Biol 141:163–174
Doxsey SJ, Stein P, Evans L, Calarco P, Kirschner M (1994) Pericentrin, a highly conserved protein of centrosomes involved in microtubule organization. Cell 76:639–650
Flory MR, Davis TN (2003) The centrosomal proteins pericentrin and kendrin are encoded by alternatively spliced products of one gene. Genomics 82:401–405
Kawaguchi S, Zheng Y (2004) Characterization of a Drosophila centrosome protein CP309 that shares homology with Kendrin and CG-NAP. Mol Biol Cell 15:37–45
Keryer G, Di Fiore B, Celati C, Lechtreck KF, Mogensen M, Delouvee A, Lavia P, Bornens M, Tassin AM (2003) Part of Ran is associated with AKAP450 at the centrosome: involvement in microtubule-organizing activity. Mol Biol Cell 14:4260–4271
Steadman BT, Schmidt PH, Shanks RA, Lapierre LA, Goldenring JR (2002) Transforming acidic coiled-coil-containing protein 4 interacts with centrosomal AKAP350 and the mitotic spindle apparatus. J Biol Chem 277(33):30165–30176
Takahashi M, Yamagiwa A, Nishimura T, Mukai H, Ono Y (2002) Centrosomal proteins CG-NAP and kendrin provide microtubule nucleation sites by anchoring gamma-tubulin ring complex. Mol Biol Cell 13:3235–3245
Ohta T, Essner R, Ryu JH, Palazzo RE, Uetake Y, Kuriyama R (2002) Characterization of Cep135, a novel coiled-coil centrosomal protein involved in microtubule organization in mammalian cells. J Cell Biol 156:87–99
Quintyne NJ, Gill SR, Eckley DM, Crego CL, Compton DA, Schroer TA (1999) Dynactin is required for microtubule anchoring at fibroblast centrosomes. J Cell Biol 147:321–334
Schroer TA (2001) Microtubules don and doff their caps: dynamic attachments at plus and minus ends. Curr Opin Cell Biol 13:92–96
Quintyne NJ, Schroer TA (2002) Distinct cell cycle-dependent roles for dynactin and dynein at centrosomes. J Cell Biol 159:245–254
Liu P, Choi Y-K, Qi RZ (2014) NME7 is a functional component of the γ-tubulin ring complex. Mol Biol Cell 25:2017–2025
Singh P, Thomas GE, Gireesh KK, Manna TK (2014) TACC3 protein regulates microtubule nucleation by affecting γ-tubulin ring complexes. J Biol Chem 289(46):31719–31735
Teixidó-Travesa N, Roig J, Lüders J (2012) The where, when and how of microtubule nucleation – one ring to rule them all. J Cell Sci 125:4445–4456
Kollman JM, Merdes A, Mourey L, Agard DA (2011) Microtubule nucleation by gamma-tubulin complexes. Nat Rev Mol Cell Biol 12:709–721. doi:10.1038/nrm3209
Mennella V, Keszthelyi B, McDonald KL, Chhun B, Kan F, Rogers GC, Huang B, Agard DA (2012) Subdiffraction-resolution fluorescence microscopy reveals a domain of the centrosome critical for pericentriolar material organization. Nat Cell Biol 14:1159–1168. doi:10.1038/ncb2597
Sonnen KF, Schermelleh L, Leonhardt H, Nigg EA (2012) 3D-structured illumination microscopy provides novel insight into architecture of human centrosomes. Biol Open 1:965–976. doi:10.1242/ bio.20122337
Lawo S, Hasegan M, Gupta GD, Pelletier L (2012) Subdiffraction imaging of centrosomes reveals higher-order organizational features of pericentriolar material. Nat Cell Biol 14:1148–1158. doi:10.1038/ncb2591
Fu J, Glover DM (2012) Structured illumination of the interface between centriole and peri-centriolar material. Open Biol 2:120104. doi:10.1098/rsob.120104
Young A, Dictenberg JB, Purohit A, Tuft R, Doxsey SJ (2000) Cytoplasmic dynein-mediated assembly of pericentrin and γ tubulin onto centrosomes. Mol Biol Cell 11:2047–2056
Gillingham AK, Munro S (2000) The PACT domain, a conserved centrosomal targeting motif in the coiled-coil proteins AKAP450 and pericentrin. EMBO Rep 1:524–529
Levy YY, Lai EY, Remillard SP, Heintzelman MB, Fulton C (1996) Centrin is a conserved protein that forms diverse associations with centrioles and MTOCs in Naegleria and other organisms. Cell Motil Cytoskeleton 33:298–323
Salisbury JL (1995) Centrin, centrosomes, and mitotic spindle poles. Curr Opin Cell Biol 7:39–45
Lutz W, Lingle WL, McCormick D, Greenwood TM, Salisbury JL (2001) Phosphorylation of centrin during the cell cycle and its role in centriole separation preceding centrosome duplication. J Biol Chem 276:20774–20780
Manandhar G, Schatten H, Sutovsky P (2005) Centrosome reduction during gametogenesis and its significance. Biol Reprod 72:2–13
Merdes A, Cleveland DA (1998) The role of NuMA in the interphase nucleus. J Cell Sci 111:71–79
Sun QY, Schatten H (2006) Multiple roles of NuMA in vertebrate cells: review of an intriguing multi-functional protein. Front Biosci 11:1137–1146
Sun Q-Y, Schatten H (2007) Centrosome inheritance after fertilization and nuclear transfer in mammals. Adv Exp Med Biol 591:58–71
Saredi A, Howard L, Compton DA (1997) Phosphorylation regulates the assembly of NuMA in a mammalian mitotic extract. J Cell Sci 110:1287–1297
Mack GJ, Ou Y, Rattner JB (2000) Integrating centrosome structure with protein composition and function in animal cells. Microsc Res Tech 49:409–419
Ou Y, Rattner JB (2004) The centrosome in higher organisms: structure, composition and duplication. Int Rev Cytol 238:119–182
Sluder G (2004) Centrosome duplication and its regulation in the higher animal cell. In: Nigg E (ed) Centrosomes in development and disease. Wiley-VCA, Weinheim, pp 167–189
Matsumoto Y, Maller JL (2002) Calcium, calmodulin, and CaMKII requirement for initiation of centrosome duplication in Xenopus egg extracts (comment). Science 295:499–502
Ohta Y, Ohba T, Miyamoto E (1990) Ca2+/calmodulin-dependent protein kinase II: localization in the interphase nucleus and the mitotic apparatus of mammalian cells. Proc Natl Acad Sci U S A 87:5341–5345
Pietromonaco SF, Seluja GA, Elias L (1995) Identification of enzymatically active Ca2+/calmodulin-dependent protein kinase in centrosomes of hematopoietic cells. Blood Cells Mol Dis 21:34–41
Fenton B, Glover DM (1993) A conserved mitotic kinase active at late anaphase-telophase in syncytial Drosophila embryos. Nature 363:637–640
Donaldson MM, Tavares AAM, Hagan IM, Nigg EA, Glover DM (2001) The mitotic roles of polo-like kinase. J Cell Sci 114:2357–2358
Wang Q, Xie S, Chen J, Fukasawa K, Naik U, Traganos F, Darzynkiewicz Z, Jhanwar-Uniyal M, Dai W (2002) Cell cycle arrest and apoptosis by human polo-like kinase 3 is mediated through perturbation of microtubule integrity. Mol Cell Biol 22(10):3450–3459
Kais Z, Parvin JD (2012) Centrosome regulation and breast cancer (Chapter 14). In: Schatten H (ed) The centrosome. Springer Science and Business Media, New York
Tugendreich S, Tomkiel J, Earnshaw W, Hieter P (1995) CDC27Hs colocalizes with CDC16Hs to the centrosome and mitotic spindle and is essential for the metaphase to anaphase transition. Cell 81:261–268
Freed E, Lacey KR, Huie P, Lyapina SA, Deshaies RJ, Stearns T, Jackson PK (1999) Components of an SCF ubiquitin ligase localize to the centrosome and regulate the centrosome duplication cycle. Genes Dev 13:2242–2257
Gstaiger M, Marti A, Krek W (1999) Association of human SCF(SKP2) subunit p19(SKP1) with interphase centrosomes and mitotic spindle poles. Exp Cell Res 247:554–562
Meraldi P, Nigg EA (2001) Centrosome cohesion is regulated by a balance of kinase and phosphatase activities. J Cell Sci 114:3749–3757
Fry AM (2002) The Nek2 protein kinase: a novel regulator of centrosome structure. Oncogene 21:6184–6194
Fry AM, Hames RS (2004) The role of the centrosome in cell cycle progression. In: Nigg E (ed) Centrosomes in development and disease. Wiley-VCA, Weinheim, pp 143–166
Jackman M, Lindon C, Nigg E, Pines J (2003) Active cyclin B1-Cdk1 first appears on centrosomes in prophase. Nat Cell Biol 5:143–148
Golsteyn RM, Mundt KE, Fry AM, Nigg EA (1995) Cell cycle regulation of the activity and subcellular localization of Plk1, a human protein kinase implicated in mitotic spindle function. J Cell Biol 129:1617–1628
Merdes A, Ramyar K, Vechio JD, Cleveland DW (1996) A complex of NuMA and cytoplasmic dynein is essential for mitotic spindle assembly. Cell 87:447–458
Fry AM, Mayor T, Meraldi P, Stierhof YD, Tanaka K, Nigg EA (1998) C-Nap1, a novel centrosomal coiled-coil protein and candidate substrate of the cell cycle-regulated protein kinase Nek2. J Cell Biol 141:1563–1574
Casenghi M, Meraldi P, Weinhart U, Duncan PI, Korner R, Nigg EA (2003) Polo-like kinase 1 regulates Nlp, a centrosome protein involved in microtubule nucleation. Dev Cell 5:113–125. doi:10.1016/S1534-5807(03)00193-X
Barr AR, Gergely F (2007) Aurora A: the maker and breaker of spindle poles. J Cell Sci 120:2987–2996
Kramer ER, Scheuringer N, Podtelejnikov AV, Mann M, Peters JM (2000) Mitotic regulation of the APC activator proteins CDC20 and CDH1. Mol Biol Cell 11:1555–1569
Huang J, Raff JW (1999) The disappearance of cyclin B at the end of mitosis is regulated spatially in Drosophila cells. EMBO J 18:2184–2195
Wakefield J, Huang J-Y, Raff JW (2000) Centrosomes have a role in regulating the destruction of cyclin B in early Drosophila embryos. Curr Biol 10:1367–1370
Wei Y, Multi S, Yang CR, Ma J, Zhang QH, Wang ZB, Li M, Wei L, Ge ZJ, Zhang CH, Ouyang YC, Hou Y, Schatten H, Sun QY (2011) Spindle assembly checkpoint regulates mitotic cell cycle progression during preimplantation embryo development. PLoS One 6(6):e21557
Krämer A, Anderhub S, Maier B (2012) Mechanisms and consequences of centrosome clustering in cancer cells (Chapter 17). In: Schatten H (ed) The centrosome. Springer Science and Business Media, New York
Schnackenberg BJ, Palazzo RE (1999) Identification and function of the centrosome centromatrix. Biol Cell 91(6):429–438
Schnackenberg BJ, Hull DR, Balczon RD, Palazzo RE (2000) Reconstitution of microtubule nucleation potential in centrosomes isolated from Spisula solidissima oocytes. J Cell Sci 113(Pt 6):943–953
Schatten H, Walter M, Mazia D, Biessmann H, Paweletz N, Coffe G, Schatten G (1987) Centrosome detection in sea urchin eggs with a monoclonal antibody against Drosophila intermediate filament proteins: characterization of stages of the division cycle of centrosomes. Proc Natl Acad Sci U S A 84:8488–8492
Kwon M, Godinho SA, Chandhok NS, Ganem NJ, Azioune A, Thery M, Pellman D (2008) Mechanisms to suppress multipolar divisions in cancer cells with extra centrosomes. Genes Dev 22:2189–2203
Sun Q-Y, Lai L, Wu G, Bonk A, Cabot R, Park K-W, Day B, Prather RS, Schatten H (2002) Regulation of mitogen-activated protein kinase phosphorylation, microtubule organization, chromatin behavior, and cell cycle progression are regulated by protein phosphatases during pig oocyte maturation and fertilization in vitro. Biol Reprod 66(3):580–588
Ou X-H, Li S, Xu B-C, Wang Z-B, Quan S, Li M, Zhang Q-H, Ouyang Y-C, Schatten H, Xing F-Q, Sun Q-Y (2010) p38α MAPK is a MTOC-associated protein regulating spindle assembly, spindle length and accurate chromosome segregation during mouse oocyte meiotic maturation. Cell Cycle 9(20):4130–4143
Schatten H, Sun Q-Y (2014) Posttranslationally modified tubulins and other cytoskeletal proteins: their role in gametogenesis, oocyte maturation, fertilization and pre-implantation embryo development. In: Sutovsky P (ed) Posttranslational protein modifications in the reproductive system. Springer Science and Business Media, New York
Janke C, Bulinski JC (2011) Post-translational regulation of the microtubule cytoskeleton: mechanisms and functions. Nat Rev Mol Cell Biol 12:773–786
Rymut SM, Kelley TJ (2015) Broader implications: biological and clinical significance of microtubule acetylation. Cell Health Cytoskelet 7:71–82
Schatten G, Simerl y C, Asai DJ, Szöke E, Cooke P, Schatten H (1988) Acetylated α-tubulin in microtubules during mouse fertilization and early development. Dev Biol 130:74–86
Asthana J, Kapoor S, Mohan R, Panda D (2013) Inhibition of HDAC6 deacetylase activity increases its binding with microtubules and suppresses microtubule dynamic instability in MCF-7 cells. J Biol Chem 288(31):22516–22526
Akella JS, Wloga D, Kim J, Starostina NG, Lyons-Abbott S, Morrissette NS, Dougan ST, Kipreos ET, Gaertig J (2010) MEC-17 is an alpha-tubulin acetyltransferase. Nature 467:218–222. doi:10.1038/nature09324
Matsuyama A, Shimazu T, Sumida Y, Saito A, Yoshimatsu Y, Seigneurin-Berny D, Osada H, Komatsu Y, Nishino N, Khochbin S et al (2002) In vivo destabilization of dynamic microtubules by HDAC6-mediated deacetylation. EMBO J 21:6820–6831, PubMed: 12486003
Hubbert C, Guardiola A, Shao R, Kawaguchi Y, Ito A, Nixon A, Yoshida M, Wang XF, Yao TP (2002) HDAC6 is a microtubule-associated deacetylase. Nature 417:455–458
North BJ, Marshall BL, Borra MT, Denu JM, Verdin E (2003) The human Sir2 ortholog, SIRT2, is an NAD+-dependent tubulin deacetylase. Mol Cell 11:437–444, PubMed: 12620231
Zilberman Y, Ballestrem C, Carramusa L, Mazitschek R, Khochbin S, Bershadsky A (2009) Regulation of microtubule dynamics by inhibition of the tubulin deacetylase HDAC6. J Cell Sci 122:3531–3541
Haggarty SJ, Koeller KM, Wong JC, Grozinger CM, Schreiber SL (2003) Domain-selective small-molecule inhibitor of histone deacetylase 6 (HDAC6)-mediated tubulin deacetylation. Proc Natl Acad Sci U S A 100:4389–4394
Butler KV, Kalin J, Brochier C, Vistoli G, Langley B, Kozikowski AP (2010) Rational design and simple chemistry yield a superior, neuro-protective HDAC6 inhibitor, tubastatin A. J Am Chem Soc 132:10842–10846
Verhey KJ, Gaertig J (2007) The tubulin code. Cell Cycle 6:2152–2160, PubMed: 17786050
Pan J, Snell W (2007) The primary cilium: keeper of the key to cell division. Cell 129:1255–1257
Wheatley DN, Wang AM, Strugnell GE (1996) Expression of primary cilia in mammalian cells. Cell Biol Int 20:73–81
D’Angelo A, Franco B (2009) The dynamic cilium in human diseases. Pathogenetics 2(1):3
Veland IR, Awan A, Pedersen LB, Yoder BK, Christensen ST (2009) Primary cilia and signaling pathways in mammalian development, health and disease. Nephron Physiol 111:39–53
Quarmby LM, Parker JDK (2005) Cilia and the cell cycle? J Cell Biol 169(5):707–710
Hildebrandt F, Otto E (2005) Cilia and centrosomes: a unifying pathogenic concept for cystic kidney disease? Nat Rev Genet 6:928–940
Davenport JR, Yoder BK (2005) An incredible decade for the primary cilium: a look at a once-forgotten organelle. Am J Physiol Renal Physiol 289:F1159–F1169
Michaud EJ, Yoder BK (2006) The primary cilium in cell signaling and cancer. Cancer Res 66:6463–6467
Satir P, Christensen ST (2008) Structure and function of mammalian cilia. Histochem Cell Biol 129:687–693
Cheng J, Türkel N, Hemati N, Fuller MT, Hunt AJ, Yamashita YM (2008) Centrosome misorientation reduces stem cell division during ageing. Nature 456:599–604
Oh J, Lee YD, Amy J, Wagers AJ (2014) Stem cell aging: mechanisms, regulators and therapeutic opportunities. Nat Med 20(8):870–880
Schatten H, Chakrabarti A, Hedrick J (1999) Centrosome and microtubule instability in cells during aging. J Cell Biochem 74:229–241
Ly DH, Lockhart DJ, Lerner RA, Schultz PG (2000) Mitotic misregulation and human aging. Science 287:2486–2492
Miao Y-L, Kikuchi K, Sun Q-Y, Schatten H (2009) Oocyte aging: cellular and molecular changes, developmental potential and reversal possibility. Hum Reprod Update 15(5):573–585
Wang Z-B, Schatten H, Sun Q-Y (2011) Why is chromosome segregation error in oocytes increased with maternal aging? Physiology 26(5):314–325
Qiao J, Wang ZB, Feng HL, Miao YL, Wang Q, Yu Y, Wei YC, Yan J, Wang WH, Shen W, Sun SC, Schatten H, Sun QY (2014) The root of reduced fertility in aged women and possible therapeutic options: current status and future perspectives. Mol Aspects Med 38:54–85
Schatten H, Rawe VY, Sun Q-Y (2012) Cytoskeletal architecture of human oocytes with focus on centrosomes and their significant role in fertilization. In: Nagy ZP, Varghese AC, Agarwal A (eds) Practical manual of in vitro fertilization: advanced methods and novel devices. Humana Press, New York
Miao Y-L, Sun Q-Y, Zhang X, Zhao J-G, Zhao M-T, Spate L, Prather RS, Schatten H (2009) Centrosome abnormalities during porcine oocyte aging. Environ Mol Mutagen 50(8):666–671
Wang HF, Takenaka K, Nakanishi A et al (2011) BRCA2 and nucleophosmin coregulate centrosome amplification and form a complex with the Rho effector kinase ROCK2. Cancer Res 71:68–77
Kim NH, Moon SJ, Prather RS, Day BN (1996) Cytoskeletal alteration in aged porcine oocytes and parthenogenesis. Mol Reprod Dev 43:513–518
Alvarez Sedó CA, Schatten H, Combelles C, Rawe VY (2011) The nuclear mitotic apparatus protein NuMA: localization and dynamics in human oocytes, fertilization and early embryos. Mol Hum Reprod 17(6):392–398. doi:10.1093/molehr/gar009
Schatten H, Sun Q-Y (2013) Chromosome behavior and spindle formation in mammalian oocytes. In: Trounson A, Gosden R, Eichenlaub-Ritter U (eds) Biology and pathology of the oocyte. Biology & pathology of the Oocyte, 2nd edn
Gardner MK, Zanic M, Howard J (2013) Microtubule catastrophe and rescue. Curr Opin Cell Biol 25:14–22
Cande Z (1990) Centrosomes: composition and reproduction. Curr Opin Cell Biol 2:301–305
Huang B (1990) Genetics and biochemistry of centrosomes and spindle poles. Curr Opin Cell Biol 2:28–32
Zhao Y, Fang X, Wang Y, Zhang J, Jiang S, Liu Z, Ma Z, Xu L, Li E, Zhang K (2014) Comprehensive analysis for histone acetylation of human colon cancer cells treated with a novel HDAC inhibitor. Curr Pharm Des 20(11):1866–1873
Glozak MA, Seto E (2007) Histone deacetylases and cancer. Oncogene 26:5420–5432
Kikuchi K, Naito K, Noguchi J, Shimada A, Kaneko H, Yamashita M, Aoki F, Tojo H, Toyoda Y (2000) Maturation/M-phase promoting factor: a regulator of aging in porcine oocytes. Biol Reprod 63:715–722
Kikuchi K, Naito K, Noguchi J, Kaneko H, Tojo H (2002) Maturation/M-phase promoting factor regulates aging of porcine oocytes matured in vitro. Cloning Stem Cells 4:211–222
Xu Z, Abbott A, Kopf GS, Schultz RM, Ducibella T (1997) Spontaneous activation of ovulated mouse eggs: time-dependent effects on M-phase exit, cortical granule exocytosis, maternal messenger ribonucleic acid recruitment, and inositol 1,4,5-trisphosphate sensitivity. Biol Reprod 57:743–750
Tian XC, Lonergan P, Jeong BS, Evans AC, Yang X (2002) Association of MPF, MAPK, and nuclear progression dynamics during activation of young and aged bovine oocytes. Mol Reprod Dev 62:132–138
Fan HY, Sun QY (2004) Involvement of mitogen-activated protein kinase cascade during oocyte maturation and fertilization in mammals. Biol Reprod 70:535–547
Tatone C, Carbone MC, Gallo R, Delle Monache S, Di Cola M, Alesse E, Amicarelli F (2006) Age-associated changes in mouse oocytes during postovulatory in vitro culture: possible role for meiotic kinases and survival factor BCL2. Biol Reprod 74:395–402
Liang CG, Su YQ, Fan HY, Schatten H, Sun QY (2007) Mechanisms regulating oocyte meiotic resumption: roles of mitogen-activated protein kinase. Mol Endocrinol 21(9):2037–2055
Lee JH, Campbell KH (2008) Caffeine treatment prevents age-related changes in ovine oocytes and increases cell numbers in blastocysts produced by somatic cell nuclear transfer. Cloning Stem Cells 10:381–390
Boveri T (1914) Zur Frage der Entstehung maligner Tumoren. G. Fisher, Jena
Schatten H, Wiedemeier A, Taylor M, Lubahn D, Greenberg NM, Besch-Williford C, Rosenfeld C, Day K, Ripple M (2000) Centrosomes-centriole abnormalities are markers for abnormal cell divisions and cancer in the transgenic adenocarcinoma mouse prostate (TRAMP) model. Biol Cell 92:331–340
Lingle WL, Salisbury JL (2000) The role of the centrosome in the development of malignant tumors. Curr Top Dev Biol 49:313–329
Lingle WL, Barrett SL, Negron VC, D'Assoro AB, Boeneman K, Liu W, Whitehead CM, Reynolds C, Salisbury JL (2002) Centrosome amplification drives chromosomal instability in breast tumor development. Proc Natl Acad Sci U S A 99:1978–1983
Boveri T (2008) Concerning the origin of malignant tumours by Theodor Boveri (Translated and annotated by Henry Harris). J Cell Sci 121(Suppl 1):1–84
Duensing S, Lee LY, Duensing A, Basile J, Piboonniyom S, Gonzalez S, Crum CP, Munger K (2000) The human papillomavirus type 16 E6 and E7 oncoproteins cooperate to induce mitotic defects and genomic instability by uncoupling centrosome duplication from the cell division cycle. Proc Natl Acad Sci U S A 97:10002–10007
Chan JY (2011) A clinical overview of centrosome amplification in human cancers. Int J Biol Sci 7(8):1122–1144
Lingle WL, Lutz WH, Ingle JN, Maihle NJ, Salisbury JL (1998) Centrosome hypertrophy in human breast tumors: implications for genomic stability and cell polarity. Proc Natl Acad Sci U S A 95:2950–2955
Liu T, Niu Y, Yu Y, Liu Y, Zhang F (2009) Increased γ-tubulin expression and P16INK4A promoter methylation occur together in preinvasive lesions and carcinomas of the breast. Ann Oncol 20:441–448
Niu Y, Liu T, Tse GM, Sun B, Niu R, Li HM, Wang H, Yang Y, Ye X, Wang Y, Yu Q, Zhang F (2009) Increased expression of centrosomal α, γ-tubulin in atypical ductal hyperplasia and carcinoma of the breast. Cancer Sci 100:580–587
Kammerer S, Roth RB, Hoyal CR, Reneland R, Marnellos G, Kiechle M, Schwarz-Boeger U, Griffiths LR, Ebner F, Rehbock J, Cantor CR, Nelson MR, Brown A (2005) Association of the NuMA region on chromosome 11q13 with breast cancer susceptibility. Proc Natl Acad Sci U S A 102(6):2004–2009
Gehmlich KL, Haren L, Merdes A (2004) Cyclin B degradation leads to NuMA release from dynein/dynactin and from spindle poles. EMBO Rep 5:97–103
Lingle WL, Salisbury JL (1999) Altered centrosome structure is associated with abnormal mitoses in human breast tumors. Am J Pathol 155:1941–1951
Korzeniewski N, Duensing S (2012) Disruption of centrosome duplication control and induction of mitotic instability by the high-risk human papillomavirus oncoproteins E6 and E7 (Chapter 12). In: Schatten H (ed) The centrosome. Springer Science and Business Media, New York
Saladino C, Bourke E, Morrison CG (2012) Centrosomes, DNA damage and aneuploidy (Chapter 13). In: Schatten H (ed) The centrosome. Springer Science and Business Media, New York
Yan B, Chng W-J (2012) The role of centrosomes in multiple myeloma (Chapter 15). In: Schatten H (ed) The centrosome. Springer Science and Business Media, New York
Ellgaard L, Molinari M, Helenius A (1999) Setting the standards: quality control in the secretory pathway. Science 286:1882–1888
Johnston JA, Ward CL, Kopito RR (1998) Aggresomes: a cellular response to misfolded proteins. J Cell Biol 143:1883–1898
Wojcik C, DeMartino GN (2003) Intracellular localization of proteasomes. Int J Biochem Cell Biol 35:579–589
Kopito RR (2000) Aggresomes, inclusion bodies and protein aggregation. Trends Cell Biol 10:524–530
Roth J, Yam GH, Fan J, Hirano K, Gaplovska-Kysela K, Le Fourn V, Guhl B, Santimaria R, Torossi T, Ziak M, Zuber C (2008) Protein quality control: the who’s who, the where’s and therapeutic escapes. Histochem Cell Biol 129:163–177
McNaught KS et al (2002) Impairment of the ubiquitin-proteasome system causes dopaminergic cell death and inclusion body formation in ventral mesencephalic cultures. J Neurochem 81:301–306
Ohki R, Nemoto J, Murasawa H, Oda E, Inazawa J, Tanaka N, Taniguchi T (2000) Reprimo, a new candidate mediator of the p53-mediated cell cycle arrest at the G2 phase. J Biol Chem 275:22627–22630
Sato N, Maitra A, Fukushima N, van Heek NT, Matsubayashi H, Iacobuzio-Donahue CA, Rosty C, Goggins M (2003) Frequent hypomethylation of multiple genes overexpressed in pancreatic ductal adenocarcinoma. Cancer Res 63:4158–4166
Katayama H, Brinkley WR, Sen S (2003) The Aurora kinases: role in cell transformation and tumorigenesis. Cancer Metastasis Rev 22:451–464
Goepfert TM, Adigun YE, Zhong L et al (2002) Centrosome amplification and overexpression of aurora A are early events in rat mammary carcinogenesis. Cancer Res 62:4115–4122
Wang X, Zhou YX, Qiao W et al (2006) Overexpression of aurora kinase A in mouse mammary epithelium induces genetic instability preceding mammary tumor formation. Oncogene 25:7148–7158
Pihan GA, Doxsey SJ (1999) The mitotic machinery is a source of genetic instability in cancer. Semin Cancer Biol 9:289–302
D’Assoro AB, Busby R, Suino K et al (2004) Genotoxic stress leads to centrosome amplification in breast cancer cell lines that have an inactive G1/S cell cycle checkpoint. Oncogene 23:4068–4075
D'Assoro AB, Busby R, Acu ID et al (2008) Impaired p53 function leads to centrosome amplification, acquired ERalpha phenotypic heterogeneity and distant metastases in breast cancer MCF-7 xenografts. Oncogene 27:3901–3911
Shao S, Liu R, Wang Y et al (2010) Centrosomal Nlp is an oncogenic protein that is gene-amplified in human tumors and causes spontaneous tumorigenesis in transgenic mice. J Clin Invest 120:498–507
Hayward DG, Clarke RB, Faragher AJ et al (2004) The centrosomal kinase Nek2 displays elevated levels of protein expression in human breast cancer. Cancer Res 64:7370–7376
Suizu F, Ryo A, Wulf G et al (2006) Pin1 regulates centrosome duplication, and its overexpression induces centrosome amplification, chromosome instability, and oncogenesis. Mol Cell Biol 26:1463–1479
Bergmann S, Royer-Pokora B, Fietze E et al (2005) YB-1 provokes breast cancer through the induction of chromosomal instability that emerges from mitotic failure and centrosome amplification. Cancer Res 65:4078–4087
Korzeniewski N, Wheeler S, Chatterjee P et al (2010) A novel role of the aryl hydrocarbon receptor (AhR) in centrosome amplification – implications for chemoprevention. Mol Cancer 9:153
Olson JE, Wang X, Pankratz VS, Fredericksen ZS, Vachon CM, Vierkant RA, Cerhan JR, Couch FJ (2011) Centrosome-related genes, genetic variation, and risk of breast cancer. Breast Cancer Res Treat 125(1):221–228. doi:10.1007/s10549-010-0950-8
Gergely F, Basto R (2008) Multiple centrosomes: together they stand, divided they fall. Genes Dev 22:2291–2296
Schatten H, Walter M, Biessmann H, Schatten G (1988) Microtubules are required for centrosome expansion and positioning while microfilaments are required for centrosome separation in sea urchin eggs during fertilization and mitosis. Cell Motil Cytoskeleton 11(4):248–259
Ganem NJ, Godinho SA, Pellman D (2009) A mechanism linking extra centrosomes to chromosomal instability. Nature 460(7252):278–282
Inoko A, Matsuyama M, Goto H, Ohmuro-Matsuyama Y, Hayashi Y, Enomoto M, Ibi M, Urano T, Yonemura S, Kiyono T, Izawa I, Inagaki M (2012) Trichoplein and Aurora A block aberrant primary cilia assembly in proliferating cells. J Cell Biol 197(3):391–405
Schiff PB, Fant J, Horwitz SB (1979) Promotion of microtubule assembly in vitro by taxol. Nature 277:665–667
Schatten G, Schatten H, Bestor T, Balczon R (1982) Taxol inhibits the nuclear movements during fertilization and induces asters in unfertilized sea urchin eggs. J Cell Biol 94:455–465
De Brabander M, Geuens G, Nuydens R, Willebrords R, De Mey J (1981) Taxol induces the assembly of free microtubules in living cells and blocks the organizing capacity of the centrosomes and kinetochores. Proc Natl Acad Sci U S A 78:5608–5612
Dimitriadis I, Katsaros C, Galatis B (2001) The effect of taxol on centrosome function and microtubule organization in apical cells of Sphacelaria rigidula (Phaeophyceae). Phycol Res 49:23–34
Shin SJ, Beech JR, Kelly KA (2012) Targeted nanoparticles in imaging: paving the way for personalized medicine in the battle against cancer. Integr Biol 5(1):29–42. doi:10.1039/C2IB20047C
Rebacz B, Larsen TO, Clausen MH, Ronnest MH, Loffler H, Ho AD, Krämer A (2007) Identification of griseofulvin as an inhibitor of centrosomal clustering in a phenotype-based screen. Cancer Res 67:6342–6350
Schatten H, Schatten G, Petzelt C, Mazia D (1982) Effects of griseofulvin on fertilization and early development of sea urchins. Independence of DNA synthesis, chromosome condensation, and cytokinesis cycles from microtubule-mediated events. Eur J Cell Biol 27:74–87
Ho YS, Duh JS, Jeng JH, Wang YJ, Liang YC, Lin CH, Tseng CJ, Yu CF, Chen RJ, Lin JK (2001) Griseofulvin potentiates antitumorigenesis effects of nocodazole through induction of apoptosis and G2/M cell cycle arrest in human colorectal cancer cells. Int J Cancer 91:393–401
Panda D, Rathinasamy K, Santra MK, Wilson L (2005) Kinetic suppression of microtubule dynamic instability by griseofulvin: implications for its possible use in the treatment of cancer. Proc Natl Acad Sci U S A 102:9878–9883
Uen YH, Liu DZ, Weng MS, Ho YS, Lin SY (2007) NF-kappaB pathway is involved in griseofulvin-induced G2/M arrest and apoptosis in HL-60 cells. J Cell Biochem 101(5):1165–1175
Marchetti F, Mailhes JB, Bairnsfather L, Nandy I, London SN (1996) Dose-response study and threshold estimation of griseofulvin induced aneuploidy during female mouse meiosis I and II. Mutagenesis 11:195–200
Schatten H (1977) Untersuchungen über die Wirkung von Griseofulvin in Seeigeleiern und in Mammalierzellen. Universität Heidelberg (Effects of griseofulvin on sea urchin eggs and on mammalian cells. University of Heidelberg)
Wehland J, Herzog W, Weber K (1977) Interaction of griseofulvin with microtubules, microtubule protein and tubulin. J Mol Biol 111:329–342
Grisham LM, Wilson L, Bensch KG (1973) Antimitotic action of griseofulvin does not involve disruption of microtubules. Nature 244:294–296
Miao YL, Zhang X, Zhao JG, Spate L, Zhao MT, Murphy CN, Prather RS, Sun QY, Schatten H (2012) Effects of griseofulvin on in vitro porcine oocyte maturation and embryo development. Environ Mol Mutagen 53(7):561–566. doi:10.1002/em.21717
Alvarez Sedó CA, Schatten H, Combelles C, Rawe VY (2011) The nuclear mitotic apparatus protein NuMA: localization and dynamics in human oocytes, fertilization and early embryos. Mol Hum Reprod 17(6):392–398
Schatten H, Sun Q-Y (2010) The role of centrosomes in fertilization, cell division and establishment of asymmetry during embryo development. Seminars in Cell and Developmental Biology 21:174–184
Schatten H, Rawe VY, Sun Q-Y (2012) Cytoskeletal architecture of human oocytes with focus on centrosomes and their significant role in fertilization. In: Practical Manual of In Vitro Fertilization: Advanced Methods and Novel Devices, edited by Zsolt Peter Nagy, Alex C. Varghese, and Ashok Agarwal. Humana Press (Springer Science+Business Media, New York, USA).
Acknowledgments
It is a pleasure to gratefully acknowledge Donald Connor’s professional help with the illustrations and support from MU’s Research Council for studies on centrosome dysfunctions.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer Science+Business Media New York
About this chapter
Cite this chapter
Schatten, H., Sun, QY. (2015). Centrosome–Microtubule Interactions in Health, Disease, and Disorders. In: Schatten, H. (eds) The Cytoskeleton in Health and Disease. Springer, New York, NY. https://doi.org/10.1007/978-1-4939-2904-7_5
Download citation
DOI: https://doi.org/10.1007/978-1-4939-2904-7_5
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4939-2903-0
Online ISBN: 978-1-4939-2904-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)