Abstract
Since its discovery well over 100 years ago (Flemming, in Sitzungsber Akad Wissensch Wien 71:81–147, 1875; Van Beneden, in Bull Acad R Belg 42:35–97, 1876) the centrosome is increasingly being recognized as a most impactful organelle for its role not only as primary microtubule organizing center (MTOC) but also as a major communication center for signal transduction pathways and as a center for proteolytic activities. Its significance for cell cycle regulation has been well studied and we now also know that centrosome dysfunctions are implicated in numerous diseases and disorders including cancer, Alstrom syndrome, Bardet–Biedl syndrome, Huntington’s disease, reproductive disorders, and several other diseases and disorders. The present review is meant to build on information presented in the previous review (Schatten, in Histochem Cell Biol 129:667–686, 2008) and to highlight functions of the mammalian centrosome in health, and dysfunctions in disorders, disease, and aging with six sections focused on (1) centrosome structure and functions, and new insights into the role of centrosomes in cell cycle progression; (2) the role of centrosomes in tumor initiation and progression; (3) primary cilia, centrosome-primary cilia interactions, and consequences for cell cycle functions in health and disease; (4) transitions from centrosome to non-centrosome functions during cellular polarization; (5) other centrosome dysfunctions associated with the pathogenesis of human disease; and (6) centrosome functions in oocyte germ cells and dysfunctions in reproductive disorders and reproductive aging.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ten years have passed since the last review on centrosomes written for the 50th anniversary of this journal (Schatten 2008) and since then, the significance of centrosomes for cell cycle regulation and multiple cellular functions has gained further recognition with important new research being conducted to understand centrosome functions and dysfunctions underlying disease.
Significant progress has been made in understanding the impact of centrosomes in cancer development and progression, building on the original experiments and brilliant interpretations by Boveri (Boveri 1901, 1914; reviewed in Schatten 2008) who identified multipolar centrosomes as hallmark characteristics of cancer cells. This knowledge combined with new insights into the significance of multipolar cancer cell centrosomes has led to a new understanding and to new therapeutic approaches to target supernumerary cancer cell centrosomes to specifically inhibit cancer cell survival and tumor growth without affecting healthy non-cancer cells. As detailed in section 2, it includes preventing cancer-specific centrosome clustering (reviewed in Krämer et al. 2011, 2012; Schatten 2013; Schatten and Sun 2015b).
It is now well understood that the significance of centrosomes reaches far beyond its role as the cell’s primary microtubule organizing center (MTOC), as we now know that centrosomes serve as a vital communication center to coordinate multiple functions with a major role in linking several signal transduction pathways. The centrosome’s diverse functions include a role in ciliogenesis (Ishikawa and Marshall 2011; Kobayashi and Dynlacht 2011), cell polarity and migration (Bornens 2012; Tang and Marshall 2012), formation of the immunological synapse (Stinchcombe and Griffiths 2014), DNA damage control, and various others (Arquint et al. 2014). The role of centrosomes in primary cilia formation and cell cycle-specific functions has seen new appreciation, as primary cilia are now being recognized as critically important components for signal transduction and for close cell cycle-specific functional coordination with centrosomes. New insights have been gained into the role of the cell’s centriole complex for primary cilia initiation and formation. New research is also being conducted to explore centrosomes as proteolytic center (reviewed in Badano et al. 2005; Schatten 2008; Fisk 2012; Prosser and Fry 2012), as discussed in section 5. Several proteolytic processes take place at or around centrosomes in which centrosomes play a major role. New experimental tools have become available during the past decade which includes new genetic manipulations and new imaging modalities such as live cell imaging and super-resolution fluorescence microscopy.
New research on the role of centrosomal proteins in cellular polarization has led to new knowledge, increasing our understanding on remodeling of non-polar cells to cellular polarization during tissue formation and embryo development while loss of cellular polarization has been associated with diseases such as cancer. Over the past decade new research has also allowed us to gain new knowledge on centrosome-Golgi interactions and on Golgi-associated centrosomal proteins with microtubule-nucleating functions that are well coordinated with centrosome functions. We have also learned that centrosome dysfunctions are implicated in neurological disorders and neurodegeneration including Alstrom syndrome, Bardet–Biedl syndrome, Huntington’s disease, lissencephaly, schizophrenia, and several others as addressed in sections 2 and 5. Furthermore, the impact of centrosome dysfunction and centrosome deconstruction during reproductive aging is now being recognized to play a role in subfertility and infertility disorders. This information has led to a new understanding of certain reproductive disorders and to advancing this area of reproduction into clinical applications aimed at stabilizing the deteriorating centrosome complex in aging oocytes to extend the fertility span of aging oocytes, thereby extending the reproductive life span for women. Research on centrosomes in aging oocytes may lead to other research on aging, as similar effects may account for cellular aging in somatic cells which may also be part of the mechanisms underlying age-related cancer cell initiation and progression.
These topics highlighted in the present review are meant to build on the information presented in the previous review paper 10 years ago (Schatten 2008). Six sections are presented to address: (1) centrosome structure and functions, and new insights into the role of centrosomes in cell cycle progression; (2) the role of centrosomes in tumor initiation and progression; (3) primary cilia, centrosome-primary cilia interactions, and consequences for cell cycle functions in health and disease; (4) transitions from centrosome to non-centrosome functions during cellular polarization; (5) other centrosome dysfunctions associated with the pathogenesis of human disease; and (6) centrosome functions in oocyte germ cells and dysfunctions in reproductive disorders and reproductive aging.
Section 1: Overview of centrosome structure and functions, and new insights into the role of centrosomes in cell cycle progression and centrosome dysfunctions underlying disease
Centrosome structure and functions
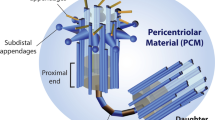
The structure and functions of mammalian somatic cell centrosomes have been detailed in the previous review (Schatten 2008), and are briefly addressed in the following. A typical mammalian somatic cell centrosome is composed of a centrally localized centriole pair with perpendicular orientation to each other, enclosed in a centrosomal matrix (Fig. 1) oftentimes also referred to as pericentriolar material (PCM) which is composed of a proteinaceous lattice of coiled-coil proteins. Numerous centrosomal proteins are localized to the centrosomal matrix including the γ-tubulin ring complexes (γ-TuRCs), pericentrin, centrin, and calcium-sensitive fibers (Salisbury 2004; reviewed in Schatten 2008; Schatten and Sun 2015a, b) while centrosome-associated proteins are localized around the centrosomal matrix.

Modified from Schatten (2013)
a A typical mammalian somatic cell centrosome consists of centrosomal material, also referred to as pericentriolar material (PCM), as it surrounds two perpendicularly oriented centrioles, termed mother and daughter centrioles. The mother centriole is distinguished from the daughter centriole by distal and subdistal appendages (shown as green necklace in b). Both centrioles are connected by interconnected fibers (shown in b). The centrosomal material consists of a meshwork of proteins embedded in a matrix of yet undetermined structural composition. Gamma-tubulin and the gamma-tubulin ring complex are embedded in the centrosomal matrix and nucleate microtubules along with associated proteins with microtubule minus ends anchored to the matrix. This diagram also shows two complexes within the centrosomal matrix: the microtubule-nucleating complex and the microtubule anchoring complex. The diagram in b shows in more detail the two centrioles (mother and daughter centriole) in perpendicular orientation to each other.
The mammalian centrosome is a multifunctional highly complex organelle with its primary well-known functions to serve as the cell’s major microtubule organizing center (MTOC) (reviewed in Schatten 2008) with several proteins participating in the nucleation (γ-tubulin, pericentrin, polo kinases, aurora kinases), anchoring (ninein, centriolin, dynactin), and release (katanin) of microtubules from the centrosome. Unlike other organelles the centrosome organelle is not membrane bound which allows dynamic changes throughout the cell cycle and close interactions with cytoplasmic components facilitated by microtubules that are organized and reorganized by centrosomes throughout the cell cycle.
Cell cycle-specific structural and functional remodeling of centrosomes takes place throughout the cell cycle to accommodate specific cellular functions which includes precise association of centrosomal proteins with the centrosomal matrix in interphase and in mitosis. While centrioles do not undergo extensive structural changes centriole duplication takes place during the S-phase and follows a semi-conservative duplication pattern during which a younger (daughter) centriole forms perpendicular to the older (mother) centriole (Fig. 1). In mammalian somatic cells, daughter and mother centrioles are composed of nine outer triplet microtubules forming a small tube that does not contain central microtubules. An important structural and functional distinction exists between mother and daughter centrioles in that the mother centriole contains appendages that are essential for anchoring microtubules and for the formation of single non-motile primary cilia in most interphase cells and post-mitotic cells, as will be discussed in section 3. Centriole functions include participation in the assembly of specific centrosome proteins and in the duplication of centrosomal material (Salisbury et al. 2002), thereby serving interconnecting centrosomal functions in a typical mammalian somatic cell centrosome.
The centrosomal matrix structure itself is still little explored, but we know that the amount and composition of centrosome proteins within the centrosome matrix is precisely regulated during normal cell cycles and that it becomes deregulated in pathological conditions such as cancer, as will be discussed in section 2. Numerous centrosomal proteins have been identified in purified centrosomes by mass spectrometric analysis (Anderson et al. 2003; Wilkinson et al. 2004) and include structural proteins [alpha-tubulin, beta-tubulin, γ-tubulin, γ-tubulin complex components 1–6, centrin 2 and 3, AKAP450, pericentrin/kendrin, ninein, pericentriolar material 1 (PCM1), ch-TOG protein, C-Nap1, Cep250, Cep2, centriole-associated protein CEP110, Cep1, centriolin, centrosomal P4.1-associated protein (CPAP), CLIP-associating proteins CLASP1 and CLASP 2, ODF2, cenexin, Lis1, Nudel, EB1, centractin, myomegalin]; regulatory molecules [cell division protein 2 (Cdc2), Cdk1, cAMP-dependent protein kinase type II-alpha regulatory chain, cAMP-dependent protein kinase-alpha catalytic subunit, serine/threonine protein kinase Plk1, serine/threonine protein kinase Nek2, serine/threonine protein kinase Sak, Casein kinase I, delta and epsilon isoforms, protein phosphatase 2A, protein phosphatase 1 alpha isoform, 14-3-3 proteins, epsilon and gamma isoforms]; motor and motor-related proteins (dynein heavy chain, dynein intermediate chain, dynein light chain, dynactin 1, p150 Glued, dynactin 2, p50, dynactin 3); and the heat shock proteins, heat shock protein Hsp90, TCP subunits, and heat shock protein Hsp73 (reviewed in Schatten 2008). Functions of these proteins have been determined and vary to some extent in different systems. Several of these proteins will be highlighted in the present review for their functions in healthy cells and dysfunctions in disorders and disease.
Currently, it is still not known how hundreds of these protein components are integrated into the highly dynamic and complex centrosome matrix being able to be regulated throughout the cell cycle (reviewed in Schatten 2008; Gopalakrishnan et al. 2011; Habermann and Lange 2012; Mennella et al. 2013; Woodruff et al. 2014; Wueseke et al. 2014) and further biochemical and new imaging modalities as well as structural analysis are still required to resolve this important questions (reviewed in Mannella et al.2013). At present, while the nature of the centrosomal matrix structure is not yet known, it is thought that it consists of cytoskeletal-like fibers that resemble intermediate filaments based on detergent-extraction experiments (Schnackenberg and Palazzo 1999; Schnackenberg et al. 2000) and on experiments in which centrosomes could be detected and brightly stained with a monoclonal antibody to invertebrate intermediate filament proteins (termed Ah6) (Schatten et al. 1987; reviewed in Schatten and Sun 2015b). Ah6 had been generated in Drosophila and it cross-reacts with a 68-kDa protein. Previous research using TEM imaging of intact centrosomes had revealed some of the structural components including an insoluble protein matrix within the centrosomal material measured as 12–15 nm structures and other unknown elements (Moritz et al. 1998; Paintrand et al. 1992; Schnackenberg and Palazzo 1999; Schnackenberg et al. 2000).
The γ-tubulin ring complex, pericentrin, centrin, and the centrosome-associated protein NuMA (Nuclear Mitotic Apparatus protein) as well as the minus-end anchoring protein ninein have been discussed in more detail in previous reviews (Schatten 2008; Schatten and Sun 2011a, b, c, 2015a, b). In brief, γ-tubulin is part of the γ-tubulin ring complexes (γ-TuRCs) that are important for microtubule nucleation. While dominantly associated with the centrosome matrix core structure it can also serve as a microtubule-nucleating protein in areas other than centrosomes which will be discussed in section 4, focused on polarized epithelial cells. In polarizing and polarized cells, γ-tubulin becomes localized to the apical and basolateral membranes for intracellular communication functions. The microtubule minus-end anchoring protein, ninein (Mogensen et al. 2000), plays an important role in microtubule anchorage at centrosomes as well as at non-centrosomal anchorage sites. The centrosomal protein pericentrin serves a role in centrosome and spindle organization (Dictenberg et al. 1998; Doxsey et al. 1994; Young et al. 2000). It forms a ca. 3-MDa complex with γ-tubulin and depends on dynein for assembly onto centrosomes (Young et al. 2000). Pericentrin is involved in recruiting γ-tubulin to centrosomes (Dictenberg et al. 1998), and it is part of the pericentrin/AKAP450 centrosomal targeting (PACT) domain (Gillingham et al. 2000). Centrins are primarily associated with centrioles and play an essential role in the duplication of centrosomes (Salisbury et al. 2002; Levy et al. 1996; Salisbury 1995; Lutz et al. 2001; reviewed in Manandhar et al. 2005).
At the onset of mitosis, a process called centrosome maturation takes place during which centrosomal material increases in size and microtubule nucleation capacity increases through an increase in the recruitment of γTuRCs from the cytoplasm resulting in an increase in the nucleation of both astral and spindle microtubules in preparation for mitosis and cell division. The centrosome-associated protein NuMA becomes important during mitosis. NuMA associates with mitotic centrosomes and cross-links spindle microtubules to precisely tether microtubules at the poles into the bipolar mitotic apparatus (Merdes and Cleveland 1998), while forming an insoluble crescent around the centrosome area facing toward the central mitotic spindle (Sun and Schatten 2006, 2007, 2011a, b). As a multifunctional protein (reviewed in Sun and Schatten 2006, 2007) NuMA functions as a nuclear matrix protein in the interphase nucleus, but it does not associate with the interphase centrosome. It becomes an important centrosome-associated protein when it moves out of the nucleus during nuclear envelope breakdown and disperses into the cytoplasm to associate with cytoplasmic microtubules for translocation to the centrosomal area in a dynein/dynactin-mediated process. NuMA dysfunctions are implicated in a variety of disorders as well as in aging and in diseases including cancer which will be discussed in sections 2 (cancer) and 6 (reproductive disorders and aging). NuMA dysfunctions are associated with mitotic dysfunctions in several cell systems (reviewed in Sun and Schatten 2006; Alvarez-Sedó et al. 2011) and play a role in mitotic abnormalities in cancer cells, including breast cancer (Kammerer et al. 2005). These major centrosomal proteins and centrosome-associated proteins are critical for centrosome functions in all mammalian cells.
New centrosome imaging
The past decade has been especially exciting for centrosome biology, as new imaging modalities have been applied to image, analyze, and elucidate centrosome structure to better understand their composition and functions as well as centrosome remodeling for cell cycle-specific regulation. Several excellent papers have been published revealing new information; in a paper published in 2013 (Mennella et al. 2013) centrosomes are addressed as “amorphous no more”, referring to the previously poorly perceived nature of centrosomal material characterized as electron-dense material when imaged with thin section TEM. In this paper, the authors used subdiffraction (super-resolution) microscopy to gain new information on pericentriolar architecture revealing a layered structure made of fibers and matrices conserved from flies to humans (Mennella et al. 2013). With the increased resolution on light microscopy levels, the authors were able to investigate the structure and dynamics of centrosomes inside cells in the native cellular environment which provides new and different information compared to proteomic analysis or use of other microscopy modalities that primarily imaged and analyzed centrosomes in fixed cells.
Centrosome-Golgi apparatus nexus and centrosome-Golgi communication
While the primary microtubule organizing center in proliferating animal cells is the centrosome, more recently, the discovery of additional microtubule nucleation capabilities by the Golgi apparatus has further increased our understanding of the microtubule network organization in interphase cells (Karanikolas and Sütterlin 2012; reviewed in Rios 2014). We now know that microtubule nucleation at the Golgi relies on multiprotein complexes, similar to those present at the centrosome, that assemble at the cis-face of the Golgi. AKAP450 plays a central role in this process, serving as a scaffold to recruit other centrosomal proteins important for microtubule nucleation and growth.
The Golgi apparatus localizes near the nucleus in most vertebrate cells, surrounding the nucleus-associated centrosome. The pericentrosomal position of the Golgi depends on microtubules and on dynein (Corthesy-Theulaz et al. 1992; Harada et al. 1998; Cole et al. 1996; Thyberg and Moskalewski 1999) which is recruited to the Golgi by the peripheral coiled-coil protein golgin 160 (Yadav et al. 2012; reviewed in Rios 2014). GMAP210 plays a role in this process which has been shown by targeting GMAP210 to mitochondria causing clustering around the centrosome while its depletion results in immotile, dispersed Golgi stacks (Yadav et al. 2009; Rios et al. 2004). GMAP210 binds microtubule minus ends and γ-tubulin (Infante et al. 1999). Molecular mechanisms involved in microtubule nucleation at the Golgi and Golgi/centrosome-based microtubule arrays communicate to ensure the correct formation of a pericentrosomal Golgi ribbon structure that is polarized and continuous as a critical feature for cell polarity in mammalian cells.
The mechanisms of microtubule nucleation at the Golgi apparatus has been revealed in recent years ascribing a new role of the Golgi in serving MTOC functions (Chabin-Brion et al. 2001). These studies showed that Golgi membranes were able to assemble and stabilize microtubules and that purified Golgi membranes contained γ-tubulin to promote microtubule assembly. Subsequent studies showed that a subset of microtubules directly grows from Golgi membranes (Efimov et al. 2007; reviewed in Karanikolas and Sütterlin 2012; Rios 2014). In addition, it was shown that siRNA-mediated depletion of γ-tubulin inhibits both Golgi and centrosome–microtubule generation, and that laser ablation of the centrosome does not affect the number of microtubules formed at the Golgi. About 50% of interphase microtubules have been estimated to originate from the Golgi and stabilization of these microtubules required CLASPs, microtubule plus-end binding proteins that are recruited to the Golgi through the interaction with the TGN-associated protein GCC185 (Efimov et al. 2007; reviewed in Karanikolas and Sütterlin 2012). Based on this knowledge and subsequent studies it was proposed that the cis-Golgi acts as a major site for microtubule nucleation by γ-TuRC using a mechanism similar to that employed by centrosomes. A hierarchy is in place concerning centrosomal protein recruitment to Golgi and centrosomes, which has been detailed in a recent review (Rios 2014). Taken together, the evidence strongly supports microtubule nucleation at the Golgi by γ-tubulin to be similar to the mechanisms used for microtubule nucleation at centrosomes and that the Golgi uses classical centrosomal proteins for its microtubule nucleation activity.
In addition to microtubule nucleation, microtubule growth and dynamics require microtubule capping and microtubule anchoring. As mentioned in the introduction, ninein is important for this process being located at the subdistal appendages of mother centrioles, a major site for microtubule anchoring where ninein targets the centriole via its C-terminus and recruits γ-tubulin-containing complexes via its N-terminus (Delgehyr et al. 2005). Ninein can function along with other anchoring proteins that are also present at the subdistal appendages of the mother centriole including the largest subunit of the dynactin complex p150Glued or the microtubule plus-end-associated protein EB1. Five of the PCM proteins, AKAP450 (Takahashi et al. 1999), CDK5Rap2 (Wang et al. 2010), myomegalin (Verde et al. 2001), CAP350 (Hoppeler-Lebel et al. 2007), and pericentrin (Oddoux et al. 2013) have been shown to be associated with the Golgi in mammalian cells.
Based on information generated so far, it is suggested that a direct nexus exists between Golgi and centrosomes to allow functional coordination although further research is needed to generate more detailed data and to validate this conclusion. However, as centrosomes are not enclosed by a membrane while the Golgi is surrounded by a membrane the interaction of microtubules at the Golgi and centrosomes are likely to be different. As will be discussed in section 4, the functional interactions of Golgi-derived microtubules and centrosome-derived microtubules during cellular polarization in epithelial cells leading to microtubule remodeling are still under-explored but it is known that functional communications are needed. Currently, still very little is known about signaling pathways between the two organelles which may impact cellular polarization, tissue formation in animal cells, as well as miscommunication leading to pathologies.
Section 2: The role of centrosomes in tumor initiation and progression
As mentioned in the introduction, the role of centrosomes in cancer has been brilliantly recognized by Theodor Boveri in a landmark paper published in 1914 (Boveri 1914). Boveri’s studies included the interpretation of dispermic fertilization resulting in multipolar mitosis and abnormal cell division (reviewed in Schatten 2008) (Fig. 2). These studies opened up an enormous field in cancer research that is growing exponentially and includes exploring the role of centrosome dysfunctions in cancer initiation and progression and finding new cures aimed at targeting cancer cell centrosome pathologies (reviewed in Schatten 2013; Schatten and Sun 2015b; Schatten and Ripple 2018).

Modified from Schatten (2013)
A (a–f) Somatic cell centrosome cycle within the cell cycle. (a) The single interphase centrosome containing a pair of centrioles is closely associated with the nucleus and nucleates an array of interphase microtubules. (b) Centriole–centrosome duplication occurs during the S phase in synchrony with DNA duplication. (c) Separation of the duplicated centriole–centrosome complex toward the opposite spindle poles takes place in the early prophase stage. (d) The bipolar mitotic apparatus becomes established when each centriole–centrosome complex has reached the opposite pole, and the nuclear envelope has broken down. During this stage interphase centrosomes mature into mitotic centrosomes acquiring mitosis-associated centrosomal proteins including NuMA that had moved out of the nucleus during nuclear envelope breakdown. (e) The metaphase centrosome becomes highly compacted to organize the metaphase spindle with microtubules attached to the kinetochores. (f) Telophase is the stage when centrosomal material becomes decompacted again before reorganizing into interphase centrosomes that associate with the nuclei of the separating daughter cells. (g, h) Centrosomal abnormalities associated with cell cycle dysfunctions. In cancer cells (g, h), centrosome and centriole numbers can amplify or over-replicate leading to aneuploidy or failure of cytokinesis. B Schematic representation of various structural centriole–centrosome abnormalities in cancer cells. Cancer cell centrosomes can undergo various mitotic configurations forming either a bipolar mitotic apparatus (a) that oftentimes contains amplified centrosomes as a result of centrosome clustering, bipolar (b) or multipolar (c, d) mitotic configurations as a result of centrosome and/or centriole overduplication. Tripolar and multipolar cells can undergo abnormal cell divisions resulting in cells with aneuploidy while cells with configurations shown in c and d may not be able to divide but rather become fragmented and disintegrate. The goal of therapies directed against the formation of abnormal centrosomes is to prevent abnormal centrosome clustering to induce fragmentation and cancer cell disintegration.
While for most cases we do not yet fully understand the cause and effect relationships concerning cancer initiation and progression we are beginning to understand the molecular changes leading to cell cycle abnormalities and subsequent cancer manifestation. The perturbation of core centrosomal or centrosome-associated proteins is linked to cell cycle mis-regulation and cancer. We now have detailed information on genetic, cellular and molecular levels underlying the manifestation of cancer and we know that several of the centrosomal proteins as well as centrosome-regulating kinases are overexpressed in cancer cells and play a pivotal role in centrosome amplification as cell and molecular hallmarks for cancer development and progression (Fukasawa et al. 1996; Carroll et al. 1999; reviewed in Schatten 2008, 2013; Schatten and Sun 2015b; Schatten and Ripple 2018). Knowledge of these specific abnormalities has offered new treatment possibilities, as will be discussed below in this section.
Abnormalities in centrosome number, size, and morphology have been reported for all human tumors studied so far, including breast, colon, liver, bone marrow, cervical, and prostate cancer (reviewed in Nigg 2002; Schatten 2013; Schatten and Sun 2015b; Schatten and Ripple 2018). Several molecular pathologies have been reported including loss of p53 or retinoblastoma tumor suppressor protein (Rb) that results in centrosome amplification; deficiency of the breast cancer gene BRCA1 and the overexpression of Aurora A as well as other mitotic kinases are implicated in cancer progression (Nigg 2002; Xu et al. 1999; Pihan et al. 1998; Fukasawa et al. 1996; Brinkley and Goepfert 1998; Boutros 2012; Fukasawa 2012).
Centrosomal dysfunctions are clearly increased in late events of tumorigenesis but they are already seen in early stages of tumor development (Pihan et al. 2003; Tarapore et al. 2014; reviewed in Schatten 2013; Schatten and Sun 2015b; Schatten and Ripple 2018). While the cause and effect relationships have not yet been determined for a variety of cancers, clear results have been reported for cervical cancer (Duensing et al. 2000; Duensing and Munger 2003). In cervical cancer, infection with ‘high-risk’ human papillomavirus (HPV) types, such as HPV16 and HPV18, is associated with more than 90% of cervical cancer cases. These studies clearly showed that the E6 and E7 oncoproteins of HPV16 induce mitotic defects by uncoupling centrosome duplication from the cell cycle. These studies also showed that the E6 and E7 proteins of low-risk HPV6 do not induce chromosomal abnormalities and are not typically associated with malignancy (Duensing and Munger 2003; Duensing et al. 2000; Schatten 2013).
Toxicants and chemicals that affect centrosomes
While the study on cervical cancer determined the cause being ‘high-risk’ human papillomavirus (HPV) types in cervical cancer initiation, new research has evolved showing that several toxicants and various drugs have been identified to affect centrosomes with consequences for cancer development and progression. Bisphenol-A (BPA), an alkylphenol and environmental estrogen-like chemical with weak estrogenic activity, is among the estrogen-like chemicals that had been identified to affect centrosomes in meiotic spindles of mammalian oocytes (reviewed in Miao et al. 2009a; and references therein), causing loss of spindle integrity and aneuploidy that has been implicated in infertility, developmental abnormalities, and early childhood cancer (Can et al. 2005; Pacchierotti et al. 2008; Eichenlaub-Ritter et al. 2008; Miao et al. 2009a, b). In cancer cells, BPA causes a time- and dose-dependent delay in cell cycle progression, primarily by interfering with centrosomal proteins that may be degraded by BPA (Tarapore et al. 2014). While the effects of toxicants and chemicals may be different in different cells, a recent study found a direct effect of BPA on centrosomes in prostate cancer cells, leading to early onset of prostate cancer (Tarapore et al. 2014). The study was conducted on 60 urology patients in which low levels of BPA exposure promoted centrosome amplification and anchorage-independent growth in vitro, thereby correlating with early onset prostate cancer. Although BPA is not classified as a carcinogen, this study suggests that it affects centrosomes, promoting early onset human prostate cancer. Previous research on animals had already shown that BPA exposure is significantly implicated in prostate cancer (Keri et al. 2007; Ho et al. 2006; Prins et al. 2011; Tang et al. 2012; Jenkins et al. 2011); the study by Tarapore et al. (2014) was the first to show a correlation of BPA to prostate cancer in humans related to the effect of BPA on the centrosome cycle contributing to prostate carcinogenesis.
Other agents and chemicals may also affect centrosomes with consequences for cancer development and progression. While the cause and effect correlation resulting in centrosome pathologies is not always clear (reviewed in Schatten 2008, 2013, 2014), it is certain that centrosome amplification is a hallmark of cancer although several factors may play a role in centrosome abnormalities and cancer development and progression.
Centrosomes as targets for cancer therapy and treatment possibilities
Our understanding of centrosome pathologies in cancer cells has stimulated new research into the development of new therapeutics targeting cancer cell centrosomes specifically without affecting non-cancer cells. These include targeting signaling pathways that play a role in centrosome abnormalities, or targeting centrosome abnormalities directly.
As mentioned above, centrosomes are abnormally phosphorylated in cancer cells which include interphase centrosomes as well as mitotic cell centrosomes. Polo-like kinases, cyclin-dependent kinases, Aurora kinases, and several others are among the overexpressed centrosome-phosphorylating kinases in cancer cells (reviewed in Schatten 2008; Boutros 2012; Fukasawa 2012). Inhibition of these overexpressed kinases is a major goal aimed at inhibiting cancer cell growth which has already advanced to some early clinical trials (Cheung et al. 2011; Schoffski 2009). Other avenues being pursued to control cancer cell centrosome abnormalities include targeting histone deacetylases such as HDAC1, HDAC5, and SIRT1, as they play a role in centrosome duplication and amplification (Ling et al. 2012).
Aryl hydrocarbon receptor agonists are also being considered as therapeutic targets to inhibit centrosome pathologies by preventing centriole overduplication which will in turn prevent centrosome duplication and amplification, as centrioles play a role in duplication of centrosomal material (Chan 2011; Korzeniewski et al. 2010).
Several known anti-cancer drugs are also known for their effects on centrosome–microtubule interactions. The well-known cancer drug paclitaxel and other taxol derivatives act primarily by inhibiting depolymerization of microtubules, thereby preventing mitosis and cell division (Schiff et al. 1979; Schatten et al. 1982a; reviewed in Schatten 2013; Schatten and Sun 2015b; Schatten and Ripple 2018). Taxol is also known for its interaction with microtubules at the centrosome–microtubule nucleation sites (De Brabander et al. 1981; Dimitriadis et al. 2001), affecting the capacity of centrosomes to nucleate microtubules in taxol-treated cells (De Brabander et al. 1981).
One new approach to directly target centrosome functions in cancer cells is through inhibition of centrosome clustering. Centrosome clustering is characteristic for multipolar cancer cells containing supernumerary centrosomes. Cancer cells have developed a mechanism to cluster their extra centrosomes into abnormal bipolar centrosomes forming bipolar spindles for survival of cancer cells containing abnormalities that may not be apparent initially on morphological levels (Krämer et al. 2012; Xiao and Yang 2016; reviewed in Schatten and Ripple 2018) but these abnormalities become manifested in subsequent cell cycles when the bipolar mitotic apparatus containing amplified centrosomes that initially can undergo cell division will result in subsequent cellular and tissue abnormalities. The mechanisms underlying centrosome clustering are still being explored and several models have been proposed in which molecular motors have been implicated (Acilan and Saunders 2008). Specific proteins required for centrosome clustering have been determined (Leber et al. 2010) and are now being explored to prevent centrosome clustering, thereby preventing the formation of abnormal bipolar centrosomes while causing cellular fragmentation as a result of multipolar centrosomes that will not be able to divide cells accurately.
One of the potent antimitotic drugs that has been identified to prevent centrosome clustering in cancer cells is griseofulvin (Rebacz et al. 2007; reviewed in Krämer et al. 2011, 2012; Schatten 2013; Schatten and Sun 2015b; Schatten and Ripple 2018). Griseofulvin had been shown to arrest cells at the G2/M transition stage in a concentration-dependent manner (Uen et al. 2007; Marchetti et al. 1996; Schatten 1977; Schatten et al. 1982b; Ho et al. 2001), and in recent years, griseofulvin has been shown to specifically inhibit clustering of supernumerary centrosomes in cancer cells (reviewed in Krämer et al. 2012). Therefore, this drug will prevent the formation of centrosomes that are abnormally clustered into a bipolar centrosome configuration to specifically control cancer cell division while not affecting non-cancer cells that do not contain multiple centrosomes and therefore do not require clustering of multipolar centrosomes. Griseofulvin is already approved as orally administered antifungal drug that interferes with microtubule functions in vivo and in vitro (Marchetti et al. 1996; Schatten 1977, 2008; Wehland et al. 1977; Schatten et al. 1982b; reviewed in Schatten and Sun 2012) but further more detailed studies are needed to determine the mechanisms by which it prevents cancer cell centrosome clustering. Earlier studies had shown that griseofulvin induces multipolar mitoses in tumor cells (Rebacz et al. 2007; Schatten 1977; Ho et al. 2001; Panda et al. 2005) which may indicate a direct effect on centrosomes or it may indicate an effect on microtubule minus ends, preventing proper centrosome organization and attachment to microtubules. As had been the case for taxol, more structural modifications of the griseofulvin molecule may be needed to develop this drug into a cancer therapeutic for clinical trials to determine the optimal medical applications and perhaps determine combination therapies to minimize potential side effects and drug resistance.
This line of research to prevent centrosome clustering in cancer cells is being pursued on several levels with the goal to develop anti-centrosome-clustering therapies, as non-clustered cancer cell centrosomes are not able to form a bipolar mitotic apparatus with amplified centrosomal components but induce cell death following cell fragmentation rather than allowing formation of aneuploid cells with consequences for genomic instability. As mentioned above, non-cancer cells will not be affected, as centrosome clustering pathways are dispensable in cells with normal centrosome numbers, but centrosome clustering is required for supernumerary centrosomes to form bipolar mitotic spindles.
Recently, new research has emerged as promising approach to prevent centrosome clustering in cancer cells by identifying proteins that are required for the clustering process. It has been shown that KIFC1, a kinesin-like protein (kinesin motor) plays a critical role in clustering the cancer-specific supernumerary centrosomes. KIFC1 is non-essential in normal somatic cells, thereby offering a most suitable target to control clustering of centrosomes into abnormal bipolar spindles without causing damaging side effects. However, it needs to be taken into account that KIFC1 also plays a role in certain vesicular and organelle trafficking, spermiogenesis, oocyte development, embryo gestation, and double-strand DNA transportation (reviewed in Xiao and Yang 2016). For possible treatment strategies aimed at targeting KIFC1, this aspect needs to be taken into account when weighing the advantages of cancer treatment targeting KIFC1. So far, several promising approaches have been reported targeting KIFC1. KIFC1 is upregulated in breast cancer including estrogen receptor negative, progesterone receptor negative, and triple-negative breast cancer (Li et al. 2015), while it is absent in normal human mammary epithelial cells. Inhibition of KIFC1 resulted in anti-breast cancer activity. In serous ovarian adenocarcinomas, KIFC1 has been shown to indicate aggressiveness of the disease, therefore serving as a biomarker to predict the stages of disease aggressiveness (Mittal et al. 2016).
Section 3: Primary cilia, centrosome-primary cilia interactions, and consequences for cell cycle functions in health and disease
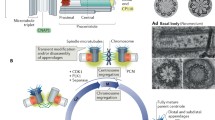
The past decade has brought us significant new insights into the important role of primary cilia for signal transduction and cell cycle regulation, its impact on cancer development and progression, as well as kidney functions and dysfunctions. One single primary cilium protrudes from almost all cells in our body and plays a significant role in cellular and cell cycle communication (reviewed in Schatten 2013) (Fig. 3). The intimate and coordinated relationship between primary cilia and centrosomes has clearly been shown during the past decade and it is now known that the mother centriole of the mitotic apparatus becomes the seed structure for the formation of non-motile primary cilia that are formed as single cilia at the surface of epithelial cells as well as most other cells in the human body. We now know the very close coordination of the primary cilium-centrosome cycle in cellular functions and dysfunctions in disease such as cancer, or disorders including obesity and others (reviewed in Pan and Snell 2007; reviewed in Schatten and Sun 2011c). As mentioned above, primary cilia formation starts when, following mitosis, the mother centriole of the centrosome complex becomes the seed structure for primary cilia formation. It undergoes extensive structural modification to form the basal body of the primary cilium (reviewed in Satir and Christensen 2008; Kobayashi and Dynlacht 2011). During G1, the distal end of the mother centriole becomes associated with a membrane vesicle (reviewed by Pan and Snell 2007; Schatten and Sun 2011c) that expands into a ciliary vesicle, surrounding the forming axoneme before fusing with the plasma membrane during primary cilia formation. The primary cilia formation cycle continues with centrioles duplicating during the subsequent S phase and primary cilium lengthening and maturing to its full length during the G2 phase. Primary cilium shortening then takes place at the G2/M transition and primary cilia completely dissemble in mitotic cells, resulting in mitotic cells devoid of primary cilia (reviewed in Schatten 2013; Schatten and Sun 2015a, b; Li and Hu 2015).

Modified from Schatten (2014)
Schematic representation of primary cilium showing the close cell cycle relationships of the primary cilium with the cell’s centrosome complex. In G1 (a) the mother centriole associates with membrane components to build the axoneme of the primary cilium. The primary cilium grows during G2 (b), duplicates during the S phase (c), and becomes disassembled during mitosis (d), when centrioles become associated again with the mitotic spindle poles.
The non-motile single primary cilium is distinguished from motile cilia by its composition of 9 outer microtubule doublets with no central microtubule pair (“9 + 0”) and the absence of dynein arms while nine doublets of microtubules surround a central microtubule pair (“9 + 2”) in motile cilia (Wheatley et al. 1996; D’Angelo and Franco 2009; Veland et al. 2009). A specialized receptor-rich plasma membrane surrounds the primary cilium which is critically important for communicating signals from the external cellular environment to its associated cell body (reviewed by Li and Hu 2015). Primary cilia dysfunctions are associated with numerous diseases such as polycystic kidney syndrome and other diseases or disorders (Yoder et al. 2002; reviewed in Hildebrandt and Otto 2005) for which the cell, molecular, and genetic aspects of primary cilia dysfunctions have been reported. Signal transduction cascades between primary cilia and the centrosome play essential roles in accurate cell cycle progression and in communicating external environmental conditions and factors to cells (Schatten 2008; D’Angelo and Franco 2009; Veland et al. 2009; Quarmby and Parker 2005; Hildebrandt and Otto 2005; Davenport and Yoder 2005; Michaud and Yoder 2006; Satir and Christensen 2008; reviewed in Li and Hu 2015).
Several major pathways are well known to require signaling through primary cilia and include the Wnt, hedgehog, and platelet-derived growth factor (PDGF) pathways (Sharma et al. 2008; Berbari et al. 2009). MAP kinase signaling between primary cilia and centrosomes is also important for centrosome functions (Schneider et al. 2005; Quarmby and Parker 2005). An intraflagellar transport (IFT) system is essential to communicate signals from the primary cilium to the cell body (reviewed in Li and Hu 2015).
Severe primary cilia dysfunctions have been reported for several diseases which in part are related to signaling dysfunctions, and to cellular, molecular and genetic disorders. Primary cilia dysfunctions play a role in cancer development and progression. In advanced tumors, the primary cilium becomes detached from the cell surface after loss of cellular polarization and loss of tissue organization. As a result, the basal body of the primary cilium becomes located in the interior cell body (Lingle and Salisbury 1999, 2000; Schatten et al. 2000c) which can result in the formation of an additional seed structure for microtubule nucleation and organization that may participate in additional spindle formations, thereby increasing the number of abnormal mitoses in cancer tissue, that can result in increases in aneuploidy and abnormal cell divisions.
Primary cilia dysfunctions can be the result of genetic factors, adverse environmental conditions, and perhaps other factors that are not yet known. Several primary cilia-associated genes are known to be mutated in cancers which includes Gli1, DNAH9, and RPGR1P1 (reviewed in Schatten and Sun 2011c). Furthermore, the oncogenic kinase Aurora A kinase (Aurora A) is localized to the basal body of primary cilia and may function in primary cilia disassembly or it may block primary cilia reassembly in coordination with other interacting proteins (Inoko et al. 2012). Aurora A may not function properly in cancer cells which may result in primary cilia–cell cycle dysfunctions.
As indicated above, primary cilia have an essential role in hedgehog signaling that is important for primary cilia-cellular communications. As hedgehog signaling abnormalities are associated with cancer progression, this knowledge offers new avenues for the design of new cancer therapeutics aimed at interfering with hedgehog signaling pathway abnormalities (Ślusarz et al. 2010; Hassounah et al. 2012).
Primary cilia dysfunctions have been reported for several cancers including prostate cancer (reviewed in Schatten and Ripple 2018) and breast cancer (reviewed in Schatten 2013; Schatten and Sun 2015b). A decrease in the percentage of ciliated cells has been reported for human prostatic intraepithelial neoplasia (PIN), invasive cancer, and perineural invasion lesions when compared to normal prostate tissue (Hassounah et al. 2012). The investigators also observed shorter cilia in PIN, cancer, and perineural invasion lesions which may affect accurate functions. Whereas primary cilia normally function to suppress the Wnt signaling pathway in epithelial cells the authors found that cilia loss may play a role in increased Wnt signaling in some prostate cancers. Therefore, targeting the Wnt signaling pathway may open up further new targeted treatment strategies related to Wnt signaling.
The PDGF signaling pathway through primary cilia has been shown to be aberrant in breast cancer as well as in prostate cancer. In breast cancer, expression of PDGFRα is a poor prognostic indicator of the disease (Jechlinger et al. 2006; Carvalho et al. 2005), and in prostate cancer, PDGFRα plays a role in survival and growth of prostate cancer cells in the bone, resulting in early metastatic foci (Liu et al. 2011).
These studies show the importance of regulated signaling through primary cilia and abnormal signaling in cancer cells after loss of primary cilia which may affect centrosome regulation and lead to centrosome pathologies as addressed in section 2.
Section 4: Transitions from centrosome to non-centrosome microtubule organization during cellular polarization
Significant changes in centrosome organization and functions take place during cellular polarization when cellular asymmetry becomes established in preparation for cellular differentiation of previously non-committed cells. These changes start out by deconstructing and decentralizing specific centrosomal components from the nucleus-associated centrosome to focal points at the apical and basolateral surfaces as studied in polarizing epithelial cells, resulting in major remodeling of the microtubule system with individual microtubules being nucleated and organized from the polarizing cell surfaces.
The underlying mechanisms of the remodeling process are still not well understood which is further complicated by the fact that there is considerable diversity in the molecular mechanisms of microtubule reorganization. However, we do know from studies in tissue culture cells that the microtubule cytoskeleton plays essential roles in establishing cellular polarity (reviewed in Müsch 2004; Muroyama and Lechler 2017) but we still do not yet have sufficient information to fully understand the polarization process and especially the reorganization of the nucleus-associated centrosome to a decentralized microtubule organization system that is typically referred to as “non-centrosomal”.
For this process to occur the centrosome gradually loses its MTOC activity while other cellular sites acquire competence to serve as microtubule organizing sites, thereby gradually assuming microtubule organizing functions (Fig. 4). Several hypotheses have been proposed to envision the loss of centrosomal MTOC activities which may include changes in transcription, RNA splicing, protein localization at the centrosome, and post-translational modifications that modify and decrease centrosomal activities to nucleate and/or anchor microtubules (reviewed in Muroyama and Lechler 2017). It is generally thought that MTOC inactivation is correlated with delocalization of centrosomal components in which decreased levels or activity of cell cycle regulators such as cyclin-dependent kinase (CDK) and PLK1 play a role which will result in dispersal of centrosomal proteins in specific tissues (Yang and Feldman 2015; Muroyama et al. 2016; Pimenta-Marques et al. 2016). Cell cycle regulators that control centrosome inactivation can include posttranslational modifications of centrosomal proteins and perhaps other modifications that are yet to be explored. Transcriptional downregulation of genes encoding centrosomal proteins also may play a role which has been shown for the differentiation process of the mammalian epidermis (Sen et al. 2010). In neurons, it has been shown that alternative splicing of the centrosomal protein ninein removes its centrosome-targeting domain, resulting in dispersal of ninein (Zhang et al. 2016).
Overview of centrosomal and non-centrosomal microtubules in mammalian cells. In non-polarized cells microtubules (red) emanate from the centrosome (blue). Microtubule minus ends (−) are anchored to the centrosome; plus ends (+) extend toward cell edges. In differentiated cells (right), microtubule minus ends do not associate with the centrosome but associate with the apical cell cortices while the plus ends are oriented toward the basal side. Gray = nucleus
Polarization and differentiation is essential for tissue formation and tissue specification. At present, we do not yet fully understand how the microtubule cytoskeleton becomes structurally and functionally reorganized to perform highly specific functions in polarizing cells. These functions differ significantly from functions in non-differentiated cells in which the nucleus-associated centrosomes facilitate and orchestrate the multitude of cellular processes. New functions in polarized cells include apical and basolateral transport of cellular components to help modify the polarizing cell surface as one of the first steps toward the formation of specific tissues.
Cellular polarization requires changes in microtubule biochemistry to achieve microtubule diversity which is facilitated by association with a variety of proteins as critical process for microtubule remodeling from being organized by the nucleus-associated centrosome to becoming organized by membrane-associated foci. Microtubules are polarized filaments with fast-growing plus ends and slower-growing minus ends that enable vectorial transport of vesicles and organelles (reviewed in Müsch 2004; Schatten and Sun 2012, 2014, 2017). As mentioned above they are organized from centrosomes in non-polarized cells and in mitotic cells (centrosomal microtubules) but they become nucleated and organized from non-centrosomal focal points in polarized epithelial cells (non-centrosomal microtubules) in a process requiring relocalization of specific centrosomal proteins. Some of these centrosomal proteins utilize microtubule-mediated translocation which has been shown for ninein (Mogensen 2004). While we do not yet have sufficient knowledge to understand the remodeling of centrosomal components during the polarization process microtubule dynamics are important for understanding how centrosomal proteins may be translocated from the centrosome along microtubules to associate with the cellular membrane. Ninein localization to apical non-centrosomal sites plays a role in anchoring microtubule minus ends. As ninein translocation from centrosomal to non-centrosomal sites takes place for the establishment of cellular polarity the centrosome is still engaged in contributing centrosomal components while its MTOC capabilities become gradually decreased (reviewed in Muroyama and Lechler 2017).
Several molecular changes take place during cellular polarization. We know that microtubule nucleation starts at the microtubule minus end which depends on the γ-tubulin ring complexes (γ-TuRCs) in cells (Moritz and Agard 2001). Ninein is also co-localized with microtubule minus ends and mediates microtubule anchoring at MTOCs. The calmodulin-regulated spectrin-associated protein (CAMSAP) plays a role in stabilizing microtubule minus ends and these proteins play a role in centrosome and in non-centrosome microtubule organization.
Among the specific centrosomal proteins that play a role in the polarization process are: the microtubule-nucleating protein γ-tubulin, the microtubule anchoring protein ninein, the microtubule minus-end stabilizing family of calmodulin-regulated spectrin-associated proteins (CAMSAP) with its members CAMSAP1, CAMSAP2, and CAMSAP3, and the microtubule-severing protein katanin, that is believed to play a role in controlling microtubule length and restricting the association of CAMSAPs to microtubules. These proteins have been shown in tissue culture cells to play a role in reorganization of microtubules from the centrosomal to the non-centrosomal organization. Microtubule reorganization at the polarizing cell surfaces starts to take place when adherens junctions form and E-cadherin-mediated cell adhesion induces development of microtubule asymmetry that may lead to different microtubule growth directions which is facilitated by γ-tubulin, ninein, CAMSAP2 and CAMSAP3 as well as katanin. In this context it is of interest that relocalization of ninein to cell junctions has been proposed to play a role in non-centrosomal microtubule formation during epithelial differentiation (Goldspink et al. 2017). The complex polarization process further requires post-translational modifications (PTMs) of microtubules to enable specific associations of cargo with microtubule motor proteins for targeted transport to remodel apical membrane domains (reviewed in Schatten and Sun 2014).
Other proteins associated with microtubules play a role in the polarization process. Microtubule plus ends are controlled by several microtubule associated proteins (MAPs) including the EB (end binding) family proteins, CLIP-170 (CLIP1), XMAP215 (CKAP5), and the CLASP family (reviewed in Akhmanova and Steinmetz 2008). Other proteins such as several MAPS including tau and MAP4 binding to microtubules along the individual microtubule and promote microtubule stabilization (Kadavath et al. 2015). The microtubule-severing proteins katanin and spastin are involved in microtubule regulation (reviewed in Roll-Mecak and McNally 2010), as are the above mentioned post-translational modifications (PTMs) of tubulin (reviewed in Schatten and Sun 2014; Song and; Brady 2015; Valenstein and Roll-Mecak 2016).
It is also possible that entire microtubules can be released from the centrosome through uncapping or severing of microtubules and stabilization at the new cellular site. The mechanisms controlling subsequent transport of microtubules to the non-centrosomal MTOC remain largely unexplored although we know from research in neurons that dynein plays a key role in trafficking microtubules and/or tubulin to their ultimate locations (He et al. 2005; del Castillo et al. 2015). The mechanisms by which centrosome-associated microtubules become remodeled to associate with their new location at the polarizing cell surface are still under recent investigation and we currently do not yet have conclusive answers. Studies using a variety of approaches to analyze microtubule nucleation are still in progress aimed at understanding the mechanisms that contribute to the formation of microtubules as cells undergo differentiation.
As mentioned above, the relocalization of centrosomal components and perhaps new organization towards establishing microtubule nucleation and polymerization from the polarizing cell surface may be different in different tissues (reviewed in Muroyama and Lechler 2017). Centrosomal proteins can undergo molecular changes. For example, the different localizations of ninein is achieved through differential splicing of the ninein transcript, eliminating the centrosome-targeting domain causing ninein dispersal (Zhang et al. 2016). We know that γ-tubulin becomes enriched at the apical cell surface which determines the apical surface as the primary microtubule nucleation side in polarizing epithelial cells. Aided by ninein microtubules become anchored and stabilized at the apical cell surface and the microtubule minus ends are protected from depolymerization by the CAMSAP/Patronin/Nezha family proteins (reviewed by Akhmanova and Hoogenraad 2015).
The stabilization of microtubule ends by CAMSAP/Patronin/Nezha family proteins has been shown for all species studied so far (Baines et al. 2009; Goodwin and Vale 2010; Meng et al. 2008). In Caco-2 cells, Nezha/CAMSAP3 was identified as an adherens junction-associated protein that regulates apicobasal microtubule organization (Meng et al. 2008). Recently, microtubule disorganization has been reported for a Camsap3 mutant mouse in which the microtubule-binding domain (the CKK domain) was deleted.
While the establishment of cell polarity is critical for coordinated tissue functions loss of cell polarity is associated with disease and it is a hallmark of cancer cells. However, the process involving loss of cellular polarization is still not well understood. Loss of microtubule organization by γ-tubulin and anchoring proteins at the apical cell surface may play a role in this process.
Section 5: Other centrosome dysfunctions associated with the pathogenesis of human disease
Centrosomes as center for proteolytic activity
As addressed above, the functional complexity and importance of the centrosome reaches well beyond its microtubule organizing capacity, as centrosomal proteins as well as centrosome-associated proteins play a role in numerous, seemingly unrelated, cellular processes that can be perturbed by centrosomal dysfunction. The role of centrosomes in the pathogenesis of human disease is intriguing and multifaceted. Human disease phenotypes and centrosome dysfunctions are linked in various ways which has resulted in newly appreciated centrosome functions in mammalian cells and dysfunctions underlying various diseases. Several centrosome-related diseases involve the basal body of motile cilia and non-motile primary cilia that share protein compositions and intertwined cellular functions including signal transduction and, indirectly, intraflagellar transport (IFT) within cilia for cellular signaling and cellular responses. Several of these dysfunctions become apparent in post-mitotic cells, such as neurons. In addition, as indicated in section 1, the centrosome plays a consequential role in the spatial organization of cellular organelles including the Golgi apparatus (Rios et al. 2004) and it also plays a critical role in the distribution of macromolecular complexes, vesicles containing various contents, and mitochondria. Several studies including those using proteomic analysis have identified a number of proteins that, when mutated, cause a wide spectrum of human genetic phenotypes as diverse as Alstrom syndrome, oro-facial-digital syndrome, kidney diseases, and others (Andersen et al. 2003; reviewed in Badano et al. 2005) as discussed below in this section.
Among the centrosome-related diseases caused directly or indirectly by centrosome dysfunctions are ubiquitin–proteasome-mediated protein degradation, neuronal migration, axonal targeting, and ciliation. The role of centrosomes in ubiquitin–proteasome-mediated protein degradation is particularly intriguing, as proteasome activity takes place close to the centrosome and the ubiquitin–proteasome degradation (UPD) pathway appears to be significantly dependent on the organizing capabilities of the centrosome.
The importance of UPD in regulating cell cycle checkpoints in eukaryotes through specific targeting of cell cycle regulators has been well documented which has been studied particularly well for UPD mediation of cyclin degradation as important part of cell cycle regulation, and it is also known that the UPD system plays an important role in cellular differentiation and development, stress response, morphogenesis of neuronal networks, and neurotransmission and apoptosis (Izzi and Attisano 2004.; DiAntonio and Hicke 2004; Peters 2002). The 26S proteasome is the major component of the UPD system targeting proteins that are modified through ubiquitination in a process requiring the combined actions of three different types of enzymes, E1, E2, E3 (Pickard and Cohen 2004). As proteasomes are concentrated at the mammalian centrosome the centrosome has also been viewed as a proteolytic center (Wojcik et al. 1996; Wigley et al. 1999; reviewed in Badano et al. 2005). The concentrated localization of proteasomal components including ubiquitin, the 20S and 19S subunits of the proteasome, as well as the E3 enzyme parkin, is especially noteworthy, as they colocalize with the centrosomal marker γ-tubulin and these proteasomal components co-purify with γ-tubulin in the centrosomal fractions after sucrose-gradient ultracentrifugation (Wigley et al. 1999).
The accumulation and concentration of proteasomal components around centrosomes appears to be microtubule-independent, as suggested by studies in which intracellular levels of misfolded proteins were experimentally increased by either proteasome inhibition with drugs such as lactacystin, or overexpression of misfolded mutant proteins, resulting in an expansion of the centrosome-associated proteasome network with expansion and recruitment of proteolytic components from the cytosol without involvement of microtubules. These studies provide experimental evidence for a critical role of centrosomes in the organization and subcellular localization of proteasomes (Wigley et al. 1999; Fabunmi et al. 2000). This area of centrosome research is still wide open for further investigations to determine the role and activities of centrosomes in monitoring and degrading misfolded proteins and perhaps signaling interactions and communications with the surrounding Golgi apparatus and endoplasmic reticulum.
Ubiquitin–proteasome degradation
Diseases related to dysfunction of ubiquitin–proteasome degradation include viability of post-mitotic neurons, as UPD dysfunctions are implicated in several neurodegenerative disorders, including Parkinson disease (Nussbaum and Ellis 2003) in which mutations of the gene that encodes parkin, an E3 ubiquitin-protein ligase, causes autosomal recessive juvenile parkinsonism (ARJP) (Ishikawa and Tsuji 1996; Takahashi et al. 1994; Kitada et al. 1998; Imai et al. 2000; Shimura et al. 2000). As mentioned above, parkin is concentrated in the centrosomal area and it binds to γ-tubulin as shown in co-immunoprecipitation assays both in vivo and in vitro using rat brains and HEK293 cells, respectively (Zhao et al. 2003; reviewed in detail by; Badano et al, 2005).
Based on research related to Parkinson and other neurological diseases centrosome dysfunctions can be implicated in the pathogenesis of protein clearance disorders, which invites further studies to gain further functional insights into still little understood diseases and their underlying mechanisms leading to pathologies.
The centrosome has further been implicated in the pathogenesis of diseases related to migration disorders, as the centrosome is critical for nuclear translocation. Several human dysplasias are related to centrosome dysfunctions including in patients with lissencephaly (smooth brain), in which the cortex appears as a smooth surface which is associated with mental retardation, seizures, and death in early childhood (Walsh 1999; Wynshaw-Boris and Gambello 2001; reviewed in Badano et al. 2005).
Vesicular transport dysfunctions and disease
Other diseases in which centrosome dysfunction are implicated are related to the centrosome’s role in vesicular transport. As the main microtubule organizing center the centrosome orchestrates and directs transport of vesicles along microtubules to their functional destinations (reviewed in Schatten 2008). Huntington disease, associated with progressive loss of cognitive function, dementia, and motor defects as a consequence of neuronal dysfunction and loss, is among the neurodegenerative disorders in which defects in microtubule-dependent vesicular transport have been reported. The huntingtin gene underlies the pathogenesis of the disease, and huntingtin is localized in the nucleus and the cytoplasm associated with vesicles and microtubules (Harjes and Wanker 2003). The molecular associations of huntingtin have been well explored and it was shown that huntingtin interacts with huntingtin-associated protein (HAP1), which binds to the p150Glued subunit of dynactin and the pericentriolar material 1 protein (PCM1), proteins that play a role in centrosome and basal-body functions. Huntingtin controls the microtubule-mediated vesicle transport of the brain-derived neurotrophic factor (BDNF) that is produced in the cortex and released in the striatum for cell survival; defective vesicle transport might play a role in the pathogenesis of Huntington disease (Gauthier et al. 2004). It is also noteworthy that fibroblast cells derived from mice and patients with Huntington disease contain abnormal centrosome numbers, reduced mitotic indices, increased frequencies of aneuploidy, and persisting midbodies (Sathasivam et al. 2001; reviewed in Badano 2005).
Basal-body dysfunctions and disease
Basal body and ciliary diseases are numerous and these are also related to centrosomal dysfunctions, as there is a close link structurally as well as functionally between centrosomes, basal bodies, and cilia (reviewed in Schatten 2008; Quarmby and Parker 2005; Li and Hu 2015) and as also addressed above in section 3.
Loss-of-function mutations affecting the basal body can result in human disorders including reversal or randomization in body symmetry, hydrocephalus, retinal degeneration, and cystic kidney and liver disease (reviewed in Badano 2005). It includes a wide spectrum of molecular dysfunctions that have been determined for several diseases causing various effects. For example, in Bardet–Biedl syndrome (BBS), a pleiotropic disorder in which retinal degeneration, obesity, learning difficulties, and polydactyly, as well as gonadal and renal malformations including cystic kidneys are observed, some of the proteins involved in the syndrome could be affected by functional signal relay between cilia, basal body, and the centrosome, related to IFT which will affect cell cycle regulation including mitosis and cell division. Trafficking of cargo between cilia to the associated cells may well be regulated by centrosomes, which has been shown for BBS. These effects are reported to be caused by centrosomal and ciliary dysfunction (Katsanis 2004; Kulaga et al. 2004; Kim et al. 2004; Blacque et al. 2004; Ansley et al. 2003). Two of the eight known BBS proteins, BBS4 and BBS8, localize to centrosomes and basal bodies as shown in mammalian cell lines.
Autosomal dominant polycystic kidney disease (ADPKD) has been clearly linked to mutations in the polycystic kidney disease genes PKD1 and PKD2 (Yoder et al. 2002), and it has been shown that the PKD1 and PKD2 proteins, polycystin 1 and 2, interact and serve as mechanosensors of extracellular fluid flow in the primary cilia of the renal epithelium that regulates intracellular Ca2+ flux (Yoder et al. 2002; Nauli et al. 2003). These studies focused attention on the important role of primary cilia as discussed in section 3.
Section 6: Centrosome functions in oocyte germ cells and dysfunctions in reproductive disorders and reproductive aging
Among the fastest aging cells in the female body are oocytes located in the female reproductive tract. As an increasing number of women have postponed the time to become pregnant oocyte aging has become a major problem and research on oocyte aging has identified several factors contributing to the aging process with significant effects on the meiotic spindle that contains the machinery for chromosome segregation during first and second meiosis, primarily consisting of a microtubule-rich spindle, spindle poles composed of centrosomal material, and chromosomes. The meiotic spindle is distinguished from the mitotic spindle by the absence of centrioles within the centrosome complex which has resulted in using different terminologies by different research groups. We prefer the term “acentriolar centrosomes”, as centrosomal material in the meiotic spindle fulfills the major functions known for mitotic cells which is to nucleate and organize microtubules that are critically important for chromosome separation and meiotic cell division. Furthermore, the meiotic centrosomal material has similar compositions as mitotic centrosomal material including the major centrosomal protein γ-tubulin and the centrosome-associated protein NuMA. As is the case for different cell systems the meiotic acentriolar centrosome can have different compositions in different species on both qualitative and quantitative levels and it also can have different regulatory enzymes although the general mechanisms are quite similar for mitotic and meiotic centrosome regulation. It should, however, be emphasized that the mouse oocyte system uses very different mechanisms for acentriolar centrosome formation and organization compared to non-rodent mammalian systems such as humans, the porcine, bovine, and most other mammalian oocytes (reviewed in Wang et al. 2011; Schatten and Sun 2013, 2015a; Qiao et al. 2014). As discussed below in this section, the mouse oocyte system also displays different characteristics during aging; furthermore, it contains acentriolar centrosomes during the first mitotic divisions of embryogenesis unlike non-rodent mammalian embryos that do contain centrioles within the centrosome complex during the first cell divisions of embryogenesis which is related to different fertilization mechanisms in rodent and non-rodent systems (reviewed in detail in Schatten and Sun 2011a, b, c; Schatten et al. 2012).
In aging oocytes, loss of meiotic spindle integrity is among the most noticeable and most consequential changes resulting in an inability of microtubules to accurately interact with meiotic chromosomes, thereby resulting in chromosomal abnormalities (aneuploidies) which can have implications in fertilization failures, arrested embryo development, childhood cancer, or embryo loss (reviewed in Miao et al. 2009a, b; Wang et al. 2011; Schatten and Sun 2013; Qiao et al. 2014) (Fig. 5). In aging non-rodent oocytes the acentriolar centrosomes become instable resulting in dispersion of centrosomal components and centrosomal proteins while numerous small aggregates become formed in the ooplasm.
MII meiotic spindle in unfertilized oocyte (left) is critically important for accurate alignment and separation of chromosomes. Center: loss of spindle integrity in aging oocytes shows disorganized microtubules (green), disorganized centrosomes (red dots), and disorganized chromosomes (blue) which will result in aneuploidy. The image on the right shows a schematic diagram of meiotic spindle organized by acentriolar centrosomes
The integrity of the highly dynamic meiotic spindle structure is actively maintained in younger oocytes by a complex set of regulatory kinases and other regulatory proteins until fertilization takes place. These include roles for CDK1/cyclin B and kinases such as PKA, AKT, MAPK, Aurora A, CaMKII, the phosphatases CDC5, CDK14s, and others that participate in the meiotic process. Alterations in regulatory kinases have been shown in aging oocytes (reviewed in Miao et al. 2009a, b; Wang et al. 2011; Qiao et al. 2014).
The dispersion of centrosomal components from the meiotic spindle poles into the cytoplasm is different in rodent and non-rodent mammals which has been discussed in several previous papers (Kim et al. 1996; George et al. 1996; Alvarez Sedó et al. 2011; reviewed in Miao et al. 2009a; Schatten et al. 2012; Schatten and Sun 2013). In brief, in non-rodent mammals, NuMA, and γ-tubulin become dispersed from the meiotic spindle, while small centrosomal aggregates are formed in the cytoplasm to nucleate and organize small cytoplasmic asters. In contrast, such small cytoplasmic asters are not seen in aging rodent oocytes, while they are seen in younger rodent (mouse) oocytes as part of the very different mechanism used for meiotic spindle formation in rodent oocytes (Schatten et al. 1988, 1985; Maro et al. 1985; reviewed in Schatten and Sun 2015a). The cytoplasmic asters typical for young mouse oocytes disintegrate in the mouse during oocyte aging (Miao et al. 2009a). These differences are important to keep in mind for correct interpretations of centrosome and microtubule dynamics in rodent (mouse) and non-rodent (human, bovine, porcine) oocytes.
Several factors account for destabilization of the meiotic spindle in aging oocytes; the inability of centrosomal proteins to accurately associate with the centrosome structure may be the result of destabilization of the centrosomal matrix structure itself or it may be the result of microtubule destabilization at the centrosome-facing microtubule areas. These microtubule areas close to centrosomes are acetylated in fresh oocytes but may not be acetylated in aging oocytes. As several centrosomal proteins as well as the centrosome-associated protein NuMA rely on motor-driven transport along microtubules to form and maintain a functional centrosome transport may be impaired in aging oocytes as a result of destabilized microtubules. Furthermore, activities of several enzymes important for centrosome maintenance and functions are decreased in aging oocytes including MAPK (reviewed in Miao et al. 2009a, b; Wang et al. 2011; Qiao et al. 2014) which contributes to microtubule and centrosome destabilization.
Knowledge about specific aspects involved in oocyte aging will help us understand the aging process and help to find countermeasures to halt or reverse aging of mammalian oocytes and perhaps other cells undergoing aging in the human body. As will be discussed below several remedies to counteract the aging process have already been identified and others are being studied to obtain more detailed information.
Centrosome dysfunctions have also been implicated in other systems during aging and senescing in which supernumerary centrosome abnormalities have been reported (Schatten et al. 1999). Aging-related abnormalities seen in mitosis become already pronounced at the transition from G2 to M when many of the cell cycle regulators are downregulated in aging cells and affect centrosome functions. The G2/M phase is especially critical for microtubule and centrosome reorganization. In aging cells, centrosomes have lower activity in centrosome-associated protein kinases (Cande 1990; Huang 1990) which includes Plk (Ly et al. 2000). It has been shown in stem cell division that centrosome mis-orientation contributes to reduced stem cell division during aging (Cheng et al. 2008), which is associated with the decline in spermatogenesis during aging.
Reversal or halting of mechanisms affected by oocyte aging to delay or prevent the aging process and to restore oocyte viability in age-affected oocytes
Because the timing of pregnancies is increasingly being postponed by women and couples for various reasons such as sociological or professional reasons numerous in vitro fertilization clinics (IVF) have been established to help overcome oocyte aging that typically occurs in women past age 35 (reviewed in Miao et al. 2009a, b; Wang et al. 2011; Qiao et al. 2014). As a result, numerous approaches have been initiated to delay or prevent the aging process and to restore oocyte viability in age-affected oocytes which includes approaches to prevent destabilization of the meiotic spindle with focus on stabilizing centrosomes and microtubules (reviewed in Schatten and Sun 2015a RFD). Supplementation of culture medium or oocyte microinjection with chemicals or factors that delay, inhibit, or reverse the oocyte aging process have been proposed and include chemicals such as caffeine (reviewed in Miao et al. 2009a), nitric oxide (NO; Goud et al. 2005a, b), dithiothreitol (DTT; Raussel et al. 2007; Tarin et al. 1998), and trichostatin A (TSA; Jeseta et al. 2008; Huang et al. 2007).
The use of caffeine to delay or prevent oocyte aging by controlling the activity of MPF has been explored by Kikuchi et al. (Kikuchi et al. 2000, 2002; reviewed in Miao et al. 2009a). As MPF and MAPK activities gradually decrease during oocyte aging (Xu et al. 1997; Tian et al. 2002; Fan and Sun 2004; Tatone et al. 2006; Liang et al. 2007) studies showed that 10 mM caffeine could prevent the decline in MPF and MAPK activity in aging bovine oocytes (Lee and Campbell 2008) and further studies showed that continuous application of caffeine is necessary to maintain the effect (Miao et al. 2009a, b, ; reviewed in). Caffeine was able to prevent aging changes of meiotic spindles when added directly after oocyte maturation or it could restore spindle integrity when added 48 h after maturation (i.e., aged for 48 h) with chromosomes, microtubules, and the centrosomal proteins γ-tubulin and NuMA displaying normal appearance like in fresh oocytes (Miao et al. 2009a, b).
Dithiothreitol (DTT) and β-mercaptoethanol both have been used to test the beneficial effects on oocyte aging and these compounds have been shown to affect oocyte aging through different mechanisms (Tarin et al. 1998); both may affect centrosome dynamics and prevent centrosome disintegration in aging oocytes, thereby preventing disorganization of the MII spindle. Other studies proposed different mechanisms by which DTT affects oocyte aging such as preventing oxidation of free thiol groups and/or altering a redox-independent signaling pathway. A direct effect on microtubules and centrosomes has not yet been established although the effect of DTT on centrosomes had been reported (Schatten 1994). β-Mercaptoethanol studies have not yet been performed specifically to analyze the effects on oocyte aging but a clear effect on centrosomes had been established in previous studies by Daniel Mazia (Mazia et al. 1960) using sea urchin eggs as model system. Subsequent studies indicated that β-mercaptoethanol may affect centrosome replication (Sluder and Begg 1985). β-Mercaptoethanol may be useful to slow deterioration of the MII spindle in aging oocytes but more research is needed to determine the specific effects on mammalian oocytes.
Nitric oxide (NO) has been used to prevent or delay oocyte aging (Goud et al. 2005a, b); NO exposure decreases the rate of spindle abnormalities, but the mechanisms underlying the effects are not entirely clear although it may indirectly preserve the integrity of microtubules in the MII spindle and therefore preserve spindle integrity in aged oocytes (Goud et al. 2005a).
Trichostatin A (TSA) has been used to show that it may have different effects on oocyte aging in different species (reviewed in Miao et al. 2009a). TSA is a non-specific inhibitor of HDAC6 and may have dual functions in aging oocytes. In addition to its role in epigenetic alterations TSA may have a stabilizing effect on microtubules that become destabilized in aging oocytes, especially those facing the centrosomal area.
Several of these chemicals or compounds may be added simultaneously to the culture medium during in vitro fertilization (IVF) procedures to promote a combinatorial effect and affect multiple mechanisms that are affected by aging. Such combined effects may also promote stabilization of the labile microtubules around the centrosomal area which may also allow targeted association of microtubule motor proteins with microtubules for transport of centrosomal proteins to the centrosome core structure. It would contribute to prevention of centrosome disintegration including NuMA that we have shown in aging human oocytes to be impaired as a result of dynein-dependent NuMA translocation to centrosomes (Alvarez Sedó et al. 2011).
We do not yet know whether or not the centrosomal matrix structure is affected by aging. We know from experiments in sea urchin eggs that this material is fibrous and has the ability to compact and decompact during the cell cycle (Schatten et al. 1987, 1992). We do not yet know whether changes in this centrosome matrix structure that is thought to be composed of intermediate filament-like structures take place during oocyte aging but we know that in non-reproductive cells intermediate filaments have been proposed to be affected by aging (Hyder et al. 2011). It is possible that centrosomal proteins cannot associate properly with the centrosomal matrix structure in aging oocytes or, alternatively, as mentioned above, that centrosomal proteins cannot be transported dynamically along microtubules to the centrosome in aging cells because transport along microtubules is impaired (Alvarez Sedó et al. 2011). In fresh oocytes, centrosome dynamics include active transport of centrosomal proteins along microtubules and active maintenance of centrosomes at the MII stage before fertilization takes place.
Taken together, the topic of aging effects on centrosomes is still under-explored in somatic cells and more research is needed on the centrosome matrix structure itself, on centrosome-regulating enzyme activity and on microtubule stability. In this context, it would also be important to investigate correlations between centrosome instability and age-related cancer in which centrosome pathologies are frequent.
Conclusion and future directions
New insights into centrosome biology has resulted in a wealth of new information on how centrosome dysfunctions are implicated in diseases and how newly gained knowledge can be utilized to remedy centrosome dysfunctions in diseases or disorders such as cancer, neurological disorders, and others. The past decade has increased our understanding on mechanisms involved in centrosome functions and mis-regulation leading to dysfunctions with new insights into the Golgi-centrosome nexus, interconnected primary cilia-centrosome functions, signal transduction, and proteolytic activities orchestrated and/or facilitated by centrosomes and new therapeutic potential to prevent centrosome clustering in cancer cells, thereby preventing abnormal cancer cell divisions. We have increased our knowledge of the role of centrosomes in polarizing cells from nucleus-associated centrosome functions to centrosome inactivation while establishing new MTOC sites during cellular polarization. This field is only at the beginning and not yet well understood, as is the effect of aging on centrosomes, both presenting wide open fields for new investigations.
While model organisms such as Drosophila, C elegans, and others have contributed good information on centrosomes that could to some extent be applied to mammalian systems, it is important to study centrosomes in mammals, as there are important differences even between different mammalian species, especially regarding centriole and centrosome organization and cell cycle-specific functions. For these reasons, we still need more specific information for mammalian centrosomes.
Taken together, as our understanding of molecular mechanisms underlying disease has increased enormously during the past few decades the role of centrosomes being implicated in orchestrating numerous cellular processes and tissue formations has further increased the appreciation for centrosomes and it can be expected that this very small but important non-membrane bound organelle will see further appreciation in future years, validating further the prediction by the classic visionary cytologist Walther Flemming (1875, 1891) who had predicted that the discovery of the centrosome would be as important as the discovery of the nucleus.
References
Acilan C, Saunders WS (2008) A tale of too many centrosomes. Cell 134:572–575
Akhmanova A, Hoogenraad CC (2015) Microtubule minus-end-targeting proteins. Curr Biol 25:R162–R171
Akhmanova A. Steinmetz MO (2008) Tracking the ends: a dynamic protein network controls the fate of microtubule tips. Nat Rev Mol Cell Biol 9:309–322
Alvarez Sedó CA, Schatten H, Combelles C, Rawe VY (2011) The nuclear mitotic apparatus protein NuMA: localization and dynamics in human oocytes, fertilization and early embryos. Mol Hum Reprod 17(6):392–398. https://doi.org/10.1093/molehr/gar009
Andersen JS, Wilkinson CJ, Mayor T, Mortensen P, Nigg EA, Mann M (2003) Proteomic characterization of the human centrosome by protein correlation profiling. Nature 426:570–574
Ansley SJ et al (2003) Basal body dysfunction is a likely cause of pleiotropic Bardet–Biedl syndrome. Nature 425:628–633
Arquint C, Gabryjonczyk AM, Nigg EA (2014) Centrosomes as signalling centres. Philos Trans R Soc Lond B Biol Sci 369
Badano JL, Teslovich TM, Katsanis N (2005) The centrosome in human genetic disease. Nat Rev Genet 6:194–207
Baines AJ, Bignone PA, King MDA, Maggs AM, Bennett PM, Pinder JC, Phillips GW (2009) The CKK domain (DUF1781) binds microtubules and defines the CAMSAP/ssp4 family of animal proteins. Mol Biol Evol 26:2005–2014
Berbari NF, O’Connor AK, Haycraft CJ, Yoder BK (2009) The primary cilium as a complex signaling center. Curr Biol 19:R526–R535
Blacque OE et al (2004) Loss of C. elegans BBS-7 and BBS-8 protein function results in cilia defects and compromised intraflagellar transport. Genes Dev 18:1630–1642
Bornens M (2012) The centrosome in cells and organisms. Science 335:422–426
Boutros R (2012) Regulation of centrosomes by cyclin-dependent kinases. In: Schatten H (ed) The centrosome, Chap. 11. Springer, New York
Boveri T (1901) Zellen-Studien: Über die Natur der Centrosomen. Jena, Germany: Fisher Z Med Naturw 28:1–220
Boveri T (1914) Zur Frage der Entstehung maligner Tumoren. G. Fisher, Jena
Brinkley BR, Goepfert TM (1998) Supernumerary centrosomes and cancer: Boveri’s hypothesis resurrected. Cell Motil Cytoskeleton 41:281–288
Can A, Semiz O, Cinar O (2005) Bisphenol-A induces cell cycle delay and alters centrosome and spindle microtubular organization in oocytes during meiosis. Mol Hum Reprod 11:389–396
Cande Z (1990) Centrosomes: composition and reproduction. Curr Opin Cell Biol 2:301–305
Carroll E, Okuda M, Horn HF, Biddinger P, Stambrook PJ, Gleich LL, Li YQ, Tarapore P, Fukasawa K (1999) Centrosome hyperamplification in human cancer: chromosome instability induced by p53 mutation and/or Mdm2 overexpression. Oncogene 18:1935–1944
Carvalho I, Milanezi F, Martins A, Reis RM, Schmitt F (2005) Overexpression of platelet-derived growth factor receptor alpha in breast cancer is associated with tumour progression. Breast Cancer Res 7:R788–R795
Chabin-Brion K, Marceiller J, Perez F, Settegrana C, Drechou A, Durand G, Pous C (2001) The Golgi complex is a microtubule-organizing organelle. Mol Biol Cell 12:2047–2060. https://doi.org/10.1091/mbc.12.7.2047
Chan JY (2011) A clinical overview of centrosome amplification in human cancers. Int J Biol Sci 7:1122–1144
Cheng J, Türkel N, Hemati N, Fuller MT, Hunt AJ, Yamashita YM (2008) Centrosome misorientation reduces stem cell division during ageing. Nature 456:599–604
Cheung CH, Coumar MS, Chang JY, Hsieh HP (2011) Aurora kinase inhibitor patents and agents in clinical testing: an update (2009–10). Expert Opin Ther Pat 21:857–884
Cole NB, Sciaky N, Marotta A, Song J, Lippincott- Schwartz J (1996) Golgi dispersal during microtubule disruption: regeneration of Golgi stacks at peripheral endoplasmic reticulum exit sites. Mol Biol Cell 7:631–650. https://doi.org/10.1091/mbc.7.4.631
Corthesy-Theulaz I, Pauloin A, Pfeffer SR (1992) Cytoplasmic dynein participates in the centrosomal localization of the Golgi complex. J Cell Biol 118:1333–1345. https://doi.org/10.1083/jcb.118.6.1333
D’Angelo A, Franco B (2009) The dynamic cilium in human diseases. PathoGenetics 2(3):1–15
Davenport JR, Yoder BK (2005) An incredible decade for the primary cilium: a look at a once-forgotten organelle. Am J Physiol Renal Physiol 289:F1159–F1169
De Brabander M, Geuens G, Nuydens R, Willebrords R, De Mey J (1981) Taxol induces the assembly of free microtubules in living cells and blocks the organizing capacity of the centrosomes and kinetochores. Proc Natl Acad Sci USA 78:5608–5612
Del Castillo U, Winding M, Lu W, Gelfand VI (2015) Interplay between kinesin-1 and cortical dynein during axonal outgrowth and microtubule organization in Drosophila neurons. eLife 4:e10140
Delgehyr N, Sillibourne J, Bornens M (2005) Microtubule nucleation and anchoring at the centrosome are independent processes linked by ninein function. J Cell Sci 118:1565–1575. https://doi.org/10.1242/jcs.02302
DiAntonio A, Hicke L (2004) Ubiquitin-dependent regulation of the synapse. Annu Rev Neurosci 27:223–246
Dictenberg J, Zimmerman W, Sparks C, Young A, Vidair C, Zheng Y, Carrington W, Fay F, Doxsey SJ (1998) Pericentrin and gamma tubulin form a protein complex and are organized into a novel lattice at the centrosome. J Cell Biol 141:163–174
Dimitriadis I, Katsaros C, Galatis B (2001) The effect of taxol on centrosome function and microtubule organization in apical cells of Sphacelaria rigidula (Phaeophyceae). Phycol Res 49:23–34
Doxsey SJ, Stein P, Evans L, Calarco P, Kirschner M (1994) Pericentrin, a highly conserved protein of centrosomes involved in microtubule organization. Cell 76:639–650
Duensing S, Munger K (2003) Centrosome abnormalities and genomic instability induced by human papillomavirus oncoproteins. Prog Cell Cycle Res 5:383–391
Duensing S, Lee LY, Duensing A, Basile J, Piboonniyom S, Gonzalez S, Crum CP, Munger K (2000) The human papillomavirus type 16 E6 and E7 oncoproteins cooperate to induce mitotic defects and genomic instability by uncoupling centrosome duplication from the cell division cycle. Proc Natl Acad Sci USA 97:10002–10007
Efimov A et al (2007) Asymmetric CLASP-dependent nucleation of noncentrosomal microtubules at the trans-Golgi network. Dev Cell 12:917–930. https://doi.org/10.1016/j.devcel.2007.04.002
Eichenlaub-Ritter U, Vogt E, Cukurcam S, Sun F, Pacchierotti F, Parry J (2008) Exposure of mouse oocytes to bisphenol A causes meiotic arrest but not aneuploidy. Mutat Res 651:82–92
Fabunmi RP, Wigley WC, Thomas PJ, DeMartin GN (2000) Activity and regulation of the centrosome-associated proteasome. J Biol Chem 275:409–413
Fan HY, Sun QY (2004) Involvement of mitogen-activated protein kinase cascade during oocyte maturation and fertilization in mammals. Biol Reprod 70:535–547
Fisk HA (2012) Many pathways to destruction: the centrosome and its control by and role in regulated proteolysis. In: Schatten H (ed) The centrosome, Chap, 8. Springer, New York
Flemming W (1875) Studien über die Entwicklungsgeschichte der Najaden. Sitzungsber Akad Wissensch Wien 71:81–147
Flemming W (1891) Verhandlungen der anatomischen Gesellschaft, Jahrg. 6, München (found as item 60 vol II in Collected Papers of Walther Flemming in M.B.L. Library, reprint collection)
Fukasawa K (2012) Molecular links between centrosome duplication and other cell cycle associated events. In: Schatten H (ed) The centrosome, Chap, 10. Springer, New York
Fukasawa K, Choi T, Kuriyama R, Rulong S, Vande Woude GF (1996) Abnormal centrosome amplification in the absence of p53. Science 271:1744–1747
Gauthier LR et al (2004) Huntingtin controls neurotrophic support and survival of neurons by enhancing BDNF vesicular transport along microtubules. Cell 118:127–138
George MA, Pickering SJ, Braude PR, Johnson MH (1996) The distribution of α- and γ-tubulin in fresh and aged human and mouse oocytes exposed to cryoprotectant. Mol Hum Reprod 2(6):445–456
Gillingham AK, Munro S (2000) The PACT domain, a conserved centrosomal targeting motif in the coiled-coil proteins AKAP450 and pericentrin. EMBO Rep 1:524–529
Goldspink DA, Rookyard C, Tyrrell BJ, Gadsby J, Perkins J, Lund EK, Galjart N, Thomas P, Wileman T, Mogensen MM (2017) Ninein is essential for apico-basal microtubule formation and CLIP-170 facilitates its redeployment to non-centrosomal microtubule organizing centres. Open Biol
Goodwin SS, Vale RD (2010) Patronin regulates the microtubule network by protecting microtubule minus ends. Cell 143:263–274
Gopalakrishnan J et al (2011) Sas-4 provides a scaffold for cytoplasmic complexes and tethers them in a centrosome. Nat Commun 2:359
Goud AP, Goud PT, Diamond MP, Abu-Soud HM (2005a) Nitric oxide delays oocyte aging. Biochemistry 44:11361–11368
Goud AP, Goud PT, Diamond MP, Van Oostveldt P, Hughes MR (2005b) Microtubule turnover in ooplasm biopsy reflects ageing phenomena in the parent oocyte. Reprod Biomed Online 11:43–52
Habermann K, Lange BM (2012) New insights into subcomplex assembly and modifications of centrosomal proteins. Cell Div 7:17
Harada A, Takei Y, Kanai Y, Tanaka Y, Nonaka S, Hirokawa N (1998) Golgi vesiculation and lysosome dispersion in cells lacking cytoplasmic dynein. J Cell Biol 141:51–59. https://doi.org/10.1083/jcb.141.1.51
Harjes P, Wanker EE (2003) The hunt for huntingtin function: interaction partners tell many different stories. Trends Biochem Sci 28:425–433
Hassounah NB, Bunch TA, McDermott KM (2012) Molecular pathways: the role of primary cilia in cancer progression and therapeutics with a focus on hedgehog signaling. Clin Cancer Res 18(9):2429–2435
He Y, Francis F, Myers KA, Yu W, Black MM, Baas PW (2005) Role of cytoplasmic dynein in the axonal transport of microtubules and neurofilaments. J Cell Biol 168:697–703
Hildebrandt F, Otto E (2005) Cilia and centrosomes: a unifying pathogenic concept for cystic kidney disease? Nat Rev Genet 6:928–940
Ho YS, Duh JS, Jeng JH, Wang YJ, Liang YC, Lin CH, Tseng CJ, Yu CF, Chen RJ, Lin JK (2001) Griseofulvin potentiates antitumorigenesis effects of nocodazole through induction of apoptosis and G2/M cell cycle arrest in human colorectal cancer cells. Int J Cancer 91:393–401
Ho SM, Tang WY, Belmonte de FJ, Prins GS (2006) Developmental exposure to estradiol and bisphenol A increases susceptibility to prostate carcinogenesis and epigenetically regulates phosphodiesterase type 4 variant 4. Cancer Res 66:5624–5632
Hoppeler-Lebel A, Celati C, Bellett G, Mogensen MM, Klein-Hitpass L, Bornens M, Tassin AM (2007) Centrosomal CAP350 protein stabilises microtubules associated with the Golgi complex. J Cell Sci 120:3299–3308. https://doi.org/10.1242/jcs.013102
Huang B (1990) Genetics and biochemistry of centrosomes and spindle poles. Curr Opin Cell Biol 2:28–32
Huang JC, Yan LY, Lei ZL, Miao YL, Shi LH, Yang JW, Wang Q, Ouyang YC, Sun QY, Chen DY (2007) Changes in histone acetylation during postovulatory aging of mouse oocyte. Biol Reprod 77:666–670
Hyder CL, Isoniemi KO, Torvaldson ES, Eriksson JE (2011) Insights into intermediate filament regulation from development to ageing. J Cell Sci 124:1363–1372
Imai Y, Soda M, Takahashi R (2000) Parkin suppresses unfolded protein stress-induced cell death through its E3 ubiquitin-protein ligase activity. J Biol Chem 275:35661–35664
Infante C, Ramos-Morales F, Fedriani C, Bornens M, Rios RM (1999) GMAP-210, A cis-Golgi networkassociated protein, is a minus end microtubulebinding protein. J Cell Biol 145:83–98. https://doi.org/10.1083/jcb.145.1.83
Inoko A, Matsuyama M, Goto H, Ohmuro-Matsuyama Y, Hayashi Y, Enomoto M, Ibi M, Urano T, Yonemura S, Kiyono T, Izawa I, Inagaki M (2012) Trichoplein and Aurora A block aberrant primary cilia assembly in proliferating cells. J Cell Biol 197(3):391–405
Ishikawa H, Marshall WF (2011) Ciliogenesis: building the cell’s antenna. Nat Rev Mol Cell Biol 12:222–234
Ishikawa A, Tsuji S (1996) Clinical analysis of 17 patients in 12 Japanese families with autosomal-recessive type juvenile parkinsonism. Neurology 47:160–166
Izzi L, Attisano L (2004) Regulation of the TGFβ signaling pathway by ubiquitin-mediated degradation. Oncogene 23:2071–2078
Jechlinger M, Sommer A, Moriggl R et al (2006) Autocrine PDGFR signaling promotes mammary cancer metastasis. J Clin Invest 116:1561–1570
Jenkins S, Wang J, Eltoum I, Desmond R, Lamartiniere CA (2011) Chronic oral exposure to bisphenol A results in a nonmonotonic dose response in mammary carcinogenesis and metastasis in MMTV-erbB2 mice. Environ Health Perspect 119:1604–1609
Jeseta M, Petr J, Krejcova T, Chmelikova E, Jilek F (2008) In vitro ageing of pig oocytes: effects of the histone deacetylase inhibitor trichostatin A. Zygote 16:145–152
Kadavath H, Hofele RV, Biernat J, Kumar S, Tepper K, Urlaub H, Mandelkow E, Zweckstetter M (2015) Tau stabilizes microtubules by binding at the interface between tubulin heterodimers. Proc Natl Acad Sci USA 112:7501–7506
Kammerer S, Roth RB, Hoyal CR, Reneland R, Marnellos G, Kiechle M, Schwarz-Boeger U, Griffiths LR, Ebner F, Rehbock J, Cantor CR, Nelson MR, Brown A (2005) Association of the NuMA region on chromosome 11q13 with breast cancer susceptibility. Proc Natl Acad Sci USA 102(6):2004–2009
Karanikolas B, Sütterlin C (2012) Functional associations between the Golgi apparatus and the centrosome in mammalian cells. In: Schatten H (ed) The centrosome, Chap. 7. Springer, New York
Katsanis N (2004) The oligogenic properties of Bardet–Biedl syndrome. Hum Mol Genet 13:R65–R71
Keri RA, Ho SM, Hunt PA, Knudsen KE, Soto AM et al (2007) An evaluation of evidence for the carcinogenic activity of bisphenol A. Reprod Toxicol 24:240–252
Kikuchi K, Naito K, Noguchi J, Shimada A, Kaneko H, Yamashita M, Aoki F, Tojo H, Toyoda Y (2000) Maturation/M-phase promoting factor: a regulator of aging in porcine oocytes. Biol Reprod 63:715–722
Kikuchi K, Naito K, Noguchi J, Kaneko H, Tojo H (2002) Maturation/M-phase promoting factor regulates aging of porcine oocytes matured in vitro. Cloning Stem Cells 4:211–222
Kim NH, Moon SJ, Prather RS, Day BN (1996) Cytoskeletal alteration in aged porcine oocytes and parthenogenesis. Mol Reprod Dev 43:513–518
Kim JC et al (2004) The Bardet–Biedl protein BBS4 targets cargo to the pericentriolar region and is required for microtubule anchoring and cell cycle progression. Nat Genet 36:462–470
Kitada T et al (1998) Mutations in the parkin gene cause autosomal recessive juvenile parkinsonism. Nature 392:605–608
Kobayashi T, Dynlacht BD (2011) Regulating the transition from centriole to basal body. J Cell Biol 193:435–444
Korzeniewski N, Duensing S (2012) Disruption of centrosome duplication control and induction of mitotic instability by the high-risk human papillomavirus oncoproteins E6 and E7. In: Schatten H (ed) The centrosome, Chap, 12. Springer, New York
Korzeniewski N, Wheeler S, Chatterjee P et al (2010) A novel role of the aryl hydrocarbon receptor (AhR) in centrosome amplification—implications for chemoprevention. Mol Cancer 9:153
Krämer A, Maier B, Bartek J (2011) Centrosome clustering and chromosomal (in)stability: a matter of life and death. Mol Oncol 5:324–335
Krämer A, Anderhub S, Maier B (2012) Mechanisms and consequences of centrosome clustering in cancer cells. In: Schatten H (ed) The centrosome, Chap, 17. Springer, New York
Kulaga HM et al (2004) Loss of BBS proteins causes anosmia in humans and defects in olfactory cilia structure and function in the mouse. Nat Genet 36:994–998
Leber B, Maier B, Fuchs F, Chi J, Riffel P, Anderhub S, Wagner L, Ho AD, Salisbury JL, Boutros M, Krämer A (2010) Proteins required for centrosome clustering in cancer cells. Sci Transl Med 2(33ra38):1–11
Lee JH, Campbell KH (2008) Caffeine treatment prevents age-related changes in ovine oocytes and increases cell numbers in blastocysts produced by somatic cell nuclear transfer. Cloning Stem Cells 10:381–390
Levy YY, Lai EY, Remillard SP, Heintzelman MB, Fulton C (1996) Centrin is a conserved protein that forms diverse associations with centrioles and MTOCs in Naegleria and other organisms. Cell Motil Cytoskeleton 33:298–323
Li Y, Hu J (2015) Small GTPases act as cellular switches in the context of Cilia. In: Schatten H (ed) The cytoskeleton in health and disease. Springer, New York
Li Y, Lu W, Chen D, Boohaker RJ, Zhai L, Padmalayam I, Wennerberg K, Xu B, Zhang W (2015) KIFC1 is a novel potential therapeutic target for breast cancer. Cancer Biol Ther 16:1316–1322
Liang CG, Su YQ, Fan HY, Schatten H, Sun QY (2007) Mechanisms regulating oocyte meiotic resumption: roles of mitogen-activated protein kinase. Mol Endocrinol 21(9):2037–2055
Ling H, Peng L, Seto E, Fukasawa K (2012) Suppression of centrosome duplication and amplification by deacetylases. Cell Cycle 11:3779–3791
Lingle WL, Salisbury JL (1999) Altered centrosome structure is associated with abnormal mitoses in human breast tumors. Am J Pathol 155:1941–1951
Lingle WL, Salisbury JL (2000) The role of the centrosome in the development of malignant tumors. Curr Top Dev Biol 49:313–329
Liu S, Ginestier C, Ou SJ et al (2011) Breast cancer stem cells are regulated by mesenchymal stem cells through cytokine networks. Cancer Res 71(2):614–624
Lutz W, Lingle WL, McCormick D, Greenwood TM, Salisbury JL (2001) Phosphorylation of centrin during the cell cycle and its role in centriole separation preceding centrosome duplication. J Biol Chem 276:20774–20780
Ly DH, Lockhart DJ, Lerner RA, Schultz PG (2000) Mitotic misregulation and human aging. Science 287:2486–2492
Manandhar G, Schatten H, Sutovsky P (2005) Centrosome reduction during gametogenesis and its significance. Biol Repro 72:2–13
Marchetti F, Mailhes JB, Bairnsfather L, Nandy I, London SN (1996) Dose-response study and threshold estimation of griseofulvin induced aneuploidy during female mouse meiosis I and II. Mutagenesis 11:195–200
Maro B, Howlett SK, Webb M (1985) Non-spindle microtubule organizing centers in metaphase II-arrested mouse oocytes. J Cell Biol 101:1665–1672
Mazia D, Harris PJ, Bibring T (1960) The multiplicity of the mitotic centers and the time-course of their duplication and separation. J Biophys Biochem Cytol 7:l–20
Meng W, Mushika Y, Ichii T, Takeichi M (2008) Anchorage of microtubule minus ends to adherens junctions regulates epithelial cell-cell contacts. Cell 135:948–959
Mennella V, Agard DA, Huang B, Laurence Pelletier L (2013) Amorphous no more: subdiffraction view of the pericentriolar material architecture. Trends Cell Biol 1–10
Merdes A, Cleveland DA (1998) The role of NuMA in the interphase nucleus. J Cell Sci 111:71–79
Miao YL, Kikuchi K, Sun QY, Schatten H (2009a) Oocyte aging: cellular and molecular changes, developmental potential and reversal possibility. Human Reprod Update 15(5):573–585
Miao YL, Sun Q-Y, Zhang X, Zhao JG, Zhao MT, Spate L, Prather RS, Schatten H (2009b) Centrosome abnormalities during porcine oocyte aging. Environ Mol Mutagen 50(8):666–671
Miao Y-L, Zhang X, Zhao JG, Spate L, Zhao MT, Murphy CN, Prather RS, Sun Q-Y, Schatten H (2012) Effects of griseofulvin on in vitro porcine oocyte maturation and embryo development. Environ Mol Mutagen 53(7):561–566. https://doi.org/10.1002/em.21717
Michaud EJ, Yoder BK (2006) The primary cilium in cell signaling and cancer. Cancer Res 66:6463–6467
Mittal K, Choi DH, Klimov S, Pawar S, Kaur R, Mitra AK, Gupta MV, Sams R, Cantuaria G, Rida PCG, Aneja R (2016) A centrosome clustering protein, KIFC1, predicts aggressive disease course in serous ovarian adenocarcinomas. J Ovar Res 9:17:1–11
Mogensen MM (2004) Microtubule organizing centers in polarized epithelial cells. In: Nigg E (ed) Centrosomes in development and disease. Wiley, Weinheim, pp 299–319
Mogensen MM, Malik A, Piel M, Bouckson-Castaing V, Bornens M (2000) Microtubule minus-end anchorage at centrosomal and non-centrosomal sites: the role of ninein. J Cell Sci 113:3013–3023
Moritz M, Agard DA (2001) Gamma-tubulin complexes and microtubule nucleation. Curr Opin Struct Biol 11:174–181
Moritz M, Zheng Y, Alberts BM, Oegema K (1998) Recruitment of the g-tubulin ring complex to Drosophila salt stripped centrosomes. J Cell Biol 142:775e786
Muroyama A, Lechler T (2017) Microtubule organization, dynamics and functions in differentiated cells. Development 144:3012–3021. https://doi.org/10.1242/dev.153171
Muroyama A, Seldin L, Lechler T (2016) Divergent regulation of functionally distinct gamma-tubulin complexes during differentiation. J Cell Biol 213:679–692
Müsch A (2004) Microtubule organization and function in epithelial cells. Traffic 5:1–9
Nauli SM et al (2003) Polycystins 1 and 2 mediate mechanosensation in the primary cilium of kidney cells. Nature Genet 33:129–137
Nigg EA (2002) Centrosome aberrations: cause or consequence of cancer progression? Nat Rev Cancer 2:815–825
Nigg EA, Raff JW (2009) Centrioles, centrosomes, and cilia in health and disease. Cell 139:663–678
Nussbaum RL, Ellis CE (2003) Alzheimer’s disease and Parkinson’s disease. N Engl J Med 348:1356–1364
Oddoux S, Zaal KJ, Tate V, Kenea A, Nandkeolyar SA, Reid E, Liu W, Ralston E (2013) Microtubules that form the stationary lattice of muscle fibers are dynamic and nucleated at Golgi elements. J Cell Biol 203:205–213. https://doi.org/10.1083/jcb.201304063
Pacchierotti F, Ranaldi R, Eichenlaub-Ritter U, Attia S, Adler ID (2008) Evaluation of aneugenic effects of bisphenol A in somatic and germ cells of the mouse. Mutat Res 651(1–2):64–70
Paintrand et al (1992) Centrosome organization their sensitivity and centriole architecture: to divalent cations. J Struct Biol 108:107e128
Pan J, Snell W (2007) The primary cilium: keeper of the key to cell division. Cell 129:1255–1257
Panda D, Rathinasamy K, Santra MK, Wilson L (2005) Kinetic suppression of microtubule dynamic instability by griseofulvin: implications for its possible use in the treatment of cancer. Proc Natl Acad Sci USA 102:9878–9883
Peters JM (2002) The anaphase-promoting complex: proteolysis in mitosis and beyond. Mol Cell 9:931–943
Pickart CM, Cohen RE (2004) Proteasomes and their kin: proteases in the machine age. Nature Rev Mol Cell Biol 5:177–187
Pihan GA et al (1998) Centrosome defects and genetic instability in malignant tumors. Cancer Res 58:3974–3985
Pihan GA, Wallace J, Zhou Y, Doxsey SJ (2003) Centrosome abnormalities and chromosome instability occur together in pre-invasive carcinomas. Cancer Res 63:1398–1404
Pimenta-Marques A, Bento I, Lopes CA, Duarte P, Jana SC, Bettencourt-Dias M (2016) A mechanism for the elimination of the female gamete centrosome in Drosophila melanogaster. Science 353:aaf4866
Prins GS, Ye SH, Birch L, Ho SM, Kannan K (2011) Serum bisphenol A pharmacokinetics and prostate neoplastic responses following oral and subcutaneous exposures in neonatal Sprague–Dawley rats. Reprod Toxicol 31:1–9
Prosser SL, Fry AM (2012) Regulation of the centrosome cycle by protein degradation. In: Schatten H (ed) The centrosome, Chap. 9. Springer, New York
Qiao J, Wang ZB, Feng HL, Miao YL, Wang Q, Yu Y, Wei YC, Yan J, Wang WH, Shen W, Sun SC, Schatten H, Sun QY (2014) The root of reduced fertility in aged women and possible therapeutic options: current status and future perspectives. Mol Aspects Med 38:54–85
Quarmby LM, Parker JDK (2005) Cilia and the cell cycle? J Cell Biol 169(5):707–710
Rausell F, Pertusa JF, Gomez-Piquer V, Hermenegildo C, Garcia-Perez MA, Cano A, Tarin JJ (2007) Beneficial effects of dithiothreitol on relative levels of glutathione S-transferase activity and thiols in oocytes, and cell number, DNA fragmentation and allocation at the blastocyst stage in the mouse. Mol Reprod Dev 74:860–869
Rebacz B, Larsen TO, Clausen MH, Ronnest MH, Loffler H, Ho AD, Krämer A (2007) Identification of griseofulvin as an inhibitor of centrosomal clustering in a phenotype-based screen. Cancer Res 67:6342–6350
Rios RM (2014) The centrosome–Golgi apparatus nexus. Phil Trans R Soc B 369:20130462. https://doi.org/10.1098/rstb.2013.0462
Rios RM, Sanchis A, Tassin AM, Fedriani C, Bornens M (2004) GMAP-210 recruits gamma-tubulin complexes to cis-Golgi membranes and is required for Golgi ribbon formation. Cell 118:323–335. https://doi.org/10.1016/j.cell.2004.07.012
Roll-Mecak A, McNally FJ (2010) Microtubule-severing enzymes. Curr Opin Cell Biol 22:96–103
Roth J, Yam GH, Fan J, Hirano K, Gaplovska-Kysela K, Le Fourn V, Guhl B, Santimaria R, Torossi T, Ziak M, Zuber C (2008) Protein quality control: the who’s who, the where’s and therapeutic escapes. Histochem Cell Biol 129:163–177
Saladino C, Bourke E, Morrison CG (2012) Centrosomes, DNA damage and aneuploidy. In: Schatten H (ed) The centrosome, Chap. 13. Springer, New York
Salisbury JL (1995) Centrin, centrosomes, and mitotic spindle poles. Curr Opin Cell Biol 7:39–45
Salisbury JL (2004) Centrosomes: Sfi1p and centrin unravel a structural riddle. Curr Biol 14:R27–R29
Salisbury JL, Suino KM, Busby R, Springett M (2002) Centrin-2 is required for centriole duplication in mammalian cells. Curr Biol 12:1287–1292
Sathasivam K et al (2001) Centrosome disorganization in fibroblast cultures derived from R6/2 Huntington’s disease (HD) transgenic mice and HD patients. Hum Mol Genet 10:2425–2435
Satir P, Christensen ST (2008) Structure and function of mammalian cilia. Histochem Cell Biol 129:687–693
Schatten H (1977) Untersuchungen über die Wirkung von Griseofulvin in Seeigeleiern und in Mammalierzellen. Universität Heidelberg; (effects of griseofulvin on sea urchin eggs and on mammalian cells. University of Heidelberg)
Schatten H (1994) Dithiothreitol prevents membrane fusion but not centrosome or microtubule organization during the first cell cycles in sea urchins. Cell Motil Cytoskel 27:59–68
Schatten H (2008) The mammalian centrosome and its functional significance. Histochem Cell Biol 129:667–686
Schatten H (2013) The Impact of Centrosome Abnormalities on Breast Cancer Development and Progression with a Focus on Targeting Centrosomes for Breast Cancer Therapy. Chapter 12. In: Cell and Molecular Biology of Breast Cancer. Edited by Heide Schatten, published by Springer Science and Business Media, LLC
Schatten H (2014) The role of centrosomes in cancer stem cell functions. In: Schatten H (ed) Cell and molecular biology and imaging of stem cells, first edition. Wiley, USA, chap 12, pp 259–279
Schatten H, Chakrabarti A (2004) Detection of centrosome structure in fertilized and artificially activated sea urchin eggs using immunofluorescence microscopy and isolation of centrosomes followed by structural characterization with field emission scanning electron microscopy. In: Schatten H (ed) Methods in molecular biology, vol 253: germ cell protocols: vol 1 sperm and oocyte analysis. Humana Press Inc, Totowa, pp 151–164
Schatten H, Ripple M (2018) The impact of centrosome pathologies on prostate cancer development and progression. In: Schatten H (ed) Cell and molecular biology of prostate cancer: updates, insights and new frontiers. Springer, New York
Schatten H, Sun QY (2010) The role of centrosomes in fertilization, cell division and establishment of asymmetry during embryo development. Semin Cell Dev Biol 21:174–184
Schatten H, Sun QY (2011a) Centrosome dynamics during meiotic spindle formation in oocyte maturation. Mol Reprod Dev 78:757–768
Schatten H, Sun QY (2011b) New insights into the role of centrosomes in mammalian fertilisation and implications for ART. Reproduction 142:793–801
Schatten H, Sun QY (2011c) The significant role of centrosomes in stem cell division and differentiation. Microsc Microanal 17(4):506–512
Schatten H, Sun Q-Y (2012) Nuclear-centrosome relationships during fertilization, cell division, embryo development, and in somatic cell nuclear transfer (SCNT) embryos. In: Schatten H (ed) The centrosome. Springer Science and Business Media, LLC
Schatten H, Sun QY (2013) Chromosome behavior and spindle formation in mammalian oocytes. In: Trounson (ed) Biology and pathology of the oocyte. Gosden & Eichenlaub-Ritter: Biology & Pathology of the Oocyte 2nd Edition. Cambridge University Press, New York
Schatten H, Sun QY (2014) Posttranslationally modified tubulins and other cytoskeletal proteins: Their role in gametogenesis, oocyte maturation, fertilization and pre-implantation embryo development. In Sutovsky P (ed) Posttranslational protein modifications in the reproductive system. Springer, New York
Schatten H, Sun QY (2015a) Centrosome and microtubule functions and dysfunctions in meiosis: implications for age-related infertility and developmental disorders. Reprod Fertil Dev 27(6):934–943. https://doi.org/10.1071/RD14493
Schatten H, Sun Q-Y (2015b) Centrosome-microtubule interactions in health, disease, and disorders. In: Schatten H (ed) The cytoskeleton in health and disease. Springer, New York
Schatten H, Sun QY (2017) Cytoskeletal functions, defects, and dysfunctions affecting human fertilization and embryo development. In: Schatten H (ed) Human reproduction: updates and new horizons. Wiley, Hoboken
Schatten G, Schatten H, Bestor T, Balczon R (1982a) Taxol inhibits the nuclear movements during fertilization and induces asters in unfertilized sea urchin eggs. J Cell Biol 94:455–465
Schatten H, Schatten G, Petzelt C, Mazia D (1982b) Effects of griseofulvin on fertilization and early development of sea urchins. Independence of DNA synthesis, chromosome condensation, and cytokinesis cycles from microtubule-mediated events. Eur J Cell Biol 27:74–87
Schatten G, Simerly C, Schatten H (1985) Microtubule configurations during fertilization, mitosis and early development in the mouse and the requirement for egg microtubule-mediated motility during mammalian fertilization. Proc Natl Acad Sci USA 82:4152–4156
Schatten H, Schatten G, Mazia D, Balczon R, Simerly C (1986) Behavior of centrosomes during fertilization and cell division in mouse oocytes and in sea urchin eggs. Proc Natl Acad Sci USA 83:105–109
Schatten H, Walter M, Mazia D, Biessmann H, Paweletz N, Coffe G, Schatten G (1987) Centrosome detection in sea urchin eggs with a monoclonal antibody against Drosophila intermediate filament proteins: characterization of stages of the division cycle of centrosomes. Proc Natl Acad Sci USA 84:8488–8492
Schatten G, Simerly C, Asai DJ, Szöke E, Cooke P, Schatten H (1988) Acetylated α-tubulin in microtubules during mouse fertilization and early development. Dev Biol 130:74–86
Schatten H, Walter M, Biessmann H, Schatten G (1992) Activation of maternal centrosomes in unfertilized sea urchin eggs. Cell Motil Cytoskel 23:61–70
Schatten H, Chakrabarti A, Hedrick J (1999) Centrosome and microtubule instability in cells during aging. J Cell Biochem 74:229–241
Schatten H, Hueser CN, Chakrabarti A (2000a) From fertilization to cancer: the role of centrosomes in the union and separation of genomic material. Microsc Res Tech 49:420–427
Schatten H, Ripple M, Balczon R, Weindruch R, Taylor M (2000b) Androgen and taxol cause cell type specific alterations of centrosome and DNA organization in androgen-responsive LNCaP and androgen-independent prostate cancer cells. J Cell Biochem 76:463–477
Schatten H, Wiedemeier A, Taylor M, Lubahn D, Greenberg MN, Besch-Williford C, Rosenfeld C, Day K, Ripple M (2000c) Centrosome-centriole abnormalities are markers for abnormal cell divisions and cancer in the transgenic adenocarcinoma mouse prostate (TRAMP) model. Biol Cell 92:331–340
Schatten H, Hueser C, Chakrabarti A (2000d) Centrosome alterations induced by formamide cause abnormal spindle pole formations. Cell Biol Internat 24(9):611–620
Schatten H, Rawe VY, Sun QY (2012) Cytoskeletal architecture of human oocytes with focus on centrosomes and their significant role in fertilization. In: Nagy ZP, Varghese AC, Agarwal A (eds) Practical manual of in vitro fertilization: advanced methods and novel devices. Humana Press (Springer), New York
Schiff PB, Fant J, Horwitz SB (1979) Promotion of microtubule assembly in vitro by taxol. Nature 277:665–667
Schnackenberg BJ, Palazzo RE (1999) Identification and function of the centrosome centromatrix. Biol Cell 91(6):429–438
Schnackenberg BJ, Hull DR, Balczon RD, Palazzo RE (2000) Reconstitution of microtubule nucleation potential in centrosomes isolated from Spisula solidissima oocytes. J Cell Sci 113(Pt 6):943–953
Schneider L, Clement CA, Teilmann SC et al (2005) PDGFR alpha signaling is regulated through the primary cilium in fibroblasts. Curr Biol 15:1861–1866
Schoffski P (2009) Polo-like kinase (PLK) inhibitors in preclinical and early clinical development in oncology. Oncologist 14:559–570
Sen GL, Reuter JA, Webster DE, Zhu L, Khavari PA (2010) DNMT1 maintains progenitor function in self-renewing somatic tissue. Nature 463:563–567
Sharma N, Berbari NF, Yoder BK (2008) Ciliary dysfunction in developmental abnormalities and diseases. Curr Top Dev Biol (85):371–427
Shimura H et al (2000) Familial Parkinson disease gene product, parkin, is a ubiquitin-protein ligase. Nat Genet 25:302–305
Sluder G, Begg DA (1985) Experimental analysis of the reproduction of spindle poles. J Cell Sci 76:35–51
Ślusarz A, Shenouda NS, Sakla MS, Drenkhahn SK, Narula AS, MacDonald RS, Besch-Williford CL, Lubahn DB (2010) Common botanical compounds inhibit the hedgehog signaling pathway in prostate cancer. Cancer Res 70(8):3382–3390
Son Y, Brady ST (2015) Post-translational modifications of tubulin: pathways to functional diversity of microtubules. Trends Cell Biol 25:125–136
Stinchcombe JC, Griffiths GM (2014) Communication, the centrosome and the immunological synapse. Philos Trans R Soc Lond B Biol Sci 369
Sun QY, Schatten H (2006) Multiple roles of NuMA in vertebrate cells: review of an intriguing multifunctional protein. Front Biosci 11:1137–1146
Sun Q-Y, Schatten H (2007) Centrosome inheritance after fertilization and nuclear transfer in mammals. In: Sutovsky P (ed) Somatic cell nuclear transfer, Landes Bioscience. Adv Exp Med Biol 591:58–71
Sütterlin C, Colanzi A (2010) The Golgi and the centrosome: building a functional partnership. J Cell Biol 188(5):621–628
Takahashi H et al (1994) Familial juvenile parkinsonism: clinical and pathologic study in a family. Neurology 44:437–441
Takahashi M, Shibata H, Shimakawa M, Miyamoto M, Mukai H, Ono Y (1999) Characterization of a novel giant scaffolding protein, CG-NAP, that anchors multiple signaling enzymes to centrosome and the Golgi apparatus. J Biol Chem 274:17:267–217 274. https://doi.org/10.1074/jbc.274.24.17267
Tang N, Marshall WF (2012) Centrosome positioning in vertebrate development. J Cell Sci 125:4951e4961
Tang WY, Morey LM, Cheung YY, Birch L, Prins GS et al (2012) Neonatal exposure to estradiol/bisphenol A alters promoter methylation and expression of Nsbp1 and Hpcal1 genes and transcriptional programs of Dnmt3a/b and Mbd2/4 in the rat prostate gland throughout life. Endocrinology 153:42–55
Tarapore P, Ying J, Ouyang B, Burke B, Bracken B, Ho S-M (2014) Exposure to bisphenol A correlates with early-onset prostate cancer and promotes centrosome amplification and anchorage-independent growth in vitro. PLoS One 9(3):e90332. https://doi.org/10.1371/journal.pone.0090332
Tarin JJ, Ten J, Vendrell FJ, Cano A (1998) Dithiothreitol prevents age-associated decrease in oocyte/conceptus viability in vitro. Hum Reprod 13:381–386
Tatone C, Carbone MC, Gallo R, Delle Monache S, Di Cola M, Alesse E, Amicarelli F (2006) Age-associated changes in mouse oocytes during postovulatory in vitro culture: possible role for meiotic kinases and survival factor BCL2. Biol Reprod 74:395–402
Thyberg J, Moskalewski S (1999) Role of microtubules in the organization of the Golgi complex. Exp Cell Res 246:263–279. https://doi.org/10.1006/excr.1998.4326
Tian XC, Lonergan P, Jeong BS, Evans AC, Yang X (2002) Association of MPF, MAPK, and nuclear progression dynamics during activation of young and aged bovine oocytes. Mol Reprod Dev 62:132–138
Uen YH, Liu DZ, Weng MS, Ho YS, Lin SY (2007) NF-kappaB pathway is involved in griseofulvin-induced G2/M arrest and apoptosis in HL-60 cells. J Cell Biochem 101(5):1165–1175
Valenstein ML, Roll-Mecak A (2016) Graded control of microtubule severing by tubulin glutamylation. Cell 164:911–921
Van Beneden E (1876) Contribution al’histoire de la vesiculaire germinative et du premier embryonnaire. Bull Acad R Belg 42:35–97
Veland IR, Awan A, Pedersen LB, Yoder BK, Christensen ST (2009) Primary cilia and signaling pathways in mammalian development, health and disease. Nephron Physiol 111:39–53
Verde I, Pahlke G, Salanova M, Zhang G, Wang S, Coletti D, Onuffer J, Jin SL, Conti M (2001) Myomegalin is a novel protein of the Golgi/centrosome that interacts with a cyclic nucleotide phosphodiesterase. J Biol Chem 276:11189–11198. https://doi.org/10.1074/jbc.M006546200
Walsh CA (1999) Genetic malformations of the human cerebral cortex. Neuron 23:19–29
Wang Z, Wu T, Shi L, Zhang L, Zheng W, Qu JY, Niu R, Qi RZ (2010) Conserved motif of CDK5RAP2 mediates its localization to centrosomes and the Golgi complex. J Biol Chem 285:22658–22665. https://doi.org/10.1074/jbc.M110.105965
Wang ZB, Schatten H, Sun QY (2011) Why is chromosome segregation error in oocytes increased with maternal. aging? Physiology 26(5):314–325
Wehland J, Herzog W, Weber K (1977) Interaction of griseofulvin with microtubules, microtubule protein and tubulin. J Mol Biol 111:329–342
Wheatley DN, Wang AM, Strugnell GE (1996) Expression of primary cilia in mammalian cells. Cell Biol Int 20:73–81
Wigley WC et al (1999) Dynamic association of proteasomal machinery with the centrosome. J Cell Biol 145:481–490
Wilkinson CJ, Andersen JS, Mann M, Nigg EA (2004) A proteomic approach to the inventory of the human centrosome. In: Nigg E (ed) Centrosomes in development and disease. Wiley, Weinheim, pp 125–142
Wojcik C, DeMartino GN (2003) Intracellular localization of proteasomes. Int J Biochem Cell Biol 35:579–589
Wojcik C, Schroeter D, Wilk S, Lamprecht J, Paweletz N (1996) Ubiquitin-mediated proteolysis centers in HeLa cells: indication from studies of an inhibitor of the chymotrypsin-like activity of the proteasome. Eur J Cell Biol 71:311–318
Woodruff JB, Wueseke O, Hyman AA (2014) Pericentriolar material structure and dynamics. Philos Trans R Soc Lond B Biol Sci 369
Wueseke O, Bunkenborg J, Hein MY, Zinke A, Viscardi V, Woodruff JB et al (2014) The Caenorhabditis elegans pericentriolar material components SPD-2 and SPD-5 are monomeric in the cytoplasm before incorporation into the PCM matrix. Mol Biol Cell 25:2984e2992
Wynshaw-Boris A, Gambello MJ (2001) LIS1 and dynein motor function in neuronal migration and development. Genes Dev 15:639–651
Xiao Y-X, Yang W-X (2016) KIFC1: a promising chemotherapy target for cancer treatment? Oncotarget 7(30):48656–48670
Xu Z, Abbott A, Kopf GS, Schultz RM, Ducibella T (1997) Spontaneous activation of ovulated mouse eggs: time-dependent effects on M-phase exit, cortical granule exocytosis, maternal messenger ribonucleic acid recruitment, and inositol 1,4,5-trisphosphate sensitivity. Biol Reprod 57:743–750
Xu X et al (1999) Centrosome amplification and a defective G2-M cell cycle checkpoint induce genetic instability in BRCA1 exon 11 isoform-deficient cells. Mol Cell 3:389–395
Yadav S, Puri S, Linstedt AD (2009) A primary role for Golgi positioning in directed secretion, cell polarity, wound healing. Mol Biol Cell 20:1728–1736. https://doi.org/10.1091/mbc.E08-10-1077
Yadav S, Puthenveedu MA, Linstedt AD (2012) Golgin160 recruits the dynein motor to position the Golgi apparatus. Dev Cell 23:153–165. https://doi.org/10.1016/j.devcel.2012.05.023
Yang R, Feldman JL (2015) SPD-2/CEP192 and CDK are limiting for microtubule-organizing center function at the centrosome. Curr Biol 25:1924–1931
Yoder BK, Hou X, Guay-Woodford LM (2002) The polycystic kidney disease proteins, polycystin-1, polycystin-2, polaris, and cystin, are co-localized in renal cilia. J Am Soc Nephrol 13:2508–2516
Young A, Dictenberg JB, Purohit A, Tuft R, Doxsey S (2000) Cytoplasmic dynein-mediated assembly of pericentrin and γ tubulin onto centrosomes. Mol Biol Cell 11:2047–2056
Zhang X, Chen MH, Wu X, Kodani A, Fan J, Doan R, Ozawa M, Ma J, Yoshida N, Reiter JF et al (2016) Cell-type-specific alternative splicing governs cell fate in the developing cerebral cortex. Cell 166:1147–1162. e1115
Zhao J, Ren Y, Jiang Q, Feng J (2003) Parkin is recruited to the centrosome in response to inhibition of proteasomes. J Cell Sci 116:4011–4019
Zhu X, Kaverina I (2013) Golgi as an MTOC: making microtubules for its own good. Histochem Cell Biol 140:361–367. https://doi.org/10.1007/s00418-013-1119-4
Zilberman Y, Ballestrem C, Carramusa L, Mazitschek R, Khochbin S, Bershadsky A (2009) Regulation of microtubule dynamics by inhibition of the tubulin deacetylase HDAC6. J Cell Science 122:3531–3541
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Schatten, H., Sun, QY. Functions and dysfunctions of the mammalian centrosome in health, disorders, disease, and aging. Histochem Cell Biol 150, 303–325 (2018). https://doi.org/10.1007/s00418-018-1698-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00418-018-1698-1