Abstract
Humic substances (HS) affect most plant metabolic processes. Regardless of their source, HS help regulate enzymatic systems related to primary, secondary, and defense metabolisms in response to environmental stress. Morphologically, the HS–plant interaction results in increased root length and the emanation of lateral roots. These morphological changes occur in response to complex regulatory and stress response processes activated by the application of HS and similar chemical fractions. Given that the roots are the main plant organs that interact with HS, HS–root interaction mechanisms are one of the most important topics in HS–plant research. Specifically, there is a known biochemical relationship between humic compounds and major plant metabolic processes. New findings about the modes of metabolite action in plants have increased our understanding of how HS help to optimize plant metabolism. Advanced technologies, such as large-scale and spectroscopy, have also increased our understanding of the modes of action of HS. The application of techniques such as amplified fragment length polymorphism (AFLP) and microarray analysis in study of HS-treated plants has demonstrated that approximately 6.1–9 % of differentially expressed genes correspond to metabolic pathways that are associated with defense mechanisms in response to stimuli. These results suggest that HS induce plant adaptive responses to environmental stress. In this study, we discuss how HS contribute to improved plant performance through complex metabolic mechanisms. We apply new findings about the modes of action of metabolites related to antioxidant mechanisms to understand HS modes of action and examine HS effects in plants by using spectroscopic techniques to study root interactions. We also propose a framework for investigating the use of HS in agriculture to improve the growth of food plants grown in high-stress environments.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
Soil organic matter is one of the most frequently discussed soil science topics in the scientific literature. Humic substances (HS) are the humified organic matter fraction in soils and play a key role in various soil and plant functions. HS influence the adaptation of plants to conditions of environmental stress by increasing the nutrient availability. They also have a direct effect on plant metabolic processes related to growth and development.
The actions of HS, both in soil and plants, are directly related to their structural characteristics. Using simple dilution procedures, three HS fractions have been identified: (a) humic acids (HA), which are soluble in basic medium and precipitate in an acidic medium (pH ~ 2.5), (b) fulvic acids (FA), which are soluble in both basic and acidic media; and (c) humins, which are not extractable from soil or sediment either in a basic or an acidic medium (Schnitzer 1978).
The effects of HS on higher plants and their development can occur either indirectly (e.g., through increased soil fertility or reduced soil compaction) or directly (by increasing total biomass production) (Nardi et al. 2002, 2007). These authors reported that humic fractions that were smaller in size exhibited greater structural flexibility and had a greater effect on the Krebs cycle in maize. Muscolo et al. (2007) suggested that the positive effects of HS in plants were more closely related to the structural characteristics of the HS than to their molecular mass. Canellas et al. (2010) argued that the molecular size of HS was not the primary factor in the stimulation of root growth in maize; rather, the hydrophobicity index was reported to be the predominant factor (Dobbss et al. 2010).
Although there may be discrepancy about the mechanisms by which HS acts on plants, still most authors are in agreement about the positive effects of HS on plants and the link between HS and increased metabolic efficiency. Some recent studies have reported that HA stimulated a phenylpropanoid metabolic system in plants and induced phenyl (tyrosine) ammonia lyase (PAL/TAL) activity (Schiavon et al. 2010). There are also reports of HA having direct effects on the generation of radical oxygen species (ROS) and the activity of catalyst enzymes in maize (Cordeiro et al. 2011). Likewise, the protective effects of HS or HS-based biostimulants have been reported for plants growing under conditions of hydric and saline stress (Vasconcelos et al. 2009; Aydin et al. 2012; García et al. 2012a).
Overall, the studies show that HS cause changes in the physiological mechanisms and adaptive processes of plants under various environmental conditions. Understanding the relationships between HS and plant physiological processes allows us to explore new techniques for managing humified organic matter in ecosystems. In agricultural systems, for example, the application of HS could be an ecologically sustainable method for improving the production of crops and other food plants. In this chapter, the scientific basis for the use of HS in agriculture is discussed.
2 Characteristics and Effects of HS
HS are recognized as the most widely distributed components of organic matter on the planet, and they are present both in terrestrial and aquatic environments. They are formed through the chemical and biological degradation of plant and animal remains and by microbial activity (Schnitzer 1978). According to Schnitzer (1978), the formation of HS can be summarized as four hypotheses: (a) the plant transformation hypothesis, (b) the chemical polymerization hypothesis, (c) the cell autolysis hypothesis, and (d) the microbial synthesis hypothesis. It is not easy to determine which hypothesis is most valid, and it is possible that all four occur simultaneously; with soil conditions determining which process is dominant. According to Ghabbour and Davies (2001), the first HS hypothesis suggests that HS include an extraordinary amount of complex, amorphous, heterogeneous, and chemically reactive molecules that are produced during biomass decomposition due to the chemical reactions that occur randomly in a large pool of organic molecules. In 2012, Nebbioso and Piccolo (2012) proposed that HS are formed through a “humeomic” process in which heterogeneous molecules associate according to shape, size, chemical affinity, hydrophobicity, and structure. The formation of HS is limited by the strength of the interactions that stabilize the associations between molecules within its supramolecular structure.
Regardless of the specific biosynthesis mechanisms involved in the formation of HS, researchers agree that HS are formed in terrestrial environments by the decomposition of plant and animal material deposited in the soil. This decomposition process helps in explaining the chemical and structural heterogeneity of humified materials. Figure 11.1 shows the structural diversity of a humified solid fraction isolated from a vermicompost. Ligninic fragments, fatty acids, and nitrogenous compounds derived from plant and animal materials can be observed.
2.1 Structural Characteristics of HS, HS Fractions, and Their Effects
Although it is possible to isolate three basic HS fractions in soil (HA, FA, and HU), HA and FA have been the most frequently studied fractions in scientific publications for examining the functions of HS. The structural characteristics of these fractions vary according to their source material and the time of transformation or formation time of organic matter for that material. Canellas et al. (2012) compared humic fractions of vermicomposts with their synthetic derivatives isolated from various Brazilian soil types. They used 13C-MNR analysis to demonstrate how structure varies according to the source material of the fraction. The authors reported that a higher amount of polar and alkylic structures was present in the vermicompost fractions compared with their derivatives and that the content of these structures was similar to that of the fractions isolated from soils. The humic fractions isolated from oxisols were more hydrophobic and aromatic than those from other soils. Moreover, the humic fractions stimulated the emission of lateral roots and the activity of H+-ATPases in corn plants (Zea mays L.), regardless of the original source of the humic material. O-alkyl, methoxy/N-alkyl, and hydrophobicity index (HB/HI) structures explained 88 % of the effect on enzyme activity.
In the same study, the structural characteristics of HA from vermicomposts at different stages of maturation and the effect of HA on plants were also examined. During vermicomposting, HA had a lower amount of carbohydrate structures and the preservation of alkylic and acrylic structures were reduced. There were no changes in the molecular weight of the HA molecules; however, increases in hydrophobic structures occurred with increased maturation time of the vermicompost. HA from 60-day vermicompost had effects on the emergence of lateral roots and proton pump induction in the plants, in spite of the structural features of HA (Aguiar et al. 2013).
Studies that characterize humic fractions from different source materials are abundant in the literature. The high variability in the structural characteristics of HS has made molecular structure a benchmark for assessing the quality of organic matter and for monitoring changes in HS that occur during the soil management or composting. Table 11.1 shows some examples of humic fractions isolated from composted sources and soil. The characterization of HS using physicochemical techniques helps explain the evolution of organic matter over time and the effects of exogenous actions, such as fertilizer application and other management activities.
2.2 Structure-Function Relations in the Interaction of HS with Metallic Elements
Another very important property of HS related to structural characteristics is their ability to interact with metal ions. Interactions between HS and ions influence soil characteristics and fertility, thereby impacting plants. HS can form complex compounds with metal ions which have varying stabilities and chemical characteristics. Interactions between HS and metals affect soil properties for the reason that some nutrient availability processes and chemical forms of metallic elements are governed by HS. Therefore, important plant development processes such as precipitation-dissolution, ion exchange, mobility, transport and accumulation, and the chemical and biochemical activity of metals are largely determined by HS (Senesi et al. 1986). One important structural feature of HS that allows for their interaction with metal ions is their high number of oxygenated functional groups (CO2H2, OH phenols, C=O). The presence of these groups allows HS to establish more stable complex bonds than those present in the soil, making metallic elements more available (Schnitzer 1978). With heavy metals, for example, HS form complex compounds in the following order of stability: Pb2+ > Cu2+ > Ni2+ > Co2+ > Zn2+ > Cd2+ > Fe2+ > Mn2+ > Mg2+ (Irving and Williams 1953).
Several studies have reported the interaction of different humic fractions with metallic elements. HA have been demonstrated to form complex compounds with Al3+ ions in soils (Gerke 1994). FA have a high capacity to form bonds with Cu2+ and Ca2+ (Iglesias et al. 2003), and HA and HU are able to form complex compounds with Cu2+ in the soil (Plaza et al. 2005; Alvarez-Puebla et al. 2004). Lead is one of the most widely studied elements with regard to the formation of complex compounds with HS (Filella and Town 2001). Some of these studies have led to the proposal of new mechanisms to explain the interaction of HS with metal ions, as shown in Fig. 11.2.
3 Interactions Between Plant Root Systems and HS
The interactions that occur between HS and plant root systems are of great importance for understanding the modes of action of HS. Two fundamental issues are important for the studies of the HS–root relationship. The first issue is about understanding what happens in plant’s environment where HS are present, and the second issue is related to the fact that most experiments examining the effects of HS in plants use root application methods. HS fractions interact directly with root structures. Studies of HA and FA marked with 14C isotopes have shown that these HS fractions are associated in greater quantities with the cell wall within the first few hours of HS–root interaction (3 h) and subsequently (18 h) become part of the soluble component of the cells. Of the different HS fractions, HA are associated in higher numbers with the cell wall at the roots, while more FA are incorporated into the cell (Vaughan and Ord 1981). Observing HS–root interactions allow us to understand how these substances are assimilated and how they affect plant processes at the leaf level. Experiments with wheat plants have shown that of the total 14C associated with HS that is assimilated by plants through roots; only 5 % is transported to leaf tissues (Vaugham and Linehan 1976).
Additionally, with regard to HS–root interactions, there are reports in the literature of physical interactions (specifically with HA) that change the functionality of cell membranes. HA supramolecular colloidal clusters in solution can migrate to the surface of the roots and cause the clogging of pores and root transpiration sites. These HA–root interactions and the formation of layers of agglomerates may be governed by electrostatic and van der Waal interactions. This phenomenon causes reductions in hydraulic conductivity, leaf organ growth, transpiration, and resistance to hydric stress and the mechanism of action is known as colloidal stress (Asli and Neumann 2010).
Evidence of the agglomeration phenomenon of HA in roots was recently captured by light microscopy, as shown in Fig. 11.3. HA–root interactions have also been demonstrated using 13C-NMR spectroscopy, which showed that HA that formed agglomerates on root surfaces had lower structural complexity than exogenously applied HA. At the same time, it was determined that these HA interaction and agglomeration events are detected by ROS generation mechanism in the leaf and root tissues, triggering the activity of antioxidative metabolic enzymes. For the first time, it was observed that this type of interaction could be related to the modes of action of HS in plants and that growth and development could be controlled through the regulation of REDOX homeostasis and other metabolic processes that are stimulated by HS (García et al. 2012b).
3.1 ROS and the Process of Root Growth and Development
In recent years, the involvement of ROS in metabolic processes associated with plant growth and development has been reported (Foreman et al. 2003; Marino et al. 2012). In contrast to what was previously thought, ROS can regulate root growth processes through independent pathways to phytohormones such as auxins. Studies conducted on Arabidopsis roots identified the transcription factor UPBEAT1 (UPB1), which regulates the balance between cell proliferation and differentiation. It was found that UBP1 directly regulates a series of peroxidases (POX) that modulate the quantity of ROS in regions of cell proliferation and elongation as soon as differentiation processes begin. UPB1 disruption results in a change in ROS balance and a delay in the root differentiation process (Tsukagoshi et al. 2010). ROS production in plant roots has also been observed when HA is applied to the roots, as demonstrated in Fig. 11.4.
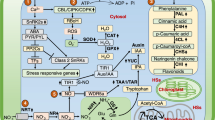
Additionally, it is now understood that signaling enzymes such as NADHP oxidases and phospholipases D are of great importance in the formation of root hairs because ROS produced by NADHP oxidases activate Ca2+ channels in the apical plasma membrane, stimulating Ca2+ influxes which are linked to lateral root growth (Šamaj et al. 2004). Knowledge about the involvement of ROS in membrane permeability and polarization processes opens new avenues for understanding the mechanisms of plant adaptation in high-stress environments. Plant signaling mechanisms in which ROS target the activation of Ca2+ channels in cell membranes have been reported (Kurusu et al. 2013). This is one of the most important ROS-mediated steps in the regulation of plant stress, hormonal signaling, polar growth, and development (Mori and Schroeder 2004). In ROS signaling processes, low OH concentrations induce Ca2+ pumping, while high OH concentrations induce Ca2+ incorporation through passive mechanisms (Zepeda-Jazo et al. 2011). With regard to apical meristem development, the maintenance and establishment of OH concentrations depend on the mechanisms that maintain ROS homeostasis. Oxidizing environments induce the reduction of cell proliferation, while less oxidizing (reducing) environments induce mitosis and cell differentiation (De Tullio et al. 2010) (Fig. 11.5).
Diagram showing a possible pathway for MPK3 induced by the action of extracellular ATP and ROS. Modified from Demidchik et al. (2009)
4 Antioxidative Response to HS in Plants
4.1 Functions, Characteristics, and Pathways of ROS Action in Plants
ROS (1O2, O2 •−, OH, and H2O2) were initially recognized in plants as toxic chemical species produced through aerobic metabolism (Mittler et al. 2011). However, in addition to being the by-products of antistress metabolism, they also have roles in signal transduction (Miller et al. 2010). Current studies show that ROS signaling functions are involved in most existing plant metabolic processes. ROS plays an important role in cellular transduction mechanisms that control metabolic processes such as growth regulation and development, biotic and abiotic stress responses, and cell death (Suzuki et al. 2012). Under conditions of homeostasis and stress, ROS are found at different concentrations in plant tissues. During normal growth processes, ROS content in plant cells is low (approximately 240 μM s−1 of O2 •− and 0.5 μM of H2O2). In contrast, in plants under stress, O2 •− content increases to 240–270 μM s−1 and H2O2 content increases to 5–15 μM (Polle 2001). The O2 •− anion is produced in the thylakoids and is the product of aerobic respiration, with approximately 1–2 % of O2 consumed by plants being transformed into O2 •−. O2 •− is one of the first ROS formed in plants, with a mean half-life of approximately 4.2 μs. H2O2 is formed by the reduction and dismutation of O2 •−. H2O2 has increasingly been recognized as the most important ROS signaling messenger due to its ability to cross cell membranes and its mean half-life (~1 ms) (Gill and Tuteja 2010; Mittler 2002).
Using ROS for signaling is advantageous for plants because plant cells have a high capacity for producing ROS and controlling internal ROS levels. A complex enzyme system, which is present in most cellular compartments, is used for this purpose. Recent studies have shown that Rbohs, a group of membrane proteins encoded by a family of ten genes in Arabidopsis (AtRboh), play a key role in ROS action and production mechanisms (Suzuki et al. 2012; Mittler 2002; Mittler et al. 2004). These findings are of great importance because Rbohs have numerous functions in plants and are known to regulate signaling mechanisms in response to abiotic stress (Kwak et al. 2003; Miller et al. 2009). Moreover, the role of Rbohs in Rboh-ROS regulation mechanisms, cell elongation processes, and root hair growth has been confirmed by Takeda et al. (2008).
4.2 ROS Generation in Response to HS
As previously discussed, the production of ROS in response to certain stimuli is clearly an efficient mechanism for regulating many metabolic pathways in plants. While there is currently a limited number of studies on plant ROS generation in response to HS, interest in HS–ROS relationship has persisted for several decades. Soil HS fractions (HA, FA, and water-soluble fractions) have been shown to stimulate the production of O2 •− anions in vitro. A study of the xanthine/xanthine-oxidase system showed that the level of O2 •− production depended on the humic fraction. FA and water-soluble fractions were the least effective in stimulating xanthine/xanthine-oxidase and the production of O2 •−. HA were the most effective fraction in stimulating the production of O2 •− (Vaughan and Ord 1982). The knowledge that HS stimulate O2 •− production through regulation of the xanthine/xanthine-oxidase system is important, given the key role that this system plays in defense mechanisms against biotic stressors (Berner and Van der Westhuizen 2010) and abiotic stress from heavy metals (Corpas et al. 2008).
The production of ROS following the application of HA was also observed in maize (Zea mays L.). HA isolated from soil were applied to the roots of maize plants grown with high and low N-NO3 − concentrations. Using fluorescence techniques, the presence of ROS was detected in the roots of plants grown under three different growth conditions (low N-NO3 −, high N-NO3 −, and no N-NO3 −). Under these conditions, the production of ROS following the application of HA did not impede the stimulation of lateral root growth and increased root biomass, suggesting that ROS production resulting from HA can act as an intermediary agent in the action processes of HS in plants (Cordeiro et al. 2011). The implications of these results led us to reconsider our understanding of the action mechanisms of HS in plants. During the growth of maize coleoptiles, ROS are released in the cell walls (specifically, OH is formed from O2 •− on the cell wall), suggesting that ROS may increase the extensibility of the wall and replace auxins as growth inducers (Schopfer et al. 2002).
ROS production following the application of HA was also observed in rice plants (Oryza sativa L.) treated with HA from manure vermicompost. Root applications of HA at concentrations of 20, 40, and 80 mg L−1 regulated ROS (O2 •− and H2O2) production in the leaves and roots at 8 and 24 h of contact. The levels of O2 •− and H2O2 in both the leaves and roots were dependent on the concentration of HA. Although root application of HS induced ROS production in these studies, lipid peroxidation was low or nonexistent in some of the treatments, suggesting that the membrane peroxidation phenomenon was not the only result of HA-induced ROS. Another important result was found in the same study. Tonoplast aquaporin genes (OsTIPs) of the leaves and roots were also regulated by the application of HA, suggesting that ROS and OsTIPs are involved in the action mechanisms of HS inside the plant cells (García et al. 2012b). Therefore, it is possible that the production of ROS in plants due to the application of HS involves the regulation of TIPs in the cell and that there is a relationship with nitrogen metabolism. Figure 11.6 shows that for OsTIP2.1 isoform, more diluted or concentrated solutions of HS exerted a repressing effect on the expression of these genes in both the leaves and roots.
Although the role of TIPs in HS action in plants is not yet completely understood, it is known that the TIP2.1 isoforms are related to N-NH4 + transport. For example, some studies report that AtTIP2.1 isoforms are induced in plants under long periods of nitrogen deficiency and short periods of N-NH4 + supplementation (Lopez et al. 2003). The role of TIPs in ammonium transport has also been noted (Loque et al. 2005). The effects of HS on the regulation of aquaporins, especially tonoplast, are of great importance because aquaporins play various roles in metabolite transport at the cellular level and are regulated in response to conditions of abiotic stress, such as hydric stress and high salinity (Li et al. 2008).
The results of studies of HS action in plants have shown that ROS production, particularly the production of H2O2, is dependent on the concentration of HS. The specific nature of HS–root interactions seems to be the key for triggering the physiological events in plants. It has been observed that in rice plants treated with moderate HS concentrations, ROS production does not cause lipid peroxidation, thereby favoring the processes of growth and lateral root formation. However, when plants are treated with elevated concentrations of HS, a high rate of ROS production can lead to lipid peroxidation and negatively affect the growth and root development, as shown in Fig. 11.7.
4.3 Relationship of ROS with Other Metabolic Pathways That Respond to HS
Understanding HS-induced ROS production in plants requires knowledge of the most current concepts related to oxidative mechanisms in plants. Given the evidence that ROS production occurs after the application of HS to plant roots, the mechanisms underlying the physiological responses to HS must also be explained. At the biochemical level, one of the most widely studied modes of action of HS in plants is similar to that of auxins. Currently, a large number of researchers have recognized that HS enter the plants through root system, where they mimic auxins and are recognized by hormonal receptors in cells. Other studies have reported the fragments of HS with structures similar to auxins that enter plants and exert auxin-like effects (Nardi et al. 2002). Root growth and an increased number of secondary roots are the most visible morphological effects of these auxin-like effects of HS. Therefore, what is the role of ROS in the biochemical-physiological mechanisms of HS action in plants?
Although auxins play the most crucial role in the regulation of growth and root development, redox regulation also plays a relevant role. The formation and preservation of the root apical meristem require ROS homeostasis in plant tissues. Redox regulation controls biochemical processes along the root and regulates the activity of auxins that influence root growth (De Tullio et al. 2010). Transcriptome studies of Arabidopsis plants have shown that auxin signaling and homeostasis are modified by ROS. In the apoplast, ROS may regulate the transcripts of auxin receptors and auxin/indole-3-acetic acid (Aux/IAA) transcriptional repressors through mechanisms that remain unknown (Blomster et al. 2011).
Other biochemical effects that are reported to have occurred as the result of HS action in plants are related to stimuli at the membrane level, with several studies reporting the stimulation of H+-ATPase activity (Canellas et al. 2010; Mora et al. 2010). However, ROS have been shown to function as signaling molecules, acting through mechanisms that involve membrane hyperpolarization, activation of Ca2+ channels, and intracellular signaling to increase the growth of secondary roots. O2 •− anions produced outside cells can be transformed to H2O2 and OH radicals. H2O2, for example, is capable of crossing the membrane and accumulating intracellularly. Through mechanisms that are not yet understood, H2O2 can stimulate Ca2+ channels intra- or extracellularly. The stimulus required to open the Ca2+ channels can increase cytosolic Ca2+, stimulating NADPH oxidases and inducing MPK3 (Demidchik et al. 2009). This type of response also occurs in different regions of the root. In the region of elongation and mature root epidermis, high concentrations of H2O2 in the apoplast and the cytosol, respectively, can activate Ca2+ channels through membrane hyperpolarization (Demidchik et al. 2007), as demonstrated in Fig. 11.8.
Effects of ROS on Ca2+ channels in plasma membrane in the region of elongation and mature epidermis roots of Arabidopsis plants. Modified from Demidchik et al. (2007)
5 Other HS-Induced Responses in Plant Metabolism
The greatest impacts of HS action in plants are due to the ability of HS to stimulate various metabolic pathways. Photosynthesis is the fundamental metabolic process underlying the production of all O2 and organic matter on the planet; therefore, studies on the influence of HS in the regulation of photosynthesis are of great importance. To study the effects of HS on photosynthesis, three humic fractions (HA, FA, and HU) were isolated from soil samples and tested in Pachira macrocarpa plants. The three HS fractions stimulated the activity of chlorophyllases (a) and (b). FA showed greater stimulation of chlorophyllase (a) activity, while HA showed greater stimulation of chlorophyllase (b) activity (Yang et al. 2004). The effects of HS on photosynthetic processes were also shown in a study of the application of HA to lettuce (Lactuca sativa L.) plants. HA isolated from forest soils were applied to the roots in nutrient solution at concentrations of 100 and 1,000 mg L−1 and were found to stimulate the photosynthetic activity and augment the chlorophyll content and conductance of mesophyll cells (Haghighi et al. 2012).
Given the effects of HS on photosynthesis, we expected that carbon (C) metabolism would also be affected as a consequence of changes in photosynthetic activity and pigment content. Four HA fractions from soils were shown to stimulate the activity of enzymes related to C metabolism in maize plants (Zea mays L.). The four HU fractions stimulated the activity of enzymes belonging to the metabolism of glycolytic pathway (glucokinase (GK), phosphoglucoisomerase (PGI), PPi-dependent phosphofructokinase (PFK), pyruvate kinase (PK)) and Krebs cycle [citrate synthase (CS), malate dehydrogenase (MDH), and isocitrate and NADP+-isocitrate dehydrogenase (NADP+-IDH)] (Nardi et al. 2007). However, in some studies, HS have been shown to modify the activity of enzymes in C and N metabolism, depending on the source material of HS. In studies evaluating the effect of humic acids from forest soils and pasture on the activity of enzymes from C and N metabolism in Pinus nigra callus, forest-derived HA were found to inhibit PGI activity and inhibit phosphoenolpyruvate carboxykinase (PEPC), glutamate dehydrogenase (GDH), MDH, and glutamine synthetase (GS) activity. On the contrary, HA from pasture soils stimulated these enzymes (Muscolo et al. 2007).
Furthermore, any actions on primary metabolism in plants can have repercussions for secondary metabolism. The primary metabolites are precursors that induce the activity of secondary metabolism enzymes. The metabolic pathway of phenylpropanoid compost synthesis is one of the most specialized linkages between metabolic functions in plants. Phenylalanine ammonia lyase (PAL) and tyrosine ammonia lyase (TAL), the two enzymes that initiate phenylpropanoid biosynthesis, use phenylalanine and tyrosine, respectively, which are substrates derived from primary metabolism (Ferrer et al. 2008). HA from vermicompost have also been shown to exert effects on the synthetic pathway of phenylpropanoids through the regulation of PAL/TAL enzymes. In maize plants, humic acid concentrations of 0.5, 1.0, and 2 mg (C) L−1 stimulated the expression of genes encoded for PAL and the enzymatic activity of PAL and TAL. After 48 h of treatment, the levels of phenols and flavonoids in the plants increased in response to the stimulus exerted on the metabolic pathway (Schiavon et al. 2010).
6 Studies of the Effect of HS Using Large-Scale Gene Expression Techniques
Some studies have used large-scale genetic analysis techniques (LGAT) to show the complex effects which HS may exert on the function and adaptive mechanisms of plants. The LGATs that have been used to study the effects of HS in plants include cDNA-AFLP and microarray analysis. Trevisan et al. (2011) applied the cDNA-AFLP technique in the study of Arabidopsis plants treated with HS. A combination of 160 primers was used and a total of 133 genes were found to be involved in the effects exerted on the plants by HS. The cDNA-AFLP technique demonstrated that, of the numerous genes involved in the HS–plant interaction, many were related to metabolic and developmental processes, as well as RNA or transcription processes. The authors showed that of all upregulated transcripts following the application of HS, 34 % belonged to metabolic processes and 9 % to stress stimuli processes. The authors noted that using of cDNA-AFLP technique allowed them to confirm that HS affect plants through the complex action mechanisms which involve both auxin-like action and other signaling mechanisms independent of auxins.
In addition, the effects of the application of HA to Brassica napus plants have also been studied using microarray techniques. Some results about the function of the group of differentially expressed genes were similar to those reported in the study that used cDNA-AFLP technique. The results of the microarray analysis study showed that four fundamental metabolic pathways (fatty acids, phytohormones, senescence, and ion development and transport) were represented by a low number of differentially expressed genes. However, other metabolic pathways were more specifically affected by the action of humic acids both in the leaves and roots. Of the total number of differentially expressed genes, 10.6 % belonged to general cellular metabolism processes, 6.6 % to nitrogen and sulfur metabolism processes, 6.1 % to carbon and photosynthesis metabolism, and 6.1 % to stress response (Jannin et al. 2012).
LGATs are robust and highly accurate techniques. The studies described above used two different LGAT techniques, HS from different sources, and different plant species. Nevertheless, both studies found that HS effects were exerted not only through known traditional pathways, such as auxin type pathways, but also through other auxin-independent pathways. Genes that are involved in the functioning of other important pathways in plants, such as nitrogen uptake and photosynthesis, are expressed in the response of plants to HS application. However, both techniques revealed that a large number of genes or transcripts that respond to HS are related to stress response. These results corroborate the findings of recent reports demonstrating the generation of ROS in plants treated with HS (García et al. 2012b; Cordeiro et al. 2011).
7 Evidence of Protective Effects of HS in Plants Growing Under High-Stress Conditions
The diverse effects of HS in plants suggest that two distinct metabolic events control their modes of action. The regulation of REDOX homeostasis, which is related to cell signaling and hormonal control, is one of the most apparent results of the application of HS to plants. However, the mechanisms explaining the link between ROS and auxins in regulating antistress responses are still not well understood (Tognetti et al. 2012). Compounds such as nitric oxide (NO) have been shown to play an intermediary role in the action of HS in plants. Zandonadi et al. (2010) reported that humic acids from vermicompost stimulated NO biosynthesis in maize plants. The authors showed that humic acids stimulated NO production at lateral root emergence sites. The stimulation of NO biosynthesis by HS suggests that this mode of action is involved in homeostatic regulation. NO has antioxidant properties and acts as a signaling molecule in the synthesis of enzymes related to ROS catalysis. NO has been shown to play an important role in plant resistance to abiotic stress (e.g., hydric stress, high salinity, or high concentrations of heavy metals) (Siddiqui et al. 2011).
NO has also been shown to be an important intermediary in the action pathways of abscisic acid (ABA) in stomata regulation (Huang et al. 2013). Similarly, two HS fractions of different molecular weights have also been shown to regulate stomata opening. Besides, HS have been shown to exert effects on the opening of stomata in Pisum sativum L. plants through an auxin-like action and phospholipase A2 stimulation (Russell et al. 2006).
7.1 Evidence of Antistress Protective Effects of HS
In addition of examining the role of HS in regulating the primary and secondary metabolism in plants, some studies have discussed the possibility of using these substances to mitigate the effects of abiotic stresses such as saline soils, hydric stress, and concentrations of heavy metals. In one study, bean plants (Phaseolus vulgaris L.) were grown in soils artificially salinized with salts from various sources, with the doses of 0.05 and 0.1 % w:w. Without the application of HA, high doses of salt caused the death of plants, while plants grown under the same saline conditions in the presence of HA survived. The application of HA improved the plant’s growth and development and the assimilation of mineral elements such as phosphorus nitrogen (Aydin et al. 2012). Leaf applications of HS in tomato plants (Lycopersicon esculentum L.) were also tested under natural levels of soil salinity. Plants that received leaf applications of HS at two different physiological stages (10 and 15 days after transplanting) showed improved conditions and internal fruit qualities, compared to plants grown in saline soils without HS application. The internal pH of the fruit, BRIX degrees, malic acid, vitamin C, and total soluble salts were all higher than in the plants not treated with HS (Pérez et al. 2011).
These results indicate that HS stimulate the enzymatic activity of peroxidases (POX), thereby decreasing the amount of H2O2 in the leaf and root tissues. These studies demonstrated the inhibition of stress-induced lipid peroxidation in plants treated with humic acids and a preservation of cell membrane permeability was observed (García et al. 2012a). The same HA were also applied to the leaves of rice plants grown in soil under drought conditions. An increase in POX enzymatic activity was observed in response to the application of HA. Plant stress tolerance increased, as shown by improvement in growth and development of the plants, even under drought conditions (Hernández et al. 2012).
The plants to which HA was applied under conditions of hydric stress, permeability of the cell membrane was similar to that of plants grown in normal conditions. Proline content in mature root tissues increased when HA was applied; however, in plants grown under conditions of hydric stress and treated with HA, proline levels were similar to the levels found in plants under normal growth conditions. Moreover, ABA production in mature root tissues was not stimulated by the application of HA, and under conditions of stress, ABA content was similar to that found in plants grown in normal conditions. These results clearly suggest that HA has protective effects in plants experiencing hydric stress. While further studies are needed to determine the mechanisms behind these effects, the protective effects appear to be exerted through ABA-independent pathways, as shown in Fig. 11.9.
Behavior patterns in rice plants treated with HA under normal growing conditions and conditions of hydric stress (due to loss of water). Level of ABA, a key hormone in stress metabolism signaling. Proline, which is an antioxidant amino acid that responds to stress events and root membrane permeability, was measured by using the conductivity of plant tissues in deionized water
8 Potential Use of HS in Agricultural Systems
Based on the results discussed so far on the impact of application of HS to plants under stress, application of HS is becoming a routine in agriculture and research on the effects of HS should be targeted at developing new technologies for sustainable agriculture. Leaf application of HS-based extracts from compost is a method that has been tested in recent years. Using vermicomposts as raw material to obtain HS in an aqueous medium is an ecological and low-cost method which is environmentally safe and readily available for agricultural applications. Most of these HS extracts are dark in color and have the additional advantage of containing minerals, natural phytohormones, and microorganisms that contribute to plant growth. Small- and medium-scale leaf applications are viable and easily implemented methods that do not require the use of large-scale technology and human resources, as shown in Fig. 11.10.
8.1 Use of Liquid Extracts of Vermicompost (Liquid Humus)
Salinity is a major problem that affects soil and consequently influences the crop yields. High soil salinity produces a condition of physiological stress in plants known as saline stress, which affects the plant’s biological productivity and reduces agricultural yields. The application of liquid humus to the leaves may be a viable alternative method for mitigating the negative effects of saline stress on food plants.
A liquid humus (Liplant®) obtained from bovine manure vermicompost was applied to the leaves of tomato plants (Lycopersicon esculentum Mill.) grown in salinized soils during the optimal and suboptimal planting seasons. Applied doses of 1:50 (v:v) of liquid humus during both seasons resulted an increased crop yields, compared to plants that did not receive humus applications. The application of liquid humus did not change the internal attributes of the fruit, demonstrating that the liquid humus was innocuous when applied under these conditions (Pérez et al. 2009, 2011). Additionally, the application of the liquid humus Liplant to tomatoes grown under normal conditions in red ferralitic soil stimulated several physiological parameters in the plants. Leaf applications of humus at a rate of 1 L ha−1 increased the number of roots per plant, root length, leaf number, and biomass (Terry et al. 2012). Other studies have shown that liquid humus increases the uptake of K, P, and N in tomato plants and enhances the photosynthetic pigment content, resulting in an increased agricultural yields and higher fruit quality (Tejada et al. 2008).
Liquid humus was applied to bean plants cultivated in oxisols using tillage system which resulted in increased leaf surface area, liquid assimilation rate, biomass, and total agricultural yield. The highest yields were obtained when the liquid humus was applied at a rate of 1:60 (v:v) (Del Valle et al. 2012). It was also used in the production of watermelon seedlings (Citrullus lanatus cv. “Crimson Sweet”). Liquid humus when applied to the leaves in 22.5 mL m−2 doses resulted in the development of seedlings with greater leaf surface area and a more developed root system (Silvia-Matos et al. 2012). In strawberry plants (Fragaria × ananassa Duch.) this product improved several physiological and agricultural parameters. The application of liquid humus was associated with increased leaf surface area (10.1–18.9 %) and dry mass (13.9–27.2 %). In addition, the application of the humus was associated with a 5.7–12.1 % reduction in fruit albinism, 8.5–11.2 % decrease in fruit malformation, and an increase in total yield to the tune of 26.5 % (Singh et al. 2010).
Several studies have examined the application of liquid humus for agricultural use. Most of the observed effects have been positive in increasing yields and improving fruit quality. The results of these studies show that the application of liquid humus is a sustainable option with numerous advantages for the production of food plants under both normal and adverse environmental conditions.
9 Conclusions and Future Prospects
The structure–function–properties relationship should be the basis of future studies of HS and their effects on the environment. Due to the heterogeneous structural characteristics and numerous sources of origin of HS, every study, whether dealing with living organisms or natural systems, must include the structural characterization of HS as its premise. Regarding the mode of action of HS in plants, we observed that in spite of experimental design, HS have effects on several different metabolic processes in plants, as demonstrated through the use of large-scale gene sequencing techniques. The main actions of HS can potentially be observed in each specific metabolic pathway. However, we still lack a complete explanation of how these actions create more effective plant responses that protect plants against possible stress.
Thus far, the identification of a relationship between the stimuli produced by HS in plant defense mechanisms and primary metabolism suggests that future studies of HS should focus on new elements in the modes of action of HS. Current ideas about mechanisms that explain the action of HS in plants need to be expanded beyond auxin-like effects and other known effects. To understand the heterogeneity of HS actions, future research must consider ROS as a chemical species of great importance in metabolic signaling processes in plants. It has been shown that HS can exert noticeable effects on the ROS production metabolism, thereby regulating the REDOX homeostasis in plants.
Finally, the results discussed in this study and the proposed framework for future studies should aid in the development of new ideas about the application of HS that will guide future studies on the use of humified organic matter in agricultural ecosystems, particularly under the conditions of high stress.
References
Aguiar NO, Olivares FL, Novotny EH, Dobbss LB, Balmori DM, Santos-Júnior LG, Chagas JG, Façanha AR, Canellas LP (2013) Bioactivity of humic acids isolated from vermicomposts at different maturation stages. Plant Soil 362:161–174
Alvarez-Puebla RA, Valenzuela-Calahorro C, Garrido JJ (2004) Cu (II) retention on a humic substance. J Colloid Interface Sci 270:47–55
Amir S, Jouraiphy A, Meddich A, Gharous M, Winterto P, Hafidi M (2010) Structural study of humic acids during composting of activated sludge-green waste: elemental analysis, FTIR and 13C NMR. J Hazard Mater 177:524–529
Asli S, Neumann PM (2010) Rhizosphere humic acid interacts with root cell walls to reduce hydraulic conductivity and plant development. Plant Soil 336:313–322
Aydin A, Kant C, Turan M (2012) Humic acid application alleviates salinity stress of bean (Phaseolus vulgaris L.) plants decreasing membrane leakage. Afr J Agric Res 7:1073–1086
Baddi GA, Hafidi M, Cegarra J, Alburquerque JA, González J, Gilard V, Revel JC (2004) Characterization of fulvic acids by elemental and spectroscopic (FTIR and 13C-NMR) analyses during composting of olive mill wastes plus straw. Bioresour Technol 93:285–290
Berner JM, Van der Westhuizen AJ (2010) Inhibition of xanthine oxidase activity results in the inhibition of Russian wheat aphid-induced defense enzymes. J Chem Ecol 36:1375–1380
Blomster T, Salojärvi J, Sipari N, Brosché M, Ahlfors R, Keinänen M, Overmyer K, Kangasjärvi J (2011) Apoplastic reactive oxygen species transiently decrease auxin signaling and cause stress-induced morphogenic response in Arabidopsis. Plant Physiol 157:1866–1883
Canellas LP, Piccolo A, Dobbss LB, Spaccini R, Olivares FL, Zandonadi DB, Façanha AR (2010) Chemical composition and bioactivity properties of size-fractions separated from a vermicompost humic acid. Chemosphere 78:457–466
Canellas LP, Dobbss LB, Oliveira AL, Chagas JG, Aguiar NO, Rumjanek VM, Novotny EH, Olivares FL, Spaccini R, Piccolo A (2012) Chemical properties of humic matter as related to induction of plant lateral roots. Eur J Soil Sci 63:315–324
Cordeiro FC, Santa-Catarina C, Silveira V, de Souza SR (2011) Humic acid effect on catalase activity and the generation of reactive oxygen species in corn (Zea Mays L). Biosci Biotechnol Biochem 75:70–74
Corpas FJ, Palma JM, Sandalio LM, Valderrama R, Barroso JB, del Río LA (2008) Peroxisomal xanthine oxidoreductase: characterization of the enzyme from pea (Pisum sativum L.) leaves. J Plant Physiol 165:1319–1330
De Tullio MC, Jiang K, Feldman LJ (2010) Redox regulation of root apical meristem organization: connecting root development to its environment. Plant Physiol Biochem 48:328–336
Del Valle GH, Hernández GO, Izquierdo FG, Fortes NA (2012) Influence of the no till and the liquid extract of vermicompost in indicators and indexes growth in common bean (Phaseolus vulgaris L.) cv. cc-25-9. Rev Cie Téc Agr 21:86–90
Demidchik V, Shabala SN, Davies JM (2007) Spatial variation in H2O2 response of Arabidopsis thaliana root epidermal Ca2+ flux and plasma membrane Ca2+ channels. Plant J 49:377–386
Demidchik V, Shang Z, Shin R, Thompson E, Rubio L, Laohavisit A, Mortimer JC, Chivasa S, Slabas AR, Glover BJ, Schachtman DP, Shabala SN, Davies JM (2009) Plant extracellular ATP signalling by plasma membrane NADPH oxidase and Ca2+ channels. Plant J 58:903–913
Dobbss LB, Rumjaneck VM, Baldotto AM, Velloso ACX, Canellas LP (2009) Caracterização química e espectroscópica de ácidos húmicos e fúlvicos isolados da camada superficial de latossolos brasileiros. R Bras Ci Solo 33:51–63
Dobbss L, Canellas LP, Olivares FL, Aguiar NO, Peres LEP, Spaccini R, Piccolo A (2010) Bioactivity of chemically transformed humic matter from vermicompost on plant root growth. J Agric Food Chem 127:1–10
Ferrari E, Francioso O, Nardi S, Saladini M, dal Ferro N, Morari F (2011) DRIFT and HR MAS NMR characterization of humic substances from a soil treated with different organic and mineral fertilizers. J Mol Struct 998:216–224
Ferrer JL, Austin MB, Stewart C Jr, Noel JP (2008) Structure and function of enzymes involved in the biosynthesis of phenylpropanoids. Plant Physiol Biochem 46:356–370
Filella M, Town RM (2001) Heterogeneity and lability of Pb(II) complexation by humic substances: practical interpretation tools. Fresenius J Anal Chem 370:413–418
Foreman J, Demidchik V, Bothwell JHF, Mylona P, Henk M, Torresk MA, Linstead P, Costa S, Brownlee C, Jonesk JDG, Davies JM, Dolan L (2003) Reactive oxygen species produced by NADPH oxidase regulate plant cell growth. Nature 422:442–446
García AC, Berbara RLL, Farias LP, Izquierdo FG, Hernández OL, Campos RH, Castro RN (2012a) Humic acids of vermicompost as an ecological pathway to increase resistance of rice seedlings to water stress. Afr J Biotechnol 11:3125–3134
García AC, Santos LA, Izquierdo FG, Sperandio MVL, Castro RN, Berbara RLL (2012b) Vermicompost humic acids as an ecological pathway to protect rice plant against oxidative stress. Ecol Eng 47:203–208
Gerke J (1994) Aluminum complexation by humic substances and aluminum species in the soil solution. Geoderma 63:165–175
Ghabbour EA, Davies G (2001) The Royal Society of Chemistry, Thomas Graham House, Science Park, Milton Road, Cambridge CB4 OW, UK Registered Charity No. 207890
Gill SS, Tuteja N (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem 48:909–930
Gondar D, Lopez R, Fiol S, Antelo JM, Arce F (2005) Characterization and acid–base properties of fulvic and humic acids isolated from two horizons of an ombrotrophic peat bog. Geoderma 126:367–374
Haghighi M, Kafi M, Fang P (2012) Photosynthetic activity and N metabolism of lettuce as affected by humic acid. J Veg Sci 18:182–189
Hernández R, García A, Portuondo L, Muñiz S, Berbara R, Izquierdo F (2012) Protección antioxidativa de los ácidos húmicos extraídos de vermicompost en arroz (Oryza sativa L.) var. IACuba30. Rev Protección Veg 27:102–110
Huang AX, Sheb XP, Zhang YY, Zhao JL (2013) Cytosolic acidification precedes nitric oxide removal during inhibition of ABA induced stomatal closure by fusicoccin. Russ J Plant Physiol 60:60–68
Iglesias A, Lopez R, Fiol S, Antelo JM, Arce F (2003) Analysis of copper and calcium–fulvic acid complexation and competition effects. Water Res 37:3749–3755
Irving H, Williams RJP (1953) The stability of transition-metal complexes. J Chem Soc 3:3192–3210
Jannin L, Arkoun M, Ourry A, Laîné P, Goux D, Garnica M, Fuentes M, San Francisco S, Baigorri R, Cruz F, Houdusse F, Garcia-Mina JM, Yvin JC, Etienne P (2012) Microarray analysis of humic acid effects on Brassica napus growth: involvement of N, C and S metabolisms. Plant Soil 359:297–319
Jerzykiewicz M (2004) Formation of new radicals in humic acids upon interaction Pb (II) ions. Geoderma 122:305–309
Kurusu T, Kuchitsu K, Nakano M, Nakayama Y, Iida H (2013) Plant mechanosensing and Ca2+ transport. Trends Plant Sci 18(4):227–233
Kwak JM, Mori IC, Pei ZM, Leonhardt N, Torres MA, Dangl JL, Bloom RE, Bodde S, Jones JD, Schroeder JI (2003) NADPH oxidase AtrbohD and AtrbohF genes function in ROS-dependent ABA signaling in Arabidopsis. EMBO J 22:2623–2633
Li G-W, Peng Y-H, Yu X, Zhang M-H, Cai W-M, Sun W-N, Su W-A (2008) Transport functions and expression analysis of vacuolar membrane aquaporins in response to various stresses in rice. J Plant Physiol 165:1879–1888
Li X, Xing M, Yang J, Huang Z (2011) Compositional and functional features of humic acid-like fractions from vermicomposting of sewage sludge and cow dung. J Hazard Mater 185:740–748
Lopez F, Bousser A, Sissoëff I, Gaspar M, Lachaise B (2003) Diurnal regulation of water transport and aquaporin gene expression in maize roots: contribution of PIP2 proteins. Plant Cell Physiol 44:1384–1395
Loque D, Ludewig U, Yuan L, von Wiren N (2005) Tonoplast intrinsic proteins AtTIP2;1 and AtTIP2;3 facilitate NH3 transport into the vacuole. Plant Physiol 137:671–680
Marino D, Dunand C, Puppo A, Pauly N (2012) A burst of plant NADPH oxidases. Trends Plant Sci 17:9–15
Miller G, Schlauch K, Tam R, Cortes D, Torres MA, Shulaev V, Dangl JL, Mittler R (2009) The plant NADPH oxidase RBOHD mediates rapid systemic signaling in response to diverse stimuli. Sci Signal 84:45
Miller G, Suzuki N, Ciftci-Yilmaz S, Mittler R (2010) Reactive oxygen species homeostasis and signaling during drought and salinity stresses. Plant Cell Environ 33:453–467
Mittler R (2002) Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci 7:405–410
Mittler R, Vanderauwera S, Gollery M, Van BF (2004) Reactive oxygen gene network of plants. Trends Plant Sci 9:490–498
Mittler R, Vanderauwera S, Suzuki N, Miller G, Tognetti VB, Vandepoele K, Gollery M, Shulaev V, Breusegem FV (2011) ROS signaling: the new wave? Trends Plant Sci 16:300–309
Mora V, Bacaicoa E, Zamarreño AM, Aguirre E, Garnica M, Fuentes M, García-Mina JM (2010) Action of humic acid on promotion of cucumber shoot growth involves nitrate-related changes associated with the root-to-shoot distribution of cytokinins, polyamines and mineral nutrients. J Plant Physiol 167:633–642
Mori IC, Schroeder JI (2004) Reactive oxygen species activation of plant Ca2+ channels. A signaling mechanism in polar growth, hormone transduction, stress signaling, and hypothetically mechanotransduction. Plant Physiol 135:702–708
Muscolo A, Sidari M, Attiná E, Francioso O, Tugnoli V, Nardi S (2007) Biological activity of humic substances is related to their chemical structure. Soil Sci Soc Am J 71:75–85
Nardi S, Pizzeghello D, Muscolo A, Vianello A (2002) Physiological effects of humic substances on higher plants. Soil Biol Biochem 34:1527–1536
Nardi S, Muscolo A, Vaccaroa S, Baiano S, Spaccini R, Piccolo A (2007) Relationship between molecular characteristics of soil humic fractions and glycolytic pathway and krebs cycle in maize seedlings. Soil Biol Biochem 39:3138–3146
Nebbioso A, Piccolo A (2012) Advances in humeomics: enhanced structural identification of humic molecules after size fractionation of a soil humic acid. Anal Chim Acta 720:77–79
Pérez JJR, Izquierdo FG, Escobar IMR, Mayoral JAL (2009) Liquid casting effect on tomato yields in saline soils of Cuban east region. Centro Agrícola 36:57–61
Pérez JJR, Izquierdo FG, Escobar IMR, Ruisánchez Y, Mayoral JAL, Amador BM, Espinoza FH, Fabré TB, Amador CA, Silvera CMO, Morales YA, Milanés JYR (2011) Efectos del humus líquido sobre algunos parámetros de calidad interna en frutos de tomate cultivados en condiciones de estrés salino [Effects of liquid humus on some parameters of internal quality of tomato fruits grown under salt stress conditions]. Centro Agrícola 38:57–61
Piccolo A, Stevenson FJ (1982) Infrared spectra of Cu2+, Pb2+ and Ca2+ complexes of soil humic substances. Geoderma 27:195–208
Plaza C, D’Orazio V, Senesi N (2005) Copper (II) complexation of humic acids from the first generation of EUROSOILS by total luminescence spectroscopy. Geoderma 125:177–186
Polle A (2001) Dissecting the superoxide dismutase–ascorbate peroxidase–glutathione pathway in chloroplasts by metabolic modeling. Computer simulations as a step towards flux analysis. Plant Physiol 126:445–462
Russell L, Stokes AR, Macdonald H, Muscolo A, Nardi S (2006) Stomatal responses to humic substances and auxin are sensitive to inhibitors of phospholipase A2. Plant Soil 283:175–185
Šamaj J, Baluška F, Menzel D (2004) New signalling molecules regulating root hair tip growth. Trends Plant Sci 9:217–220
Schiavon M, Pizzeghello D, Muscolo A, Vaccaro S, Francioso O, Nardi S (2010) High molecular size humic substances enhance phenylpropanoid metabolism in maize (Zea mays L.). J Chem Ecol 36:662–669
Schnitzer M (1978) Humic substances: chemistry and reactions. In: Schnitzer M, Khan SU (eds) Soil organic matter. Elsevier, Amsterdam
Schopfer P, Liszkay A, Bechtold M, Frahry G, Wagner A (2002) Evidence that hydroxyl radicals mediate auxin-induced extension growth. Planta 214:821–828
Senesi N, Sposito G, Martin JP (1986) Copper (ii) and iron (iii) complexation by soil humic acids: an IR and ESR study. Sci Total Environ 55:351–362
Siddiqui MH, Al-Whaibi MH, Basalah MO (2011) Role of nitric oxide in tolerance of plants to abiotic stress. Protoplasma 248:447–455
Silvia-Matos RRS, Cavalcante IHL, Júnior GBS, Albano FG (2012) Foliar spray of humic substances on seedling production of Watermelon cv. Crimson Sweet. J Agron 11:60–64
Singh R, Gupta RK, Patil RT, Sharma RR, Asrey R, Kumar A, Jangra KK (2010) Sequential foliar application of vermicompost leachates improves marketable fruit yield and quality of strawberry (Fragaria x ananassa Duch.). Sci Hortic 124:34–39
Suzuki N, Koussevitzky S, Mittler R, Miller G (2012) ROS and redox signalling in the response of plants to abiotic stress. Plant Cell Environ 35:259–270
Takeda S, Gapper C, Kaya H, Bell E, Kuchitsu K, Dolan L (2008) Local positive feedback regulation determines cell shape in root hair cells. Science 319:1241–1244
Tao Z-Y, Zhang J, Zhai J-J (1999) Characterization and differentiation of humic acids and fulvic acids in soils from various regions of China by nuclear magnetic resonance spectroscopy. Anal Chim Acta 395:199–203
Tejada M, Gonzalez JL, Hernandez MT, Garcia C (2008) Agricultural use of leachates obtained from two different vermicomposting processes. Bioresour Technol 99:6228–6232
Terry E, Díaz de Armas MM, Padrón JR, Tejeda T, Zea ME, Camacho-Ferre F (2012) Effects of different bioactive products used as growth stimulators in lettuce crops (Lactuca sativa L.). J Food Agric Environ 10:386–389
Tognetti VB, Mühlenbock P, van Breusegem F (2012) Stress homeostasis the redox and auxin perspective. Plant Cell Environ 35:321–333
Trevisan S, Botton A, Vaccaro S, Vezzaro A, Quaggiotti S, Nardi S (2011) Humic substances affect Arabidopsis physiology by altering the expression of genes involved in primary metabolism, growth and development. Environ Exp Bot 74:45–55
Tsukagoshi H, Busch W, Benfey PN (2010) Transcriptional regulation of ROS controls transition from proliferation to differentiation in the root. Cell 143:606–616
Vasconcelos FAC, Zhang X, Ervin EH, Kiehl CJ (2009) Enzymatic antioxidant responses biostimulants maize and soybean subjected to drought. Sci Agric 66:395–402
Vaugham D, Linehan DJ (1976) The growth of wheat plants in humic acid solutions under axenic conditions. Plant Soil 44:445–449
Vaughan D, Ord BG (1981) Uptake and incorporation of 14C-labelled soil organic matter by roots of Pisum sativum L. J Exp Bot 32:679–687
Vaughan D, Ord BG (1982) An in vitro effect of soil organic matter fractions and synthetic humic acids on the generation of superoxide radicals. Plant Soil 66:113–116
Yang C, Wang M, Lu Y, Chang I, Chou C (2004) Humic substances affect the activity of chlorophyllase. J Chem Ecol 8:1561–1573
Zandonadi DB, Santos MP, Dobbss LB, Olivares FL, Canellas LP, Binzel ML, Okorokova-Façanha AL, Façanha AR (2010) Nitric oxide mediates humic acids-induced root development and plasma membrane H+-ATPase activation. Planta 231:1025–1036
Zepeda-Jazo I, Velarde-Buendía AM, Enríquez-Figueroa R, Bose J, Shabala S, Muñiz-Murguía J, Pottosin II (2011) Polyamines interact with hydroxyl radicals in activating Ca2+ and K+ transport across the root epidermal plasma membranes. Plant Physiol 157:2167–2180
Acknowledgments
To the TWAS/CNPq for the grant to Andres Calderin Garcia and FAPERJ/Prioridade Rio and CNPq/UNIVERSAL to Ricardo L. Berbara.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer Science+Business Media New York
About this chapter
Cite this chapter
Berbara, R.L.L., García, A.C. (2014). Humic Substances and Plant Defense Metabolism. In: Ahmad, P., Wani, M. (eds) Physiological Mechanisms and Adaptation Strategies in Plants Under Changing Environment. Springer, New York, NY. https://doi.org/10.1007/978-1-4614-8591-9_11
Download citation
DOI: https://doi.org/10.1007/978-1-4614-8591-9_11
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4614-8590-2
Online ISBN: 978-1-4614-8591-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)