Abstract
Abiotic stresses such as salinity, drought, extreme temperature, radiation, nutrient deficiency, and heavy metals can significantly impact plant growth and yield, posing a challenge to agriculture. Various strategies have been employed to enhance plant resistance to these stresses through conventional and genetic manipulations. However, these often require extensive breeding programmes and substantial financial investments. In this regard, plant root exudates have emerged as a promising tool for improving plant performance under adverse conditions. They play a crucial role in the rhizosphere, influencing and guiding the microbial community to support plant growth. Published literature suggests that plants can adapt to abiotic stress by modifying the chemistry of their root exudates. These modified exudates can recruit beneficial microorganisms that aid in protecting plants from stress. Despite the progress made in identifying, characterizing, and detecting root exudates, there is still a substantial gap in our understanding of their significance and potential applications, particularly in addressing abiotic stress in plants. Therefore, the present review provides valuable insights into recent research progress in the field of root exudates. It covers fundamental information about root exudates, mechanisms of exudation, the impact of abiotic factors, and the interactions between roots and microbes in exudation. The review also explores how root exudates contribute to enhancing abiotic stress tolerance in plants, shedding light on the potential benefits of these compounds. The review also discusses existing challenges in the field of root exudates, particularly in their application for sustainable agriculture. It also addresses potential research gaps and outlines future directions for further investigation and practical application in agriculture for abiotic stress amelioration.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Abiotic stress is one of the prime environmental perturbations that negatively influence plant growth and development, with a significant impact on total crop production (Dwivedi and Sopory 2023; Khan et al. 2021). Generally, abiotic stresses are comprised of extreme temperature (both heat and cold), salinity, drought, flooding, nutrient deficiency, and heavy metals. These stresses have been reported to cause a significant decline (> 50%) in the total yield of almost all the major food crops grown throughout the world (Kashyap et al. 2017). It has been reported by several workers that the changing climatic conditions aggravate the negative effects of abiotic stresses on food crops with time by altering plant root growth, which further decreases crop growth by restricting the sufficient supply of nutrients and water in the phyllosphere (Fig. 1). Consequently, these will also create a major threat to the global food and nutritional security (Kosová 2018). Among various abiotic stresses, drought stress negatively impacts plant growth by altering stem elongation, leaf expansion, root proliferation, and foremost, a decline in water and nutrient uptake (Singh et al. 2023). In addition, a reduction in the leaf water potential and stomatal closing and opening, a delay in flowering, and a reduction in seed number and size are also observed in drought-stressful environments (Xu et al. 2019). Similarly, in the case of salt stress, prolonged harmful effects on glycophytes have been noticed (Zhao et al. 2021a, b; Negrão et al. 2017). The majority of the plants cannot tolerate salt concentrations beyond 200 mM (Khan et al. 2021), as it affects seed germination, seedling establishment, and vegetative growth. Further, published literature also mentioned the rise in osmotic pressure along with ion toxicity behind the oxidative damage of plants in saline environments (Subiramani et al. 2020; Kumari et al. 2019). Another category of abiotic stress, i.e., heat stress, is also reported to influence the physiology, biochemistry, and morphology of the plant and is ultimately responsible for stunted plant growth with a significant drop in plant biomass and productivity (Morcillo et al. 2021; Kashyap et al. 2018). Similarly, heavy metals have been reported to impose diverse types of direct and indirect influence on plant growth (Kushwaha and Kashyap 2021). More specifically, reduction in plant growth and commotion of various physiological and molecular phenomena, along with the culmination of chlorosis, senescence, and suppression of chlorophyll and photosynthesis activities, have been reported as a penalty of heavy metal stress (Bhat et al. 2020).
Adverse effects of abiotic stresses on root and shoot growth of plant (Khan et al. 2021)
A growing body of research literature acknowledges the rhizosphere as a hub of microbes that supports large and metabolically active groups of microbes (Pandiyan et al. 2022; Solanki et al. 2012; Kushwaha et al. 2021). Khan et al. (2021) highlighted that the rhizosphere occupies millions of microbial cells; however, their composition is significantly influenced by plant growth stages and the type of genotype. The credit for the first observation concerning the abundant presence of microbes in the rhizosphere region rather than in distant soil goes to Hiltner (1904). Later on, various indications in connection with root exudation and microbial abundance in the rhizosphere were made by Knudson (1920) and Lyon and Wilson (1921). Recently published reports indicated that in the rhizosphere, plant roots release miscellaneous types of compounds (Table S1) that serve as food and chemical attractants for diverse types of soil microbes (Kumar et al. 2022; Feng et al. 2021; Pandey et al. 2017). It has been noticed that these root exudates have the ability to manipulate the physicochemical characteristics of the soil and thus play an important role in shaping the microbial community structure in the rhizosphere (Zhang et al. 2021; Bechtold et al. 2018). Consequently, root exudates are also reported to enhance plant growth in stressful environments by convalescing the nutrients and water uptake from the soil (Ma et al. 2022a; Ahkami et al. 2017). More specifically, they increase the level of phosphate and nitrate reductase activities under water-stress regimes while reducing the Na+ accumulation in saline environments (Wang et al. 2016). Besides abiotic stress amelioration, root exudates are also reported to contain soil-borne pathogens that impede plant defence systems (Zhou et al. 2023a, b; Chen et al. 2022; Baetz and Martinoia 2014). Briefly, in the current review, attempts have been made to recapitulate the recent progress attained in the field of root exudates. The article provides fundamental information on root exudates, mechanisms of exudations, effects of abiotic factors, and root-microbe interactions in exudation, along with the role of root exudates in convalescing abiotic stress tolerance capabilities in plants. Finally, existing challenges in the field of root exudates for their application in agriculture sustainability, along with future directions, are outlined.
Root Exudation Phenomenon
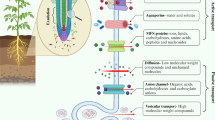
Root exudation is one of the prime events in the rhizo-deposition process and is a key source of soil organic carbon liberated by the roots of plants (Panchal et al. 2022). Chai and Schachtman (2022) mentioned that root exudation contributed up to 21% of the plant net photosynthates; however, their contribution greatly relies on the soil nutrient status, plant species, and plant growth stage (Hayat et al. 2017). Usually, root exudates represent both high molecular weight compounds (e.g., proteins and mucilage, etc.) and low molecular weight compounds, more assorted types than high molecular weight compounds (Table S1). It is important to mention here that the low-molecular-weight compounds contained a broad series of primary metabolites (e.g., amino acids, carboxylates, sugars, etc.) and secondary metabolites (e.g., coumarin, flavonoids, sorgoleone, etc.) (Gargallo-Garriga et al. 2018). Plant root exudates perform several functions, majorly linked with plant performance under stressed environments (Chai and Schachtman 2022). They also act as a vital carrier compound for the movement of material and the exchange of information between plant-soil interfaces. Moreover, they are also acknowledged as the prime players in the establishment and maintenance of the vitality and function of the rhizosphere micro-ecosystem by serving a vital part in the rhizosphere material exchange. Most importantly, they help improve the bioavailability of soil nutrients to plants (Zhao et al. 2021a, b). The carboxylates in root exudates are well recognized for their chelating properties, which help in the solubilization of phosphorus (P) for plant uptake. Besides altering soil nutrient status, root exudates also facilitate plant root-microbe interactions and help in crop growth augmentation under stressful conditions (Chai and Schachtman 2022; Solanki et al. 2022). For instance, DIMBOA (2,4-dihydroxy-7-methoxy-2H-1,4-benzoxazin-3(4H)-one), identified as one of the key maize root exudates, is reported to serve as a chemo-attractant for plant growth-promoting rhizobacterium (Pseudomonas putida KT2440) as well as offer allelopathic action against soil-borne pathogens and nearby plants (Neal et al. 2012). A list of different types of compounds released by plant roots as exudates is mentioned in Table S1.
The exudation of low molecular weight carboxylates having ≤ 3 carboxyl groups (e.g., malate, citrate, and oxalate, etc.) by roots is an important plant strategy to raise phosphorus (P) uptake by displacing insoluble P from P-containing organic and inorganic compounds in soil and making them accessible to roots (Fig. 2). The potential of carboxylates to release P is highly dependent on the concentration of carboxylates and the presence of carboxyl groups with tricarboxylates (citrate, aconitate, etc.). These have a high influence on P-desorbing, while monocarboxylates (acetate, pyruvate, etc.) have a low impact (Chai and Schachtman 2022). For instance, in the case of Arabidopsis thaliana and wheat, cultivars with higher citrate efflux have been found to be relatively more resistant to P-stress relative to genotypes displaying lower citrate efflux (Ryan et al. 2014). Further, it has been observed that the process of rhizosphere acidification operates synergistically with root exudates having carboxylates to convert the organic P (Po) and inorganic (Pi) pools. The root exudates with phenolics secrete P from the Pi pool by chelating with P-containing minerals (e.g., Fe/Al-hydroxides). In addition to this, acid phosphatases (APases) liberated P from P-containing organic matter at low pH (Chai and Schachtman 2022). However, the role of transporters responsible for phenolics and acid phosphatase efflux still needs to be investigated.
Facilitation of plant phosphorus (P) uptake by root exudates. Al aluminium, ALMT aluminium activated malate transporter, Ca calcium, Fe iron, MATE multidrug and toxic compound extrusion, PHT protonphosphate cotransporter (Chai and Schachtman 2022)
Recent research indicated that rhizosphere acidification and root exudation of carboxylates are the prime processes involved in the solubilization of P and are indirectly associated with the rise of P-solubilizing bacteria in the root zone (Chai and Schachtman 2022). Furthermore, it was also observed that phosphorus deficiency positively correlated with stimulation of the phenolic exudation in the rhizosphere (De Andrade et al. 2022). Hu et al. (2005) documented that the phenolic compounds (e.g., caffeic and protocatechuic acid) assist in P desorption by serving as a binder in the soils containing P minerals and thus help in releasing P for plant uptake, as depicted in Fig. 2. The exudation of phenolic compounds is also reported to suppress the abundance of the microbial communities dwelling in the rhizosphere and, in turn, struggle with plant roots for P nutrition. In addition to this, P deficiency is also demonstrated to persuade acid phosphatases (APases) to release into the rhizosphere (Nuruzzaman et al. 2006). At low pH, APases aid in the release of P from organic P pools (Hurley et al. 2010). APases hydrolyze the organic P content present in the rhizosphere, which later mobilises in the form of orthophosphate (after conversion from carboxylates) before its absorption by the plant roots (Fig. 2). However, the amount of APase secretion in the root zone depends on the plant species. Nuruzzaman et al. (2006) observed a higher level of secretion of APases in tomato and white lupin, while a low amount of APases was released in the case of rice, wheat, and maize.
Regulation and Factors Affecting Root Exudation
At the root-soil interface, it has been observed that the root is constantly exposed to diverse types of stress factors of both biotic and abiotic nature. In turn, roots respond to these stresses by secreting a series of diverse types of chemicals, generally mentioned in literature as root exudates. These chemicals protect roots against the adverse effects of these stresses and support favourable relationships. Mechanical impedance due to soil compaction and mild drought conditions has been reported to upset the root morphology and result in the stimulation of root exudates’ secretion (Gargallo-Garriga et al. 2018; Oliveros-Bastida et al. 2012). Under in-vitro conditions, alterations in the composition of root exudates of a particular plant species have been noticed in response to diverse types of growth media and support the notion regarding the role of released and available nutrition in the regulation of the root exudation process. Badri and Vivanco (2009) mentioned that nutrient deficiency is also positively linked with the augmentation of the exudation process of metabolites, specifically those that make the nutrients available to the roots. Besides this, temperature also serves as an important factor in the modulation of the root exudation process. This fact is well supported by the study of Bekkara et al. (1998) who reported slow exudation of tannins and phenolic compounds at low temperatures (4 °C) compared to the exudation at 30 °C in the case of Vicia faba hosts.
Elicitors are another class of molecules that are reported to encourage defence or stress-stimulated responses in plants (Meena et al. 2023; Kashyap et al. 2022). In nature, they are available in both chemical and physical forms. Badri and Vivanco (2009) mentioned that elicitors secrete phytochemicals at much higher concentrations than non-elicited plants to provide support for plant roots. Furthermore, a series of compounds normally not released in the root exudates of non-elicited plants have been observed in elicited plant root exudates. Interestingly, it has been reported that even a single elicitor has the potential to release distinct types of compounds in a single plant species (Kashyap et al. 2021). It has been noticed that the exogenous application of defence signalling molecules [e.g., salicylic acid (SA), methyl jasmonate (MeJA), nitric oxide (NO), etc.] resulted in the accumulation of a large number of secondary metabolites (Zhao et al. 2005; Kandoudi and Németh-Zámboriné, 2022). Similarly, mineral deficiency was also reported to induce the intrinsic synthesis of elicitor molecules involved in the plant signaling rejoinder. Schachtman and Shin (2007) mentioned that the deficiency of potassium leads to the induction of a JA-mediated defence mechanism. Generally, the signaling molecules are released in different parts of the plant, as SA and MeJA are noticed in the medium of cultured plant cells (Chen et al. 2001), while accumulation in the apoplastic space of plant roots has been reported in the case of nitric oxide (NO) (Stohr and Ullrich 2002). In one such study, Noritake et al. (1996) observed that NO triggers the accumulation of phytoalexin (e.g., rishitin) in the potato tuber tissues and the root exudates. Studies have also proved that the application of SA, MeJA, and NO resulted in the rise of root exudation of phytochemicals in Arabidopsis plant roots (Badri et al. 2008a). Various secondary metabolites released in response to SA, MeJA, and NO elicitors are shown in Table S2.
Mechanism of Root Secretion
Plant roots exude a series of compounds by following a passive process arbitrated by three distinct pathways (Badri and Vivanco 2009). These include diffusion, ion channels, and vesicle transport (Fig. 3). Sanders and Bethke (2000) described that during the diffusion process, small polar and uncharged molecules flow through permeable lipid membranes. However, Badri and Vivanco (2009) observed that the diffusion process is greatly influenced by membrane permeability and cytosolic pH. Besides this, other compounds such as amino acids, sugars, and carboxylate anions are also moved across the membranes with the support of proteins. The direction of the movement of the compounds is determined by their electrochemical gradient, which permits the compounds to pass from the cytoplasm of root cells (mM concentration) to the soil (µM concentration). In another similar study, Samuel et al. (1992) have shown that the high level of the cytosolic K+ diffusion potential and the proton extrusion with the help of ATPase enzymes determined the positively charged gradient, which in turn was responsible for the quick release of carboxylate anions. Besides this, membrane integrity is another factor that also influences the pace of organic acid release in the root zone. Likewise, the anion channels also play a key role in controlling the release of these compounds in the root region (Badri and Vivanco 2009). Lee et al. (2007) mentioned that the secretion of sugars, amino acids, and metals from root cells is greatly determined by a specific type of transporter. It is important to mention here that plants have a unique system of metal homeostasis that helps in avoiding the excessive accretion of free metal ions (e.g., Mn, Zn, Fe, and Cu, etc.). The metal homeostasis mechanism involves the coordination of metal ion transporters, which predominantly engage in the uptake, translocation, and compartmentalization of metal ions. For instance, in the case of graminaceous species, it has been observed that mugineic acid (MA) secreted from the roots into the rhizosphere serves as a metal-binding ligand and results in the formation of Fe (III)-MA ligand to lessen the Fe toxicity. Later, in the case of the maize host, Badri and Vivanco (2009) identified that the Fe (III)-MA ligand penetrates the root cells controlled by a specific yellow stripe-like (YSL) transporter.
Mechanisms of root exudation of compounds through the plant cell membrane (modified from Bertin et al. 2003). PM plasma membrane, TMD transmembrane domain, NBD nucleotide-binding domain
Generally, the excretion of high-molecular-weight compounds from roots depends on the workings of the vesicular transport system (Battey and Blackbourn 1993). Although the mechanism of the vesicle-mediated movement of proteins is well explained by Field et al. (2006), the transport mechanism of phytochemicals is still not completely deciphered (Grotewold 2004). However, the available information provides clear indications regarding the involvement of vesicle-mediated transport of phytochemicals in the leaf cells (Gani et al. 2021), but except for the golgi-mediated transport of mucilage polysaccharides across the root cap, the information about the mechanisms of phytochemical secretion from root cells is still under discovery (Driouich et al. 2021; Neumann and Romheld 2007).
ABC Transporters in Root Exudation Processes
Earlier published reports indicated that the ATP-binding cassette (ABC) transporters and multidrug and toxic compound extrusion (MATE) transporters are the major players responsible for the movement of defence-related phytochemicals inside the plant cells (Gani et al. 2021; Panthri and Gupta 2022). Generally, ABC transporters obtain energy for primary transport via ATP hydrolysis, while MATE transporters operate by H+ gradient-dependent secondary transport. Interestingly, both of these groups play noteworthy roles in the trafficking of flavonoids to the vacuole (Yazaki 2005). It is important to mention that the ABC transporters are one of the largest protein families and are reported in all the phyla. Sanchez-Fernandez et al. (2001) classified plant ABC proteins into 13 subfamilies based on four different parameters. These include protein size (half or full), idiotypic trans-membrane/linker domains (presence or absence), orientation (backward or forward), and sequence similarity. Based on characteristic attributes of the molecules associated with ABC transporters in plants, they are categorised into three major subfamilies and reported in the literature as pleiotropic drug resistance protein (PDR), multidrug resistance P-glycoproteins (PGP), and multidrug resistance-related protein (MRP). Later, it was reported that these ABC transporters play an essential role in various cellular processes (Stein et al. 2006). Most remarkably, they served as the determinant for seepage of potentially toxic compounds, lipid translocation, heavy metal tolerance, disease resistance, nutrient transport, salt stress, etc. (Banasiak and Jasiński 2022).
Generally, the secretion of plant secondary metabolites from plant roots is driven by ATP-dependent processes, which further indicates the involvement of ABC transporters in these processes. It has been noticed by Loyola-Vargas et al. (2007) that root secretion profiles of Arabidopsis differ significantly in composition under the influence of different types of inhibitors (e.g., potassium cyanide, nifedipine, sodium orthovanadate, verapamil, quinidine, glibenclamide, etc.), which hint towards the involvement of active transporting systems (i.e., ABC transporters and P-type ATPases) in the root secretion process because all the inhibitors used in the study possess ATP pool-deteriorating characteristics in the cell. Moreover, another independent study by Sugiyama et al. (2007) also revealed that the secretion of genistein (a signal flavonoid released by soybean roots during rhizobium symbiosis) is regulated by an ABC transporter in an ATP-dependent manner. Further, they also observed the non-repression of genistein secretion in soybean root by other inhibitors, specifically those responsible for the inhibition of plasma membrane ionophores (e.g., nigericin, valinomycin, and gramicidin). Besides this, Badri et al. (2008b) also documented the function of ABC transporters in the root secretion process by performing ABC transporter protein knock-out experiments in Arabidopsis root cells. They also noticed three different compounds transported by different ABC transporters, and among them, one such compound was documented as 3-hydroxy-4(Z), 6(Z), 8(Z), 10(Z)-tetraenoic acid, which further strengthened the notion that a single ABC transporter can shift structurally distinct compounds or that one compound can be transferred by distinct types of ABC transporters. However, we felt that further research and investigation to discover other transporting systems (e.g., MATE) and different substrates that trigger root secretion of phytochemicals are obligatory to harness the agricultural and biotechnological advantages of root exudation.
Release and Movement of Primary Metabolites at Root Tip Interface
Root exudation includes all the processes that deal with the transportation of carbon to roots and their exudation into the soil (Yin et al. 2023). The carbon produced at the photosynthesis site in plants is transported through the phloem with the assistance of the pressure-driven mechanism of phloem flow, as explained by Münch (1930). In this mechanism, the difference in turgor pressure between sink and source organs plays an essential role in the transportation of metabolites and is generally regulated by the generation of concentration gradients and source-sink relationships. De Schepper et al. (2013) mentioned that during the unloading of metabolites, low-molecular-weight proteins and solutes transferred to the phloem-pole pericycle with the help of funnel plasmodesmata, as depicted in Fig. 4. During this process, proteins travel in discrete pulses, usually called ‘batch unloading’, and their movement is limited to the phloem-pole pericycle only. However, the unloading of low-molecular-weight organic solutes takes place without restrictions, travels out of the phloem-pole pericycle, and is quite helpful for root exudation at the root tip region. Later, this mechanism was well supported by the work of Ross-Elliott et al. (2017), who also described root exudation at the root tip region as the prime course for the movement of all types of solutes during unloading and their further diffusion towards the surrounding cells because of the high degree of plasmodesmata networks in the region (Fig. 4).
Root structure and areas of root exudation. The upper figure represents the longitudinal section of a root. The two circles focus on two distinct zones, a differentiated vs. an undifferentiated area, to show the presence of a Casparian strip and low abundance of plasmodesmata in the differentiated area (left circle), and the presence of funnel plasmodesmata in the undifferentiated area (right circle). The square represents a cross section close to the meristematic area where root exudation is the highest. The lower figures represent a schematic representation of solute movement sites from phloem unloading to the soil environment, either in the differentiated zone (A) or in the undifferentiated root tip (B). A Solutes move both through the symplastic and apoplastic pathways, but then they are re-uptaken into the cytoplasm as the Casparian strip limits the apoplastic pathway. Only the cortex and epidermis are responsible for the flux of metabolites into the apoplast and consecutively into the soil (root exudation). Cortex and epidermis represent the major control point for root exudation. B At the phloem unloading site, both symplastic and apoplastic pathways are used. Because of the lack of a Casparian strip, solutes can move out of the root (root exudation) through both the apoplastic and the symplastic pathway (Cararini et al. 2019)
Principally, the symplastic pathway is one of the key channels that help in the free movement of the metabolites within the plant system. Generally, metabolites enter the soil environment with the help of the exudate plasma membrane. Yang and Hinner (2015) mentioned that the plasma membrane helps in the easy movement of small molecules (e.g., urea, glycerol, etc.) and the exchange of gas. Contrarily, they also pointed out that the penetration and transfer of ions and large-size uncharged polar molecules such as glucose do not operate through the plasma membrane, and in such cases, specific trans-membrane proteins in the plasma membrane help in their smooth movement inside the plant system. Later, Sasse et al. (2018) also observed that small pores through the lipid bilayer provide passage for large-polar or charged molecules to move across the membrane without making any physical contact with the hydrophobic fatty acid chains present on the membrane phospholipids. Further, it has been observed that the fine-tuning of the exudation flux of sugars, amino acids, and organic acids largely depends on the positive and negative regulation of the genes along with the post-translational level alteration of the specific efflux carriers and channels (Mishra et al. 2022; Badri et al. 2008a). At present, the major types of well-characterized efflux transporters of primary metabolites include amino acid transporters [UMAMIT transporters, CAT transporters, GDU transporters, sugar transporters (majorly related to the SWEET transporter family)] and organic acid transporters [ALMT/malate and MATE/citrate transporters] (Manck-Götzenberger and Requena 2016; Dinkeloo et al. 2018; Mora-Macías et al. 2017). More interestingly, the majority of these transporters are not directly coupled to ATP hydrolysis or ATP-dependent H+ pumps and H+ antiports. However, Badri et al. (2009) documented that ATP-dependent ABC transporters for secondary compounds and MATE/citrate transporters have H+-coupled antiport activity (Meyer et al. 2010), which is different from other efflux transporters and usually not coupled to ATP hydrolysis or to H+ co-transport. The most common characteristic of the majority of the primary metabolite transporters is their role as substrate-specific facilitators, which helps in rapid primary metabolite diffusion across the plasma membrane via trans-membrane carriers along the concentration gradient (Sasse et al. 2018). In this regard, Mora-Macias et al. (2017) have proved that P-deficiency or Al3+ toxicity stimulates malate exudation by up-regulating ALMT in the roots. It has been observed that after the release of metabolites from the phloem cell plasma membrane, the apoplastic transport pathway activates to release the metabolites into the soil as exudates. This observation is well supported by the study made by Vidal et al. (2018), who demonstrated that in immature wheat roots, the flow of carbon-containing compounds from the stele to the cortex follows an apoplastic pathway and is later utilised by microbial communities dwelling in the soil environment. Further, it has been noticed that the diffusion process in the apoplast is the prime process helping in the regulation of flux from plant roots to the soil environment without getting any intrusions of plasma membranes at the root tip. Somssich et al. (2016) mentioned that the endodermis cells provide a barricade against apoplastic diffusion, which is also quite evident from the fact that the meristem lacks a Casparian strip, which usually serves as an apoplastic barrier, as depicted in Fig. 4. Overall, we felt that the knowledge of the contributing efflux carriers for the synthesis of primary metabolites of the exudates at the soil-root interaction zone can help in generating new information about the root exudation regulation mechanisms.
Role of Root-Rhizosphere Interactions in Root Exudates Exudation
The root tip is the prime place, where the majority of the root exudation activities take place (Sasse et al. 2018). It is important to mention here that the root tip is the foremost important plant part that is in direct contact with the new soil environment, which in turn plays an important function in regulating root responses under the pursuance of environment-driven stimuli (Tedone et al. 2020). The root-soil interaction zone in the rhizosphere is the prime place for plant-microbial interactions, where both advantageous and detrimental microorganisms reside together. Pérez-de-Luque et al. (2017) mentioned that the microbial community structure in the rhizosphere is highly dynamic and influenced by myriads of factors, including soil nutrition, plant root architecture, type of plant species, environment, etc. Furthermore, it has been noticed that the rhizobacteria interactions in the root zone (i.e., rhizosphere) introduce various types of intuitive impact on soil health by affecting the soil nutrient status, heavy metal remediation processes, soil immune responses, and root growth (Badri et al. 2009). Later, it was reported that the interactions between roots, rhizosphere, and microbial communities modulate plant root growth and their proliferation, which ultimately play a significant role in regulating the exchange of resources between the soil environment and the upper section of plants, i.e., the shoot (Zhang et al. 2017). The root-root-rhizobacterial interactions also promote root exudation, which promotes the attraction of microorganisms towards the root vicinity (Semchenko et al. 2014). Besides, root exudates also mediate plant–microbe interactions by acting as food and chemo-attractants for different types of microbial associations, which further assists in root colonisation and plant growth promotion. In another independent study, Neal and co-workers (2012) noticed that the presence of Pseudomonas putida in the maize rhizosphere helps in benzoxazinone removal from the rhizosphere. Later, Dilnashin et al. (2020) documented that microbial soil diversity is greatly influenced by root exudate composition. The list of selected bacteria with a role in root exudation enrichment and amelioration of abiotic stress in cereals has been presented in Table 1.
A series of published reports indicated that organic acids are one of the prime components of root exudates (Xiong et al. 2023; Fujii et al. 2021; Sharma et al. 2020). Organic acid not only serves as a source of energy for regulating microbial metabolism at the cellular level but also serves as one of the intermediary compounds for regulating the bio-geochemical processes of various cycles operating in the rhizosphere. It has been noticed that low-molecular-weight carbon (C) compounds of root exudates play an important role as a precursor in the biosynthesis of rhizobacterial phytohormones. For instance, tryptophan (Trp) is one such compound that is mostly present in the root tip region and helps in IAA production as a precursor (Li et al. 2018a, b). Similarly, the oozing of aminocyclopropane-1-carboxylic acid (ACC) from plant roots has been mentioned in earlier published reports (Xiang et al. 2019). It is important to mention that this compound serves as a precursor for ethylene synthesis and is utilised as a carbon and nitrogen source by the bacteria dwelling in the rhizosphere. Besides the aforementioned compounds, the oozing of flavonoids from the roots of leguminous crops and their significance in inducing transcription of Rhizobia Nod factors (NFs) have been established (Sugiyama 2019). Functionally, NFs are required by the roots of leguminous crops for the formation of root hairs and nodule initiation. It is evident from various reports that root-rhizosphere and rhizobacterial interactions operate in a mutual communication mode between plants, soil, and microorganisms (Wang et al. 2020). Further, it has been noticed that all the aforementioned interactions and communications are of a convoluted type and play an indispensable role in determining the positive outcome in terms of soil health and plant fitness. Rahimi et al. (2020) explained that the root-rhizosphere and rhizobacterial interactions are highly advantageous to plants in terms of nutrient acquisition from soil and significant enhancements in plant growth, grain quality, and total yield. In addition to this, plant rhizobacteria help to improve nutrient cycling in the rhizosphere and tolerance capabilities in plants to resist unfavourable environmental conditions.
The term ‘DefenseBiome’ is widely used in published literature in connection to the plant-associated microbes that boost plant growth in stressed environments. Further, Liu et al. (2020) also pointed out that the scope of ‘DefenseBiome’ is much wider and currently widely used in the context of the development and design of functionally consistent and reliable synthetic microbial communities for better and more recuperative plant health. In recent times, DefenseBiome’s concept has been explored to gain deep insight into plant-microbial interactions as well as to invent new and novel plant probiotics (Liu et al. 2020). This concept got strength from the findings of Sharp et al. (2011), where they noticed lesser emanation of ethylene from mature foliage of Cytisus × praecox under drought stress post-inoculation of Variovorax paradoxus 5C-2. In addition, they also observed a significant reduction in the abscission of mature leaves. Similar, favourable effects of the rhizobacterium inoculation have been reported in Fargesia murielae against wounding or injury due to divisional propagation, as well as in the case of Aquilegia × hybrids under drought stress regimes. The affectivity of microbial partners in plant–microbe interactions and their usefulness in supporting plant growth under abiotic stress conditions depend upon several biochemical and physiological features of the interacting microbes, and a major among them is their survivability. Pathania et al. (2020) mentioned that Bacillus MT7 associated with the maize rhizosphere offered better survivability as well as supporting tomato growth under a series of abiotic stressors such as drought, temperature, heavy metals, salt, etc. Further, they also noticed that the colonisation ability of Bacillus MT7 was also greatly influenced by their potential to act in response to tomato root exudates and biofilm formation for better establishment and proliferation in the tomato rhizosphere, along with the expression of diverse types of plant growth-promoting attributes (Zhou et al. 2016). More specifically, under alkaline conditions, Paenibacillus polymyxa BFKC01 facilitates Fe mobilisation by stimulating the production of phenolic compounds in the root exudates of Arabidopsis. It also produces auxin, which improves the plant root system and consequently enhances Fe acquisition from the soil. Consequently, it leads to higher endogenous Fe content in plants and enhanced photosynthesis in an alkaline environment (Zhou et al. 2016). Later, Woo et al. (2020) recorded that Bacillus subtilis strain GOT9 stimulates the expression of drought and salt-inducible genes and, as a result, plays a significant role in promoting lateral root growth and development under drought and salt stress in the case of Arabidopsis and Brassica hosts (Woo et al. 2020). Similarly, the transcriptome analysis of Photorhabdus luminescens performed by Regaiolo et al. (2020) also indicated the role of a series of genes involved in chitin degradation, biofilm regulation, the formation of flagella, and the type VI secretion system while interacting with the root exudates. They also noticed that P. luminescens suppressed the growth of plant pathogens and exhibited specific interactions with plant roots (Regaiolo et al. 2020).
Several research documents indicated that the root exudate composition is greatly influenced by the genotypic characteristics of the plant species and stress type (Vives-Peris et al. 2020; Ulrich et al. 2022). Further, it has been noticed that root exudate composition also plays a significant role in regulating the growth of rhizobacteria in the rhizosphere (Feng et al. 2021; Sun et al. 2021a, b). For instance, the study of Vives-Peris et al. (2018a, b) highlighted that the root exudates from tolerant genotypes for salt and drought stresses stimulate the growth and colonization of Pseudomonas putida KT2440 and Novosphingobium sp. HR1a in comparison to the exudates released by salt-sensitive genotypes. The two bacteria isolated from tolerant genotypes for two stresses also displayed a high level of β-galactosidase activity, proline, and salicylates in root exudates of tolerant genotypes, further strengthening the potential of these rhizobacteria to serve as bio-indicators of stress in the citrus host. Moreira et al. (2020) observed that the application of P. reactans EDP28, Pantoea alli ZS 3–6, and Rhizoglomus irregulare resulted in a remarkable increase in biomass and nutrient status of maize. It is ascribed to the significant rise of K+ and Na+ reduction in corn plants under saline stress, revealing the salinity-abating attributes of these tested microbes (Moreira et al. 2020). Besides, the plant–microbe interactions help the plant develop a rhizosheath on the root tip, which helps the plant protect against abiotic stresses that include soil acidity, nutrient deficiency (N and P), and water stress. However, the rhizosheath characteristics are greatly influenced by the physiology and morphology of plant root systems. Besides this, the interactions between plants and microbes on the root surface also play a significant role in regulating rhizosheath characteristics. The role of exopolysaccharide-producing bacteria (EPB) in modulating root exudates and soil aggregation has also been highlighted by Ilyas et al. (2020). Further, they also mentioned that, as a consequence of this, the size of the rhizosheath increased, which further enhanced the minerals and water absorption abilities of the plants. Based on the aforementioned information, it can be concluded that any strategy based upon the association of gene networks regulating rhizosheath formation and root traits can become a prospective strategy for managing the adverse effects of climate change on agriculture.
Previous published literature indicated that under abiotic stress, plants make alterations in their root architecture by exudating specific metabolites through roots in the rhizosphere, which helps them combat the stress situation (Salem et al. 2022; Koprivova and Kopriva 2022). In one such situation of phosphorus deficiency, the tall fescue in the presence of Neotyphodium spp. as an endophyte has been found to abet the P-deficiency stress by making changes in the chemical composition and root architecture of the rhizosphere by exuding out phenolic-like compounds (Verma et al. 2021). Furthermore, the phenolic-like compounds were found to have Al-chelating activity, which promoted aluminium sequestration on the root surface (Malinowski and Belesky 2000). In the case of Capsicum annuum, inoculation with the mycorrhizal fungus (Glomus mosseae) increased the root area by approximately 50%, induced high numbers of lateral roots, and promoted plant growth. Further, mycorrhization helped the plants cope with glyphosate-induced abiotic stress by plummeting mitotic activity and mitotic index as well as cell length in root apices (Ronco et al. 2008).
A large array of mechanisms operating during plant–microbe interactions and offering tolerance against abiotic stresses has a significant contribution to the promotion of sustainable agriculture. In one such transcriptomic study involving Trichoderma parareesei and tomato root exudates, the results of the spatio-temporal analysis revealed the priming effect of T. parareesei strain T6 in stimulating tomato defence responses towards biotic and abiotic stresses by upregulating the expression levels of lipoxygenase 1 (LOX1), ethylene-insensitive protein 2 (EIN2), SOS1 and the salicylic acid-related PR-1 gene (Rubio et al. 2014). Further, they also noticed that the interaction was beneficial for both of the partners. In another independent study by Mendoza-Mendoza et al. (2018), it has also been observed that the presence of sucrose in root exudates plays a vital function in Trichoderma-root interactions. They also noticed that the production of sucrose in root exudates as a consequence of Trichoderma-root interactions stimulates induced systemic tolerance (IST) against diseases and insect pest infestation by triggering oxidative bursts, enhancing salicylic acid synthesis by plants, and rapid secretion of elicitor-like proteins by Trichoderma spp. A similar type of mutualistic endosymbiosis has also been noticed in the case of Piriformospora indica, which offers protection against drought in Andrographis paniculata under laboratory conditions and has also been found to support higher production of diterpenoid lactones (e.g., andrographolide) with strong pharmacological actions (Nair and Manjula 2020). Under drought stress conditions, the plants colonized with P. indica have been recorded to support plant growth as well as release high levels of antioxidant enzymes, high proline content, and low malondialdehyde accumulation in contrast to control plants.
Role of Root Exudates in Recruiting Beneficial Microorganisms
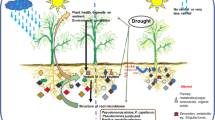
Recent studies emphasise the role of root exudates in plant–microbe interactions, specifically as a “cry for help” to recruit beneficial microorganisms and enhance plant defence under stress conditions (Rolfe et al. 2019; Yuan et al. 2023; Upadhyay et al. 2022; Liu et al. 2021; Bakker et al. 2018). It has been observed that the secretion of vanillic acid, fumaric acid, and p-coumaric acid from barley roots plays a role in persuading the colonization of Pseudomonas fluorescens CHA0 strains. This colonization triggers the rapid synthesis of 2,4-diacetylphloroglucinol (DAPG), contributing to the protection of barley from the invasion of Pythium ultimum (Jousset et al. 2011). Similarly, malic acid secretion by Arabidopsis thaliana roots is noted for recruiting Bacillus subtilis FB17, which aids in protecting Arabidopsis from Pseudomonas syringae infection (Yuan et al. 2018). Scopoletin is another metabolite that is identified as a crucial root exudate in the interaction between Arabidopsis thaliana and P. simiae WCS417. This compound helps to protect the host from fungal infections caused by Verticillium dahliae and Fusarium oxysporum (Stringlis et al. 2018a, b). Root secretion of benzonitrile, benzothiazole, dimethyl trisulfide, formic acid, and terpene-like compounds from tomato plants is observed under Fusarium oxysporum stress. These secreted compounds augment the root interactions with Bacillus sp., contributing to better resilience and defense mechanisms in tomato plants (Gulati et al. 2020). More recently, it has been documented that the roots of Panax notoginseng secrete cinnamic acid, 2-dodecenoic acid, and 12-oxo-phytodienoic acid metabolites under autotoxic ginsenoside stress. These secreted metabolites promote chemotaxis and colonisation of Burkholderia sp. B36 in the rhizosphere, ultimately enhancing the survival rate of P. notoginseng (Deng et al. 2023). These aforementioned points highlight the sophisticated and dynamic interplay between plants and their associated microbes, showcasing how root exudates play a crucial role in recruiting beneficial microorganisms and bolstering plant defence mechanisms.
Root Exudates in Mitigating Abiotic Stresses in Crop Plants
The role of root exudates in combating different abiotic stresses has been studied in several host plants (Table 2). Sun et al. (2021a, b) documented that the composition and quantity of root exudates were not only determined by the plant species and genotype but also relied on external environmental parameters. The impact of different abiotic stresses on root exudate production and their function in enhancing plant performance under different stress conditions is described in the following sections.
Drought
Drought is observed as one of the major abiotic factors that negatively limit plant growth and crop yield (Singh et al. 2023). It is estimated that agricultural land under extreme drought could rise to 30% by the end of the century under the current situation of climate change (Burke et al. 2006). Recently published reports indicated that drought stress greatly influences the root exudates and root-associated microbiomes in nature (Chen et al. 2022; Ulrich et al. 2022). Several published studies have revealed that the number of constituents (e.g., soluble sugars, amino acids, and organic acids) in root exudate composition increased with the rise in drought stress gradients (Ulrich et al. 2019; Song et al. 2012). Analogous reports of a significant rise in the composition of organic acids in maize root exudates (e.g., succinic acid, acetic acid, citric acid, lactic acid, and maleic acid, etc.) under drought stress have also been observed (Chen et al. 2022; Hummel 2010). Besides this, roots extract K+ from the soil, and it is exchanged with organic acids produced as root exudates. Several pieces of published literature have highlighted that potassium (K+) and organic acid in plant root exudates play a prime role in regulating osmotic stress under drought conditions (Chai and Schachtman 2022; Carvalhais et al. 2011). In the case of Quercus ilex, Gargallo-Garriga et al. (2018) have reported that a rise in the level of drought severity influenced 71% of the total metabolites in root exudates, where the majority of the secondary metabolites belonged to the alkaloids and terpenoids categories. It is interesting to reveal that the composition of the root exudates reallocates towards primary metabolites (~ 81% of the total metabolites) during the drought recovery stage. However, in severe drought situations, the alterations in the composition of root exudates become irretrievable, resulting in plant death. Increased terpenoid production has also been recorded in Camellia sinensis after exposing plants to diverse types of abiotic stress situations, including drought (Han-Chen et al. 2020). Rhizosheath, which serves as a vital aspect of plant endurance under drought stress, helps in providing strength to plants under drought conditions and is the consequence of the mucilage exudation by the plant roots. Mucilage helps in assembling the soil particles as well as in soil aggregate stabilisation around the roots (Ndour et al. 2020). Besides this, exopolysaccharides produced by several rhizobacteria also have a role in strengthening rhizosheath formation (Kaci et al. 2005). Canarini et al. (2016) demonstrated that sunflower plants under drought produce large amounts of exudates, but their composition remained unchanged. Contrarily, in the case of soybean plants, the profile of root exudates altered under drought stress, but the exudation rate remained unchanged. The drought stress was also reported to enhance root exudation of phytohormones (e.g., ABA) and organic osmolytes (e.g., proline, betaine, trehalose, and pinitol) (Gargallo-Garriga et al. 2018; Calvo et al. 2017). It is worth mentioning here that ABA regulates plant responses under both drought and saline stresses (Sah et al. 2016). Published reports also indicated that the exogenous treatment of ABA in the root tissues of rice and barley alters the expression of water channel aquaporins and root hydraulic conductivity (Ding et al. 2016; Sharipova et al. 2016). Besides this, plants minimise water loss under drought stress and sustain cell turgor through the intracellular accumulation of osmolytes (Sharma et al. 2019a, b). Various workers documented that the exudation of osmolytes and ABA in root exudates is due to their leakage from roots under drought conditions (Shi et al. 2015; Serraj et al. 2002). In the case of maize root exudates, an increase in the content of organic acids (succinic acid, malic acid, lactic acid, acetic acid, maleic acid, and citric acid) under drought has been observed by Song et al. (2012). Later on, Chen et al. (2022) also noticed a similar role of organic acids in providing osmotic stress tolerance in plants under drought. Besides these, a significant rise (~ 71%) in the release of organic carbon in the rhizosphere with major components of organic acids (succinic, fumaric, and malic acids) has also been documented by Vives-Peris et al. (2020). Similarly, in the case of Agropyron cristatum, it has been noticed that drought promotes a 71% higher release of organic carbon in the rhizosphere with higher quantities of succinic, fumaric, and malic acids. Besides the aforementioned reports, drought stress is also reported to enhance organic acid production by the corn roots, with malic acid as the major contributor, and to contribute to phosphate solubilization and plant growth promotion under drought stress conditions (Henry et al. 2007).
Salinity
Salinity stress is one of the prime concerns for sustainable crop production and is predicted to influence 20 and 30% of the total global arable and irrigated agricultural land, respectively (Bhardwaj et al. 2022; Kashyap et al. 2020). By 2050, it has been forecast that more than 50 percent of the global fertile agricultural soil will be salinized (Sharma et al. 2019a, b). Salinity negatively affects various aspects of plant development, including seed germination, plant growth, development, and yield (Zhang et al. 2020). Saline stress hampers the plant’s ability to carry out photosynthesis effectively. This is due to a decrease in the content of chlorophyll and carotenoids, distortion of the chloroplast ultrastructure, and damage to the photosystem II (PSII) system. These factors collectively reduce the plant’s capacity for photosynthesis (Pan et al. 2021). It has been observed that salinity also affects transpiration and gaseous exchange in plants, which reduces stomatal conductance, which is the ability of stomata to open and close, leading to decreased gas exchange between the plant and the atmosphere. Besides this, the presence of high salt levels in the soil lowers soil water potential and leaf water potential. This disrupts plant water relations and reduces the turgor pressure within plant cells, ultimately causing osmotic stress (Navada et al., 2020). Salinity also increases the production of reactive oxygen species (ROS) within plant cells, which can cause oxidative stress and lead to various forms of damage within plant cells, including lipid peroxidation, membrane deterioration, and damage to DNA and proteins (El Ghazali 2020). Generally, it has been found that saline soils represent a blend of salts of sodium, chloride, sulphate, magnesium, and calcium ions, with sodium chloride being the major player. Salinity causes the accumulation of ions such as sodium (Na+) and chloride (Cl) in plant tissues. This disrupts the uptake of essential ions such as potassium (K+) and calcium (Ca2+), leading to an ionic imbalance within the plant. This imbalance can have detrimental effects on various physiological processes (Isayenkov and Maathuis 2019). Yildirim et al. (2006) mentioned that salinity stress created a metabolic disorder in plants by enhancing Na+ uptake and lessening K+ and Ca2+ uptake. Besides this, the influence of salinity stress on the root exudation pattern of the plants is also observed (Vives-Peris et al. 2020). For instance, the level of flavonoids in root exudates of Phaseolus vulgaris and Glycine max increased when grown under saline stress (Dardanelli et al. 2010, 2012). Similarly, citrus root exudates exhibit enhanced levels of salicylates and proline in a saline environment (Vives-Peris et al. 2017). Singh and Jha (2016) also mentioned that the plants accumulate a series of osmolytes, for instance, proline, antioxidative enzymes, glycine betaine, and total soluble sugars, for acclimatisation under saline stress. Xie et al. (2020) conducted a study to investigate the impact of saline stress, specifically caused by sodium chloride (NaCl), on the amino acids present in the root exudates of Phragmites australis. The results of their research revealed that the plant may undergo biochemical changes in its root exudates, possibly altering the composition and secretion of total amino acids, as part of its strategy to cope with saline environments. Kumawat et al. (2022) reinforce the importance of organic molecules found in the root exudates of P. australis (e.g., trehalose, proline, acetylated glutamine dipeptides, carboxylamines, etc.) in helping halophilic microorganisms cope with salt stress. Under salt stress conditions, root exudates promote Ensifer meliloti CL 09 growth, which suggests that root exudates might be one of the factors for rhizosphere and endosphere microbial selection under salt stress (Moghaddam et al. 2016). In another study conducted by Lombardi et al. (2018), it was noticed that the root exudates released by plants under salt stress stimulate the growth of the beneficial rhizosphere fungus Trichoderma harzianum and act as chemoattractants.
Temperature
Temperature is another major factor that negatively influences plant growth and development (Walne and Reddy 2022; Hatfield and Prueger 2015). Several reports indicated that a rise of 3–4 °C in temperature could lead to a significant reduction of crop yield in the range of 15–35% in Asia, Africa, and the Middle East countries (Kashyap et al. 2018; Ortiz et al. 2008). A series of recent publications indicated that temperature influences both the quality and quantity of root exudates by regulating respiration, translocation, and photosynthesis in plants (Leuschner et al. 2022; Vives-Peris et al. 2020). Vives-Peris et al. (2018a) documented that the root exudates derived from heat-stressed plants grown at 30 °C do not reflect any major changes in the growth pattern of P. putida KT2440 and Novosphingobium sp. HR1a. However, root exudates derived from the plants grown at 40 °C showed a stimulatory impact on the growth of both of these bacteria. It was more pronounced in root exudates from the heat-responsive Macrophylla (Vives-Peris et al. 2018a). Besides this, the thermo-tolerant bacterium (P. putida AKMP7) also showed an array of plant growth-promoting attributes (e.g., production of HCN, ammonia, siderophore, phytohormones, P-solubilization, etc.) in addition to their contribution in conferring tolerance against heat stress in plants (Ali et al. 2011). It is worth mentioning that the plant growth-promoting bacteria release diverse types of growth regulators at the plant root interface that in turn help in stimulating root development and better absorption of nutrients and water from the soil surface (Zimmer et al. 1995). Recently, Tiziani et al. (2022) noticed that the composition of maize root exudates endures diverse types of modulation under heat stress in the root zone but is not significantly influenced by maize root types. Additionally, they also noticed that heat stress-specific exudates also alter the relative abundance of specific bacterial taxa, including beneficial microorganisms, in the host plants.
Nutrient Deficiecy
Plant developmental processes are greatly influenced by the availability of essential mineral nutrients in the soil. Plants primarily acquire mineral nutrients through the rhizosphere, which is the soil zone influenced by plant roots. In this region, microorganisms interact with plant products released in root exudates (Pantigoso et al. 2023). Mei et al. (2012) provided conclusive evidence regarding the role of root exudates in the symbiotic system of legumes, which usually requires a huge pool of P. They reported that the citrate secreted by the root chelates has the potential to enhance the N fixation capacity of root nodules in P-deprived soil (Mei et al. 2012). Similarly, Xia et al. (2016) noticed that the exudates of Michelia macclurei promote root growth and the establishment of Cunninghamia lanceolata by manipulating rhizosphere microflora for P acquisition. Other secondary metabolites, such as flavonoids and strigolactones, are also reported to facilitate plant association with mycorrhizal fungi under phosphorus-deficient conditions (Chai and Schachtman 2022). It has been noticed that in a nitrogen-deprived environment, root exudates assist plants in building strong associations with nitrogen-fixing microbes to check soil nitrogen losses through inhibition of the bacteria engaged in nitrification and denitrification processes. A series of research publications also indicated the potential of organic acid and acid phosphatase secretions from faba bean and chickpea roots in enhancing the absorption of P in maize plants (Zhang et al. 2020; Li et al. 2004). Similarly, Flavin’s exudation from Beta vulgaris also helps to improve Fe accessibility (Sisó-Terraza et al. 2016).
Organic acids (OAs) in root exudates are another vital player that contributes to protecting against plant stress because they can modify pH, behave as metal chelators, and also offer nutrients in the form of carbon sources for microbes (Panchal et al. 2021). Yang et al. (2019) observed that the high-potassium tobacco cultivar ND202 exuded a significantly higher level of available K content and organic acids in root exudates in comparison to common varieties, viz., K326 and NC89. Further, they also noticed that the ND202 cultivar maintained normal root vigour even under K-deficiency stress due to a rise in the production of stress-resistant related enzymes (e.g., POD, SOD, and CAT). The study by Gomez-Zepeda and colleagues (2021) also reported alternations in exudation rate and localization patterns of organic acids under P stress in the roots of Arabidopsis plants by using very advanced techniques able to detect organic acids at sub-micromolar levels. The authors felt that these findings are relevant for developing plant genotypes with augmented Pi-uptake. Besides the aforementioned reports, Javed et al. (2020) also documented the function of silicon (Si) application in abating the negative impact of cadmium (Cd) injury in Ajwain (Trachyspermum ammi). They also noticed that Si treatment also helps in enhancing the plant biomass, growth, antioxidant enzymes, photosynthetic apparatus, and mineral uptake in T. ammi. Besides this, a reduction in the exudation of organic acids and oxidative stress indicators in the stem and roots of T. ammi plants has also been noticed. In summary, the aforementioned reports established the significance of root exudates in stress amelioration and further highlighted the need for a better understanding of the pathways and mechanisms involved in the root exudate secretion that help in ameliorating plant stresses. Besides this, the merits of primary and secondary metabolites released from root exudates also need to be established as one of the prospective options for crop improvement as well as for abiotic stress amelioration for agricultural sustainability under climate change.
Light
Light has an important role in the root exudation process (Guo et al. 2023; Martin et al. 2018). Vives-Peris et al. (2020) mentioned that during the photosynthesis process, a part of the carbon-dioxide fixed is exuded out as root exudates because the process of photosynthesis is greatly influenced by light wavelength and photoperiod. For instance, Crocus sativus exposed to a longer photoperiod (14 h) released a higher amount of organic acids (e.g., benzoic and 4-hydroxybenzoic acids) in comparison to plants exposed to a short photoperiod of 10 h (Pramanik et al. 2000). Whereas, the circadian rhythm does not influence the root exudation process (Badri et al. 2010). He et al. (2016) noticed that the enhanced UV-B exposure led to a reduction in the resource allocation to the below-ground biomass and root exudation in the case of mire plants. Similarly, a decrease in the levels of malic acid and tartaric acid and an increase in the levels of succinic acid and oxalic acid in rice root exudates have been reported by Rinnan et al. (2006) in response to UV-B exposure. Recently, Zhou et al. (2022) experimentally proved that the total organic carbon (TOC) content of root exudates declines with the rise in light intensity when lettuce plants are grown under a hydroponic solution. Contrarily, increased light intensity (white light of 250 μmol m−2 s−1) significantly reduced the secretion of autotoxins (e.g., ferulic acid, benzoic acid, tannic acid, and gallic acid). Hence, the authors believe that little information is available on how and whether LED light quality impacts the root exudates of hydroponic leafy vegetables in a plant factory. Overall, the above-mentioned research strengthens the notion of the significant role of light in the root exudation process, and the authors think that there is a need to obtain a better understanding of plant exudates and soil microbiomes’ behaviour under light stress for the development of proficient and specific microbiota management strategies for agricultural sustainability.
Toxic Ions
Toxic ions, especially those of heavy metals, disturb the integrity of various cellular structures, including the cell wall and cell membrane. Toxic ions, especially in the root and leaf tissues, were reported to affect the activity of mitochondria and the cytoplasm, which further led to the generation of malondialdehyde (MDA) and reactive oxygen species (ROS) (Ghuge et al. 2023). They also influence the composition of fatty acids in plants, including linoleic acid and linolenic acid, and negatively influence membrane fluidity and function (Gill et al. 2022). It has been observed that ion toxicity disrupts the plant’s ability to take up water and essential nutrients from the soil. This can lead to water stress and nutrient deficiencies in the plant (Angulo-Bejarano et al. 2021). Ion toxicity can interfere with the plant’s transportation system, affecting the movement of sugars and nutrients from the upper to lower parts of the plant as well as posing a reduction in the chlorophyll content, negatively impacting photosystem II in chloroplasts, and resulting in decreased photosynthesis (Alengebawy et al. 2021). The presence of toxic ions in the soil can also influence the root exudation process (Vives-Peris et al. 2020). It has been noticed that aluminium toxicity in A. thaliana hosts helps in the rapid release of citrate and malate in root exudates in addition to their role in plant growth-promoting rhizobacteria recruitment (Kobayashi et al. 2013, Liu et al. 2009). The same observation has also been reported in the case of cadmium stress, which also alters the composition of root exudates in the Sedum alfredii host with the major involvement of trehalose, naphthalene, erythritol, n-octacosane, and d-pinitol in the Cd stabilisation process. Contrarily, the release of glycine, tetradecanoic acid, phosphoric acid, oxalic acid, and threonic acid is involved in the Cd mobilization process at the soil root interface (Luo et al. 2014). Recently, Jogawat et al. (2021) documented that heavy metal (e.g., Cd, Cu, and Pb) exposure leads to the production of phenolic acids, rutin, and chlorogenic acid in the case of corn plants. Comparative metabolic profiling analysis of root exudates of Sedum alfredo under Pb stress showed a significant change in the concentrations and species of root exudates, where l-proline, l-alanine, and oxalic acid help in the activation of Pb in the soil while glyceric acid and 2-hydroxyacetic acid have not much influence on Pb activation at the soil-root interface (Luo et al. 2017). Besides this, Sun et al. (2020) also documented that Cd stress showed notable alterations in the organic acids, lipids, and amino acid profiles of the root exudates of S. plumbizincicola. Further, it has also been observed that Cd stress suppresses organic acid metabolism and lipid metabolism by influencing the amino acid metabolic pathway of S. plumbizincicola under Cd stress. Leng et al. (2022) demonstrated that the phenolic root exudates have the potential to enhance the CD tolerance of Avicennia marina. They also noticed that the phenolic root exudates hinder functional bacteria-mediated iron and sulphur cycles and support Cd immobilisation in mangrove sediments. Besides this, flavonoids have also been reported to enhance Cd tolerance and offer a simulative influence on the symplasmic movement of Cd in A. marina roots (Li et al. 2015). Kidd (2001) documented the role of root exudates in Al resistance and Si-triggered amelioration of Al toxicity in three varieties (HS701b, Clavito, and Sikuani) of maize. In another independent study, Huang et al. (2023) demonstrated that amino acids and organic acids in the root exudates of Nicotiana tabacum play a crucial role in Cd uptake. However, their contribution is highly dependent on genotype type. Malate has also been reported to play a vital role in Al tolerance, and its levels in the root apex of Arabidopsis are regulated by NADP-ME1, while in its absence, the organic acid content increases. Besides this, malate is also reported to influence the signalling processes involved in the generation of reactive oxygen species (ROS) and glutamate, which are critical factors for root growth under aluminium stress (Badia et al. 2020). From the aforementioned reports, it is clear that further research is essential to get a true picture of the mechanisms operating behind root exudate secretion in response to ion toxicity at the soil-root interface.
Molecular Advances in the Understanding of the Functions of the Root Exudates
Several research publications suggested that genomics and metabolomic technologies have made a great impact and proved their relevance in crop improvement (Salem et al. 2022; Pang et al. 2021). Besides this, a noteworthy level of research has been made in the area of abiotic stress mitigation in plants, which can be accomplished by performing an in-depth analysis of the plant–microbe interactome. Different omics tools help in unzipping myriads of mechanisms associated with plant–microbe networks (Fig. 5). Hoekenga et al. (2006) also indicated that the Al-activated root malate efflux transporter (AtALMT1) performs a vital function in offering Al tolerance to the Arabidopsis host. It has been noticed that Al-toxicity in Arabidopsis thaliana hosts helps in the rapid release of citrate and malate in root exudates by activating the AtALMT1 and AtMATE1 genes, which act as Al3+ ion chelators, in addition to their role in plant growth-promoting rhizobacteria recruitment (Kobayashi et al. 2013, Liu et al. 2009). The genetic complementation studies have proved that two alleles, i.e., Ler and Col, are equally effective in providing Al tolerance and the liberation of Al-activated malate. However, it is important to mention here that although AtALMT1 is an indispensable aspect of Al tolerance, it does not contain a major QTL for Al tolerance. The Trichoderma-plant interaction (T. atroviride and T. harzianum) study performed by Tucci et al. (2011) was successful in revealing the significance of plant genotypic characters on the modulation of plant–microbe interactions, along with their influence on plant growth and stress mitigation. Similarly, Kumari et al. (2015) observed induced systemic tolerance (IST) in soybeans against salinity stress in response to the exogenous application of Pseudomonas sp. AK-1 and Bacillus sp. SJ-5 strains. Further, they also noticed that the enhancement in salt stress tolerance in plants is due to the rapid accumulation of proline and a rise in lipoxygenase activity. The role of OAs in enhancing aluminium (Al) tolerance in the roots of legume crops has been attributed to the reversal of Al-induced modifications in proteins and the metabolic regulation of plant-secreted microRNAs (Jaiswal et al. 2018). Further, it has also been observed that in the case of symbiotic rhizobia, Al tolerance is enhanced because of the production of siderophores, exopolysaccharides, activation of heavy metal-resistant efflux pumps, and up-regulation of the metal-inducible (dmeRF) gene clusters (Jaiswal et al. 2018). Zhalnina et al. (2018) used an integrated approach of comparative genomics and metabolomics to establish that the pre-programmed developmental processes in Avena barbata play an important role in defining the chemical composition of root exudates. In a similar fashion, by using an integrated microbiome and metabolomic analysis approach, Li et al. (2023) established that both drought and genotype play important roles in altering the compositions of rice rhizosphere bacterial communities and root exudates. Further, they also pointed out that suppression of amino acid exudation and organic acid exudation will serve as efficient approaches to selecting specific rhizosphere bacterial communities for rice to survive under drought stress. Wu et al. (2023) conducted a comprehensive investigation into the response of Stylosanthes guianensis (stylo) to low-phosphorus (Pi) conditions by integrating phenotypic, transcriptomic, metabolomic, and gene function analysis. They found upregulation of phosphate starvation response (PSR) genes of FLA, EXP, XTH, PAP, ABCG, and MATE families in the roots of stylo under low-Pi conditions. Further, they concluded that the PSR genes may contribute to the growth of roots, the increase in root-secreted APase activity, and the efflux of root-secreted flavonoids and organic acids in stylo, thereby promoting the recycling and utilization of soil insoluble-P and organic-P.
Research Gap, Challanges and the Way Foward
Most of the published literature indicated that phytochemical and protein secretions via roots are one of the prime and adaptive modes for plants to respond to and modify the microenvironment around roots. It has emerged from the research published over several decades that genomic and metabolic-driven technology revolutions help in generating novel information and offer a better understanding of the mechanisms or processes associated with root exudate-mediated communication among plants, soil, and other organisms dwelling in the soil. Several pieces of evidence indicate that the primary and secondary metabolites of root exudates can be employed in agricultural systems to augment crop production by activating plant defense systems against all forms of abiotic stress. However, emerging root exudation research advances in connection with serving as a strategy for plants to cope with abiotic stress indeed highlight several research gaps and crucial questions: (i) Does the composition and quantity of root exudates change under abiotic stress conditions?; (ii) How do different severity levels of abiotic stresses affect the dynamics of root exudation?; (iii) If there is a change in the root exudate profile, what are the specific compounds released by plants in response to abiotic stresses?; (v) Are these compounds known to have a role in salt stress tolerance, and do they interact with the soil environment?; (v) How does a plant regulate the production and release of root exudates in response to abiotic stresses?; (vi) Are there specific signaling pathways or molecular mechanisms involved in the plant’s ability to sense and respond to abiotic stresses stress through root exudation?; (vii) How do the altered root exudates influence the composition and function of the soil microbiome under abiotic stress conditions?; (viii) Are there specific microorganisms that are attracted or repelled by these exudates, and how does this affect the plant’s ability to cope with abiotic stresses? (ix) Do root exudates enhance nutrient availability or provide other benefits to soil microorganisms that, in turn, contribute to the plant’s abiotic stress tolerance? Answering these questions and filling the aforementioned research gap requires a multidisciplinary approach involving techniques from molecular biology, metabolomics, soil microbiology, and plant physiology. Advanced analytical methods, such as gas chromatography-mass spectrometry (GC–MS), liquid chromatography-mass spectrometry (LC–MS), capillary electrophoresis-mass spectrometry (CE-MS), Fourier transform near-infrared (FT-NIR), and nuclear magnetic resonance (NMR), could be useful for analysing the diverse classes of metabolites released by plant roots under different types of abiotic stresses. Similarly, for distinguishing plant-specific metabolites from those released by associated microbiomes, techniques such as mass spectrometry imaging (MSI), matrix-assisted laser desorption ionisation (MALDI), laser ablation electrospray ionisation (LAESI), and live single-cell mass spectrometry (LSC-MS) that offer metabolite analysis at the individual cell level will be useful, as metabolites are stable molecules and closer to the phenotype than genes and proteins. Moreover, an amalgamation of these analytical techniques may offer additional information for a comprehensive analysis of metabolites. Network analysis, machine learning, artificial intelligence, and ecological modelling can aid in establishing relationships among root exudates, microbial taxa, functional genes, and myriads of abiotic stress factors. New and innovative tools beyond metabolite-based communication, such as volatiles and small RNA, offer additional insights into the functionality and significance of root exudates at the plant-soil interface under abiotic stress conditions. Besides these, another big hurdle is the dissection and characterization of new transport systems to decode the potential regulatory mechanisms functionally involved in the process of root secretions. Genome-wide association studies (GWAS) combined with gene editing tools can identify and validate genes and metabolites influencing root exudate secretions and microbiome interactions in stressful environments. Therefore, multifaceted molecular and bioinformatic approaches should be explored to discover the interaction molecules of root exudates and rhizosphere microbiomes from a broad perspective. Besides the aforementioned points, the amalgamation of multi-omics and imaging technologies with a synthetic communities approach will be quite useful in pin-pointing and getting an accurate picture regarding the function of exudates in the interaction network between the phyllosphere (above-ground) and rhizosphere (below-ground) and vice versa. Altogether, it can be concluded that fundamental knowledge and emerging research concepts on root exudates’ behaviour, i.e., ‘cry of help’ (Rolfe et al. 2019), are necessary to have a better ecological and evolutionary understanding of the multitrophic interactions (both biotic and abiotic) for the sustainable development of agriculture. Recently, Ma et al. (2022a) have highlighted the ecological role of root exudates in promoting the rhizosphere priming effect (RPE). Root exudates contribute to RPE by directly supplying a carbon source for microorganisms and indirectly by destabilizing organic carbon. Besides direct involvement of root exudates in material cycles, they also serve as signaling agents. Signal substances such as phenols, flavins, and other secondary metabolites act as operators, establishing a communication bridge between plants and microorganisms, as well as between different plants (van der Heijden et al. 2008; Wardle 2004). Several ecological studies also reveled that plant allelopathy, replant disease, and interspecific facilitation in intercropping systems are influenced by the integrative effects of plant–microbe interactions mediated by root exudates (Zhou et al. 2023a, b; Mwendwa et al. 2021; Xu et al. 2021). For instance, root exudates of Rehmannia glutinosa are reported to stimulate the proliferation of Fusarium oxysporum. This, in turn, alters the expression patterns of Leucine-rich repeat receptor-like protein kinases, disrupts the growth and development of R. glutinosa, and leads to the formation of replant disease (Yang et al. 2023). The negative effects of replant disease in R. glutinosa are alleviated by intercropping with Achyranthes bidentata. This alleviation is attributed to the modulation of root exudates and improvement of the rhizosphere microenvironment. Li et al. (2020) investigate bacterial and fungal communities in various niches of Casuarina equisetifolia finding that ecological niche selection plays a role in shaping the assembly and diversity of microbial communities. Published literature also suggests that despite numerous initiatives to develop effective microbial consortia, there are still challenges to achieving consistent results in the field. The focus on root exudates and their functional interplay with beneficial microbes, both directly and through gene expression, will be one of the potential solutions. Therefore, researchers should emphasize the recruitment of plant growth-promoting microbes through root exudates that can enhance root colonization and establish sustainable relationships between the microbes and plants under abiotic stress conditions. This hypothesis of specific recruitment could be one of the most promising techniques to improve the performance of plant growth-promoting microbes in real-world agricultural settings.
Conclusion
Root exudates, which account for about 21% of the net photosynthates, are not a wasteful activity as their composition (quality and quantity) is regulated by crop stage, plant genotype, soil nutrient status, environmental conditions, etc. The plant adapts under adverse conditions by exuding out myriads of primary and secondary metabolites, which in turn attract a new set of microorganisms towards the root surface, facilitate nutrient cycles, or induce changes in root architecture that ultimately contribute to the higher availability of nutrients and water to the plant. The induction of root exudation by environmental conditions and their positive role in imparting abiotic stress tolerance further opens new vistas for developing new crop varieties. Root exudate profiles and exogenous use of selected exudates allow manipulation of the field conditions, which further help in supporting plant growth under abiotic stress conditions. Moreover, the recruitment of plant growth-promoting microbes through root exudates that can enhance root colonization and establish sustainable relationships between the microbes and plants under abiotic stress conditions would be a promising technique for enhancing abiotic stress tolerance in plants in real-world agricultural settings.
References
Ahkami AH, WhiteHandakumbura RAPP, Jansson C (2017) Rhizosphere engineering: enhancing sustainable plant ecosystem productivity. Rhizosphere 3:233–243
Alengebawy A, Abdelkhalek ST, Qureshi SR, Wang MQ (2021) Heavy metals and pesticides toxicity in agricultural soil and plants: ecological risks and human health implications. Toxics 9(3):42. https://doi.org/10.3390/toxics9030042
Ali Q, Ashraf M (2011) Induction of drought tolerance in maize (Zea mays L.) due to exogenous application of trehalose: growth, photosynthesis, water relations and oxidative defence mechanism. J Agron Crop Sci 197:258–271
Ali SZ, Sandhya V, Grover M, Linga VR, Bandi V (2011) Effect of inoculation with a thermotolerant plant growth promoting Pseudomonas putida strain AKMP7 on growth of wheat (Triticum spp.) under heat stress. J Plant Interact 6:239–246
Ali Q, Anwar F, Ashraf M, Saari N, Perveen R (2013) Ameliorating effects of exogenously applied proline on seed composition, seed oil quality and oil antioxidant activity of maize (Zea mays L.) under drought stress. Int J Mol Sci 14:818–835
Angulo-Bejarano PI, Puente-Rivera J, Cruz-Ortega R (2021) Metal and metalloid toxicity in plants: an overview on molecular aspects. Plants (Basel) 10(4):635. https://doi.org/10.3390/plants10040635
Anwer S, Ashraf MY, Hussain M, Ashraf M, Jamil A (2012) Citric acid mediated phytoextraction of cadmium by maize (Zea mays L.). Pak J Bot 44:1831–1836
Badia MB, Maurino VG, Pavlovic T, Arias CL, Pagani MA, Andreo CS, Saigo M, Drincovich MF (2020) Loss of function of Arabidopsis NADP-malic enzyme 1 results in enhanced tolerance to aluminium stress. Plant J 101:653–665
Badri DV, Vivanco JM (2009) Regulation and function of root exudates. Plant Cell Environ 32:666–681
Badri DV, Loyola-Vargas VM, Broeckling CD, De-la-Peña C, Jasinski M, Santelia D, Martinoia E, Sumner LW (2008a) Altered profile of secondary metabolites in the root exudates of Arabidopsis ATP-binding cassette transporter mutants. Plant Physiol 146:762–771
Badri DV, Loyola-Vargas VM, Du J, Stermitz FR, Broeckling CD, Iglesias-Andreu L, Vivanco JM (2008b) Transcriptome analysis of Arabidopsis roots treated with signaling compounds: a focus on signal transduction, metabolic regulation and secretion. New Phytol 179:209–223
Badri DV, Weir TL, Van der Lelie D, Vivanco JM (2009) Rhizosphere chemical dialogues: plant–microbe interactions. Curr Opin Biotechnol 20:642–650
Badri DV, Loyola-Vargas VM, Broeckling CD, Vivanco JM (2010) Root secretion of phytochemicals in Arabidopsis is predominantly not influenced by diurnal rhythms. Mol Plant 3:491–498
Baetz U, Martinoia E (2014) Root exudates: the hidden part of plant defense. Trends Plant Sci 19(2):90–98
Bakker PAHM, Pieterse CMJ, de Jonge R, Berendsen RL (2018) The soil-borne legacy. Cell 172:1178–1180
Banasiak J, Jasiński M (2022) ATP-binding cassette transporters in nonmodel plants. New Phytol 233(4):1597–1612
Battey NH, Blackbourn HD (1993) The control of exocytosis in plant cells. New Phytol 125:307–308
Bechtold U, Field B (2018) Molecular mechanisms controlling plant growth during abiotic stress. J Exp Bot 69:2753–2758
Bekkara F, Jay M, Viricel MR, Rome S (1998) Distribution of phenolic compounds within seed and seedlings of two Vicia faba cvs differing in their see tannin content and study of their seed and root phenolic exudations. Plant Soil 203:27–36
Bertin C, Yang X, Weston LA (2003) The role of root exudates and allelochemicals in the rhizosphere. Plant Soil 256:67–83
Bhardwaj AK, Arya G, Kumar R, Hamed L, Pirasteh-Anosheh H, Jasrotia P, Kashyap PL, Singh GP (2022) Switching to nanonutrients for sustaining agroecosystems and environment: the challenges and benefits in moving up from ionic to particle feeding. J Nanobiotechnol 20:19. https://doi.org/10.1186/s12951-021-01177-9
Bharti N, Pandey SS, Barnawal D, Kumar V, Kalra A (2016) Plant growth promoting rhizobacteria Dietzia natronolimnaea modulates the expression of stress responsive genes providing protection of wheat from salinity stress. Sci Rep 6:34768
Bhat MA, Kumar V, Bhat MA, Wani IA, Dar FL, Farooq I, Bhatti F, Koser R et al (2020) Mechanistic insights of the interaction of plant growth-promoting rhizobacteria (PGPR) with plant roots toward enhancing plant productivity by alleviating salinity stress. Front Microbiol 11:1952
Burke EJ, Brown SJ, Christidis N (2006) Modeling the recent evolution of global drought and projections for the twenty-first century with the Hadley centre climate model. J Hydrometeorol 7:1113–1125
Calvo OC, Franzaring J, Schmid I, Müller M, Brohon N, Fangmeier A (2017) Atmospheric CO2 enrichment and drought stress modify root exudation of barley. Glob Change Biol 23:1292–1304
Canarini A, Merchant A, Dijkstra FA (2016) Drought effects on Helianthus annuus and Glycine max metabolites: from phloem to root exudates. Rhizosphere 2:85–97
Canarini A, Kaiser C, Merchant A, Richter A, Wanek W (2019) Root exudation of primary metabolites: mechanisms and their roles in plant responses to environmental stimuli. Front Plant Sci 10:157. https://doi.org/10.3389/fpls.2019.00157
Carvalhais LC, Dennis PG, Fedoseyenko D, Hajirezaei MR, Borriss R, von Wiren N (2011) Root exudation of sugars, amino acids, and organic acids by maize as affected by nitrogen, phosphorus, potassium, and iron deficiency. J Plant Nutr Soil Sci 174:3–11. https://doi.org/10.1002/jpln.201000085
Chai YN, Schachtman DP (2022) Root exudates impact plant performance under abiotic stress. Trends Plant Sci 27:80–91
Chandra D, Srivastava R, Glick BR, Sharma AK (2020) Rhizobacteria producing ACC deaminase mitigate water-stress response in finger millet (Eleusine coracana (L.) Gaertn.). Biotech 10:65
Chen HJ, Hou WC, Kuc J, Lin YH (2001) Ca2+-dependent and Ca2+-independent excretion modes of salicylic acid in tobacco cell suspension culture. J Exp Bot 52:1219–1226
Chen Y, Yao Z, Sun Y, Wang E, Tian C, Sun Y, Liu J, Sun C (2022) Current studies of the effects of drought stress on root exudates and rhizosphere microbiomes of crop plant species. Int J Mol Sci 23:2374
Daei G, Ardekani MR, Rejali F, Teimuri S, Miransari M (2009) Alleviation of salinity stress on wheat yield, yield components, and nutrient uptake using arbuscular mycorrhizal fungi under field conditions. J Plant Physiol 66:617–625
Dardanelli MS, Manyani H, González-Barroso S, Rodríguez-Carvajal MA, Gil-Serrano AM, Espuny MR, López-Baena J, Bellogín RA (2010) Effect of the presence of the plant growth promoting rhizobacterium (PGPR) Chryseobacterium balustinum Aur9 and salt stress in the pattern of flavonoids exuded by soybean roots. Plant Soil 328:483–493
Dardanelli MS, de Córdoba FJF, Estévez J, Contreras R, Cubo MT, Rodríguez-Carvajal MA, Gil-Serrano AM, López-Baena FJ (2012) Changes in flavonoids secreted by Phaseolus vulgaris roots in the presence of salt and the plant growth-promoting rhizobacterium Chryseobacterium balustinum. Appl Soil Ecol 57:31–38
De Andrade SAL, Borghi AA, De Oliveira VH, Gouveia LdM, Martins API, Mazzafera P (2022) Phosphorus shortage induces an increase in root exudation in fifteen Eucalypts species. Agronomy 12(9):2041. https://doi.org/10.3390/agronomy12092041
De Schepper V, De Swaef T, Bauweraerts I, Steppe K (2013) Phloem transport: a review of mechanisms and controls. J Exp Bot 64:4839–4850
Deng L, Luo L, Li Y, Wang L, Zhang J, Zi B, Ye C, Liu Y, Huang H, Mei X, Deng W, He X, Zhu S, Min Yang M (2023) Autotoxic ginsenoside stress induces changes in root exudates to recruit the beneficial Burkholderia Strain B36 as revealed by transcriptomic and metabolomic approaches. J Agric Food Chem 71(11):4536–4549. https://doi.org/10.1021/acs.jafc.3c00311
Dilnashin H, Birla H, Hoat TX, Singh HB, Singh SP, Keswani C (2020) Applications of agriculturally important microorganisms for sustainable crop production. Molecular aspects of plant beneficial microbes in agriculture. Academic Press, Cambridge, pp 403–415
Ding L, Li Y, Wang Y, Gao L, Wang M, Chaumont F, Shen Q, Guo S (2016) Root ABA accumulation enhances rice seedling drought tolerance under ammonium supply: interaction with aquaporins. Front Plant Sci 7:1206
Dinkeloo K, Boyd S, Pilot G (2018) Update on amino acid transporter functions and on possible amino acid sensing mechanisms in plants. Semin Cell Dev Biol 74:105–113
Doneva D, Pal M, Brankova L, Szalai G, Tajti J, Khalil R, Ivanovska B, Velikova V (2021) The effects of putrescine pre-treatment on osmotic stress responses in drought-tolerant and drought-sensitive wheat seedlings. Physiol Plant 171:200–216
Driouich A, Gaudry A, Pawlak B, Moore JP (2021) Root cap-derived cells and mucilage: a protective network at the root tip. Protoplasma 258(6):1179–1185. https://doi.org/10.1007/s00709-021-01660-y
Dwivedi P, Sopory SK (2023) Unravelling the mechanism of stress responses in crop plants. J Plant Growth Regul. https://doi.org/10.1007/s00344-023-11104-x
El-Daim IAA, Bejai S, Meijer J (2014) Improved heat stress tolerance of wheat seedlings by bacterial seed treatment. Plant Soil 379:337–350
ElGhazali GEB (2020) Suaeda vermiculata Forssk ex. J.F. Gmel.: structural characteristics and adaptations to salinity and drought: a review. Intermt J Sci 9:28–33. https://doi.org/10.18483/ijSci.2268
El-Hawary M, Nashed ME (2019) Effect of foliar application by some antioxidants on growth and productivity of maize under saline soil conditions. J Plant Prod 10:93–99
Elkeilsh A, Awad YM, Soliman MH, Abu-Elsaoud A, Abdelhamid MT, El-Metwally IM (2019) Exogenous application of b-sitosterol mediated growth and yield improvement in water-stressed wheat (Triticum aestivum) involves up-regulated antioxidant system. J Plant Res 132:881–901
Farooq M, Wahid A, Lee DJ (2009) Exogenously applied polyamines increase drought tolerance of rice by improving leaf water status, photosynthesis and membrane properties. Acta Physiol Plant 31:937–945
Feng H, Fu R, Hou X, Lv Y, Zhang N, Liu Y, Xu Z, Miao Y (2021) Chemotaxis of beneficial rhizobacteria to root exudates: the first step towards root–microbe rhizosphere interactions. Int J Mol Sci 22(13):6655. https://doi.org/10.3390/ijms22136655
Field B, Jordan F, Osbourn A (2006) First encounters—deployment of defence-related natural products by plants. New Phytol 172:193–207
Fujii K, Hayakawa C (2021) Root exudation and biodegradation of organic acids in a tropical forest soil under dipterocarp and pioneer trees. Plant Soil 469:213–226. https://doi.org/10.1007/s11104-021-05132-3
Gani U, Vishwakarma RA, Misra P (2021) Membrane transporters: the key drivers of transport of secondary metabolites in plants. Plant Cell Rep 40:1–18. https://doi.org/10.1007/s00299-020-02599-9
Gargallo-Garriga A, Preece C, Sardans J, Oravec M, Urban O, Penuelas J (2018) Root exudate metabolomes change under drought and show limited capacity for recovery. Sci Rep 8:1–15
Ghafoor R, Akram NA, Rashid M, Ashraf M, Iqbal M, Lixin Z (2019) Exogenously applied proline induced changes in key anatomical features and physio-biochemical attributes in water stressed oat (Avena sativa L.) plants. Physiol Mol Biol Plants 25:1121–1135
Ghuge SA, Nikalje GC, Kadam US, Suprasanna P, Hong JC (2023) Comprehensive mechanisms of heavy metal toxicity in plants, detoxification, and remediation. J Hazard Mater 450:131039
Gill RA, Kanwar MK, Rodrigues dos Reis A, Ali B (2022) Editorial: heavy metal toxicity in plants: recent insights on physiological and molecular aspects. Front Plant Sci 12:830682. https://doi.org/10.3389/fpls.2021.830682
Gomez-Zepeda D, Frausto M, Nájera-González HR, Herrera-Estrella L, Ordaz-Ortiz JJ (2021) Mass spectrometry-based quantification and spatial localization of small organic acid exudates in plant roots under phosphorus deficiency and aluminium toxicity. Plant J 106:1791–1806
Grotewold E (2004) The challenges of moving chemicals within and out of cells: Insights into the transport of plant natural products. Planta 219:906–909
Grover M, Madhubala R, Ali SZ, Yadav SK, Venkateswarlu B (2014) Influence of Bacillus spp. strains on seedling growth and physiological parameters of sorghum under moisture stress conditions. J Basic Microbiol 54:951–961
Gulati S, Ballhausen MB, Kulkarni P, Grosch R, Garbeva P (2020) A non-invasive soil-based setup to study tomato root volatiles released by healthy and infected roots. Sci Rep 10(1):1–11. https://doi.org/10.1038/s41598-020-69468-z
Guo Z, Qin Y, Lv J, Wang X, Ye T, Dong X, Du N, Zhang T, Piao F, Dong H, Shen S (2023) High red/far-red ratio promotes root colonization of Serratia plymuthica A21–4 in tomato by root exudates-stimulated chemotaxis and biofilm formation. Plant Biochem. https://doi.org/10.1101/2023.07.06.547930
Han-Chen Z, Ferdinand SL, Xing-Kai Y, Yan Z, Shu W (2020) Analysis of terpene synthase family genes in Camellia sinensis with an emphasis on abiotic stress conditions. Sci Rep 10:933
Hasanuzzaman M, Nahar K, Rahman A, Inafuku M, Oku H, Fujita M (2018) Exogenous nitric oxide donor and arginine provide protection against short-term drought stress in wheat seedlings. Physiol Mol Biol Plants 24:993–1004
Hassan N, Ebeed H, Aljaarany A (2020) Exogenous application of spermine and putrescine mitigate adversities of drought stress in wheat by protecting membranes and chloroplast ultra-structure. Physiol Mol Biol Plants 26:233–245
Hatfield JL, Rueger JH (2015) Temperature extremes: effect on plant growth and development. Weather Clim Extremes 10:4–10
Hayat S, Faraz A, Faizan M (2017) Root exudates: composition and impact on plant-microbe interaction. In: Ahmad I, Husain FM (eds) Biofilms in plant and soil health. John Wiley & Sons Ltd., Hoboken, pp 179–193
He YM, Zhan FD, Li Y, Xu WW, Zu YQ, Yue M (2016) Effect of enhanced UV-B radiation on methane emission in a paddy field and rice root exudation of low-molecular-weight organic acids. Photochem Photobiol Sci 15(6):735–737
Henry A, Doucette W, Norton J, Bugbee B (2007) Changes in crested wheat grass root exudation caused by food, drought, and nutrient stress. J Environ Qual 36:904–912
Hiltner L (1904) Uber neure erfahrungen und probleme auf dem Gebiet der Boden-bakteriologie und unter besondere Berucksichtigung der grundungung und Bracke. Arbeit Deut Landw Ges Berlin 98:59–78
Hoekenga OA, Maron LG, Piñeros MA, Cançado GM, Shaff J, Kobayashi Y, Ryan PR, Dong B (2006) AtALMT1, which encodes a malate transporter, is identified as one of several genes critical for aluminium tolerance in Arabidopsis. Proc Natl Acad Sci USA 103:9738–9743
Hu H, Tang C, Rengel Z (2005) Role of phenolics and organic acids in phosphorus mobilization in calcareous and acidic soils. J Plant Nutr 28:1427–1439
Huang H, Lu R, Zhan J, He J, Wang Y, Li T (2023) Role of root exudates in cadmium accumulation of a low-cadmium-accumulating tobacco line (Nicotiana tabacum L.). Toxics 11(2):141. https://doi.org/10.3390/toxics11020141
Hummel I, Pantin F, Sulpice R, Piques M, Rolland G, Dauzat M, Christophe A, Pervent M (2010) Arabidopsis plants acclimate to water deficit at low cost through changes of carbon usage: an integrated perspective using growth, metabolite, enzyme, and gene expression analysis. Plant Physiol 154:357–372
Hurley BA, Tran HT, Marty NJ, Park J, Snedden WA, Mullen RT, Plaxton WC (2010) The dual-targeted purple acid phosphatase isozyme AtPAP26 is essential for efficient acclimation of Arabidopsis to nutritional phosphate deprivation. Plant Physiol 153:1112–1122
Ilyas N, Mumtaz K, Akhtar N, Yasmin H, Sayyed RZ, Khan W, Enshasy HAE, Dailin DJ, Elsayed EA, Ali Z (2020) Exopolysaccharides producing bacteria for the amelioration of drought stress in wheat. Sustainability 12(21):8876. https://doi.org/10.3390/su12218876
Isayenkov SV, Maathuis FJM (2019) Plant salinity stress: many unanswered questions remain. Front Plant Sci 10:80
Jaiswal SK, Naamala J, Dakora FD (2018) Nature and mechanisms of aluminium toxicity, tolerance and amelioration in symbiotic legumes and rhizobia. Biol Fertil Soils 54:309–318
Javed MT, Saleem MH, Aslam S, Rehman M, Iqbal N, Begum R, Ali S, Alsahli AA (2020) Elucidating silicon-mediated distinct morpho-physio-biochemical attributes and organic acid exudation patterns of cadmium stressed Ajwain (Trachyspermum ammi L.). Plant Physiol Biochem 157:23–37
Jogawat A, Yadav B, Chhaya NOP (2021) Metal transporters in organelles and their roles in heavy metal transportation and sequestration mechanisms in plants. Physiol Plant 173:259–275
Jousset A, Schulz W, Scheu S et al (2011) Intraspecific genotypic richness and relatedness predict the invasibility of microbial communities. ISME J 5:1108–1114. https://doi.org/10.1038/ismej.2011.9
Kaci Y, Heyraud A, Barakat M, Heulin T (2005) Isolation and identification of an EPS producing Rhizobium strain from arid soil (Algeria): characterization of its EPS and the effect of inoculation on wheat rhizosphere soil structure. Res Microbiol 156:522–531
Kandoudi W, Németh-Zámboriné É (2022) Stimulating secondary compound accumulation by elicitation: Is it a realistic tool in medicinal plants in vivo? Phytochem Rev 21:2007–2025. https://doi.org/10.1007/s11101-022-09822-3
Kashyap PL, Rai P, Srivastava AK, Kumar S (2017) Trichoderma for climate resilient agriculture. World J Microbiol Biotechnol 33:155. https://doi.org/10.1007/s11274-017-2319-1
Kashyap PL, Rai P, Kumar R, Sharma S, Jasrotia P, Srivastava AK et al (2018) Microbial nanotechnology for climate resilient agriculture. Microbes for climate resilient agriculture. Wiley, Hoboken, pp 279–344
Kashyap PL, Solanki MK, Kushwaha P, Srivastava KS (2020) Biocontrol potential of salt-tolerant Trichoderma and hypocrea isolates for the management of tomato root rot under saline environment. J Soil Sci Plant Nutr 20:160–176. https://doi.org/10.1007/s42729-019-00114-y
Kashyap PL, Kumar S, Aggarwal SK, Kaul N, Jasrotia P, Gupta A, Singh GP (2021) Resistance inducers and their role in reinforcing wheat defense system against fungal pathogens. J Cereal Res 13(3):229–254. https://doi.org/10.25174/2582-2675/2022/112810
Kashyap PL, Kumar S, Jasrotia P, Kumar RS, Sharma A, Tripathi R, Singh DP, Singh GP (2022) Induced resistance for sustainable management of wheat diseases. In: Sengar R, Chaudhary R, Bhadauriya H (eds) Handbook of research on green technologies for sustainable management of agricultural resources. IGI Global, Hershey, pp 385–408
Khan N, Ali S, Shahid MA, Mustafa A, Sayyed RZ, Curá JA (2021) Insights into the interactions among roots, rhizosphere, and rhizobacteria for improving plant growth and tolerance to abiotic stresses: a review. Cells 10:1551
Kidd P, Llugany M, Poschenrieder C, Gunse B, Barcelo J (2001) The role of root exudates in aluminium resistance and silicon-induced amelioration of aluminium toxicity in three varieties of maize (Zea mays L.). J Exp Bot 52:1339–1352
Knudson L (1920) The secretion of invertase by plant roots. Am J Bot 7:371–379
Kobayashi Y, Lakshmanan V, Kobayashi Y, Asai M, Iuchi S, Kobayashi M, Bais HP, Koyama H (2013) Overexpression of AtALMT1 in the Arabidopsis thaliana ecotype Columbia results in enhanced Al-activated malate excretion and beneficial bacterium recruitment. Plant Signal Behav 8:e25565
Koprivova A, Kopriva S (2022) Plant secondary metabolites altering root microbiome composition and function. Curr Opin Plant Biol 67:102227
Kosová K, Vítámvás P, Urban MO, Prášil IT, Renaut J (2018) Plant abiotic stress proteomics: the major factors determining alterations in cellular proteome. Front Plant Sci 9:122
Kubi J (2005) The effect of exogenous spermidine on superoxide dismutase activity, H2O2 and superoxide radical level in barley leaves under water deficit conditions. Acta Physiol Plant 27:289–295
Kumar R, Kumar S, Srivastava S, Kashyap PL, Kumar A, Shekhar RK, Singh GP (2022) Rhizosphere microbes and wheat health management. In: Singh UB, Sahu PK, Singh HV, Sharma PK, Sharma SK (eds) Rhizosphere microbes: microorganisms for sustainability. Springer, Singapore, pp 223–242
Kumari S, Vaishnav A, Jain S, Varma A, Choudhary DK (2015) Bacterial-mediated induction of systemic tolerance to salinity with expression of stress alleviating enzymes in soybean (Glycine max L. Merrill). J Plant Growth Regul 34:558–573
Kumari B, Mallick MA, Solanki MK, Solanki AC, Hora A, Guo W (2019) Plant growth promoting rhizobacteria (PGPR): modern prospects for sustainable agriculture. In: Ansari R, Mahmood I (eds) Plant health under biotic stress. Springer, Singapore, pp 109–127
Kumawat C, Kumar A, Parshad J, Sharma SS, Patra A, Dogra P, Yadav GK, Dadhich SK, Verma R, Kumawat GL (2022) Microbial diversity and adaptation under salt-affected soils: a review. Sustainability 14:9280
Kushwaha P, Kashyap PL (2021) A review of advances in bioremediation of heavy metals by microbes and plants. J Nat Resour Conserv Manag 2(1):65–80
Lee YH, Foster J, Chen J, Voll LM, Weber AP, Tegeder M (2007) AAP1 transports uncharged amino acids into roots of Arabidopsis. Plant J 50:305–319
Leng Z, Wu Y, Li J, Nie Z, Jia H, Yan C, Hong H, Wang X, Du D (2022) Phenolic root exudates enhance Avicennia marina tolerance to cadmium under the mediation of functional bacteria in mangrove sediments. Mar Pollut Bull 185(Pt A):114227. https://doi.org/10.1016/j.marpolbul.2022.114227
Leuschner C, Tückmantel T, Meier IC (2022) Temperature effects on root exudation in mature beech (Fagus sylvatica L.) forests along an elevational gradient. Plant Soil 481:147–163. https://doi.org/10.1007/s11104-1800022-05629-5
Li SM, Li L, Zhang FS, Tang C (2004) Acid phosphatase role in chickpea/maize intercropping. Ann Bot 94(2):297–303
Li J, Lu H, Liu J, Hong H, Yan C (2015) The influence of flavonoid amendment on the absorption of cadmium in Avicennia marina roots. Ecotoxicol Environ Saf 120:1–6. https://doi.org/10.1016/j.ecoenv.2015.05.004
Li GX, Wu XQ, Ye JR, Yang HC (2018a) Characteristics of organic acid secretion associated with the interaction between Burkholderia multivorans WS-FJ9 and poplar root system. Bio Med Res Int 2018:9619724
Li L, Gu W, Li J, Li C, Xie T, Qu D, Meng Y, Li C (2018b) Exogenously applied spermidine alleviates photosynthetic inhibition under drought stress in maize (Zea mays L.) seedlings associated with changes in endogenous polyamines and phytohormones. Plant Physiol Biochem 129:35–55
Li Q, Wu Y, Wang J, Yang B, Chen J, Wu H, Zhang Z, Lu C, Lin W, Wu L (2020) Linking short-chain N-acyl homoserine lactone-mediated quorum sensing and replant disease: a case study of rehmannia glutinosa. Front Plant Sci 11:787. https://doi.org/10.3389/fpls.2020.00787
Li G, Wang K, Qin Q, Li Q, Mo F, Nangia V, Liu Y (2023) Integrated microbiome and metabolomic analysis reveal responses of rhizosphere bacterial communities and root exudate composition to drought and genotype in rice (Oryza sativa L.). Rice 16:19. https://doi.org/10.1186/s12284-023-00636-1
Liu J, Magalhaes JV, Shaf J, Kochian LV (2009) Aluminium-activated citrate and malate transporters from the MATE and ALMT families function independently to confer Arabidopsis aluminium tolerance. Plant J 57:389–399
Liu H, Brettell LE, Qiu Z, Singh BK (2020) Microbiome-mediated stress resistance in plants. Trends Plant Sci 25:733–743
Liu H, Li J, Carvalhais LC, Percy CD, Prakash Verma J, Schenk PM et al (2021) Evidence for the plant recruitment of beneficial microbes to suppress soil-borne pathogens. New Phytol 229(5):2873–2885. https://doi.org/10.1111/nph.17057
Lombardi N, Vitale S, Turrà D, Reverberi M, Fanelli C, Vinale F, Marra R, Ruocco M, Pascale A, d’Errico G, Woo SL, Lorito M (2018) Root exudates of stressed plants stimulate and attract Trichoderma soil fungi. Mol Plant Microbe Interact 31(10):982–994. https://doi.org/10.1094/MPMI-12-17-0310-R
Loyola-Vargas VM, Broeckling CD, Dayakar BV, Vivanco JM (2007) Effect of transporters on the secretion of phytochemicals by the roots of Arabidopsis thaliana. Planta 225:301–310
Luo Q, Sun L, Hu X, Zhou R (2014) The variation of root exudates from the hyperaccumulator Sedum alfredii under cadmium stress: metabolomics analysis. PLoS ONE 9:e115581
Luo Q, Wang S, Sun LN, Wang H (2017) Metabolic profiling of root exudates from two ecotypes of Sedum alfredii treated with Pb based on GC-MS. Sci Rep 7:39878. https://doi.org/10.1038/srep39878
Lyon TL, Wilson JK (1921) Liberation of organic matter by roots of growing plants. Cornell University, New York
Ma W, Tang S, Dengzeng Z, Zhang D, Zhang T, Ma X (2022a) Root exudates contribute to belowground ecosystem hotspots: a review. Front Microbiol 13:937940. https://doi.org/10.3389/fmicb.2022.937940
Malinowski DP, Belesky DP (2000) Adaptations of endophyte-infected cool-season grasses to environmental stresses. Crop Sci 40:923–940
Manck-Götzenberger J, Requena N (2016) Arbuscular mycorrhiza symbiosis induces a major transcriptional reprogramming of the potato SWEET sugar transporter family. Front Plant Sci 7:487
Marcinska I, Dziurka K, Waligorski P, Janowiak F, Skrzypek E, Warchoł M, Juzon K, Kapłoniak K (2020) Exogenous polyamines only indirectly induce stress tolerance in wheat growing in hydroponic culture under polyethylene glycol-induced osmotic stress. Life 10:151
Martin BC, Gleeson D, Statton J, Siebers AR, Grierson P, Ryan MH et al (2018) Low light availability alters root exudation and reduces putative beneficial microorganisms in seagrass roots. Front Microbiol 8:02667
Marulanda A, Azcón R, Chaumont F, Ruiz-Lozano JM, Aroca R (2010) Regulation of plasma membrane aquaporins by inoculation with a Bacillus megaterium strain in maize (Zea mays L.) plants under unstressed and salt-stressed conditions. Planta 232:533–543
Meena M, Yadav G, Sonigra P, Nagda A, Mehta T, Swapnil P, Harish MA (2023) Role of elicitors to initiate the induction of systemic resistance in plants to biotic stress. Plant Stress 5:100103. https://doi.org/10.1016/j.stress.2022.100103
Mei PP, Gui LG, Wang P, Huang JC, Long HY, Christie P, Li L (2012) Maize/faba bean intercropping with rhizobia inoculation enhances productivity and recovery of fertilizer P in a reclaimed desert soil. Field Crops Res 130:19–27. https://doi.org/10.1016/j.fcr.2012.02.007
Mendoza-Mendoza A, Zaid R, Lawry R, Hermosa R, Monte E, Horwitz BA, Mukherjee PK (2018) Molecular dialogues between Trichoderma and roots: role of the fungal secretome. Fungal Biol Rev 32:62–85
Meyer S, De Angeli A, Fernie AR, Martinoia E (2010) Intra- and extra-cellular excretion of carboxylates. Trends Plant Sci 15:40–47
Mishra AK, Sudalaimuthuasari N, Hazzouri KM, Saeed EE, Shah I, Amiri KMA (2022) Tapping into plant-microbiome interactions through the lens of multi-omics techniques. Cells 11(20):3254. https://doi.org/10.3390/cells11203254
Moghaddam JA, Boehringer N, Burdziak A, Kunte HJ, Galinski EA, Schäberle TF (2016) Different strategies of osmoadaptation in the closely related marine myxobacteria Enhygromyxa salina SWB007 and Plesiocystis pacifica SIR-1. Microbiology 162:651–661
Mora-Macías J, Ojeda-Rivera JO, Gutiérrez-Alanís D, Yong-Villalobos L, Oropeza-Aburto A, Raya-González J, Jiménez-Domínguez G, Chávez-Calvillo G (2017) Malate-dependent Fe accumulation is a critical checkpoint in the root developmental response to low phosphate. Proc Natl Acad Sci USA 25:E3563–E3572
Morcillo RJ, Manzanera M (2021) The effects of plant-associated bacterial exopolysaccharides on plant abiotic stress tolerance. Metabolites 11:337
Moreira H, Pereira SI, Vega A, Castro PM, Marques AP (2020) Synergistic effects of arbuscular mycorrhizal fungi and plant growth-promoting bacteria benefit maize growth under increasing soil salinity. J Environ Manag 257:109982
Münch E (1930) Die stoffbewegungen in der pflanze (Gustav Fischer, Jena). Curr Opin Plant Biol 43:36–42
Mwendwa JM, Weston PA, Weidenhamer JD, Fomsgaard IS, Wu HW, Saliya G et al (2021) Metabolic profling of benzoxazinoids in the roots and rhizosphere of commercial winter wheat genotypes. Plant Soil 466:467–489. https://doi.org/10.1007/s11104-021-04996-9
Nair DS, Manjula S (2020) Induction of root endosymbiosis as a highly sustainable and efficient strategy for overproduction of the medicinally important diterpenoid lactone-andrographolide in Andrographis paniculata (Burm. F.) Wall. ex Nees. Ind Crops Prod 156:112835
Navada S, Vadstein O, Gaumet F, Tveten AK, Spanu C, Mikkelsen Ø, Kolarevic J (2020) Biofilms remember: osmotic stress priming as a microbial management strategy for improving salinity acclimation in nitrifying biofilms. Water Res 176:115732. https://doi.org/10.1016/j.watres.2020.115732
Naveed M, Mitter B, Reichenauer TG, Wieczorek K, Sessitsch A (2014) Increased drought stress resilience of maize through endophytic colonization by Burkholderia phytofirmans PsJN and Enterobacter sp. FD17. Environ Exp Bot 97:30–39
Ndour PMS, Heulin T, Achouak W, Laplaze L, Cournac L (2020) The rhizosheath: from desert plants adaptation to crop breeding. Plant Soil 456:1–13
Neal AL, Ahmad S, Gordon-Weeks R, Ton J (2012) Benzoxazinoids in root exudates of maize attract Pseudomonas putida to the rhizosphere. PLoS ONE 7:e35498
Negrão S, Schmöckel SM, Tester M (2017) Evaluating physiological responses of plants to salinity stress. Ann Bot 119:1–11
Neumann G, Romheld V (2007) The release of root exudates as affected by the plant’s physiological status. In: Pinton R, Varanini Z, Nannipieri P (eds) The rhizosphere: biochemistry and organic substances at the soil-plant interface. Marcel Dekker, New York, pp 41–93
Noritake T, Kawakita K, Doke N (1996) Nitric oxide induces phytoalexin accumulation in potato tuber tissues. Plant Cell Physiol 37:13–116
Nuruzzaman M, Lambers H, Bolland MDA, Veneklaas EJ (2006) Distribution of carboxylates and acid phosphatase and depletion of different phosphorus fractions in the rhizosphere of a cereal and three grain legumes. Plant Soil 281:109–120
Oliveros-Bastidas AJ, Macías FA, Molinillo JMG (2012) Mechanical impedance effects on growth, phenolics and 2,4-dihydroxy-7-methoxy-2H-1,4-benzoxazin-3-one (DIMBOA) in root exudates of Zea mays L. Int J Res Agric for 2:225–234
Ortiz R, Braun HJ, Crossa J, Crouch JH, Davenport G, Dixon J (2008) Wheat genetic resources enhancement by the international maize and wheat improvement center (CIMMYT). Genetics Resour Crop Evol 55:1095–1140
Pan T, Liu M, Kreslavski VD, Zharmukhamedov SK, Nie C, Yu M, Kuznetsov VV, Allakhverdiev SI, Shabala S (2021) Non-stomatal limitation of photosynthesis by soil salinity. Crit Rev Environ Sci Technol 51(8):791–825. https://doi.org/10.1080/10643389.2020.1735231
Panchal P, Miller AJ, Giri J (2021) Organic acids: versatile stress-response roles in plants. J Exp Bot 72(11):4038–4052. https://doi.org/10.1093/jxb/erab019
Panchal P, Preece C, Peñuelas J, Giri J (2022) Soil carbon sequestration by root exudates. Trends Plant Sci 27:749–757
Pandey P, Irulappan V, Bagavathiannan MV, Senthil-Kumar M (2017) Impact of combined abiotic and biotic stresses on plant growth and avenues for crop improvement by exploiting physio-morphological traits. Front Plant Sci 8:537
Pandiyan K, Kushwaha P, Srivastava R, Kashyap PL (2022) Metatranscriptomics of plant rhizosphere: a promising tool to decipher the role of microorganisms in plant growth and development. In: Singh UB, Rai JP, Sharma AK (eds) Re-visiting the rhizosphere eco-system for agricultural sustainability rhizosphere biology. Springer, Singapore, pp 491–509
Pang Z, Chen J, Wang T, Gao C, Li Z, Guo L, Xu J, Cheng Y (2021) Linking plant secondary metabolites and plant microbiomes: a review. Front Plant Sci 12:621276. https://doi.org/10.3389/fpls.2021.621276
Panthri M, Gupta M (2022) An insight into the act of iron to impede arsenic toxicity in paddy agro-system. J Environ Manag 316:115289. https://doi.org/10.1016/j.jenvman.2022.115289
Pantigoso HA, Manter DK, Fonte SJ, Vivanco JM (2023) Root exudate-derived compounds stimulate the phosphorus solubilizing ability of bacteria. Sci Rep 13:4050. https://doi.org/10.1038/s41598-023-30915-2
Pathania P, Bhatia R, Khatri M (2020) Cross-competence and affectivity of maize rhizosphere bacteria Bacillus sp. MT7 in tomato rhizosphere. Sci Hortic 272:109480
Pérez-de-Luque A, Tille S, Johnson I, Pascual-Pardo D, Ton J, Cameron DD (2017) The interactive effects of arbuscular mycorrhiza and plant growth-promoting rhizobacteria synergistically enhance host plant defences against pathogens. Sci Rep 7:1–10
Pramanik MHR, Nagai M, Asao T, Matsui Y (2000) Effects of temperature and photoperiod on phytotoxic root exudates of cucumber (Cucumis sativus) in hydroponic culture. J Chem Ecol 26:1953–1967
Rahimi S, Talebi M, Baninasab B, Gholami M, Zarei M, Shariatmadari H (2020) The role of plant growth-promoting rhizobacteria (PGPR) in improving iron acquisition by altering physiological and molecular responses in quince seedlings. Plant Physiol Biochem 155:406–415
Rauf M, Awais M, Ud-Din A, Ali K, Gul H, Rahman MM, Hamayun M, Arif M (2021) Molecular mechanisms of the 1-aminocyclopropane-1-carboxylic acid (ACC) deaminase producing Trichoderma asperellum map1 in enhancing wheat tolerance to waterlogging stress. Front Plant Sci 11:2213
Regaiolo A, Dominelli N, Andresen K, Heermann R (2020) The biocontrol agent and insect pathogen Photorhabdus luminescens interacts with plant roots. Appl Environ Microbiol 86:e00891-e920
Rinnan R, Gehrke C, Michelsen A (2006) Two mire species respond differently to enhanced ultraviolet B radiation: effects on biomass allocation and root exudation. New Phytol 169(4):809
Rolfe SA, Griffiths J, Ton J (2019) Crying out forhelp with root exudates: adaptive mechanisms by whichstressed plants assemble health-promoting soilmicrobiomes. Curr Opin Microbiol 49:73–82. https://doi.org/10.1016/j.mib.2019.10.003
Ronco MG, Ruscitti MF, Arango MC, Beltrano J (2008) Glyphosate and mycorrhization induce changes in plant growth and in root morphology and architecture in pepper plants (Capsicum annuum L.). J Hortic Sci Biotechnol 83:497–505
Ross-Elliott TJ, Jensen KH, Haaning KS, Wager BM, Knoblauch J, Howell AH, Mullendore DL, Monteith AG (2017) Phloem unloading in Arabidopsis roots is convective and regulated by the phloem-pole pericycle. E Life 6:24125
Rubio MB, Quijada NM, Perez E, Dominguez S, Monte E, Hermosa R (2014) Identifying beneficial qualities of Trichoderma parareesei for plants. Appl Environ Microbiol 80:1864–1873
Ryan PR, James RA, Weligama C, Delhaize E, Rattey A, Lewis DC, Bovill WD, McDonald G (2014) Can citrate efflux from roots improve phosphorus uptake by plants? Testing the hypothesis with near-isogenic lines of wheat. Physiol Plant 151:230–242
Sah SK, Reddy KR, Li J (2016) Abscisic acid and abiotic stress tolerance in crop plants. Front Plant Sci 7:571
Salem MA, Wang JY, Al-Babili S (2022) Metabolomics of plant root exudates: from sample preparation to data analysis. Front Plant Sci 13:1062982. https://doi.org/10.3389/fpls.2022.1062982
Samuel AL, Fernando M, Glass ADM (1992) Immunofluorescent localization of plasma membrane H+-ATPase in barley roots and effects of K nutrition. Plant Physiol 99:1509–1514
Sanchez-Fernandez R, Davies TGE, Coleman OD, Rea PA (2001) The Arabidopsis thaliana ABC protein superfamily, a complete inventory. J Biol Chem 276:30231–30244
Sanders D, Bethke P (2000) Membrane transport. In: Buchanan BB, Gruisham W, Jones RL (eds) Biochemistry and molecular biology of plants. ASPP, Rockville, pp 110–158
Sasse J, Martinoia E, Northen T (2018) Feed your friends: do plant exudates shape the root microbiome? Trends Plant Sci 23:25–41
Schachtman DP, Shin R (2007) Nutrient sensing and signaling: NPKS. Annu Rev Plant Biol 58:47–69
Sebastian A, Prasad M (2018) Exogenous citrate and malate alleviate cadmium stress in Oryza sativa L.: probing role of cadmium localization and iron nutrition. Ecotoxicol Environ Saf 166:215–222
Semchenko M, Saar S, Lepik A (2014) Plant root exudates mediate neighbour recognition and trigger complex behavioural changes. New Phytol 204:631–637
Serraj R, Sinclair TR (2002) Osmolyte accumulation: can it really help increase crop yield under drought conditions? Plant Cell Environ 25:333–341
Sezgin A, Altuntasx C, Demiralay M, Cinemre S, Terzi R (2019) Exogenous alpha lipoic acid can stimulate photosystem II activity and the gene expressions of carbon fixation and chlorophyll metabolism enzymes in maize seedlings under drought. J Plant Physiol 232:65–73
Sharipova G, Veselov D, Kudoyarova G, Fricke W, Dodd IC, Katsuhara M, Furuichi T, Ivanov I (2016) Exogenous application of abscisic acid (ABA) increases root and cell hydraulic conductivity and abundance of some aquaporin isoforms in the ABA-deficient barley mutant Az34. Ann Bot 118:777–785
Sharma A, Kashyap PL, Srivastava AK, Bansal YK, Kaushik R (2019a) Isolation and characterization of halotolerant bacilli from chickpea (Cicer arietinum L.) rhizosphere for plant growth promotion and biocontrol traits. Eur J Plant Pathol 153:787–800. https://doi.org/10.1007/s10658-018-1592-7
Sharma A, Shahzad B, Kumar V, Kohli SK, Sidhu GPS, Bali AS, Handa N, Kapoor D (2019b) Phytohormones regulate accumulation of osmolytes under abiotic stress. Biomolecules 9:285
Sharma M, Saleh D, Charron J-B, Jabaji S (2020) A crosstalk between Brachypodium root exudates, organic acids, and Bacillus velezensis B26, a growth promoting bacterium. Front Microbiol 11:575578. https://doi.org/10.3389/fmicb.2020.575578
Sharp R, Chen L, Davies WJ (2011) Inoculation of growing media with the rhizobacterium Variovorax paradoxus 5C–2 reduces unwanted stress responses in hardy ornamental species. Sci Hortic 129:804–811
Shaw AK, Bhardwaj PK, Ghosh S, Roy S, Saha S, Sherpa AR, Saha SK, Hossain Z (2016) β-Aminobutyric acid mediated drought stress alleviation in maize (Zea mays L.). Environ Sci Pollut Res 23:2437–2453
Shi L, Guo M, Ye N, Liu Y, Liu R, Xia Y, Cui S, Zhang J (2015) Reduced ABA accumulation in the root system is caused by ABA exudation in upland rice (Oryza sativa L. var. Gaoshan1) and this enhanced drought adaptation. Plant Cell Physiol 56:951–964
Singh RP, Jha PN (2016) Mitigation of salt stress in wheat plant (Triticum aestivum) by ACC deaminase bacterium Enterobacter sp. SBP-6 isolated from Sorghum bicolor. Acta Physiol Plant 38:110
Singh R, Singh P, Sharma R (2014) Microorganism as a tool of bioremediation technology for cleaning environment: a review. Proc Int Acad Ecol Environ Sci 4:1–6
Singh N, Chattopadhyay D, Gupta SK (2023) Updating the impact of drought on root exudation: a strigolactones perspective. J Plant Growth Regul 42:5131–5151. https://doi.org/10.1007/s00344-023-11061-5
Sisó-Terraza P, Rios JJ, Abadia J, Abadia A, Álvarez-Fernández A (2016) Flavins secreted by roots of iron-deficient Beta vulgaris enable mining of ferric oxide via reductive mechanisms. New Phytol 209(2):733–745
Solanki MK, Kumar S, Pandey AK, Srivastava S, Singh RK, Kashyap PL, Srivastava AK, Arora DK (2012) Diversity and antagonistic potential of Bacillus spp. associated to the rhizosphere of tomato for the management of Rhizoctonia solani. Biocontrol Sci Technol 22(2):203–217
Solanki MK, Solanki AC, Rai S, Srivastava S, Kashyap BK, Divvela PK, Kumar S, Yandigeri MS, Kashyap PL, Shrivastava AK, Ali B, Khan S, Jaremko M, Qureshi KA (2022) Functional interplay between antagonistic bacteria and Rhizoctonia solani in the tomato plant rhizosphere. Front Microbiol 13:990850. https://doi.org/10.3389/fmicb.2022.990850
Somssich M, Khan GA, Persson S (2016) Cell wall heterogeneity in root development of Arabidopsis. Front Plant Sci 7:1242
Song F, Han X, Zhu X, Herbert SJ (2012) Response to water stress of soil enzymes and root exudates from drought and non-drought tolerant corn hybrids at different growth stages. Can J Soil Sci 92:501–507
Stein M, Dittgen J, Sanchez-Rodriguez C, Hou BH, Molina A, Schulze-Lefert P, Lipka V, Somerville S (2006) Arabidopsis PEN3/PDR8, an ATP binding cassette transporter, contributes to non-host resistance to inappropriate pathogens that enter by direct penetration. Plant Cell 18:731–746
Stohr C, Ullrich WR (2002) Generation and possible roles of NO in plant roots and their apoplastic space. J Exp Bot 53:2293–2303
Stringlis IA, Yu K, Feussner K, de Jonge R, Van Bentum S, Van Verk MC, Berendsen RL, Bakker PAHM (2018a) MYB72-dependent coumarin exudation shapes root microbiome assembly to promote plant health. Proc Natl Acad Sci USA 115:E5213–E5222
Stringlis IA, Yu K, Feussner K, de Jonge R, Van Bentum S, Van Verk MC, Berendsen RL, Bakker PAHM, Feussner I, Pieterse CMJ (2018b) MYB72-dependent coumarin exudation shapes root microbiome assembly to promote plant health. Proc Natl Acad Sci USA 115(22):E5213–E5222. https://doi.org/10.1073/pnas.1722335115
Su F, Jacquard C, Villaume S, Michel J, Rabenoelina F, Clement C, Barka AB, Dhondt-Cordelier S (2015) Burkholderia phytofrmans PsJN reduces impact of freezing temperatures on photosynthesis in Arabidopsis thaliana. Front Plant Sci 6:810
Subiramani S, Ramalingam S, Muthu T, Nile SH, Venkidasamy B (2020) Development of abiotic stress tolerance in crops by plant growth-promoting rhizobacteria (PGPR). Phyto-microbiome in stress regulation. Environmental and microbial biotechnology. Springer, Singapore, pp 125–145
Sugiyama A (2019) The soybean rhizosphere: metabolites, microbes, and beyond—a review. J Adv Res 19:67–73
Sugiyama A, Shitan N, Yazaki K (2007) Involvement of a soybean ATP-binding cassette-type transporter in the secretion of genistein, a signal flavanoid in legume–Rhizobium symbiosis. Plant Physiol 144:2000–2008
Sun Q, Wang X, Ding S, Yuan X (2005) Effects of exogenous organic chelators on phytochelatins production and its relationship with cadmium toxicity in wheat (Triticum aestivum L.) under cadmium stress. Chemosphere 60:22–31
Sun L, Cao X, Tan C, Deng Y, Cai R, Peng X, Bai J (2020) Analysis of the effect of cadmium stress on root exudates of Sedum plumbizincicola based on metabolomics. Ecotoxicol Environ Saf 205:111152. https://doi.org/10.1016/j.ecoenv.2020.111152
Sun H, Jiang S, Jiang C, Wu C, Gao M, Wang Q (2021a) A review of root exudates and rhizosphere microbiome for crop production. Environ Sci Pollut Res 28:54497–54510. https://doi.org/10.1007/s11356-021-15838-7
Sun Y, Tian L, Chang J, Shi S, Zhang J, Xie H, Cai Y, Chen D (2021b) Rice domestication influences the composition and function of the rhizosphere bacterial chemotaxis systems. Plant Soil 466:81–99
Tedone F, Dottore ED, Palladino M, Mazzolai B, Marcati P (2020) Optimal control of plant root tip dynamics in soil. Bioinspir Biomim 15:056006. https://doi.org/10.1088/1748-3190/ab9a15
Timmusk S, El-Daim IAA, Copolovici L, Tanilas T, Kännaste A, Behers L, Nevo E, Seisenbaeva G (2014) Drought-tolerance of wheat improved by rhizosphere bacteria from harsh environments: enhanced biomass production and reduced emissions of stress volatiles. PLoS ONE 9:e96086
Tiziani R, Miras-Moreno B, Malacrinò A, Vescio R, Lucini L, Mimmo T, Cesco S, Sorgonà A (2022) Drought, heat, and their combination impact the root exudation patterns and rhizosphere microbiome in maize roots. Environ Exp Bot 203:105071. https://doi.org/10.1016/j.envexpbot.2022.105071
Tucci M, Ruocco M, De Masi L, De Palma M, Lorito M (2011) The beneficial effect of Trichoderma spp. on tomato is modulated by the plant genotype. Mol Plant Pathol 12:341–354
Ulrich DEM, Sevanto S, Ryan M, Albright MBN, Johansen RB, Dunbar JM (2019) Plant-microbe interactions before drought influence plant physiological responses to subsequent severe drought. Sci Rep 9:249
Ulrich DEM, Clendinen CS, Alongi F, Mueller RC, Chu RK, Toyoda J, Gallegos-Graves V, Goemann HM, Peyton B, Sevanto S, Dunbar J (2022) Root exudate composition reflects drought severity gradient in blue grama (Bouteloua gracilis). Sci Rep 12:12581. https://doi.org/10.1038/s41598-022-16408-8
Upadhyay SK, Srivastava AK, Rajput VD, Chauhan PK, Bhojiya AA, Jain D, Chaubey G, Dwivedi P, Sharma B, Minkina T (2022) Root exudates: mechanistic insight of plant growth promoting rhizobacteria for sustainable crop production. Front Microbiol 13:916488. https://doi.org/10.3389/fmicb.2022.916488
van der Heijden MGA, Bardgett RD, vanStraalen NM (2008) The unseen majority: soil microbes as drivers of plant diversity and productivity in terrestrial ecosystems. Ecol Lett 11:296–310
Verma SK, Sahu PK, Kumar K, Pal G, Gond SK, Kharwar RN, White JF (2021) Endophyte roles in nutrient acquisition, root system architecture development and oxidative stress tolerance. J Appl Microbiol 131(5):2161–2177
Veroneze-Junior V, Martins M, Mc Leod L, Souza K, Santos-Filho PR, Magalhaes PC, Carvalho DT, Santos MH (2020) Leaf application of chitosan and physiological evaluation of maize hybrids contrasting for drought tolerance under water restriction. Braz J Biol 80:631–640
Vidal A, Hirte J, Bender SF, Mayer J, Gattinger A, Höschen C, Schädler S, Iqbal TM (2018) Linking 3D soil structure and plant-microbe-soil carbon transfer in the rhizosphere. Front Environ Sci 6:9
Vives-Peris V, Gómez-Cadenas A, Pérez-Clemente RM (2017) Citrus plants exude proline and phytohormones under abiotic stress conditions. Plant Cell Rep 36:1971–1984
Vives-Peris V, Gómez-Cadenas A, Pérez-Clemente RM (2018a) Salt stress alleviation in citrus plants by plant growth-promoting rhizobacteria Pseudomonas putida and Novosphingobium sp. Plant Cell Rep 37:1557–1569
Vives-Peris V, Molina L, Segura A, Gomez-Cadenas A, Pérez-Clemente RM (2018b) Root exudates from citrus plants subjected to abiotic stress conditions have a positive effect on rhizobacteria. J Plant Physiol 228:208–217
Vives-Peris V, de Ollas C, Gómez-Cadenas A, Pérez-Clemente RM (2020) Root exudates: from plant to rhizosphere and beyond. Plant Cell Rep 39:3–17
Walne CH, Reddy KR (2022) Temperature effects on the shoot and root growth, development, and biomass accumulation of corn (Zea mays L.). Agriculture 12(4):443. https://doi.org/10.3390/agriculture12040443
Wang Q, Dodd IC, Belimov AA, Jiang F (2016) Rhizosphere bacteria containing 1-aminocyclopropane-1-carboxylate deaminase increase growth and photosynthesis of pea plants under salt stress by limiting Na+ accumulation. Funct Plant Biol 43:161–172
Wang G, Xiao Y, Deng X, Zhang H, Li T, Chen H (2018) Exogenous hydrogen peroxide contributes to heme oxygenase-1 delaying programmed cell death in isolated aleurone layers of rice subjected to drought stress in a cGMP-dependent manner. Front Plant Sci 9:84
Wang D, Gao Y, Li M, Sturrock CJ, Gregory AS, Zhang X (2020) Change in hydraulic properties of the rhizosphere of maize under different abiotic stresses. Plant Soil 452:615–626
Wardle DA, Bardgett RD, Klironomos JN, Setala H, van der Putten WH, Wall DH (2004) Ecological linkages between aboveground and belowground biota. Science 304(5677):1629–1633
Woo OG, Kim H, Kim JS, Keum HL, Lee KC, Sul WJ, Lee JH (2020) Bacillus subtilis strain GOT9 confers enhanced tolerance to drought and salt stresses in Arabidopsis thaliana and Brassica campestris. Plant Physiol Biochem 148:359–367
Wu Y, Zhao C, Zhao X, Yang L, Liu C, Jiang L, Liu G, Liu P, Luo L (2023) Multi-omics-based identification of purple acid phosphatases and metabolites involved in phosphorus recycling in stylo root exudates. Int J Biol Macromol 241:124569
Xia ZC, Kong CH, Chen LC, Wang P, Wang SL (2016) A broadleaf species enhances an autotoxic conifers growth through belowground chemical interactions. Ecology 97:2283–2292
Xiang G, Ma W, Gao S, Jin Z, Yue Q, Yao Y (2019) Transcriptomic and phosphoproteomic profiling and metabolite analyses reveal the mechanism of NaHCO3-induced organic acid secretion in grapevine roots. BMC Plant Biol 19:383
Xie T, Gu W, Wang M, Zhang L, Li C, Li C, Li W, Li L (2019) Exogenous 2-(3,4-dichlorophenoxy) triethylamine ameliorates the soil drought effect on nitrogen metabolism in maize during the pre-female inflorescence emergence stage. BMC Plant Biol 19:107
Xie E, Wei X, Ding A, Zheng L, Wu X, Anderson B (2020) Short-term effects of salt stress on the amino acids of Phragmites australis root exudates in constructed wetlands. Water 12(2):569. https://doi.org/10.3390/w12020569
Xiong D, Huang J, Lin TC, Liu X, Xu C, Chen S, Yang Z, Chen G, Yang Y (2023) Warming increased metabolite composition and pathways in root exudates of chinese fir saplings in subtropical China. J Soil Sci Plant Nutr 23:2545–2565. https://doi.org/10.1007/s42729-023-01212-8
Xu K, Lee YS, Li J, Li C (2019) Resistance mechanisms and reprogramming of microorganisms for efficient bio-refinery under multiple environmental stresses. Synth Syst Biotechnol 4:92–98
Xu Y, Yang M, Yin R, Wang L, Luo L, Zi B et al (2021) Autotoxin Rg1 induces degradation of root cell walls and aggravates root rot by modifying the rhizospheric microbiome. Microbiol Spectr 9:e0167921. https://doi.org/10.1128/spectrum.01679-21
Yang NJ, Hinner MJ (2015) Getting across the cell membrane: an overview for small molecules, peptides, and proteins. In: Gautier A, Hinner M (eds) Site-specific protein labelling: methods in molecular biology. Humana Press, New York
Yang Y, Yang Z, Yu S, Chen H (2019) Organic acids exuded from roots increase the available potassium content in the rhizosphere soil: a rhizobag experiment in Nicotiana tabacum. Hort Sci 54:23–27
Yang S, Liu H, Xie P, Wen T, Shen Q, Yuan J (2023) Emerging pathways for engineering the rhizosphere microbiome for optimal plant health. J Agric Food Chem 71:4441–4449. https://doi.org/10.1021/acs.jafc.2c08758
Yazaki K (2005) Transporters of secondary metabolites. Curr Opin Plant Biol 8:301–307
Yildirim E, Taylor AG, Spittler TD (2006) Ameliorative effects of biological treatments on growth of squash plants under salt stress. Sci Hortic 111:1–6
Yin S, Wang C, Lv C, Zhou Z (2023) Short-term responses of root traits and carbon exudation to drought in a Larix gmelinii plantation. Plant Soil 484:393–405. https://doi.org/10.1007/s11104-022-05800-y
Yuan J, Zhao J, Wen T et al (2018) Root exudates drive the soil-borne legacy of aboveground pathogen infection. Microbiome 6:156. https://doi.org/10.1186/s40168-018-0537-x
Yuan T, Ren W, Wang Z, Fry EL, Tang S, Yin J, Zhang J, Jia Z (2023) How does the pattern of root metabolites regulating beneficial microorganisms change with different grazing pressures? Front Plant Sci 14:1180576. https://doi.org/10.3389/fpls.2023.1180576
Zhalnina K, Louie KB, Hao Z, Mansoori N, da Rocha UN, Shi S, Cho H, Karaoz U (2018) Dynamic root exudate chemistry and microbial substrate preferences drive patterns in rhizosphere microbial community assembly. Nat Microbiol 3:470–480
Zhang R, Vivanco JM, Shen Q (2017) The unseen rhizosphere root–soil–microbe interactions for crop production. Curr Opin Microbiol 37:8–14
Zhang DS, Lyu Y, Li HB, Tang XY, Hu R, Rengel Z, Zhang FS, Whalley WR, Davies W, Cahill JF Jr, Shen JB (2020) Neighbouring plants modify maize root foraging for phosphorus: coupling nutrients and neighbours for improved nutrient-use efficiency. New Phytol 226(1):244–253
Zhang W, Gao W, Whalley WR, Ren T (2021) Physical properties of a sandy soil as affected by incubation with a synthetic root exudate: strength, thermal and hydraulic conductivity, and evaporation. J Soil Sci 72:782–792
Zhao J, Davis LC, Verpoorte R (2005) Elicitor signal transduction leading to production of plant secondary metabolites. Biotechnol Adv 23:283–333
Zhao M, Zhao J, Yuan J, Hale L, Wen T, Huang Q, Vivanco JM, Zhou J (2021a) Root exudates drive soil-microbe-nutrient feedbacks in response to plant growth. Plant Cell Environ 44:613–628
Zhao S, Zhang Q, Liu M, Zhou H, Ma C, Wang P (2021b) Regulation of plant responses to salt stress. Int J Mol Sci 22(9):4609. https://doi.org/10.3390/ijms22094609
Zhou C, Guo J, Zhu L, Xiao X, Xie Y, Zhu J, Ma Z, Wang J (2016) Paenibacillus polymyxa BFKC01 enhances plant iron absorption via improved root systems and activated iron acquisition mechanisms. Plant Physiol Biochem 105:162–173
Zhou C, Wang Q, Liu W, Li B, Shao M, Zhang Y (2022) Effects of red/blue versus white LED light of different intensities on the growth and organic carbon and autotoxin secretion of hydroponic lettuce. Hortic Environ Biotechnol 63:195–205. https://doi.org/10.1007/s13580-021-00394-3
Zhou X, Zhang J, Urahman MK, Gao D, Wei Z, Wu F, Dini-Andreote F (2023a) Interspecific plant interaction via root exudates structures the disease suppressiveness of rhizosphere microbiomes. Mol Plant 16(5):849–864. https://doi.org/10.1016/j.molp.2023.03.009
Zhou XG, Zhang JY, Rahman MKU, Gao DM, Wei Z, Wu FZ et al (2023b) Interspecific plant interaction via root exudates structures the disease suppressiveness of rhizosphere microbiomes. Mol Plant 16:849–864. https://doi.org/10.1016/j.molp.2023.03.009
Zimmer W, Kloos K, Hundeshagen B, Niederau E, Bothe H (1995) Auxin biosynthesis and denitrification in plant growth promoting bacteria. In: Fendrik I, Del Gallo M, Vanderleyden J, de Zamaroczy M (eds) Azospirillum VI and related microorganisms, genetics-physiology-ecology. Springer, Berlin, pp 121–128
Funding
The work is funded by Science and Engineering Research Board, Dept. of Science and Technology, Govt. of India via sanction order CRG/2019/000414 dated 27th January 2020.
Author information
Authors and Affiliations
Contributions
OPA: Conceptualization, drafting and editing; DY: Drafting, figures making; NW: Reference setting and editing PLK: Drafting and editing; PS: Editing and RT: Editing.
Corresponding authors
Ethics declarations
Conflict of interest
The authors report no conflict of interest.
Additional information
Handling Editor: Boon Chin Tan.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ahlawat, O.P., Yadav, D., Walia, N. et al. Root Exudates and Their Significance in Abiotic Stress Amelioration in Plants: A Review. J Plant Growth Regul 43, 1736–1761 (2024). https://doi.org/10.1007/s00344-024-11237-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-024-11237-7