Abstract
It is widely reported that some humic substances behave as exogenous auxins influencing root growth by mechanisms that are not yet completely understood. This study explores the hypothesis that the humic acids’ effects on root development involve a nitric oxide signaling. Maize seedlings were treated with HA 20 mg C L−1, IAA 0.1 nM, and NO donors (SNP or GSNO), in combination with either the auxin-signaling inhibitor PCIB, the auxin efflux inhibitor TIBA, or the NO scavenger PTIO. H+-transport-competent plasma membrane vesicles were isolated from roots to investigate a possible link between NO-induced H+-pump and HA bioactivity. Plants treated with either HA or SNP stimulated similarly the lateral roots emergence even in the presence of the auxin inhibitors, whereas NO scavenger diminished this effect. These treatments induced H+-ATPase stimulation by threefold, which was abolished by PTIO and decreased by auxin inhibitors. HA-induced NO synthesis was also detected in the sites of lateral roots emergence. These data depict a new scenario where the root development stimulation and the H+-ATPase activation elicited by either HA or exogenous IAA depend essentially on mechanisms that use NO as a messenger induced site-specifically in the early stages of lateral root development.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Humic substances (HS) are complex mixtures of biologically transformed organic debris and the most abundant organic materials in the environment (Hayes and Malcolm 2001). Structurally, HS are defined as supramolecular associations of relatively smaller organic molecules, clustered basically by hydrophobic interactions and hydrogen bonds, which can be disrupted by organic acids (Piccolo 2002) exuded by plant roots (Canellas et al. 2008). These substances are naturally present in soils or in processed organic residues and control a variety of biological, chemical and physical processes that contribute directly or indirectly to plant growth and environmental adaptability, improving the soil aggregate stability (Piccolo and Mbagwu 1999), the ion fluxes and nutrient uptake (Pinton et al. 1999; Quaggiotti et al. 2004), and carbon metabolism (Nardi et al. 2007). There is a growing body of evidence that HS isolated as humic acids (HA) are endowed with auxin-like activities that influence root morphology and metabolism (Canellas et al. 2002, 2008; Zandonadi et al. 2007), a property that had also been described to HS characterized as fulvic acids (Muscolo et al. 1999). Furthermore, these works provided evidence that HS with distinct characteristics and isolated by different procedures can be considered as environmental sources of indole-3-acetic acid (IAA), which could account for exogenous auxin signaling that regulates root system architecture by an as yet unclear mechanism (Zandonadi et al. 2007).
Auxin is the most studied class of phytohormones that has been implicated in practically all aspects of plant development, including cell division and expansion (Davies 1995). Auxins also play a crucial role in plant development by driving the construction of a branched root system rich in lateral roots responsible for providing water and nutrients (Malamy and Benfey 1997). Lateral root (LR) initiation is highly influenced by environmental signals such as nutrient availability, drought, salinity and organic matter (Malamy 2005; Dobbss et al. 2007). It is well established that the auxin mechanism of action involves the induction of the plasma membrane H+-ATPase (Hager et al. 1991; Frías et al. 1996), which is a housekeeping enzyme responsible for regulating key functions of plant cells, including nutrient uptake and stress responses. The acid growth mechanism based on IAA-induced cell wall loosening is also related to the H+-ATPase activation and the consequent decrease of apoplastic pH (Hager 2003). It has been proposed that HS could promote root growth, in a way similar to auxin by modulating not only the plasmalemma (Canellas et al. 2002; Quaggiotti et al. 2004) but also the tonoplast proton pumps (Zandonadi et al. 2007).
Although previous studies demonstrated that HS can induce root development in leaf explants by affecting auxin transport (Nardi et al. 1994), other fractions of HS appear to exert their effects on roots in a way somewhat independent of some auxin response elements (Schmidt et al. 2007). For instance, HA from different soils and organic residues usually stimulate the root growth and morphology better than exogenous IAA, suggesting the presence of other unidentified HA-derived bioactive molecules (Zandonadi et al. 2007). In fact, it remains still unknown whether the HA fraction could operate by influencing auxin transport or signaling, or even through other hormonal pathways.
Nitric oxide (NO) is a free radical reactive gas for which multiple roles in plant physiology have been described (Besson-Bard et al. 2008). Gouvêa et al. (1997) suggested that NO donors and auxin could share common steps in a signal transduction pathway during root cell expansion in maize. It was demonstrated that NO is involved in the auxin-induced lateral root formation (Correa-Aragunde et al. 2004). These authors showed NO production in the pericycle cells, which give place to LR initiation, indicating that NO is required during the early stages of LR development. Two enzymes have been claimed to be responsible for NO production during auxin-induced lateral development: nitrate reductase (Kolbert et al. 2008a) and a putative nitric oxide synthase (Flores et al. 2008). But a non-enzymatic mechanism was earlier described for production of NO from NO2 −, occurring on the plant cell surface in response to apoplast acidification (Stöhr and Ullrich 2002; Bethke et al. 2004).
Since the PM H+-ATPase activation is essential for apoplastic acidification during root growth (Hager 2003), and the activity and expression of this enzyme was proved to be enhanced not only by IAA and HA (Canellas et al. 2002) but also by NO (Zhao et al. 2004), we postulate and study an as yet unexplored mechanism of action by which HA and exogenous auxins could regulate root growth and morphology by specific cross activations of H+-pumps and NO production pathways. For this propose, in the present work we take advantage of auxin inhibitors and NO donor and scavenger substances to shed light on the participation of the auxin and NO signaling pathways involved in HA bioactivity.
Materials and methods
Extraction of humic acids
Humic acids (HA) were isolated from vermicompost obtained with cattle manure and chemically characterized as previously described by Canellas et al. (2002). Standard purification procedures were used. Briefly, 10 vol of 0.5 M NaOH was mixed with 1 vol vermicompost, under N2 atmosphere. After 12 h, the suspension was centrifuged at 5,000g and acidified to pH 1.5 using 6 M HCl. The solubilization and precipitation of HA were repeated three times. HA were mixed with 10 vol of a diluted mixture of HF–HCl solution [5 mL HCl (12 M) + 5 mL HF (48%)]. After centrifugation (5,000g) for 15 min, the sample was repeatedly washed with water until a negative test against AgNO3 was obtained, followed by dialyzing against deionized water using a 12–14 kDa cutoff membrane (Thomas Scientific Inc.). The dialyzed sample was lyophilized and characterized chemically. Then, the HA powder was solubilized with 50–100 mL of 0.05 M NaOH and the pH was adjusted to 5.5 with 0.1 M HCl.
Plant growth and treatments
Maize seeds (Zea mays L., var. UENF 506/6, from Universidade Estadual do Norte Fluminense Darcy Ribeiro, Rio de Janeiro, Brazil) were surface disinfested by soaking in 0.5% NaClO for 30 min, followed by rinsing and then soaking in water for 2 h. Afterward, the seeds were sown on wet filter paper and germinated in the dark at 25 ± 1°C. Three-day-old maize seedlings with primary roots approximately 3.0–3.5 cm long were placed in autoclaved moist filter paper, vertically oriented into a 50 mL tube containing an aqueous solution of 2 mM CaCl2 and the treatments were supplemented with vermicompost humic acids (HA, 20 mg C L−1), auxin (IAA, 0.1 nM) or the NO donor sodium nitroprusside (SNP, 200 μM). In addition, 200 μM n-nitrosoglutathione (GSNO) was used as an alternative NO donor in some experiments. However, SNP was the NO donor used in majority of the assays due its relatively low cost and well-documented application as NO donor (e.g., Gouvêa et al. 1997; Correa-Aragunde et al. 2004; Ederli et al. 2009).
Treatments were supplemented with or without p-chlorophenoxyisobutyric acid (PCIB, 20 μM), 2,3,5-triiodobenzoic acid (TIBA, 20 μM) or 2-phenyl-4,4,5,5-tetramethyl-imidazoline-1-oxyl-3-oxide (PTIO, 200 μM). Preliminary dose–response assays showed that the root elongation rate was inhibited approximately 50% at 20 μM TIBA or PCIB (data not shown), as previously reported (Collings et al. 1992; Rahman et al. 2007). Seedlings were grown in a chamber at 25 ± 1°C and a 14:10 h (light:dark) photoperiod. In order to exclude any chemical interaction among auxin inhibitors or the NO scavenger within the treatments with HA, IAA or SNP, the seedlings were pretreated with 200 μM PTIO or auxin inhibitors (20 μM TIBA or 20 μM PCIB and TIBA plus PCIB) for 24 h and then shifted to a minimum media (2 mM CaCl2), and subjected to treatment with 20 mg C L−1 HA or 0.1 nM IAA or 200 μM SNP for another 72 h. The results were similar to those treatments in which the inhibitors were added together with the HA or IAA (data not shown). Therefore, we decide to use the inhibitors combined with HA, IAA and SNP for future studies. The chemicals TIBA, PCIB, IAA, SNP and PTIO were purchased from Sigma-Aldrich (St Louis, MO, USA). Seedlings (20 replicates in three independent experiments) were harvested after 4 days of treatment and the number of LRs per seedling and the length of the primary root were quantified. The density of LRs along the primary root was calculated by dividing the number of LRs by the primary root length and expressed as number of LRs per centimeter.
Plasma membrane-enriched vesicles
Plasma membrane (PM) vesicles were isolated from roots using differential centrifugation as described by De Michelis and Spanswick (1986), with some modifications (Zandonadi et al. 2007). Briefly, about 15 g (fresh weight) of maize roots was homogenized using a mortar and pestle in 30 mL of ice-cold buffer containing 250 mM sucrose, 10% (w/v) glycerol, 0.5% (w/v) PVP (PVP-40, 40 kDa), 2 mM EDTA, 0.2% (w/v) BSA, and 0.1 M Tris–HCl buffer, pH 7.6. Just prior to use, 150 mM KCl, 2 mM DTT, and 1 mM PMSF were added to the buffer. The homogenate was strained through four layers of cheesecloth and centrifuged at 1,500g for 10 min. The supernatant was centrifuged at 8,000g for 10 min and then at 100,000g for 40 min. The pellet was resuspended in a small volume of ice-cold buffer containing 10 mM MES–BTP, pH 7.5, 10% (v/v) glycerol, 1 mM DTT and 1 mM EGTA. The suspension containing membrane vesicles was layered over a 25/42% (w/w) discontinuous sucrose gradient that contained, in addition to sucrose, 10 mm MES–BTP buffer, pH 7.5, 1 mM DTT, and 1 mM EDTA. After centrifugation at 100,000g for 3 h in a swinging bucket, the vesicles that sedimented at the interface between 25 and 42% sucrose were collected and centrifuged at 100,000g for 40 min. The pellet was resuspended in a buffer containing 10 mM MES–BTP, pH 7.6, 10% (v/v) glycerol, 1 mM DTT, and 1 mM EGTA. The vesicles were either used immediately or frozen under liquid N2 and stored at −70°C until use. All procedures were carried out below 4°C. Protein concentrations were determined by the method of Bradford (1976).
Plasma membrane H+-ATPase activity
The hydrolytic H+-ATPase activity in PM vesicles was determined by measuring the release of Pi colorimetrically (Fiske and Subbarow 1925). Between 70 and 80% of the PM vesicle H+-ATPase activity measured at pH 6.5, was inhibited by 0.1 mM sodium orthovanadate (Na3VO4), a very effective inhibitor of PM H+-ATPase (Sze 1985). The assay medium consisted of 1 mM ATP–BTP, 5 mM MgSO4, 10 mM MOPS–BTP (pH 6.5), 100 mM KCl, 0.2 mM Na2MoO4 and 0.05 mg mL−1 vesicle protein. The membrane sidedness and permeability were verified using 0.01% (w/v) Brij 58 (polyoxyethylene 20 cetyl ether) as previously described (Palmgren et al. 1990). Nearly 60% of the plasma membrane fraction was composed by tight sealed inside-out oriented vesicles, independently of the treatment (data not shown). In all experiments, the enzyme activity was measured at 35°C, with or without vanadate, and the difference between these two activities was attributed to the PM H+-ATPase. The vanadate sensitivity observed here (around 75%) is in agreement with other work with maize roots (De Michelis and Spanswick 1986; Fischer-Schliebs et al. 1994; Canellas et al. 2002).
Plasma membrane ATPase H+-pumping
The electrochemical H+-gradient generated by the PM H+-ATPase was estimated from the initial rate of quenching of the fluorescent pH probe 9-amino-6-chloro-2-methoxyacridine (ACMA) (415/485 nm, excitation/emission), and expressed as percentage over control. The assay medium contained 10 mM HEPES-BTP (pH 6.5), 100 mM KCl, 5 mM MgCl2, 2 μM ACMA and 0.05 mg L−1 PM vesicle protein. Addition of vanadate to the medium reduced the H+ transport and the difference between vanadate-insensitivity and sensitivity was calculated and attributed to the P-type H+-ATPase. The reaction was initiated by the addition of 1 mM ATP. The addition of either 3 μM FCCP or 2 μM NH4Cl abolished the H+ gradient created by ATP hydrolysis.
Rhizospheric pH measurements
Roots of maize seedlings treated for 72 h were placed on glass plate covered by a 5 mm layer of a medium containing 1% agar, 10 mM CaSO4 and 0.03% (w/v) bromocresol purple indicator at pH 6.5. Purple to yellow color shift of the medium corresponds the region acidified by roots (yellow indicates pH below 6.0). Images were captured after 70 min of incubation and control plates containing vanadate (500 μM Na3VO4), a P-type ATPase inhibitor, exhibited no color shift at this time. Rhizospheric pH changes were quantified by potentiometric pH measurements using an electrode coupled to a chamber containing 2 g of 2 cm long lateral roots excised from 12 plants immersed in 25 mL of low ionic strength pH buffer solution (10 mM HEPES-BTP, pH 7.0), with or without 500 μM Na3VO4.
NO measurement and localization
Nitric oxide was imaged using 4,5-diaminofluorescein diacetate (DAF-2 DA) and fluorescence microscopy. Root transverse sections from mature zones treated for 72 h were loaded with 10 μM DAF-2 DA in 10 mM HEPES-BTP buffer, pH 7.5, for 40 min, washed three times in fresh buffer and analyzed microscopically (excitation 488 nm, emission 495–575 nm). The transversal root sections had approximately 5 μm and were performed using the Table Microtome (LPC model, Rolemberg e Bhering Trading and Import, Belo Horizonte, Brazil). Images acquired from the light microscope (Zeiss Axioplan coupled with Canon A640 digital camera) were analyzed using ImageJ software (NIH) in the LR zone (20–30 mm from root–seed junction). Maize roots without DAF-2 DA addition were used as a blank control. The same camera settings were recorded for each digital image and not processed further. At least five samples were measured in each treatment in four independent experiments.
Statistical analysis
Data were analyzed by performing ANOVA and Dunnett’s test to determine the differences between treatments and controls (P < 0.05 considered as statistically significant).
Results
NO is related to IAA and HA effects on root development
The effects of HA, IAA and SNP on both LR initiation and primary root (PR) growth of maize seedlings were examined. In order to investigate the involvement of auxin-like mechanisms of action on LR stimulation by HA and SNP the seedlings were treated with 20 μM TIBA (inhibitor of auxin transport) or 20 μM PCIB (inhibitor of auxin signaling) and TIBA plus PCIB for 4 days with or without 0.1 nM IAA, 20 mg C L−1 HA or 200 μM SNP (Fig. 1). The relevance of NO production during LR and PR growth promoted by IAA, HA, and SNP was accessed using 200 μM PTIO. The treatments with IAA, HA or SNP enhanced the number of LRs around 2.5-fold (Fig. 1). The treatments either with the auxin response inhibitor PCIB or auxin efflux inhibitor TIBA decreased the LR formation by nearly 50%, and the use of both auxin inhibitors combined inhibited about 60% of LR emission (Fig. 1). Both inhibitors were competent to impair the endogenous auxin effects on LR initiation. When exogenous IAA was added, the inhibitory effect of auxin inhibitors was slightly reduced. In the presence of HA, neither TIBA nor PCIB was able to reduce the number of LRs below the control level. The effects of IAA and HA on LRs were impaired by PTIO. Even in the presence of auxin inhibitors, a high number of LRs due to SNP treatment was observed (Fig. 1).
Root growth measurements. Effect of 20 mg C L−1 humic acid (HA), 0.1 nM indole-3-acetic acid (IAA), 20 μM auxin efflux inhibitor (TIBA), 20 μM auxin signaling inhibitor (PCIB), 200 μM sodium nitroprusside (SNP) and 200 μM specific NO scavenger 2-phenyl-4,4,5,5-tetramethyl-imidazoline-1-oxyl-3-oxide (PTIO) on root growth pattern evaluated by quantification of PR length (white bars) and LR emergence (black bars). Data represent means from three independent experiments ± SD (n = 12 in each experiment). Columns designated with asterisks indicate a significant difference with respect to the control at P < 0.05 (Dunnett’s test)
Although the average values of PR length of seedlings treated with IAA, HA and SNP have been higher than that of control, under this experimental condition no statistical difference was found. However, the PR length was significantly inhibited by PCIB and TIBA (55 and 45%, respectively), and no additive effect was observed in treatments, in which both inhibitors were added together (Fig. 1). Even in the presence of exogenous IAA, the PR length was significantly reduced by auxin inhibitors used singly as well as combined. On the other hand, SNP was able to antagonize the inhibitory effect of PCIB and TIBA on PR growth, similar to that observed in plants treated with HA in the presence of these auxin inhibitors. No significant effect was induced by the NO scavenger PTIO on the PR, but in the presence of both SNP and PTIO some increase in PR elongation was observed, suggesting that residual amounts of NO might be related to this phenomenon.
It is worth noting that even without significant changes promoted by IAA, HA and SNP on PR growth, these treatments induced two times greater LR density (Fig. 2), a feature related to the number of LR emergence events per area of the parent root. The auxin inhibitors did not affect the LR density in control, but both IAA and HA effects were antagonized by TIBA and PCIB, whereas neither TIBA nor PCIB affected the SNP-induced LR density. On the other hand, the LR density was dramatically reduced using PTIO (~4 times) and all treatment effects on LR density were antagonized whenever this NO scavenger was used. These results clearly demonstrate that NO is a key element for LR proliferation and that the NO-stimulatory effect can not be diminished upon inhibition of endogenous auxin pathways and transport.
Lateral root density. Effect of 20 mg C L−1 humic acid (HA), 0.1 nM indole-3-acetic acid (IAA), 20 μM auxin efflux inhibitor (TIBA), 20 μM auxin signaling inhibitor (PCIB), 200 μM sodium nitroprusside (SNP) and 200 μM specific NO scavenger 2-phenyl-4,4,5,5-tetramethyl-imidazoline-1-oxyl-3-oxide (PTIO) on LRs density (LRs per centimeter). Data represent means from three independent experiments ± SD (n = 12 in each experiment). Columns designated with asterisks indicate a significant difference with respect to the control at P < 0.05 (Dunnett’s test)
Plasma membrane H+-ATPase activity is enhanced by IAA, HA and SNP and reduced by auxin inhibitors and NO scavenger
Plasma membrane vesicles isolated from maize roots treated for 4 days with 20 mg C L−1 HA, 200 μM SNP and IAA 0.1 nM exhibited nearly 1.5-fold stimulation of the vanadate-sensitive ATP hydrolysis (Fig. 3). The reduction of the H+-ATPase activity by TIBA, PCIB or PTIO was not statistically different (P < 0.05). The stimulatory effect of HA on this enzyme was almost abolished by TIBA and PCIB, recovering the control level. The stimulatory effect of IAA was inhibited more effectively by PCIB, while the stimulation promoted by SNP was reduced by PTIO, although the inhibition was less severe than that observed for auxin inhibitors over both HA and IAA treatments. The addition of PTIO with HA or IAA resulted in ATP hydrolysis near to control.
Hydrolytic H+-ATPase activity. Effects of 20 mg C L−1 humic acid (HA), 0.1 nM indole-3-acetic acid (IAA), 20 μM auxin efflux inhibitor (TIBA), 20 μM auxin signaling inhibitor (PCIB), 200 μM sodium nitroprusside (SNP) and 200 μM specific NO scavenger 2-phenyl-4,4,5,5-tetramethyl-imidazoline-1-oxyl-3-oxide (PTIO) on initial rate of vanadate-sensitive ATP hydrolysis from root-derived plasma membrane vesicles. Data represent means from three independent experiments ± SD (n = 12 in each experiment). Columns designated with asterisks indicate a significant difference with respect to the control at P < 0.05 (Dunnett’s test)
The H+-pumping activity of the plasma membrane H+-ATPase was evaluated by measuring the rate of ACMA quenching, sensitive to 0.1 mM Na3VO4. Figure 4 shows that the initial velocity (V 0) of ATP-dependent H+-pumping of plasma membrane vesicles was only slightly inhibited (~20%) by TIBA, while the use of PCIB resulted in a greater inhibition (~50%). A similar profile was observed in the H+-gradient formation, as calculated by the total extent of the ACMA fluorescence quenching (ΔF). However, the NO scavenger inhibited 90% of the V 0 and only 20% of the ΔF, suggesting that the catalytic turnover of this enzyme is affected by endogenous NO. The V 0 of ATP-dependent H+ accumulation of IAA- and SNP-treated seedlings was around 2.5-times higher than that of the control, while HA increased the V 0 around threefold (Fig. 4). The ΔF was around threefold greater in root vesicles treated with IAA and HA, while the SNP treatment was about 2.5-times greater than that of control. Even in the presence of TIBA, both HA- and IAA-treated plants held both V 0 and ΔF stimulation at least twice of control. On the other hand, PCIB strongly diminished the IAA effects on these parameters. Although in the presence of the PCIB HA effects on V 0 and ΔF was at least 60% greater than that of control, this auxin response inhibitor diminished from 20 to 30%, the stimulatory effects of HA on the H+-pumping activity. The data also revealed a higher coupling between the ATP hydrolysis and the ATP-dependent H+-pumping activity in response to HA, IAA and SNP treatments. All these treatments promoted higher stimulatory effects on the proton pumping than on the ATPase hydrolytic activity. Conversely, auxin inhibitors diminished the coupling of the pump, and PTIO affected it to a greater extent. The V 0 was dramatically reduced in relation to control by PTIO even in the presence of SNP, while ΔF remained at control level. The stimulatory effect of IAA and HA on V 0 and ΔF was reduced more than half in the presence of PTIO.
Plasma membrane ATPase proton pumping activity. Effects of 20 mg C L−1 humic acid (HA), 0.1 nM indole-3-acetic acid (IAA), 20 μM auxin efflux inhibitor (TIBA), 20 μM auxin signaling inhibitor (PCIB), 200 μM sodium nitroprusside (SNP) and 200 μM specific NO scavenger 2-phenyl-4,4,5,5-tetramethyl-imidazoline-1-oxyl-3-oxide (PTIO) on initial velocity (V 0; white bars) and steady state (ΔF; black bars) of ATP-dependent H+ gradient across plasma membrane vesicles monitored by the fluorescence quenching of the pH-sensitive dye 9-amino-6-chloro-2-methoxyacridine (ACMA). Data represent means from three independent experiments ± SD (n = 12 in each experiment). Columns designated with asterisks indicate a significant difference with respect to the control at P < 0.05 (Dunnett’s test)
Using the pH indicator bromocresol purple and potentiometric pH measurements of a medium containing excised LRs, it was possible to show that the acidification promoted by IAA, HA and SNP are vanadate sensitive, revealing that the increase of the H+ extrusion was related to the H+-ATPase activation (Fig. 5).
Rhizospheric pH measurements. Effects of 20 mg C L−1 humic acid (HA), 0.1 nM indole-3-acetic acid (IAA), 200 μM sodium nitroprusside (SNP) on the acidification of maize roots exposed to treatments for 72 h. After incubation for 70 min in the media containing the pH indicator bromocresol purple the images were recorded. The yellow regions in rhizosphere correspond to acidification bellow 6.0. Parallel assays monitoring pH in solution was carried out with and without 500 μM Na3VO4. The rhizospheric pH changes are quantified by potentiometric pH measurements in the presence or not of 500 μM Na3VO4 (Graphs, stars (*) indicate a significant difference with respect to the control at P < 0.05 by Dunnett’s test). Inset shows representative image of roots treated with HA in the presence of 500 μM Na3VO4
HA, IAA and SNP induces NO accumulation in specific maize root cell types
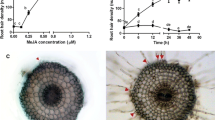
Nitric oxide was detected in situ using the fluorescent probe DAF-2 DA at the mature root zone. The endogenous NO fluorescence was more pronounced at endodermal layer and vascular tissue system with an increased signal around 230, 160, and 225% by treatments with HA, IAA and SNP, respectively (Fig. 6). The cell types exhibiting prominent fluorescence were endodermal layer, protoxylem bundles, and parenchymatic xylem layer boundaring metaxylem and medullar parenchyma (Fig. 6). In addition, pericycle layer, metaxylem vessels, medullar parenchyma and other cell types belonging to the central cylinder showed less prominent signal (Figs. 6, 7). The presence of PTIO diminished 50% of the endogenous NO fluorescence and reduced the signals obtained with the HA, IAA and SNP treatments to levels similar to that of untreated seedlings. Despite the qualitative differences all treatments showed the same anatomical pattern of fluorescent signal localization. Lateral root formation was shown to occur in the mature zone of whole transverse maize root sections (Fig. 7). Besides the vascular tissue pattern demonstrated a strong polarized fluorescent signal was clearly observed at the base of the newly formed lateral root in conjunction with vascular tissue cell types around the emergence site (Fig. 6). Such polarized signal and that observed at the outer cortex from the mother and lateral root epidermis have been expressed only in root sections exhibiting visible lateral roots.
Nitric oxide production in root endodermal layer and vascular tissues. Maize transverse root sections loaded with DAF-2 DA and observed by bright-field and fluorescence microscopy as described in “Materials and methods”. Effects of 20 mg C L−1 humic acid (HA), 0.1 nM indole-3-acetic acid (IAA), 200 μM sodium nitroprusside (SNP) and 200 μM specific NO scavenger 2-phenyl-4,4,5,5-tetramethyl-imidazoline-1-oxyl-3-oxide (PTIO) on NO production. An alternative NO donor, 200 μM GSNO (n-nitrosoglutathione), was also used as a control to verify the specificity of NO effect. Control blank with no DAF-2 DA addition is shown. Fluorescence intensity of the images was estimated by measuring the pixel intensity and analyzed with the ImageJ (NIH) software. Data are expressed as arbitrary units (AU). Mean ± SD of four independent experiments performed in triplicate is shown. Scale bars 50 μm
Nitric oxide production in the site of lateral root emergence. Maize transverse root sections were treated and visualized as described in Fig. 6 and “Materials and methods”. Fluorescence of roots treated with HA is shown in detail. Arrow indicates the DAF-2 DA fluorescence at the outer cortex parenchymatic cells and epidermis; arrowhead indicates the asymmetric distribution of NO production around metaxylem vessel boundaring medullar parenchyma; open diamond indicates the NO production at protoxylem vessel; star indicates the NO production at endodermis layer. Scale bars 50 μm
Although, widely used as NO donor, SNP can also release cyanide and nitrate. Therefore, an alternative NO donor (GSNO) was used, which cannot generate cyanide as by-product (Fig. 6). A possible nitrate side effect could be ruled out since the oxidation process that generates NO2 −/NO -3 from SNP is effective (Beligni and Lamattina 2000) and a nitrate concentration needed to produce any significant effect should be greater than 1 mM (for review see Zhang and Forde 1998), impossible to be generated by 200 μM SNP. Thus, these data and the antagonizing effects of PTIO provide strong evidence in support of the hypothesis that NO is involved in the processes of HA-induced root growth, H+-ATPase activity and DAF fluorescence.
Discussion
Root development is a key requirement for the ability of plants to adapt and survive in adverse conditions and therefore, the LR number and placement dramatically influenced by external factors (Leyser and Fitter 1998). Auxins are traditionally recognized to provide a fundamental signal during LR development (Casimiro et al. 2003). It has long been recognized that soils, particularly those rich in decaying organic matter, contain auxins (Whitehead 1963; Albuzio et al. 1989), specially the indole-3-acetic acid (IAA), which is the most abundant naturally occurring auxin. In fact, IAA molecules were revealed in the structure of humic substances by different authors using diverse methods (Muscolo et al. 1999; Canellas et al. 2002). The HA herein studied have been characterized previously (Canellas et al. 2002) and presented IAA-like molecules identified by gas chromatography–mass spectrometry associated to HA structure, presumably by means of hydrophobic interactions in clusters intrinsic to their supramolecular arrangement. Correa-Aragunde et al. (2004) reported that NO mediates auxin LR development and the hypothesis of a NO signal downstream of auxin signaling during LR formation has been strongly supported (Kolbert et al. 2008a; Flores et al. 2008). This work shows that maize root architecture is modified by IAA, HA, and SNP, in a similar way, leading to an increase in the number of LRs, a higher root density and a PM H+-ATPase activation.
In order to address the dependence of the HA effects on IAA signaling and transport during LR development, we have used two auxin inhibitors with different modes of action. The p-chlorophenoxyisobutyric acid (PCIB) is a widely used auxin inhibitor that impairs auxin-signaling pathway by regulating Aux/IAA protein stability and thereby influencing the auxin-regulated root physiology, but it does not inhibit auxin transport (Oono et al. 2003). However, the auxin transport also controls several developmental events in plants, including LR initiation (Reed et al. 1998). Therefore, we also used 2,3,5-triiodobenzoic acid (TIBA), an auxin efflux inhibitor (Sussman and Goldsmith 1981; Poupart et al. 2005), and also reported to impair both PIN1 and PM H+-ATPase vesicles trafficking (Geldner et al. 2001).
Plant root architecture regulation is a complex issue that may differ between and within plant species. Our data showed that auxin inhibitors diminished the number of LR stimulated by exogenous IAA or HA treatments, resulting in a lower LR density (Figs. 1, 2). These inhibitory effects were more pronounced in IAA-treated plants than on the HA-treated ones, suggesting that part of the HA effects could activate other pathways beyond that of auxins. Although, IAA and HA induced only a non-statistically significant increase (20–30%) of PR length, this tendency is in agreement with previous data, which showed that auxin concentrations as low as 10−10 M could induce this trait in maize roots (Zhao et al. 2002; Zandonadi et al. 2007). On the other hand, the LR density was clearly enhanced in plants treated with IAA and HA, indicating that the average number of LR emergence events per area of the parent root increased. Since the HA bioactivity was only partially impaired by TIBA or PCIB and that the NO scavenger PTIO was much more effective in antagonizing the HA effects, it seems likely that the remaining HA activity (pathway insensitive to the auxin inhibitors) can also be mediated by NO. Neither TIBA nor PCIB could counteract the stimulation on LR development promoted by the treatment with SNP (Figs. 1, 2, 6). Previously, it was shown that even in the presence of the auxin efflux inhibitor 1-naphthylacetic acid, SNP was able to enhance the LR number in tomato (Correa-Aragunde et al. 2004). Our data corroborate and extend these and other previous data (Kolbert et al. 2008a) suggesting an essential role of NO production during auxin-dependent LR development.
Taken together the data from Figs. 3, 5, 6, it is possible to postulate that the HA-induced activation of the plasma membrane H+-ATPase could be related to HA-mediated NO production activating the acid-growth mechanism for LR development. As we already demonstrated the induction of LR by IAA and HA occurred along with the H+-pump induction in the site of LR emergence (Canellas et al. 2002; Zandonadi et al. 2007). This model is in line with the fact that root primordia are rich in PM H+-ATPase (Moriau et al. 1999) and that overexpression of H+-PPase leads to an increase in of activity of the PM H+-ATPase and auxin transport, both correlating with the higher number of LR (Li et al. 2005).
Classical works showed that maize roots with high elongation rate exhibited more intense acid efflux than regions with lower growth rate, corroborating to the acid-growth mechanism (Pilet et al. 1983; Versel and Mayor 1985). Furthermore, the PR elongation of maize seedlings could be either stimulated or inhibited by, respectively, low (at nM range) or high (µM range) auxin concentrations, concomitantly to a pH decreasing or increasing of the external medium (Evans and Vesper 1980; Moloney et al. 1981). The pH shift triggered by low auxin concentration has been linked to the H+ efflux mediated by the PM H+-ATPase (Wright and Rayle 1983; Gouvêa et al. 1997). In agreement, we demonstrated that IAA, HA and SNP can enhance acidification around maize LR (Fig. 5), in a way consistent with the PM H+-ATPase activation (Figs. 3, 4). Although, at this stage, it is still not clear if there will be any causal relationship between this acidification and LR induction, it seems unlikely that the two phenomena may be triggered independently of each other by all the treatments (HA, IAA, and SNP).
We have also analyzed the presence of NO in maize root segments using the fluorescent probe DAF-2 DA. Both SNP and GSNO stimulated fluorescence in roots to the same extent (Fig. 7). The use of a free-cyanide NO donor rules out the possibility of extra interfering factors other than the NO gas per se influence the characteristics evaluated here. The specific green fluorescence was partially prevented by using PTIO and stimulated by HA, IAA, and SNP treatments as compared to the control roots. Higher levels of NO were detected at the epidermis, outer cortical parenchymatic layers, protoxylem bundles and parenchymatic metaxylem cells facing the medullar region. Interestingly, Jahn et al. (1998) have described a very similar profile for the abundance of the PM H+-ATPase in epidermal, protoxylem, and outer cortical layers and xylem parenchyma cells. Nonetheless, the most remarkable NO signal was specifically located at the vascular tissue region representing the junction between the newly formed and the mother root in early stages of LR development, in agreement with previous report by Correa-Aragunde et al. (2004) and the HA was the most effective treatment to promote this effect.
Among the numerous cellular processes controlled by auxins, apoplast acidification due to the PM H+-ATPase enhanced activity and expression is one of the most important to the plant growth (Hager et al. 1991; Frías et al. 1996). It was previously reported that TIBA inhibits acid efflux in roots (Mulkey et al. 1981) and shoots possibly related to the decrease in vanadate-sensitive H+ efflux (Wright and Rayle 1983). Further, the inhibitory effect of vanadate on maize root elongation induced by 1 nM IAA was reported (Gouvêa et al. 1997). Auxin can enhance PR elongation in maize seedlings at very low concentrations (Zhao et al. 2002), possibly by H+-pump activation (Zandonadi et al. 2007).
Assuming the pH reflects information about an ongoing or preceding process (Felle 2001), the pH could act as a messenger in situations, where pH changes are preconditions for certain processes, such as drought, gravitropism and root growth, all process related to NO (Hu et al. 2005; Correa-Aragunde et al. 2004; Kolbert et al. 2008b). From this point of view, the H+-ATPase could transcend its classical functions as an energy transducer system to also assume a key role as signaling transducers by mediating cross-interactions between HA, IAA, pH and NO signals among other messengers. In fact, this proton pump is involved in many physiological responses and is also tightly regulated upon many endogenous and environment signals (Ramos et al. 2008 and references therein). Specifically in root cells, a Ca2+-dependent phosphorylation of H+-ATPase has been related to the cytoplasm and apoplast pH control (Schallern and Sussman 1988; Lino et al. 1998). Since NO donors can induce cytosolic Ca2+ transients (Lamotte et al. 2006), possible interactions between NO, Ca2+ and pH signals associated to the H+-ATPase differential activation need to be further explored in future researches.
Not much is known about the NO-mediated transduction cascades in plants (Kasprowicz et al. 2009). The majority of enzymes affected by NO are regulated by S-nitrosylation of cysteine residues, including ATP synthases (Lindermayr et al. 2005). It is now thought that NO is part of an universal redox-based signaling mechanism, which seems to represent important post-translational modifications induced by NO on target proteins (Kasprowicz et al. 2009). Erdei and Kolbert (2008) reported that dithiothreitol, which creates a reducing environment affecting S-nitrosylation, delayed LR emission and NO production. It could involve S-nitrosylation of proteins modulating the H+-ATPase or the enzyme itself, since a possible down-regulation of this H+-pump could affect the rhizosphere acidification, and then the LR emission as well as the acid-induced NO production. Interestingly, there are evidences suggesting that protein kinases (PK) act as targets of NO signaling (Pagnussat et al. 2004) and it is well known that the PM H+-ATPase activity is highly regulated by a PK-dependent phosphorylation of its C-terminal autoinhibitory domain (reviewed in Palmgren 2001). The NO signaling was also proved to involve cGMP, cADPR, and Ca2+ signals (Neill et al. 2008) and cytoskeleton changes (Kasprowicz et al. 2009), all of which could influence directly or indirectly the H+-pump regulation. Future studies integrating the present experimental system and identification of S-nitrosylated proteins using mass spectrometry (Lindermayr et al. 2005) may be useful to clarify the mechanisms of NO-induced H+-ATPase regulation.
In any case, the PM H+-ATPase appears to be a target of NO-mediated HA action (Figs. 3, 4). Based on the classical chemiosmotic hypothesis of auxin transport (Lomax et al. 1985; Li et al. 2005), H+ extrusion induced by NO could assume a crucial role in auxin distribution in roots. On the other hand, the IAA and HA-induced H+-ATPase activity could account for the activation of the non-enzymatic pH-dependent mechanism of NO production in the apoplast. In addition, to complete this scenario, it is tempting to speculate that the phenolic components reported to be present in these HA (Canellas et al. 2002) could contribute to this process, as it was previously described for other kinds of phenolic agents (Stöhr and Ullrich 2002; Bethke et al. 2004).
To the best of our knowledge, these are the first evidences on NO-induced PM ATPase hydrolytic activity and H+-pumping monitored during LR development. However, further studies will be needed to clarify whether the relationship between the enzyme activation and LR development is causal or casual. Nevertheless, the present data on H+-pump activation, root acidification and growth are too consistent with the acid-growth mechanism to be coincidental and yet, it cannot be better explained from any alternative proposed model. The auxin inhibitors data reinforced previous evidence suggesting that HA could act as environmental derived auxins, and goes further by showing the increase of NO production during HA-induced LR development. Taking together these data concerning the NO-activation of the H+-pump, the HA and IAA-induced acid growth mechanism (Zandonadi et al. 2007), and the acidifying-dependent apoplastic NO production (e.g. Stöhr and Ullrich 2002), it is possible to postulate an intriguing NO-induced NO-release amplifier mechanism for the HA/auxin signaling, by which the root system architecture could be modulated depending on the signals derived from the organic matter of its environment.
Abbreviations
- IAA:
-
Indole-3-acetic acid
- HA:
-
Humic acids
- NO:
-
Nitric oxide
- LR:
-
Lateral root
- SNP:
-
Sodium nitroprusside
- DAF-2 DA:
-
4,5-Diaminofluorescein diacetate
- HS:
-
Humic substances
- PCIB:
-
p-Chlorophenoxyisobutyric acid
- TIBA:
-
2,3,5-Triiodobenzoic acid
- PTIO:
-
2-Phenyl-4,4,5,5-tetramethyl-imidazoline-1-oxyl-3-oxide
- PM:
-
Plasma membrane
- Na3VO4 :
-
Sodium orthovanadate
- ACMA:
-
9-Amino-6-chloro-2-methoxyacridine
- PR:
-
Primary root
- V0 :
-
Initial velocity
- ΔF :
-
Fluorescence quenching
- DTT:
-
Dithiothreitol
- MES:
-
2-(N-Morpholino)ethanesulfonic acid
- BIS:
-
1,3-Bis[tris(hydroxymethyl)-methyloamino]
- MOPS:
-
4-Morpholinepropanesulfonic acid
References
Albuzio A, Nardi S, Gulli A (1989) Plant growth regulator activity of small molecular size humic fractions. Sci Total Environ 81(82):671–674
Beligni MV, Lamattina L (2000) Nitric oxide stimulates seed germination and de-etiolation, and inhibits hypocotyl elongation, three light-inducible responses in plants. Planta 210:215–221
Besson-Bard A, Pugin A, Wendehenne D (2008) New insights into nitric oxide signaling in plants. Annu Rev Plant Physiol Biol 59:21–39
Bethke P, Badger MR, Jones RL (2004) Apoplastic synthesis of nitric oxide by plant tissues. Plant Cell 16:332–341
Bradford MM (1976) A rapid and sensitive method for the quantification of micrograms quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Canellas LP, Olivares FL, Okorokova-Façanha AL, Façanha AR (2002) Humic acids isolated from earthworm compost enhance root elongation, lateral root emergence, and plasma membrane H+-ATPase activity in maize roots. Plant Physiol 130:1951–1957
Canellas LP, Teixeira Junior LRL, Dobbss LB, Silva CA, Médici LO, Zandonadi DB, Façanha AR (2008) Humic acids crossinteractions with root and organic acids. Ann Appl Biol 153:157–166
Casimiro I, Beeckman T, Graham N, Bhalerao R, Zhang H, Casero P, Sandberg G, Bennett MJ (2003) Dissecting Arabidopsis lateral root development. Trends Plant Sci 8:165–171
Collings DA, White RG, Overall RL (1992) Ionic current changes associated with the gravity-induced bending response in roots of Zea mays L. Plant Physiol 100:1417–1426
Correa-Aragunde N, Graziano M, Lamattina L (2004) Nitric oxide plays a central role in determining lateral root development in tomato. Planta 218:900–905
Davies PJ (1995) The plant hormones: their nature, occurrence and functions. In: Davies PJ (ed) Plant hormones: physiology, biochemistry and molecular biology. Kluwer, Dordrecht, Netherlands, pp 1–12
De Michelis MI, Spanswick RM (1986) H+-pumping driven by vanadate sensitive ATPase in membrane vesicles from corns roots. Plant Physiol 81:542–547
Dobbss LB, Medici LO, Peres LEP, Pino-Nunes LE, Rumjanek VM, Façanha AR, Canellas LP (2007) Changes in root development of Arabidopsis promoted by organic matter from oxisols. Ann Appl Biol 151:199–211
Ederli L, Reale L, Madeo L, Ferranti F, Gehring C, Fornaciari M, Romano B, Pasqualini S (2009) NO release by nitric oxide donors in vitro and in planta. Plant Physiol Biochem 47:42–48
Erdei L, Kolbert Z (2008) Nitric oxide as a potent signalling molecule in plants. Acta Biol Szeged 52:1–5
Evans ML, Vesper MJ (1980) An improved method for detecting auxin-induced hydrogen ion efflux from corn coleoptile segments. Plant Physiol 66:561–565
Felle HH (2001) pH: signal and messenger in plant cells. Plant Biol 3:577–591
Fischer-Schliebs E, Varanini Z, Lüttge U (1994) Isolation of H+-transport-competent plasma membrane vesicles from corn roots by discontinuous sucrose gradient centrifugation: effect of membrane protectant agents. J Plant Physiol 144:505–512
Fiske CF, Subbarow Y (1925) The colorometric determination of phosphorus. J Biol Chem 66:375
Flores T, Todd CD, Tovar-Mendez A et al (2008) Arginase-negative mutants of Arabidopsis exhibit increased nitric oxide signaling in root development. Plant Physiol 147:1936–1946
Frías I, Caldeira MT, Perez CJR et al (1996) A major isoform of the maize plasma membrane H+-ATPase: characterization and induction by auxin in coleoptiles. Plant Cell 8:1533–1544
Geldner N, Friml JK, York-Dieter S, Jürgens G, Palme K (2001) Auxin transport inhibitors block PIN1 cycling and vesicle trafficking. Nature 413:425–428
Gouvêa CM, Souza CP, Magalhães CAN, Martin IS (1997) NO-releasing substances that induce growth elongation in maize root segments. Plant Growth Regul 21:183–187
Hager A (2003) Role of the plasma membrane H+-ATPase in auxin-induced elongation growth: historical and new aspects. J Plant Res 116:483–505
Hager A, Debus G, Edel HG, Stransky H, Serrano R (1991) Auxin induces exocytosis and rapid synthesis of a high-turnover pool of plasma-membrane H+-ATPase. Planta 185:527–537
Hayes MH, Malcolm R (2001) Consideration of composition and aspects of the structures of humic substances. In: Humic substances and chemical contaminants. Soil Science Society of America, Madison, pp 3–39
Hu X, Neill SJ, Tang Z, Cai W (2005) Nitric oxide mediates gravitropic bending in soybean roots. Plant Physiol 137:663–670
Jahn T, Baluska F, Michalke W, Harper JF, Volkmann D (1998) Plasma membrane H+-ATPase in the root apex: evidence for strong expression in xylem parenchyma and asymmetric localization within cortical and epidermal cells. Physiol Plant 104:311–316
Kasprowicz A, Szuba A, Volkmann D, Baluska F, Wojtaszek P (2009) Nitric oxide modulates dynamic actin cytoskeleton and vesicle trafficking in a cell type-specific manner in root apices. J Exp Bot 60:1605–1617
Kolbert Z, Bartha B, Erdei L (2008a) Exogenous auxin-induced NO synthesis is nitrate reductase-associated in Arabidopsis thaliana root primordia. J Plant Physiol 165:967–975
Kolbert Z, Bartha B, Erdei L (2008b) Osmotic stress- and indole-3-butyric acid-induced NO generation are partially distinct processes in root growth and development in Pisum sativum. Physiol Plant 133:406–416
Lamotte O, Courtois C, Dobrowolska G, Besson A, Pugin A, Wendehenne D (2006) Mechanisms of nitric-oxide induced increase of free cytosolic Ca2+ concentration in Nicotiana plumbaginifolia cells. Free Radic Biol Med 40:1369–1376
Leyser O, Fitter A (1998) Roots are branching out in patches. Trends Plant Sci 3:203–204
Li J, Yang H, Peer WA et al (2005) Arabidopsis H+-PPase AVP1 regulates auxin mediated organ development. Science 310:121–125
Lindermayr C, Saalbach G, Durner J (2005) Proteomic identification of S-nitrosylated proteins in Arabidopsis. Plant Physiol 137:921–930
Lino B, Baizabal-Aguirre VM, de la Vara LE Gonzalez (1998) The plasma-membrane H+-ATPase from beet root is inhibited by a calcium-dependent phosphorylation. Planta 204:352–359
Lomax TL, Mehlhorn RJ, Briggs WR (1985) Active auxin uptake by zucchini membrane vesicles: quantitation using ESR volume and ΔpH determinations. Proc Natl Acad Sci USA 82:6541–6545
Malamy JE (2005) Intrinsic and environmental response pathways that regulate root system architecture. Plant Cell Environ 28:67–77
Malamy JE, Benfey PN (1997) Down and out in Arabidopsis: the formation of lateral roots. Trends Plant Sci 2:390–396
Moloney MM, Elliott MC, Cleland RE (1981) Acid-growth effects in maize roots: evidence for a link between auxin-economy and proton extrusion in the control of root growth. Planta 152:285–291
Moriau L, Michelet B, Bogaerts P, Lambert L, Oufattole M, Boutry M (1999) Expression analysis of two gene subfamilies encoding the plasma membrane H+-ATPase in Nicotiana plumbaginifolia reveals the major transport functions of this enzyme. Plant J 19:31–41
Mulkey TJ, Kuzmanoff KM, Evans ML (1981) The agar-dye method for visualizing acid efflux patterns during tropistic curvatures. What’s new plant physiol 12:9–12
Muscolo A, Bovalo F, Gionfriddo F, Nardi S (1999) Earthworm humic matter produces auxin-like effects on Daucus carota cell growth and nitrate metabolism. Soil Biol Biochem 31:1303–1311
Nardi S, Panuccio MR, Abenavoli MR, Muscolo A (1994) Auxin-like effect of humic substances from faeces of Allolobophora caliginosa and A. rosea. Soil Biol Biochem 26:1341–1346
Nardi S, Muscolo A, Vaccaro S, Baiano S, Spaccini R, Piccolo A (2007) Relationship between molecular characteristics of soil humic fractions and glycolytic pathway and Krebs cycle in maize seedlings. Soil Biol Biochem 39:3138–3146
Neill S, Bright J, Desikan R, Hancock J, Harrison J, Wilson I (2008) Nitric oxide evolution and perception. J Exp Bot 59:25–35
Oono Y, Chiharu O, Rahman A, Asppuria ET, Hayashi K, Tanaka A, Uchimiya H (2003) p-Chlorophenoxyisobutyric acid impairs auxin response in Arabidopsis root. Plant Physiol 134:1135–1147
Pagnussat GC, Lanteri ML, Lombardo MC, Lamattina L (2004) Nitric oxide mediates the indole acetic acid induction activation of a mitogen-activated protein kinase cascade involved in adventitious root development. Plant Physiol 135:279–286
Palmgren MG (2001) Plant plasma membrane H+-ATPases: powerhouses for nutrient uptake. Annu Rev Plant Physiol Plant Mol Biol 52:817–845
Palmgren MG, Sommarin M, Ulvskov P, Larsson C (1990) Effect of detergents on the H+-ATPase activity of inside-out and right-side-out plant plasma membrane vesicles. Biochim Biophys Acta 1021:133–140
Piccolo A (2002) The supramolecular structure of humic substances: a novel understanding of humus chemistry and implications in soil science. Adv Agron 75:57–133
Piccolo A, Mbagwu JSC (1999) Role of hydrophobic components of soil organic matter in soil aggregate stability. Soil Sci Soc Am J 63:1801–1810
Pilet PE, Versel JM, Mayor G (1983) Growth distribution and surface pH patterns along maize roots. Planta 158:398–402
Pinton R, Cesco S, Iacolettig G, Astolfi S, Varanini Z (1999) Modulation of NO3- uptake by water-extractable humic substances: involvement of root plasma membrane H+-ATPase. Plant Soil 215:155–161
Poupart J, Rashotte AM, Muday GK, Waddell CS (2005) The RIB1 mutant of Arabidopsis has alterations in indole-3-butyric acid transport, hypocotyl elongation, and root architecture. Plant Physiol 139:1460–1471
Quaggiotti S, Rupert B, Pizzeghello D, Francioso O, Tugnoli V, Nardi S (2004) Effect of low molecular size humic substances on nitrate uptake and expression of genes involved in nitrate transport in maize (Zea mays L.). J Exp Bot 55:803–813
Rahman A, Bannigan A, Sulaman W, Pechter P, Blancaflor EB, Baskin TI (2007) Auxin, actin and growth of the Arabidopsis thaliana primary root. Plant J 50:514–528
Ramos AC, Facanha AR, Feijó JA (2008) Proton (H+) flux signature for the presymbiotic development of the arbuscular mycorrhizal fungi. New Phytol 178:177–188
Reed RC, Brady SR, Muday GK (1998) Inhibition of auxin movement from the shoot into the root inhibits lateral root development in Arabidopsis. Plant Physiol 118:1369–1378
Schallern GE, Sussman MR (1988) Phosphorylation of the plasma membrane H+-ATPase of higher plants by a calcium-stimulated protein kinase. Planta 173:509–518
Schmidt W, Santi S, Pinton R, Varanini Z (2007) Water-extractable humic substances alter root development and epidermal cell pattern in Arabidopsis. Plant Soil 300:259–267
Stöhr C, Ullrich WR (2002) Generation and possible roles of NO in plant roots and their apoplastic space. J Exp Bot 53:2293–2303
Sussman MR, Goldsmith MHM (1981) The action of specific inhibitors of auxin transport on uptake of auxin and binding of n-1-naphthylphthalamic acid to a membrane site in maize coleoptiles. Planta 152:13–18
Sze H (1985) H+-translocating ATPases: advances using membrane vesicles. Annu Rev Plant Physiol 36:175–208
Versel JM, Mayor G (1985) Gradients in maize roots: local elongation and pH. Planta 164:96–100
Whitehead DC (1963) Some aspects of the influence of organic matter on soil fertility. Soil Fert 26:217–223
Wright LZ, Rayle DL (1983) Evidence for a relationship between H+ excretion and auxin shoot gravitropism. Plant Physiol 72:99–104
Zandonadi DB, Canellas LP, Façanha AR (2007) Indolacetic and humic acids induce lateral root development through a concerted plasmalemma and tonoplast H+ pumps activation. Planta 225:1583–1595
Zhang H, Forde BG (1998) An Arabidopsis MADS box gene that controls nutrient-induced changes in root architecture. Science 279:407–409
Zhao H, Hertel R, Ishikawa H, Evans ML (2002) Species differences in ligand specificity of auxin-controlled elongation and auxin transport: comparing Zea and Vigna. Planta 216:293–301
Zhao LQ, Zhang F, Guo JK, Yang YL, Li BB, Zhang LX (2004) Nitric oxide functions as a signal in salt resistance in the calluses from two ecotypes of reed. Plant Physiol 134:849–857
Acknowledgments
This work was supported by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ) and International Foundation for Science (IFS).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zandonadi, D.B., Santos, M.P., Dobbss, L.B. et al. Nitric oxide mediates humic acids-induced root development and plasma membrane H+-ATPase activation. Planta 231, 1025–1036 (2010). https://doi.org/10.1007/s00425-010-1106-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-010-1106-0