Abstract
The role of xanthine oxidase (XO) in the defense response of wheat (Triticum aestivum L.) against the Russian wheat aphid (RWA) (Diuraphis noxia) was studied. Xanthine oxidase catalyzes the oxidation of hypoxanthine to xanthine to uric acid, and oxygen radicals that are formed as a by-product at both of these oxidation steps may participate in plant defense reactions. A resistant wheat cultivar (Tugela DN), and a close isogenic susceptible cultivar (Tugela), were infested with 20–30 aphids per plant before inhibiting xanthine oxidase by adding allopurinol as a soil drench to the resistant plants. Increases in H2O2 content were detected early after infestation in the resistant wheat, and the downstream defense related responses, chitinase and peroxidase activities, were selectively induced by RWA infestation. These downstream defense responses were substantially inhibited in allopurinol treated-resistant wheat, presumably as a response to inhibition of XO, and superoxide dismutase (SOD). We conclude that the production of active oxygen species through the XO system plays an important role in the induction of defense reactions in wheat against the RWA.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Russian wheat aphid (RWA), Diuraphis noxia (Mordvilko) is a serious wheat pest in many countries including South Africa (Du Toit and Walters, 1984). Control of this pest has been achieved by using insecticides (Du Toit, 1989) and resistant cultivars (Marasas et al., 1997). Although resistance or tolerance has been obtained by breeding (Du Toit, 1989), our understanding of the biochemical resistance mechanism in wheat against the RWA is incomplete. The new biotype 2 has overcome the natural resistance mechanism of wheat (Haley et al., 2004; Tolmay et al., 2007). This new biotype poses a great threat for wheat producing areas.
According to Bellefant-Miller et al., (1994), the resistance response to RWA infestation is a typical hypersensitive response (HR). The damage caused by the RWA, a phloem feeder, is probably due to a phytotoxin secreted during feeding (Burd, 2002). The resistance response in wheat against the RWA is characterized by the accumulation of apoplastic proteins that are serologically related to pathogenesis-related proteins (PR-proteins), and increased β-1,3-glucanase, chitinase, and peroxidase (POD) activities (Van der Westhuizen et al., 1998a, b). Induction of these proteins is characteristic of the hypersensitive response (Mur et al., 2008).
The generation of active oxygen intermediates such as superoxide (O2 ●), hydrogen peroxide (H2O2), and hydroxyl radical (OH●) is an early event during pathogenesis, and forms part of a complex signaling network that triggers the overall defense response that may include the HR (Apel and Hirt, 2004; Hung et al., 2005). Hydrogen peroxide is directly toxic to microorganisms (Labas et al., 2008) and drives rapid, peroxidase-mediated oxidative cross-linking of structural proteins in the cell wall (Karlsson et al., 2005). Several enzymes located inside and on the surface of plant cells are probably involved in the synthesis of H2O2 (Apel and Hirt, 2004). Membrane-bound NADPH oxidase (Levine et al., 1994; Bolwell, 1999), an apoplastic peroxidise (POD) (Bolwell et al., 1995), and peroxisomal xanthine oxidase (XO) (Sandalio et al., 1997) have been implicated in hydrogen peroxide production. Xanthine oxidase produces O2 ●, which is converted to H2O2 by superoxide dismutase (SOD) (Montalbini, 1992a). The oxidative purine catabolic pathway, which includes XO, is selectively induced in wheat plants infected with Puccinia recondita (Montalbini, 1992b). Increases in XO activity found in beans upon infestation with Uromyces phaseoli were successfully inhibited by allopurinol treatment, and the resultant hypersensitive cell collapse also was inhibited when infected with pathogens (Montalbini, 1992a).
Moloi and Van der Westhuizen (2006) demonstrated that NADPH-oxidase is involved in the defense response of wheat to the RWA. In this study, we demonstrated the importance of the xanthine/xanthine oxidase system for the induction of resistance to Russian wheat aphid infestation.
Methods and Materials
Plant and Aphid Material
Wheat (Triticum aestivum) plants, resistant (cv. Tugela DN) and susceptible (close isogenic cv. Tugela) (Du Toit, 1989) to the RWA (biotype SA1) Diuraphis noxia (Mordvilko) were grown in a glasshouse at day and night temperatures of 25 º C and 21 º C respectively.
A colony of RWA was obtained from the Agricultural Research Station in Bethlehem, South Africa. The aphids were maintained on Tugela wheat until experimental wheat plants were infested. Plants were infested at the second-leaf growth stage with 25 fourth instar aphids per plant.
All the leaves of the plant were collected at specific time intervals after infestation and immediately frozen in liquid nitrogen. They were then stored at −20 º C for subsequent analyses.
Enzyme Extraction
Enzymes were extracted by grinding 1 g of frozen leaf tissue with 100 mg acid-washed sand in 10 ml of 100 mM potassium phosphate buffer (pH 7.5) containing 1 mM EDTA and 1% PVP (Rao et al., 1997). After centrifugation (25 000 × g, 20 min), the supernatant was used for the enzyme assays.
Protein concentrations were determined with the Biorad Microplate method (Rybutt and Parish, 1982). The assay mixture consisted of 160 μl distilled water, 40 μl BioRad, and 10 μl standard (0.5 μg. μl−1 γ-globulin) or sample (Bradford, 1976).
Determination of Hydrogen Peroxide Content
Hydrogen-peroxide was determined via formation of the colored titanium-hydrogen peroxide complex (Brennan and Frenkel, 1977). Leaf tissue (100 g) was homogenized in 200 ml cold acetone, filtered, and brought to 300 ml with distilled water. Two ml of 20% titanium tetrachloride in concentrated HCl (v/v) were added to 20 ml of the extract, followed by the addition of 4 ml concentrated NH4OH to precipitate the peroxide-titanium complex. After centrifugation (5 min at 10 000 g), the precipitate was dissolved in 15 ml of 2 N H2SO4, washed repeatedly with acetone, and brought to a final volume of 20 ml with distilled water. The absorbance was measured at 415 nm against a water blank. The concentration of hydrogen peroxide in the extracts was determined by comparing the absorbance to a standard curve representing titanium-H2O2 complex.
Determination of Superoxide Dismutase Activity
Following the method of Keppler and Novacky (1987), SOD activity was measured by measuring the inhibition of the nitroblue tetrazolium (NBT) reduction. The reaction mixture consisted of 50 mM phosphate buffer (pH 7.8), 13 mM methionine, 75 μM NBT, 2 μM riboflavin, and 50 μl sample. The reaction mixture was irradiated by placing it 30 cm below two fluorescent lamps (2 × 40 W) for 10 min. A non-irradiated duplicate was used as a control (reference cuvette). After irradiation, the absorbance of the reaction mixture was measured at 560 nm (Hitachi U-2000 double-beam spectrophotometer). In addition, a sample without crude extract was irradiated and used to measure the maximum attainable absorbance at 560 nm. The SOD activity is expressed as log [A560 (with crude extract) mg−1/A560 (without crude extract)] (Keppler and Novacky, 1987).
Determination of Peroxidase Activity
The peroxidase assay mixture consisted of 40 mM potassium buffer (pH 5.5), 5 mM guaiacol, and 8.2 mM H2O2. The change in absorbance was measured at 470 nm for 180 sec at 30°C (Hitachi U-2000 double-beam spectrophotometer) (Zieslin and Ben-Zaken, 1991).
Determination of Chitinase Activity
Chitinase activity was measured according to the method of Wirth and Wolf (1990), with slight modifications. The method is based on the precipitability of carboxymethyl-chitin-remazol brilliant violet 5R (CM-Chitin-RBV) (Loewe Biochemica GmbH) from buffered solutions with HCl.
The reaction mixture for determining chitinase activity consisted of 66.6 mM Na-acetate buffer (pH 5.0), and 100 μl CM-chitin-RBV (2 mg. ml−1) and sample. The reaction mixture was incubated at 37˚C, and after 30 min the reaction was stopped by the addition of 100 μl of 2 M HCl. The sample was put on ice for 10 min to allow complete precipitation and subsequently centrifuged at 12 000 g for 7 min. Before measuring the absorbence at 550 nm, the sample was diluted 4× with distilled water (Wirth and Wolf, 1990).
In Vivo Treatment with Allopurinol
The effect of allopurinol (4-hydroxypyrazolo(3,4-d)pyrimidine) on the HR was evaluated according to the method described by Montalbini (1992b). Allopurinol was applied to plants as a soil drench (250 ml of 0.4 mM solution daily). Application was started 7 d after the wheat was planted, and continued until the last sampling date. Allopurinol is a purine analogue and a competitive inhibitor of XO. It tightly binds to the reduced molybdenum component of the enzyme, thus inhibiting activity and the production of oxygen radicals cannot take place (Massey et al., 1970; Hille and Massey, 1981).
In Vitro Treatment with Allopurinol
Allopurinol was added to the respective reaction mixtures for SOD, POD and chitinase to test for in vitro inhibition. Final concentration of allopurinol in the reaction mixtures was 0.4 mM.
Statistical Analysis
Six leaf samples of each treatment were collected. Enzyme activities were repeated four times for every sample. Statistical analyses were performed using STATISTICA, version 9, from StatSoft®. The normality of the data was checked using the Shapiro-Wilkes test. A one-way ANOVA, using the Turkey HSD test, was performed at a significance level of 95%. The purpose of the ANOVA was to check whether there is a statistical difference in the enzyme activities between the infested resistant wheat and the corresponding controls.
Results
Hydrogen Peroxide Content
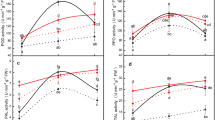
An increase (P < 0.05) in H2O2 was detected in the infested, resistant wheat as early as 8 h after infestation (Fig. 1a). Significantly increased H2O2 levels were observed over a 48 h period (Fig. 1b) with the highest levels detected 15 h after infestation in the infested, resistant wheat. At 15 h post infestation, the H2O2 was 112% higher in the infested, resistant wheat than in the non-infested resistant wheat. After 48 h, the H2O2 was 85% higher in the infested, resistant wheat compared to the infested, susceptible wheat. There were no significant differences between the H2O2 content of the non-infested, resistant, and susceptible wheat leaves.
In Vitro Effect of Allopurinol on Enzyme Activities
The in vitro effect of allopurinol was tested on the activities of SOD, POD, and chitinase. Allopurinol did not inhibit the activities of SOD, POD, or chitinase in vitro (results not shown).
Effect of Allopurinol on Superoxide Dismutase Activity In Vivo
RWA infestation significantly increased (P < 0.05) SOD activity in infested, resistant wheat (Fig. 2). SOD activity did not change during the investigated period in susceptible, infested, or resistant wheat. SOD activity reached a peak 10 h after infestation in infested, resistant wheat. The allopurinol drench significantly (P < 0.05) inhibited the induced SOD activity by 70% in infested, resistant wheat (Fig. 2).
Time course of SOD activity for a 48 h period and the inhibitory effect of drenching the plants with allopurinol (#) on SOD activity. Error bars indicate standard deviation. Asterisks denote significant increases above the infested control (P < 0.05), diamonds denote significant decreases (P < 0.05) from the corresponding controls
Effect of Allopurinol on Peroxidase Activity
RWA infestation significantly (P < 0.05) induced POD activity in infested, resistant wheat, peaking at 24 h. Little change occurred in the controls where the activity remained constant. The allopurinol drench significantly (P < 0.05) inhibited the induced (24 h) POD activity by 80% (Fig. 3) in infested, resistant wheat.
Time course study of POD activity for a 48 h period and the inhibitory effect of drenching the plants with allopurinol (#) on POD activity. Error bars indicate standard deviation. Asterisks denote significant increases above the infested control (P < 0.05), diamonds denote significant decreases (P < 0.05) from the corresponding controls
Effect of Allopurinol on Chitinase Activity
Aphid infestation induced chitinase activity (P < 0.05) in infested, resistant wheat (Fig. 4). The selectively induced chitinase activity was significantly (P < 0.05) inhibited by the allopurinol drench to levels similar to those of controls.
Time course of chitinase activity for a 48 h and the inhibitory effect of drenching the plants with allopurinol (#) on chitinase activity. Error bars indicate standard deviation. Asterisks denote significant increases above the infested control (P < 0.05), diamonds denote significant decreases (P < 0.05) from the corresponding controls
Discussion
H2O2 is responsible for induction of the defense genes as well as for cell death in the development of restricted lesions (Pellinen et al., 2002; Apel and Hirt, 2004). Felton et al. (1994, 1999) demonstrated differential increases in H2O2 in the incompatible interaction between plants and insects. During the incompatible interaction between wheat and the RWA, H2O2 elicited the accumulation of salicylic acid (Mohase and Van der Westhuizen, 2002), which is responsible for systemic acquired resistance.
A possible mechanism for the production of active oxygen species such as H2O2 during the HR is via the enzyme XO, which converts hypoxanthine xanthine, and xanthine to uric acid (Montalbini 1992a, b). Enhanced activity in XO and uricase preceded and accompanied the HR induced by Uromyces phaseoli infection in beans (Montalbini, 1991). Furthermore, active oxygen species drive the synthesis of cell-membrane structural proteins; oxidize cinnamyl alcohols to free radicals by peroxidase/H2O2, leading to the formation of lignin (Karlsson et al., 2005); trigger programmed cell death; and diffuse to neighboring cells to induce defense genes (Mur et al., 2008).
In this study, the authors wanted to determine if oxygen radicals and, in particular, H2O2, are responsible for inducing the downstream defense response in wheat that is resistant to RWA. Allopurinol, which is an inhibitor of XO activity (Montalbini 1991, 1992a, b) was used to inhibit the production of O −2 and consequently H2O2. The interaction between resistant wheat and the RWA was manifested as a differential increase in H2O2 that reached peak values quite soon after infestation (8 h).
The activity of SOD was dramatically reduced (Fig. 2a) in the infested, resistant wheat growing in the soil drenched with allopurinol, thus suggesting that no H2O2 was produced. The low SOD activity could be due to the disappearance of its substrate (O −2 ), or it may be that the genes encoding for SOD were not induced.
It is well established that POD (Van der Westhuizen et al., 1998a, b) and chitinase (Van der Westhuizen et al., 1998b) activities are involved in the downstream defense response of wheat against the RWA, and they were used in this study to indicate whether the downstream defense response is activated or not. This study confirmed the selective induction of POD (Fig. 3) and chitinase (Fig. 4) activities in the infested, resistant wheat upon infestation with the RWA. Allopurinol drenching prevented differential induction of POD (Fig. 3). Loss of POD activity may be the result of insufficient substrate (H2O2) and/or insufficient gene induction due to low H2O2 levels. For this reason, the inhibition of POD activity by allopurinol does not necessarily prove that the H2O2 was necessary to induce the downstream defense response. The authors, therefore, additionally investigated the effect of allopurinol inhibition on the chitinase defense response.
The expression of chitinase has a defensive role when plants are infected with pathogens. Some chitinases have lysozymal activity and can hydrolyze the peptidoglycans in bacterial cell walls, whereas others have exohydrolytic activity (Punja and Zhang, 1993). Wheat infested with the RWA showed differential increases in chitinase activity (Van der Westhuizen et al., 1998b). In this study, we confirmed the differential induction of chitinase activity (Fig. 4). Allopurinol drenching resulted in a 97% decline in activity (Fig. 4). The successful induction of chitinase is, therefore, dependent on the activity of xanthine oxidase. Allopurinol applied in vitro had no effect on the activity of chitinase in infested resistant wheat.
Surprisingly, the leaves of resistant wheat displayed signs of rolling when allopurinol was applied (results not shown), a phenomenon seen only in susceptible plants when infested with the RWA. This can be explained because of the suppressed defense responses that resulted from the allopurinol drenching. Montalbini (1992a, Montalbini and Torre, 1996) has shown that defense responses associated with the HR are suppressed after allopurinol treatment.
Resistant wheat infected with the RWA showed differential increases in H2O2 levels and SOD activity. The importance of H2O2 during the defense response to induce the downstream defense mechanism was demonstrated by inhibiting XO activity with allopurinol. Allopurinol drenching resulted in suppressed SOD, POD, and chitinase activities, thus indicating that the generation of H2O2 via XO is necessary for the activation of downstream defense responses.
References
Apel, K., and Hirt, H. 2004. Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 55:373–399.
Bellefant-Miller, H., Porter, D. R., Pierce, M. L., and Mort, A. J. 1994. An early indicator of resistance in barley to Russian wheat aphid. Plant Physiol. 105:1289–1294.
Bolwell, G. P. 1999. Role of reactive oxygen species and NO in plant defense responses. Curr. Opin. Plant Biol. 2:287–294.
Bolwell, G. P., Butt, V. S., Davies, D. R., and Zimmerlin, A. 1995. The origin of the oxidative burst in plants. Free Radical Res. 23:517–532.
Bradford, M. M. 1976. A rapid and sensitive method for the quantification of micron quantities of protein utilizing the principle of protein dye binding. Anal. Biochem. 72:248–254.
Brennan, T., and Frenkel, C. 1977. Involvement of hydrogen peroxide in the regulation of senescence in pear. Plant Physiol. 59:411–416.
Burd, J. D. 2002. Physiological modifications of the host feeding site by cereal aphids (Homoptera: Aphididae). J. Econ. Entomol. 95:463–468.
Du Toit, F. 1989. Inheritance in two Triticum aestivum lines to Russian wheat aphid (Homoptera: Aphididae). J. Econ. Entomol. 82:1251–1253.
Du Toit, F., and Walters, M. C. 1984. Damage assessment and economic threshold values for the chemical control of the Russian wheat aphid Diuraphis noxia (Mordvilko), on winter wheat. pp 58–62, in M. C. Walters (ed.). Technical Communication, Department of Agriculture, RSA 191.
Felton, G. W., Bi, J. L., Summers, C. B., Mueller, A. J., and Duffey, S. S. 1994. Potential role of lipoxygenase in defense against insect herbivory. J. Chem. Ecol. 20:541–666.
Felton, G. W., Korth, K. L., Bi, J. L., Wesley, S. V., Huhman, D. V., Mathews, M. C., Murphy, J. B., Lamb, C., and Dixon, R. A. 1999. Inverse relationship between systemic resistance of plants to microorganisms and to insect herbivory. Curr. Biol. 9:317–320.
Haley, S. D., Peairs, F. B., Walker, C. B., Rudolph, J. B., and Randolph, T. L. 2004. Occurrence of a new Russian wheat aphid biotype in Colorado. Crop Sci. 44:1589–1592.
Hille, R., and Massey, V., 1981. Tight binding inhibitors of xanthine oxidase. Pharmacol. Ther. 14:249–263.
Hung, S-H., Yu, C-H., and Lin, C. H. 2005. Hydrogen peroxide functions as a stress signal in plants. Bot. Bull. Acad. Sin. 46:1–10.
Karlsson, M., Melzer, M., Prokhorenko, I., and Wingsle, G. 2005. Hydrogen peroxide and expression of hipl-superoxide dismutase are associated with the development of secondary cell walls in Zinnia elegans. J. Exp. Bot. 56:2085–2093.
Keppler, L. D., and Novacky, A. 1987. The initiation of membrane lipid peroxidation during bacteria-induced hypersensitive reaction. Physiol. Mol. Plant Pathol. 30:233–245.
Labas, M. D., Zalazar, C. S., Brandi, R. J., and Cassano, A. E. 2008. Reaction kinetics of bacteria disinfection employing hydrogen peroxide. Biochem. Engineering. J. 38:78–87.
Levine, A., Tenhaken, R., and Lamb, C. 1994. H2O2 from the oxidative burst orchestrates the plant hypersensitive disease resistance response. Cell 79:583–593.
Marasas, C., Anandajayasekeram, P., Tolmay, V., Martella, D., Purchase, J., and Prinsloo, G. 1997. Socio–economic impact of the Russian wheat aphid control Res. program. Published by: South African Centre for Cooperation in Agricultural and Natural Resources Research & Training 1–103.
Massey, V., Komai, H., Palmer, G., and Elion, G. 1970. The existence of nonfunctional sites in milk xanthine oxidase: reaction with functional active site inhibitors. Vitamins and Hormones (NY) 28:505–531.
Mohase, D., and Van Der Westhuizen, A. J. 2002. Salicylic acid is involved in resistance responses in the Russian wheat aphid-wheat interaction. J. Plant Physiol. 159:585–590.
Moloi, M. J., and Van Der Westhuizen, A. J. 2006. The reactive oxygen species are involved in resistance responses of wheat to the Russian wheat aphid. J. Plant Physiol. 163:1118–1125.
Montalbini, P. 1991. Levels of ureides and enzymes of ureide synthesis in Vicia faba leaves infected by Uromyces fabae and effect of allopurinol on biotrophic fungal growth. Phytopathol. Medit. 30:83–92.
Montalbini, P. 1992a. Changes in xanthine oxidase activity in bean leaves induced by Uromyces phaseoli infection. J. Phytopathol. 134:63–74.
Montalbini, P. 1992b. Inhibition of hypersensitive response by allopurinol applied to the host in the incompatible relationship between Phaseolus vulgaris and Uromyces phaseoli. J. Phytopathol. 134:218–228.
Montalbini, P., and Torre, G. D. 1996. Evidence of a two-fold mechanism responsible for the inhibition by allopurinol of the hypersensitive response induced in tobacco by tobacco necrosis virus. Physiol. Mol. Plant Pathol. 48:273–287.
Mur, L. A. J., Kenton, P., Lloyd, A. J., Ougham, H., and Prats, E. 2008. The hypersensitive response; the centenary is upon us but how much do we know? J. Exp. Bot. 59:501–520.
Pellinen, R. I., Korkonen, M-S., Taurianen, A. A., Palva, E. T., and Kangasjärvi, J. 2002. Hydrogen peroxide activates cell death and defense gene expression in Birch. Plant Physiol. 130:549–560.
Punja, Z. K., and Zhang, Y-Y. 1993. Plant chitinases and their roles in resistance to fungal diseases. J. Nematol. 25:526–540.
Rao, M. V., Paliyath, G., Ormond, D. G., Murr, D. P., and Watkins, C. B. 1997. Influence of salicylic acid on H2O2 production, oxidative stress, and H2O2-mediating enzymes. Plant Physiol. 115:137–149.
Rybutt, D. B., and Parish, C. R. 1982. Protein determination on an automatic spectrophotometer. Anal. Biochem. 121:213–214.
Sandalio, L. M., Lopez-Huertas, E., Bueno, P., and Del Rio, L. A. 1997. Immuno cytochemical localization of copper, zinc superoxide dismutase in peroxisomes from waterlemon (Citrullus vulgaris Schrad) cotyledons. Free Rad. Res. 26:187–194.
Tolmay, V. L., Lindeque, R. C., and Prinsloo, G. J. 2007. Preliminary evidence of a resistance-breaking biotype of the Russian wheat aphid Diuraphis noxia (Kurdjumov) (Homoptera: Aphididae), in South Africa. Afr. Entomol. 15:228–230.
Van Der Westhuizen, A. J., Qian, X-M., and Botha, A-M. 1998a. β-1,3-glucanase in wheat and resistance to the Russian wheat aphid. Physiol. Plant. 103:125–131.
Van Der Westhuizen, A. J., Qian, X-M., and Botha, A-M. 1998b. Differential induction of apoplastic peroxidase and chitinase activities in susceptible and resistant wheat cultivars by Russian wheat aphid infestation. Plant Cell Rep. 8:132–137.
Wirth, S. J., and Wolf, G. A. 1990. Dye-labeled substrates for assay and detection of chitinase and lysozyme activity. J. Microbiol. Meth. 12:197–205.
Zieslin, N., and Ben-Zaken, R. 1991. Peroxidase, phenylalanine ammonia-lyase and lignification in peduncles of rose flowers. Plant Physiol. and Biochem. 29:147–151.
Acknowledgements
We wish to acknowledge the financial support from the National Research Foundation and the University of the Free State towards this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Berner, J.M., Van der Westhuizen, A.J. Inhibition of Xanthine Oxidase Activity Results in the Inhibition of Russian Wheat Aphid-Induced Defense Enzymes. J Chem Ecol 36, 1375–1380 (2010). https://doi.org/10.1007/s10886-010-9879-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-010-9879-y