Abstract
Modularity is defined as the ability to combine variable components of an implant in order to accommodate clinical hip, knee, or shoulder cases where standard monoblock designs may not offer optimum outcomes. Modular designs have been used for decades in adult reconstruction surgery. However, recent innovations, such as a second neck-stem taper junction in hip implants or multi-modular revision implants for hip, knees, and shoulder cases, have been presented and favored in clinical use for their advantages in facilitating the anatomic restoration of the defective joints. Intraoperative adjustment of limb length, head-neck angle, neck-shaft version in hip and shoulder cases, and accurate reestablishment of joint line in knee arthroplasties, all provide flexibility and a variety of available options.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Tibial Component
- Ultrahigh Molecular Weight Polyethylene
- Polyethylene Wear
- Glenoid Component
- Tibial Insert
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Introduction
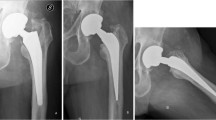
Modularity is defined as the ability to combine variable components of an implant in order to accommodate clinical hip, knee, or shoulder cases where standard monoblock designs may not offer optimum outcomes [1]. Modular designs have been used for decades in adult reconstruction surgery [1]. However, recent innovations, such as a second neck-stem taper junction in hip implants (Fig. 18.1) or multi-modular revision implants for hip (Fig. 18.2), knees, and shoulder cases, have been presented and favored in clinical use for their advantages in facilitating the anatomic restoration of the defective joints [2]. Intraoperative adjustment of limb length, head-neck angle, neck-shaft version in hip and shoulder cases, and accurate reestablishment of joint line in knee arthroplasties, all provide flexibility and a variety of available options [2–6].
However, new problems have also arisen from the presence of additional metal interfaces. Catastrophic fractures at the junction sites, cold, welding, corrosion and fretting as well as the clinical implications of early implant loosening, and systemic immune reactions have been noted [2, 7–10].
With regard to the above statements and concerns, we present in this chapter up-to-date experimental and clinical data about the use of modular implants in hip, knee, and shoulder arthroplasties. We show the advantages and disadvantages associated with their use and known future directions.
Total Hip Replacement
Acetabular Components
Modular acetabular components have a history of almost 30 years [1]. Although clinical reports did not show a clear advantage of the primary cemented modular over monoblock implants in terms of longevity and loosening, their ability to replace the liner without disrupting the prosthesis-bone interface in future procedures has been a significant evolution in implant design [1]. New cementless metal-backed implant designs with different surface porous coatings have been used, showing at least an outcome equal to that of cemented cups, while in revision cases modular implants have outperformed monoblock-cemented components [11–13]. The major advantage of modular metal-backed acetabular components lies in the option of screw placement through holes in the metal shell [11–13]. These screws, especially in the setting of revision surgery, provide adjunctive fixation when primary scratch fit is not considered adequate. Moreover, multi-hole implants increase screw placement options, when bone loss and poor bone quality limit the available sites of screw insertion [11–13]. Another potential advantage of modular acetabular components is the interchangeability of liners, according to clinical demands [1, 14]. Standard, high-lip, high-offset, or constraint liners can be selected on the basis of trial reduction and tests of the stability and range of motion. Moreover, the ability to exchange a liner years after insertion because of excessive wear is an occasional advantage [1, 2]. A number of potential complications associated with the use of modular acetabular components have also been reported. Simple liner exchange is not always feasible. Concomitant acetabular shell loosening and damage, and insufficiency of locking mechanism are often a problem [13, 15]. Of greater concern is the possibility of increased polyethylene wear at the interface of the acetabular shell and rear of the liner, the so-called backside wear [16, 17]. Production of particulate debris due to micromotion and wear may occur, and subsequent bone lysis may be observed [16, 17]. The magnitudes of micromotion vary among different implant designs, ranging from 5 to 311 μm. The linear wear rate is estimated to range between 0.03 and 0.42 mm/year [18–21]. Other concerns are the abrasion of polyethylene from protruding screw heads, the cold flow of polyethylene into screw holes, and the perforation of a congruent liner by sharp metal components of the locking mechanism [16–20]. Several predisposing factors related to increased wear have been identified [22]. Inadequate thickness of the polyethylene, ineffective metal backing, and liner-metal surfaces incongruities are the most commonly reported [19, 20, 23, 24]. Kurtz et al., using a finite analysis model, showed that backside nonconformity and locking restraints substantially influence relative motion as well as load transfers at the liner-shell modular interface [25]. With regard to the liner thickness, it is generally recommended that current implant designs should include a minimum thickness for conventional polyethylene liners of 6–8 mm, with adequate congruency, and ability to bottom out at physiologic loads [22]. However, new ultrahigh molecular weight polyethylene and ceramic liners show better wear resistance and conformity, allowing for thinner liners to be used, and greater sizes of ball heads to be accommodated from smaller-diameter acetabular shells [26, 27]. Examination of retrieved specimens and laboratory testing suggest that improving implant design could eliminate most of these potential problems [16, 17, 28]. Since the shell is now appreciated to represent a wear interface with the backside of the liner, it should be highly conforming as well as smooth and the surface treated, like any other weight-bearing surface [19, 29, 30]. Hemispheric cups have been shown to have the best conformity between the shell and the liner [31, 32]. The locking mechanism should be strong enough to resist levering out. The force necessary for dissociation of the modular liner from acetabular shell has been reported to be extremely variable ranging from 14.9 to 1,380 lb [19, 24, 30]. However, novel liner locking mechanisms have shown efficient pullout and lever-out strength (399 ± 53 N) (28.03 ± 2.8 Nm) for up to ten million cycles of loading of 5 Nm, without significant reduction in strength, no detectable fretting wear and substantial sealing [15, 19, 24, 29, 30]. Liners may also rotate within the shell cavity without dissociation, causing impingement on the femoral neck, especially when high-lip liners are used [33, 34]. When the relative lack of conformity is combined with the empty space for screw holes, the actual surface area supported by metal varies from 25 to 75 % [35–37]. Therefore, screw holes should be as few as possible to minimize the risk of debris generation and to give effective joint space; non-used screw holes should be tapped before the fixation of the liner to eliminate this problem. In revision components, making provision for adjunctive screw fixation is still advisable in most cases [35–37]. Several finite element models support the improved stress distribution in the subchondral bone through the metal-backed implants [22, 25, 38]. This is also confirmed by histologic analyses of early retrieved porous-coated acetabular components indicating that adequate bone ingrowth is present when adjunctive fixation is utilized [22, 25, 38].
Modular Stems
Clinical Advantages and Disadvantages
Modular implants have a number of advantages comparing to monoblock implants. Variability in femoral head length allows for better restoration of limb length inequalities and femoral offset, resulting in improvement of hip stability and hip biomechanics (Fig. 18.3) [2]. Blaha in 2006 presented his theory of a “sweet spot” on the femur and the need to duplicate it during reconstruction as accurately as possible [39]. Optimum neck height and anteversion can be achieved independently of the femoral neck position using modular neck and head implants [39]. Moreover, different implant materials can now be combined, giving several options in bearing surface selection, according to the patient’s specific needs and/or surgeon’s preferences [1, 2]. In revision cases, in which only an acetabular component is being replaced, modular heads can be removed facilitating hip exposure. Intraoperative variability and flexibility provided by choices of different diameter stem lengths, fixation types, proximal metaphyseal sizes, and orientation enable the establishment of a stable hip joint [1, 22]. Additionally, stem modularity enhances fit and fill, provides greater initial fixation, and more uniform stress distribution while minimizing stress shielding, bone loss, and incidence of thigh pain (Fig. 18.4) [1, 22]. Proponents of stem modularity believe that the modular components offer optimal proximal metaphyseal fill and proximal stress transfer with distal fit for initial torsional stability [1, 22, 40–42]. Modularity potentially provides an adequate number of proximal and distal geometry combinations to facilitate the achievement of maximal direct bone contact with porous coating proximally and stem contact with endosteal cortex distally [40–42].
However, problems with femoral stem-head modularity had been recognized early. Dissociation of the head, corrosion at the modular head-neck interface, and fractures at the base of the modular trunnion have been extensively reported [43–48]. Negative effects on range of motion have also been recognized especially whenever skirted femoral heads are used. This is owing to the reduction of head-neck ratio, which induces earlier impingement of the neck onto the acetabular rim, excessive polyethylene wear, and liner dissociation [49, 50]. Head-liner mismatch is another effect of head-stem modularity. The large available number of component combinations increases the potential risk of mismatch. Head-taper mismatch has also been reported and is shown to be related to increased micromotion and development of corrosion [51, 52]. Awareness is therefore needed when combining components from different manufacturers, which is not unusual especially in revision cases.
Mechanisms of Corrosion, Fretting, Cracking, and Failure of Modular Interface
Corrosion products and wear debris generated at the head-neck (Fig. 18.5) and neck-stem interfaces are well documented in the current literature [53–56]. It is generally agreed that the surface damage seen at the head-neck taper is initiated by fretting. Fretting has been demonstrated in 100 % of test specimens in vitro and in over 50 % of retrieved implants [46, 55, 56]. Fretting increases the development of crevice and galvanic corrosion by disrupting the passive oxide layer of the taper interface. Gilbert et al. tried to document the taper corrosion processes better using metallurgical sectioning techniques and scanning electron microscopy [54]. They showed that a pitting attack on both sides of the taper interface evolves into plunging pits. The latter ultimately develop into cracks where the crack propagation process is one of corrosion resulting in oxide formation and subsequent reorganization. The oxide that forms has a complex evolving structure including a network of transport channels that provide access of fluid to the crack tip. This emergent behavior does not appear to require continued fretting corrosion to propagate the pitting and cracking. This mechanism is similar to stress corrosion cracking where the crack tip stresses arise from the oxide formation in the crack and not externally applied tensile stresses. Rodriguez et al. investigated the surface of hip implants with Ti-6Al-4V/Ti-6Al-4V modular taper interfaces and showed that an in vivo hydrogen embrittlement is the mechanism of degradation in modular connections, which results from electrochemical reactions induced in the crevice environment of the tapers during fretting-crevice corrosion [57]. Hardening by nitriding or nitrogen implantation also can improve the strength and wear resistance of the Morse taper [57].
Several parameters are associated with corrosion and fretting of modular taper surfaces. The impact of different material combinations, flexural rigidity, head and neck moment arm, neck length, and implantation time has been evaluated [46, 56, 58]. Material combination, head offset, and assembling conditions are reported by different authors as independent causative factors in fretting [59]. In single modular implants (modularity at the head-neck junction), stainless steel/cobalt-chromium and titanium/cobalt alloy couplings have shown increased corrosion compared to cobalt alloy/cobalt alloy ones. Moreover, metal/metal junctions induce significantly higher cobalt and chromium metal releases and fretting compared to ceramic/metal junctions [46, 56, 58, 60]. Double modular implants (modularity at both head-neck and neck-stem junctions) fretting and crevice corrosion are expected to be increased due to increased modular interfaces. Fretting and corrosion have been shown to be common at both head-neck junction (54 % showing corrosion; 88 % showing fretting) and stem-sleeve junction (88 % corrosion ; 65 % fretting) in a series of 78 retrieved hip implants [53]. Metal ion and particulate debris generation is increased [53]. Titanium releases measured from titanium (Ti6A14V) modular interfaces are extremely low. However, titanium neck adapters show larger micromotions than cobalt-chrome neck adapters [61]. Neck adapters made of cobalt-chrome alloy show significantly reduced micromotions especially in the case of contaminated cone connection. Grupp et al. demonstrated that with cobalt-chromium neck, the micromotions can be reduced by a factor of 3 compared to the titanium neck [46]. The incidence of fretting corrosion was also lessened with cobalt-chrome necks. Modular titanium alloy neck adapters may fail due to decreased stiffness and increased surface micromotion and should be used with great caution on patients with an average weight over 100 kg [46].
In revision implants, distal modularity has been associated with erosion of the shaft and migration of the distally modular component in some cases. This raises the concern of wear debris and lysis originating at this interface [1]. Implant geometry and neck-shaft angle also play a significant role in fretting and corrosion at the junction of head and neck. Higher offset is associated with increased fretting damage. Corrosion and fretting is higher for heads than necks. Larger-diameter necks increase neck stiffness and therefore could possibly reduce fretting and corrosion of the taper interface regardless of the alloy used. Carlson et al. showed that small-diameter femoral stems with large offsets have an increased risk of stem fracture [53].
Debris and Wear
Every combination of materials may generate the production of millions of particles in the 1- to 2-μm range. The most important factor in increasing the particle count is dimensional mismatch. Roughened and nitrogen-implanted surfaces produce fewer particles, while heads larger than 10 mm produce more particles [62, 63]. It is now agreed that metal particles may act as a third body to accelerate polyethylene wear and subsequently cause bone lysis and implant loosening [62, 63]. Corrosion products from modular head and neck tapers increase the particulate debris in the joint and migrate along membranes at the bone-implant interface to sites remote from their origin. Urban et al. showed that these particles could also migrate to the prosthetic bearing surface inducing third-body wear [64, 65]. The increased production of polyethylene debris from third-body wear could contribute to periprosthetic bone loss and aseptic loosening, with implications for possible systemic toxicity [64, 65].
Stress Distribution and Micromotion
Stress distribution within components and the micromotion of the interface significantly influence the function of the taper lock in the long term [51, 61, 63]. Bending-induced gap opening between the cone and the sleeve in double modular implants (head and neck modularity) can lead to an inflow of biological fluids and thus accelerate implant corrosion [51, 61, 63]. Local areas of high stress can accelerate the corrosive process and initiate local yielding. This may lead to fracture in one of the modular components, especially when high-offset necks are selected for heavy-weighting individuals [46]. However, Chu et al. observed that for titanium (Ti6A14V) components, cortical bone bridging and ingrowth occurs across the taper lock gap, which induces a reduction in the peak stress by 45 % and in the contact interface separation by 55 % [44]. Such tissue formation around the taper lock joint could also form a closed capsule to restrict the migration of wear particles and prevent bone resorption and implant loosening.
Assembling Process
Special attention should be paid during the assembling process. If the prosthesis is cemented, the head should be impacted with several firm blows on the back table prior to implantation. Forceful blows shortly after cement polymerization can damage the implant-cement interface. Assembly prior to insertion is therefore advisable [66, 67]. When implanting an uncemented stem, the head should be impacted onto the trunnion after implantation of the stem, because the vibration of striking the implant can disrupt the lock of the Morse taper. In either case, extreme care should be taken to keep the interface clean, dry, and free of any debris [67, 68]. Contaminated surfaces exhibit significantly larger micromotion comparing to meticulously cleaned ones. Even a fraction of a millimeter of blood can substantially adversely affect the taper lock and accelerate corrosion and wear [61]. There are several studies reporting on optimizing the assembling technique of modular components. Rehmer et al. tried to assess the influence of assembly force, assembly tool, and number of hammer strokes on the taper junction strength of various metal combinations [66]. The authors showed that taper strength linearly increased with assembly forces. Multiple impactions did not increase taper strength. A single impact is sufficient to achieve fixation. Ceramic and cobalt-chromium heads showed similar fixation patterns on titanium tapers. It was also suggested that impaction forces of at least 4kN achieve sufficient head-taper junction strength in all bearing conditions. Pallini et al., on the other hand, tried to determine the disassembly force and showed that blows to the proximal end of the neck-stem coupling should be avoided as this could compromise the cleanliness of the head-neck modularity and damage the bearing surfaces [68]. They also reported that disassembly force after manual insertion followed by the first small postoperative loads imposed by the patient during walking was as high as that obtained with hammer blows, and therefore application of hammer blows to fix neck-stem coupling is unnecessary. Nganbe et al. assessed the distraction forces after in vitro cycling in bovine serum and showed that the neck-stem pull-off force initially increases during cycling and reaches a maximum value of 5.704 kN at 100,000 cycles [67].
Total Knee Replacement
Tibial Inserts
The benefit of modularity in total knee arthroplasty implants is widely recognized and includes the ability to fine-tune soft tissue balance and reestablish more accurately the height of the joint line. Modular tibial components offer a variety of options especially for the difficult revision cases with significant bone loss. Modular inserts provide a number of choices of thickness as well as the degree of constraint of the articular surface. This increases intraoperative variability, mainly by providing the option of switching from a posterior cruciate ligament – retaining (CR) to a posterior stabilized (PS) insert utilizing the same tibial baseplate. The use of modular inserts is also useful for these cases of excessive polyethylene wear, without implant loosening, that a simple polyethylene insert exchange with a thicker and/or more constrained liner could be sufficient [1]. However, modularity of tibial components has not shown any superiority in terms of implant survivorship comparing to non-modular implants. Several disadvantages of modularity have been reported. The unintended bearing surface between the back surface of the tibial implant and the metallic tray results in micromotion that increases polyethylene wear [69, 70]. The main contributing factors include the following: insufficiency of locking mechanism, failure of thin polyethylene modular inserts, abrasion of the tibial spine with secondary wear, impingement of the locking pin against the femoral component, and corrosion between screws and the baseplate [71]. A membrane invariably forms at this interface, and concern has been expressed about the possibility that this increases the potential for late infection [1]. To date, there is no evidence to support this concern.
The clinical relevance of micromotion and backside wear is now well understood. Parks et al. investigated the anteroposterior and mediolateral motion between the tibial inserts and baseplate that were measured with an extensometer placed across the modular interface [72]. The authors observed hundreds of microns of motion even under a 100 N load and variability between implants of the same design, showing that more efforts should be made in the improvement of locking mechanisms in modular knee implants. Engh et al. highlighted the insufficiency of the capture mechanisms of some modular fixed-bearing tibial components [73]. In this study, a uniaxial mechanical testing machine was used to evaluate a variety of total knee components applying loads along the anteroposterior and mediolateral axes of the tibial component. It is significant that motion between the polyethylene insert and the metal baseplate increases after a period of in vivo loading. The same study group tried to quantify the relative motion of the modular interface, which was measured in the transverse plane, and correlate it to the backside wear that was observed. For this purpose they used these measurements to compute the insert motion index, which served to quantify unrestricted motion of the insert with respect to the baseplate. It was shown that the mean insert motion index for the tibial components was 416 μm, ranging from 104 to 760 μm. This insert motion was positively correlated with backside polyethylene wear (p = 0.003) and baseplate wear (p < 0.001). Moreover, baseplate wear was found to be strongly correlated with backside polyethylene wear (p < 0.001). Wasielewski also observed a micromotion between 2 and 25 μm in the shear plane relative to metal backing, suggesting that undersurface motion may be inevitable [74]. The author demonstrated that forces at the modular interface, created during physiologic loading, are influenced by the insert type, the articular design, and the surgical technique. Increasing articular insert constraint can increase the forces at the main articulation to be resisted and transferred to this and the other interfaces. Designs with a cam-post mechanism that force rollback at a certain flexion angle create a significant force in this shear plane. Inserts with highly conforming articular geometries can have a similar effect. Component alignment and position, and ligament balance also may influence backside wear as suggested by the great variability of wear patterns seen on similar insert retrievals and by kinematic differences observed in fluoroscopic studies of the same implant design [69, 71, 75].
Several studies have found that micromotion at the tibial tray-polyethylene interface is associated with increased risk for increased particulate debris generation. Conditt et al. found that pitting, burnishing, and measurable polyethylene protrusions may occur on the backside of polyethylene inserts [71]. Li et al. showed that the amount of polyethylene wear found after examining 55 retrieved tibial inserts with four different locking mechanisms was as high as 591 mg from the inferior surface [76]. This corresponded to a polyethylene wear rate from the backside of the tibial insert of greater than 100 mg, which is two to four times higher than wear rates associated with total hip replacements. Debris from backside wear combined with wear from the articular side might account for the increasing prevalence of osteolysis since modular components have become widely used [70]. Peters et al. reported that the incidence of osteolysis in an uncemented modular tibial component is 16 % [77]. Surace et al. found that in the anteroposterior profile of the polyethylene insert, a concave deformation of the back surface is developed in 96 % of the retrieved implants they examined, using a stereomicroscope with a digital optical system [78]. Akisue et al. reported that the backside deformation is associated with polyethylene thickness and the type of locking mechanism [79]. This concave deformation may facilitate accumulation and transportation of wear debris to the tibial bone-implant interface.
Augmentation Devices
The use of metal augmentation devices to reconstruct femoral or tibial bone deficiencies during a revision knee arthroplasty has been another impetus to increase the modularity of total knee replacement components [80–82]. Utilization of these devices is generally faster and technically easier when compared with the reconstructive techniques that use autograft or allograft bone segments [80, 83]. Metal augments are better indicated for small- and medium-sized structural bone defects. Metal blocks and wedges have both been utilized. However, there is some evidence that the block configuration is biomechanically superior, as it distributes the load more evenly than does wedge augmentation [5, 80, 84]. Trabecular metal augmentation has added new treatment options for severe proximal tibial bone defects in revision knee arthroplasty [5, 80]. Porous tantalum tibial cones provide mechanical support for the tibial component and have the potential for long-term biologic fixation [80, 85, 86]. The major disadvantage of adding modular components is the potential for fretting or failure of the interface, although these events have not yet been reported. In order to prevent this type of complication, most modular revision implant designs have tried to reduce the number of modular parts to a minimum by using components that require assembly and providing a large inventory of one-piece integral components with wedge or block augments incorporated into the tibial baseplate [80].
Stems
Modular stems add additional fixation, which is often necessary because of bone loss in revision knee replacement [80]. A press fit can be obtained in the femoral and tibial canals by utilizing a wide range of lengths, diameters, and offsets [5, 80, 87]. Options of both straight and curved stems are also available. Hybrid type of fixation with cementing of the articular surfaces and press fitting of the stems in the medullary canals is usually applied. Improved results with press fitting of stems and cementing of only the surface of the tibia and femur have been reported [88, 89]. One major advantage is that press-fit stems are easier to revise when necessary, since cement does not have to be inserted into the medullary canal of the tibia or femur [88, 89]. Disadvantages include increased potential for fracture of the tibial or femoral shaft in an attempt to achieve a press fit with large stems. Stress shielding due to the stiffness of the stems may cause bone resorption of the distal femur and proximal tibia [90, 91]. In addition, there is an increased concern regarding fretting corrosion and the generation of particulate debris from the modular connection or failure of the connection [1].
Shoulder Arthroplasty
In recent years there has been an increasing interest in humeral component modularity. Modular shoulder implants offer a wide variety of diameters and sizes in both humeral and glenoid components [92, 93]. The modularity of total shoulder arthroplasty implants has demonstrated several advantages compared to monoblock implants [92, 93]. Humeral stem insertion is much easier without the attached humeral head component. Diameter and offset may be varied according to the desired soft tissue tension, thus maximizing stability and range of motion. Moreover, the glenoid component and the humeral head may be revised without removal of the humeral stem, and conversion to inverse type prosthesis can now be done [92–94]. At the glenoid side, modularity of polyethylene and metal backing can also facilitate simple exchange of the insert without the need to remove the metal-back component [95]. Potential disadvantages of modular shoulder implants include instability or stiffness when the selected humeral head is too small or too large, respectively, corrosion and fretting at the head-stem interface, component dissociation (head-stem and polyethylene-metal back), and stress shielding at the glenoid side [92, 96].
Humeral Head-Stem
Dissociation of the humeral component has been of great concern [97, 98]. Improper taper fit caused by contamination of the head-stem interface with blood is reported as the most likely factor responsible for in vivo dissociations in types of commercially available implants. Blevins et al. conducted a biomechanical and implant retrieval study investigating the effect of loading rate, load amplitude, and the number of impactions on fixation of the humeral head component [98]. These authors demonstrated that the dissociation force is linearly proportional to the impaction force. However, repetitive loading beyond two impactions does not significantly increase taper strength. Chao and Kasman noted only a 6 % increase in dissociation force after 1,000 loading cycles with a maximum sliding distance for the shank inside the socket of 0.1 mm [99]. The mean dissociation force after two impactions with a mallet was 2,926 ± 955 N [98]. Cooper and Brems measured a mean force of 2,996 N to dissociate a retrieved Biomet humeral component [100]. Asglan et al. reported dissociation forces in excess of 4,000 N after the loading of an 8° included angle titanium taper [101]. Chao and Kasman reported dissociation forces of approximately 1,300 N after an impaction force of 2,225 N (4° included cone angle titanium taper) [99]. It is shown that contamination of the taper with as little as 0.4 ml of fluid could lessen the fixation strength of the taper. Contamination with liquid (water, oil, and blood) and solid debris (polymerized, morselized polymethyl methacrylate cement) may affect the fixation of the taper [98]. With regard to the effect of the taper material, Blevins et al. showed that the coefficient of friction for the cobalt-chrome-titanium taper (0.7 ± 2.5) is not statistically different from that of the titanium tapers but does show considerable variation (range from −8.60 to 8.06) [98]. Regression analysis between the impaction force and coefficient of friction for titanium-titanium and cobalt-chrome-titanium tapers shows no significant effect. However, Chao and Kasman found that titanium tapers had a higher dissociation force than those of stainless steel [99]. This difference between studies may be due to the wide variation in the measured coefficients of friction for cobalt-chrome-titanium tapers.
Metal-Backed Glenoid Components
At the glenoid side, when compared with the cemented all-polyethylene components, the uncemented modular metal-backed components display lower subchondral stresses. This effect is more pronounced during eccentric loading. However, high polyethylene stress regions are present at the polyethylene-metal interface in relation to the all-polyethylene components. This result suggests that this interface will be the site of initial polyethylene yielding and ultimately, component failure, at loads that are lower than those necessary to cause failure in the all-polyethylene component. In a 3D finite element analysis model, Gupta et al. showed that, although the indications of stress shielding and separation of modular parts of the prosthesis are apparent, the implant-bone interface seems less likely to fail as compared to cemented designs [102]. Once initial fixation of the implant is achieved, the uncemented modular design appears to have better prospects than cemented non-modular ones. The use of highly stiff (5 mm) metal backing offers rigidity to the implant and therefore causes reduction of stresses in the polyethylene cup and the underlying bone. One the one hand, stresses in the polyethylene cup are reduced by 20 % as compared to the cemented total polyethylene design, thereby decreasing the risk of polyethylene wear [102]. On the other hand, the use of thicker metal-backing results in higher metal-bone and polyethylene-metal interface stresses. These high stresses indicate potential interface disruption, separation of the prosthesis from bone, or separation of polyethylene cup from the metal backing. A thicker polyethylene cup (7 mm) with a thinner circular metal backing (3 mm) might result in lower stresses in the polyethylene cup as well as reduction in the weight of the glenoid component. Stresses in polyethylene cups of thinner metal-backed designs are also reduced when cement is used (8 %), but these reductions are less compared to the thicker metal-backed non-cemented cup (20 %) [102, 103]. As with modular hip and knee components, the potential for generation of wear debris is a concern [1]. Lysis has not been reported to date; however, experience with these modular components is of relatively short duration. Long-term implications are yet to be determined.
Conclusions
The introduction of modular implants has been revolutionary in reconstructive surgery of the hip, knee, and shoulder. Implant modularity allows for more anatomical restoration of limb length inequalities, better implant fit and fill, improved soft tissue tensioning, increased stability, and better overall restoration of joint biomechanics. It facilitates surgical exposure in revision cases and permits the exchange of only the parts that need to be revised, thus preserving a patient from any additional bone loss which may be created during well-fixed implant removal. However, new problems have been recognized in the presence of additional metal interfaces. Dissociation of modular parts, corrosion, fretting, cracking, and failure of the modular interfaces have been presented, and the mechanisms thereof have been extensively studied. Improvements in stress distribution and micromotion between surfaces have been achieved through better manufacturing and machining processes aiming at a reduction of the wear products. Technical features regarding the combination of different materials and the assembling process have also been well studied, and useful recommendations for the everyday clinical practice have been presented.
In conclusion, modularity is a significant renovation in the field of adult reconstruction surgery. Surgical options have been increased, and the variety of random unexpected intraoperative events and problems may now be addressed easily. Acknowledgment of the particular technical specifications and problems related to the presence of additional modular interfaces is of paramount importance, and therefore, it is recommended limiting their use where appropriate.
References
Barrack RL. Modularity of prosthetic implants. JAAOS. 1994;2(1):16–25.
Srinivasan A, Jung E, Levine BR. Modularity of the femoral component in total hip arthroplasty. JAAOS. 2012;20(4):214–22.
Benazzo F, Rossi SM. Modular tibial plate for minimally invasive total knee arthroplasty. Knee Surgery Sports Traumatol Arthrosc. 2012;20(9):1796–802.
Archibeck MJ, et al. A comparison of two implant systems in restoration of hip geometry in arthroplasty. Clin Orthop. 2011;469:443–6.
Lombardi AV, Berend KR, Adams JB. Management of bone loss in revision TKA: it’s a changing world. Orthopedics. 2010;33(9):662.
Cuff D, et al. Torsional stability of modular and non-modular reverse shoulder humeral components in a proximal humeral bone loss model. J Shoulder Elbow Surg. 2011;20(4):646–51.
Lakstein D, et al. Fracture of cementless femoral stems at the mid-stem junction in modular revision hip arthroplasty systems. J Bone Joint Surg Am. 2011;93A:57–65.
Fraitzl CR, et al. Corrosion at the stem-sleeve interface of a modular titanium alloy femoral component as a reason for impaired disengagement. J Arthroplasty. 2011;26(1):113–9.
Kretzer JP, et al. Metal release and corrosion effects of modular neck total hip arthroplasty. Int Orthop. 2009;33(6):1531–6.
Engh GA, et al. Analysis of wear in retrieved mobile and fixed bearing knee inserts. J Arthroplasty. 2009;24(6):S28–32.
Markel D, et al. Deformation of metal-backed acetabular components and the impact of liner thickness in a cadaveric model. Int Orthop. 2011;35(8):1131–7.
Hallan G, et al. Metal-backed acetabular components with conventional polyethylene: a review of 9113 primary components with a follow-up of 20 years. J Bone Joint Surg Br. 2010;92B:196–201.
Werle J, et al. Polyethylene liner dissociation in Harris-Galante acetabular components: a report of 7 cases. J Arthroplasty. 2002;17(1):78–81.
Callaghan JJ, et al. Concerns and improvements with cementless metal-backed acetabular components. Clin Orthop. 1995;311:76–84.
Poole CE, et al. Early follow-up for a hybrid total hip arthroplasty using a metal-backed acetabular component designed to reduce “backside” polyethylene wear. HSS J. 2005;1(1):31–4.
Bradford L, et al. Wear and surface cracking in early retrieved highly cross-linked polyethylene acetabular liners. J Bone Joint Surg Am. 2004;86A:1271–82.
Chen PC, et al. Polyethylene wear debris in modular acetabular prostheses. Clin Orthop. 1995;317:44–56.
Amirouche F, et al. Study of micromotion in modular acetabular components during gait and subluxation: a finite element investigation. J Biomech Eng. 2008;130(2):021002.
Wasielewski RC, et al. The acetabular insert-metal backing interface: an additional source of polyethylene wear debris. J Arthroplasty. 2005;20(7):914–22.
von Schewelov T, et al. Catastrophic failure of an uncemented acetabular component due to high wear and osteolysis: an analysis of 154 omnifit prostheses with mean 6-year follow-up. Acta Orthop Scand. 2004;75(3):283–94.
Fehring TK, et al. Motion at the modular acetabular shell and liner interface. A comparative study. Clin Orthop. 1999;367:306–14.
Kurtz SM, Edidin AA, Bartel DL. The role of backside polishing, cup angle, and polyethylene thickness on the contact stresses in metal-backed acetabular components. J Biomech. 1997;30(6):639–42.
Young AM, et al. Effect of acetabular modularity on polyethylene wear and osteolysis in total hip arthroplasty. J Bone Joint Surg Am. 2002;84A:58–63.
Gonzalez della Valle A, et al. Dislodgment of polyethylene liners in first and second-generation Harris-Galante acetabular components. A report of eighteen cases. J Bone Joint Surg Am. 2001;83A:553–9.
Kurtz SM, et al. Backside nonconformity and locking restraints affect liner/shell load transfer mechanisms and relative motion in modular acetabular components for total hip replacement. J Biomech. 1998;31(5):431–7.
Orradre Burusco I, et al. Cross-linked ultra-high-molecular weight polyethylene liner and ceramic femoral head in total hip arthroplasty: a prospective study at 5 years follow-up. Arch Orthop Trauma Surg. 2011;131(12):1711–6.
Kim YH, Kim JS. Tribological and material analyses of retrieved alumina and zirconia ceramic heads correlated with polyethylene wear after total hip replacement. J Bone Joint Surg Br. 2008;90B:731–7.
Teeter MG, et al. Technique to quantify subsurface cracks in retrieved polyethylene components using micro-CT. J Long Term Eff Med Implants. 2010;20(1):27–34.
Akbari A, et al. Minimal backside surface changes observed in retrieved acetabular liners. J Arthroplasty. 2011;26(5):686–92.
Kyle RF, et al. Factors influencing the initial micromotion between polyethylene acetabular cups and titanium alloy shells. J Arthroplasty. 2006;21(3):443–8.
D’Angelo F, et al. Failure of dual radius hydroxyapatite-coated acetabular cups. J Orthop Surg Res. 2008;3:35.
Weber D, et al. Cementless hemispheric acetabular component in total hip replacement. Int Orthop. 2000;24(3):130–3.
Min BW, et al. Polyethylene liner failure in second-generation Harris-Galante acetabular components. J Arthroplasty. 2005;20(6):717–22.
Shon WY, et al. Impingement in total hip arthroplasty a study of retrieved acetabular components. J Arthroplasty. 2005;20(4):427–35.
Kurtz SM, et al. Simulation of initial frontside and backside wear rates in a modular acetabular component with multiple screw holes. J Biomech. 1999;32(9):967–76.
Mantell SC, et al. A parametric study of acetabular cup design variables using finite element analysis and statistical design of experiments. J Biomech Eng. 1998;120(5):667–75.
Bartel DL, Bicknell VL, Wright TM. The effect of conformity, thickness, and material on stresses in ultra-high molecular weight components for total joint replacement. J Bone Joint Surg Am. 1986;68A:1041–51.
Kummer FJ, et al. Loading of the acetabulum by polyethylene and all-ceramic inserts in metal-backed acetabular cups. Bull Hosp Jt Dis. 2003;61(3–4):132–4.
Blaha JD. The modular neck: keystone to functional restoration. Orthopedics. 2006;29(9):804–5.
De la Torre BJ, et al. 10 years results of an uncemented metaphyseal fit modular stem in elderly patients. Indian J Orthop. 2011;45(4):351–8.
Goyal N, Hozack WJ. Neck-modular femoral stems for total hip arthroplasty. Surg Technol Int. 2010;20:309–13.
Khmelnitskaya E, et al. Optimizing for head height, head offset, and canal fit in a set of uncemented stemmed femoral components. Hip Int. 2008;18(4):286–93.
Buttaro M, Comba F, Piccaluga F. Modular femoral head dissociation after dislocation and entrapment in reconstruction ring: a case report. Hip Int. 2007;17(1):49–51.
Chu CM, Wang SJ, Lin LC. Dissociation of modular total hip arthroplasty at the femoral head-neck interface after loosening of the acetabular shell following hip dislocation. J Arthroplasty. 2001;16(6):806–9.
Skendzel JG, Blaha JD, Urquhart AG. Total hip arthroplasty modular neck failure. J Arthroplasty. 2011;26(2):338.e1–4.
Grupp TM, et al. Modular titanium alloy neck adapter failures in hip replacement – failure mode analysis and influence of implant material. BMC Musculoskelet Disord. 2010;11:3.
Dangles CJ, Altstetter CJ. Failure of the modular neck in a total hip arthroplasty. J Arthroplasty. 2010;25(7):1169.e5–7.
Shiga T, et al. Disassembly of a modular femoral component after femoral head prosthetic replacement. J Arthroplasty. 2010;25(4):659.e17–19.
Malik A, Maheshwari A, Dorr LD. Impingement with total hip replacement. J Bone Joint Surg Am. 2007;89A:1832–42.
Chandler DR, et al. Prosthetic hip range of motion and impingement. The effects of head and neck geometry. Clin Orthop. 1982;166:284–91.
Shareef N, Levine D. Effect of manufacturing tolerances on the micromotion at the Morse taper interface in modular hip implants using the finite element technique. Biomaterials. 1996;17(6):623–30.
Lieberman JR, et al. An analysis of the head-neck taper interface in retrieved hip prostheses. Clin Orthop Relat Res. 1994;(300):162–7.
Huot Carlson JC, et al. Femoral stem fracture and in vivo corrosion of retrieved modular femoral hips. J Arthroplasty. 2012;27(7):1389–96.e1.
Gilbert JL, et al. In vivo oxide-induced stress corrosion cracking of Ti-6Al-4V in a neck-stem modular taper: emergent behavior in a new mechanism of in vivo corrosion. J Biomed Mater Res B Appl Biomater. 2011 Nov 24. doi:10.1002/jbm.b.31943. [Epub ahead of print].
Kop AM, Keogh C, Swarts E. Proximal component modularity in THA-At what cost? An implant retrieval study. Clin Orthop Relat Res. 2012;470(7):1885–94.
Kop AM, Swarts E. Corrosion of a hip stem with a modular neck taper junction: a retrieval study of 16 cases. J Arthroplasty. 2009;24(7):1019–23.
Rodriguez D, et al. Low cycle fatigue behavior of Ti6Al4V thermochemically nitrided for its use in hip prostheses. J Mater Sci. 2001;12(10–12):935–7.
Gilbert JL, Mehta M, Pinder B. Fretting crevice corrosion of stainless steel stem-CoCr femoral head connections: comparisons of materials, initial moisture, and offset length. J Biomed Mater Res B. 2009;88(1):162–73.
Chandra A, et al. Life expectancy of modular Ti6Al4V hip implants: influence of stress and environment. J Mech Behav Biomed Mater. 2011;4(8):1990–2001.
Dalmigli M, et al. The effect of surface treatments on the fretting behavior of Ti-6Al-4V alloy. J Biomed Mater Res B. 2008;86(2):407–16.
Jauch SY, et al. Influence of material coupling and assembly condition on the magnitude of micromotion at the stem-neck interface of a modular hip endoprosthesis. J Biomech. 2011;44(9):1747–51.
MacQuarrie RA, et al. Wear-particle-induced osteoclast osteolysis: the role of particulates and mechanical strain. J Biomed Mater Res B. 2004;69(1):104–12.
Chu Y, et al. Stress and micromotion in the taper lock joint of a modular segmental bone replacement prosthesis. J Biomech. 2000;33(9):1175–9.
Urban RM, et al. Accumulation in liver and spleen of metal particles generated at nonbearing surfaces in hip arthroplasty. J Arthroplasty. 2004;19(8 Suppl 3):94–101.
Urban RM, et al. Dissemination of wear particles to the liver, spleen, and abdominal lymph nodes of patients with hip or knee replacement. J Bone Joint Surg Am. 2000;82A:457–76.
Rehmer A, Bishop NE, Morlock MM. Influence of assembly procedure and material combination on the strength of the taper connection at the head-neck junction of modular hip endoprostheses. Clin Biomech. 2012;27(1):77–83.
Nganbe M, et al. Retrieval analysis and in vitro assessment of strength, durability, and distraction of a modular total hip replacement. J Biomed Mater Res A. 2010;95(3):819–27.
Pallini F, et al. Modular hip stems: determination of disassembly force of a neck-stem coupling. Artif Organs. 2007;31(2):166–70.
Conditt MA, et al. Backside wear of polyethylene tibial inserts: mechanism and magnitude of material loss. J Bone Joint Surg Am. 2005;87(2):326–31.
Engh GA, Ammeen DJ. Epidemiology of osteolysis: backside implant wear. Instr Course Lect. 2004;53:243–9.
Conditt MA, Stein JA, Noble PC. Factors affecting the severity of backside wear of modular tibial inserts. J Bone Joint Surg Am. 2004;86A:305–11.
Parks NL, et al. The Coventry Award. Modular tibial insert micromotion. A concern with contemporary knee implants. Clin Orthop. 1998;356:10–5.
Engh GA, et al. In vivo deterioration of tibial baseplate locking mechanisms in contemporary modular total knee components. J Bone Joint Surg Am. 2001;83A:1660–5.
Wasielewski RC, et al. Tibial insert undersurface as a contributing source of polyethylene wear debris. Clin Orthop. 1997;345:53–9.
Conditt MA, et al. Backside wear of modular ultra-high molecular weight polyethylene tibial inserts. J Bone Joint Surg Am. 2004;86A:1031–7.
Li S, et al. Assessment of backside wear from the analysis of 55 retrieved tibial inserts. Clin Orthop. 2002;404:75–82.
Peters Jr PC, et al. Osteolysis after total knee arthroplasty without cement. J Bone Joint Surg Am. 1992;74A:864–76.
Surace MF, et al. Coventry Award paper. Backsurface wear and deformation in polyethylene tibial inserts retrieved postmortem. Clin Orthop. 2002;404:14–23.
Akisue T, et al. “Backside” polyethylene deformation in total knee arthroplasty. J Arthroplasty. 2003;18(6):784–91.
Qiu YY, et al. Review article: treatments for bone loss in revision total knee arthroplasty. J Orthop Surg. 2012;20(1):78–86.
Engh GA, Ammeen DJ. Bone loss with revision total knee arthroplasty: defect classification and alternatives for reconstruction. Instr Course Lectures. 1999;48:167–75.
Haas SB, et al. Revision total knee arthroplasty with use of modular components with stems inserted without cement. J Bone Joint Surg Am. 1995;77A:1700–7.
Kuchinad RA, et al. The use of structural allograft in primary and revision knee arthroplasty with bone loss. Adv Orthop. 2011;2011:578952.
Frehill B, et al. Initial stability of type-2 tibial defect treatments. Proc Inst Mech Eng H. 2010;224(1):77–85.
Haidukewych GJ, Hanssen A, Jones RD. Metaphyseal fixation in revision total knee arthroplasty: indications and techniques. JAAOS. 2011;19(6):311–8.
Wilson DA, et al. Continued stabilization of trabecular metal tibial monoblock total knee arthroplasty components at 5 years-measured with radiostereometric analysis. Acta Orthop Scand. 2012;83(1):36–40.
Pandit H, et al. Total knee arthroplasty: the future. J Surg Orthop Adv. 2006;15(2):79–85.
Marx R, et al. Surface pretreatment for prolonged survival of cemented tibial prosthesis components: full- vs. surface-cementation technique. Biom Eng Online. 2005;4:61.
Peters CL, et al. Tibial component fixation with cement: full- versus surface-cementation techniques. Clin Orthop. 2003;409:158–68.
Completo A, Fonseca F, Simoes JA. Strain shielding in proximal tibia of stemmed knee prosthesis: experimental study. J Biomech. 2008;41(3):560–6.
Lonner JH, et al. Changes in bone density after cemented total knee arthroplasty: influence of stem design. J Arthroplasty. 2001;16(1):107–11.
Mileti J, et al. Monoblock and modular total shoulder arthroplasty for osteoarthritis. J Bone Joint Surg Br. 2005;87(4):496–500.
van de Sande MA, Rozing PM. Modular total shoulder system with short stem. A prospective clinical and radiological analysis. Int Orthop. 2004;28(2):115–8.
Groh GI, Wirth MA. Results of revision from hemiarthroplasty to total shoulder arthroplasty utilizing modular component systems. J Shoulder Elbow Surg. 2011;20(5):778–82.
Cheung EV, Sperling JW, Cofield RH. Polyethylene insert exchange for wear after total shoulder arthroplasty. J Shoulder Elbow Surg. 2007;16(5):574–8.
Churchill RS, et al. Humeral component modularity may not be an important factor in the outcome of shoulder arthroplasty for glenohumeral osteoarthritis. Am J Orthop. 2005;34(4):173–6.
Skirving AP. Total shoulder arthroplasty – current problems and possible solutions. J Orthop Sci. 1999;4(1):42–53.
Blevins FT, et al. Dissociation of modular humeral head components: a biomechanical and implant retrieval study. J Shoulder Elbow Surg. 1997;6(2):113–24.
Chao EY, Kasman R. Conical press-fit in tumor prosthesis design. Trans Orthop Res Soc. 1983;8:107.
Cooper RA, Brems JJ. Recurrent disassembly of a modular humeral prosthesis. A case report. J Arthroplasty. 1991;6(4):375–7.
Asglan CGI, Hori R. Fatigue of tapered joints. In: Transactions of the second world congress on biomaterials tenth annual meeting of the society of biomaterials, Washington, DC, 1984.
Gupta S, van der Helm FC, van Keulen F. Stress analysis of cemented glenoid prostheses in total shoulder arthroplasty. J Biomech. 2004;37(11):1777–86.
Gupta S, van der Helm FC, van Keulen F. The possibilities of uncemented glenoid component – a finite element study. Clin Biomech. 2004;19(3):292–302.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer-Verlag London
About this chapter
Cite this chapter
Babis, G.C., Sakellariou, V.I. (2014). Modular Interfaces. In: Karachalios, T. (eds) Bone-Implant Interface in Orthopedic Surgery. Springer, London. https://doi.org/10.1007/978-1-4471-5409-9_18
Download citation
DOI: https://doi.org/10.1007/978-1-4471-5409-9_18
Published:
Publisher Name: Springer, London
Print ISBN: 978-1-4471-5408-2
Online ISBN: 978-1-4471-5409-9
eBook Packages: MedicineMedicine (R0)