Abstract
Pure tin is currently the most widely employed lead-free finish for plating of component terminals despite its propensity to spontaneous whisker formation. Whiskers are filamentary crystals, conductive and mechanically strong, measuring up to a few millimetres, though the common variety observed on matt tin finish on copper substrate was hardly ever reported to exceed 0.5 mm. A positive stress gradient within the Sn layer, that is either a lowering compressive or an increasing tensile stress towards the root of a whisker, is reputed as the driving force for whisker formation. The formation of whisker is a major reliability concern for the electronic industry. Whisker-related failures in electric and electronic hardware have been reported since the 1940, and the failure risk cannot be overlooked especially in modern electronic systems. Understanding the tin whisker phenomenon and further developing mitigation strategies and test methods for evaluating whisker performance are all important tasks to be fulfilled in the future.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
6.1 Introduction
Tin-based finishes are the dominant solution for replacing tin–lead alloys used in lead-frame plating due to economic and manufacturing reasons. Pure tin layers are also extensively used as contact surface and protective coatings for connectors, relays and other electronic parts, as well as solderable finish on printed circuit boards.
Outstanding characteristics of corrosion resistance, low melting point, high electrical conductivity, solderability, non-toxicity and large availability have motivated this choice.
However, tin-rich finishes pose a reliability risk due to potential growth of whiskers, i.e. filamentary crystals which are conductive and mechanically strong. In fact, pure tin and tin-rich finishes are all, in different degree, prone to whiskering.
The tin whisker is an old problem for the electronic industry and an enduring challenge for physical metallurgists. Following the approval of the RoHS directive by the European Parliament, the whisker problem was unexpectedly revived and continues to stand up as a major technical issue raised by the lead-free legislation.
It is about 60 years since the first time whiskers were recognized as a potential threat to electric and electronic equipment. During this relatively long lapse of time, indeed a long time from the perspective of technology development, innumerable experimental investigations and a few theoretical studies were conducted, thanks to which a significant progress in understanding was achieved. In more recent years, whisker studies have been strongly intensified because of the urgent need of mastering this insidious threat for the sake of industrial implementation. A review compiled by Galyon [1] is a detailed account of the history of this troublesome and fascinating phenomenon up to 2004.
The prevailing attitude towards the whisker issue is that the current state of knowledge is inadequate to provide a comprehensive picture of the phenomenon. In particular, the mechanistic details so far uncovered and the physical models developed are still unable to provide a solid base for the assessment of the kinetics of whisker growth. Moreover, the technique of mitigating whisker growth is today not much ahead of the level of efficacy and technological confidence of some decades ago. Nevertheless, tin whisker mitigation practices have seen some refinement and consolidation over the last 10 years, and, most importantly, new plating processes have been developed ensuring lower propensity to whiskering.
Therefore, despite a persistent uncertainty, the present level of understanding, the development of mitigation practices and, finally, the advances in plating processes, all these elements have contributed to give confidence to users in adopting tin-based finishes for electronic products with short service life and low-reliability requirements. Still, given the present state of knowledge, the use of tin finish in mission critical applications is unanimously considered as a high-risk scenario. However, both management and market factors may leave no choice but using tin base finish also in high-reliability segments of the microelectronic industry. That is the simple reason why continued research on tin whisker and mitigation is a must.
6.2 Failure Mechanisms
Tin whiskers are reputed responsible of failure events by a number of different mechanisms. However, it should be stressed that not all of these mechanisms are either documented or undoubtedly ascertained.
-
Electrical shorts at relays and connectors. This is the best documented failure occurrence caused by whisker formation; a number of reported cases are included in the list of whisker failures compiled by Leidecker and Brusse [2].
-
Electrical shorting between neighbouring terminations of lead-frame. In low-voltage, high-impedance circuits, the current may be insufficient to fuse a whisker open. According to Arnold, the fusing current for a 2 μm diameter, 2-mm-long whisker is in excess of 10 mA [3]. The fusing current of a whisker depends on its diameter and its structure, if hollow. According to Dunn [4], the short-circuit current ranges from 10 to 30 mA as the diameter increases from 1 to 3 μm. A current range of 7–15 mA to fuse open a tin whisker depending on its geometry was reported in a recent study [5]. A burnout maximum current of 75 mA was reported by Hada et al. [6]. An empirical model for quantifying the probability of failure by short circuit caused by tin whiskers as a function of voltage was developed by Courey et al. [7, 8].
-
Shorts caused by metal debris, bridging the gap between conductors remote from the whisker origin. Such an event of failure requires a whisker to break loose from the surface because of mechanical shock or vibrations. This failure risk should be weighted in the light of the available information about whisker mechanical strength. Dunn performed mechanical shock and vibration testing on tin whiskers [9], concluding that, in his experimental conditions (vibration frequency in the range 10–2,000 Hz, shock loading in the range from 20 to 2,000×g), neither vibration nor mechanical shock caused any visible damage to whiskers. The same conclusion emerged from a study where similar conditions of vibration test were used [10]. In the later study, mechanical shock tests were also performed according to the drop impact test method for mobile phone giving as well a negative response.
-
Loose whiskers may interfere with sensitive optics functioning and actuators operation. See comments above.
-
In the presence of high field, the risk exists of spark formation at the tip, possibly initiating metal vapour arching in vacuum and combustion.
-
Interference in high-frequency devices. According to a recent publication [11], the effect of whiskers on high-frequency circuits performance, in the range 1–20 GHz, and its interference with high-speed signals is deemed negligible. This conclusion is based on a modelling work. On the other hand, Galyon and Gedney [12] state that whiskers become an issue above 6 GHz and that whisker length needs to be less than 75 μm in order to avoid affecting high-speed circuits.
In summary, electrical shorting caused by whiskers bridging conductors could represent the highest risk event. This is a concern particularly for fine pitch components and for applications in static environments. In a dynamic mechanical environment, whiskers may be expected to break off if long enough, in which case the whisker risk would be that of a shorting event caused by loose filaments. However, this failure risk needs to be further assessed. Not only whiskers seem to have quite a strong endurance to vibrations and mechanical shocks but, in case of breaking, the risk of shorting may be significantly reduced if not nullified, because of the high contact resistance between the mating surfaces [13]. In fact, short circuits caused by whiskers bridging closely spaced circuit elements require a minimum relatively high contact pressure or, alternatively, local breaking of the surface oxide. According to Hilty and Corman [5], contact pressure in the range of 10−3–10−2 N was necessary in order to overcome the contact resistance. In the same study, voltage breakdown was observed on applying voltage higher than 3 V to the contact interface.
6.3 Whisker Morphology
There are two main morphological types of spontaneous crystal growth, which are usually observed on tin-based finish and which can be classified as whiskers.
The first type is the hair-like or filamentary crystal, which is the archetype and the icon of the whisker growth phenomenon (see Figs. 6.1, 6.2). Filamentary crystals are characterized by a small diameter, in the range of 1–3 μm, length from a few tenths to several hundreds of micrometres and either a smooth or tapered lateral surface, e.g. whiskers on bright pure Sn and Sn(Cu) layers [14]. The second type is the columnar crystal, which is taking the place as the more familiar look of whiskers, being the most common whisker crystal shape observed on matt tin finishes (Fig. 6.3). Columnar whisker crystals, with diameter usually in the range of 2–6 μm, depending on the grain size of the tin layer, have length rarely exceeding 0.2 mm and a streaked surface. There are however reports of whisker growth on matt tin surfaces having length up to 0.45 mm under high temperature and humidity conditions, that is 60°C and 93% RH [15], or with a thin tin layer, namely 2.5 μm, on copper lead-frame under ambient storage conditions [16].
Whiskers on bright Sn/Ni/CuFeP (Ni barrier nominal thickness: 2 μm). Ageing: 55°C/uncontrolled humidity storage/3,400 h after conditioning [17]
A common and characteristic morphological feature of both filamentary and columnar whisker crystals is a constant cross section from the base to the tip.
A different class of morphological features, still referable to spontaneous crystal growth or recrystallization growth behaviour of a tin surface, engenders a number of different growth features, which are named by different locutions, such as nodule or bud and flower or simply eruption (see Fig. 6.4).
On the left, a linear array of nodules along a surface scratch on a 5-μm bright tin layer on brass substrate, after 1,000-h ageing at room temperature and uncontrolled humidity. On the right, a tin nodule or flower from which a whisker is sprouting (10-μm-thick bright tin coating on CuFeP substrate)
Nodules and, more generally, eruptions may be whisker growth warning signs. All these features may be seen on either matt or bright tin coatings, though odd-shape eruptions are more common on bright tin surfaces.Footnote 1
Whiskers should be straightforwardly identified by their peculiar morphology, i.e. the hair-like or columnar shape of growth. However, from time to time, there are reports of abnormal crystal growth phenomena observed on tin layers as well as other metals, which are hastily classified as whisker. Unusual morphology includes conical, pyramidal whiskers and the like. A recent example of such odd growth morphology is the report of ribbon-like whiskers observed on matt tin layer on Cu–5%Sn–0.35%P substrate after exposure to 55°C/100% RH [18]. Unusual, rarely observed, growth morphologies are possibly relevant to mechanism, but cannot be deemed relevant to most in-use conditions.
The user should be aware that the plating process itself can produce a rather impressive gallery of peculiar growth features, including dendrites (i.e. branched crystals), needle-like crystals usually in thick bundles, conical or acicular protrusions and even ribbon-like crystals. These abnormal growth features can be produced during the plating process and are associated with conditions of either mass transport polarization effects or out of control plating bath chemistry (a library of such abnormal morphological features was produced by dosing a tin electrolyte with a wetting agent in conjunction with lead impurity [19]).
Besides the consequences of process control deficiency, it should also be mentioned that corrosive environment may induce the growth of seemingly whisker crystals, likewise the special case of whisker growth on silver surface exposed to sulphide-containing environment.Footnote 2 Caution should be used in drawing conclusions from corrosion-induced tin whisker growth, meaning by this expression the formation of whisker crystals in the presence of a specific chemical participating in their formation, since field conditions are, for most applications, far less aggressive than those required for such an occurrence on tin finish.
6.4 Mechanisms
The detailed mechanism of whisker growth is still controversial and, as a result, the ultimate theory of whisker formation has yet to be written. Despite this state of affairs, there is a wide consensus that whisker formation is a stress relief phenomenon—a discovery now more than 50 years old [21, 22]. Namely, it is now generally recognized that this peculiar crystal growth phenomenon is driven by compressive mechanical stress in the tin deposit on which the whisker crystals grow. A well-informed account and an historical perspective of the tin whisker theory development are given in reference [1].
One of the arguments that is frequently put forward to justify the impossibility to define a solid theoretical framework and find a general agreement on the underlying mechanism of whisker formation is the inconsistency of the results reported in the plethora of whisker studies available, conducted during more than half a century. Indeed, it is common experience of those who have carried out whisker testing that the consistency of the results is by itself an achievement. Indeed, there are many different factors from which whisker growth may be influenced. Among these, the plating process itself is a critical and hard to control variable.
Thanks to the recent development of test methods, notwithstanding the fact that they are still under scrutiny, this seemingly inherent and frustrating characteristic of the whisker growth phenomenon seems to have been alleviated if not set aside.
However, the key reason why whisker formation is still not completely understood is its challenging inherent complexity. As any other crystal growth phenomenon, whisker formation is a two-stage process, involving nucleation and growth. In addition, whiskers usually grow only after a dormancy period, which can range from a few weeks to years.
Recently, there has been a strong resurgence of interest in the fundamental mechanism of whisker growth. The outcome of this intense activity has been twofold. On one hand, the concept that whisker growth may be associated with a multiplicity of mechanisms is gaining ground. On the other hand, the key mechanistic aspects have been identified and nowadays are also largely agreed on.
6.4.1 Stress Sources
From an industrial perspective, the most important aspect in whisker studies is the suppression or mitigation of whisker formation and growth. In this respect, as well as for the purpose of product qualification, the development of acceleration methods is an essential requisite. Obviously, a detailed knowledge of the parameters affecting whisker growth is key to advances in acceleration and mitigation.
It has been speculated that the driving force for whisker growth is a state of compressive strain within the Sn layer or a positive strain gradient in the direction from the base to the surface [23] and/or in the direction from the surroundings to the whisker root parallel to the surface of the layer [24]. Synchrotron radiation micro-diffraction analysis of the stress state in the region surrounding a whisker provided some piece of evidence to this principle (on Sn(Cu) deposit) [25]. The details of this mechanism are being developed so that it is still not possible to formulate a quantitative growth model effectively relating the growth kinetics to relevant physical and experimental factors, such as material properties, process parameters and part design.
The nature and origin of this strain should first be defined. Basically, the factors unanimously recognized as the most common determining conditions for whisker formation and growth are the following:
-
1.
Formation of an intermetallic compound between the Sn-based finish and the substrate material, inducing compressive strain into the Sn or Sn alloy layer.
-
2.
Temperature cycling generating stresses due to thermal expansion mismatch between coating and substrate materials.
Other sources of stress possibly having an impact on whisker growth, either as determining driving force or as an accelerating factor, were identified over the years, including the following:
-
Mechanical stress and surface damage, an issue particularly for connectors [26].
-
Surface chemical modification, primarily oxidation but also corrosion.
-
Electric bias.
Some of these are discussed in more details in the following.
A mechanism of stress generation is a necessary condition for whisker growth but it is not sufficient to explain the formation of whisker. A further condition is the inhibition of a uniform strain relaxation in the tin layer or, analogously, the local relaxation of the strain resulting in the formation of a whisker.
6.4.1.1 Diffusion in the Copper–Tin System
The reference metallurgical system for the whisker issue is the metal couple Sn/Cu, the tin layer being a matt tin finish. The most widely accepted view is that chemical affinity between members of the couple triggers the room temperature inter-diffusion of the two metals, resulting in the formation of an irregular intermetallic compound layer and in the following build-up of compressive stress into the tin layer. The basic model of internal stress generation by a chemical reaction in the Sn/Cu couple is schematically illustrated in Fig. 6.5.
What is particularly relevant to the whisker problem is the identification of the dominant diffusing species at low temperature, the microstructural evolution of the interface interphases and the related stress evolution resulting from inter-diffusion and phase transformation.
A complete and unambiguous description of the behaviour of the Cu/Sn diffusion couple is still lacking. In particular, there are conflicting views on the kinetic law governing the growth of the intermetallic layer and confusing results concerning diffusion parameters. For a critical overview of the investigations into the Cu/Sn diffusion couple carried out during the last decades, the reader is referred to a paper of Laurila et al. [27].
The available experimental evidence indicates that copper is the fast diffusant of the couple at room temperature [27], though a conclusive marker study of the room temperature inter-diffusion in the Cu–Sn system, directly relevant to the whisker case study, has yet to be performed. A room temperature marker study of a thin-film Cu/Sn diffusion couple was conducted by Tu and Thompson [28]. The Cu/Sn couple was prepared by consecutive deposition of the metals by electron beam evaporation, using a discontinuous, island-like film of W as diffusion marker and measuring its depth position by Rutherford backscattering spectroscopy. According to this study, Cu atoms were the main diffusing species at room temperature. The other important finding of this work was that by isothermal annealing at T lower than 60°C only Cu6Sn5 formed and, since Cu was identified as the fast diffusant, the interface reaction was the Cu6Sn5/Sn. The formation of the intermetallic Cu3Sn was observed together with the Cu6Sn5 phase following isothermal annealing at temperature in excess of 60°C.
According to the analysis of Laurila et al. [27], the low temperature behaviour of the Cu/Sn diffusion couple (T < 60°C) can be rationalized assuming that it is determined by the operation of short-circuit diffusion paths, namely interstitial diffusion of Cu atoms in the Sn and grain boundary diffusion of Cu atoms through the Cu6Sn5 reaction layer. This behaviour changes progressively with the increase in temperature above about 60°C, as a result of volume diffusion becoming predominant. In fact, different studies agree on the observation that Sn is the fast diffusant during formation of the Cu6Sn5 phase at high temperature [29–32] and that both Cu and Sn are mobile in Cu3Sn, the fast diffusant being Cu [31, 32]. The Cu flux was found to be as much as three times greater than the Sn flux at 200°C. As pointed out by Ho et al. [33], the latter finding is in agreement with the observation that in the Cu/Sn diffusion couple Kirkendall voiding occurs at the Cu/Cu3Sn interface or within the Cu3Sn layer.
The room temperature diffusion behaviour and the reactivity of the Cu/Sn couple is particularly important to the whisker phenomenon: without the unbalance in the room temperature global diffusivity between Cu and Sn atoms and the simultaneous formation of an intermetallic layer—which takes place within the tin deposit, therefore inducing a compressive strain into the tin grains—a necessary condition for whisker growth would be lacking.
6.4.1.2 Thermal Cycling
Thermal stress arises as a result of CTE mismatch between Sn and substrate material (for stress being compressive on heating: α sub < α Sn—e.g. Alloy 42, ceramic substrate as for multi-chip module ceramic capacitors). This mechanism of stress generation is relevant to temperature cycling testing. In thermal cycling conditions of −55 to 85°C, whisker growth is reported to reach saturation after 1,500–2,000 cycles [34, 35]. The maximum tin whisker length observed on matt tin coatings over copper alloy lead-frame materials is about 50 μm at saturation. Similar observations are reported for test up to 8,000 cycles [36] and 10,000 cycles [37]. On the contrary, the saturation length of whiskers on alloy 42 lead-frame is in excess of 100 μm [38]. Tin whiskers of length exceeding 100 μm were also observed on ceramic chip capacitors after 500 temperature cycles (−40°C/90°C) [39].
6.4.1.3 Oxidation and Corrosion
The recognition of the accelerating influence on whisker growth by atmospheric O2 and water vapour was an early observation of whisker studies [20]; the effect of air exposure on whisker growth on Cd was noted even earlier [40]. Kehrer and Kadereit [41] reported that 30–100 nm thick Sn films deposited on glass in a moist oxygen atmosphere of 10−4 Torr formed many whiskers after annealing at 60°C, while when the Sn films were deposited at 10−6 Torr, no whiskers were observed. Similarly, in ultra-high vacuum, no surface hillocks were found to grow on Al surfaces under compression [42]. Both these observations pointed to a specific mechanism of whiskering driven by oxidation.
The involvement of oxidation in the whisker mechanism of tin coatings on brass was also demonstrated, though in this case, the dominant interaction was that between oxygen and zinc out-diffusing from the base metal at the surface [43]. A similar behaviour, i.e. where a different element appears responsible for increased susceptibility to whisker growth induced or triggered by oxidation, emerges from the results of different studies, including Sn–Mn electrodeposits [44], Sn–3Ag–0.5Cu–1Ce [45] and Sn–Zn solder [46] and bulk Sn–Al alloy [47].
The accelerating effect of humidity on whisker growth was discussed in relation to oxidation/corrosion first by Oberndorff et al. [48, 49] and later by Osenbach et al. [50]. The interpretation emerging from these works was that the influence of oxidation on whisker formation is through the induction of compressive stress due to the volume expansion accompanying the formation of oxidation products.
The issue of corrosion during storage in high temperature and humidity environment was carefully considered and studied in the frame of the activities carried out within the iNEMI Tin Whisker Accelerated Test Project, due to its particular relevance to the development of test methods for evaluating tin whiskers. Corrosion of the tin, possibly enhanced by galvanic contact with the underlying copper [49] or alloy 42 [16] substrate, is now generally acknowledged as a promoting factor for whisker growth, though its relevance to in-use condition is still debated. An example of intense whiskering in a corroded zone on a matt tin layer on Cu lead-frame is displayed in Fig. 6.6.
The relevance of corrosion as a potential synergetic factor in whiskering was raised in the PROTIN project, in the light of the results of whisker growth testing on tin-plated copper lead-frame at 60°C/93% RH storage conditions [51]. This phenomenon was later observed also by Osenbach et al. [52] and described as a “condensation-induced corrosion-assisted whisker growth”. In this study, trim and formed components were found to be particularly vulnerable to corrosion-assisted whisker formation compared to non-trim and formed devices, which may be explained in terms of acceleration due to galvanic coupling between the Sn layer and the Cu substrate, according to previous studies by Oberndorff et al. [48]. In addition, it is worth noting that the morphology of the growth features, called by these authors “flower clusters of Sn whiskers”, was quite different from the conventional tin whisker filamentary or columnar growth and that also tin-based materials usually considered practically immune from whiskering may grow whisker if exposed to corrosive environment or contamination during processing, e.g. by flux residues [53].
The occurrence of corrosion-assisted whisker formation may continue to be a concern for damp-heat testing of component finishes for whisker growth, where the temperature/humidity condition may incidentally get close to the condensation point or as a result of incidental contamination. Control over this phenomenon does not seem to be easily manageable, since it does not depend exclusively on storage conditions but also on surface contamination and other accidental factors. The influence of corrosion is currently explained according to the same interpretation scheme used to rationalize the influence of oxidation [49, 50].
6.4.1.4 Electric Bias
Another possible stressing/accelerating factor is electric bias; its evident connection to in-use conditions is the reason why the Phase 4 evaluation of the iNEMI Tin Whisker Accelerated Test Project was designed to investigate the effects of electrical bias on whisker formation on matte tin-plated components, assembled with both lead-free and tin–lead solder.
Published information is relatively scarce and somewhat confusing, possibly not comparable due to different bias level or stress (current density). Early investigation performed by Arnold seemed to rule out the influence of bias on whisker growth [3]. These findings were confirmed in recent works. According to Osenbach et al., there is no effect of electric bias on the propensity for whisker growth [52]. Similarly, Hilty et al. [54] found no appreciable influence of applied bias on whisker growth. The latter authors followed the test conditions suggested by iNEMI (5 V bias, with variable electric field intensity). Tests performed on samples continuously exposed to an 50°C/50% RH environment showed that whiskers grow both at the anode and cathode end; the whisker density was observed to decrease in the presence of bias, while the length of whisker was observed to increase [55]. According to a report from Texas Instruments (TI) [56], based on results of combined environmental (51°C/85% RH) and bias tests, bias is a relevant additional stress condition, producing more consistent whisker growth results, though no evidence of acceleration was actually reported. The 5 V bias suggested by the iNEMI consortium for testing was later adopted by TI and Alcatel [55].
The electromigration behaviour and its relationship with whisker growth was studied in pure tin using Blech structure by Liu et al. [57]. Tests were performed at current density of 7.5 × 104 and 1.5 × 105 A cm−2, i.e. two to three orders of magnitude higher than in standard electronic applications, at room temperature and 50°C. They found that whiskers grew on the anode, while voids were observed on the cathode, concluding that whisker growth occurred as a result of compressive stress induced by the Sn atoms electromigration flux.
The influence of bias on whisker growth is still to be completely clarified. There seems to be no conclusive evidence that bias or current flow has an influence on whiskering of matt tin; on the other hand, there is some evidence suggesting that whisker formation on bright tin finishes may be enhanced and/or accelerated by the presence of bias [58, 59].
6.5 Theory of Whisker Formation
Early theories of whisker growth were based on dislocation dynamics and reached their final stage of development with the models proposed by Lindborg [60] and Lee [61]. The mechanism of whisker growth based on dislocation theory was first questioned by Ellis [62] and has been the object of much criticism since then. It can be now considered superseded by the recent developments. The experimental evidence disproving dislocation-based theories is reviewed and discussed in references [1, 14, 63].
Briefly speaking, there are two main theoretical approaches to the whisker growth phenomenon, with significant overlapping [14]. The first has its origin in a reference theoretical study by Tu [64] and is generally known as the Cracked Oxide Theory. The second one, largely based on recrystallization concepts, can be traced back to early studies of the whisker phenomenon, stemming from the work of Ellis [62], Glazunova [65] and Furuta [66]. It was then revitalized and developed by the Tin Whisker Modelling Group of the iNEMI consortium and is currently being further refined by group members, e.g. [67, 68].
The key difference between the two approaches lies in the explanation of the initiation of whisker formation, namely while in the former, a key role is attributed to the surface oxide, in the latter, the mechanism relies completely on the recrystallization behaviour of the material, namely in the formation of recrystallized grains. Grains acting as initiation site for whiskers are expected to have peculiar characteristics, such as oblique angle grain boundaries [63] or yield stress lower than that of as-deposited grains [69]. The detailed mechanism of whisker development will change accordingly.
6.5.1 Cracked Oxide Theory
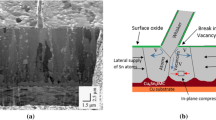
According to the analysis of Tu [64], the driving force for whisker formation is the compressive stress generated by the interface reaction between the tin film and the copper substrate to form the Cu6Sn5 intermetallic, as shown schematically in Fig. 6.7.
A schematic diagram illustrating the Cracked Oxide Theory [64]. Copper migration is driven by chemical potential gradient to form the intermetallic layer Cu6Sn5 in grain boundaries. This induces a compressive stress in the Sn layer. To relieve the stress a flux of tin atoms driven by the stress gradient (creep) diffuses away and supplies Sn atoms to a growing whisker
The mechanism proposed requires three conditions: (1) the room temperature interstitial diffusion of Cu atoms into the Sn lattice and the reaction between the two metals to form Cu6Sn5; (2) the room temperature self-grain boundary diffusion of Sn atoms, that is the diffusional process enabling stress relief; (3) a stable and protective surface oxide, which is the condition required to prevent a uniform relaxation of the compressive stress.
It is important to stress that chemical reactions provide a continuous driving force, as long as reactants are available, contrary to sources of thermal and mechanical stresses, which are not usually constantly active, though there are exceptions (e.g. connectors). The Cu6Sn5 intermetallic compound formed by the reaction between the solder finish and the substrate continues to grow overtime in the Sn layer grain boundaries, generating and maintaining, like a wedge [61], the compressive stress. Stress relieving is made possible by Sn grain boundary diffusion down the stress gradient, feeding the growth of a whisker. In order for this stress relief process to result in whisker formation, a further condition is necessary, the localized fracture of the oxide layer on the tin surface, enabling the extrusion of the needle like crystal. In the absence of a protective surface oxide, whisker growth would be impossible, since stress could be uniformly relieved. Similar to much older concepts theorizing the inhibition of general extrusion for whiskers to grow [21, 70], the cracked oxide theory presumes that free deformation in the direction normal to the film plane is restrained by the tin surface oxide. On the other hand, this surface oxide must not be thick so that it can be broken at certain weak spots on the surface.
This weakness of the oxide layer allowing local breaching is explained as a consequence of the tin lattice elastic anisotropy, resulting in strain in the direction normal to the film plane varying with the orientation of grains. It is therefore assumed that difference in strain along different crystallographic directions will give rise to shear cracking of the surface oxide [61]. A weak spot in the protective layer can be identified with a structural discontinuity, such as the misorientation of a grain to the adjoining grains (e.g. (210) grain in a (321) + (211) matrix) [71].
According to Galyon: « The weak oxide approach remains viable, but no direct proof has yet been published » [72]. In fact, recent results, though based on a small base of data, show that there is no apparent crystallographic relationship between whiskers and adjacent grains [73].
Experimental evidence against the involvement of oxidation in whisker formation was claimed by Moon et al. [74]. Sn(Cu) coatings were cleaned using an Ar+ ion beam and aged in the 2 × 10−9 Pa Auger system chamber. Whiskers and other features present during Ar+ ion cleaning left visible shadows on the surface. During ageing in the ultrahigh vacuum system, new whiskers, identified by the absence of the shadows, nucleated and grew. According to the authors, this was the demonstration that whiskers can be formed in the absence of a surface oxide. However, it is known from an early study that ion beam bombardment of a tin surface may induce whisker growth [75]. Therefore, the observation of Moon and colleagues does not seem to be conclusive in this respect.
On the other hand, there are several observations that do support the relevance of oxidation in whisker formation, as already noted previously. Moreover, if grain boundary diffusion and grain boundary pinning are both taken as necessary conditions for whisker growth, the role of the surface oxide would become decisive, for both the initiation (the latency period) and the nucleation of whiskers [76]. In fact, given that premise, the opening of local discontinuities (breaches) in the oxide layer is needed for allowing a vacancy flow into the material.
An alternative explanation for the so-called weakness of the oxide layer—not relying on either the elastic anisotropy influence [61, 71] nor the oxidation rate difference between adjoining grains with different orientation [50, 52]—was proposed on the basis of the electrochemical behaviour of the Sn metal, that is the fast ion-exchange kinetics and active–passive behaviour of the metal [17]. This concept could help in clarifying the accelerating effect of humidity on whisker growth and also the interplay between corrosion and whisker formation.
6.5.2 Recrystallization Theory
The existence of a latency period preceding the growth suggests that whisker formation may take place only after the achievement of a yet to define critical state, which most likely depends on the microstructure evolution of the material. The view of the dormancy period as an incubation time for a recovery and recrystallization stage [77] is currently an accepted interpretation [78]. As defects and impurities pile up at grain boundaries, the dormancy period goes towards its end, since recrystallization becomes inhibited. The time length of the dormancy period depends therefore on the initial level of the excess energy stored into the material in the as-plated state. As already noted earlier, these principles were resumed by the NEMI Tin Whisker Modelling Group and reawakened in the frame of the current recrystallization theory of whisker growth. A recent paper attempts to gain direct evidence to support this interpretation, presenting interesting results [79].
Within the iNEMI Modelling Group, the basic elements for a recrystallization theory were first discussed by Boguslavsky and Bush [80], in connection with a dislocation mechanism, later completely abandoned. The general framework of the so-called Integrated Theory was outlined in a paper by Galyon [72] and later complemented by a paper by Smetana [63].
Special whisker grains are assumed to form during the initiation stage of whisker growth. According to Smetana [63], whisker grains are recrystallized grains characterized by oblique grain boundaries, contrary to as-grown grains, which have vertical boundaries. This is the key feature of such whisker grains, since, while the stress state in columnar grains is biaxial—i.e. there is no stress component normal to the layer plane—on the contrary, the stress state in a grain with oblique boundaries is triaxial at the root—there is a stress component along the oblique boundary—and biaxial at the free surface. This shear stress at oblique grain boundaries is thought to be essential to whisker development. Based on a simple geometric model, the oblique grain boundary is described as a lower stress interface compared to vertical grain boundaries, thus accounting for a positive stress gradient towards the whisker root. The theory further assumes that Sn atoms diffuse towards this lower stress zone activating grain boundary sliding, the final stage leading to whisker growth.
Probably, the most appreciated result of the Integrated Theory has been the attempt to provide a simple and effective description of the metal couples of interest for lead-frame components (namely, the Sn/Cu, its variants Sn/Ni/Cu and the couple Sn/Fe–Ni42), which notoriously show different propensity to develop whisker as well as different sensitivity to stress factors in terms of the stress state induced into their layered structure.
The Sn/Cu couple is described by the zone model reproduced in Fig. 6.8. According to Gaylon’s analysis [72], grain boundary diffusion of Cu atoms into the Sn creates a vacancy-rich zone in the Cu substrate, which may eventually cause the formation of microvoids in the reaction layer. Because of the excess volume made available, a tensile stress develops in the Cu-depleted zone; on the other hand, the formation of Cu6Sn5 at grain boundaries results in compressive stress within the tin layer. This is the driving force responsible for Sn diffusion towards the surface and, given the correct conditions, for whisker growth.
Schematic diagram of the 4-zone structure of a Sn/Cu couple after intermetallic formation as depicted by Gaylon [72]
Similar zone models were also proposed for the other systems, providing a persuasive tool to explain the build-up of internal stress into the different systems, their sign—compressive/tensile—and their influence on the whisker formation [81]. In support to his thesis, Galyon published a series of SEM micrographs showing the presence of microvoids at the Cu6Sn5/Cu interface [72]. As to the stress state developing in the metal couple after formation of the Cu6Sn5 layer by room temperature annealing, Tu [82] reported that the strain in the remaining Cu and Sn layer was tensile and compressive, respectively, in agreement with the zonal concept proposed by Galyon.
Nevertheless, this pictorial view of the dynamics of whisker growth, though persuasive, is not actually based on strong experimental evidence. The zone structure of the Sn coating/Cu substrate has yet to be firmly supported by convincing evidence. According to the classical theory of diffusion in the solid state [83], vacancy concentration may not reach equilibrium in a reactive diffusion couple with a strong unbalance of the members flux, possibly resulting in the formation of voids either into the reaction layer or at the interface between the product phase and the fast diffusant member. In principle, the Sn/Cu couple could show a similar behaviour on ageing, in conditions leading to the formation of only the Cu6Sn5 phase, provided that Cu is the fast diffusant in these conditions. However, this room temperature morphological evolution of the Sn/Cu interface has not been demonstrated. Actually, Kirkendall void formation in the Sn/Cu diffusion couple was reported to occur only when the product phase was Cu3Sn not with Cu6Sn5 [27, 33]. The kinetics of formation of the Cu3Sn phase is diffusion controlled, and the Sn atoms are a much slower diffusant with respect to the Cu atoms in the Cu3Sn compound. This behaviour may account for the absence of local equilibrium of point defects, the consequent rising of a supersaturation in vacancy concentration, and the actual observation of voiding.
Finally, the very possibility of Kirkendall void formation in the Sn/Cu couple, even for the case of Cu3Sn formation, was also questioned based on the observation that the presence of impurities—such as into an electroplated Cu pad—seems to be a condition for its occurrence [27].
6.6 Mitigation
Mitigation strategies are processes performed during manufacturing to reduce the risk of whisker growth by minimizing compressive stress induced into tin-based surface finishes.
There is no mitigation practice that will guarantee a zero whisker risk; that is, there is no proof that any of the mitigation techniques so far developed can provide exemption from whiskers for tin-based finishes.
Mitigation strategies currently used in the industry are largely based on previous experimental and theoretical achievements, though they have been thoroughly re-examined and proven effective on a much larger and more reliable base of data only in recent years.
Mitigation practices are addressed in the JEDEC/IPC joint publication JP-002, “Current Tin Whisker Theory and Mitigation Practices Guideline” [78].
The final scope of a mitigation technique should be the suppression of the driving force for whisker growth. In the real world, the achievement of this objective is not straightforward. In fact, the whisker growth theory itself is not mature enough to provide the basic elements necessary for the confident design of effective measures of mitigation. According to another view, a further obstacle against the safe development of mitigation techniques is that there is not a single whisker mechanism. In other words, if the assumption is made that compressive internal stress or stress gradient is the root cause of whisker formation and that there are different stress-generating mechanisms, then mitigation techniques may be effective only with respect to one of these different mechanisms. That is the reason why mitigation techniques should not be regarded as prevention techniques, rather as whisker risk reduction methods.
There are basically two different strategies of mitigation, having the largest diffusion in the industry and the highest acceptance among users:
-
Recourse to an underlayer as diffusion barrier, usually a nickel layer (silver was also shown to be an effective barrier [51, 84]).
-
Annealing shortly after plating (so-called post-bake treatment).
Both these mitigation techniques address but one stress source, i.e. that associated with the otherwise uncontrolled interface reaction between the plated Sn and the substrate material. Indeed, as noted by Tu et al. [85], the use of a barrier layer to slow down or prevent the chemical reaction at the interface is inherently inadequate to completely suppress whisker growth, since it has no effect on the self-grain boundary diffusion of tin, which is the transport process responsible for feeding atoms to a growing whisker. The relevance of this remark is not diminished by recent reports questioning the key role of intermetallic formation at the deposit/substrate interface as the major stress source for whisker growth, based on the observation of the effects of co-deposited impurities [86]; neither could it be disputed were the interface reactivity disproved as a necessary condition for a tin layer/substrate system to initiate whisker growth, as recently reported [87].
Eventually, from the point of view of industrial applications, intermetallic formation in the Sn/Cu couple remains the most important stress-generating factor, and then the first to be targeted by any mitigation technique; and the more so, since it operates continuously over a long time span contrary to other possible stress sources.
6.6.1 Nickel Barrier
The use of nickel plate as a barrier layer has an established industrial tradition for both matt and bright Sn electrodeposits. With regard to the whisker risk, there are about 15 years of experience in surface mount ceramic capacitor. Interestingly, this mitigation practice was proven ineffective against thermal cycling in laboratory testing on ceramic chip capacitors [39]. This suggests that the results of whiskers acceleration tests should be carefully scrutinized since they may not be straightforwardly related to in-use conditions.
Mitigation is primarily achieved by eliminating compressive stress build-up due to Cu diffusion and the concurrent formation of the Cu6Sn5 intermetallic at the Sn/Cu interface. A further benefit is claimed in the frame of the integrated theory of Gaylon [72, 81], based on a four-zone model of the Sn/Ni/Cu system, where diffusion of Sn into Ni is thought to cause tensile stress build-up in the Sn at the interface with the Ni underlayer, counteracting the compressive growth stress in the Sn layer. Internal stress in Sn deposits over a Ni barrier on copper alloy substrate was actually reported to be tensile for thickness of the tin layer in the range up to 2 μm and after 40 days room temperature ageing [88]. Accordingly, it was also shown that a Sn/Ni finish on copper may also perform effectively against whisker growth in thermal cycling conditions, provided the thickness of the Sn layer is suitably tailored in order to maintain an average tensile stress in the Sn also during the heating cycle.
A few warning remarks should be noted in connection with the use of the nickel barrier: first, barrier films may fail if porous or thin, may be damaged or cracked; during reflow, solder may react with nickel and form Ni3Sn4 within the Sn, causing compressive stress. A minimum thickness of 1.27 μm is usually specified for the nickel barrier, following iNEMI recommendation.
6.6.2 Annealing or Post-Plate Bake
Tin whisker mitigation by annealing is a strategy that has a quite good reputation and has been in use as long as the nickel barrier solution. The post-plate treatment of matt tin-plated lead-frame (1 h at 150°C within 24 h of plating) has been adopted as mitigation technique by the major electronic components producers. This mitigation technique must be used in combination with a minimum tin layer thickness of 7 μm or 10 μm nominal [78].
Some 35 years ago, it was already established that a post-plate annealing is effective in increasing the incubation time and decreasing the maximum whisker length [89]. Recent results, see e.g. [52], corroborate this conclusion.
According to Oberndorff et al. [84, 90], the post-plating annealing (1 h at 150°C, directly after plating) promotes the formation of a more uniform and even intermetallic layer (see Fig. 6.9), consisting of Cu3Sn and Cu6Sn5, inhibiting if not preventing the formation of the compound at grain boundaries. Such an intermetallic layer is believed to be a durable and effective diffusion barrier against grain boundary diffusion at ambient conditions, slowing down the otherwise continuing growth of a discontinuous layer of the Cu6Sn5 intermetallic. The improved planarity of the layer, possibly in connection with microstructural modifications of the tin coating, is believed to reduce compressive stresses and more particularly localized stresses in the interface region.Footnote 3 These effects of the post-bake treatment on the structure and morphology of the intermetallic layer and on the internal stress state of the tin deposit were fully confirmed in a recent work by Sobiech et al. [92].
A schematic diagram illustrating the structure of a post-plate annealed Sn/Cu couple (below) compared to the structure formed on ageing at room temperature (above). By annealing an uniform and even intermetallic layer can be formed, acting as an effective diffusion barrier. This reactive barrier has a two-layer structure, due to the high-temperature reaction of Cu and Sn resulting in the formation of both Cu3Sn and Cu6Sn5
The annealing treatment needs to be applied shortly after plating, because intermetallic particles may form under ambient conditions and penetrate deep into grain boundary, growing to the height of about 1 μm within a few weeks. The thickness of the intermetallic layer that is formed by the post-bake procedure is about 1 μm thick [78].
While the key feature of the post-plating bake treatment is believed to be the formation of an intermetallic two-layer Cu6Sn5/Cu3Sn barrier at the Sn/Cu interface, further beneficial effects of an annealing treatment may be residual stress relief, grain growth and recrystallization [51, 61, 76, 93].
The modification of the stress state of the tin coating due to post-plating annealing was purposely investigated by Sobiech et al. [92] using a methodology to perform X-ray stress measurements with controlled depth of penetration. According to the results of this study, a residual tensile stress persists in the surface region of tin coatings subjected to post-plate annealing even after prolonged ageing, while compressive stresses appear concentrated in the interface region. This depth-dependent stress distribution is believed to result in the absence of a significant stress gradient in the near surface region of the tin deposit, which in turn is claimed as the reason for whisker suppression [92]. In this respect, it should be noted that, according to the analysis of Osenbach et al. [76], the improvement is one of degree not of kind that is the increasing of the incubation time not the elimination of the potential for whisker growth.
6.6.3 Chemical Etching or Roughening Copper Substrate
Recent work has shown that surface roughening of the copper-based alloys before plating could reduce tin whisker growth, when the etch depth is in the range of 3–4 μm. However, there is limited evidence so far. Deep etch (~3.5) of copper lead-frames was reported to result in even growth of intermetallic layer and whisker-free deposits [94]. The mitigating effect of surface roughening was reported also for fine pitch connectors, though whisker testing was not performed according to standard methods [95]. According to Zhang et al. [94], whisker growth is affected by texture and roughness/topography of copper substrate. The higher the surface roughness, the lower is the whisker density and the average length. A similar result was also reported for sputtered tin films on brass substrate of varying roughness conditions [96].
This influence is apparently related to the growth characteristics of the intermetallic layer. Reasonably, the rougher the surface, the higher the nucleation intensity for intermetallic formation; this leads to the growth of a fine grained and relatively more smooth and regular intermetallic layer. This, in turn, may favourably affect the stress state brought about by intermetallic growth.
6.6.4 Fusing and Hot Dipping
Fusing is performed by immersion in a hot oil bath, shortly after plating. In principle, it may eliminate any residual stress, cause grain growth and possibly thermal tensile stress build-up. For tin deposits on copper substrate, fusing causes the formation of an intermetallic layer of Cu6Sn5 and Cu3Sn [97]. This technique has a quite good reputation, but is not used for components.
Hot dipping is a molten tin process, which is used for components. Hot dipping with pure tin is not effective as mitigation technique, based on both industry experience and testing results [37], though the latter refer to the environmental conditions of 60°C/93% RH, i.e. testing conditions that were later abandoned because of the risk of corrosion. Hot dip Sn–Ag and Sn–Ag–Cu are reputed an effective mitigation [78].
6.6.5 Conformal Coatings
The application of conformal coatings aims at retarding and/or containing whisker growth within the coat by sealing the plated surface. Creating a mechanical barrier against whisker growth should prevent whiskers from causing shorts between adjacent conductors.
Actually, whiskers may penetrate also relatively thick conformal coating, but the risk of a whisker penetrating the coating on a facing surface or the event of two whiskers growing from near surfaces and making contact in between to create a short can be reasonably deemed negligible. Other issues with conformal coatings may be coverage, depending on the material of choice and the method of application, cost and reworkability.
Papers focused on the application of conformal coatings as a mitigation practice against tin whisker growth are relatively few in the literature [98–100] and all from the aerospace industry. A paper from Boeing presents a detailed investigation into different coating materials and changing thickness [101]. Among the different materials tested, Parylene C and silicone were found to be the most effective. Test conditions were as follows: 278 days at ambient +336 days at 50°C/50% RH. No obvious correlation was found between mechanical properties of the coatings and ability to suppress whisker growth; no obvious correlation between oxygen and water vapour permeability and ability to suppress whisker growth. Generally, thin coatings, with thickness below about 60–80 μm, are penetrated by whiskers. Acrylic coatings were penetrated at thickness up to about 100 μm. Thicker coatings, with thickness in the range 100–150 μm, were not penetrated.
According to Livingston [102], the use of conformal coatings or foam encapsulation over tin finish encounters some limitations. First, there may be variability in the quality and thickness of the coating coverage. Provision should be made to avoid that the coating does not bridge the gap from one surface to another, since this could provide a direct path for whisker. The application of the coating under mounted components may be difficult. If the coating is applied by a spray process, care should be taken to avoid shadowing effects due to high-profile components. Livingston also reports results concerning the evaluation of conformal coatings (Uralane 5750), according to which whiskers grew through a 6.3-μm-thick coating after 2.5 years of storage at room temperature. On the other hand, no whisker growth occurred through a 50-μm-thick coating over the time span of 3 years.
Other characteristics, such as modulus and coefficient of thermal expansion, should be considered to prevent cracking. Finally, conformal coatings permeability to moisture poses an additional reliability concern, i.e. electrolytic migration that may be trigged by surface contamination (e.g. flux residue [103]).
6.6.6 Mitigation by Alloying with Lead
The use of Sn–Pb solder alloy finish, with Pb content usually in the range 3–10%, was the industry standard mitigation measure against the whisker risk since the 1960. This peculiar virtue of Pb alloyed Sn finish was first discussed by Arnold [104] and is still the subject of investigation and speculation. In fact, the reasons why Sn–Pb is whisker free or, if not exactly so, less prone to whiskering are still not well understood.
The Sn–Pb binary system stands by itself among the phase diagrams of the binary Sn alloys, also when compared to other Sn eutectic binary systems. The room temperature mutual solubility is limited, about 2% Sn in Pb and practically nil for Pb in SnFootnote 4; however, at the eutectic temperature, the solubility of Sn into Pb is quite remarkable, about 19% (29% at) [105]. This means that some degree of supersaturation may be obtained in the electrodeposited alloy [106] and in fact Raub reported evidence of formation by electrodeposition of metastable Pb–Sn solid solution with Sn content up to 8% [107]. Since the solubility of Pb in Sn is very limited, the electrodeposited alloy shows a two-phase structure with an almost pure Sn matrix and a Pb(Sn) solution. By room temperature recrystallization or after heat treatment, the near room temperature equilibrium concentration of Sn in the Pb-rich phase, about 2%, is re-established [108], resulting in Pb segregation at grain boundaries. Grain boundary segregation of the Pb-rich phase is apparently a characteristic of deposit growth and not only a result of transformation taking place after deposition. In other words, the electrodeposition of Sn–Pb alloy is likely to proceed by a process of simultaneous nucleation of the two-phase alloy [109], so that the observed two-phase structure is the intrinsic growth structure of the alloy and not only the result of the structural rearrangement of a supersaturated solid solution taking place during or shortly after the deposition.
The peculiarity of the electrocrystallization process of Sn–Pb electrodeposits is also responsible for the microstructure of the layer (in particular of matt Sn–Pb coatings), which is not columnar or field oriented, as invariably is for either matt or bright Sn deposits. In fact, because of the simultaneous deposition of the two phases, grain growth of the Sn matrix may be prevented by the formation of the Pb reach phase, and new grains must be nucleated and grow so that the final microstructure shows an equiaxed rather than columnar type of structure. It should be noted that the formation of a well-developed equiaxed (not columnar) microstructure depends on both the Pb content in the alloy, the type of bath and the deposition conditions, as may be inferred from results reported in [109], [110] and [111].
The deformation behaviour of the Pb reach intergranular phase should play an important role in determining the stress relaxation of the deposit. First, contrary to the precipitation of Cu6Sn5 particles, Pb segregation causes tensile stress build-up. Lead has in fact higher density than tin and, given that the alloy is formed as a two-phase supersaturated solid solution, the following separation of almost pure Pb and its segregation at grain boundary causes the build-up of tensile residual stress in the Sn–Pb coatings. Over longer time, the evolution of internal stress may also be influenced by the formation of Cu6Sn5 at the coating/substrate interface. On the other hand, the almost equiaxed grain structure of some Sn–Pb deposits permits a uniform creep deformation contrary to the case of columnar microstructure such as those of pure matt Sn and Sn–Cu, where creep is localized, giving rise to hillocks and whiskers. Hillocks may occur when grain boundary is relatively mobile (pure Sn), and whiskers when grain boundary motion is impeded (Sn–Cu) [111].
Lead does not seem to be replaceable by any other metal as an alloying element of Sn in solder finishes. First, the systems that can be considered viable for solder finish are not many. Several requirements must be simultaneously satisfied: the alloying element must be electrochemically co-deposited; it should not impair solderability to any appreciable extent; it should not be a toxic or hazardous material. Based on such requirements, it is easily seen that a number of possible alloying elements are ruled out and that there are but a few options. Not surprisingly, these have all already been tested and/or are in use: Ag–Sn, Bi–Sn, Cu–Sn, Sn–Zn. A further option could be Sn–Sb; however, antimony raises concerns as to its toxicity.
6.7 Test Methods and Specification Standards
Standardized test methods and specification standards enabling the assessment of susceptibility and defining acceptance requirements for whisker growth are key to a save implementation of tin-based lead-free finishes in components manufacturing and electronic assembly.
Standard test methods are essential for providing the industry with a methodological framework and a set of operating procedures for process and technology qualification; standard tests are also vital to the ongoing activities in the development and qualification of tin plating processes with low whisker propensity.
The final goal is to develop an accelerated test enabling the qualification of products to serve in a given application.
Industry consortiums concerned with the tin whisker issue have been working intensely to identify and evaluate environmental test conditions for the assessment of whisker growth propensity of tin coating: Soldertec Global and the E4 group in Europe,Footnote 5 the iNEMI consortium in the USFootnote 6 and the JEITA whisker test method subcommittee in Japan. In addition, a Tin Whisker Team established in the CALCE consortium conducted research in this field, operating in test plans development and mitigation practices qualification.Footnote 7
Test methods for whisker growth currently in use are still under examination and liable to modification, in particular, the high temperature/high relative humidity test. The following remarks should be taken into account as a common sense guidance for any further development.
-
Not a single test is capable of simulating the action of all—so far known and agreed on—driving factors for whisker growth. This is the reason why different tests are needed to assess susceptibility. In this respect, the final application may be of special relevance in dictating the dominant stress source (e.g. tin coating for connectors).
-
Conflicting results were reported in the early literature and are still being reported, most likely because of the multi-mechanism nature of whisker growth and the practical impossibility to control all the chemical and physical parameters influencing this phenomenon. The only way to master this inherent uncertainty is to provide for a sample size statistically meaningful.
-
Whiskers growth goes through an incubation time which is unpredictable. A suitable test duration and/or an appropriate pre-conditioning must be allowed.
-
There is no definitive consensus on mechanism, so that test conditions and possibly evaluation criteria may be still subject to revision.
6.7.1 JEDEC Test Method Standard
The JEDEC Standard JESD22A121 “Measuring Whisker Growth on Tin and Tin Alloy Surface Finishes” was released in May 2005 [112], after an intense work carried out under the guidance of the iNEMI Whisker Test Method Committee.
It recommends three test conditions, namely: two isothermal conditions with controlled humidity and a thermal cycling condition.
It is clearly stated that the information provided by the tests are inadequate to quantitatively predict whisker growth over long time period. This means that there is no correlation between the results of accelerated tests described in the Standard and in-service environmental conditions. The scope of the standard is in fact to provide a suite of test methods for measurement and comparison of whisker propensity for different finish processes, a consistent inspection protocol for tin whisker examination and a standard reporting format.
Test conditions have already experienced significant changes with respect to the initial proposal. When the “NEMI Test Method for Evaluating Tin Whisker Growth on Plated Surfaces”, revision 6.1, of June 2004, was submitted as a new standard proposal to JEDEC, three tests were recommended to evaluate whisker growth propensity: storage at room ambient conditions: 30 ± 2°C/60 ± 3% RH; storage at 60 ± 5°C/93 ± 3% RH; air to air temperature cycling: −55 to 85°C or −40 to 85°C, 3 cycles/h.
Test conditions for high-temperature/humidity storage were later revised, due to concern about the risk of corrosion and its playing as a potential synergetic factor in whiskering, as pointed out by E3 members in the PROTIN project [51]. Damp-heat test conditions were then slightly mitigated and set to 60 ± 5°C, 87 +3/−2% RH, as given in the October 2005 revision of the Test Method. The influence of humidity and the potential synergy between corrosion and whisker formation was further investigated. This concern was stressed during the iNEMI Tin Whisker Workshop held on the 31st of May 2005 in conjunction with the 2005 ECTC conference. As stated in the press release: “a new factor in tin whisker generation that needs to be understood is heavy oxidation or «corrosion» of the tin in humid environments”.Footnote 8 As new data became available [37], the iNEMI user group changed the high T/H test conditions, namely adjusting the temperature to 55°C and the relative humidity to 85%. The temperature cycling test duration was also changed to a total of 1,500 cycles, since this was shown to saturate whisker growth on matt Sn on copper lead-frame [113]. These changes were introduced in the Specification Standard JESD-201 [114].
The JEDEC Standard JESD22A121 provides methods and procedures to accelerate and characterize whisker growth on tin-based finishes; it does not define acceptance criteria for the purpose of technology/process qualification. Test conditions and inspection intervals, as defined in the Standard and later revised as in the Specification Standard JESD-201, are reported in Table 6.1. Optional test sample preconditioning treatments are also defined according to four options (paragraph 5.1.2 of the standard): none; ambient conditions storage (15–30°C, 30–80% RH) up to 4 weeks; simulated Sn–Pb assembly process; simulated Pb-free assembly process. Preconditioning should be agreed upon between user and supplier before testing.
It should be emphasized that the present test methods are not relevant for applications where mechanical stress and other potentially harmful effects play a role.
6.7.2 IEC Test Method Standard
The intention to propose a draft Tin Whisker Test Method to the IEC International Electrotechnical Commission was announced in the occasion of the Tin Whisker Joint Meeting held in Tokyo on May 15, 2003, by Soldertec, NEMI and JEITA.
The standard proposal was submitted by JEITA in March 2004 [115]; it was approved and introduced as new work item in the IEC/TC 91 work programme in January 2005.
The committee draft was circulated at the end of April 2005 and discussed at the TC91/WG3 meeting in Frankfurt, in September 2005. Based on the discussion results, a Committee Draft for Vote was prepared and circulated in December 2005, approved as of May 2006 and registered as FDIS, Final Draft International Standard, in November 2006.
The standard “IEC 60068-2-82 Ed. 1.0: Environmental Testing- Part 2-82: Test–Test Tx: Whisker test methods for electronic and electric terminals” was finally released in June 2007.
This document addresses the need for standardized whisker test methods: it does not define acceptance criteria for tin-based finishes on components, though it does provide guidelines for acceptance but only as informative material (Annex C and D).
Three test methods are proposed as the basis for a full protocol of whisker test conditions, similarly to the JEDEC Standard: an ambient test; a damp-heat test; a temperature cycling test. Test methods were defined on the basis of the tin whisker growth mechanism conceived by the JEITA Whisker subcommittee [115].
Preconditioning treatments are dictated for SMD and other components intended for soldering, namely a simulate reflow or wave-soldering process, respectively. Besides, the influence of all manufacturing processes should be considered, e.g. trim and form of lead-frames, and a suitable preconditioning is required to this purpose prior to testing. Test methods and conditions are summarized in Table 6.2.
The test method shall be selected depending on type of plating, underlayer and substrate material of components, according to a defined decision matrix. For example, see Table 6.2, the duration of the thermal cycling test shall be (P) for Fe–Ni substrate alloy plated with Sn or Sn alloy; (Q) for all other substrate/underlayer and plating combination.
The IEC standard does not specify tests for whisker growth under external mechanical stressFootnote 9 and more generally cannot be applied for the assessment of whiskering on other components of electronic and electrical equipment unless it is ascertained that the material system and the whisker growth mechanism are comparable to those addressed and assumed for the tests specified in the standard.
As already noted earlier, this standard is not a specification standard, and guidelines for acceptance are included only for informative purpose. Acceptance should be based on maximum whisker length observed on test samples, based on the assumption that the whisker risk is only related to short circuit, i.e. to the proximity of conductive elements and surfaces in electric or electronic circuitry. In fact, since there is no evidence of any specific risk related to whisker density, in addition, nor evidence of any correlation between whisker density and whisker length, there is no reason to introduce an acceptance criterion based on whisker density.
As default criterion, it is suggested that, unless otherwise specified in the relevant specification, the maximum whisker length at the end of the test period should not exceed 50 μm for any of the prescribed tests; however, according to the application, i.e., for higher packing density, higher value may also be specified.
On 21 October 2005, the JEDEC Standard JESD22A121 was submitted to TC47 Semiconductor Devices as PAS, Publicly Available Specification, and approved on January 27, 2006. The first edition of the IEC PAS 62483 was issued in September 2006. The agreement is apparently that as the test method standard was done by TC91, the acceptance standard will be done by TC47. In this frame, the approval of A121 as PAS was intended as a temporary solution waiting for the TC91 test method standard.
6.7.3 JEDEC Acceptance Standard
The main issue to face with tin whisker was the lack of a clear understanding of the mechanism. This primarily meant that it was not possible to rely on accelerated growth results obtained under test conditions (such as those of JESD22A121) for determining acceleration factors and, consequently, there was no room for the development of a qualification specification. However, under the pressure of the lead-free changeover, the need to develop a strategy to minimize the reliability risk brought about by the introduction of tin-based finishes was mandatory.
In this context, the E4 group established minimum requirements for product qualification to meet customer’s specifications.Footnote 10
The iNEMI Tin Whisker User Group was formed to work on the assessment of mitigation practices and definition of reliability criteria. During the development of the acceptance standard, this working group released two specification documents: “Recommendations on Lead-Free Finishes for Components Used in High-Reliability Products”, first released in May 2005, and updated in December 2006 [116]; and “Tin Whisker Acceptance Test Requirements” (July 28, 2004).
The scope of these documents was, and still is in their more recent versions, to provide the industry with recommendations for components used in high-reliability products and to list alternatives and mitigation practices for eradicating or lowering the tin whisker risk on finished components, connectors and PCBs.
The above-mentioned document, in its original version, also served as the basis for the draft of the JEDEC/IPC joint publication JP-002, “Current Tin Whisker Theory and Mitigation Practices Guideline” (March 2006), which is a valuable source of background information on Tin Whiskers [78].
The draft document titled “Tin Whisker Acceptance Test Requirements” was submitted to JEDEC for formal standard creation and formed the basis of the JEDEC Standard JESD201 “Environmental Acceptance Requirements for Tin Whisker Susceptibility of Tin and Tin Alloy Surface Finish”, released March 2006 [114]. An explanatory introduction to the JEDEC Acceptance Standard was later given in a short article authored by Joe Smetana and Rod Gedney [113].
The specification indicates the test methods for measuring tin whisker growth, the acceptance procedures for tin-based surface finishes, the acceptance criteria and an in-progress tin whisker evaluation programme. In particular, test duration and acceptance criteria are defined according to four different product classes, which should be agreed to between supplier and user. In addition, different test duration is prescribed for technology and process change acceptance.
Compared to the original user group recommendations, a few revisions were introduced during industry review of the standard proposal. In particular, as already mentioned previously, the high temperature/humidity storage test conditions were changed from 60 ± 5°C/87 +3/−2% RH to 55 ± 3°C/85 ± 3% RH in order to minimize the risk of condensation in the test chamber. This demonstrates that there was a general recognition of the necessity to preserve test samples from corrosion.
Recently, the iNEMI Tin Whisker Accelerated Test group completed its Phase 5 Evaluation, specifically addressing the influence of temperature and humidity in a wide range of conditions [117]. The final objective was the definition of phenomenological acceleration factors for whisker growth to provide ground for both the optimum choice of test conditions and the assessment of failure rate in field service. Additional points of debate were the storage tests duration and the sample size. Test duration was initially open since growth saturation was required for the test being completed. This condition was removed fixing at 4,000-h test duration for both storage conditions. As to sample size, a minimum of 18 samples per test type is prescribed, unless the number of terminations is less than 16, since a minimum number of inspected terminations is also prescribed. Moreover, additional samples shall be included for the high-temperature/humidity test to allow for terminations or components being skipped because of corrosion.
The outcome of Phase 5 evaluation was that both whisker growth and initiation of corrosion can be represented as a function of temperature and humidity. The development of such a phenomenological model is believed to allow the assessment of whisker growth in other temperature/humidity conditions based on the results of iNEMI tests. This extensive study also lead to the conclusion that a model for the incubation time of whisker growth, either in corroded or not corroded area, can be established, though with caution. A further significant result of the Phase 5 evaluation was also the remark that two distinct temperature/humidity test conditions are not necessary for the purpose of process qualification and acceptance requirements; in this respect, according to the iNEMI Tin Whisker Accelerated Test Project the optimal high-temperature/high-humidity test conditions for Sn-plated copper substrates are 60°C/87% RH.
6.7.4 GEIA Standard
The GEIA (now TechAmerica) standard GEIA-STD-0005-2, “Standard for Mitigating the Effects of Tin Whiskers in Aerospace and High Performance Electronic Systems”, released June 2006, describes processes for mitigating the effects of tin finish in electronic systems used in military, aerospace and other high-reliability applications; it does not provide a formal risk assessment of whisker formation.Footnote 11 Several Control Levels are defined according to the type and extent of tin finish use restriction, as shortly outlined in Table 6.3, and guidance on selecting the appropriate level and product examples is provided in the Standard. Requirements are differentiated and detailed for each Control Level with reference to product and material documentation, monitoring plan and procedure, mitigation measures and risk assessment. Suppliers must meet the relevant requirements for the applicable Control Level.
The final normative section of the document is devoted to implementation techniques. It specifies control procedures over the introduction of tin finishes into production, methods for monitoring and testing for tin whiskers and mitigation strategies.
6.8 Closing Remarks
Tin-based solder finishes have been safely implemented in the consumer segment of the electronic industry. However, the tin whisker problem remains a potential risk that cannot be overlooked. Current and future research efforts in this field should address different and so far neglected aspects, both at the fundamental and applied level.
Fundamental understanding of whisker growth is essential to the development of reliable mitigation practices and prevention strategies, enabling larger use of tin-based finishes; it is also necessary to support and validate work on tin whiskers acceleration factors and risk assessment.
Chemical modification appears as the key factor in whisker formation, either if it is the case of intermetallic formation at interface, bulk phase transformation, surface or inter-granular oxide formation. According to the current interpretation, all these factors are ultimately responsible for inducing a compressive stress (gradient) into the tin or tin alloy layer; this compressive stress (gradient) is almost unanimously considered as the driving force for whisker growth. However, the current theory of whisker formation is not complete. In particular, for the sake of fundamental knowledge as well as for potential practical benefit, basic research should now concentrate on incubation and nucleation mechanisms.
From the industrial perspective, the need of long-term performance results and field studies for current accepted mitigation strategies is an obvious priority. Moreover, a unified set of test methods and acceptance criteria should be adopted globally. Eventually, special attention should be given to risk assessment. In this respect, material properties, such as internal stress in tin deposits, strength and resistance to mechanical shock and vibration of whisker crystals, should be further investigated.
Notes
- 1.
Tin is electrodeposited with either a matt or bright finish depending on the type and load of additives in the plating solution. The type of finish is basically characterized by the surface grain size, which is usually in the range of 3–5 μm for matt Sn (but may range from 1 to 10 μm scaling with layer thickness) and less than 1 μm for bright Sn deposits. The level of compressive residual stress (higher for bright, lower for matt tin) and the carbon content in the layer (less than 0.05% for matt Sn and in the range of 0.1–1% for bright tin) are also usually referred to as qualifying properties of the type of finish.
- 2.
Many other metal surfaces grow either metal or compound whiskers, in different and possibly quite special conditions [20] but, in most cases, with the notable exceptions of zinc, cadmium and silver whiskers, it is hard to say if this is of any practical relevance.
- 3.
An early view on the effect of post-plating annealing of electroplated tin, recently revived in a paper by Pinsky [91], attributes its mitigation efficacy to hydrogen relief. However, it is hard to say if there is any direct and uncontroversial evidence of the involvement of hydrogen codeposition in whiskering of tin finish.
- 4.
The opposite occurs in the Sn-Bi binary system which is practically the only other simple eutectic system of Sn relevant to application as solder finish. The Sn–Zn system is also a simple eutectic system with essentially no mutual solubility [105].
- 5.
Formerly E3: Philips Semiconductors, ST Microelectronics, and Infineon Technologies; Freescale Semiconductor joined in 2004.
- 6.
Formerly NEMI, National Electronics Manufacturing Initiative; the i prefix was inserted when becoming International, from January 1, 2005.
- 7.
Computer Aided Life Cycle Engineering (CALCE) Electronic Products and Systems Centre, a consortium linked to aerospace and military industries in the US. Currently the CALCE Tin Whiskers team (http://www.calce.umd.edu/lead-free/tin-whiskers/. Accessed 13 October 2009) is engaged in assisting consortium members in the implementation of Sn-based finish. Expression of this commitment is also the recent release of a software for assessing the tin whisker failure risk in electronic hardware [59].
- 8.
See for meeting presentations: http://www.inemi.org/cms/newsroom/Presentations/tin_whisker_workshop_may_2005.html. Accessed 13 October 2009.
- 9.
At the time of this writing a standard proposal has been submitted to IEC for a whisker test method designed for connectors of electronic equipment, based on the application of an external mechanical stress.
- 10.
E4 presentation: Lead-free packaging for semiconductor devices, 24 March 2005; available at http://www.st.com/stonline/leadfree/e4fsl_OnlinePDF.pdf. Accessed 13 October 2009.
- 11.
For an outline of the scope and content of this document see: http://www.empf.org/empfasis/2006/mar06/geia-tin_whiskers.html. Accessed 13 October 2009.
References
Galyon GT (2005) Annotated tin whisker bibliography and anthology. IEEE Trans Electron Pack Manuf 28:94–122
Leidecker H and Brusse J (2006) Tin whiskers: a history of documented electrical system failures. Technical presentation to Space Shuttle Program Office. http://nepp.nasa.gov/whisker/reference/tech_papers/2006-Leidecker-Tin-Whisker-Failures_OnlinePDF.pdf. Accessed 30 Sept 2009
Arnold SM (1956) The growth and properties of metal whiskers. Technical proceedings of the 43rd annual convention of the American electroplaters society, pp 26–31
Dunn BD (1987) A laboratory study of tin whiskers growth. ESA Sci Tech Rev 223:1–50
Hilty RD, Corman N (2007) An electrical characterization of tin whisker shorting. Proceedings of the 2007 materials research society spring meeting 993:E02-02
Hada Y, Morikawa O, Togami H (1978) Study of tin whiskers on electromagnetic relay parts. 26th Annual National Relay Conference, 9.1–9.15
Courey KJ, Asfour SS, Bayliss JA, Ludwig LL, Zapata MC (2008) Tin whisker electrical short circuit characteristics—part I. IEEE Trans Electron Pack Manuf 31:32–40
Courey KJ, Asfour SS, Onar A, Bayliss JA, Ludwig LL, Wright MC (2008) Tin whisker electrical short circuit characteristics—Part II. IEEE Trans Electron Pack Manuf 32:41–48
Dunn BD (1987/1988) Mechanical and electrical characteristics of tin whiskers with special reference to spacecraft systems. ESA J 11–12:1–17
Okada S, Higuchi S, Ando T (2003) Field reliability estimation of tin whiskers generated by thermal cycling stress. Capacitor and resistor technology symposium (CARTS) Europe
Evans R (2006) Analysis of the effect of tin whiskers at high frequencies. IEEE Trans Electron Pack Manuf 29:274–279
Galyon GT, Gedney R (2004) Avoiding tin whisker reliability problems. Circuits Assembly 15(8):26–31
Fang T, Mathew S, Osterman M, Pecht M (2007) Assessment of risk resulting from unattached tin whisker bridging. Circuit World 33:5–8
Choi WJ, Galyon G, Tu KN, Lee TY (2004) The structure and kinetics of tin whisker formation and growth on high tin content finishes. In: Puttlitz KJ, Stalter KA (eds) Handbook of lead-free solder technology for microelectronic applications. Marcel Dekker, New York
Hilty RD (2009) Tin whiskers in electronic components. Tyco electronics. http://www.tycoelectronics.com/customersupport/rohssupportcenter/pdf/tyco_lf_customer_packet_whiskers_OnlinePDF.pdf. Accessed 10 October 2009
Nakadaira Y, Jeong S, Shim J, Seo J, Min S, Cho T, Kang S, Oh S (2008) Growth of tin whiskers for lead-free plated leadframe packages in high humid environments and during thermal cycling. Microelectron Reliab 48:83–104
Vicenzo A, Cavallotti PL, Crema P (2002) Electrochemical control of whisker growth on electrodeposited tin coatings. Trans IMF 80(3):79–84
Jiang B, Xian A-P (2007) Observations of ribbon-like whiskers on tin finish surface. J Mater Sci Mater Electron 18:513–518
Rinne CL, Hren JJ, Fedkiw PS (2002) Electrodeposition of tin needle-like structures. J Electrochem Soc 149:C150–C158
Nabarro FRN, Jackson PJ (1958) Growth of crystal whiskers. In: Doremus RH, Roberts BW, Turnbull D (eds) Growth and perfection of crystals. Wiley, New York
Hasiguti RR (1953) A tentative explanation of the accelerated growth of tin whiskers. Acta Metall 3:200–201
Franks J (1958) Growth of whiskers in the solid phase. Acta Metall 6:103–109
Sobiech M, Welzel U, Mittemeijer EJ, Hugel W, Seekamp A (2008) Driving force for Sn whisker growth in the system Cu–Sn. Appl Phys Lett 93:011906/1–011906/3
Sobiech M, Wohlschlögel M, Welzel U, Mittemeijer EJ, Hügel W, Seekamp A, Liu W, Ice GE (2009) Local, submicron strain gradients as the cause of Sn whisker growth. Appl Phys Lett 94:221901/1–221901/3
Choi WJ, Lee TY, Tu KN, Tamura N, Celestre RS, MacDowell AA, Bong YY, Nguyen L (2003) Tin whiskers studied by synchrotron radiation scanning X-ray micro-diffraction. Acta Mater 51:6253–6261
Lal S, Moyer TD (2005) Role of intrinsic stresses in the phenomena of tin whiskers in electrical connectors. IEEE Trans Electron Pack Manuf 28:63–74
Laurila T, Vuorinen V, Kivilahti JK (2005) Interfacial reactions between lead-free solders and common base materials. Mater Sci Eng R49:1–60
Tu KN, Thompson RD (1982) Kinetics of interfacial reaction in bimetallic Cu–Sn thin films. Acta Metall 30:947–952
Bhedwar H, Ray K, Kulkarni S, Balasubramanian V (1972) Kirkendall effect studies in copper–tin diffusion couples. Scr Metall 6:919–922
Onishi M, Fujibuchi H (1975) Reaction-diffusion in the Cu–Sn system. Trans Jpn Inst Met 16:539
Oh M (1994) Growth kinetics of intermetallic phases in the Cu–Sn binary and the Cu–Ni–Sn ternary systems at low temperatures. Doctoral dissertation, Lehigh University
Paul A (2004) The Kirkendall effect in solid state diffusion. PhD thesis, Eindhoven University of Technology
Ho CE, Yang SC, Kao CR (2007) Interfacial reaction issues for lead-free electronic solders. J Mater Sci Mater Electron 18:155–174
Vo N, Kwoka M, Bush P (2005) Tin whisker test standardization. IEEE Trans Electron Pack Manuf 28:3–9
Reynolds HL, Lee CJ, Smetana J (2007) Tin whiskers: mitigation strategies and testing. In: Bradley E, Handwerker CA, Bath J, Parker RD, Gedney RW (eds) Lead-free electronics. Wiley, Hoboken, NJ
The Facts About Tin Whiskers (2009) EIA Engineering Bulletin, August 2004. http://www.ec-central.org/pdf/Engineering/bulletins/EIA-ECA-CB19_13AUG04_OnlinePDF.pdf. Accessed 30 September 2009
Schroeder V, Bush P, Williams M, Vo NN, Reynolds HL (2006) Tin whisker test methods development. IEEE Trans Electron Pack Manuf 29:231–238
Dittes M, Oberndorff P, Crema P, Schroeder V (2003) The effect of temperature cycling on tin whisker formation. Proceedings of the electronics packaging technology conference, pp 183–188
Brusse J (2002) Tin whisker observations on pure tin-plated ceramic chip capacitors. Proceedings of the AESF SUR/FIN, pp 45–61
Compton KG, Mendizza A, Arnold SM (1951) Filamentary growths on metal surfaces whiskers. Corrosion 7:327–334
Kehrer H-P, Kadereit HG (1970) Tracer experiments on the growth of tin whiskers. J Appl Phys 16:411–412
Chang CY, Vook RW (1993) The effect of surface aluminium oxide films on thermally induced hillock formation. Thin Solid Films 228:205–209
Kawanaka R, Fujiwara K, Nango S, Hasegawa T (1983) Influence of impurities on the growth of tin whiskers. Jpn J Appl Phys 22:917–922
Keming C, Wilcox GD (2005) Observations of the spontaneous growth of tin whiskers on tin-manganese alloy electrodeposits. Phys Rev Lett 94:066104/1–066104/4
Chuang T-H (2006) Rapid whisker growth on the surface of Sn–3Ag–0.5Cu–1.0Ce solder joints. Scr Mater 55:983–986
Kim K-S, Matsuura T, Suganuma K (2006) Effects of Bi and Pb on oxidation in humidity for low-temperature lead-free solder systems. J Electron Mater 35:41–47
Hoffman EN, Barsoum MW, Wang W, Doherty RD, Zavaliangos A (2005) On the spontaneous growth of soft metallic whiskers. Proceedings of the 51st IEEE holm conference on electrical contacts, pp 121–126
Oberndorff P, Dittes M, Crema P, Chopin S (2005) Whisker formation on matte Sn influencing of high humidity. Proceedings of the electronic components and technology conference, pp 429–433
Oberndorff P, Dittes M, Crema P, Su P, Yu E (2006) Humidity effects on Sn whisker formation. IEEE Trans Electron Pack Manuf 29:239–245
Osenbach JW, De Lucca JM, Potteiger BD, Amin A, Shook RL, Baiocchi FA (2007) Sn corrosion and its influence on whisker growth. IEEE Trans Electron Pack Manuf 30:23–35
Dittes M, Oberndorff P, Crema P (2004) PROTIN final report
Osenbach JW, Shook RL, Vaccaro BT, Potteiger BD, Amin AN, Hooghan KN, Suratkar P, Ruengsinsub P (2005) Sn whiskers: material, design, processing, and post-plate reflow effects and development of an overall phenomenological theory. IEEE Trans Electron Pack Manuf 28:36–62
Handwerker C, Pedigo A, Blendell J (2007) Whisker to hillock transition: stress relaxation mechanisms in Sn, Sn–Cu, and Sn–Pb films. Tin Whisker workshop, Electronic components and technology conference, Reno
Hilty RD, Corman NE, Herrmann H (2005) Electrostatic fields and current-flow impact on whisker growth. IEEE Trans Electron Pack Manuf 28:75–84
Fukuda Y, Osterman M, Pecht M (2007) The impact of electrical current, mechanical bending, and thermal annealing on tin whisker growth. Microelectron Eng 47:88–92
Romm DW, Abbot DC, Grenney S, Khan M (2003) Whisker evaluation of tin-plated logic component leads. Application report SZZA037A. http://focus.ti.com/lit/an/szza037a/szza037a_OnlinePDF.pdf. Accessed 31 Aug 2009
Liu SH, Chen C, Liu PC, Chou T (2004) Tin whisker growth driven by electrical currents. J Appl Phys 95:7742–7747
Oberndorff P, Dittes M, Crema P (2004) Whisker testing: reality or fiction. Proceedings of the IPC/Soldertec 2nd international conference on lead free electronics, Amsterdam
Fukuda Y, Fang T, Ganesan S, Osterman M, Pecht M (2006) Tin whiskers in electronics. In: Ganesan S, Petch M (eds) Lead-free electronics. Wiley, Hoboken
Lindborg U (1976) A model for the spontaneous growth of zinc, cadmium, and tin whiskers. Acta Metall 24:181–186
Lee B-Z, Lee DN (1998) Spontaneous growth mechanism of tin whiskers. Acta Mater 46:3701–3712
Ellis WC, Gibbons DF, Treuting RJ (1958) Growth of metal whiskers from the solid. In: Doremus RH, Roberts BW, Turnbull D (eds) Growth and perfection of crystals. Wiley, New York
Smetana J (2007) Theory of tin whisker growth: the end game. IEEE Trans Electron Pack Manuf 30:11–22
Tu KN (1994) Irreversible process of spontaneous whisker growth in bimetallic Cu–Sn thin-film reactions. Phys Rev B 49:2030–2034
Glazunova VK, Kudryavtsev NT (1963) An investigation of the conditions of spontaneous growth of filiform crystals on electrolytic coatings. Zhur Priklad Khim 36:543–550 (translated from Russian)
Furuta N, Hamamura K (1969) Growth mechanism of proper tin-whisker. Jpn J Appl Phys 8:1404–1410
Vianco PT, Rejent JA (2009) Dynamic recrystallization (DXR) as the mechanism for Sn whisker development. Part I: a model. J Electron Mater 38:1815–1825
Vianco PT, Rejent JA (2009) Dynamic recrystallization (DXR) as the mechanism for Sn whisker development. Part II: experimental study. J Electron Mater 38:1826–1837
Buchovecky EJ, Du N, Bower AF (2009) A model of Sn whisker growth by coupled plastic flow and grain boundary diffusion. J Appl Phys 94:191904/1–191904/3
Franks J (1956) Metal whiskers. Nature 177:984
Tu KN, Li JCM (2005) Spontaneous whisker growth on lead-free solder finishes. Mater Sci Eng A 409:131–139
Galyon GT, Palmer L (2005) An integrated theory of whisker formation: the physical metallurgy of whisker formation and the role of internal stresses. IEEE Trans Electron Pack Manuf 28:17–30
Frye A, Galyon GT, Palmer L (2007) Crystallographic texture and whiskers in electrodeposited tin films. IEEE Trans Electron Pack Manuf 30:2–10
Moon K-W, Johnson CE, Williams ME, Kongstein O, Stafford GR, Handwerker CA, Boettinger WJ (2005) Observed correlation of Sn oxide film to Sn whisker growth in Sn–Cu electrodeposit for Pb-free solders. J Electron Mater 34:L31–L33
Poker DB, Schubert J, Stritzker B (1987) Ion-induced growth of whiskers on Sn films. MRS symposia proceedings, 74 beam-solid interactions and transient processes, Boston, pp 529–534
Osenbach JW, De Lucca JM, Potteiger BD, Amin A, Baiocchi FA (2007) Sn-whiskers: truths and myths. J Mater Sci Mater Electron 18:283–305
Kakeshita T, Shimizu K, Kawanaka R, Hasegawa T (1982) Grain size effect of electroplated tin coatings on whisker growth. J Mater Sci 17:2560–2566
JEDEC/IPC Joint Publication JP002 (2006) Current tin whiskers theory and mitigation practices guidelines. http://www.jedec.org/DOWNLOAD/search/JP002_OnlinePDF.pdf. Accessed 1 Aug 2009
Sakuyama S, Kutami M (2005) Substitute materials for complete elimination of hazardous substances. Study of whisker growth on lead-free plating. Fujitsu Sci Tech J 41(2):217–224
Boguslavsky I, Bush P (2003) Recrystallization principles applied to whisker growth in tin. IPC SMEMA Council APEX: S12-4, pp 1–10
Galyon GT, Xu C, Lal S, Notohardjono B, Palmer L (2005) The integrated theory of whisker formation—a stress analysis. Proceedings of the electronic components and technology conference, pp 421–428
Tu KN (1973) Interdiffusion and reaction in bimetallic Cu–Sn thin films. Acta Metall 21:347–354
Porter DA, Easterling KE (1992) Phase transformations in metals and alloys. CRC, New York
Dittes M, Oberndorff P, Petit L (2003) Tin whisker formation: results, test methods and countermeasures. Proceedings of the 53 IEEE electronic components and technology conference, pp 822–826
Tu K-N, Suh J-o, Wu A T-C, Tamura N, Tung C-H (2005) Mechanism and prevention of spontaneous tin whisker growth. Mater Trans 46:2300–2308
Moon K-W, Williams ME, Johnson CE, Stafford GR, Handwerker CA, Boettinger WJ (2001) The formation of whiskers on electroplated tin containing copper. In: Hanada S, Zhong Z, Nam SW, Wright RN (eds) Proceedings of the fourth pacific Rim international conference on advanced materials and processing. The Japanese Institute of Metals, pp 1115–1118
Williams ME, Moon K-W, Boettinger WJ, Josell D, Deal AD (2007) Hillock and whisker growth on Sn and SnCu electrodeposits on a substrate not forming interfacial intermetallic compounds. J Electron Mater 36:214–219
Xu C, Zhang Y, Fan C, Abys JA (2005) Driving force for the formation of Sn whiskers: compressive stress—pathways for its generation and remedies for its elimination and minimization. IEEE Trans Electron Pack Manuf 28:31–35
Sabbagh NAJ, McQueen HJ (1975) Tin whiskers: causes and remedies. Met Finish 73:27–31
Oberndorff P, Chen CC, Yu E, van de Water J (2004) Whisker mitigation by postbake: test results. Proceedings of the IPC/Soldertec 2nd international conference on lead free electronics, Amsterdam
Pinsky DA (2008) The role of dissolved hydrogen and other trace impurities on propensity of tin deposits to grow whiskers. Microelectron Reliab 48:675–681
Sobiech M, Welzel U, Schuster R, Mittemeijer EJ, Hügel W, Seekamp A, Müller V (2007) The microstructure and state of stress of Sn thin films after post-plating annealing: an explanation for the suppression of whisker formation? Proceedings of the 57th IEEE electronic components and technology conference, pp 192–197
Wei CC, Liu PC, Chen C, Lee JCB, Wang IP (2007) Relieving Sn whisker growth driven by oxidation on Cu leadframe by annealing and reflowing treatments. J Appl Phys 102:043521/1–043521/7
Zhang W, Egli A, Schwager F, Brown N (2005) Investigation of Sn–Cu intermetallic compounds by AFM: new aspects of the role of intermetallic compounds in whisker formation. IEEE Trans Electron Pack Manuf 28:85–93
Takeuchi M, Kamiyama K, Suganuma K (2006) Suppression of tin whisker formation on fine pitch connectors by surface roughening. J Electron Mater 35:1918–1925
Rodekohr CL, Flowers GT, Suhling JC, Bozack MJ (2008) Influence of substrate surface roughness on tin whisker growth. Proceedings of the 54th IEEE holm conference on electrical contacts, pp 245–248
Gorbunova KM, Glazunova VK (1984) Present state of the problem of spontaneous whisker crystals on electrolytic coatings. Prot Met 20:269–282
Britton SC (1974) Spontaneous growth of whiskers on tin coatings: 20 years of observation. Trans IMF 52:95–102
Kadesch JS, Brusse J (2001) The continuing dangers of tin whiskers and attempts to control them with conformal coat. NASA’s EEE Links Newsletter, July 2001. http://nepp.nasa.gov/whisker/reference/tech_papers/kadesch2001-article-dangers-of-tin-whiskers-and-conformal-coat-study_OnlinePDF.pdf. Accessed 30 Sept 2009
Kadesch JS, Leidecker H (2000) Effects of conformal coat on tin whisker growth. Proceedings of the 37th IMAPS Nordic annual conference, pp 108–116
Woodrow TA, Ledbury EA (2005) Evaluation of conformal coatings as a tin whisker mitigation strategy. IPC/JEDEC 8th international conference on lead-free electronic components and assemblies, San Jose
GEIA Engineering Bulletin GEB-0002 (2003) Reducing the risk of tin whisker induced failures in electronic equipment
Novak J (1994) Physical analysis of dendrites within conformal coat. Proceedings of the IEEE aerospace applied conference, pp 381–390
Arnold SM (1959) The growth of metal whiskers on electrical components. Proceedings of the IEEE electronic components conference, pp 75–82
ASM Handbook (1992) Alloy phase diagrams, vol 3. ASM, Cleveland
Cavallotti PL, Nobili L, Vicenzo A (2005) Phase structure of electrodeposited alloys. Electrochim Acta 50:4557–4565
Raub B, Blum W (1955) Electrodeposition of Pb–Sn alloys. Metalloberflache 9A:54–57 (in German). English translation by Duffy G (1961) NIST report AD0843305
Raub E, Elser F (1978) Die inhibierte Elektrokristallisation von Zinn-bleilegierungen. J Less Common Met 62:431–445
Jordan M (1995) The electrodeposition of tin and its alloys. Eugen G. Leuze Publishers, Bad Saulgau, pp 219–226
Zhang W, Schwager F (2006) Effects of lead on tin whisker elimination. J Electrochem Soc 153:C337–C343
Boettinger WJ, Johnson CE, Bendersky LA, Moon K-W, Williams ME, Stafford GR (2005) Whisker and hillock formation on Sn, Sn–Cu and Sn–Pb electrodeposits. Acta Mater 53:5033–5050
JEDEC Standard JESD22-A121A (2008) Test method for measuring whisker growth on tin and tin alloy surface finishes. http://www.jedec.org/download/search/22a121A_OnlinePDF.pdf. Accessed 30 Sept 2009
Smetana J, Gedney R (2005) Tin whisker management guidelines. Printed Circuit Design Manuf 22(12):14–17
JEDEC Standard JESD201A (2008) Environmental acceptance requirements for tin whisker susceptibility of tin and tin alloy surface finish. http://www.jedec.org/download/search/JESD201A_OnlinePDF.pdf. Accessed 30 Sept 2009
Sakamoto I (2005) Whisker test methods of JEITA whisker growth mechanism for test methods. IEEE Trans Electron Pack Manuf 28:10–16
iNEMI user group recommendations on lead-free finishes for components used in high-reliability products, Version 4, 1 Dec, 2006. http://thor.inemi.org/webdownload/projects/ese/tin_whiskers/Pb-Free_Finishes_v4_OnlinePDF.pdf. Accessed 30 Sept 2009
Reynolds HL (2007) Accelerated tin whisker test committee update: Phase 5 evaluation. Tin whisker workshop, Electronic components and technology conference, Reno
Acknowledgments
I am indebted to Pascal Oberndorff for his criticism and remarks to the draft manuscript. I am especially grateful to Jacob Klerk for his considerate encouragement during the short life of the Elfnet Tin Whisker Group.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2011 Springer-Verlag London Limited
About this chapter
Cite this chapter
Vicenzo, A. (2011). Tin Whiskers. In: Grossmann, G., Zardini, C. (eds) The ELFNET Book on Failure Mechanisms, Testing Methods, and Quality Issues of Lead-Free Solder Interconnects. Springer, London. https://doi.org/10.1007/978-0-85729-236-0_6
Download citation
DOI: https://doi.org/10.1007/978-0-85729-236-0_6
Published:
Publisher Name: Springer, London
Print ISBN: 978-0-85729-235-3
Online ISBN: 978-0-85729-236-0
eBook Packages: EngineeringEngineering (R0)