Abstract
Pyrolysis–gas chromatography–mass spectrometry (Py–GC–MS) has confirmed to be a versatile technique that benefits food and environmental analyses. It has been used for the chemical characterization of materials and compounds that are not suitable for traditional gas chromatography (GC) because of their large size. The controlled thermal degradation carried out during pyrolysis is able to break down macromolecules into volatile fragments easier to identify because they become separable by GC and detectable by mass spectrometry (MS). A wide array of applications has been reported using Py–GC–MS, from characterization of macromolecules (polymers, paints, lacquers, adhesives, plastic, synthetic fibers, organic matter, etc.) in a variety of disciplines including forensics, history, engineering, and, of course, food and environmental sciences. In recent years, this technique has experienced an important increase due to its capability for the chemical fingerprinting of organic matter, and the identification and characterization of nano-, micro-plastics used for food package and present in environmental and food samples. In this chapter, we describe current Py–GC–MS instrumentation and working modes and summarize recent applications in food and environmental analysis with special emphasis on its strengths and limitations.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Pyrolysis–gas chromatography–mass spectrometry (Py–GC–MS) can characterize macromolecules [1] because pyrolysis transforms non-volatile macromolecules into their structural units, which are normally small and volatile, while GC provides resolving power and MS identification capability, broadening the range of applications [2]. The International Union of Pure and Applied Chemistry (IUPAC) defines Py–GC as “a version of reaction chromatography in which a sample is thermally decomposed to simpler fragments before entering the column,” and Py–GC–MS as “the characterization in an inert atmosphere of a material or a chemical process in which chemical degradation reaction(s) is/are induced by thermal energy” [3].
Pyrolysis provides the thermal decomposition of the sample at relatively low pressure and at temperatures between 500 and 1,400°C originating characteristics volatile units in the presence of an inert gas (the most common is helium). Chemical changes in the macromolecules are induced by high temperatures with progressive bond breakages going from the weakest to the strongest. Among reactions implicated are depolymerization (resulting in basic units and/or oligomers), random excision (resulting in random small fragments as all the C-C bonds have the same energy), and the elimination of side groups (cleaved of the polymer bond). Other possible but minor reactions include chard formation, oxidation cross-linking, cyclization, isomerization, and hydrogenation [2]. The fragmentation of molecules caused by pyrolysis depends on the relative strength of the bonds and the ability of the formed free radicals to provide steady products [4, 5]. For instance, big complex hydrocarbon molecules can be broken down during pyrolysis into simpler and smaller molecules of char, liquid, and gas (Fig. 1) [6].
Decomposition of large hydrocarbon molecules into smaller ones during pyrolysis [6]
The units formed in the pyrolyzers are separated by chromatography, using fused silica capillary columns, and identified by MS (using mass spectral libraries or selecting few characteristic ions). Py–GC–MS is well-matched with most of the treatments used with environmental and food samples but can also eliminate these processes as it is capable to analyze directly macromolecules and/or polymers [7,8,9]. One important advantage of this technique is the small quantity of sample used (5–200 μg) although samples must be dried.
Py–GC–MS enables the analysis of macromolecules as polymers, copolymers and additives, packaging materials, fibers, coatings, electronic intermediates, paints, lacquers, paper or wood derivatives, bio-oils and biomass, food, drugs, surfactants, fragrances, etc. [9,10,11,12,13,14,15]. The applications of Py–GC–MS include research, quality control, and characterization of materials as well as forensic analyses, conservation and restoration of cultural heritage, biotechnology, geology, and agriculture among others. Publications on Py–GC–MS are mostly focused on its applications such as the authentication of extra virgin olive oils [11] or the characterization, for example, of bio-oils [4, 12, 14, 16], polymers [1, 17, 18], lacquer films [9, 15], or lignite tars [19]. There are also a number of publications, including several reviews, dealing with soil organic matter and its chemical fingerprint [8, 20,21,22,23,24,25,26], and specially with the occurrence and fate of micro- and nano-plastics (MPs and NPs, respectively) in the environment [27,28,29,30,31,32,33,34,35], which has gained great attention recently.
In this chapter, we highlight new instrumental advances that combined with the multiple workflows of Py–GC–MS have broad food and environmental applications, mainly in relation to compositional analysis, quality control of authenticity, adulteration, and contamination detection (especially by plastic polymers), and characterization of organic matter.
2 Py–GC–MS: Instrumentation and Working Modes
2.1 Working Modes in Py–GC–MS
The technological evolution of Py–GC–MS has mostly be focused on devices design, inertness of their components, versatility of the working modes, and reproducibility of the obtained data, and it has already been reported in several review articles [2, 36]. However, there are several important new developments in the pyrolysis devices (microfurnace chambers, quartz liners for the sample, etc.) as well as in the GC–MS process itself (time-of-flight (TOF) MS in the low- or high-resolution mode, comprehensive GCxGC, etc.) that deserve to be highlighted.
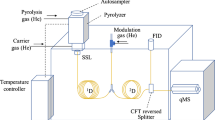
Currently, there are different possibilities to work with Py–GC–MS depending on the analyses’ objectives summarized as (Fig. 2):
Evolved gas analysis (EGA-MS) (Fig. 2a): the degradation products are separated from macromolecules based on the temperature at which they are formed rather than at their volatilization temperature. This is achieved using a slow temperature ramp in the pyrolyzer and a short and narrow (2.5 m, 0.15 mm i.d.) deactivated capillary tube without a stationary phase, which connects directly the GC injector and the MS-detector. During the GC oven is held constant at 300°C, the pyrolyzer is heated by a temperature program from 40 to 500°C. The resulting chart is called an EGA thermogram. EGA-MS is a previous step in which the number of peaks and the temperature zone for each peak are vital information in determining correct temperatures for the other working modes (i.e., single-shot, double-shot, and heart-cut).
Single-shot analysis (Fig. 2b): the pyrolysis is performed at a single temperature (normally >500°C depending on the sample analyzed), which increases as fast as possible (in current instruments <20 ms). The macromolecules are fragmented in the pyrolyzer, and the volatile pyrolysis products, known as pyrolyzates, are separated in the chromatographic column.
Double-shot analysis (Fig. 2c): it includes two stages, the first one involves thermal desorption of the volatile compounds (of low molecular weight analyzed at low temperature, ~80–350°C) examined then by GC–MS. In the most recent instruments, these volatile compounds are cryo-trapped temporarily at the head of a separation column by liquid nitrogen. In the second step, the residual sample left after desorption (in which the non-volatile macromolecules remain) is pyrolyzed at high temperatures (~500–800°C), and the pyrolyzate is again analyzed by GC–MS.
Heart-cut analysis (HC/EGA-GC-MS) (Fig. 2d): it is the two-dimensional way of working in Py–GC. The EGA is used to produce a thermogram, and each temperature zone of interest is analyzed independently by heart cutting evaporating components that are selectively introduced to the GC column (by means of a selective sampler), where they are temporarily trapped prior to the analysis by GC–MS (e.g., cryo-trapped as described above). This method allows searching both the specific components in a highly complex matrix and the whole composition of a complex system. While extremely useful, this technique can add complexity and required-time for the analysis.
Reactive Pyrolysis–GC–MS: with this technique the sample undergoes a chemical derivatization reaction in the pyrolysis chamber avoiding extensive sample preparation (the most used derivatizing agent is tetramethylammonium hydroxide). This derivatization may be instead of, or in addition to, heat-induced pyrolysis of the macromolecule into smaller fragments.
In addition to these analytical techniques, modern pyrolyzers can also be used only for thermal desorption of analytes (TD/Py–GC–MS) as in the first step of double-shot analyses. The system configurations for the four analytical techniques can also include a vent-free GC–MS adapter enabling column exchange without venting the MS.
2.2 Recent Advances in Py–GC–MS Instrumentation
Py–GC–MS can deliver quantitative results with high accuracy and precision. As in any other analytical method, quantification of determined compounds requires standards, isotopically labeled internal standards, and the selection of the proper ions [12, 26]. The main disadvantage attributed to Py–GC–MS is its poor reproducibility because of factors such as sample heterogeneity, slow transfer of the pyrolyzate to the chromatographic column, and catalytic events in the pyrolyzer that may alter the chemical nature of the sample. Other drawbacks of the technique are its destructive character, and the difficulty of interpreting the pyrograms obtained due to the large number of reactions that might take place and the density of peaks in the pyrograms [36]. Consequently, recent advances have been focused on developing new approaches to overcome these problems by improving the reproducibility of the pyrolyzer, enhancing the separation of the compounds, and improving the identification of the reactions products.
2.2.1 Pyrolyzer
Pyrolysis systems have been classified according to their heating mechanisms as: pulse-mode pyrolyzers (filament, Curie-point or induction, and laser) and continuous-mode pyrolyzers (furnace or microfurnace pyrolyzer) (Fig. 3). Current environmental and food applications of Py–GC–MS are mostly focused on microfurnace pyrolyzers because this type of pyrolyzer can work with higher amounts of samples, which is important for the analysis of minority compounds. Improvements in this field have been related to the control of the pyrolysis temperature (rapid rise, reproducibility) and the connection of the pyrolyzer with the GC, which must be as direct as possible (to prevent pyrolyzates back into the injector where they can undergo secondary pyrolysis).
Pyrolysis systems (pyrolyzers) classified according to their heating mechanisms: (a) filament, (b) Curie-point, (c) microfurnace [2]
The filament type pyrolyzer heats a sample tube using a filament (Fig. 3a). This technique has some drawbacks in temperature accuracy and reproducibility since some types of samples may be not uniformly heated. Moreover, high boiling compounds may be absorbed in the transfer line carrying the pyrolyzates to GC, which make difficult to introduce them to a GC column. Some progress has been done in developing better and more effective sample holders able to short the analyte diffusion path and to reduce peak broadening. Considerable effort has also been made in the implementation of a technology ensuring accurate monitoring of the filament resistance to guarantee truthful temperature control (heat-transfer variations can affect the temperature of the sample). Modern instruments can perform programmed heating in addition to pulsed pyrolysis, to obtain sequential pyrolysis of a sample combining different temperature ramps, and velocities of heating. Other improvements search to avoid evaporation of the volatile and semi-volatile compounds and denaturation, degradation, or thermofixation of the samples because of sample-preheating (essential in this technique). Consequently, thermal desorption in last instruments is carried out by modular systems or complicated trapping sequences [37, 38].
The Curie-point pyrolyzer (Fig. 3b) is similar to the filament pyrolyzer (preheating of the sample is also necessary) but different temperature steps cannot be programmed as required for EGA-MS analysis, since the temperature ceases to rise when the Curie-point of the metal holding the sample is reached. Then, the EGA analysis is impossible.
The vertical furnace/microfurnace pyrolyzer is a hollow quartz tube covered by a heater device for rapid heating and cooling (Fig. 3c). Modern microfurnaces are equipped with hollow ceramic heaters and powerful cooling fans, able to achieve rapid heating and cooling, together with temperature sensors for the sample. Changes in the sample cup materials for inertness have been made recently such as the use of quartz or chemically bonded quartz thin layers. Microfurnaces avoid the problems caused by the preheating of the filament and Curie-point pyrolyzers by placing the sample in an inert deactivated cup, held at ambient temperature (in helium) at the same time that the furnace is preheated to the pyrolysis temperature, and then letting drop the cup into the microfurnace. The sample is purged of oxygen before heated avoiding oxidation reactions [5, 39, 40].
The microfurnace pyrolyzer has several advantages over other types of techniques: a) accurate furnace set temperature in a wide-ranging temperature or continuous ramp-up heating, b) direct interface to the GC injector, which allows the complete introduction of all molecular weight compounds (low to high), and c) the sample just needs to be simply placed in a sample cup and it is ready for analysis. Worth mentioning is the Multi-Shot Pyrolyzer EGA/PY-3030D with a low mass ceramic heater allowing pyrolysis temperatures up to 1,050°C, rapid heating (600°C/min) and cooling (100°C/min), very precise temperature control in the microfurnace (40–1,050°C ± 0.1°C), simple handling of samples through weighing in sample cup (approx. 50–200 μg), and no condensation of gases at cold spots (no dead volumes) during transition to GC due to an interface-heating.
In relation to the connection of the pyrolyzer to the GC, the main concern is to transfer the analytes in a tight “plug.” This means heating the sample as fast as possible, in order to have the resulting volatiles in a very short time with narrow and well separated peaks. Cryo-trapping capabilities using liquid nitrogen are available for Py–GC–MS in both pulse- and continuous-mode pyrolyzers [41, 42].
2.2.2 Gas Chromatography–Mass Spectrometry
Although most Py–GC–MS applications have been made with conventional GC separation, there is no limitation in terms of their possible modes. There are two major trends in the use of this technique: (ultra-)fast chromatography and comprehensive two-dimensional GC (GCxGC) [4, 12, 14, 16, 19]. Fast GC seeks to reduce the analysis time by using different combinations of narrower and shorter columns, with lower phase thickness and/or higher carrier gas flow, as well as by high temperature heating ramp rates (up to 250°C/min) and oven cool downs (450 to 50°C in <3 min). However and probably due to the complexity of pyrolyzates, there are few publications reporting this combination [4, 12]. On the other hand, GCxGC provides better resolution power and higher peak capacity by adding a second GC column, with different polarity and connected through a modulator (usually a cryo-modulator), which traps the eluting fractions coming from the first column and injects them into the second one achieving true two-dimensional separations [14, 16, 19]. In the future, this technique could add a third dimension of separation if it is used together with heart-cut EGA [2]. Nevertheless, it is important to highlight that the improvement possibly gained by the combination of these techniques will require enhanced data processing tools due to the great amount of information generated and the time needed to interpret it.
The detection technique of pyrolyzates is typically mass spectrometry (MS) often accomplished by a single quadrupole mass analyzer due to its robustness and capacity to detect the fragmentation products. The molecules detected by MS are identified through their mass spectra using commercial libraries (e.g., NIST/EPA/NIH, Wiley, MPW, Norman Mass Bank, m/z Cloud) or by means of reference substances [4]. Despite triple quadrupole instruments (QqQ) are nowadays the most recommended systems for the analysis of target compounds (because of their higher sensitivity and specificity), it is not commonly used in combination with Py–GC since the compounds formed in the pyrolysis are mostly unknown and the application of the QqQ is less evident [7]. In contrast, high-resolution mass spectrometry (HRMS) and mainly (quadrupole) time-of-flight (TOF or QqTOF) has become essential in Py–GC–MS analyses since these detectors provide information on the most probable empirical formula of both the analyzed molecule and its characteristic fragments. Although HRMS application focuses on using selected ion monitoring, the analysis of the full spectra in the development of the method is mandatory [12, 26].
3 Applications of Py–GC–MS in Food and Environmental Analysis
Py–GC–MS can be used in a wide array of applications from characterization of materials (polymers, lacquer films, etc.) to quality control (of food and daily commodities) including also forensic analyses in environmental sciences among others. In recent years, the most interesting applications of Py–GC–MS in environmental samples are (1) the chemical fingerprinting of organic matter (OM) [8, 20,21,22,23,24,25,26] and (2) the identification, characterization, and fate of MPs and NPs [28,29,30,31,32, 34, 35, 43, 44]. Furthermore, Py–GC–MS main application in food are related to (1) the determination of geographical origin, (2) detection of fraudulently added substances, and (3) quality control.
3.1 Characterization of Food
Because of its versatility, Py–GC–MS offers ample possibilities to be exploited in multiple applications related to food industry, from food authentication to the analysis of composition, quality, and additives, including contaminants as well. One of the first studies using Py–GC–MS in food analysis was published in 1999 [10]. It presented the results obtained by the European Commissions’ Joint Research Centre for the detection of added whey proteins in milk, the ripening stages of cheese, the characterization of vinegar and wine, as well as the assessment of the geographical origin of cocoa butters.
Nowadays, the isotope ratio mass spectroscopy (IRMS) for determination of the stable isotope composition of light elements is an interesting approach to discriminate production areas and to validate the origin of vegetables and fruits. The analysis of δ13C in discrete oil components using a compound-specific isotope analysis (CSIA) is an accurate method compared to the isotope assessment in bulk samples. Direct analytical Py-CSIA was used for the authentication of extra virgin olive oils avoiding chemical and/or physical pre-treatments [11]. The δ2H value in nine pyrolysis compounds detected in all extra virgin olive oils ranged from −112 to −267 mUr. These compounds were chosen as likely surrogate descriptors of the olive oil geographic origin as they were significantly correlated with longitude and annual temperature by means of Principal Component Analysis and Multiple Linear Regression. Similarly, and because of its capacity to analyze macromolecules, Curie-point-Py–GC–MS has been used to characterize the solvent extracts (ethyl-acetate, n-butanol, raw theabrownin) from a type of Pu-erh tea and Dian Hong black tea. Results showed substantial different chemical compositions among solvent extracts from both teas, and proved the great characterization capacity of the technique [45].
Py–GC–MS has demonstrated to be a useful tool able to provide precise fingerprinting but also detailed information about quality, composition, and additives in active packages of biogenic polymers employed in food industry. One of these materials is the oregano essential oil, which is used due to its bioactive properties. However, because of oregano essential oils high volatility, characteristics like aging and preparation conditions of films can produce losses of active agent. Py–GC–MS was used to characterize a bioactive polylactic acid with polybutylene succinate (95:5) film extruded with variable amounts of oregano essential oil [13]. Bioplastic pyrolysis enables identifying lactide enantiomers and monomer units from the polylactic acid and unambiguous molecular markers from the polybutylene succinate fraction. Oregano essential oil pyrolysis revealed aromatics and terpenes with terpinene, cymene, and thymol/carvacrol peaks that were identified as diagnostic peaks showing significant linear correlation coefficients with the amount of oregano essential oil included in the bioplastic matrix.
Other applications of Py–GC–MS in food industry are focused on the determination of contaminants. Using this technique, the thermal stability of five pharmaceuticals commonly detected in food (chloramphenicol, florfenicol, lincomycin hydrochloride, diphenhydramine hydrochloride, and carbamazepine) was analyzed [46]. Results showed a number of volatile thermal degradation products (some of them previously unidentified) with a technique simpler than others reported earlier. The test was conducted at 250°C (i.e., above actual cooking temperatures of 180–220°C) for 20 s only, a very short period of time (compared to cooking times). However, it seems to reveal information about the degradation products generated at lower temperatures or longer cooling times. Authors concluded that in future studies, lower pyrolysis temperatures should be explored to simulate the general cooking conditions and provide some insight in the fate of drug residues in food during processing. In this regard, the effect of pyrolysis temperature from 150 to 550°C with 100°C-increments was studied in raw or cooked fish muscle samples (Oreochromis niloticus) to detect cylindrospermopsin and its degradation products by both (1) applying each temperature to a different sample (single-shot pyrolysis) or (2) sequentially applying each temperature to the same sample (multi-shot pyrolysis) (Fig. 4) [47]. Relative abundance of degradation products was found to be different depending on the cooking process (microwaving, broiling, boiling, and steaming) being boiling the only cooking technique that showed to decrease the relative percentage of cylindrospermopsin compared to the control group.
Analytical pyrolysis of tilapia muscle. Total current ion chromatograms (TIC) performed at (a) single-shot mode, i.e. different sample at different temperatures and (b) multi-shot mode, i.e. same sample at increasing temperatures. Chromatograms are divided in sections of preferential compound classes and major compounds detected are labeled on the corresponding peaks. FA: fatty acid, PUFA: polyunsaturated fatty acid, Me: methyl [47]
Thermal desorption using Py–GC–MS was used together with a novel sorbent developed for sorptive enrichment of dilute analytes from liquid samples [48]. The extraction element is made of titanium open tubular tube (30 mm × 1.2 mm i.d. × 1.6 mm o.d.) coated with a chemically bonded layer of polydimethylsiloxane (PDMS 500 μm in thickness). This sorbent was used to develop a method to extract and analyze bethrodine (a herbicide) in water samples over a concentration range from 2.5 to 2.5 × 104 ng/L. A detection limit of 0.5 ng/L was achieved with fairly good reproducibility of the measurements (relative standard deviation, R.S.D., below 7.5% at 10 ng/L). This procedure was also applied for the simultaneous determination of five preservatives – benzoic acid, sorbic acid, and methyl, ethyl, and propyl esters of p-hydroxybenzoic acid – in soft drinks, yogurts, and sauces [49]. The method attained limits of detection between 0.002 and 0.2 mg/L, and it was applied to real samples spiked at levels of between 20 and 100 mg/L showing good recoveries (92–106%) and precisions (R.S.D.s of 0.9–4.6% (n = 5)).
3.2 Determination of MPs and NPs
Environmental contamination by MPs and NPs is a global problem with a worldwide-recognized dimension as proven by the large number of studies published in recent years [28,29,30,31,32,33,34,35, 43, 50,51,52,53,54,55]. Py–GC–MS has become one of the most promising techniques to assess MPs and NPs in environmental samples since it is able to detect low MP dimensions, and even NPs (the most difficult ones because of their size). It is more sensitive and less affected by sample impurities than other methods. Py–GC–MS has been used to identify MPs in soil [56, 57], soil amended with solid waste compost [58], plants [27], bio solids [59], river sediments [40, 60], beach sediments [61], coastal sediments [30, 62], tidal flat sediment [63], suspended particulate matter [40], wastewater [64], sea water [65, 66], surface water [28, 52, 67, 68], salt [63, 69], bivalves [66, 70,71,72], fish [30, 43, 47, 51, 73], and other types of biota [52, 73]. Py–GC–MS has also been applied to determine NPs in water [74,75,76].
MPs can be directly assessed from the samples, such as soil [58], but they are usually separated and concentrated from the matrix by wet digestion (using oxidants, acids, or enzymes [62, 77]), or by density differences (with solutions of NaCl [61], NaI, ZnCl2, NaBr [69], or sodium polytungstate [60]), with a final filtration. In samples with high OM content, as wastewater, sequential filtration can help to isolate the MPs without clogging the pore filters [64]. Very recently, pressurized liquid extraction has been applied to solubilize MPs in solvents such as dichloromethane or tetrahydrofuran [44, 59]. NPs can also be extracted from aqueous samples by filtration working in the range of nanoscale (24 nm–52 nm) [75]. Ultrafiltration has also been used to separate MPs and NPs from water attaining lower sizes (5–50 nm) [74] Other techniques as Triton X-45-based cloud-point extraction has been tested to pre-concentrate NPs providing an enrichment factor of 500 without disturbing their actual morphology and sizes [76].
In the determination of MPs and NPs by Py–GC–MS, the pyrolyzer most frequently used is the microfurnace [59,60,61,62,63, 69, 76], because of its higher sample capacity, though filament [57, 58, 75] and Curie-point [57, 58, 75] pyrolyzers have also been applied. A comparison of micro furnace and Curie-point pyrolyzers to assess MPs in the environment proved that the former can process more sample quantity due to its larger sample cups allowing the transfer of pre-concentrated MPs directly from the glass fiber filters [63]. The double-shot pyrolysis is the preferred mode since the first-shot can act as a clean-up step avoiding interferences as those of OM [40, 57, 59, 62, 76], despite none of the OM compounds can interfere with the indicators selected for polypropylene (PP), polyethylene terephthalate (PET), polycarbonate (PC), poly-(methyl methacrylate) (PMMA), and polystyrene (PS).
The GC–MS is commonly used with a quadrupole mass analyzer although TOF analyzer has also been reported in studies of MPs and NPs in water [75] and wastewater samples [34]. In the former study, TOF was operated in nominal mass units not in accurate mass, similar to a quadrupole but offering an increased sensitivity because all the ions accumulated are transmitted to the TOF detector. In most cases, selected ion monitoring (SIM) mode is the only working mode that attains sufficient sensitivity to detect MPs at low concentrations. It is important to keep in mind that other organic molecules (e.g., organic matter) can be present in the extract/sample leading to a number of non-volatile pyrolysis products, which enhance the risk of interferences with specific indicators of compounds, boost the maintenance frequency, and hamper a reliable quantification. As it has been explained, compounds in the pyrograms can be identified by means of mass spectral databases, custom databases containing pre-acquired pyrograms of plastic references, or by comparison with the published scientific literature [61, 68]. In general, to identify and quantify MPs and NPs, specific indicator compounds are selected for each type of plastic as presented extensively by Picó and Barceló [2]. These indicator compounds were chosen after assessing their specificity against a number of natural materials as chitin, wood, pine needles, cellulose, humic acid, etc. [59]. However, this method of data processing is very time consuming. In this sense, the research moves toward the design of software for automatic qualitative analysis of this information as an automated algorithm developed and implemented in the F-Search software [18]. The process is based on the generation of summated mass spectra (SMS) for each polymer, obtained by extracting specific m/z and retention index coordinates of the characteristic pyrolysis products. The identification of a polymer is performed by comparing its SMS with those of a built-in library. After validation, the algorithm was tested on a reference sample containing 11 types of synthetic polymers providing relative standard deviations around 10%. The lowest estimated amount of polymer detectable in a sample, was lower than 1 μg for most polymers.
Reactive pyrolysis after TMAH derivatization has been able to reduce organic interferences and because this technique improves the detection sensitivity for PET and PC [60, 69, 77]. When pyrolysis is used in combination with TMAH, the pyrolytic behavior of polyethylene (PE), PP, PS, and polyvinylchloride (PVC) remained unaffected while that of PET, PMMA, PC, and polyamide (PA6) changed. As an example of the complexity of the technique and the problems originated by interferences, determination of PS requires the selection of a characteristics PS trimer (m/z 312), even after derivatization, because chitin (widely present in the natural environment) releases styrene (m/z 104) during pyrolysis (Fig. 5) [76].
Py–GC–MS chromatograms of PS- and PMMA-nanoplastic samples and standards: (a) Total ion chromatogram (TIC) and selected-indicator-ion chromatograms (SIC) of the PS-nanoplastic sample. (b) Mass spectrum of the PS-nanoplastic-sample pyrolytic product. (c) TIC and SIC of PMMA-nanoplastic sample. (d) Mass spectrum of the PMMA-nanoplastic-sample pyrolytic product. (e) TIC and SIC of the PS-nanoplastic standard. (f) Mass spectrum of the PS-nanoplastic-standard pyrolytic product. (g) TIC and SIC of the PMMA-nanoplastic standard. (h) Mass spectrum of the PMMA-standard pyrolytic product [76]
The first international attempt (17 laboratories from eight different countries) to compare the suitability of the methods commonly used in MPs analysis showed the performance of Py–GC–MS within the field as well as the main obstacles faced by researchers [78]. Different techniques as microscopy, Fourier-transform infrared microspectroscopy (μ-FTIR), Raman microspectroscopy (μ-Raman), thermal extraction and desorption or Py–GC–MS, scanning electron microscopy, and particle counter were compared in relation to the total particle number, polymer type, number of particles, and/or the particle mass of each polymer type. For the identification of polymer type, μ-Raman and Py–GC–MS performed best. The quantification of polymer mass for identified polymer types was questionable for Py–GC–MS, whereas other methods failed to determine the correct polymer mass. Quantification of particle number per identified polymer type was evaluated successful for μ-FTIR and the quantification of total particle numbers was best for microscopy and to a lesser extent for μ-FTIR [78].
The performance of Py–GC–MS has also been compared to μ-FTIR and μ-Raman, as most successful complementary approaches in the identification of MPs [60]. Py–GC–MS identified copolymers as PE-PP or PE-PP-PA6 that could be difficult to distinguish with μ-Raman without chemometrics approach leading to results with a finer identification [70]. Chemical information provided by Raman and FTIR spectra is limited compared to the one of Py–GC–MS. This is because the selectivity of vibrational spectroscopy is not enough to identify specific monomers and co-monomers, mixtures, additives, and degradation products [79]. Py–GC–MS is able to characterize MPs at a molecular level identifying synthetic polymers as well as the possible presence of additives in contrast to the second common limitation of Raman spectroscopy and ATR-FTIR that provided spectra relative to a surface portion of the MP fragments [60, 70]. The advantage of Py–GC–MS over FTIR spectroscopy is that both polymer types and organic plastic additives can be studied in a single run [80]. Due to Py–GC–MS important advantages, new statistical approaches to tackle polymer variability and matrix interference are being developed [79]. This augurs that pyrolysis will exponentially increase its applicability for analyzing MPs and NPs in the environment.
3.3 Characterization of Organic Matter
Py–GC–MS has also demonstrated to be a powerful tool for analyzing the molecular fingerprint of organic matter (OM) in soils and sediments [5, 23, 24, 39, 40, 81,82,83], as well as to analyze changes in the dissolved organic matter (DOM) fraction [84,85,86]. While in water the sample should be filtrated through a glass filter to later isolate and enrich the DOM, by reverse phase mechanisms, as SPE [84] and pre-HPLC [85], in soil and sediment the sample just need to be dried, sieved, and pulverized [5, 39, 40]. DOM can also be extracted from soils using a lysimeter-pump or by shaking soil with water [87].
The study of OM includes the characterization of a diversity of compounds including polysaccharides, amino sugars, proteins, polyhydroxy aromatics, lipids, lignin, etc. Other compounds as humic acids and fulvic acids need prior extraction and clean-up following standard methods (these include elimination of the free OM by flotation with H3PO4, demineralization with Na4P2O7 to release the humic substances complexed with oxides, then, extraction with basic solutions as NaOH, and separation by precipitation in an acidified solution, pH < 2). Humic acids are later purified by washing with several acidic solutions [83] and/or dialyzed into cellophane bags [20, 88]. The three types of pyrolyzers described previously have been applied to characterize OM: micro furnace [39, 40, 83, 89, 90], platinum heated filament pyrolyzer [5, 20, 86, 87, 91], and Curie-point [85, 88]. Most of these studies have only used the single-shot at temperatures between 500°C and 800°C [5, 20, 39, 40, 83, 85, 87,88,89,90,91]. The double-shot pyrolysis that makes accessible both free volatiles and high molecular weight structures has been hardly used to characterize OM (probably because OM is formed by non-volatile compounds). The reactive pyrolysis has also been tested in few studies [87, 92] since it simplifies the pyrogram providing less fragments. However, a comparison of the ability of both techniques to characterize DOM in soil from the Three Gorges Reservoir area stated that Py–GC–MS fingerprinting is more helpful to quantify microbial DOM than reactive pyrolysis [86], which is useful to assess the molecular features of polyphenolic (cinnamic acids, lignin, and tannin) and aliphatic (cutin and suberin) fractions.
OM characterization by GC–MS is generally performed using a 30 m capillary column and a simple quadrupole in scan mode. Compounds detected in a pyrogram can range between 100 and 400 and are identified by means of a mass spectral database or library as previously described. Their relative abundance can be calculated by normalizing the peak areas of each individual compound to the total area of all the peaks detected. In general, to avoid the high number of pyrolyzates in a sample, the best is to focus the study on a few good biomarkers giving information about the process of interest. For instance, reactive Py–GC–MS has been used to identify branched-chain fatty acids in humic acids to describe microbial activities occurring during composting processes [92]. Methoxyphenols (12 major guaiacyl- and syringyl-type compounds) have also been detected by Py–GC–MS in topsoil samples since they are distinctive molecules advising on the occurrence and degree of alteration of lignin, and have also been proposed as indicators of the soil’s capacity to storage C [82, 89].
Studies about changes in OM after different disturbances as forest fires [21,22,23, 81] or land use changes [5, 82] have also been performed. The thermal transformation of soil OM caused the thermal breakdown and cracking of n-alkane compounds, as was revealed by the increase of the ratio of short-to-long chains and the alteration of the typical odd-over-even carbon predominance indexes [21, 22]. Analyses carried out by using a vertical microfurnace type double-shot pyrolyzer indicated that the fire resulted in an enrichment of aromatic compounds, nitrogen (N) constituents, lignin-derived compounds, and polysaccharides [23, 81]. The effect of land use changes in OM compounds has been studied using different approaches including Py–GC–MS. Zhang et al. [5] used a heated platinum filament pyrolyzer directly connected to a GC–ion trap MS to analyze the effects of freshwater wetlands conversion to sugarcane (Saccharum officinarum) on the soil OM cycling and processes. These authors concluded that the land use change not only reduced total OM contents but also significantly increased the aliphatic fractions while lignin fractions were almost completely exhausted. In another study, Campo et al. [82] by means of Curie-point pyrolysis with tetramethylammonium hydroxide assessed the effects of afforestation on the soil lignin content (based on the vanillyl, syringyl, and cinnamyl contents). According to them, afforestation with Pinus nigra was the best practice for increasing the soil organic carbon stock and the lignin content in soil.
Py–GC–MS using two ionization systems, electron-ionization (EI) and photon-ionization (PI), with different mass selective analyzers (quadrupole- and a time-of-fight, TOF-MS) has been performed for the analysis of DOM in water [84]. In this research, resonance-enhanced-multi-photon-ionization (REMPI) provided information on the molecular weight and a high sensitivity and selectivity for aromatic hydrocarbons. Consequently, the characterization of natural samples by EI-quadrupole/MS and their aromatic fingerprint (REMPI-TOF-MS) were accomplished (Fig. 6). In general, there are few studies incorporating the recent developments of GC–MS, possibly because these analyses are complex and time consuming besides needing highly specialized technicians to deal with the high amount of data generated. In order to overcome this difficulty, statistical tests have been applied to improve and schematize the visualization/interpretation of the information obtained. As pyrolyzates can fluctuate among samples in their presence and intensity, a lot of effort is devoted nowadays to develop automated identification and quantification software that can help to process all the information obtained from the pyrographs and to visualize the results [20, 93].
Direct comparison of the chromatograms resulting from the REMPI-TOFMS (above) and EI-QMS (below) detection for At4 station (Bothnian Bay). The TD step is shown on the left and the pyrolysis step on the right. On the ordinate the GC-time and -temperature are plotted. On the abscissa the mass-to-charge ratio is depicted. Graduated in color are the intensities [84]
4 Conclusions
Py–GC–MS is recognized as a valuable technique in food and environmental chemistry as proved by the increasing number of studies using it. Because of its versatility, Py–GC–MS offers ample possibilities to be exploited in multiple applications related to food industry, from food authentication to the analysis of composition, quality, and additives, including contaminants as well. It should be kept in mind that pyrolysis is a time-consuming and complex technique, then, it will be used only in those cases where the other chromatographic techniques failed, such as in the characterization of macromolecules. In recent years, it has been applied to the chemical characterization of organic matter in water, soil, and sediment but also, to assess the modifications that disturbances as forest fires or land use changes produced. Evaluation of the contamination by MPs and NPs of environmental samples has also become a hot topic addressed by the application of this technique. Py–GC–MS has demonstrated important advantages over others, such as μRaman and μFTIR to evaluate MPs, since it has been the only one able to detect NPs. The use of very small quantities of sample and the lack, in most cases, of pre-treatment have also added value to this technique. However, the large amount of results obtained and the difficulty in interpreting them are constraints that also need to be considered. On the other hand, most of the studies are focused on the robustness of the pyrolyzer and therefore, latest innovations in GC–MS have not been yet introduced in most applications. In this sense, it is expected that Py–GC–MS takes advantage of the great separation power of GCxGC, the speed of rapid GC, and the identification capability that HRMS and HRMS in tandem can provide. Furthermore, environmental analyses can also be enhanced by the application of EGA or shot-cut-EGA that would add a further dimension to multidimensional techniques. Py–GC–MS is a robust, multipurpose, adaptable, and useful technique with a promising future to overcome new challenges as those depicted in this chapter.
References
Obst V, Steinhaus J, Knupp G, Schroeder-Obst D, Fink W, Kusch P (2014) Application of pyrolysis–gas chromatography–mass spectrometry for the identification of polymeric materials. LCGC N Am 32:210–217
Picó Y, Barceló D (2020) Pyrolysis gas chromatography-mass spectrometry in environmental analysis: focus on organic matter and microplastics. Trends Anal Chem 130:115964. https://doi.org/10.1016/j.trac.2020.115964
McNaught AD, Wilkinson A (eds) (1997) Compendium of chemical terminology.2nd edn. Blackwell Scientific Publications, Oxford
Schena T, Farrapeira R, Erle TRB, Krause LC, von Muhlen C, Caramao EB (2019) Fast two-dimensional gas chromatography applied in the characterization of bio-oil from the pyrolysis of coconut fibers. Separation Sci Plus 2(3):89–99. https://doi.org/10.1002/sscp.201800129
Zhang Z, Wang JJ, Lyu X, Jiang M, Bhadha J, Wright A (2019) Impacts of land use change on soil organic matter chemistry in the Everglades, Florida – a characterization with pyrolysis-gas chromatography–mass spectrometry. Geoderma 338:393–400. https://doi.org/10.1016/j.geoderma.2018.12.041
Basu P (2018) Chapter 5 – pyrolysis. In: Basu P (ed) Biomass gasification, pyrolysis and torrefaction3rd edn. Academic Press, pp 155–187
Gómez X, Meredith W, Fernández C, Sánchez-García M, Díez-Antolínez R, Garzón-Santos J et al (2018) Evaluating the effect of biochar addition on the anaerobic digestion of swine manure: application of Py-GC/MS. Environ Sci Pollut Res 25(25):25600–25611. https://doi.org/10.1007/s11356-018-2644-4
Laskar DD, Ke J, Zeng J, Gao X, Chen S (2013) Py-GC/MS as a powerful and rapid tool for determining lignin compositional and structural changes in biological processes. Curr Anal Chem 9(3):335–351. https://doi.org/10.2174/1573411011309030003
Ma XM, Lu R, Miyakoshi T (2014) Application of pyrolysis gas chromatography/mass spectrometry in lacquer research: a review. Polymers 6(1):132–144. https://doi.org/10.3390/polym6010132
Guillou C, Lipp M, Radovic B, Reniero F, Schmidt M, Anklam E (1999) Use of pyrolysis–mass spectrometry in food analysis: applications in the food analysis laboratory of the European Commissions’ Joint Research Centre. J Anal Appl Pyrolysis 49(1):329–335. https://doi.org/10.1016/S0165-2370(98)00121-1
Jiménez-Morillo NT, Cabrita MJ, Dias CB, González-Vila FJ, González-Pérez JA (2020) Pyrolysis-compound-specific hydrogen isotope analysis (δ2H Py-CSIA) of Mediterranean olive oils. Food Control 110:107023. https://doi.org/10.1016/j.foodcont.2019.107023
Lima NK, Lopes AR, Guerrero Jr PG, Yamamoto CI, Hansel FA (2018) Determination of volatile organic compounds in eucalyptus fast pyrolysis bio-oil by full evaporation headspace gas chromatography. Talanta 176:47–51. https://doi.org/10.1016/j.talanta.2017.08.008
Llana-Ruiz-Cabello M, Pichardo S, Morillo NJ, Bermudez JM, Aucejo S, Gonzalez-Vila F et al (2015) Fingerprinting (Py–GC/MS) of a bio-film active food package with Origanum vulgare L. essential oil. Toxicol Lett 238(2 Suppl):S72. https://doi.org/10.1016/j.toxlet.2015.08.251
Onorevoli B, Machado ME, Polidoro AS, Corbelini VA, Caramao EB, Jacques RA (2017) Pyrolysis of residual tobacco seeds: characterization of nitrogen compounds in bio-oil using comprehensive two-dimensional gas chromatography with mass spectrometry detection. Energy Fuel 31(9):9402–9407. https://doi.org/10.1021/acs.energyfuels.7b00405
Fu Y, Xiao Q, Zong S, Wei S (2020) Characterization and quantitation study of ancient lacquer objects by NIR spectroscopy and THM-Py-GC/MS. J Cult Herit 46:95–101. https://doi.org/10.1016/j.culher.2020.06.015
Primaz CT, Schena T, Lazzari E, Caramao EB, Jacques RA (2018) Influence of the temperature in the yield and composition of the bio-oil from the pyrolysis of spent coffee grounds: characterization by comprehensive two dimensional gas chromatography. Fuel 232:572–580. https://doi.org/10.1016/j.fuel.2018.05.097
Duemichen E, Eisentraut P, Celina M, Braun U (2019) Automated thermal extraction-desorption gas chromatography mass spectrometry: a multifunctional tool for comprehensive characterization of polymers and their degradation products. J Chromatogr A 1592:133–142. https://doi.org/10.1016/j.chroma.2019.01.033
Matsui K, Ishimura T, Mattonai M, Iwai I, Watanabe A, Teramae N et al (2020) Identification algorithm for polymer mixtures based on Py-GC/MS and its application for microplastic analysis in environmental samples. J Anal Appl Pyrolysis 149:104834. https://doi.org/10.1016/j.jaap.2020.104834
Mao F, Fan H, Wang J (2019) Biogenic oxygenates in lignite pyrolysis tars and their thermal cracking revealed by two-dimensional gas chromatography/time-of-flight mass spectrometry (GC x GC-TOFMS). J Anal Appl Pyrolysis 139:213–223. https://doi.org/10.1016/j.jaap.2019.02.008
Almendros G, Hernández Z, Sanz J, Rodríguez-Sánchez S, Jiménez-González MA, González-Pérez JA (2018) Graphical statistical approach to soil organic matter resilience using analytical pyrolysis data. J Chromatogr A 1533:164–173. https://doi.org/10.1016/j.chroma.2017.12.015
Campo J, Nierop KGJ, Cammeraat E, Andreu V, Rubio JL (2011) Application of pyrolysis-gas chromatography/mass spectrometry to study changes in the organic matter of macro- and microaggregates of a Mediterranean soil upon heating. J Chromatogr A 1218(30):4817–4827. https://doi.org/10.1016/j.chroma.2011.03.038
Faria SR, De la Rosa JM, Knicker H, González-Pérez JA, Keizer JJ (2015) Molecular characterization of wildfire impacts on organic matter in eroded sediments and topsoil in Mediterranean eucalypt stands. Catena 135:29–37. https://doi.org/10.1016/j.catena.2015.07.007
Jiménez-Morillo NT, Almendros G, De la Rosa JM, Jordán A, Zavala LM, Granged AJP et al (2020) Effect of a wildfire and of post-fire restoration actions in the organic matter structure in soil fractions. Sci Total Environ 728:138715. https://doi.org/10.1016/j.scitotenv.2020.138715
Ma S, Chen Y, Lu X, Wang X (2018) Soil organic matter chemistry: based on pyrolysis-gas chromatography-mass spectrometry (Py-GC/MS). Mini-Rev Org Chem 15(5):389–403. https://doi.org/10.2174/1570193X15666180108152845
Santoiemma G (2018) Recent methodologies for studying the soil organic matter. Appl Soil Ecol 123:546–550. https://doi.org/10.1016/j.apsoil.2017.09.011
Yamamoto S, Yoshioka H, Ishiwatari R (2007) Pyrolysis- and chemical degradation-GC/MS analyses of environmental kerogen and humic substances and their applications to geochemistry. Bunseki Kagaku 56(2):71–91. https://doi.org/10.2116/bunsekikagaku.56.71
Allouzi MMA, Tang DYY, Chew KW, Rinklebe J, Bolan N, Allouzi SMA et al (2021) Micro (nano) plastic pollution: the ecological influence on soil-plant system and human health. Sci Total Environ 788:147815. https://doi.org/10.1016/j.scitotenv.2021.147815
Barcelo D, Pico Y (2019) Microplastics in the global aquatic environment: analysis, effects, remediation and policy solutions. J Environ Chem Eng 7(5). https://doi.org/10.1016/j.jece.2019.103421
Fu W, Min J, Jiang W, Li Y, Zhang W (2020) Separation, characterization and identification of microplastics and nanoplastics in the environment. Sci Total Environ 721:137561. https://doi.org/10.1016/j.scitotenv.2020.137561
Jiang Y, Yang F, Hassan Kazmi SSU, Zhao Y, Chen M, Wang J (2022) A review of microplastic pollution in seawater, sediments and organisms of the Chinese coastal and marginal seas. Chemosphere 286:131677. https://doi.org/10.1016/j.chemosphere.2021.131677
Peñalver R, Arroyo-Manzanares N, López-García I, Hernández-Córdoba M (2020) An overview of microplastics characterization by thermal analysis. Chemosphere 242:125170. https://doi.org/10.1016/j.chemosphere.2019.125170
Pico Y, Alfarhan A, Barcelo D (2019) Nano- and microplastic analysis: focus on their occurrence in freshwater ecosystems and remediation technologies. Trac-Trends Anal Chem 113:409–425. https://doi.org/10.1016/j.trac.2018.08.022
Shi W, Cui T, Wu H, LeBlanc GA, Wang F, Lihui AN (2021) A proposed nomenclature for microplastic contaminants. Mar Pollut Bull 172:112960. https://doi.org/10.1016/j.marpolbul.2021.112960
Vilakati B, Sivasankar V, Nyoni H, Mamba BB, Omine K, Msagati TAM (2021) The Py – GC-TOF-MS analysis and characterization of microplastics (MPs) in a wastewater treatment plant in Gauteng Province. S Afr Ecotoxicol Environ Saf 222:112478. https://doi.org/10.1016/j.ecoenv.2021.112478
Yu Z-f, Song S, Xu X-l, Ma Q, Lu Y (2021) Sources, migration, accumulation and influence of microplastics in terrestrial plant communities. Environ Exp Bot 192:104635. https://doi.org/10.1016/j.envexpbot.2021.104635
Sobeih KL, Baron M, Gonzalez-Rodriguez J (2008) Recent trends and developments in pyrolysis-gas chromatography. J Chromatogr A 1186(1–2):51–66. https://doi.org/10.1016/j.chroma.2007.10.017
Fan X, Wei S, Zhu M, Song J, Peng P (2018) Molecular characterization of primary humic-like substances in fine smoke particles by thermochemolysis–gas chromatography–mass spectrometry. Atmos Environ 180:1–10. https://doi.org/10.1016/j.atmosenv.2018.02.033
Zhang Z, Xu G, Wang Q, Cui Z, Wang L (2019) Pyrolysis characteristics, kinetics, and evolved gas determination of chrome-tanned sludge by thermogravimetry–Fourier-transform infrared spectroscopy and pyrolysis gas chromatography-mass spectrometry. Waste Manag 93:130–137. https://doi.org/10.1016/j.wasman.2019.05.034
Girona-García A, Badía-Villas D, Jiménez-Morillo NT, González-Pérez JA (2019) Changes in soil organic matter composition after Scots pine afforestation in a native European beech forest revealed by analytical pyrolysis (Py-GC/MS). Sci Total Environ 691:1155–1161. https://doi.org/10.1016/j.scitotenv.2019.07.229
Mazzetto JML, Melo VF, Bonfleur EJ, Vidal-Torrado P, Dieckow J (2019) Potential of soil organic matter molecular chemistry determined by pyrolysis-gas chromatography/mass spectrometry for forensic investigations. Sci Justice 59(6):635–642. https://doi.org/10.1016/j.scijus.2019.07.003
Wu X, Ba Y, Wang X, Niu M, Fang K (2018) Evolved gas analysis and slow pyrolysis mechanism of bamboo by thermogravimetric analysis, Fourier transform infrared spectroscopy and gas chromatography-mass spectrometry. Bioresour Technol 266:407–412. https://doi.org/10.1016/j.biortech.2018.07.005
Zhong L, Ni R, Zhang L, He Z, Zhou H, Li L (2019) Determination of total arsenic in soil by gas chromatography after pyrolysis. Microchem J 146:568–574. https://doi.org/10.1016/j.microc.2019.01.057
Pico Y, Barcelo D (2019) Analysis and prevention of microplastics pollution in water: current perspectives and future directions. Acs Omega 4(4):6709–6719. https://doi.org/10.1021/acsomega.9b00222
Dierkes G, Lauschke T, Becher S, Schumacher H, Foeldi C, Ternes T (2019) Quantification of microplastics in environmental samples via pressurized liquid extraction and pyrolysis-gas chromatography. Anal Bioanal Chem 411(26):6959–6968. https://doi.org/10.1007/s00216-019-02066-9
Gong J-S, Tang C, Peng C-X (2012) Characterization of the chemical differences between solvent extracts from Pu-erh tea and Dian Hong black tea by CP–Py–GC/MS. J Anal Appl Pyrolysis 95:189–197. https://doi.org/10.1016/j.jaap.2012.02.006
Tian L, Bayen S, Yaylayan V (2017) Thermal degradation of five veterinary and human pharmaceuticals using pyrolysis-GC/MS. J Anal Appl Pyrolysis 127:120–125. https://doi.org/10.1016/j.jaap.2017.08.016
Prieto AI, Guzmán-Guillén R, Jos Á, Cameán AM, de la Rosa JM, González-Pérez JA (2020) Detection of cylindrospermopsin and its decomposition products in raw and cooked fish (Oreochromis niloticus) by analytical pyrolysis (Py-GC/MS). Chemosphere 244:125469. https://doi.org/10.1016/j.chemosphere.2019.125469
Wang L, Hosaka A, Watanabe C, Ohtani H, Tsuge S (2004) Development of a novel solid-phase extraction element for thermal desorption gas chromatography analysis. J Chromatogr A 1035(2):277–279. https://doi.org/10.1016/j.chroma.2004.02.059
Wang L, Zhang X, Wang Y, Wang W (2006) Simultaneous determination of preservatives in soft drinks, yogurts and sauces by a novel solid-phase extraction element and thermal desorption-gas chromatography. Anal Chim Acta 577(1):62–67. https://doi.org/10.1016/j.aca.2006.06.030
Huang Y, Qing X, Wang W, Han G, Wang J (2020) Mini-review on current studies of airborne microplastics: analytical methods, occurrence, sources, fate and potential risk to human beings. Trends Anal Chem 125:115821. https://doi.org/10.1016/j.trac.2020.115821
Li J, Liu H, Paul Chen J (2018) Microplastics in freshwater systems: a review on occurrence, environmental effects, and methods for microplastics detection. Water Res 137:362–374. https://doi.org/10.1016/j.watres.2017.12.056
Mai L, Bao LJ, Shi L, Wong CS, Zeng EY (2018) A review of methods for measuring microplastics in aquatic environments. Environ Sci Pollut Res 25(12):11319–11332. https://doi.org/10.1007/s11356-018-1692-0
Rios Mendoza LM, Balcer M (2019) Microplastics in freshwater environments: a review of quantification assessment. Trends Anal Chem 113:402–408. https://doi.org/10.1016/j.trac.2018.10.020
Silva AB, Bastos AS, Justino CIL, da Costa JP, Duarte AC, Rocha-Santos TAP (2018) Microplastics in the environment: challenges in analytical chemistry – a review. Anal Chim Acta 1017:1–19. https://doi.org/10.1016/j.aca.2018.02.043
Kusch P (2017) Chapter 7 – application of pyrolysis-gas chromatography/mass spectrometry (Py-GC/MS). In: Rocha-Santos TAP, Duarte AC (eds) Comprehensive analytical chemistry, vol 75. Elsevier, pp 169–207
David J, Steinmetz Z, Kučerík J, Schaumann GE (2018) Quantitative analysis of poly(ethylene terephthalate) microplastics in soil via thermogravimetry-mass spectrometry. Anal Chem 90(15):8793–8799. https://doi.org/10.1021/acs.analchem.8b00355
Steinmetz Z, Kintzi A, Muñoz K, Schaumann GE (2020) A simple method for the selective quantification of polyethylene, polypropylene, and polystyrene plastic debris in soil by pyrolysis-gas chromatography/mass spectrometry. J Anal Appl Pyrolysis. https://doi.org/10.1016/j.jaap.2020.104803
Watteau F, Dignac MF, Bouchard A, Revallier A, Houot S (2018) Microplastic detection in soil amended with municipal solid waste composts as revealed by transmission electronic microscopy and pyrolysis/GC/MS. Front Sustain Food Syst 2. https://doi.org/10.3389/fsufs.2018.00081
Okoffo ED, Ribeiro F, O'Brien JW, O'Brien S, Tscharke BJ, Gallen M et al (2020) Identification and quantification of selected plastics in biosolids by pressurized liquid extraction combined with double-shot pyrolysis gas chromatography-mass spectrometry. Sci Total Environ 715:136924. https://doi.org/10.1016/j.scitotenv.2020.136924
Käppler A, Fischer M, Scholz-Böttcher BM, Oberbeckmann S, Labrenz M, Fischer D et al (2018) Comparison of μ-ATR-FTIR spectroscopy and Py-GCMS as identification tools for microplastic particles and fibers isolated from river sediments. Anal Bioanal Chem 410(21):5313–5327. https://doi.org/10.1007/s00216-018-1185-5
Doyen P, Hermabessiere L, Dehaut A, Himber C, Decodts M, Degraeve T et al (2019) Occurrence and identification of microplastics in beach sediments from the Hauts-de-France region. Environ Sci Pollut Res 26(27):28010–28021. https://doi.org/10.1007/s11356-019-06027-8
Gomiero A, Øysæd KB, Agustsson T, van Hoytema N, van Thiel T, Grati F (2019) First record of characterization, concentration and distribution of microplastics in coastal sediments of an urban fjord in south West Norway using a thermal degradation method. Chemosphere 227:705–714. https://doi.org/10.1016/j.chemosphere.2019.04.096
Fischer M, Scholz-Boettcher BM (2019) Microplastics analysis in environmental samples – recent pyrolysis-gas chromatography-mass spectrometry method improvements to increase the reliability of mass-related data. Anal Methods 11(18):2489–2497. https://doi.org/10.1039/c9ay00600a
Funck M, Yildirim A, Nickel C, Schram J, Schmidt TC, Tuerk J (2019) Identification of microplastics in wastewater after cascade filtration using Pyrolysis-GC-MS. MethodsX 7:100778. https://doi.org/10.1016/j.mex.2019.100778
Lv L, He L, Jiang S, Chen J, Zhou C, Qu J et al (2020) In situ surface-enhanced Raman spectroscopy for detecting microplastics and nanoplastics in aquatic environments. Sci Total Environ 728. https://doi.org/10.1016/j.scitotenv.2020.138449
Yu J, Wang P, Ni F, Cizdziel J, Wu D, Zhao Q et al (2019) Characterization of microplastics in environment by thermal gravimetric analysis coupled with Fourier transform infrared spectroscopy. Mar Pollut Bull 145:153–160. https://doi.org/10.1016/j.marpolbul.2019.05.037
Ravit B, Cooper K, Buckley B, Yang I, Deshpande A (2019) Organic compounds associated with microplastic pollutants in New Jersey, U.S.A. surface waters. AIMS Environ Sci 6(6):445–459. https://doi.org/10.3934/environsci.2019.6.445
Hendrickson E, Minor EC, Schreiner K (2018) Microplastic abundance and composition in Western Lake superior as determined via microscopy, Pyr-GC/MS, and FTIR. Environ Sci Technol 52(4):1787–1796. https://doi.org/10.1021/acs.est.7b05829
Fischer M, Goßmann I, Scholz-Böttcher BM (2019) Fleur de Sel—an interregional monitor for microplastics mass load and composition in European coastal waters? J Anal Appl Pyrolysis 144. https://doi.org/10.1016/j.jaap.2019.104711
Hermabessiere L, Himber C, Boricaud B, Kazour M, Amara R, Cassone AL et al (2018) Optimization, performance, and application of a pyrolysis-GC/MS method for the identification of microplastics. Anal Bioanal Chem 410(25):6663–6676. https://doi.org/10.1007/s00216-018-1279-0
Fabbri D, Rombolà AG, Vassura I, Torri C, Franzellitti S, Capolupo M et al (2020) Off-line analytical pyrolysis GC–MS to study the accumulation of polystyrene microparticles in exposed mussels. J Anal Appl Pyrolysis 149:104836. https://doi.org/10.1016/j.jaap.2020.104836
Diez-Quijada L, de Oliveira FL, Jos Á, Cameán AM, Aparicio-Ruiz R, Vasconcelos V et al (2020) Alterations in Mediterranean mussel (Mytilus galloprovincialis) composition exposed to cyanotoxins as revealed by analytical pyrolysis. J Anal Appl Pyrolysis 152:104970. https://doi.org/10.1016/j.jaap.2020.104970
Dehaut A, Hermabessiere L, Duflos G (2019) Current frontiers and recommendations for the study of microplastics in seafood. Trends Anal Chem 116:346–359. https://doi.org/10.1016/j.trac.2018.11.011
Mintenig SM, Bäuerlein PS, Koelmans AA, Dekker SC, Van Wezel AP (2018) Closing the gap between small and smaller: towards a framework to analyse nano- and microplastics in aqueous environmental samples. Environ Sci Nano 5(7):1640–1649. https://doi.org/10.1039/c8en00186c
Sullivan GL, Gallardo JD, Jones EW, Hollliman PJ, Watson TM, Sarp S (2020) Detection of trace sub-micron (nano) plastics in water samples using pyrolysis-gas chromatography time of flight mass spectrometry (PY-GCToF). Chemosphere 249:126179. https://doi.org/10.1016/j.chemosphere.2020.126179
Zhou X-x, Hao L-t, Wang H-y-z, Li Y-j, Liu J-f (2019) Cloud-point extraction combined with thermal degradation for nanoplastic analysis using pyrolysis gas chromatography-mass spectrometry. Anal Chem 91(3):1785–1790. https://doi.org/10.1021/acs.analchem.8b04729
Fischer M, Scholz-Böttcher BM (2017) Simultaneous trace identification and quantification of common types of microplastics in environmental samples by pyrolysis-gas chromatography-mass spectrometry. Environ Sci Technol 51(9):5052–5060. https://doi.org/10.1021/acs.est.6b06362
Müller YK, Wernicke T, Pittroff M, Witzig CS, Storck FR, Klinger J et al (2020) Microplastic analysis—are we measuring the same? Results on the first global comparative study for microplastic analysis in a water sample. Anal Bioanal Chem 412(3):555–560. https://doi.org/10.1007/s00216-019-02311-1
La Nasa J, Biale G, Fabbri D, Modugno F (2020) A review on challenges and developments of analytical pyrolysis and other thermoanalytical techniques for the quali-quantitative determination of microplastics. J Anal Appl Pyrolysis 149:104841. https://doi.org/10.1016/j.jaap.2020.104841
Akoueson F, Chbib C, Monchy S, Paul-Pont I, Doyen P, Dehaut A et al (2021) Identification and quantification of plastic additives using pyrolysis-GC/MS: a review. Sci Total Environ 773:145073. https://doi.org/10.1016/j.scitotenv.2021.145073
Chen H, Rhoades CC, Chow AT (2020) Characteristics of soil organic matter 14 years after a wildfire: a pyrolysis-gas-chromatography mass spectrometry (Py-GC-MS) study. J Anal Appl Pyrolysis 152:104922. https://doi.org/10.1016/j.jaap.2020.104922
Campo J, Stijsiger RJ, Nadal-Romero E, Cammeraat ELH (2019) The effects of land abandonment and long-term afforestation practices on the organic carbon stock and lignin content of Mediterranean humid mountain soils. Eur J Soil Sci 70(5):947–959. https://doi.org/10.1111/ejss.12799
Banach-Szott M, Debska B, Tobiasova E, Pakula J (2019) Structural investigation of humic acids of forest soils by pyrolysis-gas chromatography. Pol J Environ Stud 28(6):4099–4107. https://doi.org/10.15244/pjoes/97392
Otto S, Streibel T, Erdmann S, Klingbeil S, Schulz-Bull D, Zimmermann R (2015) Pyrolysis–gas chromatography–mass spectrometry with electron-ionization or resonance-enhanced-multi-photon-ionization for characterization of polycyclic aromatic hydrocarbons in the Baltic Sea. Mar Pollut Bull 99(1):35–42. https://doi.org/10.1016/j.marpolbul.2015.08.001
Park J, Choi M, Cho J, Chon K (2018) Transformation of dissolved organic matter in a constructed wetland: a molecular-level composition analysis using pyrolysis-gas chromatography mass spectrometry. Environ Eng Res 23(4):390–396. https://doi.org/10.4491/eer.2018.043
Jiang T, Kaal J, Liang J, Zhang Y, Wei S, Wang D et al (2017) Composition of dissolved organic matter (DOM) from periodically submerged soils in the Three Gorges Reservoir areas as determined by elemental and optical analysis, infrared spectroscopy, pyrolysis-GC–MS and thermally assisted hydrolysis and methylation. Sci Total Environ 603–604:461–471. https://doi.org/10.1016/j.scitotenv.2017.06.114
Taube PS, Silva DS, Vasconcelos AA, Rebellato L, Madureira LAS, Hansel FA (2018) Exploratory on-line pyrolysis and thermally assisted hydrolysis and methylation for evaluating non-hydrolyzable organic matter in anthropogenic soil from central Brazilian Amazon. Brazilian. J Anal Chem 5(19):38–53. https://doi.org/10.30744/brjac.2179-3425.2018.5.19.38-53
Dorado J, Almendros G, González-Vila FJ (2016) Response of humic acid structure to soil tillage management as revealed by analytical pyrolysis. J Anal Appl Pyrolysis 117:56–63. https://doi.org/10.1016/j.jaap.2015.12.016
Jimenez-Gonzalez MA, Alvarez AM, Carral P, Gonzalez-Vila FJ, Almendros G (2017) The diversity of methoxyphenols released by pyrolysis-gas chromatography as predictor of soil carbon storage. J Chromatogr A 1508:130–137. https://doi.org/10.1016/j.chroma.2017.05.068
Schellekens J, Almeida-Santos T, Macedo RS, Buurman P, Kuyper TW, Vidal-Torrado P (2017) Molecular composition of several soil organic matter fractions from anthropogenic black soils (Terra Preta de Índio) in Amazonia — a pyrolysis-GC/MS study. Geoderma 288:154–165. https://doi.org/10.1016/j.geoderma.2016.11.001
Heslop J, Walter Anthony K, Zhang M (2017) Utilizing pyrolysis GC-MS to characterize organic matter quality in relation to methane production in a thermokarst lake sediment core. Org Geochem 103:43–50. https://doi.org/10.1016/j.orggeochem.2016.10.013
Fukushima M, Tu X, Aneksampant A, Tanaka A (2018) Analysis of branched-chain fatty acids in humic substances as indices for compost maturity by pyrolysis-gas chromatography/mass spectrometry with tetramethylammonium hydroxide (TMAH-py-GC/MS). J Mater Cycles Waste Manag 20(1):176–184. https://doi.org/10.1007/s10163-016-0559-z
Chen H, Blosser GD, Majidzadeh H, Liu X, Conner WH, Chow AT (2018) Integration of an automated identification-quantification pipeline and statistical techniques for pyrolysis GC/MS tracking of the molecular fingerprints of natural organic matter. J Anal Appl Pyrolysis 134:371–380. https://doi.org/10.1016/j.jaap.2018.07.002
Acknowledgements
This work has been supported by Grant RTI2018-097158-B-C31 funded by MCIN/AEI/10.13039/501100011033 and by “ERDF A way of making Europe,” and project ANTROPOCEN@ (PROMETEO/2018/155) funded by Generalitat Valenciana.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Campo, J., Picó, Y. (2022). Thermal Desorption and Pyrolysis Combined with Gas Chromatography–Mass Spectrometry in Food and Environmental Chemistry. In: Picó, Y., Campo, J. (eds) Mass Spectrometry in Food and Environmental Chemistry. The Handbook of Environmental Chemistry, vol 119. Springer, Cham. https://doi.org/10.1007/698_2022_887
Download citation
DOI: https://doi.org/10.1007/698_2022_887
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-19092-6
Online ISBN: 978-3-031-19093-3
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)