Abstract
An increasing number of reports have been published concerning microplastic (MP) pollution in aquatic environments. Methods used in these studies continue to be updated and lack standardization, so that an up-to-date review pertaining methods for MP research is needed. This critical review examines the analytical methods, including sampling, identification, and quantitation, for MP research. Samples are generally collected from water, sediment, and biota gastrointestinal tract. Manta nets or trawls are prevalently used in surface water sampling, while direct shoveling or box-corer grab are commonly applied in sediment sampling. Microplastics in biota are generally obtained by dissecting organisms and separating livers, gills, and guts. Density separation is frequently chosen to separate MPs from sample matrices. Chemical digestion can dissolve other organic materials and isolate MPs for further identification. Visual sorting should be combined with chemical composition analysis to better identify the polymer type. Pyrolysis or thermal decomposition gas chromatography coupled with mass spectrometry, Fourier transform infrared spectroscopy, and Raman spectroscopy are currently the main technologies for MP identification. Units prevalently used to express MP abundance in water, sediment, and biota are “particles per m3,” “particles per m2,” and “particles per individual,” respectively. As MP abundances often varied with the methods used, we recommend that analytical protocols of MPs should better be standardized and optimized. Despite the important progress in analysis of MPs, detection technologies for identifying nano-sized plastic particles are still lacking, and therefore should be developed swiftly.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Ever since plastic particles in the sea were first reported in 1972 (Carpenter and Smith 1972), plastic pollution in aquatic environments has drawn increasing worldwide concern (Thompson et al. 2004). In particular, plastic particles, designated as “microplastics” in 2004, have been the focus of recent research interests (Thompson et al. 2004). Although there is no agreeable standard, microplastics (MPs) are commonly defined as plastic particles with sizes less than 5 mm (Betts 2009; Cole et al. 2011). Browne et al. (2007) defined MPs as plastic particles with particle size ≤ 1 mm, while those smaller than 100 nm have been dubbed “nanoplastics” (Andrady 2011; Koelmans et al. 2015). Potential sources (primary and secondary sources) of MPs are well documented (Andrady 2011; Cole et al. 2011; Duis and Coors 2016). Primary MPs are created as particulates in commercial products in which they are found, typically facial cleansers (Fendall and Sewell 2009). Other manufactured microscopically sized particles such as in drilling fluids and industrial abrasives are also considered primary MPs (Cole et al. 2011). Fragmentation of large plastic debris through physical, chemical, or biological processes can create smaller plastic particles, which are defined as secondary MPs (Andrady 2011; Cole et al. 2011; Duis and Coors 2016). It has remained a challenge to track the transformation of MPs and accurately evaluate MP concentrations in environmental matrices. Analytical methods continue to evolve and need to be standardized eventually.
As an emerging environmental contaminant, MPs pose possible risks to the aquatic ecosystem due to their widespread occurrence and potential biological effects (Barnes 2002). The accumulation of MPs in the marine environment typically starts through estuaries, which receive large amounts of plastics from anthropogenic activities (Browne et al. 2010, 2011). Because most MPs are durable and buoyant, they can transport and persist in the environment for long duration and therefore float to remote open waters, where their presence in marine systems is a global concern (Ballent et al. 2012; Cole et al. 2011). Within the marine environment, MPs occurred in marine organisms (Frias et al. 2014; Watts et al. 2014), beach and benthic sediments (Claessens et al. 2011; Stolte et al. 2015; van Cauwenberghe et al. 2013), and water column (Desforges et al. 2014). Ingestion of plastic particles by fish was first reported decades ago (Carpenter et al. 1972). Attention has been paid to the effects of plastic pellets on aquatic organisms, since plastics were reported to be a vector for toxic chemical transportation in marine environment (Browne et al. 2013; Cole and Galloway 2015; Mato et al. 2001). Physical damage (e.g., esophageal blockage) and chemical hazards (e.g., immobilization of Daphnia magna) of MPs have been widely investigated (Rehse et al. 2016; Rochman et al. 2013b; Tourinho et al. 2010; Wright et al. 2013b). Sorption of persistent organic pollutants (POPs) (Rios et al. 2007; Van et al. 2012) and metals (Rochman et al. 2014; Turner and Holmes 2015) to MPs may aggravate the toxicology of plastic particles, once ingested (Besseling et al. 2013).
To understand better the distribution and effects of MPs, identification and quantification are the main concerns of most studies to date. Visual sorting and instrumental analysis can yield considerably different results. For example, only 20–70% of MPs identified by visual sorting as plastics are typically confirmed as such by instrumental identification (Lenz et al. 2015). Therefore, inaccurate monitoring methods may greatly under/overestimate MP pollution (Eriksen et al. 2013; Hidalgo-Ruz et al. 2012). A lack of standard protocols for MP sampling and analysis leads to unreliable or incomparable data on MP concentrations and polymer compositions among studies (Hidalgo-Ruz et al. 2012). Therefore, an up-to-date and comprehensive evaluation for analytical methods of MPs in the aquatic environment is necessary, which may be useful in support of establishing criteria for effective monitoring and comparison.
Herein we present a critical review of the most commonly used methodologies for sampling, identifying, and quantifying MPs from various environmental media. Based on an integrated assessment of the utility and limitation of these methodologies, areas for improvements are suggested. Small-sized MPs are the most important for future investigations; hence, MPs larger than 5 mm are not discussed further in this review.
Literature assimilation
An extensive literature review was conducted upon database of Web of Science for publications up to 2017 via searching subjects of “microplastic” OR “microplastics” AND “environment.” These literatures were further classified into several topics, such as toxicology, analytical methods for identification and quantification, and environmental matrices (e.g., water, sediment, and biota). The number of publications pertaining MP studies increased rapidly over the last 5 years, especially for analytical methods (Fig. 1).
Literature search results from Web of Science displayed as the number of publications on microplastics from 2013 to 2017, via searching subjects of “microplastic” OR “microplastics” AND “environment.” The published papers were further classified into toxicology, analytical methods for identification and quantification and environmental matrices (e.g., water, sediment, and biota)
Field sampling
As the first step of field research, appropriate sampling methods are needed to provide comprehensive and representative MP samples at the locale of interest. Additionally, aerial fallout may be a source of MPs in the environment (Dris et al. 2016); thus, it is suggested that background contamination should be always taken into consideration during the whole study process. Hidalgo-Ruz et al. (2012) summarized three generally recognized sampling approaches: selective, bulk, and volume-reduced sampling methods for sea surface, water column, and sediment. In the present review, updated sampling methods of MPs in environmental matrices (including water, sediment, and biota) were included and the efficiencies of different sampling methods were compared.
Water samples
Microplastics are generally buoyant and durable, which allow them to float and persist in water. Surface water sampling is prevalently used by researchers to investigate the occurrence of MPs (Eriksen et al. 2013; Yu et al. 2016; Zhao et al. 2014). There is no standardized or specified depth for water sampling, so various sampling depths are used by researchers (Table S1). For instance, using a rotate drum sampler, Ng and Obbard (2006) detected a large number of MP aggregates in the surface microlayer water of Singapore’s marine environment. Song et al. (2014) applied a metal sieve by taping the neuston layers at depth of 150–400 μm, which are typical depths of the microlayer (Cunliffe et al. 2013), to take advantage of surface tension to sample the microlayer water. Water trapped in the mesh of the metal sieve was collected and stored in bottles for further analysis. Using this method, Song et al. (2015b) investigated the distribution of MPs in the seawater microlayer in South Korea, and the MP concentration they detected was much higher than that using other sampling methods (such as trawling and pumping). However, this is a time-consuming method and is more suitable for small-scale surface water sampling rather than for open sea operation.
Given the advantages of large surface areas of open seas and lakes as well as the small volume of final samples, neustonic/manta trawls or nets are prevalently employed for surface water sampling at the depths of 0–0.5 m (Table S1), depending on the submerged height of the trawls used (Anderson et al. 2017; Eriksen et al. 2013; Ivar Do Sul et al. 2014). Almost all surface water samplings with trawls are conducted during research cruises in the sea (Eriksen et al. 2013; Zhang et al. 2017b). The mesh size of trawls ranges from 100 to 500 μm. An obvious technical challenge for trawling is that MPs smaller than the net aperture size may pass through trawls, resulting in underestimation of MP abundance. Because nets with smaller mesh sizes may get clogged with other particulates (e.g., plankton), the most appropriate and widely accepted trawl mesh size for MP sampling is around 300 μm. Particles smaller than 300 μm can be obtained through bulk water sampling using pumps or other techniques (Enders et al. 2015).
Setälä et al. (2016) compared two water sampling methods: trawling (333 μm) and pumping (300 or 100 μm). A higher MP concentration was obtained using a pump with a 100-μm filter than a pump with a 300-μm filter or a trawl. To include plastic samples smaller than a manta trawl mesh size, a submergible pump was used to collect subsurface water at a depth of 4.5 m with a 5-mm aperture size filter (Desforges et al. 2014). They found that size fractions between 100 and 500 μm had the highest MP concentration among all size classes. Similarly, Enders et al. (2015) detected marine MPs down to 10 μm by pumping subsurface water from a depth of 3 m. The greatest number of MPs was observed in the size fraction of 10–20 μm. To prevent clogging, a polytetrafluoroethylene pump was used by Zhao et al. (2014) to collect bulk water samples at 1 m from the water surface, with further filtration in the laboratory using a 32-μm steel sieve. Pumping water from the subsurface may fail to collect MPs floating on the air-water interface.
Although most MPs are buoyant, high-density plastic particles with additives or other attachments tend to sink into deeper aquatic environments. Hence, MPs in sediment and benthic community are also widely studied (van Cauwenberghe et al. 2013). Taking surface water alone may underestimate the abundance of MPs, although the MP concentration gradient decreases exponentially with increasing water depth (Reisser et al. 2015). Doyle et al. (2011) sampled water at 212 m under the water surface, which required specialized and expensive equipment, but plastic particles (> 0.5 mm) were detected at such depth from only one of the six cruises. To lower the cost for deep-sea MP monitoring, a novel autonomous sensor was developed by Edson and Patterson (2015) for in situ measurement of MPs in the deep water column. This sensor was able to continuously collect seawater from 28 discrete temporal sampling points and measure salinity and temperature at the same time. To improve the reliability of deep-sea sampling, more applicable technologies (e.g., remote sensors) should be developed.
In conclusion, manta trawls or nets are strongly recommended for large-scale surface water sampling in lakes or seas, as they can filter a large quantity of water to collect floating MPs. The aperture size, trawling speed, and sampling time should be standardized, so as to acquire comparable data from different locations. Galgani et al. (2013) recommended a trawl with mesh size of 333 μm and a duration of 30 min for surface seawater sampling. Additionally, a lightweight coarse mesh aluminum cover may strengthen the trawls and prevent them from being broken after a long sampling time. To include MPs sized smaller than the net mesh size, pumping of bulk water is also suggested to complement trawling.
Porous media samples
Porous media can either be sampled for MPs from the surficial layer of coastal/lakeshore beaches or from the seafloor/lake bottom. Because sampling on beaches is easy and convenient, sampling along shorelines has been heavily used worldwide to monitor MP contamination, such as on the coastlines of Singapore (Ng and Obbard 2006), the Tamar Estuary in the UK (Browne et al. 2010), the Belgian coast (Claessens et al. 2011), the German North Sea (Dekiff et al. 2014), Hong Kong (Fok and Cheung 2015), the Bohai Sea (Yu et al. 2016), and even a remote lake in China (Zhang et al. 2016). However, there is no standardized procedure for beach sampling, for which criteria such as tidelines, sampling depth, and sampling volume/area varied (Table 1). Some researchers randomly selected sampling sites on the beach (Nuelle et al. 2014), while others collected samples by tide (Mathalon and Hill 2014). For the latter, the high strandline or tideline was the most favored beach sampling site. Claessens et al. (2011) demonstrated that the higher water marks might accumulate more MPs than the lower ones.
A stainless steel tool (e.g., shovel and spoon), coupled with latex gloves and cotton clothes, is often used to minimize procedural contamination in beach sampling. The sampling depth varied among studies, with the first 5 cm being the most common (Hidalgo-Ruz et al. 2012). A quadrat (sampling area) of typically but not always 25 cm2 (Table 1) is frequently used. Either many such squares are sampled, evenly dispersed along the coastline (Van et al. 2012), or a few representative quadrats are selected (Liebezeit and Dubaish 2012). While bulk sampling of beaches may better reflect the occurrence of MPs than representative squares, the workload to characterize bulk samples can be enormous (Nel and Froneman 2015).
With accumulation of biofilms, floating low-density MPs will start to sink, and may end up at the seafloor/lake bottom sediment along with high-density MPs. Woodall et al. (2014) reported that the deep sea might be a major sink for plastics. For sediment sampling, the most frequently used tool is box-corer grab (Vianello et al. 2013), and petite Ponar grabs also have been used in rivers (Castañeda et al. 2014). Superficial (0–10 cm) sediments are generally collected in freshwater, and the top sediment layer (~ 10 cm) is collected from the continental shelf, at a depth of 3500 m (Woodall et al. 2014). A sediment core with a surface area of 25 cm2 was collected and the top 1 cm was separated for further analysis of MPs (van Cauwenberghe et al. 2013). Turner and Holmes (2015) sampled the top 10 cm of a river sediment core representing sediment accumulation of approximately the most recent 10 years, as radiometric chronology indicated sediment accumulation rates of 0.8–1.6 cm year−1.
With constant deposition of particulate matter and fluctuation of near-bottom and pore water, MPs can be accumulated in sediment with depth variability. Thus, stratified sediment samples collected with cores may be used to reconstruct the depositional history of MPs (Hidalgo-Ruz et al. 2012). Carson et al. (2011) reported that the top 15 cm accounted for 95% of the total detected MPs, with more than half in the top 5 cm. The lack of a standardized protocol for sediment sampling would result in difficulties in comparing studies using different sampling methods and parameters. In addition, the geochronology of sediment should be taken into account when considering the sediment sampling depth, because MPs in sediment can be transported by bioturbation (e.g., activity of crabs, worms, and other benthic organisms). Currently, surface sediment sampling can only obtain data on recent MP contamination, but no information about accumulation patterns and/or potential sources. Future studies on MP abundance in sediment should also provide geochronology from radionuclides, contaminant profiles, or other means, which can indicate the sediment accumulation rate. Besides, to ensure the representability, at least five sample replicates (5 m between replicates) should be collected from the target strandline (Galgani et al. 2013).
Biota samples
As MPs can be ingested by organisms, biota samples can be used to monitor MP contamination in aquatic environments. The most frequently monitored biota are fish (Peters and Bratton 2016), sea turtles (Wedemeyer-Strombel et al. 2015), seabirds (e.g., fulmars) (van Franeker et al. 2011), bivalves (Li et al. 2015), marine worms (Wright et al. 2013a), and plankton (Setälä et al. 2014). Some biota are sampled from the wild environment, while others are from farmed specimens. In an attempt to determine the effect of the inhabited environment, Davidson and Dudas (2016) found no significant difference between ingested MP abundances in wild versus farmed clams. Generally, biota is dissected to collect MPs from various tissues and organs. Depending on organism size, the digestive tract can be separated and stored for large individuals, while small individuals can be analyzed entirely (Galgani et al. 2013). Unsurprisingly given their recalcitrance, MPs are often found in digestive systems, which are separated for further treatment and extraction.
In addition, it remains a challenge to obtain a large number of biota samples, especially for top predators, which may hinder accurate measurements of MP contamination in organisms. For example, Wedemeyer-Strombel et al. (2015) found no anthropogenic debris in two leatherback sea turtles. Given the small sample number, it is not at all certain that leatherback sea turtles are free of MP contamination. Considering this scenario, foods ingested by organisms that cannot be sampled in large quantity can be used to indicate any potential MP contamination.
Separation and purification
Flotation
Density separation is widely used to isolate low-density particles from higher-density sand, mud, sediment, and other sample matrices (Dillon 1964). Many MPs, such as polypropylene (PP) and polyethylene (PE), have lower densities than sea water (~ 1.10 g cm−3). High-density plastics, e.g., polyvinyl chloride (PVC), have densities up to 1.40 g cm−3 or greater depending on additives and attached biofilms. Various high-density solutions have been employed to isolate MPs from environmental matrices (Table 2). The most favored solution is saturated NaCl, which is cheap and nonhazardous and has a density of approximately 1.20 g cm−3 (Fries et al. 2013). The drawback of NaCl solution is that some plastic particles (e.g., PVC) with density higher than 1.20 g cm−3 may not be completely extracted, even though Browne et al. (2010) extracted high-density microplastics using saturated NaCl solution. To overcome this deficit, Nuelle et al. (2014) developed a two-step method, using the air-induced overflow with NaCl solution for pre-extraction and NaI solution (1.80 g cm−3) for further flotation. Recovery rates ranged from 67% (expended polystyrene, EPS) to 99% (PE) for different plastic classifications (Nuelle et al. 2014). Another widely used flotation solution is ZnCl2 (1.50–1.70 g cm−3), which can extract almost all MPs (Liebezeit and Dubaish 2012) but is relatively toxic. Although a range of solutions are used, general procedures for density separation are similar. Briefly, the sample matrix is mixed with a flotation solution, typically by shaking to homogenize the slurry. This slurry is then allowed to sit for several hours so that denser materials (e.g., sand) are settled. Supernatant factions are normally collected for further analysis. To improve extraction efficiency, the solution above the sediment or all solution is subsequently separated by filtration (Stolte et al. 2015; Zobkov and Esiukova 2017a). Sequential extraction was also suggested to improve efficiency (Hidalgo-Ruz et al. 2012). Besides these density solutions, oil can also be used to extract MPs from environmental samples, and an average recovery of 96.1% (± 1.4%) was reported by Crichton et al. (2017).
Technical instruments have been designed for extraction by density separation. Recovery rates for MPs were up to 98 and 100% using an elutriation column and a Munich Plastic Sediment Separator, respectively (Claessens et al. 2013; Imhof et al. 2012). Following the research by Imhof et al. (2012), Zobkov and Esiukova (2017b) evaluated the efficiency of Munich Plastic Sediment Separator for extracting MPs from bottom sediment in the Baltic Sea, and high efficiencies (97.1 ± 2.6%) were achieved. Additionally, Fuller and Gautam (2016) successfully applied pressurized fluid extraction to extract MPs from other matrices, such as glass beads, soil, and municipal waste. However, the recovery decreased with decreasing plastic particle size, which was 97.9% for 30 μm PS and 93.6% for 10 μm PS particles (Claessens et al. 2013). More efficient instruments or solutions should be developed to achieve better recovery with low cost and environmentally friendly consequences. Because most MPs are hydrophobic, surfactants can be used to separate MPs from water (Shen et al. 2002). A combination of flotation and other assistant extraction methods is suggested for future research.
Sieving and filtration
Generally, stainless steel sieves or glass fiber filters instead of plastic tools are used to minimize procedural contamination, and rinsing is always required after each sieving or filtration. A wet sieving process was suggested by Masura et al. (2015), who poured samples through a 5.6-mm stainless steel mesh sieve and then a 0.3-mm sieve. With this step, target MPs were separated for further sorting and identification. To minimize clogging of sieve apertures, a medium coarse mesh sieve of 1 mm is suggested and typically used (Nuelle et al. 2014, van Cauwenberghe et al. 2013). A 500-μm sieve is often used to obtain size fractions of > 500 and < 500 μm. Such fractionation is reasonable, because MPs in the > 500 μm fraction can be identified visually (Hidalgo-Ruz et al. 2012). Other studies employed fine aperture sieves or filters (e.g., 38, 65, and 63 μm) directly to retain target MPs (Claessens et al. 2011; Nel and Froneman 2015; Vianello et al. 2013). Disadvantages of this sieving method include, but are not limited to, easy blocking of sieve holes, difficulty in obtaining wide-range size fractions, and lengthy sample processing time. To save filtration time, stacks of sieves or filters with different aperture sizes may be used to fractionate MPs. Wu et al. (2016) fractionated paint flakes in sediment into four sizes, i.e., < 30, 30–63, 63–200, and 200–2000 μm. This size fraction method is also suitable for MPs.
Size fractionation has varied in different studies. Lambert and Wagner (2016) divided MP samples into three size fractions with a 1-mm sieve and a filter paper of 10-μm pore size. Mathalon and Hill (2014) sieved MPs smaller than 500 μm into several fractions using a series of sieves (500, 355, 250, 150, 106, and 63 μm), yielding detailed size dependency of MPs. However, more fractions may mean less abundant MPs in each fraction. A standardized series of reasonable size fractions is urgently needed to better compare the occurrence of MPs from the same size fraction. Browne et al. (2010) reported that MPs smaller than 1 mm accounted for 65% of the total detected plastic items in sediment along the estuarine shorelines in the UK. A study on the ingestion of MPs by biota found that MPs with sizes smaller than 250 μm amounted to 17−79% of the total (Li et al. 2016). However, accurately identifying and quantifying MPs remaining on the filter paper remains a challenge.
Purification
Organic matter attached to the surface of MPs in environmental samples should be removed to allow clear identification of the type of plastics. A solution of 30% H2O2 has been frequently used for that purpose (Liebezeit and Dubaish 2012; Mathalon and Hill 2014; Stolte et al. 2015; Zhao et al. 2014). Nuelle et al. (2014) demonstrated that 35% H2O2 performed better than other solutions (e.g., 30% H2O2, 20% HCl, and 20–50% NaOH) in dissolving organic matter, although it could cause color fading on MPs. Enzymatic digestion has also been used to dissolve biogenic matter from environmental MPs (Cole et al. 2014). For biota samples, 10% KOH solution has been applied to isolate MPs from the contents of digestive tracts (Foekema et al. 2013; Zhang et al. 2017a). Collard et al. (2015) also successfully extracted MPs by immersing fish stomach extracts in 9% NaClO and then further treating the filtrate with a mixture of NaClO and 65% HNO3. To accelerate the digestion process, Roch and Brinker (2017) developed a novel procedure combining NaOH and HNO3 to yield recovery rates higher than 95% with few changes to the characteristics of MPs isolated. High recovery rates (93 ± 10%) of MPs in the soft tissues of mussels were achieved with an enzyme digestion method (Catarino et al. 2017).
Purification is the key to accurate identification of MPs. Although the effects of these solutions on MP characteristics have been demonstrated, potential influences on organic chemicals affiliated to MPs remain unknown. Future efforts should be directed toward the effects of pretreatment on environmental MPs. Without successful pretreatment, the recovery rate of MPs may be lower than predicted. Multi-step pretreatments are suggested to ensure complete removal of biofilms and other organic materials attached on the surfaces of MPs. For example, proteinase K and H2O2 are capable of dissolving biofilms and organic materials, while a mixture of H2O2 and FeSO4 in Fenton is often used to oxidize organic compounds (Anderson et al. 2017; Masura et al. 2015). Because MPs are a good sorbent for POPs, Fenton-based treatment would destroy POPs in MPs, undermining any attempt to measure POPs affiliated with MPs. This can be circumvented by cleaning of MPs with distilled water when POPs associated with MPs are measured (Van et al. 2012).
Identification
Visual sorting
After separation and purification, target MPs need to be sorted from the remaining matrix. Large plastics can be sorted out directly, while smaller-sized ones need further observation under a dissection microscope (typically a stereomicroscope) after the samples are dried under cover in an oven, generally at 60 °C (de Carvalho and Baptista Neto 2016; Fok and Cheung 2015; Mathalon and Hill 2014). Potential target MPs are thus magnified, sorted, and counted. Norén (2007) suggested criteria for visual sorting of MPs, while this method is suitable for the sorting of large MPs; thus, it is not always applicable and is necessary to use other instrumental techniques (such as FT-IR and Raman spectroscopy) for further identification (Cheung et al. 2016). Lenz et al. (2015) reported that only 68% of visually sorted particles were MPs after identification by Raman spectroscopy, and the success rates of different particles or fibers were dependent on the particle color (Fig. 2). Fibers are easier to be identified than particles, with higher success rates were obtained for fibers (Lenz et al. 2015).
Percentage of plastics with different colors identified by Raman spectroscopy in the total potential plastics after visually sorting. The percentage is shown as success rate. Data were adopted from Lenz et al. (2015)
Visual identification may not be accurate enough in this case and may under- or overestimate the abundance of MPs. Although visual sorting is a time-saving method for enumeration of MPs, more reliable technologies are urgently desirable for evaluating the abundance and chemical composition of MPs (Table S2). Song et al. (2015b) found that more MP particles were observed under FT-IR than using an optical microscope. Thus, the number of plastic particles may vary with different methods, e.g., Pyr-GC/MS, TDS-GC/MS, FT-IR, and Raman spectroscopy.
Pyrolysis-GC/MS and TDS-GC/MS
A key benefit of Pyr-GC/MS is that it can simultaneously analyze both the polymer type and organic additives of MPs. Upon being extracted from environmental matrices, plastic particles are thermally deconstructed before the polymer composition of each particle is determined with GC/MS (Fries et al. 2013). This technique allows only one particle at a time to go through the pyrolysis tube, which is both time-consuming and limited by the aperture size of the tube (< 1 mm). The TDS-GC/MS method has been used in analysis of MP composition in environmental samples (Dümichen et al. 2015; Dekiff et al. 2014). Compared with Pyr-GC/MS, TDS-GC/MS can process larger sample mass and measure more complex matrices (Dümichen et al. 2015).
To identify and quantify trace amounts of MPs in environmental samples with Pyr-GC/MS, Fischer and Scholz-Böttcher (2017) employed characteristic fragment ions from each type of plastic as markers. No pre-sorting was required, and the data were robust as r2 was 0.86–0.99 for the calibration curve (Fischer and Scholz-Böttcher 2017). However, this approach could yield only the mass of each type of MPs but not the particle number count. FT-IR or Raman spectroscopy may be an alternative for MP determination without destroying target particles. Pyr-GC/MS or TDS-GC/MS may be complementary with FT-IR or Raman spectroscopy for comprehensive analysis of MPs (Fischer and Scholz-Böttcher 2017).
FT-IR spectroscopy
FT-IR and its optimized technologies, such as micro FT-IR (Vianello et al. 2013), attenuated total reflectance (ATR) FT-IR (Cheung et al. 2016), and focal plane array detector-based micro FT-IR imaging (Löder et al. 2015), are also used in MP studies. Harrison et al. (2012) compared reflectance micro FT-IR and ATR FT-IR in analysis of polyethylene MPs. Both showed satisfactory performance in identifying polymer compositions (Harrison et al. 2012). ATR FT-IR was better in obtaining spectra of MPs with irregular shapes than micro FT-IR, but was only suitable for analyzing particles larger than 500 μm (Löder and Gerdts 2015). To deal with this issue, Löder et al. (2015) applied focal plane array-based micro FT-IR imaging to determine MPs in environmental samples. Compared to traditional FT-IR, this technology can detect plastic particles down to 20 μm in size and cover a large filter surface area (> 10 mm diameter) (Löder et al. 2015).
Using FT-IR at a resolution of 8 cm−1, Song et al. (2015a) identified and counted MP particles directly on filter paper after drying at 60 °C. Löder et al. (Löder and Gerdts 2015) suggested that a resolution of 8 cm−1 was optimal for micro FT-IR, to produce high-quality data and save measurement time. This resolution was also applied by Vianello et al. (2013) to determine MPs from the bottom sediment of Lagoon of Venice, Italy. Nevertheless, it remains a challenge to apply FT-IR in analyzing ultra-fine plastic particles (e.g., particle sizes < 1 μm) and classifying polymer type from complex environmental samples. Although manual sorting is also required before FT-IR analysis (Claessens et al. 2011), FT-IR is a promising technology but further optimization is required for accurate MP analysis.
Raman spectroscopy
Raman spectroscopy is another promising analytical technique frequently used for MP detection (van Cauwenberghe et al. 2013; Zhang et al. 2016). The main benefits of Raman spectroscopy are that small particles down to 1 μm can be examined and that it has better responses to non-polar plastic functional groups than other analytical methods (Lenz et al. 2015). To minimize false signals with Raman micro-spectrometry, rigorous sample purification is strongly recommended. A suitable wavelength for Raman laser spectroscopy is essential for balancing the enhancement of signal intensity and suppression of sample fluorescence (Löder and Gerdts 2015). In addition, a typical Raman laser spectrometer is much more expensive than a FT-IR, e.g., USD 250,000 versus USD 50,000.
Aside from the three most popular technologies (Pyr-GC/MS or TDS-GC/MS, FT-IR, and Raman spectroscopy), time-of-flight secondary ion mass spectrometry (TOF-SIMS)-based analysis and imaging has also been used for MP detection (Jungnickel et al. 2016). This method can directly detect plastic particles smaller than 10 μm in sea sand with a fragment ion of m/z 113, which showed sufficient power to discriminate polyethylene from environmental matrices (Jungnickel et al. 2016). However, fragment ions from other plastics may overlap with those from environmental matrices, making it difficult to distinguish them. An optimized analytical procedure is still urgently needed to conquer the drawbacks of the methods discussed above, and simultaneously provide comprehensive images of polymer types and numbers in a broad particle size range. Recently, Shim et al. (2016) successfully applied a Nile Red staining method for MP detection in organics-rich samples and achieved a recovery of 98%. This method can also be used for MP quantification following identification (Maes et al. 2017).
Quantification
Quantitative data are needed to illustrate the abundance of MPs in environmental matrices. Upon identification, plastic particles are either counted manually with the assistance of microscope or weighted with a scale. The concentration units of “items/particles per m2” and “items/particles per m3” are most commonly used to characterize MPs in surface water sampled by trawling, while MP concentrations in water column or bulk surface water using various aperture size cutoffs are usually quantified as “items/particles per m3” (Table S2). For sediment samples, sampling quadrate areas are calculated to present MP concentrations as “items/particles per m2” and “grams/mg per m2.” “items/particles per kilogram dry/wet weight” and “items/particles per m2 sampling area” are also commonly used for sediment samples. If the shape of sampling area and sampling depth are standardized, sediment MP concentrations may be easily converted from per square meter to per cubic meter. Numbers of plastic particles in sea water (167 ± 138 items per thousand m3) are typically much smaller than those in nearby estuarine water (4.14 ± 2.46 × 103 items per m3) (Zhao et al. 2014). As noted previously, an estuarine environment is often impacted by heavy anthropogenic activities from inland (Zhao et al. 2015). To better understand the origins and sources of MPs, more efforts should be directed toward terrestrial environments, as a large portion of land-derived MPs will end up in the oceans. Abundances of MPs ranged from 97 ± 208 items per hundred kg dry sediment in beach sediment of the northern South China Sea (Peng et al. 2017) to 49,600 items per kg dry sediment in the beaches of the East Frisian islands (Liebezeit and Dubaish 2012). When calculated as items per square meter, the number of sediment MP particles ranged from 12 items in beaches of Guanabara Bay of Southeast Brazil (de Carvalho and Baptista Neto 2016) to 13.8 ± 13.7 × 103 items in the St. Lawrence River of Canada (Castañeda et al. 2014). These values are much greater than those in surface water, ranging from 5 (0–17) items per km2 in the northern South China Sea (Zhou et al. 2011) to 0.055–34.2 × 105 items per m2 of water surface in Xiangxi Bay of the Three Gorges Reservoir in China (Zhang et al. 2017a). Higher MP concentrations in sediment than in water may be resulted from accumulation of MPs in sediment through regular tides. The variability of MP abundances in different regions is derived not only from actual environmental factors (e.g., extent of contamination and population difference) but also from sampling and processing issues such as different filtration pore sizes as previously discussed.
The abundance of MPs may vary with sampling sites, which is not necessarily reflective of the magnitudes of MP pollution, but may also be related to the pore sizes of sampling tools. For instance, sampling in North East Pacific Ocean, Desforges et al. (2014) observed much higher MP abundance using filtration pore size of 0.062 mm than that obtained by Doyle et al. (2011) using a trawl size of 0.5 mm (Table S1). Besides, some studies also collected both coastline beach sediment and adjacent water samples simultaneously. As such, it is not surprising that abundances of MPs were cohesive in beach and adjacent water samples (Table 3). Discharge and washout from terrestrial environments are most likely the main sources of MPs to the water. Jambeck et al. (2015) estimated that 4.80–12.7 million metric tons of plastic debris entered the oceans from land in 2010. They also predicted that this number might increase tenfold by 2025. It is thus important to monitor the riverine flux of MPs to the oceans, and establishing a uniform and convertible set of metrics to quantify MP abundance in environmental matrices will definitely help.
Microplastics tend to transfer in the aquatic food web (Setälä et al. 2014), as larger biota are shown to accumulate more MPs than smaller ones (Fig. 3). Although they are not in the same food chain, the result can be an indication for bioaccumulation tendency of MPs. The highest average abundance of MPs was found in a whale with 88 items per individual (Lusher et al. 2015), which may be attributed to the large quantity of food in the whale’s diet. Approximately 50 fish were found in the digestive tract of this whale, indicating that MPs accumulated in the whale may have partially originated from these fish that may have ingested MPs themselves. Because most plastic wastes are generated inland, discharged to the marine environment, and gradually dispersed to remote oceans, semi-pelagic fish such as the bogue (Boops boops) are more closely associated with MPs than pelagic fish (e.g., swordfish), with an average abundance per fish of four items (Nadal et al. 2016) and one item (Romeo et al. 2015), respectively. Because there is no significant correlation between biota weight or size and the number of plastic particles present (Foekema et al. 2013; Tourinho et al. 2010), we suggest the use of items per individual as the unit of MP abundance in biota samples.
Summarized numbers (average ± SD) of plastic items per individual organism from literature surveys. Data were adopted from the studies of Kaposi et al. (2014) for marine larvae; Devriese et al. (2015) for shrimp; Li et al. 2015, 2016) for bivalves; Possatto et al. (2011), Romeo et al. (2015), Miranda and de Carvalho-Souza (2016), Nadal et al. (2016), and Peters and Bratton (2016) for fish; Tourinho et al. (2010) and van Franeker et al. (2011) for seabirds; Barnes (2002) and Tourinho et al. (2010) for sea turtles; and Lusher et al. (2015) for a whale
Manual counting of MPs is time-consuming, so automatic counting software coupled with identification techniques should be developed for the quantification of MPs. A novel method using Nile Red staining for MP quantification is promoted, which can rapidly detect small MPs (down to 20 μm), and the capability of this method has been demonstrated (Erni-Cassola et al. 2017; Maes et al. 2017). In addition, the units of weight and count are suggested to be standardized or exchangeable for comparison of MP abundances between studies.
Quality assurance and quantity control
During the whole sample process, quality assurance and quantity control (QA/QC) is essential and of great importance for data accuracy. In field sampling, procedural blanks (containing pure water) and spiked blanks (containing pure water and a certain amount of MPs of known composition and abundance) should be prepared (Galgani et al. 2013). Non-plastic sampling tools, latex gloves, and cotton clothes should be used during sampling. During the extraction process in laboratory, recovery rates of standard MPs (with the similar sizes and classifications of field samples) using the applied extraction method should always be provided (Stolte et al. 2015). Since there are some synthetic fibers in the atmospheric fallout (Dris et al. 2016), blanks and inter-laboratory tests can be performed to minimize the effect of the experimental environment. In addition, Zobkov and Esiukova (2017a) suggested that an internal standard and occasional empty runs can ensure the reliability of extraction efficiency in processing sediment samples. For MP identification, reference data of standard MPs from the instrument data base should be used to compare with field collected MPs (Ng and Obbard 2006). When analyzing MP samples from heavy contaminated sites (more than thousands of particles), it is hard to count all particles one by one. Grouping/pooling is always performed to roughly estimate the total MP concentration, and at least 10% of particles should be instrumentally identified and counted (Mahon et al. 2017). Besides, the percentage of identified MPs in the total suspected particles should also be provided; otherwise, the concentration of MPs would be overestimated.
Recommendations
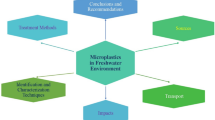
This review has provided an overview of MP detection in aquatic environments (Fig. 4), and discussed numerous methods used in sampling, extraction, identification, and quantification of MPs. Existing data are often incomparable because of the various approaches used. To better compare MP contamination worldwide, procedures and methodologies should be standardized so that they are practical over the entire analytical process. Amid the limitations of existing methods and technologies that have been discussed in this review, recommendations are given as follows.
-
a)
For sediment sampling, selection of sampling sites is of high importance to provide truly representative MP contamination in the target location. The sampling depth and location (e.g., high, mid, or low tide line) should be specified and standardized for sediment sampling.
-
b)
Manta trawls or nets are strongly recommended for large-scale surface water sampling in lakes or seas, since they can filter a large quantity of water to collect floating MPs during sampling. To include size range of MPs smaller than the net mesh size, pumping of bulk water is also suggested to complement trawling.
-
c)
For biota sampling, a certain food web of samples should be better collected, as done in chemical contaminant studies (Kidd et al. 1995; Xu et al. 2014).
-
d)
Density separation is still strongly recommended to float MPs from sediment samples. Technologies (e.g., separator) based on density separation or assistant reagents (e.g., surfactants) complementary to density separation should be promoted.
-
e)
During the purification stage, 30% H2O2 solution combined with FeSO4 (Fenton) is highly recommended to remove organic matter. The effects of purification solutions on other target organic compounds should be fully investigated in future research.
-
f)
It remains a challenge to identify plastic particles with sizes smaller than 1 μm. This is the limit of a visual microscope and is necessary for sorting purposes before instrumental identification. Complementary technologies, such as X-ray diffraction, X-ray photoelectron spectroscopy, and energy disperse spectroscopy, are encouraged to be used for small particle identification.
-
g)
Manual counting is generally used for MP quantification; however, this method is time-consuming. Automated quantification technology (e.g., flow cytometry and imaging technologies coupled with statistical analysis) could be adapted to efficiently obtain the concentrations of MPs in environmental samples. Dynamic light scattering is suggested to provide the size distribution of MPs, although nano-sized MPs will be difficult to detect using this technique against the scattering provided by much larger MPs.
Given the current knowledge discussed in this review, efficient detection methods could become a solid foundation for research to understand MP distributions worldwide. Because plastic wastes have been classified as hazardous materials (Rochman et al. 2013a), policies should be built to better manage plastic pollution (Tibbetts 2015). This would rely on the availability of more comparable and robust data on the distribution, fate, transport, and effects of MPs in the marine environment. With such efforts, contamination of MPs may be reduced or eliminated.
References
Anderson PJ, Warrack S, Langen V, Challis JK, Hanson ML, Rennie MD (2017) Microplastic contamination in Lake Winnipeg, Canada. Environ Pollut 225:223–231
Andrady AL (2011) Microplastics in the marine environment. Mar Pollut Bull 62:1596–1605
Ballent A, Purser A, Mendes PDJ, Pando S (2012) Physical transport properties of marine microplastic pollution. Biogeosci Discuss 4:54–56
Barnes DKA (2002) Invasions by marine life on plastic debris. Nature 416:808–809
Besseling E, Wegner A, Foekema EM, Koelmans AA (2013) Effects of microplastic on fitness and PCB bioaccumulation by the lugworm Arenicola marina (L.) Environ Sci Technol 47:593–600
Betts K (2009) Why small plastic particles may pose a big problem in the oceans. Environ Sci Technol 42:8995–8995
Browne MA, Galloway T, Thompson R (2007) Microplastic—an emerging contaminant of potential concern? Integr Environ Assess Manag 3:559–561
Browne MA, Galloway TS, Thompson RC (2010) Spatial patterns of plastic debris along estuarine shorelines. Environ Sci Technol 44:3404–3409
Browne MA, Crump P, Niven SJ, Teuten E, Tonkin A, Galloway T, Thompson R (2011) Accumulation of microplastic on shorelines woldwide: sources and sinks. Environ Sci Technol 45:9175–9179
Browne MA, Niven SJ, Galloway TS, Rowland SJ, Thompson RC (2013) Microplastic moves pollutants and additives to worms, reducing functions linked to health and biodiversity. Curr Biol 23:2388–2392
Carpenter EJ, Smith KL (1972) Plastics on the Sargasso Sea surface. Science 175:1240–1241
Carpenter EJ, Anderson SJ, Harvey GR, Miklas HP, Peck BB (1972) Polystyrene spherules in coastal waters. Science 178:749–750
Carson HS, Colbert SL, Kaylor MJ, McDermid KJ (2011) Small plastic debris changes water movement and heat transfer through beach sediments. Mar Pollut Bull 62:1708–1713
Castañeda RA, Suncica A, Anouk S, Anthony R (2014) Microplastic pollution in St. Lawrence River sediments. Can J Fish Aquat Sci 71:21–40
Catarino AI, Thompson R, Sanderson W, Henry TB (2017) Development and optimization of a standard method for extraction of microplastics in mussels by enzyme digestion of soft tissues. Environ Toxicol Chem 36:947–951
Cheung PK, Cheung LTO, Fok L (2016) Seasonal variation in the abundance of marine plastic debris in the estuary of a subtropical macro-scale drainage basin in South China. Sci Total Environ 562:658–665
Claessens M, Meester SD, Landuyt LV, Clerck KD, Janssen CR (2011) Occurrence and distribution of microplastics in marine sediments along the Belgian coast. Mar Pollut Bull 62:2199–2204
Claessens M, Van Cauwenberghe L, Vandegehuchte MB, Janssen CR (2013) New techniques for the detection of microplastics in sediments and field collected organisms. Mar Pollut Bull 70:227–233
Cole M, Galloway TS (2015) Ingestion of nanoplastics and microplastics by Pacific oyster larvae. Environ Sci Technol 49:14625–14632
Cole M, Lindeque P, Halsband C, Galloway TS (2011) Microplastics as contaminants in the marine environment: a review. Mar Pollut Bull 62:2588–2597
Cole M, Webb H, Lindeque PK, Fileman ES, Halsband C, Galloway TS (2014) Isolation of microplastics in biota-rich seawater samples and marine organisms. Sci Rep 4:2231–2236
Collard F, Gilbert B, Eppe G, Parmentier E, Das K (2015) Detection of anthropogenic particles in fish stomachs: an isolation method adapted to identification by Raman spectroscopy. Arch Environ Contam Toxicol 69:331–339
Corcoran PL, Biesinger MC, Grifi M (2009) Plastics and beaches: a degrading relationship. Mar Pollut Bull 58:80–84
Crichton EM, Noël M, Gies EA, Ross PS (2017) A novel, density-independent and FTIR-compatible approach for the rapid extraction of microplastics from aquatic sediments. Anal Methods 9:1419–1428
Cunliffe M, Engel A, Frka S, Gasparovic B, Guitart C, Murrell J, Salter M, Stolle C, Upstill-Goddard R, Wurl O (2013) Sea surface microlayers: a unified physicochemical and biological perspective of the air-ocean interface. Prog Oceanogr 109:104–116
Davidson K, Dudas SE (2016) Microplastic ingestion by wild and cultured Manila clams (Venerupis philippinarum) from Baynes Sound, British Columbia. Arch Environ Contam Toxicol 71:147–156
de Carvalho DG, Baptista Neto JA (2016) Microplastic pollution of the beaches of Guanabara Bay, Southeast Brazil. Ocean Coast Manag 128:10–17
Dekiff JH, Remy D, Klasmeier J, Fries E (2014) Occurrence and spatial distribution of microplastics in sediments from Norderney. Environ Pollut 186:248–256
Desforges J-PW, Galbraith M, Dangerfield N, Ross PS (2014) Widespread distribution of microplastics in subsurface seawater in the NE Pacific Ocean. Mar Pollut Bull 79:94–99
Devriese LI, van der Meulen MD, Maes T, Bekaert K, Paul-Pont I, Frère L, Robbens J, Vethaak AD (2015) Microplastic contamination in brown shrimp (Crangon crangon, Linnaeus 1758) from coastal waters of the Southern North Sea and Channel area. Mar Pollut Bull 98:179–187
Dillon WP (1964) Flotation technique for separating fecal pellets and small marine organisms from sand. Limnol Oceanogr 9:601–602
Doyle MJ, Watson W, Bowlin NM, Sheavly SB (2011) Plastic particles in coastal pelagic ecosystems of the Northeast Pacific Ocean. Mar Environ Res 71:41–52
Dris R, Gasperi J, Saad M, Mirande C, Tassin B (2016) Synthetic fibers in atmospheric fallout: a source of microplastics in the environment? Mar Pollut Bull 104:290–293
Duis K, Coors A (2016) Microplastics in the aquatic and terrestrial environment: sources (with a specific focus on personal care products), fate and effects. Environ Sci Eur 28:1–25
Dümichen E, Barthel A-K, Braun U, Bannick CG, Brand K, Jekel M, Senz R (2015) Analysis of polyethylene microplastics in environmental samples, using a thermal decomposition method. Water Res 85:451–457
Edson EC, Patterson MR (2015) MantaRay: a novel autonomous sampling instrument for in situ measurements of environmental microplastic particle concentrations, OCEANS 2015 - MTS/IEEE Washington, pp 1–6
Enders K, Lenz R, Stedmon CA, Nielsen TG (2015) Abundance, size and polymer composition of marine microplastics ≥10 μm in the Atlantic Ocean and their modelled vertical distribution. Mar Pollut Bull 100:70–81
Eriksen M, Mason S, Wilson S, Box C, Zellers A, Edwards W, Farley H, Amato S (2013) Microplastic pollution in the surface waters of the Laurentian Great Lakes. Mar Pollut Bull 77:177–182
Erni-Cassola G, Gibson MI, Thompson RC, Christie-Oleza J (2017) Lost, but found with Nile red; a novel method to detect and quantify small microplastics (20 μm-1 mm) in environmental samples. Environ Sci Technol 51:13641–13648
Fendall LS, Sewell MA (2009) Contributing to marine pollution by washing your face: microplastics in facial cleansers. Mar Pollut Bull 58:1225–1228
Fischer M, Scholz-Böttcher BM (2017) Simultaneous trace identification and quantification of common types of microplastics in environmental samples by pyrolysis-gas chromatography-mass spectrometry. Environ Sci Technol 51:5052–5060
Fischer EK, Paglialonga L, Czech E, Tamminga M (2016) Microplastic pollution in lakes and lake shoreline sediments—a case study on Lake Bolsena and Lake Chiusi (central Italy). Environ Pollut 213:648–657
Foekema EM, De GC, Mergia MT, van Franeker JA, Murk AJ, Koelmans AA (2013) Plastic in north sea fish. Environ Sci Technol 47:8818–8824
Fok L, Cheung PK (2015) Hong Kong at the Pearl River Estuary: a hotspot of microplastic pollution. Mar Pollut Bull 99:112–118
Frère L, Paulpont I, Rinnert E, Petton S, Jaffré J, Bihannic I, Soudant P, Lambert C, Huvet A (2017) Influence of environmental and anthropogenic factors on the composition, concentration and spatial distribution of microplastics: a case study of the Bay of Brest (Brittany, France). Environ Pollut 225:211–222
Frias JPGL, Otero V, Sobral P (2014) Evidence of microplastics in samples of zooplankton from Portuguese coastal waters. Mar Environ Res 95:89–95
Fries E, Dekiff JH, Willmeyer J, Nuelle M-T, Ebert M, Remy D (2013) Identification of polymer types and additives in marine microplastic particles using pyrolysis-GC/MS and scanning electron microscopy. Environ Sci Process Impacts 15:1949–1956
Fuller S, Gautam A (2016) A procedure for measuring microplastics using pressurized fluid extraction. Environ Sci Technol 50:5774–5780
Galgani F, Hanke G, Werner S, Oosterbaan L, Nilsson P, Fleet D (2013) Guidance on monitoring of marine litter in European seas. European Commission
Harrison JP, Ojeda JJ, Romero-González ME (2012) The applicability of reflectance micro-Fourier-transform infrared spectroscopy for the detection of synthetic microplastics in marine sediments. Sci Total Environ 416:455–463
Harrison JP, Schratzberger M, Sapp M, Osborn AM (2014) Rapid bacterial colonization of low-density polyethylene microplastics in coastal sediment microcosms. BMC Microbiol 14:1–15
Hidalgo-Ruz V, Gutow L, Thompson RC, Thiel M (2012) Microplastics in the marine environment: a review of the methods used for identification and quantification. Environ Sci Technol 46:3060–3075
Imhof HK, Schmid J, Niessner R, Ivleva NP, Laforsch C (2012) A novel, highly efficient method for the separation and quantification of plastic particles in sediments of aquatic environments. Limnol Oceanogr Methods 10:524–537
Ivar Do Sul JA, Costa MF, Fillmann G (2014) Microplastics in the pelagic environment around oceanic islands of the Western Tropical Atlantic Ocean. Water Air Soil Pollut 225:1–13
Jambeck JR, Geyer R, Wilcox C, Siegler TR, Perryman M, Andrady A, Narayan R, Law KL (2015) Plastic waste inputs from land into the ocean. Science 347:768–771
Jungnickel H, Pund R, Tentschert J, Reichardt P, Laux P, Harbach H, Luch A (2016) Time-of-flight secondary ion mass spectrometry (ToF-SIMS)-based analysis and imaging of polyethylene microplastics formation during sea surf simulation. Sci Total Environ 563:261–266
Kaposi KL, Mos B, Kelaher BP, Dworjanyn SA (2014) Ingestion of microplastic has limited impact on a marine larva. Environ Sci Technol 48:1638–1645
Kidd KA, Schindler DW, Hesslein RH, Muir DC (1995) Correlation between stable nitrogen isotope ratios and concentrations of organochlorines in biota from a freshwater food web. Sci Total Environ 160–161:381
Koelmans AA, Besseling E, Shim WJ (2015) Nanoplastics in the aquatic environment. Critical review. In: Bergmann M, Gutow L, Klages M (eds) Marine anthropogenic litter. Springer International Publishing, Cham, pp 325–340
Lambert S, Wagner M (2016) Characterisation of nanoplastics during the degradation of polystyrene. Chemosphere 145:265–268
Lenz R, Enders K, Stedmon CA, Mackenzie DMA, Nielsen TG (2015) A critical assessment of visual identification of marine microplastic using Raman spectroscopy for analysis improvement. Mar Pollut Bull 100:82–91
Li J, Yang D, Li L, Jabeen K, Shi H (2015) Microplastics in commercial bivalves from China. Environ Pollut 207:190–195
Li J, Qu X, Su L, Zhang W, Yang D, Kolandhasamy P, Li D, Shi H (2016) Microplastics in mussels along the coastal waters of China. Environ Pollut 214:177–184
Liebezeit G, Dubaish F (2012) Microplastics in beaches of the East Frisian Islands Spiekeroog and Kachelotplate. Bull Environ Contam Toxicol 89:213–217
Löder MGJ, Gerdts G (2015) Methodology used for the detection and identification of microplastics—a critical appraisal. In: Bergmann M, Gutow L, Klages M (eds) Marine anthropogenic litter. Springer International Publishing, Cham, pp 201–227
Löder MGJ, Kuczera M, Mintenig S, Lorenz C, Gerdts G (2015) Focal plane array detector-based micro-Fourier-transform infrared imaging for the analysis of microplastics in environmental samples. Environ Chem 12:563–581
Lusher AL, Hernandez-Milian G, O'Brien J, Berrow S, O'Connor I, Officer R (2015) Microplastic and macroplastic ingestion by a deep diving, oceanic cetacean: the True’s beaked whale Mesoplodon mirus. Environ Pollut 199:185–191
Maes T, Jessop R, Wellner N, Haupt K, Mayes AG (2017) A rapid-screening approach to detect and quantify microplastics based on fluorescent tagging with Nile Red. Sci Rep 7:44501
Mahon AM, Connell BO, Healy MG, Connor IO, Officer R, Nash R, Morrison L (2017) Microplastics in sewage sludge: effects of treatment. Environ Sci Technol 51:810–818
Martins J, Sobral P (2011) Plastic marine debris on the Portuguese coastline: a matter of size? Mar Pollut Bull 62:2649–2653
Masura J, Baker J, Foster G, Arthur C (2015) Laboratory methods for the analysis of microplastics in the marine environment: recommendations for quantifying synthetic particles in waters and sediments. NOAA Techical Memorandum NOS-OR&R-48
Mathalon A, Hill P (2014) Microplastic fibers in the intertidal ecosystem surrounding Halifax Harbor, Nova Scotia. Mar Pollut Bull 81:69–79
Mato Y, Isobe T, Takada H, Kanehiro H, Ohtake C, Kaminuma T (2001) Plastic resin pellets as a transport medium for toxic chemicals in the marine environment. Environ Sci Technol 35:318–324
Miranda DA, de Carvalho-Souza GF (2016) Are we eating plastic-ingesting fish? Mar Pollut Bull 103:109–114
Nadal MA, Alomar C, Deudero S (2016) High levels of microplastic ingestion by the semipelagic fish bogue Boops boops (L.) around the Balearic Islands. Environ Pollut 214:517–523
Nel HA, Froneman PW (2015) A quantitative analysis of microplastic pollution along the south-eastern coastline of South Africa. Mar Pollut Bull 101:274–279
Ng KL, Obbard JP (2006) Prevalence of microplastics in Singapore’s coastal marine environment. Mar Pollut Bull 52:761–767
Norén F (2007) Small plastic particles in coastal Swedish waters. Sweden, KIMO
Nuelle MT, Dekiff JH, Remy D, Fries E (2014) A new analytical approach for monitoring microplastics in marine sediments. Environ Pollut 184:161–169
Peng G, Zhu B, Yang D, Su L, Shi H, Li D (2017) Microplastics in sediments of the Changjiang Estuary, China. Environ Pollut 225:283–290
Peters CA, Bratton SP (2016) Urbanization is a major influence on microplastic ingestion by sunfish in the Brazos River Basin, Central Texas, USA. Environ Pollut 210:380–387
Possatto FE, Barletta M, Costa MF, Ivar do Sul JA, Dantas DV (2011) Plastic debris ingestion by marine catfish: an unexpected fisheries impact. Mar Pollut Bull 62:1098–1102
Rehse S, Kloas W, Zarfl C (2016) Short-term exposure with high concentrations of pristine microplastic particles leads to immobilisation of Daphnia magna. Chemosphere 153:91–99
Reisser J, Slat B, Noble K, Plessis KD, Epp M, Proietti M, De Sonneville J, Becker T, Pattiaratchi C (2015) The vertical distribution of buoyant plastics at sea: an observational study in the North Atlantic Gyre. Biogeosciences 12:1249–1256
Rios LM, Moore C, Jones PR (2007) Persistent organic pollutants carried by synthetic polymers in the ocean environment. Mar Pollut Bull 54:1230–1237
Roch S, Brinker A (2017) A rapid and efficient method for the detection of microplastic in the gastrointestinal tract of fishes. Environ Sci Technol 51:4522–4530
Rochman CM, Browne MA, Halpern BS, Hentschel BT, Hoh E, Karapanagioti HK, Rios-Mendoza LM, Takada H, Teh S, Thompson RC (2013a) Policy: classify plastic waste as hazardous. Nature 494:169–171
Rochman CM, Hoh E, Kurobe T, Teh SJ (2013b) Ingested plastic transfers hazardous chemicals to fish and induces hepatic stress. Sci Rep 3:3263
Rochman CM, Hentschel BT, Teh SJ (2014) Long-term sorption of metals is similar among plastic types: implications for plastic debris in aquatic environments. PLoS One 9:1–10
Romeo T, Pietro B, Pedà C, Consoli P, Andaloro F, Fossi MC (2015) First evidence of presence of plastic debris in stomach of large pelagic fish in the Mediterranean Sea. Mar Pollut Bull 95:358–361
Setälä O, Fleming-Lehtinen V, Lehtiniemi M (2014) Ingestion and transfer of microplastics in the planktonic food web. Environ Pollut 185:77–83
Setälä O, Magnusson K, Lehtiniemi M, Noren F (2016) Distribution and abundance of surface water microlitter in the Baltic Sea: a comparison of two sampling methods. Mar Pollut Bull 110:177–183
Shen H, Pugh RJ, Forssberg E (2002) Floatability, selectivity and flotation separation of plastics by using a surfactant. Colloids Surf A Physicochem Eng Asp 196:63–70
Shim WJ, Song YK, Hong SH, Jang M (2016) Identification and quantification of microplastics using Nile Red staining. Mar Pollut Bull 113:469–476
Song YK, Hong SH, Mi J, Kang JH, Kwon OY, Han GM, Shim WJ (2014) Large accumulation of micro-sized synthetic polymer particles in the sea surface microlayer. Environ Sci Technol 48:9014–9021
Song YK, Hong SH, Jang M, Han GM, Rani M, Lee J, Shim WJ (2015a) A comparison of microscopic and spectroscopic identification methods for analysis of microplastics in environmental samples. Mar Pollut Bull 93:202–209
Song YK, Hong SH, Mi J, Han GM, Shim WJ (2015b) Occurrence and distribution of microplastics in the sea surface microlayer in Jinhae Bay, South Korea. Arch Environ Contam Toxicol 69:279–287
Stolte A, Forster S, Gerdts G, Schubert H (2015) Microplastic concentrations in beach sediments along the German Baltic coast. Mar Pollut Bull 99:216–229
Thompson RC, Olsen Y, Mitchell RP, Davis A, Rowland SJ, Anthony WGJ, McGonigle D, Russell AE (2004) Lost at sea: where is all the plastic? Science 304:838–838
Tibbetts JH (2015) Managing marine plastic pollution: policy initiatives to address wayward waste. Environ Health Perspect 123:A90–A93
Tourinho PS, Ivar do Sul JA, Fillmann G (2010) Is marine debris ingestion still a problem for the coastal marine biota of southern Brazil? Mar Pollut Bull 60:396–401
Turner A, Holmes LA (2015) Adsorption of trace metals by microplastic pellets in fresh water. Environ Chem 12:600–610
van Cauwenberghe L, Vanreusel A, Mees J, Janssen CR (2013) Microplastic pollution in deep-sea sediments. Environ Pollut 182:495–499
van Franeker JA, Blaize C, Danielsen J, Fairclough K, Gollan J, Guse N, Hansen P-L, Heubeck M, Jensen J-K, Le Guillou G, Olsen B, Olsen K-O, Pedersen J, Stienen EWM, Turner DM (2011) Monitoring plastic ingestion by the northern fulmar fulmarus glacialis in the North Sea. Environ Pollut 159:2609–2615
Van A, Rochman CM, Flores EM, Hill KL, Vargas E, Vargas SA, Hoh E (2012) Persistent organic pollutants in plastic marine debris found on beaches in San Diego, California. Chemosphere 86:258–263
Vianello A, Boldrin A, Guerriero P, Moschino V, Rella R, Sturaro A, Da Ros L (2013) Microplastic particles in sediments of Lagoon of Venice, Italy: first observations on occurrence, spatial patterns and identification. Estuar Coast Shelf Sci 130:54–61
Watts AJR, Lewis C, Goodhead RM, Beckett SJ, Moger J, Tyler CR, Galloway TS (2014) Uptake and retention of microplastics by the shore crab carcinus maenas. Environ Sci Technol 48:8823–8830
Wedemeyer-Strombel KR, Balazs GH, Johnson JB, Peterson TD, Wicksten MK, Plotkin PT (2015) High frequency of occurrence of anthropogenic debris ingestion by sea turtles in the North Pacific Ocean. Mar Biol 162:2079–2091
Woodall LC, Sanchezvidal A, Canals M, Paterson GL, Coppock R, Sleight V, Calafat A, Rogers AD, Narayanaswamy BE, Thompson RC (2014) The deep sea is a major sink for microplastic debris. R Soc Open Sci 1:1–8
Wright SL, Rowe D, Thompson RC, Galloway TS (2013a) Microplastic ingestion decreases energy reserves in marine worms. Curr Biol 23:R1031–R1033
Wright SL, Thompson RC, Galloway TS (2013b) The physical impacts of microplastics on marine organisms: a review. Environ Pollut 178:483–492
Wu C-C, Bao L-J, Tao S, Zeng EY (2016) Significance of antifouling paint flakes to the distribution of dichlorodiphenyltrichloroethanes (DDTs) in estuarine sediment. Environ Pollut 210:253–260
Xu J, Guo CS, Zhang Y, Meng W (2014) Bioaccumulation and trophic transfer of perfluorinated compounds in a eutrophic freshwater food web. Environ Pollut 184:254–261
Yu X, Peng J, Wang J, Wang K, Bao S (2016) Occurrence of microplastics in the beach sand of the Chinese inner sea: the Bohai Sea. Environ Pollut 214:722–730
Zhang K, Su J, Xiong X, Wu X, Wu C, Liu J (2016) Microplastic pollution of lakeshore sediments from remote lakes in Tibet plateau, China. Environ Pollut 219:450–455
Zhang K, Xiong X, Hu H, Wu C, Bi Y, Wu Y, Zhou B, Lam PKS, Liu J (2017a) Occurrence and characteristics of microplastic pollution in Xiangxi Bay of Three Gorges Reservoir, China. Environ Sci Technol 51:3794–3801
Zhang W, Zhang S, Wang J, Wang Y, Mu J, Wang P, Lin X, Ma D (2017b) Microplastic pollution in the surface waters of the Bohai Sea, China. Environ Pollut 231:541–548
Zhao SY, Zhu L, Wang T, Li D (2014) Suspended microplastics in the surface water of the Yangtze Estuary System, China: first observations on occurrence, distribution. Mar Pollut Bull 86:562–568
Zhao S, Zhu L, Li D (2015) Characterization of small plastic debris on tourism beaches around the South China Sea. Reg Stu Mar Sci 62:55–62
Zhou P, Huang C, Fang H, Cai W, Li D, Li X, Yu H (2011) The abundance, composition and sources of marine debris in coastal seawaters or beaches around the northern South China Sea (China). Mar Pollut Bull 62:1998–2007
Zobkov M, Esiukova E (2017a) Microplastics in Baltic bottom sediments: quantification procedures and first results. Mar Pollut Bull 114:724–732
Zobkov MB, Esiukova EE (2017b) Evaluation of the Munich plastic sediment separator efficiency in extraction of microplastics from natural marine bottom sediments. Limnol Oceanogr Methods 15:967–978
Acknowledgements
We would like to thank W. Tyler Mehler for assistance with editing the manuscript.
Funding
The present study was financially supported by the Ministry of Science and Technology of China (Nos. 2016YFC1402200 and 2017ZX07301005), the Natural Sciences and Engineering Research Council of Canada, and the Canada Research Chairs Program.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Electronic supplementary material
ESM 1
(DOC 118 kb)
Rights and permissions
About this article
Cite this article
Mai, L., Bao, LJ., Shi, L. et al. A review of methods for measuring microplastics in aquatic environments. Environ Sci Pollut Res 25, 11319–11332 (2018). https://doi.org/10.1007/s11356-018-1692-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-018-1692-0