Abstract
We describe and discuss the different components necessary for the construction of a microfluidic system including micropump, microvalve, micromixer and detection system. For the microfluidic detector, we focus on carbon nanotube (CNTs) based electrochemical sensors. The properties, structure and nomenclature of CNTs are briefly reviewed. CNT modification and the use of CNTs in conjunction with electrochemical microfluidic detection are then extensively discussed.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
In recent years there has been great interest in miniaturized analysis systems for chemical and biological sensing applications. These systems and devices, also well known as lab-on-a-chip (LOC), offer several advantages over conventional scale analytical methods, including low sample/reagent consumption, fast analysis, high throughput and automation capability [1–16]. In this chapter, microfluidic systems and the various microfluidic components are concisely described and discussed. Finally, we discuss the properties which make carbon nanotubes (CNTs) ideal modifiers for improving the sensitivity and/or selectivity of electrochemical detectors, and describe how such detectors can be integrated into microfluidic systems.
2 Microfluidic System
2.1 Classes of Microfluidic Systems
Microfluidic systems can generally be divided into two main classes, continuous-flow and droplet-based [17–19]. Continuous-flow is based on the manipulation of a continuous flow of fluid in micron-sized channels, which may be driven by mechanical fluid pressure [20, 21] or by capillary forces and electrokinetic actuation [22, 23]. In a typical continuous flow system, fluids including carrier fluid, buffer and analytes from reservoirs are driven into nanoport inlets by either external pumps or integrated on-chip micropumps through microchannels at controlled flow rates [24]. Fluid manipulations such as mixing and focusing are then conducted using microvalves, micromixers and other microfluid manipulators depending on the application [25]. In some specific applications such as the polymerase chain reaction, fluid will be confined in reaction microchambers that include special-purpose components such as a microheater and microsensors [26]. Processed fluids will be then analyzed by either on-chip or off-chip detectors [27]. In advanced biochemical applications, fluids may be separated along a microcolumn by methods including capillary electrophoresis and liquid chromatography using a specially treated microcolumn and microelectrodes [28, 29]. Finally, analyzed fluids will exit outlets and be collected at waste sinks.
Continuous microfluidic operation is a common technique that is easy to implement, suitable for definite and uncomplicated biochemical applications and is necessary for particular flow-based functions such as separation. However, it suffers from several limitations including relatively high sample/analyte consumption, limited flexibility/reconfigurability, poor fault tolerance capability and poor scalability because of the permanent closed-channel configuration and flow-component-system interdependency. Droplet-based microfluidics has recently been developed to overcome these shortcomings. In these systems, droplets of samples/reagent are formed instead of a continuous stream, which results in lower sample/reagent consumption.
There are two main types of droplet-based systems, namely continuous and discrete [30]. In the continuous type, droplets are generated and transported along microfluidic channels by flowing sample/reagent into an immiscible carrier fluid that separates and encapsulates the continuous solution stream into microdroplets. This system can be considered an extension of the continuous flow scheme and hence it is essentially the continuous flow systems that include a special mixer specially designed to generate a stream of micro-droplets. The main benefit of this method is very high throughput and lower sample/reagent consumption. However, it also requires additional carrier fluids and it still suffers from similar shortcoming to a continuous flow system, particularly complicated fluid manipulation, limited flexibility/reconfigurability, poor fault tolerance capability, and poor scalability.
In the discrete droplet-based approach, samples/reagent are formed, manipulated and analyzed as isolated microdroplets with no need of carrier and microchannel [19, 30, 31]. The use of discrete droplets can greatly reduce sample/reagent consumption when compared to continuous systems. Due to the architectural similarities with digital microelectronic systems, droplet-based systems are often referred to as “digital microfluidics”. In typical digital systems, individual droplets are generated, moved, merged, split and mixed by a system of structures operated based on various droplet actuation mechanisms including electrowetting-on-dielectric (EWOD), surface acoustic wave (SAW), dielectrophoresis, thermocapillary forces and magnetic forces. These techniques allow parallel processing ability, high architectural flexibility, scalability and dynamic reconfigurability because the force driving the droplets may be controlled by software-driven electronics and the droplets can be freely manipulated in an open platform. In addition, the fabrication of the digital systems is relatively simple and lower priced because complex microchannels and mechanical components are eliminated. Thus, digital microfluidic systems have increasingly gained interest in various applications. Nevertheless, droplet-based systems also have some limitations. For instance, they cannot provide some important functions such as chemical separation, which is very important for biochemical sensing applications. In addition, integration of detection components in the system requires further technological development. Therefore, we will focus here on continuous flow microfluidic systems as they are still more widely used in biochemical sensing applications.
2.2 Microfluidic Components
The key microfluidic components for a general integrated microfluidic system are concisely discussed below.
2.2.1 Micropump
A micropump is a miniaturized pumping device that can be integrated into a microfluidic system for driving fluid at a desired flow rate. Micropumps can be divided into two main classes, mechanical or non-mechanical [32].
2.2.1.1 Mechanical Micropump
Mechanical micropump operation is based on mechanical moving parts such as membranes or diaphragms and check valves, which are periodically actuated by one or more physical mechanisms to generate fluid flow. Major types of mechanical micropumps include reciprocating diaphragm, peristaltic, syringe and rotary structures [20]. A reciprocating diaphragm micropump typically contains a pumping chamber, flexible actuating diaphragm, mechanical actuator, inlet valve and outlet valve as illustrated in Fig. 1a. Fluid flow is generated by the periodic movement of the diaphragm, which draws in fluid through the inlet valve when the diaphragm retracts (under pressure state) and drives it out when diaphragm extends (over pressure state) [33]. The actuators move the diaphragm back and forth between the two states, causing a volume change called the stroke volume, which is proportional to the generated pumping pressure and pumping rate. The actuation must go against the remaining dead volume of fluid in the chamber and thus the pumping efficiency is proportional to the ratio of stroke to dead volume, called the compression ratio (ε).
Passive inlet and outlet check valves [34], allowing flow only in one direction, are normally used to dictate the flow direction as shown in Fig. 1a. These check valves may be replaced by nozzle/diffuser elements to yield valveless micropumps as illustrated in Fig. 1b. These elements are taper pipes that allow more fluid to enter via the inlet than to leave at the outlet during the under pressure state and vice versa during over pressure state [35]. The main advantage of a valveless structure is simpler fabrication, while the key disadvantage is relatively lower efficiency due to some backward flow.
A peristaltic micropump [36, 37] is a valveless bi-directional micropump containing three actuating diaphragms arranged in series as illustrated in Fig. 1c. Sequential peristaltic movement of diaphragms can be programmed to transfer the fluid from one port to the other with no valve or diffuser/nozzle element and the flow direction can be inverted by reversing the order of diaphragm movement [33, 38]. In contrast, syringe micropumps utilize actuators located in a reservoir chamber to drive the fluid into a nozzle as shown in Fig. 1d. The key feature of syringe micropump is ability to provide accurate, steady and non-turbulent flow control [39, 40]. Rotary micropumps employ a rotating microgear motor to transfer fluid from inlet to outlet, which may employ nozzle/diffuser elements, as illustrated in Fig. 1e. Micromotor is typically driven by either electromagnetic or electrostatic actuation [41]. A rotary micropump can provide a large pumping rate, however its fabrication involves a complicated micromachining process.
The mechanical actuator is the most critical part of a mechanical micropump because it primarily dictates the pumping performance. Important actuation mechanisms employed in mechanical micropumps include piezoelectric, shape memory alloy (SMA), bimorph, ionic conductive polymer film (ICPF), electrostatic, electromagnetic, pneumatic, thermopneumatic and phase change [26, 32]. Table 1 summarizes the main structures, advantages and disadvantages of mechanical micropumps. These mechanical actuators can be further divided into two groups according to application method.
In the first group, comprising piezoelectric, SMA, bimorph and ICPF, the actuator is employed in the micropump by direct attachment of the whole actuator body to the diaphragm. A piezoelectric actuator comprises a piezoelectric layer sandwiched between two metallic films, which are deformed due to strain induced in the piezoelectric crystals under high applied voltage [42–44]. An SMA actuator consists of a microheater and a layer of special metal alloy that has shape memory effect, such as Au/Cu, In/Ti, and Ni/Ti [45, 46]. With this effect, the SMA structure is deformed and reformed between two states as a result of phase transformation between the austenite phase at high temperature and martensitic phase at low temperature. An SMA structure that is mechanically constrained by a diaphragm will exert a large actuation force and displacement by heating and cooling. A bimorph actuator contains a microheater and a bilayer of two dissimilar materials. The bilayer structure will expand or contract upon heating or cooling due to the difference in the thermal expansion coefficients of the two materials [47]. An ICPF actuator is a polyelectrolyte film with both sides chemically plated with platinum and can be actuated by a stress gradient due to ionic movement under an applied electric field [48]. During ionic movement, cations attached to polymer molecules move and simultaneously take solvent shell water molecules to the negative electrode (cathode), causing cathode expansion and anode shrinkage.
In the second group of micropumps, the diaphragm of the micropump is a part of the actuator system. Electrostatic actuators are used in micropumps by making the diaphragm the movable electrode of an electrostatic capacitor. The movable electrode is separated from a fixed plate by a micron-sized air gap and is deflected by Coulomb attraction forces under high applied voltage between the two electrodes [49, 50]. Similarly, electromagnetic actuation can be used for micropumping by bonding a permanent magnet to the diaphragm. The magnetic diaphragm is then actuated by an electromagnetic field induced by applying current to coils around soft magnetic cores [51, 52]. Pneumatic micropump utilizes a compliant diaphragm that is a wall of actuation chamber and is expanded or contracted by air/vacuum pressure, which is manipulated by external solenoid-valve-controlled oil-less diaphragm vacuum pump/compressors [53, 54]. Typically, PDMS membrane, an elastomer with low Young’s modulus and high reversible strain, is employed as diaphragm for pneumatic actuation. PDMS provides large stroke, low leakage performance in close position and excellent adherence to glass/silicon substrate. The use of an external air/vacuum system makes the overall system become large. Thermopneumatic principle that uses thermally-induced volume change of air in the sealed chamber has been utilized to replace the external air/vacuum part with integrated microheater/refrigerator [55, 56]. Thermopneumatic system is thus better in term of system integration. Phase-change actuation is similar to thermopneumatic actuation except that it uses a liquid-phase fluid and an external heater (outside actuation chamber). The diaphragm is expanded and contracted by vaporization and condensation of the working fluid [57, 58].
From Table 1, it can be seen that actuation mechanisms which require heating, including SMA, bimorph, thermopneumatic and phase change will require high power and give a slow response but can produce a large actuation stroke. Electromagnetic actuation is a special category in that it has high power consumption due to high current but offers a fast response. In contrary, actuation mechanisms that are based on electric fields, including electrostatic, piezoelectric and ICPF have low power consumption and provide a fast response but have a small actuation stroke. The choice of mechanical micropump depends on flow rate, pumping fluid force, power consumption, operating frequency and fabrication/cost requirements.
2.2.1.2 Non-Mechanical Micropump
Non-mechanical micropumps are based on micro-scale phenomena that convert non-mechanical energy to kinetic fluidic energy. Geometry design and fabrication techniques for these kinds of pump are simpler due to the absence of moving parts. Non-mechanical phenomena used in micropumps include magnetohydrodynamic (MHD), ferrofluidic, electrohydrodynamic (EHD), electroosmotic, electrowetting, thermo bubble, flexural planar wave (FPW), electrochemical and evaporation [26, 32]. The structure, advantages and disadvantages of non-mechanical micropumps are summarized in Table 2.
MHD actuation is based on the Lorentz force acting on electrically conductive fluids (conductivity >1 s/m) under magnetic and electrical fields [59, 60]. A typical MHD micropump comprises of a rectangular microchannel with two opposite walls being electrodes for applying an electric field and the other two walls being permanent magnets of opposite polarity for providing the magnetic field. Ferrofluid magnetic actuation utilizes the movement of ferrofluid plug, which is a carrier fluid such as water, hydrocarbons or fluorocarbons with suspended ferromagnetic nanoparticles. The ferrofluid movement is induced by moving magnetic field mechanically using motor controlled magnet [61].
EHD actuation, an electrokinetic effect, relies on Coulombic force acting on electric-field-induced charges in a low conductivity or dielectric fluid [62, 63]. Since charges must be induced, the pumping liquid is directly affected by EHD actuation. In an EHD micropump, ions in the fluid move between two permeable electrodes (emitters and collectors directly in contact with fluid), resulting in an EHD pressure gradient. Electroosmosis is another electrokinetic effect, in which ionic liquid is moved by viscous force as a result of boundary ion movement relative to the stationary charged surfaces of a microchannel under an applied electric field [64, 65]. Stationary negative charges are induced due to interaction between an ionic solution and a microchannel surface such as fused silica and PDMS. The induced charged surface attracts the positively charged ions to form electric double layers, which comprise a fixed-charge Stern layer and cation-rich Guoy–Chapman boundary layer, in which ions can move under an electric field. The movement of these ions leads to viscous motion of neutral fluid in the channel (Table 2). In contrast, electrowetting provides fluid movement through a change in surface tension under applied electric potential via the dielectric layer. The change in electrical potential results in a change in interfacial energy between the liquid and dielectric interface [66, 67]. A continuous electrowetting micropump utilizes the electrowetting effect to manipulate two immiscible liquids such as liquid-phase mercury droplet/electrolyte The surface tension difference between left and right droplet surfaces, due to potential drop across the electric double layer, pushes the droplet from left and right (Table 2). The droplet movement in electrolyte is then used to actuate a micropump diaphragm.
Thermo bubble is the generation and expansion of a bubble by heating under a fluid [68]. The bubble expansion creates a large pressure and actuation force that can be used to drive fluid in a micropump. It can be used to form a diaphragm less and valveless micropump when used along with nozzle/diffuser element (see Table 2). A net flow is generated from nozzle to diffuser by bubble expansion and extinction periodically controlled by heating voltage. Electrochemical reactions such as the electrolysis of water can be used instead to generate gas bubbles to provide the fluid driving force [40, 69]. In an electrochemical micropump, an electrode containing water in an actuation chamber is used to generate bubbles that drive the diaphragm to pump fluid into the main chamber (Table 2).
The FPW micropump utilizes ultrasonically driven acoustic streaming to initiate fluid flow. FPW produced by an array of piezoelectric or SAW transducers propagate along a thin wall of microchannel to transfer its momentum to fluid [70, 71]. Lastly, evaporation-based micropumps exploit controlled liquid evaporation through a membrane into a gas space containing a sorption agent and flow-induced capillary forces, which are generated as a result of evaporation [72]. The vapor pressure in the gas chamber is kept below saturation by gas absorber and evaporated liquid is continuously replaced by the flow of liquid through the microfluidic system.
All non-mechanical micropumps share the common advantages of no moving parts making design and fabrication relatively simple, while there are different pros/cons for each type of micropump. From Table 2, it can be seen that mechanisms involving the application of an electrical potential, including MHD, ferrofluidic, EHD, electroosmosis, electrowetting and electrochemical, provide fast responses, low power consumption and are simple to fabricate but are limited to conducting fluids. Thermo-bubble requires high power consumption and gives a slow response but can produce a large actuation stroke. FPW is a unique method that requires low operating power and can work with any fluid but suffers from a low actuation force and has a complex fabrication process. Evaporation is a simple and novel method that requires no external energy but is limited to suction operation. The choice of non-mechanical micropump depends on type of application and other operational parameters such as flow rate, pumping fluid, power consumption, operating frequency and cost. For example, an electroosmosis pump is essential for capillary electrophoresis.
2.2.2 Microvalves
A microvalve is a miniaturized switching device that can be integrated into microfluidic systems for valving fluid flow direction, and is an important element of mechanical micropumps as well as fully integrated microfluidic chips. Microvalves can be divided into two main classes, passive and active microvalves and each class may be further subdivided into mechanical or non-mechanical types. They are summarized in Table 3 and discussed below.
2.2.2.1 Passive Microvalves
Passive microvalves, which are often used in mechanical micropumps, do not employ any kind of external actuation. Most common passive microvalves are mechanical flap valves with various structures including cantilever, disc and spherical ball (Table 3) [58, 73]. Disc and ball structures are more preferred for low leakage during closing operation. In addition, valve seat having a mesa pit structure may be used in these structures to reduce any pressure-drop during opening operation by diminishing the structural stiction on the valve anchor. They use a pressure difference between the inlet and outlet of the valve to overcome spring force and control the valve opening. In contrast, passive plug (PP) mechanical microvalve, consisting of snug and airtight stub and stem components, utilizes stub weight and flow control elevation mechanism inside the access port to control fluid flow [74]. The fluid flow is controlled by relative input/output pressure, height of elevation and stub weight. In addition, tight seal (fully close) operation may be attained manually using the snug fit and additional weight above the PP stub.
In-line polymerized gel microvalve is a mechanical passive valve that utilizes gel photopolymerization by a thermally induced pressure difference to create local gel plugs to prevent bulk liquid flow [75, 76]. The microfluidic gel valve is typically fabricated from a mixture of Tris–HCl, acrylamide/bic and 1-hydroxyl-cyclohexylphenylketone (HCPK) photoinitiator. Polymerization will occur when passing fluid provides a suitable temperature and pH to the gel. The leakage characteristic of the gel plug mainly depends on the degree of gel cross-linking, which can be optimized to yield minimal leakage at high hydrostatic pressure. Hydrophobic microvalve is a non-mechanical passive valve that utilizes hydrophobic patches in otherwise hydrophilic microchannels to confine fluid within hydrophilic regions by surface tension force [77]. Hydrophobic patches can be formed by coating the regions selectively with a hydrophobic substance such as Teflon and EGC-1700. For instance, passive stop valves can be made by coating access ports with a hydrophobic film. The hydrophobic microvalve is easy to fabricate but it seriously suffers from poor stability due to possible desorption of the hydrophobic film, or reaction with the working fluid.
2.2.2.2 Active Microvalves
Active microvalves are operated by actuating force and offer higher performance than passive ones but they also require more complexity and fabrication cost. The majority of active microvalves are mechanical valves that employ a sealing diaphragm actuated by various mechanical mechanisms to interpose between the two ports of microchannel as illustrated in Table 3. When the diaphragm is not actuated, fluid flows normally under a driving pressure between inlet and outlet ports. Under actuation, the diaphragm is pressed onto the valve seat and stops fluid flow. Mechanical actuation mechanisms utilized in micropumps such as electrostatic, electromagnetic, piezoelectric, SMA and thermopneumatic can all be used in microvalves [78–82]. Diaphragm based mechanical microvalve offers simple and effective operation with low leakage in close mode and low resistance in open mode but they require relatively high fabrication cost. The speed and power consumption are dependent on actuation mechanisms, which can be selected and optimized according to application requirements.
Another important group of active microvalves is non-mechanical phase change type operated based on solid–liquid phase transition effects [83]. The phase-change microvalves typically consist of a microchannel, a cavity, a phase change plug material and a thermoelectric (TE) microheater/refrigerator as depicted in Table. 3. In operation, phase change material including hydrogel, sol–gel, paraffin and ice are thermally actuated to transform their phase from liquid to solid and vice versa to stop and pass fluid flow. The advantages of this type of microvalves include very low leakage, simple fabrication and ease of large-scale integration. However, they suffer from high power consumption and slow response.
A stimuli-responsive, or smart, hydrogel is an effective phase change material that can change its volume reversibly and reproducibly by more than one order of magnitude from a very small change of input parameters such as pH, glucose, temperature, electric field, light, carbohydrates or antigen [84]. At temperatures above the hydrogel critical temperature T c (32°C), the hydrogel is unexpanded and the microchannel is open. When the temperature is decreased below T c by a thermoelectric (TE) refrigerator, the hydrogel swells and blocks the flow. Hydrogel plugs offer perfect sealing, relatively high pressure tolerance and self-actuated open-loop control abilities. Pluronics sol–gel is another promising phase change material [85]. The pluronic polymer forms a cubic liquid crystalline solid gel plug that stop fluid flow at room temperature. When cooled below the pluronics gel transition point (5°C), the gel is liquefied, allowing fluid flow. The advantages of pluronics gel include good sealing and simple implementation by one-shot injection.
Paraffin is another attractive phase change material due to low cost. In paraffin microvalve, the paraffin plug is liquefied by thermal heating, then moved in the microchannel to a fluid port by upstream fluid pressure or external pneumatic air/vacuum actuation and conformally solidified on the port wall, forming a leak-proof solid seal [86, 87]. Heating can be done globally or locally by isothermal or spatial gradient actuation, respectively. Local heating allows much shorter actuation time and lower power consumption than global heating. For reversible close–open operation, external pneumatic air/vacuum actuation is required to move the molten paraffin between fluid ports, making the system fairly complicated. Lastly, water can be used as phase transformation material. Ice plugs can be formed by TE cooling below water freezing point (0°C) to non-invasively close aqueous solution flow, and ice melting by heating can restore the flow [88]. Ice valves are advantageous for bio/chemical microfluidic systems due to biocompatibility and contamination-less property, but require high power consumption and have long response times. These may be reduced by pre-cooling at the expense of power consumption.
2.2.3 Micromixer
A micromixer is a miniaturized merging device that can be integrated in microfluidic systems for mixing two or more fluid flow. Fluid mixing in microchannels is generally difficult due to the low Reynolds number laminar flow effects of fluids in micron-sized conduits [23, 89]. Thus, various strategies have been developed to achieve effective micromixing in fully integrated microfluidic chips. Micromixers can be divided into two main classes, passive and active [26].
2.2.3.1 Passive Micromixers
The mixing process of passive micromixers mainly relies on chaotic diffusion and convection, which may be achieved by a properly designed microchannel’s geometry and surface topography [90]. The Y/T-type flow configurations are the simplest mixing structures that combine two or more Y and T mixing junctions to induce chaotic diffusion [91–93]. For instance, four T-type and one Y-type micromixer combination have been shown to provide high mixing efficiency. However, large combinations of Y–T junctions are not desirable due to size, complexity and cost.
To avoid using large Y/T-type micromixers, a single Y or T mixing junction may be used in combination with other passive micromixing strategies including droplet formation and movement with recirculation flow [94–96]. Droplet formation from two or more fluids to be mixed increases the fluid interface between them, while droplet movement generates an internal flow field inside the turning and elongated droplet, resulting in internal mixing and enhanced mixing efficiency. For successful recirculation, multi-phase droplets must move as one with non-slip boundary conditions. Transient adsorption of droplets to the microchannel walls should be prevented by the use of a suitable surfactant. Droplets can be generated by pressure-driven mixing of multiple immiscible phases with large differences in surface tension, such as an aqueous solution and oil in a microchannel or by fluid mixing driven by capillary effects including thermocapillary or electrocapillary (electrowetting) that employ temperature difference or electric field to bring two or more fluids to mix at a junction. After droplet formation, thermocapillaries or electrocapillaries will then be used again to move droplets around the microchannel to induce recirculation mixing. Another effective droplet mixing strategy is to use the shear force between an additional immiscible carrier liquid and the sample droplet to accelerate the mixing process.
2.2.3.2 Active Micromixers
Active micromixers employ some external agitating energy to create turbulent mixing and are considerably more effective than passive mixers, but high cost and complexity make them less attractive for practical microfluidic systems. Common actuation mechanisms employed in an active micromixer include electrokinetic, acoustic, MHD and mechanical actuation by micropumps/microvalves. Electrokinetically-driven micromixing provides significant diffusion enhancement from local circulation zones and stretching/folding buck flow induced by a fluctuating applied electric potential that results in a variation of zeta potential on the microchannel walls [97, 98]. The electrokinetic method offers very high mixing speed but it requires a high operating voltage. Acoustic agitation or ultrasonication is a well-known and effective fluid mixing technique that utilizes an acoustic field to induce frictional forces at air/liquid interface of an air bubble in a liquid medium, resulting in bulk fluid flow called cavitation or acoustic microstreaming [99]. The bubble-induced streaming will be most effective if bubbles are excited near their resonance frequency, which depends mainly on bubble size and distribution. Acoustic fields can be induced by an integrated bulk wave or SAW actuator. Acoustic microstreaming offers many advantages including fast operation, simple apparatus, ease of implementation, low power consumption (~2 mW) and low cost.
MHD micromixing use Lorentz force to roll and fold electrolyte solutions in a mixing chamber [100]. In contrast to a MHD micropump, MHD electrode arrays are deposited on the microchannel’s surface in the transverse direction, instead of parallel to the microchannel walls. In addition, complex flow fields such as cellular motion, which provide enhanced fluid mixing interface, can be induced under applied AC potential differences across pairs of electrodes with appropriately designed patterns. Mechanical actuation by integrated microvalves/micropumps can also provide highly effective micromixing [54]. Various type of actuation mechanisms have been reported for micromixing. For example, a pneumatic peristaltic rotary micropump has been used for fixed volume as well as continuous-flow on-chip mixing. In fixed volume mixing, fluid injected into the chamber are circulated and quickly mixed with the rotary pump. In a circular loop, different fluids are moving at different velocities with the parabolic profile of the Poiseuille flow and their interfaces will stretch into long and thin streams and finally wrap around into a complete mix. In continuous-flow mixing, the mixing efficiency increases as the overall fluid flow rate decreases and the rotary mixer loop increases.
2.2.4 Detection System
Detection system is an analysis apparatus that may be applied to and/or integrated in microfluidic systems for chemical and biosensing. It is a very important part of micro total analysis system. Sensors or detectors in microfluidic systems must be much more sensitive than conventional methods because of small sample volume, short sampling time and low analyte concentration. The most widely used detection strategies are electrochemical, optical and mass spectroscopic [101, 102].
Electrochemical detections including potentiometric [103, 104], voltametric [105–107], amperometric [108–110], coulometric [111] and AC impedance [112–114] techniques utilize potential, current or charge signals from reducing and/or oxidizing reaction at electrodes, which can be either two-electrode system that comprises working and counter electrodes or three-electrode system that includes an additional reference electrode. Electrochemical detectors are the easiest to integrate into microfluidic devices, due to the fact that the technology for the fabrication of microelectrodes and the placement of microelectrodes within or at the outlets of microchannels is now well established [115–118]. Additionally, electrochemical detection can provide relatively low limits of detection without requiring expensive transduction methods [119]. A number of microfluidic systems have been integrated with on-chip micro-electrochemical sensors using different working electrode materials, including gold, platinum, polymer, CNTs and other nanocomposites. Conventional metal thin film electrodes tend to suffer from insufficient sensitivity and poor limit of detection. The limit of detection for electrochemical measurement can often be improved by modifying the electrode surface with a catalyst of some kind. The improvement can be due to either increasing the rate of the electrode reaction, or by lowering the working potential and thus lowering the background current. The electrode surface can also be modified by a biological recognition element, which can be used to improve the selectivity of detection and/or extend detection to electro-inactive analytes when used in conjunction with an electrochemical label (for DNA or antibody-based detection). As outlined in the following sections, CNTs are ideally suited both to use as electrocatalysts and as nano-dimensional supports for the construction of electrochemical labels. As will be detailed below, both the CNT modification of electrodes and the covalent and non-covalent modification of CNTs by chemical or biological catalysts, have been successfully reported. Another type of electrochemical sensor, which is relatively less commonly used, is ion-sensitive field effect transistor (ISFET). It is relatively difficult to be integrated in microfluidic system due to relatively complicated fabrication process. Nevertheless, its integration in microfluidic systems has been successfully demonstrated [120–122].
Optical detections including fluorescence [123–127], chemiluminescence [128–131], electrochemiluminescence [132, 133], bioluminescence [134–136], surface plasmon resonance (SPR) [137–145], surface enhanced Raman scattering (SERS) [146–150], absorbance [151], transmittance [152] and other microscopic imaging methods [153, 154] have widely been applied for biochemical sensing in microfluidic systems [155]. Typical optical detection system consists of laser, lens/mirror system and photodetectors. In general, optical components are normally located off-chip but may be assembled or attached to a chip. However, they have relatively large size and may not be fabricated directly on microfluidic chips. The integration still has not been widely attainable and optical-based microfluidic systems remain relatively expensive and bulky. In addition to miniaturization issue, optical signal amplification for sensitivity and limit of detection improvement are also challenging for optical microfluidic based systems [123, 156, 157].
Mass spectroscopic (MS) detection is a promising alternative due to its high detection capability. Typically, MS system comprises ionization chamber, quadruple mass selector and ion detectors. Similar to optical methods, its integration with microfluidic systems is difficult because it is challenging to miniaturize and integrate MS components with microfluidic system. Nevertheless, there have been several microfluidic systems with MS detection [158–163]. In addition to these common methods, many other sensors and detection methods have been demonstrated in microfluidic systems. For instance, Microfluidic chip calorimeter has been developed based on catalytic enzyme reaction detected by calorimetry [164, 165]. In addition, nanomechanical resonant sensors has also recently been integrated within microfluidic channels [166, 167]. Capacitively-coupled contactless conductivity detection is another new scheme recently developed in microfluidic systems [168, 169]. Nuclear magnetic resonance [170] as well as acoustic [171] based sensors have also been recently employed and integrated microfluidic platforms. Furthermore, multiple detection strategies, which combine more than one detection methods such as optical and electrochemical methods for selective and multiplexed detections, have also been widely studied [172–175].
2.3 Fabrication of Microfluidic System
Fabrication of microfluidic systems may be mainly divided into traditional processes based on microelectromechanical system (MEMS) or other alternative processes. Traditional MEMS processes include thick/thin deposition by physical vapor deposition (PVD) such as sputtering and evaporation or chemical vapor deposition (CVD) such as low-pressure and plasma enhanced CVD, photolithographic patterning, wet chemical etching, dry etching such as reactive ion etching (RIE) and bonding [176]. These processes are mainly used for fabrication of silicon- and glass-based continuous or digital microfluidic chip. Although silicon-based microfluidic devices offer full functional capability, their high fabrication/material cost and poor biocompatibility makes them less attractive for chemical and biosensing applications. Glass, polymer and paper are much more attractive materials for microfluidics due to low price, biocompatility and low cost fabrication. Fabrication of microfluidic chip based on these material involve the combination of some standard technology such as photolithography and other alternative processes including solution processing, replication by casting, embossing or injection molding, oxygen-plasma-assisted bonding, adhesive bonding, tape grafting, and inkjet or offset printing. The fabrication process for glass/polymer-based [37, 56, 177, 178] and paper-based devices [179–181] has been rapidly developed to realize cheap, portable and high throughput microfluidic chips. However, most of these microfluidic systems still cannot be implemented with full functionality due to low-temperature, low-structural-complexity and other fabrication limitations. Thus, new fabrication approaches and hybrid technologies are still being developed to achieve low-cost and fully functional integrated microfluidic devices.
3 Carbon Nanotubes
3.1 Introduction
Since the first report of their synthesis by Iijima [182, 183], CNTs have generated much interest as electrode surface modifiers in the construction of bio and chemical sensors. This interest comes from the fact that they have a high tensile strength, a high value of Young’s modulus (a measure of stiffness), often good electrical conductivity, exhibit electrocatalytic properties to a number of analytes, can undergo chemical fictionalization, and can be used as carriers for redox mediators, metal nanomaterials or biological recognition elements. CNTs can be made by arc discharge [182], laser ablation [184] or CVD [185]. The later technique provides lower yields than the two former, but has the advantage of both producing high quality CNTs and allowing control of CNT position and alignment.
Structurally, the CNTs is a graphene sheet rolled into a cylinder. Hence, the carbon atoms in the nanotube are all sp 2 hybridized. Iijima initially reported the synthesis of structures consisting of tens of concentric graphitic cylinders [182], referred to as multi-walled carbon nanotubes (MWNTs). These typically have an internal cylinder spacing of 0.3–0.4 nm and external diameters of 2–500 nm. Iijima and Ichihashi [183] and Bethune et al. [186] later synthesized (single-walled carbon nanotubes (SWNTs), consisting of individual graphitic cylinders. More recently, structures consisting of two cylinders, double-walled carbon nanotubes (DWNTs), have been reported [187]. Recent reviews can be found for both the general properties of CNTs [188–192] and their use in sensors [193–196].
3.2 Structure of CNTs
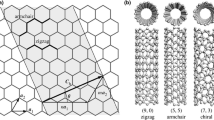
As noted above, if a single graphene sheet is rolled into a cylinder the product is a CNTs. Although this is not physically how CNTs are made, as shown in Fig. 2, the process can be imagined as a means to understanding the different possible CNT structures. These are:
-
1.
Zig-zag carbon nanotubes: These are produced by rolling up the graphene sheet parallel to the horizontal vector a 1. The resulting nanotube has a “zig-zag”-shaped edge.
-
2.
Armchair carbon nanotubes: In this case the graphene sheet is rolled on an axis 30° to a 1. The result is that the edge of the nanotube consists of the sides of one row of six-membered rings.
-
3.
Chiral carbon nanotubes: These are produced by rolling the graphene sheet at an angle θ to a 1 such that 0° < θ < 30°. A line drawn parallel to a 1 would then spiral upwards around the tube. Hence there are two enantiomeric forms of any given chiral CNTs.
The easiest and most common way to describe the structure of a given nanotube is by means of vector notation. As shown in Fig. 2, we can specify a vector C which will join two equivalent points on the graphene plane, i.e. when the graphene plane is rolled into the cylinder the two end-points of C will be superimposed. The structure of the resulting nanotube is then described by the direction and length of C, expressed as:
where a 1 and a 2 are the unit vectors shown in Fig. 2. As illustrated in Fig. 3, the integer pairs (n, m) correspond to points on the graphene lattice, following the notation of Dresselhaus and co-workers [197]. Hence, in Fig. 2 a vector C drawn to any (n, m) coordinate represents a particular nanotube structure. All nanotubes of the form (n,0) must be zig-zag, while all nanotubes where n = m must be armchair. All other (n, m) coordinates will be chiral. Based on the C–C bond lengths in graphene a 1 = a 2 = 0.246 nm. From which the dimensions of the nanotube can be calculated using:
Graphene sheet with atoms labeled according to the (n, m) notation of Dresselhaus and co-workers [197]
All armchair SWNTs are metallic conductors, while for zig-zag and chiral SWNTs, approximately one-third of the possible structures are metallic and the other two thirds are semi-conducting.
3.3 Properties of Carbon Nanotubes
3.3.1 Inherent Electrode Properties
Due to the fact that CNTs conduct, a CNT film on an electrode will increase the effective area available to the analyte, and hence the sensitivity of the response can be expected to increase. For example, comparative cyclic voltammograms with Fe(CN6)3− showed that modification of a glassy carbon electrode with SWNTs increased the electrode area by a factor of approximately 1.8 [198]. In addition to this, CNTs have been shown to have an electrocatalytic effect to the oxidation of many analytically-important substances, including hydrogen peroxide [199], NADH [200], dopamine [201], ascorbic acid [201, 202], uric acid [202] and norepinephrine [203]. Crompton and co-workers have concluded this catalytic effect occurs via reaction at the ends of the CNTs. This is based on the fact that edge-plane pyrolytic graphite electrodes (pyrolytic graphite cut so that the graphite layers are perpendicular to the electrode surface) show similar electrochemistry and catalytic effects to CNTs, where as basal plane graphite (pyrolytic graphite cut so that the graphite layers are parallel to the surface) shows quite different properties, but can be made to behave like edge-plane electrodes if coated with a CNT film [204].
Another useful inherent property of CNTs is that they can promote direct electron transfer between a redox protein and an electrode surface. This has been reported for peroxidases [205], cytochrome c [206], myoglobin [207], catalase [208] and glucose oxidase [209]. The last case is exceptional as the FAD/FADH2 redox couple is buried deep within the enzyme shell, and therefore direct communication with an electrode is usually difficult to achieve. The realization of electron transfer was probably assisted by the fact that the nanotube arrays were vertically aligned on the electrode surface, as Gooding et al. have shown in the case of peroxidases that direct electrochemistry came predominantly from the enzyme immobilized at the ends of the nanotubes, and that the electron transfer rates were independent of nanotube length [205].
3.3.2 Properties Through Modification
As described below, CNTs can be modified to improve dispersion in a solvent, to impart redox properties, and to allow binding to a particular biological recognition element such as an enzyme, antibody or DNA sequence.
3.3.2.1 Noncovalent Modification
Various ionic and non-ionic polymers can be “wrapped” around CNTs due to a combination of Van der Waals forces and hydrophobic interactions. Typically the polymer has a recurring functional group which interacts with the wall of the CNT, causing the wrapping to occur. Examples of such polymers include poly(p-phenylenevinylene) [210], poly{(m-phenylenevinylene)-co-[2,5-dioctyloxy-(−p-phenylenevinylene]} [211] and biopolymers such as amylose [191]. Essentially, the forces of attraction which occur between the CNTs, causing bundle formation, are replaced by forces of attraction to the polymer. Hence, the wrapping process solubilised the nanotubes. This is extremely useful if the nanotubes are to be deposited on an electrode in an uniform and reproducible manner. If the solubilisation is performed by surfactant molecules such as sodium dodecyl sulphate (SDS) [212] or sodium dodecyl benzene sulphate (SDBS) [213] then the nanotubes can be dispersed in aqueous solutions. In the case of Nafion-solublisation of CNTs, it was shown that the presence of the Nafion did not prevent the CNTs catalytic effect on the redox of hydrogen peroxide [199].
Redox behavior can be imparted to CNTs by the adsorption of aromatic compounds. Due to consisting of sp 2 hybridized carbon atoms, a CNT will possess an extended system of delocalized π electrons and this can interact with the π-system of an aromatic molecule. However, the molecule needs to be larger than a single benzene ring for adsorption to occur. If the molecule possesses redox properties then these will hence be imparted to the nanotube conjugate. For example, methylene blue (MB) has been strongly adsorbed onto CNTs purely by sonication [214], and the resulting structures showed a fast rate of electron transfer when adsorbed on glassy carbon electrodes [214–216]. Acridine orange (the MB molecule with the sulphur atom replaced by carbon) has also been adsorbed onto CNTs by the same method [216].
3.3.2.2 Covalent Modification
The covalent modification of CNTs is a widely researched field and a detailed review of all the methods of covalent nanotube attachment is beyond the scope of this chapter. Some comprehensive reviews are given in refs [217–219]. When nanotubes are treated with strong acids [220], or strong acids mixed with an oxidizing agent [221], for purification and opening, the nanotube ends and defects become functionalized by carboxyl groups. These groups can then undergo further reaction [222–225].
In contrast to tube-end modification, reaction with the side walls of CNTs proceeds by fictionalization of “pristine”, i.e. unoxidised, tubes. Highly reactive reagents are usually necessary. Nanotubes with smaller diameters are likely to be more amenable to reaction, due to their greater curvature causing a greater distortion of the π-electron cloud, thus presenting a richer π-electron surface. Methods of fictionalization include fluorination [226], addition reactions with carbenes, such as dipyridyl imidazolium carbene [227], or with nitrenes [228], reaction with ylides [229], solution phase ozonolysis [230] or silylation [231]. Electrochemistry can also be used as a means of fictionalization, either by performing the electrode reaction of a compound (e.g. a diazonium salt [232]) which then reacts with the nanotube, or by electrooxidation of the nanotubes themselves. All of these methods can in principle be used to attach an appropriately modified redox molecule to the nanotube, and hence there is huge scope for modifying nanotube redox behavior.
3.3.2.3 Biological Modification
The biological modification of CNTs covers both covalent and noncovalent methods. The first such modifications were performed in the mid to late 1990s [233, 234] and since then a large amount of research has been performed in this field. Some recent reviews are given in ref [235, 236]. In the case of modification by enzymes, both covalent and non-covalent attachment are possible, although non-covalent attachment is thought to cause less change to the enzyme confirmation [237], and is thus more likely to retain enzyme activity. The simplest form of non-covalent attachment is direct adsorption to the nanotube. The forces responsible for the attachment can be a combination of hydrophobic interactions and π–π stacking between the delocalized π-system of the nanotube and residues on the enzyme containing aromatic groups (tryptophan, phenylalanine etc.) [237]. Electrostatic attraction can also promote direct adsorption [238] in cases where a nanotube has been carboxylated, provided the protons of the carboxylic acids are dissociated and the pH is below the enzyme isoelectric point, causing it to have a positive charge. Carboxylation of CNTS can also promote direct adsorption via hydrogen bonding [239]. Note that although structural changes upon adsorption can be expected to be less than with covalent attachment, they still occur. For example, horseradish peroxidase has been shown to lose about 35% of the helical content of its secondary structure when adsorbed on SWNTs [240].
Alternative to direct adsorption, the CNTs can first be modified by polymers or surfactants as described in Sect. 3.3.2.1. This has the advantage of imparting good aqueous dispersibility to the nanotubes and thus allowing them to be well mixed with the enzymes to be immobilized. The enzymes are taken up mainly through electrostatic attractions [241–243]. In some cases, such as the adsorption of glucose oxidase onto polyaniline-coated MWNTs, this method has enabled direct electrochemistry [242]. Surfactants which have been used for this purpose include SDS and Eastman AQ (for HRP) [242] and Triton X-100 (for biliverdin IXβ reductase) [244].
A popular method of modifying surfaces with polymers is the layer-by-layer (l-b-l) technique first described by Decher and co-workers [245]. This involves the deposition of alternating layers of oppositely charged polyelectrolytes. The adsorption of each layer causes a charge over-compensation to occur, making it possible to then adsorb a layer of the opposite charge [246]. The method can be adapted to the deposition of enzymes, provided the pH used is not equal to the enzymes’ isoelectric point, i.e. provided the enzyme has an overall charge. Based on this method sensitive glucose sensors were developed using the immobilization of glucose oxidase (GOx) on SWNTs [247] as well as sensitive immunoassays using HRP with chemiluminescence detection [248], and alkaline phosphatase (ALP) with electrochemical detection [249].
In terms of covalent attachment of enzymes to nanotubes, this can be performed either by directly attaching the enzyme, usually by forming an amide bond between enzyme amine groups and carboxyl groups on the CNT sidewalls [250], or by using a linking molecule. The linking molecule is adsorbed to the CNT through hydrophobic and π–π interactions, and can then be bound to the enzyme through amino groups [251] or reaction with succinimidy ester groups [252], for example.
Since antibodies are also proteins, much of the above discussion for the enzyme modification of CNTs can also be applied to modification by antibodies. One of the simplest methods of antibody attachment is direct adsorption [253–255]. This is thought to proceed by hydrophobic interactions [256]. Probably due to the similarity in hydrophobicities, CNTs coated by aromatic redox compounds, such as methylene blue, can also take up antibodies by direct adsorption. This enables the modified CNTs to act as immunoassay redox labels. Not all of the CNTs communicate with the electrode in such a configuration, but when a solution phase mediator is added, to carry charge from CNT to electrode, fg mL−1 concentrations can be detected [257]. Alternatively, antibodies can be coated onto CNTs by the l-b-l method [258] or by using a succinimidyl link [259, 260].
A simple demonstration of DNA immobilization on CNTs can be performed by sonicating bundles of CNTs in water in the presence of single strand DNA (ssDNA). The result is that the CNTs are dispersed, due to the helical wrapping of the ssDNA around the nanotubes. The bases are attracted to the nanotube sidewalls by π–π interactions, and so the ionized sugar-phosphate backbone points outwards, imparting solubility in aqueous media [261]. This type of immobilization can be used to form sensors, based on the fact that metal ions induce a structural change in the DNA, which can be detected as a change in IR spectra [262]. A spectral change is also associated with hybridization, and hence the complementary DNA strand can be detected [263]. CNTs can also be covalently attached to DNA. Acid treatment can be used to produce carboxylic acid groups on CNT sidewalls and these can be reacted with amine-tagged DNA to form amide bonds [264]. If the CNTs are modified by a redox couple then the DNA hybridization can be detected, as has been demonstrated using ferrocene carboxaldehyde [265]. Alternatively, streptavidin can be adsorbed onto the CNTs and then attached to DNA bearing a biotin tag [266]. In this case hybridization detection came from the fact that the CNT was also functionalized by ALP. Thus, enzyme immobilization on a nanotube can be combined with either antibody or DNA immobilization, to produce a highly sensitive electrochemical label.
3.4 Carbon Nanotube Modification of Electrodes
If CNTs are dispersed in solution by one of the coating methods described above, then they can be cast directly onto the electrode. The advantage of this procedure is its simplicity, the disadvantage is the lack of uniformity that will occur over the electrode surface and the degree of variation from one electrode to the next. Casting can also be used to deposit composites of nanotubes mixed with other electroactive materials. A thorough review of this is given by Agui et al. [267]. The most commonly used materials for composite production are conducting polymers and metal nanoparticles. In the case of conducting polymers, the main purpose if to provide the CNTs with good electrode adhesion within a conducting matrix. If the conducting polymer is electrochemically deposited onto the CNT layer, then the polymer quantity can be fairly accurately regulated by controlling the charge passed during the deposition. Analyte reaction can possibly occur at the polymer as well as at the CNTs, depending on the potential and polymer used. Examples of composites of this kind include polyaniline/MWNT films on Au for nitrite oxidation [268], polypyrrole/SWNT films on glassy carbon for ascorbic acid, dopamine and uric acid [269], and poly3-methylthiophene/MWNT composites for NADH oxidation [270].
The construction of nanoparticle (NP)/CNTs composites is mainly performed to increase electrocatalytic activity to a particular analyte. After casting a nanotube film, metal nanoparticles can be electrochemically deposited onto the film, as has been demonstrated for Pt-NP/MWNT coatings for estrogen detection [271]. Alternatively, the metal nanoparticles can be synthesised in the presence of CNTs, which results in adsorption onto the nanotube surface. Examples of this include FeCo-NP/MWNT composites for cathodic hydrogen peroxide detection [272] and Ag/MWNT composites for thiocyanate detection [273]. Nanotube electrode coatings bearing multiple species of nanoparticle are also possible [274]. Finally, after a nanoparticle–CNT composite has been synthesised and deposited on an electrode, a conducting polymer can be electropolymerised over the film. This has been demonstrated for the electropolymerisation of polythionine onto a Au-NP/MWNT coating for an electrode detecting DNA by guanine and adenine oxidation [275].
Another relatively simple electrode modification is to mix the nanotubes with mineral oil and pack then into a cavity above an electrical contact [276], analogous to the way carbon paste electrodes are made. This has been done using unmodified nanotubes for the detection of drugs such as pentoxifylline [277], urapidil [278], sulfamethoxazole [279], and clinically important analytes such as theophylline [280] and homocysteine [281]. Nanotubes have also been mixed with ionic liquids [282] and with a number of different mediators, which act as redox catalysts for an analyte. These have included hydroquinone [283] and a number of different metal complexes [284–289]. The combination in the paste of redox mediator, nanotube and enzyme has also been used to demonstrate biosensors for glucose [290] and lactate [291]. Nanotube pastes have also been mixed with a metal chelator for stripping analysis [292] and with Ag nanoparticles for electrocatalytic oxidation [293]. It has been found that if the metallic impurities on the nanotubes are removed prior to forming the paste, then the electrodes have better between batch precision [294]. This is due to the active role of the impurities in many electrochemical reactions, and the fact that the quantity of impurities can change from batch to batch. However, variations can still occur regarding the electrode surface, and similarly to carbon paste electrodes, we can expect a large amount of time will be required smoothing the electrode over a flat surface before use, to ensure reproducibility. An easier way to achieve reproducibility from roughly the same nanotube matrix is to use the CNTs to form an ink which can be screen printed [295].
A greater level of reproducibility can be achieved by modifying the electrodes with vertically aligned nanotubes. This has the advantage of presenting the highly catalytic end groups for reaction, and as noted in Sect. 3.3.1, can allow direct electrochemistry from enzymes. Alignment can be achieved by first generating carboxylic acid groups at the ends of the CNTs [296] and then reacting these to form functionalities that can be attached to an electrode surface. Examples of this include thiol-tagging for alignment on Au [297], metal ions as bridging agents between end groups and a modified surface [296], alignment to a modified surface by electrostatic attraction [298], and alignment through complexation [299]. Alternatively, aligned nanotubes can be grown onto a surface by photolithography [300].
3.5 Carbon Nanotube-Modified Electrodes in Microfluidics
CNTs have been used in microfluidic systems for the construction of both chemical and biosensors. The field has been reviewed relatively recently by Chen [301]. The simplest method of introducing CNTs to a microfluidic system is to deposit a CNT dispersion onto a working electrode that can be fitted into a microchip-based detector. CNT dispersions in Nafion have been deposited onto screen printed electrodes to form a chemical sensor for the detection, after separation, of hydrazine, phenol, purine and some amino acids [302], and deposited onto a glassy carbon electrode for use as an immunosensor. In the latter case the HRP label on an antibody specific to prostate specific antigen (PSA) was detected via the oxidation and then re-reduction at the electrode of 4-tert-butylcatechol [303]. Other electrodes modified by CNT deposition include indium tin oxide (ITO)-coated glass for dopamine measurement (CNTs dispersed in SDS and then spin coated onto the electrode) [304], Au electrodes for the oxidation of antibiotics [305], phenols [306], and catecholamines and their metabolites [307].
The use of CNT-composite electrodes has also been popular with microfluidic detectors. These have included CNTs mixed with Teflon [200], epoxy (thiols) [308], copper (carbohydrates) [309], dihydropyran (insulin) [310, 311], polystyrene (rutin) [311], and the polymer EpoTek H77A (free chlorine) [312]. The CNT composite can be cast onto a glassy carbon electrode surface [310, 311], but is more usually fitted into a cavity above an electrical contact. The same packing in a cavity procedure is used when CNT/carbon paste electrodes, made by mixing CNTs with mineral oil, are used in microfluidics [313]. Alternatively, covalent immobilization of an enzyme onto CNTs which are then formed into a specific microscale shape can be used to form a bioreactor which can then be fitted into a microfluidic device, as shown in Fig. 4 for co-immobilized glucose oxidase and HRP [314].
Microreactor fabricated from polydimethylsiloxane (PDMS), incorporating microreactor of SWNTs functionalised with glucose oxidase and horseradish peroxidase. Taken with permission from [314] © Elsevier Ltd
As noted in Sect. 3.1, CVD production of CNTs can allow control of position and alignment, and this has been used to grow CNTs directly on the sensing portion of the microchip [315–317]. Interest in this method stems from the fact that it is thought to provide a higher CNT density, better alignment and stronger substrate adhesion than casting CNT dispersions or forming CNT pastes. The technique has been applied to biosensor construction by immobilizing cholesterol oxidase (ChOx) onto the aligned nanotubes and measuring the oxidation of the redox mediator Fe(CN) 4−6 [315]. The CNTs were grown on a Au film that had been sputter-coated in the form of a strip across the microchannel of the device. ChOx was immobilized on the nanotubes by using the microchannel to deliver a ChOx/Fe(CN) 4−6 /poly(vinyl alcohol) solution to the working electrode. A similar CNT deposition method, onto a Au strip sputter-coated across a microchannel, was used to construct a microfluidic device to measure salbutamol by its irreversible oxidation at the nanotubes [316]. As well as direct growth onto sputtered Au electrodes, CVD can also be used to form CNTs within the nanopores of a preformed layer of Al oxide. When used to detect iodide this method was found to have better signal to noise characteristics that that of aligned CNTs alone [317].
4 Concluding Remarks
The use of microfluidic-based analytical devices has a number of advantages over analysis performed on a conventional scale. These include: only a low volume of samples and reagents are needed, reducing the cost of the analysis and the amount of waste generated; the large surface-to-volume ratio of the fluids used enhances mass and heat transfer, thus shortening analysis time; the portability of the device allows on-site analysis; multiple samples can be analyzed in parallel, allowing a high throughput. Of the detection systems used with microfluidics, electrochemistry is attractive due to providing relatively high sensitivities at relatively low cost. Also, where needed, microelectrode fabrication techniques are now well established. As described in this chapter, CNTs can be used to modify electrodes and can improve the response to particular analytes. This can be by: increasing the effective electrode area; catalyzing the electrode reaction of an analyte; enabling direct electron transfer from an enzyme that reacts with a particular analyte; acting as a conductive support for a redox mediator which can react with the analyte; forming the base of an electrochemical label to provide detection of an antibody-antigen or a DNA target-probe binding event. The well-characterized methods of chemical and adsorptive CNT modification mean that there is a wide scope for the further improvement of microfluidic capabilities by the use of CNTs in electrochemical detectors.
References
Bi H, Weng X, Qu H et al (2005) Strategy for allosteric analysis based on protein-patterned stationary phase in microfluidic chip. J Proteome Res 4(6):2154–2160
Blanco-Gomez G, Glidle A, Flendrig LM et al (2009) Integration of low-power microfluidic pumps with biosensors within a laboratory-on-a-chip device. Anal Chem 81(4):1365–1370
Chatrathi MP, Collins GE, Wang J (2007) Microchip-based electrochemical enzyme immunoassays. Methods Mol Biol (Clifton, NJ) 385:215–224
Dong H, Li CM, Zhang YF et al (2007) Screen-printed microfluidic device for electrochemical immunoassay. Lab Chip 7(12):1752–1758
Ferguson BS, Buchsbaum SF, Swensen JS et al (2009) Integrated microfluidic electrochemical DNA sensor. Anal Chem 81(15):6503–6508
Ferguson BS, Buchsbaum SF, Wu TT et al (2011) Genetic analysis of h1n1 influenza virus from throat swab samples in a microfluidic system for point-of-care diagnostics. J Am Chem Soc 133(23):9129–9135
He P, Greenway G, Haswell SJ (2010) Development of enzyme immobilized monolith micro-reactors integrated with microfluidic electrochemical cell for the evaluation of enzyme kinetics. Microfluid Nanofluidics 8(5):565–573
Huang CJ, Chien HC, Chou TC et al (2011) Integrated microfluidic system for electrochemical sensing of glycosylated hemoglobin. Microfluid Nanofluidics 10(1):37–45
Jiang JH, Bo ML, Jiang DC et al (2009) A novel fabrication route to integrating label-free detection of DNA hybridization in microfluidic channel. In: IFMBE proceedings, pp 140–143
Kwakye S, Baeumner A (2003) A microfluidic biosensor based on nucleic acid sequence recognition. Anal Bioanal Chem 376(7):1062–1068
Lin L, Cai Y, Lin R et al (2011) New integrated in vivo microdialysis-electrochemical device for determination of the neurotransmitter dopamine in rat striatum of freely moving rats. Microchim Acta 172(1):217–223
Liu RH, Yang J, Lenigk R et al (2004) Self-contained, fully integrated biochip for sample preparation, polymerase chain reaction amplification, and DNA microarray detection. Anal Chem 76(7):1824–1831
Njoroge SK, Chen HW, Witek MA et al (2011) Integrated microfluidic systems for DNA analysis. Top Curr Chem 304:203–260
Park S, Zhang Y, Lin S et al (2011) Advances in microfluidic PCR for point-of-care infectious disease diagnostics. Biotechnol Adv 29:830–839
Pavlovic E, Lai RY, Wu TT et al (2008) Microfluidic device architecture for electrochemical patterning and detection of multiple DNA sequences. Langmuir 24(3):1102–1107
Pereira SV, Bertolino FA, Messina GA et al (2011) Microfluidic immunosensor with gold nanoparticle platform for the determination of immunoglobulin g anti-Echinococcus granulosus antibodies. Anal Biochem 409(1):98–104
Abdelgawad M, Watson MWL, Wheeler AR (2009) Hybrid microfluidics: a digital-to-channel interface for in-line sample processing and chemical separations. Lab Chip 9(8):1046–1051
Srinivasan V, Pamula VK, Fair RB (2004) Droplet-based microfluidic lab-on-a-chip for glucose detection. Anal Chim Acta 507(1):145–150
Zhao Y, Chakrabarty K (2011) Fault diagnosis in lab-on-chip using digital microfluidic logic gates. J Electron Test 27(1):69–83
Laser DJ, Santiago JG (2004) A review of micropumps. J Micromech Microeng 14(6):R35–R64
Roman GT, Kennedy RT (2007) Fully integrated microfluidic separations systems for biochemical analysis. J Chromatogr A 1168(1–2):170–188
Pennathur S (2008) Flow control in microfluidics: are the workhorse flows adequate? Lab Chip 8(3):383–387
Squires TM, Quake SR (2005) Microfluidics: fluid physics at the nanoliter scale. Rev Mod Phys 77(3):977–1026
Atalay YT, Vermeir S, Witters D et al (2011) Microfluidic analytical systems for food analysis. Trends Food Sci Technol 22(7):386–404
Nguyen NT, Wu Z (2005) Micromixers – a review. J Micromech Microeng 15(2):R1–R16
Zhang C, Xing D, Li Y (2007) Micropumps, microvalves, and micromixers within PCR microfluidic chips: advances and trends. Biotechnol Adv 25(5):483–514
Crevillen AG, Avila M, Pumera M et al (2007) Food analysis on microfluidic devices using ultrasensitive carbon nanotubes detectors. Anal Chem 79(19):7408–7415
Jayarajah CN, Skelley AM, Fortner AD et al (2007) Analysis of neuroactive amines in fermented beverages using a portable microchip capillary electrophoresis system. Anal Chem 79(21):8162–8169
Revermann T, Götz S, Künnemeyer J et al (2008) Quantitative analysis by microchip capillary electrophoresis – current limitations and problem-solving strategies. Analyst 133(2):167–174
Karuwan C, Sukthang K, Wisitsoraat A et al (2011) Electrochemical detection on electrowetting-on-dielectric digital microfluidic chip. Talanta 84(5):1384–1389
Paik P, Pamula VK, Fair RB (2003) Rapid droplet mixers for digital microfluidic systems. Lab Chip 3(4):253–259
Nisar A, Afzulpurkar N, Mahaisavariya B et al (2008) Mems-based micropumps in drug delivery and biomedical applications. Sens Actuators B Chem 130(2):917–942
Smits JG (1990) Piezoelectric micropump with three valves working peristaltically. Sens Actuators A Phys 21(1–3):203–206
Van de Pol FCM, Van Lintel HTG, Elwenspoek M et al (1990) A thermopneumatic micropump based on micro-engineering techniques. Sens Actuators A Phys 21(1–3):198–202
Stemme E, Stemme G (1993) A valveless diffuser/nozzle-based fluid pump. Sens Actuators A Phys 39(2):159–167
Hsu YC, Lin SJ, Hou CC (2008) Development of peristaltic antithrombogenic micropumps for in vitro and ex vivo blood transportation tests. Microsyst Technol 14(1):31–41
Jeong OC, Park SW, Yang SS et al (2005) Fabrication of a peristaltic PDMS micropump. Sens Actuators A Phys 123–124:453–458
Teymoori MM, Abbaspour-Sani E (2004) Design and simulation of a novel electrostatic peristaltic micromachined pump for drug delivery applications. Sens Actuators A Phys 117(2):222–229
Lee SW, Sim WY, Yang SS (2000) Fabrication and in vitro test of a microsyringe. Sens Actuators A Phys 83(1):17–23
Suzuki H, Yoneyama R (2002) A reversible electrochemical nanosyringe pump and some considerations to realize low-power consumption. Sens Actuators B Chem 86(2–3):242–250
Dapper J, Clemens M, Ehrfeld W et al (1997) Micro gear pumps for dosing of viscous fluids. J Micromech Microeng 7(3):230–232
Feng GH, Kim ES (2005) Piezoelectrically actuated dome-shaped diaphragm micropump. J Microelectromech Syst 14(2):192–199
Kan J, Yang Z, Peng T et al (2005) Design and test of a high-performance piezoelectric micropump for drug delivery. Sens Actuators A Phys 121(1):156–161
Koch M, Harris N, Evans AGR et al (1998) A novel micromachined pump based on thick-film piezoelectric actuation. Sens Actuators A Phys 70(1–2):98–103
Benard WL, Kahn H, Heuer AH et al (1997) Titanium-nickel shape-memory alloy actuated micropump. In: Proceedings of the international conference on solid-state sensors and actuators, vol 1, pp 361–364
Benard WL, Kahn H, Heuer AH et al (1998) Thin-film shape-memory alloy actuated micropumps. J Microelectromech Syst 7(2):245–251
Zhan C, Lo T, Liu L et al (1996) A silicon membrane micropump with integrated bimetallic actuator. Chin J Electron 5(2):33–35
Guo S, Nakamura T, Fukuda T et al (1997) Development of the micro pump using ICPF actuator. In: Proceedings of the IEEE international conference on robotics and automation, vol 1, pp 266–271
Bourouina T, Bosseboeuf A, Grandchamp JP (1997) Design and simulation of an electrostatic micropump for drug-delivery applications. J Micromech Microeng 7(3):186–188
MacHauf A, Nemirovsky Y, Dinnar U (2005) A membrane micropump electrostatically actuated across the working fluid. J Micromech Microeng 15(12):2309–2316
Gong Q, Zhou Z, Yang Y et al (2000) Design, optimization and simulation on microelectromagnetic pump. Sens Actuators A Phys 83(1):200–207
Yamahata C, Lotto C, Al-Assaf E et al (2005) A PMMA valveless micropump using electromagnetic actuation. Microfluid Nanofluidics 1(3):197–207
Liao CS, Lee GB, Liu HS et al (2005) Miniature RT-PCR system for diagnosis of RNA-based viruses. Nucleic Acids Res 33(18)
Lien KY, Lee WC, Lei HY et al (2007) Integrated reverse transcription polymerase chain reaction systems for virus detection. Biosens Bioelectron 22(8):1739–1748
Jeong OC, Yang SS (2000) Fabrication and test of a thermopneumatic micropump with a corrugated p+ diaphragm. Sens Actuators A Phys 83(1):249–255
Kim JH, Na KH, Kang CJ et al (2005) A disposable thermopneumatic-actuated micropump stacked with PDMS layers and ito-coated glass. Sens Actuators A Phys 120(2):365–369
Boden R, Lehto M, Simu U et al (2005) A polymeric paraffin micropump with active valves for high-pressure microfluidics. In: Digest of technical papers – international conference on solid state sensors and actuators and microsystems, TRANSDUCERS ’05, vol 1, pp 201–204
Sim WY, Yoon HJ, Jeong OC et al (2003) A phase-change type micropump with aluminum flap valves. J Micromech Microeng 13(2):286–294
Huang L, Wang W, Murphy MC et al (2000) Liga fabrication and test of a dc type magnetohydrodynamic (MHD) micropump. Microsyst Technol 6(6):235–240
Jang J, Lee SS (2000) Theoretical and experimental study of MHD (magnetohydrodynamic) micropump. Sens Actuators A Phys 80(1):84–89
Munchow G, Dadic D, Doffing F et al (2005) Automated chip-based device for simple and fast nucleic acid amplification. Expert Rev Mol Diagn 5(4):613–620
Darabi J, Rada M, Ohadi M et al (2002) Design, fabrication, and testing of an electrohydrodynamic ion-drag micropump. J Microelectromech Syst 11(6):684–690
Fuhr G, Hagedorn R, Muller T et al (1992) Pumping of water solutions in microfabricated electrohydrodynamic systems. 25–30
Chen L, Wang H, Ma J et al (2005) Fabrication and characterization of a multi-stage electroosmotic pump for liquid delivery. Sens Actuators B Chem 104(1):117–123
Zeng S, Chen CH, Mikkelsen JC Jr et al (2001) Fabrication and characterization of electroosmotic micropumps. Sens Actuators B Chem 79(2–3):107–114
Yun KS, Cho IJ, Bu JU et al (2002) A surface-tension driven micropump for low-voltage and low-power operations. J Microelectromech Syst 11(5):454–461
Zahn JD, Deshmukh A, Pisano AP et al (2004) Continuous on-chip micropumping for microneedle enhanced drug delivery. Biomed Microdevices 6(3):183–190
Tsai JH, Lin L (2001) A thermal bubble actuated micro nozzle-diffuser pump. In: Proceedings of the IEEE micro electro mechanical systems (MEMS), pp 409–412
Yoshimi Y, Shinoda K, Mishima M et al (2004) Development of an artificial synapse using an electrochemical micropump. J Artif Organs 7(4):210–215
Luginbuhl P, Collins SD, Racine GA et al (1997) Microfabricated lamb wave device based on PZT sol–gel thin film for mechanical transport of solid particles and liquids. J Microelectromech Syst 6(4):337–346
Nguyen NT, Meng AH, Black J et al (2000) Integrated flow sensor for in situ measurement and control of acoustic streaming in flexural plate wave micropumps. Sens Actuators A Phys 79(2):115–121
Effenhauser CS, Harttig H, Kramer P (2002) An evaporation-based disposable micropump concept for continuous monitoring applications. Biomed Microdevices 4(1):27–32
Carrozza MC, Croce N, Magnani B et al (1995) A piezoelectric-driven stereolithography-fabricated micropump. J Micromech Microeng 5(2):177–179
Prakash R, Kaler KVIS (2007) An integrated genetic analysis microfluidic platform with valves and a PCR chip reusability method to avoid contamination. Microfluid Nanofluidics 3(2):177–187
Fan ZH, Ricco AJ, Tan W et al (2003) Integrating multiplexed PCR with CE for detecting microorganisms. Micro Total Anal Syst 849
Koh CG, Tan W, Zhao MQ et al (2003) Integrating polymerase chain reaction, valving, and electrophoresis in a plastic device for bacterial detection. Anal Chem 75(17):4591–4598
Gong H, Ramalingam N, Chen L et al (2006) Microfluidic handling of PCR solution and DNA amplification on a reaction chamber array biochip. Biomed Microdevices 8(2):167–176
Kohl M, Dittmann D, Quandt E et al (2000) Thin film shape memory microvalves with adjustable operation temperature. Sens Actuators A Phys 83(1):214–219
Zdeblick MJ, Anderson R, Jankowski J et al (1994) Thermopneumatically actuated microvalves and integrated electro-fluidic circuits. In: Solid-state sensor and actuator workshop, pp 251–255
Felton MJ (2003) The new generation of microvalves. Anal Chem 75(19):429A–432A
Esashi M, Shoji S, Nakano A (1989) Normally closed microvalve and mircopump fabricated on a silicon wafer. Sens Actuators 20(1–2):163–169
Fahrenberg J, Bier W, Maas D et al (1995) A microvalve system fabricated by thermoplastic molding. J Micromech Microeng 5(2):169–171
Pal R, Yang M, Johnson BN et al (2004) Phase change microvalve for integrated devices. Anal Chem 76(13):3740–3748
Wang J, Chen Z, Mauk M et al (2005) Self-actuated, thermo-responsive hydrogel valves for lab on a chip. Biomed Microdevices 7(4):313–322
Liu Y, Rauch CB, Stevens RL et al (2002) DNA amplification and hybridization assays in integrated plastic monolithic devices. Anal Chem 74(13):3063–3070
Liu RH, Bonanno J, Yang J et al (2004) Single-use, thermally actuated paraffin valves for microfluidic applications. Sens Actuators B Chem 98(2–3):328–336
Pal R, Yang M, Lin R et al (2005) An integrated microfluidic device for influenza and other genetic analyses. Lab Chip 5(10):1024–1032
Gui L, Liu J (2004) Ice valve for a mini/micro flow channel. J Micromech Microeng 14(2):242–246
Stone HA, Stroock AD, Ajdari A (2004) Engineering flows in small devices: microfluidics toward a lab-on-a-chip. Annu Rev Fluid Mech 36:381–411
Stroock AD, Dertinger SKW, Ajdari A et al (2002) Chaotic mixer for microchannels. Science 295(5555):647–651
Hashimoto M, Barany F, Soper SA (2006) Polymerase chain reaction/ligase detection reaction/hybridization assays using flow-through microfluidic devices for the detection of low-abundant DNA point mutations. Biosens Bioelectron 21(10):1915–1923
Legendre LA, Bienvenue JM, Roper MG et al (2006) A simple, valveless microfluidic sample preparation device for extraction and amplification of DNA from nanoliter-volume samples. Anal Chem 78(5):1444–1451
Mastrangelo CH, Burns MA, Burke DT (1999) Integrated microfabricated devices for genetic assays. In: Microprocesses and nanotechnology conference, Yokohama, pp 1–2
Chang YH, Lee GB, Huang FC et al (2006) Integrated polymerase chain reaction chips utilizing digital microfluidics. Biomed Microdevices 8(3):215–225
Curcio M, Roeraade J (2003) Continuous segmented-flow polymerase chain reaction for high-throughput miniaturized DNA amplification. Anal Chem 75(1):1–7
Mohr S, Zhang YH, Macaskill A et al (2007) Numerical and experimental study of a droplet-based PCR chip. Microfluid Nanofluidics 3(5):611–621
Jacobson SC, McKnight TE, Ramsey JM (1999) Microfluidic devices for electrokinetically driven parallel and serial mixing. Anal Chem 71(20):4455–4459
Lee CY, Lee GB, Lin JL et al (2005) Integrated microfluidic systems for cell lysis, mixing/pumping and DNA amplification. J Micromech Microeng 15(6):1215–1223
Liu RH, Yang J, Pindera MZ et al (2002) Bubble-induced acoustic micromixing. Lab Chip 2(3):151–157
Bau HH, Zhong J, Yi M (2001) A minute magneto hydro dynamic (MHD) mixer. Sens Actuators B Chem 79(2–3):207–215
Gotz S, Karst U (2007) Recent developments in optical detection methods for microchip separations. Anal Bioanal Chem 387(1):183–192
Newman CID, Giordano BC, Cooper CL et al (2008) Microchip micellar electrokinetic chromatography separation of alkaloids with UV-absorbance spectral detection. Electrophoresis 29(4):803–810
Ibanez-Garcia N, Mercader MB, Mendes Da Rocha Z et al (2006) Continuous flow analytical microsystems based on low-temperature co-fired ceramic technology. Integrated potentiometric detection based on solvent polymeric ion-selective electrodes. Anal Chem 78(9):2985–2992
Rashid M, Dou YH, Auger V et al (2010) Recent developments in polymer microfluidic devices with capillary electrophoresis and electrochemical detection. Micro Nanosyst 2(2):108–136
Wang J, Polsky R, Tian B et al (2000) Voltammetry on microfluidic chip platforms. Anal Chem 72(21):5285–5289
Mishra NN, Retterer S, Zieziulewicz TJ et al (2005) On-chip micro-biosensor for the detection of human CD4+ cells based on ac impedance and optical analysis. Biosens Bioelectron 21(5):696–704
Triroj N, Lapierre-Devlin MA, Kelley SO et al (2006) Microfluidic three-electrode cell array for low-current electrochemical detection. IEEE Sens J 6(6):1395–1402
Moon BU, Koster S, Wientjes KJC et al (2010) An enzymatic microreactor based on chaotic micromixing for enhanced amperometric detection in a continuous glucose monitoring application. Anal Chem 82(16):6756–6763
Schwarz MA (2004) Enzyme-catalyzed amperometric oxidation of neurotransmitters in chip-capillary electrophoresis. Electrophoresis 25(12):1916–1922
Moreira NH, De Jesus De Almeida AL, De Oliveira Piazzeta MH et al (2009) Fabrication of a multichannel PDMS/glass analytical microsystem with integrated electrodes for amperometric detection. Lab Chip 9(1):115–121
Sassa F, Laghzali H, Fukuda J et al (2010) Coulometric detection of components in liquid plugs by microfabricated flow channel and electrode structures. Anal Chem 82(20):8725–8732
Wang R, Lin J, Lassiter K et al (2011) Evaluation study of a portable impedance biosensor for detection of avian influenza virus. J Virol Methods 178(1–2):52–58
Segerink LI, Sprenkels AJ, Ter Braak PM et al (2010) On-chip determination of spermatozoa concentration using electrical impedance measurements. Lab Chip 10(8):1018–1024
Sabounchi P, Morales AM, Ponce P et al (2008) Sample concentration and impedance detection on a microfluidic polymer chip. Biomed Microdevices 10(5):661–670
Crevillen AG, Pumera M, Gonzalez MC et al (2009) Towards lab-on-a-chip approaches in real analytical domains based on microfluidic chips/electrochemical multi-walled carbon nanotube platforms. Lab Chip 9(2):346–353
Hervas M, Lopez MA, Escarpa A (2011) Integrated electrokinetic magnetic bead-based electrochemical immunoassay on microfluidic chips for reliable control of permitted levels of zearalenone in infant foods. Analyst 136(10):2131–2138
Kovaehev N, Canals A, Escarpa A (2010) Fast and selective microfluidic chips for electrochemical antioxidant sensing in complex samples. Anal Chem 82(7):2925–2931
Shiddiky MJA, Park H, Shim YB (2006) Direct analysis of trace phenolics with a microchip: in-channel sample preconcentration, separation, and electrochemical detection. Anal Chem 78(19):6809–6817
Fritsch I, Aguilar ZP (2007) Advantages of downsizing electrochemical detection for DNA assays. Anal Bioanal Chem 387(1):159–163
Yang SY, DeFranco JA, Sylvester YA et al (2009) Integration of a surface-directed microfluidic system with an organic electrochemical transistor array for multi-analyte biosensors. Lab Chip 9(5):704–708
Zhang Q, Jagannathan L, Subramanian V (2010) Label-free low-cost disposable DNA hybridization detection systems using organic TFTS. Biosens Bioelectron 25(5):972–977
Sharma S, Buchholz K, Luber SM et al (2006) Silicon-on-insulator microfluidic device with monolithic sensor integration for μTAS applications. J Microelectromech Syst 15(2):308–313
Shin KS, Lee SW, Han KC et al (2007) Amplification of fluorescence with packed beads to enhance the sensitivity of miniaturized detection in microfluidic chip. Biosens Bioelectron 22(9–10):2261–2267
Mecomber JS, Stalcup AM, Hurd D et al (2006) Analytical performance of polymer-based microfluidic devices fabricated by computer numerical controlled machining. Anal Chem 78(3):936–941
Darain F, Gan KL, Tjin SC (2009) Antibody immobilization on to polystyrene substrate – on-chip immunoassay for horse IgG based on fluorescence. Biomed Microdevices 11(3):653–661
Matsubara Y, Murakami Y, Kobayashi M et al (2004) Application of on-chip cell cultures for the detection of allergic response. Biosens Bioelectron 19(7):741–747
Chandrasekaran A, Acharya A, You JL et al (2007) Hybrid integrated silicon microfluidic platform for fluorescence based biodetection. Sensors 7(9):1901–1915
Al Lawati HAJ, Suliman FEO, Al Kindy SMZ et al (2010) Enhancement of on chip chemiluminescence signal intensity of tris(1,10-phenanthroline)-ruthenium(ii) peroxydisulphate system for analysis of chlorpheniramine maleate in pharmaceutical formulations. Talanta 82(5):1999–2002
Lv Y, Zhang Z, Chen F (2002) Chemiluminescence biosensor chip based on a microreactor using carrier air flow for determination of uric acid in human serum. Analyst 127(9):1176–1179
Thongchai W, Liawruangath B, Liawruangrath S et al (2010) A microflow chemiluminescence system for determination of chloramphenicol in honey with preconcentration using a molecularly imprinted polymer. Talanta 82(2):560–566
Heyries KA, Loughran MG, Hoffmann D et al (2008) Microfluidic biochip for chemiluminescent detection of allergen-specific antibodies. Biosens Bioelectron 23(12):1812–1818
Hosono H, Satoh W, Fukuda J et al (2007) On-chip handling of solutions and electrochemiluminescence detection of amino acids. Sens Actuators B Chem 122(2):542–548
Hosono H, Satoh W, Fukuda J et al (2007) Microanalysis system based on electrochemiluminescence detection. Sens Mater 19(4):191–201
Roda A, Guardigli M, Michelini E et al (2009) Bioluminescence in analytical chemistry and in vivo imaging. Trends Analyt Chem 28(3):307–322
Tran TH, Chang WJ, Kim YB et al (2007) The effect of fluidic conditions on the continuous-flow bioluminescent detection of ATP in a microfluidic device. Biotechnol Bioprocess Eng 12(5):470–474
Maehana K, Tani H, Kamidate T (2006) On-chip genotoxic bioassay based on bioluminescence reporter system using three-dimensional microfluidic network. Anal Chim Acta 560(1–2):24–29
Liu J, Eddings MA, Miles AR et al (2009) In situ microarray fabrication and analysis using a microfluidic flow cell array integrated with surface plasmon resonance microscopy. Anal Chem 81(11):4296–4301
Escobedo C, Vincent S, Choudhury AIK et al (2011) Integrated nanohole array surface plasmon resonance sensing device using a dual-wavelength source. J Micromech Microeng 21(11)
Hsu WT, Hsieh WH, Cheng SF et al (2011) Integration of fiber optic-particle plasmon resonance biosensor with microfluidic chip. Anal Chim Acta 697(1–2):75–82
Huang C, Bonroy K, Reekmans G et al (2009) Localized surface plasmon resonance biosensor integrated with microfluidic chip. Biomed Microdevices 11(4):893–901
Okan M, Balci O, Kocabas C (2011) A microfluidic based differential plasmon resonance sensor. Sens Actuators B Chem 160(1):670–676
Hiep HM, Nakayama T, Saito M et al (2008) A microfluidic chip based on localized surface plasmon resonance for real-time monitoring of antigen-antibody reactions. Jpn J Appl Phys 47(2 Pt 2):1337–1341
Chien WY, Khalid MZ, Hoa XD et al (2009) Monolithically integrated surface plasmon resonance sensor based on focusing diffractive optic element for optofluidic platforms. Sens Actuators B Chem 138(2):441–445
Huang C, Bonroy K, Reekman G et al (2009) An on-chip localized surface plasmon resonance-based biosensor for label-free monitoring of antigen-antibody reaction. Microelectron Eng 86(12):2437–2441
Hemmi A, Usui T, Moto A et al (2011) A surface plasmon resonance sensor on a compact disk-type microfluidic device. J Sep Sci 34(20):2913–2919
Ashok PC, Singh GP, Tan KM et al (2010) Fiber probe based microfluidic raman spectroscopy. Opt Express 18(8):7642–7649
Lim C, Hong J, Chung BG et al (2010) Optofluidic platforms based on surface-enhanced Raman scattering. Analyst 135(5):837–844
Quang LX, Lim C, Seong GH et al (2008) A portable surface-enhanced Raman scattering sensor integrated with a lab-on-a-chip for field analysis. Lab Chip 8(12):2214–2219
Ackermann KR, Henkel T, Popp J (2007) Quantitative online detection of low-concentrated drugs via a sers microfluidic system. Chemphyschem 8(18):2665–2670
Chen L, Choo J (2008) Recent advances in surface-enhanced Raman scattering detection technology for microfluidic chips. Electrophoresis 29(9):1815–1828
Chandrasekaran A, Packirisamy M (2006) Absorption detection of enzymatic reaction using optical microfluidics based intermittent flow microreactor system. IEE Proc Nanobiotechnol 153(6):137–143
Gordon R, Sinton D, Kavanagh KL et al (2008) A new generation of sensors based on extraordinary optical transmission. Acc Chem Res 41(8):1049–1057
Zhao L, Cheng P, Li J et al (2009) Analysis of nonadherent apoptotic cells by a quantum dots probe in a microfluidic device for drug screening. Anal Chem 81(16):7075–7080
Stewart ME, Yao J, Maria J et al (2009) Multispectral thin film biosensing and quantitative imaging using 3D plasmonic crystals. Anal Chem 81(15):5980–5989
Luan L, Evans RD, Jokerst NM et al (2008) Integrated optical sensor in a digital microfluidic platform. IEEE Sens J 8(5):628–635
Fang X, Liu Y, Kong J et al (2010) Loop-mediated isothermal amplification integrated on microfluidic chips for point-of-care quantitative detection of pathogens. Anal Chem 82(7):3002–3006
Lagally ET, Simpson PC, Mathies RA (2000) Monolithic integrated microfluidic DNA amplification and capillary electrophoresis analysis system. Sens Actuators B Chem 63(3):138–146
Nguyen TH, Pei R, Qiu C et al (2009) An aptameric microfluidic system for specific purification, enrichment, and mass spectrometric detection of biomolecules. J Microelectromech Syst 18(6):1198–1207
Wei H, Li H, Mao S et al (2011) Cell signaling analysis by mass spectrometry under coculture conditions on an integrated microfluidic device. Anal Chem 83(24):9306–9313
Bai HY, Lin SL, Chan SA et al (2010) Characterization and evaluation of two-dimensional microfluidic chip-HPLC coupled to tandem mass spectrometry for quantitative analysis of 7-aminoflunitrazepam in human urine. Analyst 135(10):2737–2742
Thorslund S, Lindberg P, Andrén PE et al (2005) Electrokinetic-driven microfluidic system in poly(dimethylsiloxane) for mass spectrometry detection integrating sample injection, capillary electrophoresis, and electrospray emitter on-chip. Electrophoresis 26(24):4674–4683
Sikanen T, Tuomikoski S, Ketola RA et al (2007) Fully microfabricated and integrated SU-8-based capillary electrophoresis-electrospray ionization microchips for mass spectrometry. Anal Chem 79(23):9135–9144
Lazar IM, Grym J, Foret F (2006) Microfabricated devices: a new sample introduction approach to mass spectrometry. Mass Spectrom Rev 25(4):573–594
Leifheit M, Bergmann W, Greiser J (2008) Application of exchangeable biochemical reactors with oxidase-catalase-co-immobilizates and immobilized microorganisms in a microfluidic chip-calorimeter. Eng Life Sci 8(5):540–545
Zhang Y, Tadigadapa S (2004) Calorimetric biosensors with integrated microfluidic channels. Biosens Bioelectron 19(12):1733–1743
Waggoner PS, Tan CP, Craighead HG (2010) Microfluidic integration of nanomechanical resonators for protein analysis in serum. Sens Actuators B Chem 150(2):550–555
Ricciardi C, Canavese G, Castagna R et al (2010) Integration of microfluidic and cantilever technology for biosensing application in liquid environment. Biosens Bioelectron 26(4):1565–1570
Mahabadi KA, Rodriguez I, Lim CY et al (2010) Capacitively coupled contactless conductivity detection with dual top-bottom cell configuration for microchip electrophoresis. Electrophoresis 31(6):1063–1070
Pumera M, Wang J, Opekar F et al (2002) Contactless conductivity detector for microchip capillary electrophoresis. Anal Chem 74(9):1968–1971
Lee H, Sun E, Ham D et al (2008) Chip-NMR biosensor for detection and molecular analysis of cells. Nat Med 14(8):869–874
Mitsakakis K, Gizeli E (2011) Detection of multiple cardiac markers with an integrated acoustic platform for cardiovascular risk assessment. Anal Chim Acta 699(1):1–5
Liu C, Yy M, Zg C et al (2008) Dual fluorescence/contactless conductivity detection for microfluidic chip. Anal Chim Acta 621(2):171–177
Yi C, Zhang Q, Li CW et al (2006) Optical and electrochemical detection techniques for cell-based microfluidic systems. Anal Bioanal Chem 384(6):1259–1268
Wellman AD, Sepaniak MJ (2007) Multiplexed, waveguide approach to magnetically assisted transport evanescent field fluoroassays. Anal Chem 79(17):6622–6628
James CD, McClain J, Pohl KR et al (2010) High-efficiency magnetic particle focusing using dielectrophoresis and magnetophoresis in a microfluidic device. J Micromech Microeng 20(4)
van Lintel HTG, van De Pol FCM, Bouwstra S (1988) A piezoelectric micropump based on micromachining of silicon. Sens Actuators 15(2):153–167
Pan T, McDonald SJ, Kai EM et al (2005) A magnetically driven PDMS micropump with ball check-valves. J Micromech Microeng 15(5):1021–1026
Gervais L, Delamarche E (2009) Toward one-step point-of-care immunodiagnostics using capillary-driven microfluidics and PDMS substrates. Lab Chip 9(23):3330–3337
Martinez AW, Phillips ST, Whitesides GM et al (2010) Diagnostics for the developing world: microfluidic paper-based analytical devices. Anal Chem 82(1):3–10
Nie Z, Nijhuis CA, Gong J et al (2010) Electrochemical sensing in paper-based microfluidic devices. Lab Chip 10(4):477–483
Carvalhal RF, Kfouri MS, De Piazetta MHO et al (2010) Electrochemical detection in a paper-based separation device. Anal Chem 82(3):1162–1165
Iijima S (1991) Helical microtubes of graphitic carbon. Nature 354:56–58
Iijima S, Ichihashi T (1993) Single-shell carbon nanotubes of 1-mm diameter. Nature 363:603–605
Thess A, Lee R, Nikolaev H et al (1998) Crystalline ropes of metallic carbon nanotubes. Science 273:483–487
Yacaman M, Yoshida M, Rendon L et al (1993) Catalytic growth of carbon microtubules with fullerene structure. Appl Phys Lett 62:202–204
Bethune D, Kiang C, de Vries M et al (1993) Cobalt-catalysed growth of carbon nanotubes with single-atomic-layer walls. Nature 363:605–607
Ci L, Rao Z, Zhou Z et al (2002) Double wall carbon nanotubes promoted by sulfur in a floating iron catalyst CVD system. Chem Phys Lett 359:63–67
Lehman J, Terrones M, Mansfield E et al (2011) Evaluating the characteristics of multiwall carbon nanotubes. Carbon 49:2581–2602
Popov V (2004) Carbon nanotubes: properties and application. Mat Sci Eng R Rep 43:61–102
Burghard M (2005) Electronic and vibrational properties of chemically modified single-wall carbon nanotubes. Surf Sci Rep 58:1–109
Krueger A (2010) Carbon materials and nanotechnology. Wiley, Weinheim, pp 123–281
Harris P (2009) Carbon nanotube science. Synthesis, properties and applications. Cambridge University Press, Cambridge
Ye J-S, Shen F-S (2007) Carbon nanotube-based sensor. In: Kumar C (ed) Nanomaterials for biosensors. Wiley, Weinheim
Fam D, Palaniappan A, Tok A (2011) A review on the technological aspects influencing commercialization of carbon nanotube sensors. Sens Actuators B Chem 157:1–7
Vashist S, Zheng D, Al-Rubeaan K et al (2011) Advances in carbon nanotube based electrochemical sensors for bioanalytical applications. Biotechnol Adv 29:169–188
Yanez-Sedeno P, Pingarron J, Riu J et al (2010) Electrochemical sensing based on carbon nanotubes. Trends Analyt Chem 29:939–953
Saito R, Fujita M, Dresselhaus G et al (1992) Electronic structure of chiral graphene tubules. Appl Phys Lett 60:2204–2206
Phanthong C, Somasundrum M (2009) Enhanced sensitivity of 4-chlorophenol detection by the use of nitrobenzene as a liquid membrane over a carbon nanotube-modified glassy carbon electrode. Electroanalysis 20:1024–1027
Wang J, Mussameh M, Lin Y (2003) Solubilization of carbon nanotubes by nafion toward the preparation of amperometric biosensors. J Am Chem Soc 125:2408–2409
Wang J, Musameh M (2003) Carbon nanotube/teflon composite electrochemical sensors and biosensors. Anal Chem 75:2075–2079
Wang Z, Liu J, Liang Q et al (2002) Carbon nanotube-modified electrodes for the simultaneous determination of dopamine and ascorbic acid. Analyst 127:653–658
Rubianes M, Rivas G (2003) Carbon nanotubes paste electrode. Electrochem Commun 5:689–694
Wang J, Li M, Shi Z et al (2002) Electrocatalytic oxidation of norepinephrine at a glassy carbon electrode modified with single wall carbon nanotubes. Electroanalysis 14:225–230
Banks C, Compton R (2006) New elements from old: from carbon nanotubes to edge plane pyrolytic graphite. Analyst 131:15–21
Gooding J, Wibowo R, Lin J et al (2003) Protein electrochemistry using aligned carbon nanotube arrays. J Am Chem Soc 125:9006–9007
Davies J, Coles R, Hill H (1997) Protein electrochemistry at carbon nanotube electrodes. J Electroanal Chem 440:279–282
Zhao G, Zhang L, Wei X et al (2003) Myoglobin on multi-walled carbon nanotubes modified electrode: direct electrochemistry and electrocatalysis. Electrochem Commun 5:825–829
Wang L, Wang J, Zhou F (2004) Direct electrochemistry of catalase at a gold electrode modified with single-wall carbon nanotubes. Electroanalysis 16:627–632
Patolsky F, Weizmann Y, Willner I et al (2004) Long-range electrical contacting of redox enzymes by SWCNT connectors. Angew Chem Int Ed 43:2113–2117
Star A, Stoddart J, Steurman D et al (2001) Preparation and properties of polymer-wrapped single-walled carbon nanotubes. Angew Chem Int Ed 40:1721–1775
Riggs J, Guo Z, Carroll D et al (2000) Strong luminescence of solubilized carbon nanotubes. J Am Chem Soc 122:5879–5880
Manne S, Cleveland J, Gaub H (1994) Direct visualization of surfactant hemimicelles by force microscopy of the electrical double layer. Langmuir 10:4409–4413
Islam M, Rojas E, Bergey D et al (2003) High weight fraction surfactant solubilization of single-wall nanotubes in water. Nano Lett 3:269–273
Yan Y, Zhang M, Gong K et al (2005) Adsorption of methylene blue dye onto carbon nanotubes:? A route to an electrochemically functional nanostructure and its layer-by-layer assembled nanocomposite. Chem Mater 17:3457–3463
Palangsuntikul R, Somasundrum M, Surareungchai W (2000) Kinetic and analytical comparison of horseradish peroxidase on bare and redox-modified single-walled carbon nanotubes. Electrochim Acta 56:470–475
Liu C-H, Li J-J, Zhang H-L et al (2008) Structure dependent interaction between organic dyes and carbon nanotubes. Colloids Surf A 313–314:9–12
Hirsch A, Vostrowsky O (2005) Functionalization of carbon nanotubes. Top Curr Chem 245:193–237
Tasis D, Tagmatarchis N, Bianco A (2006) Chemistry of carbon nanotubes. Chem Rev 106:1105–1136
Prato M, Kostarelos K, Bianco A (2008) Functionalized carbon nanotubes in drug design and discovery. Acc Chem Res 41:60–68
Tsang S, Chen Y, Harris P et al (1994) A simple chemical method of opening and filling carbon nanotubes. Nature 372:159–162
Hiura H, Ebbesen T, Tanigaki K (1995) Opening and purification of carbon nanotubes in high yields. Adv Mater 7:275–276
Chen J, Hamon M, Hu H et al (1998) Solution properties of single-walled carbon nanotubes. Science 282:95–98
Liu J, Rinzler A, Dai H et al (1998) Fullerene pipes. Science 280:1253–1256
Coleman K, Chakraborty A, Bailey S et al (2007) Iodination of single-walled carbon nanotubes. Chem Mater 19:1076–1081
Aizawa M, Shaffer M (2003) Silylation of multi-walled carbon nanotubes. Chem Phys Lett 368:121–124
Mickelson E, Huffman C, Rinnzler A (1998) Fluorination of single-wall carbon nanotubes. Chem Phys Lett 296:188–194
Holzinger M, Vostrowsky O, Hirsch A (2001) Sidewall functionalization of carbon nanotubes. Angew Chem Int Ed 40:4002–4005
Holzinger M, Abraham J, Whelan P (2003) Functionalization of single-walled carbon nanotubes with R-oxycarbonyl nitrenes. J Am Chem Soc 125:8566–8580
Georgakilas V, Kordatos K, Prato M (2002) Organic functionalization of carbon nanotubes. J Am Chem Soc 124:760–761
Banerjee S, Wong S (2002) Rational sidewall functionalization and purification of single-walled carbon nanotubes by solution-phase ozonolysis. J Phys Chem B 106:12144–12151
Hemraj-Benny T, Wong S (2006) Silylation of single-walled carbon nanotubes. Chem Mater 18:4827–4839
Bahr J, Yang J, Kosynkin D et al (2001) Functionalization of carbon nanotubes by electrochemical reduction of aryl diazonium salts: a bucky paper electrode. J Am Chem Soc 123:6536–6542
Tsang S, Davis J, Green M et al (1995) Immobilization of small proteins in carbon nanotubes: high resolution transmission electron microscopy study and catalytic activity. Chem Commun 1803–1804
Davis J, Green M, Hill H et al (1998) The immobilization of proteins in carbon nanotubes. Inorg Chim Acta 272:261–266
Feng W, Ji P (2011) Enzymes immobilized on carbon nanotubes. Biotechnol Adv. doi:101016/jbiotechadv201107007
Cui D (2007) Advances and prospects on biomolecules functionalized carbon nanotubes. J Nanosci Nanotechnol 7:1298–1314
Matsuura K, Saito T, Okazaki T et al (2006) Selectivity of water-soluble proteins in single-walled carbon nanotube dispersions. Chem Phys Lett 429:497–502
Nepal D, Geckeler K (2006) PH-sensitive dispersion and debundling of single-walled carbon nanotubes: lysozyme as a tool. Small 2:406–412
Yu C, Yen M, Chen L (2010) A bioanode based on MWCNT/protein assisted co-immobilization of glucose oxidase and 2,5-dihydroxybenzaldehyde for glucose fuel cells. Biosens Bioelectron 25:2515–2521
Das D, Das P (2009) Superior activity of strucurally deprived enzyme-carbon nanotube hybrids in cationic reverse micelles. Langmuir 25:2515–2521
Lee C, Tsai Y (2009) Preparation of multiwalled carbon nanotube-chitosan-alcohol dehydrogenase nanobiocomposite for amperometric detection of ethanol. Sens Actuators B 138:518–523
Lee K, Komathi S, Nam N et al (2010) Sulfonated polyaniline network grafted multiwall carbon nanotubes for enzyme immobilization, direct electrochemistry and biosensing of glucose. Microchem J 95:74–79
Wu X, Zhao B, Wu P et al (2009) Effects of ionic liquids on enzymatic catalysis of the glucose oxidase toward the oxidation of glucose. J Phys Chem B 113:13365–13373
Panhius M, Salvador-Morales C, Franklin E et al (2003) Characterization of an interaction between functionalized carbon nanotubes and an enzyme. J Nanosci Nanotechnol 3:209–213
Decher G (1997) Fuzzy nanoassemblies. Towards layered polymeric multicomposites. Science 277:1232–1237
Nagoka Y, Shiratori S, Einaga Y (2008) Photo-control of adhesion properties by detachment of the outermost layer in layer-by-layer assembled multilayer films of preyssler-type polyoxometalate and polyethyleneimine. Chem Mater 20:4004–4010
Wang Y, Joshi P, Hobbs K (2006) Nanostructured biosensors built by layer-by-layer electrostatic assembly of enzyme-coated single-walled carbon nanotubes and redox polymers. Langmuir 22:9776–9783
Bi S, Zhou H, Zhang S (2009) Multilayers enzyme-coated carbon nanotubes as biolabel for ultrasensitive chemiluminescence immunoassy of cancer biomarker. Biosens Bioelectron 24:2961–2966
Munge B, Liu G, Collins G et al (2005) Multiple enzyme layers on carbon nanotubes for electrochemical detection down to 80 DNA copies. Anal Chem 77:4662–4666
Huang W, Taylor S, Fu K et al (2002) Attaching proteins to carbon nanotubes via diimide-activated amide-activated amidation. Nano Lett 2:311–314
Pang H, Liu J, Hu D et al (2010) Immobilization of laccase onto l-aminopyrene functionalized carbon nanotubes and their electrocatalytic activity for oxygen reduction. Electrochim Acta 55:6611–6616
Kim B, Kang B, Bahk Y et al (2009) Immobilization of horseradish peroxidase on multi-walled carbon nanotubes and its enzymatic stability. Curr Appl Phys 9:e263–e265
Teker K, Sirdeshmukh R, Sivakumar K et al (2005) Applications of carbon nanotubes for cancer research. Nanobiotechnology 1:171–182
Liu G, Chen H, Peng H et al (2011) A carbon nanotube-based highly-sensitive electrochemical immunosensor for rapid and portable detection of clenbuterol. Biosens Bioelectron. doi:101016/jbios201107037
O’Conor M, Kim S, Killard A et al (2004) Mediated amperometric immunoassay using single walled carbon nanotube forests. Analyst 129:1176–1180
Villamizar R, Maroto A, Ruis F et al (2008) Fast detection of Salmonella infantis with carbon nanotube field effect transistors. Biosens Bioelectron 24:279–283
Chunglok W, Khownarumit P, Rijiravanich P et al (2011) Electrochemical immunoassay platform for high sensitivity protein detection based on redox-modified carbon nanotube labels. Analyst 136:2969–2974
Leng C, Wu J, Xu Q et al (2011) A highly sensitive disposable immunosensor through direct electro-oxidation of oxygen catalyzed by palladium nanoparticle decorated carbon nanotube label. Biosens Bioelectron 27:71–76
Liu S, Lin Q, Zhang X et al (2011) Electrochemical immunosensor for salbutamol detection based on CS-Fe3O4-PAMAM-GNPS nanocomposites and HRP-MWCNTS-Ab bioconjugates for signa amplification. Sens Actuators B Chem 156:71–78
Shao N, Lu S, Wickstrom E et al (2007) Integrated molecular targeting of IGF1R and HER2 surface receptors and destruction of breast cancer cells using single wall carbon nanotubes. Nanotechnology 18:315101
Zheng M, Jagota A, Strano M et al (2003) DNA-assisted dispersion and separation of carbon nanotubes. Nat Mater 2:338–342
Heller D, Jeng E, Yeung T et al (2006) Optical detection of DNA conformational polymorphism on single-walled carbon nanotubes. Science 311:508–511
Jeng E, Moll A, Roy A et al (2006) Detection of DNA hybridization using the near-infrared band-gap fluorescence of single-walled carbon nanotubes. Nano Lett 6:371–375
Dwyer C, Guthold M, Falvo M et al (2002) DNA-functionalized single-walled carbon nanotubes. Nanotechnology 13:601–604
He P, Li S, Dai L et al (2005) DNA-modified carbon nanotubes for self-assembling and biosensing applications. Synth Met 154:17–20
Wang J, Liu G, Jan M (2004) Ultrasensitive electrical biosensing of proteins and DNA: carbon nanotube derived amplification of the recognition and transduction events. J Am Chem Soc 126:3010–3011
Agui L, Yanez-Sedeno P, Pingarron JM (2008) Role of carbon nanotubes in electroanalytical chemistry. A review. Anal Chim Acta 622:11–47
Guo M, Chen J, Li J et al (2005) Fabrication of polyaniline/carbon nanotube composite modified electrode and its electrocatalytic property to the reduction of nitrite. Anal Chim Acta 532:71–77
Li Y, Wang P, Wang L et al (2007) Overoxidized polypyrrole film directed single-walled carbon nanotubes immobilization on glassy carbon electrode and its sensing applications. Biosens Bioelectron 22:3120–3125
Tang Q, Luo X, Wen R (2005) Construction of a heteropolyanion-containing polypyrrole/carbon nanotube modified electrode and its electrocatalytic property. Anal Lett 38:1445–1456
Lin X, Li Y (2006) A sensitive determination of estrogens with a Pt nano-clusters/multi-walled carbon nanotubes modified glassy carbon electrode. Biosens Bioelectron 22:253–259
Yuan PS, Wu HQ, Xu HY et al (2007) Synthesis characterization and electrocatalytic properties of FeCo alloy nanoparticles supported on carbon nanotubes. Mater Chem Phys 105:391–394
Yang P, Wei W, Tao C (2007) Determination of trace thiocyanate with nano-silver coated multi-walled carbon nanotubes modified glassy carbon electrode. Anal Chim Acta 585:331–336
Dai X, Wildgoose GG, Compton RG (2006) Designer electrode interfaces simultaneously comprising three different metal nanoparticle (Au, Ag, Pd)/carbon microsphere/carbon nanotube composites: progress towards combinatorial electrochemistry. Analyst 131:1241–1247
Liu H, Wang G, Chen D et al (2008) Fabrication of polythionine/NPAu/MWNTs modified electrode for simultaneous determination of adenine and guanine in DNA. Sens Actuators B Chem 128:414–421
Valentini F, Amine A, Orlanducci S et al (2003) Carbon nanotube purification: preparation and characterization of carbon nanotube paste electrodes. Anal Chem 75:5413–5421
Abbar JC, Malode SJ, Nandibewoor ST (2012) Electrochemical detection of a hemorheologic drug pentoxifylline at a multi-walled carbon nanotube paste electrode. Bioelectrochemistry 83:1–7
Zheng L, Song J (2007) Voltammetric behaviour of urapidil and its determination at multi-wall carbon nanotube paste electrode. Talanta 73:943–947
Arvand M, Ansari R, Heydari L (2011) Electrocatalyic oxidation and differential pulse voltammetric detection of sulfamethoxazole using carbon nanotube paste electrode. Mat Sci Eng C 31:1819–1825
Malode SJ, Shetti NP, Nandibewoor ST (2012) Voltammetric behaviour of theophylline and its determination at multi-wall carbon nanotube paste electrode. Colloids Surf B Biointerfaces 97:1–6
Lawrence NS, Deo RP, Wang J (2004) Determination of homocysteine at carbon nanotube paste electrodes. Talanta 63:443–449
Tavana T, Khalilzadeh MA, Karimi-Maleh H et al (2012) Sensitive voltammetric determination of epinephrine in the presence of acetaminophen at a novel ionic liquid modified carbon nanotubes paste electrode. J Mol Liq 168:69–74
Mazloum-Ardakani M, Ganjipour B, Beitollahi H et al (2011) Simultaneous determination of levodopa, carbidopa and tryptophan using nanostructured electrochemical sensor based on novel hydroquinone and carbon nanotubes: application to the analysis of some real samples. Electrochim Acta 56:9113–9120
Beitollahi H, Sheikhshoaie I (2011) Electrocatalytic and simultaneous determination of isoproterenol uric acid and folic acid at molybdenum (VI) complex-carbon nanotube paste electrode. Electrochim Acta 56:10259–10263
Patrascu D, David I, David V et al (2011) Selective voltammetric determination of electroactive neuromodulating species in biological samples using iron (III) phthalocyanine modified multi-wall carbon nanotubes paste electrode. Sens Actuators B Chem 156:731–736
Balan I, David IG, David V et al (2011) Electrocatalytic voltammetric determination of guanine at a cobalt phthalocyanine modified carbon nanotubes paste electrode. J Electroanal Chem 654:8–12
Zheng L, Song J-F (2009) Nickel (II)-baicalein complex modified multiwall carbon nanotube paste electrode and its electrcatalytic oxidation toward glycine. Anal Biochem 391:56–63
Shahrokhian S, Kamazadeh Z, Bezaatpour A et al (2008) Differential pulse voltammetric determination of N-acetylcysteine by the electrocatalytic oxidation at the surface of carbon nanotube-paste electrode modified with cobalt salophen complexes. Sens Actuators B Chem 133:599–606
Qu J, Zou X, Liu B et al (2007) Assembly of polyoxmetalates on carbon nanotubes paste electrode and its catalytic behaviours. Anal Chim Acta 599:51–57
Antiochia R, Gorton L (2007) Development of a carbon nanotube paste electrode osmium polymer-mediated biosensor for determination of glucose in alcoholic beverages. Biosens Bioelectron 22:2611–2617
Pereira AC, Aguiar M, Kisner A et al (2007) Amperometric biosensor for lactate based on lactate dehydrogenase and meldola blue coimmobilized on multi-wall carbon nanotube. Sens Actuators B Chem 124:269–276
Janegitz BC, Marcolino-Junior LH, Campana-Filho SP et al (2009) Anodic stripping voltammetric determination of copper (II) using a functionalized carbon nanotubes paste electrode modified with crosslinked chitosan. Sens Actuators B Chem 142:260–266
Tashkhourian J, Nezhad MRH, Khodavesi J et al (2009) Silver nanoparticles modified carbon nanotube paste electrode for simultaneous determination of dopamine and ascorbic acid. J Electroanal Chem 633:85–91
Merisalu M, Kruusma J, Banks CE (2010) Metallic impurity free carbon nanotube paste electrodes. Electrochem Commun 12:144–147
Wang J, Musameh M (2004) Screen-printed carbon nanotube paste electrodes. Analyst 129:512–515
Chattopadhyay D, Galeska I, Papadimitrakopoulos F (2001) Metal-assisted organization of shortened carbon nanotubes in monolayer and multilayer forest assemblies. J Am Chem Soc 26:9451–9452
Liu Z, Shen Z, Zhu T et al (2000) Organizing single-walled carbon nanotubes on gold using a wet chemical self-assembling technique. Langmuir 16:3569–3573
Kim B, Sigmund W (2003) Self-alignment of shortened multiwall carbon nanotubes on polyelectrolyte layers. Langmuir 19:4848–4851
Wu B, Zhang J, Zhong W (2001) Chemical alignment of oxidatively shortened single-walled carbon nanotubes on silver surface. J Phys Chem B 105:5075–5078
Yang Y, Huang S, He H et al (1999) Patterned growth of well-aligned carbon nanotubes: a photolithographic approach. J Am Chem Soc 121:10832–10833
Chen G (2007) Carbon nanotube and diamond as electrochemical detectors in microchip and conventional capillary electrophoresis. Talanta 74:326–332
Wang J, Chen G, Chatrathi M et al (2004) Capillary electrophoresis microchip with a carbon nanotube-modified electrochemical detector. Anal Chem 76:298–302
Panini N, Messina G, Salinas E et al (2008) Integrated microfluidic systems with an immunosensor modified with carbon nanotubes for detection of prostate specific antigen (PSA) in human serum samples. Biosens Bioelectron 23:1145–1151
Shi B-X, Wang Y, Zhang K et al (2011) Monitoring of dopamine release in single cell using ultrasensitive ito microsensors modified with carbon nanotubes. Biosens Bioelectron 26:2917–2921
Hong C-C, Wang C-Y, Peng K-T et al (2011) A microfluidic chip plat-form with electrochemical carbon nanotubes electrodes for pre-clinical evaluation of antibiotic nanocapsules. Biosens Bioelectron 26:3620–3626
Pumera M, Llopis X, Merkoci A et al (2006) Microchip capillary electrophoresis with a single-wall carbon nanotube/gold electrochemical detector for de-termination of aminophenols and neurotransmitters. Microchim Acta 152:261–265
Vlakova M, Schwarz M (2007) Determination of cationic neurotransmitters and metabolites in brain homogenates by microchip electrophoresis and carbon nanotube-modified amperometry. J Chromatogr A 1142:214–221
Chen G, Zhang L, Wang J (2004) Miniaturized capillary electrophoresis system with a carbon nanotube microelectrode for rapid separation and detection of thiols. Talanta 64:1018–1023
Wang J, Chen G, Wang M et al (2004) Carbon-nanotube/copper composite electrodes for capillary electrophoresis microchip detection of carbohydrates. Analyst 129:512–515
Snider R, Ciobanu M, Rue A et al (2008) A multiwall carbon nanotube/dihydropyran composite film electrode for insulin detection in a microphysiometer chamber. Anal Chim Acta 609:44–52
Xu J, Zhang H, Chen G (2007) Carbon nanotube/polystyrene composite electrode for microchip electrophoretic determination of rutin and quercetin in flos Sophorae immaturus. Talanta 73:932–937
Olive-Monllau R, Martinez-Cisneros C, Bartoli J et al (2011) Integration of a sensitive carbon nanotube composite electrode in a ceramic microanalyzer for the amperometric determination of free chlorine. Sens Actuators B Chem 151:416–422
Chicharro A, Sanchez E, Bermejo A et al (2005) Carbon nanotubes paste electrodes as new detectors for capillary electrophoresis. Anal Chim Acta 543:84–91
Kim J, Baek J, Kim H et al (2006) Integration of enzyme immobilised single-walled carbon nanotubes mass into the microfluidic platform and its application for the glucose detection. Sens Actuators A Phys 128:7–13
Wisitsoraat A, Sritongkham P, Karuwan C et al (2010) Fast cholesterol detection using flow injection microfluidic device with functionalized carbon nanotubes based electrochemical sensor. Biosens Bioelectron 26:1514–1520
Karuwan C, Wisitoraat A, Maturos T et al (2009) Flow injection based microfluidic device with carbon nanotube electrode for rapid salbutamol detection. Talanta 79:995–1000
Phokharatkul D, Karuwan C, Lomas T et al (2011) AAO-CNTs electrode on microfluidic flow injection system for rapid iodide sensing. Talanta 84:1390–1395
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2012 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Sritongkham, P., Wisitsoraat, A., Tuantranont, A., Somasundrum, M. (2012). Integration of CNT-Based Chemical Sensors and Biosensors in Microfluidic Systems. In: Tuantranont, A. (eds) Applications of Nanomaterials in Sensors and Diagnostics. Springer Series on Chemical Sensors and Biosensors, vol 14. Springer, Berlin, Heidelberg. https://doi.org/10.1007/5346_2012_42
Download citation
DOI: https://doi.org/10.1007/5346_2012_42
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-36024-4
Online ISBN: 978-3-642-36025-1
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)