Abstract
This study aims to evaluate the performance of a bioreactor at an upflow packed bed with synthetic wastewater containing phenol under continuous operation. The used microorganism was formed by a mixed culture isolated from a wastewater treatment plant. The mean residence time (tm = 3.92 h) in the bioreactor was obtained experimentally by following the tracer NaCl 0.5 M concentration in a continuous effluent stream. For the first time, we calculated the superficial velocities of liquid (uL) and gas (uG) in a three-phase medium (air-water-sand) in the absence of biomass; these velocities vary from 5.40 × 10–3 to 5.84 × 10–3 and from 0.36 × 10–3 to 3.67 × 10–3 m/s, respectively. Afterwards, the effects of airflow (AF = 10, 20, and 30 L/h), liquid flow rates (LF = 3, 5, and 9 mL/min), and initial phenol concentration (C0 = 100, 200, 300, 500, and 700 mg/L) on the biodegradation rate of phenol were studied. The results indicate that the rate of continuous biodegradation increases with the initial phenol concentration and decreases both with the AF and LF rates. The biofilm concentration uniformly increases with increasing the height of the sand bed. The process in the continuous model also contributed to a high elimination efficiency of 100%.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
Phenol is distributed in the form of natural or artificial mono-aromatic compounds in different environments (soil, air, and water) [1]. It is considered to be quite toxic to humans via oral exposure and is discharged by many industries such as petrochemical, paint, paper, and coal conversion [2]. The pollution of water by phenol is a worldwide problem because of its toxicity to plants and aquatic life [3]. Phenol is corrosive and causes severe chemical burns on contact. Its exposure also irritates the eyes, throat, and nervous system. Many expensive and difficult physicochemical methods have been used to extract phenolic compounds from industrial wastewater [4]. Additionally, these methods can produce environmentally unfriendly by-products. On the other hand, the biological treatment of wastewater containing organic pollutants is a cost-efficient method as compared to physicochemical techniques to prevent this type of pollutant [5]. Hence, the biological methods are appropriate for the removal of phenol and its derivative compounds [6]. It has been reported that some microorganisms are capable of mineralizing phenol as a source of carbon and energy for their growth and functioning of cellular processes [7]. The first isolation of microorganisms used for the degradation of phenol was reported in 1908 [7]. The used microorganisms are commonly aerobes capable of degrading the organic matter [8] and are widely active in degrading toxic compounds as they grow faster than anaerobes and mineralize organic compounds. For this reason, mixed populations of microorganisms in activated sludges can degrade phenol in most effluents [9]. A mixed culture is a miniature microorganism population in nature. Several works have been reported in the literature on phenol biodegradation using pure bacteria or mixed culture. The degradation of phenol by Psuedomonasputida P8 and Cryptococcus elinovii H1 was studied in [10]. At the same time, the elimination of water pollution by a mixed culture of known bacteria (Pseudomonas putida Tan-1 and Staphylococcus aureus Tan-2) was reported in [11]. Studies regarding research subjects on the biodegradation of phenol-based compounds using mixed microbial culture are summarized in Table 1. It includes pH, concentrations of phenol, the type of microorganisms, the type of reactor, and the removal efficiency.
This work presents a novel packed bed bioreactor composed of baffles of prismatic shape inclined at 30° for the upflow sense. The reactor architecture relies critically on the hydraulic retention time which determines how long polluting compounds remain within the reactor for their effective degradation. Also, the effects of reactor and sand bed design on the liquid and gas holdups are incorporated in this study. The bioreactor efficiently operates at room temperature using the phenol biodegradation on a mixed microbial culture, isolated from an activated sludge of a wastewater treatment plant after acclimation. The study also evaluates the operational stability of the reactor at different concentrations of C0.
EXPERIMENTAL
Bioreactor Design
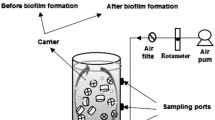
The experimental setup of the upflow packed bed bioreactor is illustrated in Fig. 1. It is built of Plexiglas with a parallelepiped shape and a capacity of 2 L. The reactor is composed of two plates (35 × 7 cm) which consist of prismatic triangular baffles with inclination angles of 30°. Aeration of the reactor was provided by an air pump in the base part by using a sand distributor at 0.5 cm2 and 1 cm in height. The sand particles with a density of 2.32 g/cm3 and a porosity of 0.25 were employed as packing and supporting material for the microorganisms in the bioreactor. To facilitate homogenization, the liquid inside the reactor is continuously pumped from the base to the top (upflow) of the reactor. A multichannel peristaltic pump (ISMATIC) was used to pump a fixed amount of synthetic wastewater. The prepared mineral synthetic medium (MSM) was pumped upward through the packed bed with a fixed flow rate and a hydraulic retention time (HRT) of 6 h.
Sterilization
The glassware was washed with deionized water and autoclaved at 120°C for 20 min to eliminate microorganisms. The bioreactor was cleaned between cycles in a closed circuit using diluted HCl, followed by alcohol and finally washed with hot distilled water.
Microorganisms and Culture Conditions
MSM used in this study is characterized in Table 2, it is dissolved in distilled water. The phenol solution was added after sterilization at 121°C for 20 min, and the pH was adjusted to 7.0 with NaOH (2 M) solution [11]. The microorganisms were formed by a mixed population of organisms. They were isolated and enriched from the hazardous activated sludge collected from a wastewater treatment plant located in the Boumerdes area (50 km east of Algiers). It is an elective aerobic which is used for the phenol biodegradation as a sole organic carbon source [16].
Analytical Methods
The reactor was initiated by inoculation of mixed bacteria previously isolated in activated sludge and put in a Broth fluid (BF) [18]. After 18 h of incubation in an incubator at 30°C, the contents of the flask were transferred to round bottom tubes and vigorously centrifuged (3000 rpm, 30 min). The supernatant was removed, and the pellet (cell deposit) was rapidly dissolved in an aqueous NaCl solution (0.5 M) [19]. The bacterial cells were centrifuged again according to the conditions explained above. The final concentrations of the cells were titrated by measurement of the absorbance at a wavelength of 600 nm (DO600) [20]. The floating cell pellet is carefully recovered and suspended in the MSM solution containing phenol at a concentration C0 of 100 mg/L. In the presence of oxygen, the solution containing the mixed culture circulates in the reactor in an ascending flow and a closed loop for 2 days. This culture uses the phenol present in the solution to grow, multiply, and attach to the support (sand bed) until they are completely covered [21]. The cell density is monitored by a HACH DR 2800 spectrophotometer. The theoretical HRT is fixed at 6 h (flow rate 3 mL/min). The system is maintained at ambient temperature (28 ± 3°C), and the concentration of phenol in suspension is determined by the standard method [22].
Process Model
The mean residence time (tm) in the reactor was determined by the tracer injection technique. A 5-mL portion 3% NaCl solution (0.5 M) was injected into the lower part, and the conductivity was measured at the outlet using a conductivity meter (Consort C3010). The tm was determined as:
where C is the tracer concentration inside the bioreactor, ti is the time at instant i, and n is the instantaneous total number of equations.
The mathematical model describing the liquid holdup (εL) in a fixed bed bioreactor in the absence of biomass is calculated from the following equation:
where uL (m/s) and HL (cm) represent the superficial velocity and the height of the bed, respectively.
For incompressible flow, the liquid superficial velocity in a porous medium is calculated according to the Ergun equation:
where \(\frac{{\Delta P}}{{{{H}_{{\text{L}}}}}}\) (bar/cm) is the fixed bed pressure drop, μL is the liquid dynamic viscosity (g/cm s or cP), ρL is the liquid density (g/mL), φV is the coefficient of sphericity, dp is the mean diameter of solid particles (cm) and є is the bed porosity. The constants A and B are equal, respectively, to 150 and 1.75 [23].
The gas hold-up (εG) is one of the essential parameters for the design of a three-phase reactor and \(~{{\varepsilon }_{{\text{G}}}}\) measures the quantity (or volume) of gas present in a three-phase mixture. A reduction in the retention rate is accompanied by a reduction in the interfacial area and the material transfer rate [24].
Below we present the equation related to the gas hold-up:
where VG, VL, and VS represent the gas phase, the liquid phase, and the solid phase volume (mL), respectively.
We represent below the equation for the gas hold-up of the air-water-sand phases involved in our bioreactor.
where qL is the LF rate (mL/min), ms is the sand mass (g).
The degradation rate can be expressed with respect to the phenol concentration by the following expression:
where C0 is the initial phenol concentration (mg/L), Ct is the concentration of phenol at time t.
RESULTS AND DISCUSSION
In the Absence of Biomass
The Effect of Superficial Liquid Velocity on Liquid Holdup
In the multiphase reactor (solid-liquid-gas), it is important to determine the liquid hold-up (εL), a dimensionless parameter which is a parameter of interest for the characterization of fixed bed reactors. It affects the liquid-solid contact, the particle wetting rate, the material and heat transfer (which varies with pore velocity and film thickness), and most obviously the residence time (Eq. 1) of the liquid in the reactor. The effects of superficial liquid velocity on liquid holdup were determined in a prismatic baffles bioreactor at an updraft packed bed (sand). The water holdup in the sand bed calculated from Eq. 2 was improved in paper [25] as shown in Fig. 2, which shows the variation of the liquid holdup phase (εL) as a function of the superficial liquid velocity (uL). The uL values are calculated from the different LF rates studied (3, 5, 7, and 9 mL/min) using Eq. 3 based on the pressure losses (ΔP/HL). In this study, experimental data of ΔP/HL were evaluated by using a 40 mbar “PRECIman” pressure gauge. It results from Fig. 2 that εL presents a maximum value of 0.65 for a low superficial liquid velocity uL (at LF = 3 mL/min), then slowly decreases until reaching a minimal value of 0.32. The arc of the curve obtained is due to the decrease in the residence time of the liquid phase which results in a slight increase in the surface speed, it is also due to the increase in the LF rate. Following these results, the best working interval is obtained at values of uL ranging from 5.40 × 10–3 to 5.84 × 10–3 m/s.
Effect of Superficial Gas Velocity on Gas Holdup
Gas holdup or void fraction is the relative volume of gas present during the gas-liquid dispersion in the bioreactor and determines the transfer from gas to the liquid phase. From the liquid holdup results obtained, velocity uL was set at 5.40 × 10–3 m/s (LF = 3 mL/min). The εG holdup studied in the air-water-sand system is evaluated from our data (uL) by using Eq. 5 to understand the extent of its application to the system air-water-sand phases studied. Figure 3 shows the effect of velocity uG on the gas holdup phase (εG). According to the paper [26], the air holdup results are shown in Fig. 3, noting that an increase in the rate of εG induced an increase of uL. All εG holdup studied present low values varying from 0.94 to 0.98, i.e. the holdup of ~100%. This could also be due to the slight increase in the superficial gas holdup (airflow). In other words, the geometry of the bioreactor (parallelepiped shape and prismatic triangular baffles), height bed as well and the porosity of the sand can influence the linearity of air holdup. Following these results, the best working interval for the superficial gas velocity is obtained in the range (0.36 × 10–3 to 3.67 × 10–3 m/s).
In the Presence of Biomass
Effect of Airflow Rate
Phenol is a dangerous molecule that causes multiorgan failure and is suspected of being carcinogenic. It is easily absorbed by ingestion or inhalation and distributes rapidly throughout the body. Therefore, its elimination is critically important. The presence of oxygen is essential for the smooth running of the aerobic phenol biodegradation.
The AF effect for continuous phenol biodegradation was studied for three flow rates 10, 20, and 30 L/h. The initial phenol concentration and the LF rate were set at 100 mg/L and 3 mL/min (uL = 5.40 × 10–3 m/s), respectively. The data on phenol biodegradation at ambient temperature for different AF rates during the biodegradation process is presented in Fig. 4. The AF rates from 10 to 30 L/h and the mean residence times (Eq. 1) from 2.76 to 5.77 h were maintained during the phenol biodegradation. The results clearly show that the phenol degradation decreases with increasing the AF because of the insufficient residence time; this observation is similar to those obtained elsewhere [27–29]. Also, an increase in the AF rate caused the mass transfer limitation so the detachment of the biofilm was fixed on the sand bed. For a better biodegradation of phenol, the aeration flow meter was set at 10 L/h (uG = 0.36 × 10–3 m/s).
Effect of Liquid Flow Rate
The effect of the rate of the liquid feed on the phenol biodegradation is studied at three values: 3, 5, and 9 mL/min and the initial phenol concentration (C0) is set at 100 mg/L. The evolution of the phenol concentration over time for different flow rates, illustrated in Fig. 5, clearly indicates that the biodegradation is influenced by the change in LF rate.
Continuous Phenol Biodegradation
The phenol biodegradation by the bacterial mixture has also been acclimatized and evaluated by the single organic charge in the same reactor. The efficiency (Eq. 6) of the bioreactor fed continuously by MSM showed that the removal rate of phenol varied with the number of tests.
The biodegradation tests were conducted during the summer period (2015 and 2016, July−September). These experiments were carried out until the formation of biofilm where the microorganisms reached an inhibition phase of microbial growth. The first stage of our test started with the reactor at a concentration of 100 mg/L. For other tests, the phenol concentration was gradually increased to 200, 300, 500, and 700 mg/L and the response to the reactor exit for all concentrations was controlled at every transition step as illustrated in Fig. 6. The biodegradation time profile (Fig. 6) shows that the phenol concentrations 100, 200, and 300 mg/L demonstrate a complete degradation in 19, 28, and 63 days, respectively. The biodegradation of phenol is almost total, close to 100%, for each concentration tested.
The reactor performance for the phenol degradation at relatively high concentrations is confirmed by the obtained results. Indeed, an increase in the C0 concentration to 300 mg/L increases the biodegradation time of phenol. Beyond this concentration, a decline phase in growth rate is observed as shown in Figs. 4d and 4e; this is represented by a shrinkage of the degradation, and this observation is similar to that reported elsewhere [30]. Finally, the maximum rate of phenol biodegradation is 0.44, 0.87, 1.30, 2.12, and 3.03 g/L per day for waters containing 100, 200, 300, 500, and 700 mg/L phenol, respectively.
Effect of Packing Height on Baffles Reactor
The packing height on the baffles bioreactor has a strong effect on both the bacteria fixation and the biofilm formation. To verify the efficiency of the reactor, the sand bed is subdivided into three heights (HL): H1, H2, and H3, equal to 4, 8, and 12 cm, respectively. The biofilm cells were determined for all heights of the packed bed, and the phenol elimination efficiency was investigated. The heights are measured from the packing bottom to the sampling points, while the flow ratio of water/air in the sand packing bed is fixed at 1.08. The phenol concentration increases with raising the packing height owing to the increment of contact time liquid/air. After complete phenol degradation and biofilm formation, three samples are taken in the middle of each level of the bioreactor at H1, H2, and H3, and this is for each phenol concentration (C0: 100 to 700 mg/L). The biomass of the superficial part (above H3) is not taken into consideration because the solution excess is evacuated at this level of the reactor. The samples are washed several times with hot distilled water (~70°C) under manual stirring to recover the biomass (biofilm).
The supernatant obtained (biomass detached from the sand) is recovered in a beaker and dried in an oven at 100 ± 5°C until total water evaporation. Figure 7 shows the amount of washed and dried biomass (total suspended solids (TSS)) for phenol biodegradation of 300 mg/L.
Different TSS concentrations (the biofilm mass (mg), fixed on 1 g of sand) (Cbf) obtained (100–700 mg/L) at heights (H1, H2, and H3) are illustrated in Fig. 8. The latter indicates that Cbf gradually increases from one level to another along the reactor: a higher concentration is noted in the upper part and similar results have been reported by others [31]. In addition, it has been shown in paper [32] that the rate of degradation increases with an increase of AF to ensure air distribution concerning the bed height. The results indicate that the bioreactor packing height and baffles play an important role in the overall performance and separation of pollutant compounds in the sand bed [33].
CONCLUSIONS
Continuous phenol degradation was carried out in a prismatic-shaped baffle bioreactor by using a bacterial mixed culture. Various issues were explored, such as the startup period, the effect of the phenol concentration feed, and the long-term performance of the bioreactor. The effect of the LF rate, AF, and initial phenol concentrations were investigated under continuous operations. The mean residence time after setting the AF rate at 10 L/h (uG = 0.36 × 10–3 m/s) and the LF rate at 3 mL/min (uL = 5.40 × 10–3 m/s) was 3.92 h. The evaluation of the bioreactor performance was based on the amount of phenol degraded per unit of time. When it operated in continuous mode, the phenol biodegradation exhibited a high elimination efficiency (~100%). It was also found that the rate of continuous biodegradation of phenol increased with raising its concentration and or decreasing the LF rate and AF rate. The results proved that a continuous operation of the bioreactor can be conducted by a sum of operating conditions resulting in a complete biodegradation of phenol. Finally, it has been demonstrated that at long-term continuous operation in a prismatic-shaped baffles bioreactor may allow even distribution of liquids and air along the bed with uniform formation of the biofilm.
REFERENCES
Abd-El-Haleem, D., Beshay, U., Abdelhamid, A.O., Moawad, H., and Zaki, S., Effects of mixed nitrogen sources on biodegradation of phenol by immobilized Acinetobacter sp. strain W-17, Afr. J. Biotechnol., 2003, vol. 2, no. 1, pp. 8–12.
Dursun, A.Y. and Tepe, O., Internal mass transfer effect on biodegradation of phenol by Caalginate immobilized Ralstonia eutropha, J. Hazard. Mater., 2005, vol. 126, nos. 1–3, pp. 105–11.
Moreno, L., Nemati, M., and Predicala, B., Biodegradation of phenol in batch and continuous flow microbial fuel cells with rod and granular graphite electrodes, Environ. Technol., 2017, vol. 39, pp.144–156. https://doi.org/10.1080/09593330.2017.1296895
Jalayeri, H., Ardejani, F.D., and Marandi, R., Biodegradation of phenol from a synthetic aqueous system using acclimatized activated sludge, Arab. J. Geosci., 2013, vol. 6, no. 10, pp. 3847–3852.
Hsien, T.Y. and Lin, Y.H., Biodegradation of phenolic wastewater in a fixed biofilm reactor, Chem. Eng. J., 2005, vol. 27, no. 2, pp. 95–103.
Wang, G., Wen, J., Li, H., and Qiu, C., Biodegradation of phenol and m-cresol by Candida albicans PDY-07 under anaerobic condition, J. Ind. Microbiol. Biotechnol., 2009, vol. 36, pp. 809–814.
Reshma, J.K., Thanga, S.G., and Mathew, A., Phenol degradation by bacteria isolated from coir retting beds of south Kerala coast, J. Global Biosci., 2014, vol. 3, no. 6, pp. 935–940.
Kim, J.H., Oh, K.K., Lee, S.T., Kim, S.W., and Hong, S.I., Biodegradation of phenol and chlorophenols with defined mixed culture in shake-flasks and a packed bed reactor, Process. Biochem., 2002, vol. 37, no. 12, pp. 1367–1373.
Marrot, B., Martinez, A.B., Moulin, P., and Roche, N., Biodegradation of high phenol concentration by activated sludge in an immersed membrane bioreactor, Biochem. Eng. J., 2006, vol. 30, no. 2, pp. 174–183.
Zache, G., Rehm, H.–J., Degradation of phenol by a coimmobilized entrapped mixed culture, Appl. Microbiol. Biotechnol., 1989, vol. 30, no. 4, pp. 426–432.
Senthilvelan, T., Kanagaraj, J., Panda, R.C., and Mandal, A.B., Biodegradation of phenol by mixed microbial culture: An eco-friendly approach for the pollution reduction, Clean Technol. Environ., 2014, vol. 16, no. 1, pp. 113–126.
Bajaj, M., Gallert, C., and Winter, J., Biodegradation of high phenol containing synthetic wastewater by an aerobic fixed bed reactor, Bioresour. Technol., 2008, vol. 99, no. 17, pp. 8376–8381.
Fernandez, I., Suarez-Ojeda, M. E., and Perez, J., Aerobic biodegradation of a mixture of monosubstituted phenols in a sequencing batch reactor, J. Hazard. Mater., 2013, vol. 260, pp. 563–568.
Tay, J.H., Jiang, H.L., and Tay, S.T.L., High-rate biodegradation of phenol by aerobically grown microbial granules, J. Environ. Eng., 2004, vol.130, no. 12, pp. 1415–1423.
Singh, N. and Balomajumder, C., Batch growth kinetic studies for elimination of phenol and cyanide using mixed microbial culture, J. Water Process Eng., 2016, vol. 11, pp. 130–137.
Saravanan, P., Pakshirajan, K., and Saha, P., Biodegradation of phenol and m-cresol in a batch and fed batch operated internal loop airlift bioreactor by indigenous mixed microbial culture predominantly Pseudomonas sp., Bioresour. Technol., 2008, vol. 99, no. 18, pp. 8553–8558.
Hamitouche, A., Bendjama, Z., Amrane, A., Kaouah, F., and Hamane, D., Phenol biodegradation by mixed culture in batch reactor—Optimization of the mineral medium composition, Ann. Microbiol., 2012, vol. 62, no. 2, pp. 581–586.
Jiang, Y., Wen, J., Lan, L., and Hu, Z., Biodegradation of phenol and 4-chlorophenol by the yeast Candida tropicalis, Biodegradation, 2007, vol. 18, no. 6, pp. 719–729.
Dower, W.J., Miller, J.F., and Ragsdale, C.W., High efficiency transformation of E. coli by high voltage electroporation, Nucleic Acids Res., 1988, vol. 16, no. 13, pp. 6127–6145.
Holben, W.E., Jansson, J.K., Chelm, B.K., and Tiedjel, J.M., DNA Probe method for the detection of specific microorganisms in the soil bacterial community, Appl. Environ. Microbiol., 1988, vol. 54, no. 3, pp. 703–711.
Paca, J., Jr., Kosteckova, A., Stiborova, M., Sobotka, M., Gerrard, A.M., and Soccol, C.R., Continuous aerobic phenol degradation by defined mixed immobilized culture in packed bed reactors, Folia Microbiol., 2005, vol. 50, no. 4, pp. 301–308.
APHA Standard Methods for the Examination of Water and Wastewater, Washington, DC: Am. Public Health Assoc., 1998, 20th ed.
Macdonald, I.F., El-Sayed, M.S., Mow, K., and Dullien, F.A.L., Flow through porous media—The Ergun equation revisited, Ind. Eng. Chem. Fundam., 1979, vol. 18, no. 3, pp. 199–208.
Trambouze, P. and Euzen, J.-P., Les Reacteurs chimique: De la conception a la mise en œuvre, Paris: TechNIP, 2002.
Bayraktar, E. and Mehmetoglu, U., Back mixing and liquid hold-up in a cocurrent up-flow packed bed bioreactor, Chem. Eng. Commun., 2007, vol. 185, no. 1, pp. 125–140.
Larachi, F., Laurent, A., Wild, G., and Midoux, N. Some experimental liquid saturation results in fixed-bed reactors operated under elevated pressure in concurrent upflow and downflow of the gas and the liquid, Ind. Eng. Chem. Res., 1991, vol. 30, no. 11, pp 2404–2410.
El-Naas, M.H., Al-Zuhair, S., and Makhlouf, S., Continuous biodegradation of phenol in a spouted bed bioreactor (SBBR), Chem. Ing. J., 2010, vol. 160, no. 2, pp. 565–570.
Sheeja, R.Y. and Murugesan, T., Mass transfer studies on the biodegradation of phenols in up-flow packed bed reactors, J. Hazard. Mater., 2002, vol. 98, nos. 2−3, pp. 287–301.
Vinod, A.V. and Kumar, R.N., Dynamic and gas holdup studies in a bubble column bioreactor, Inl. Conf. Environ. Sci. Technol., 2011, vol. 6, part 2, pp. 384–387.
Agrawal, C.M., McKinney, J.S., Lanctot, D., and Athanasiou, K.A., Effects of fluid flow on the in vitro degradation kinetics of biodegradable scaffolds for tissue engineering, Biomaterials, 2000, vol. 21, no. 23, pp. 2443–2452.
Sa, C.S.A. and Boaventura, R.A.R., Biodegradation of phenol by Pseudomonas putida DSM 548 in a trickling bed reactor, Biochem. Eng. J., 2001, vol. 9, no. 3, pp. 211–219.
Hannaford, A.M. and Kuek, C., Aerobic batch degradation of phenol using immobilized Pseudomonas putida, J. Ind. Microb. Biotechnol., 1999, vol. 22, no. 2, pp. 121–126.
Gopinath, M., Mohanapriya, C., Sivakumar, K., Baskar, G., Muthukumaran, C., and Dhanasekar, R., Biodegradation of toluene vapor in coir based upflow packed bed reactor by Trichoderma asperellum isolate, Environ. Sci. Pollut. Res. Int., 2015, vol. 23, no. 5, pp. 4129–4137.
ACKNOWLEDGMENTS
The authors are grateful the Faculty of Mechanical and Processes Engineering supported this work, in terms of providing the materials as well as the analysis equipment mentioned in this article. The authors are also thankful to Pr. Z. Bendjama, the retired faculty member, for her worthwhile advice, as well as Dr. C. Nekaa and Dr. N. Doufene for technical assistance.
Funding
The authors are grateful to the Directorate General for Scientific Research and Technological Development “DGRSDT Algeria” for financial support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors of this work declare that they have no conflicts of interest.
Additional information
Publisher’s Note.
Allerton Press remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Nadji Bouakaz, Semssoum, A., Boumaza, S. et al. Continuous Phenol Biodegradation in a Bioreactor at Upflow Packed Bed by Mixed Microbial Culture. J. Water Chem. Technol. 46, 201–211 (2024). https://doi.org/10.3103/S1063455X24020048
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3103/S1063455X24020048