Abstract
Biodegradation of phenol and 4-chlorophenol (4-cp) using a pure culture of Candida tropicalis was studied. The results showed that C. tropicalis could degrade 2,000 mg l−1 phenol alone and 350 mg l−1 4-cp alone within 66 and 55 h, respectively. The capacity of the strain to degrade phenol was obviously higher than that to degrade 4-cp. In the dual-substrate system, 4-cp intensely inhibited phenol biodegradation. Phenol beyond 800 mg l−1 could not be degraded in the presence of 350 mg l−1 4-cp. Comparatively, low-concentration phenol from 100 to 600 mg l−1 supplied a sole carbon and energy source for C. tropicalis in the initial phase of biodegradation and accelerated the assimilation of 4-cp, which resulted in the fact that 4-cp biodegradation velocity was higher than that without phenol. And the capacity of C. tropicalis to degrade 4-cp was increased up to 420 mg l−1 with the presence of 100–160 mg l−1 phenol. In addition, the intrinsic kinetics of cell growth and substrate degradation were investigated with phenol and 4-cp as single and mixed substrates in batch cultures. The results illustrated that the models proposed adequately described the dynamic behaviors of biodegradation by C. tropicalis.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Phenol widely exists in many industry wastewaters and effluents (Bielefeldt and Stensel 1999; Aleksieva et al. 2002; Kavitha and Palanivelu 2004). Its derivative chlorophenol, especially 4-chlorophenol (4-cp) as a typical example of an environmentally significant compound, has been detected in several industries, such as wood preservatives, waste incineration, uncontrolled use of pesticides, fungicides and herbicides, as well as byproducts formed during bleaching of pulp with chlorine and in chlorination disinfection of drinking water (Saéz and Rittmann 1993; Kim et al. 2002; Yuan and Lu 2005). Because of improper treatment of these materials, they have widely contaminated soil and groundwater, and their toxicity seriously affects living organisms. Once wastewater containing phenolic compounds is discharged into the receiving body of water, it endangers fish life, even at a relatively low concentration e.g. 5–25 mg l−1 (Kibret et al. 2000; Chung et al.2003; Kumar et al. 2005). For drinking water, a guideline concentration of 1 μg l−1 has been prescribed (WHO 1994). Due to the toxic properties of both phenol and chlorophenol (Müller and Babel 1994; Wang and Loh 2000; Ai et al. 2005), the efficient removal of these compounds from industrial aqueous effluents is of great practical significance for environmental protection.
To treat phenolic compounds, recently developed biodegradation techniques are preferable for their economical advantages (Bux et al. 1999; Zumriye et al. 1999; Fialová et al. 2004), and low possibility of the production of byproducts (Wang et al. 2000). However, the use of such technology is confined due to phenolic toxic properties, especially 4-cp, for microorganisms (Ai et al. 2005; Yuan and Lu 2005). Some organisms have been proven to mineralize 100–1,200 mg l−1 phenol or/and chlorophenol under aerobic conditions as the sources of carbon and energy (Hofmann and Krueger 1985; Vallini et al. 2001; Alexievaa et al. 2004; Santos and Linardi 2004). Such biodegradation capacity limits the progress of biodegradation techniques. The development of highly active strain has become crucial for the biological method to treat industrial wastewater containing phenolic compounds. Fialová et al. (2004) reported the yeast Candida maltosa for its phenol-degrading potential up to 1,700 mg l−1. It’s noteworthy recently that Jiang et al. (2005) isolated a strain of yeast C. tropicalis, able to degrade phenol up to 2,000 mg l−1 from acclimated activated sludge. However, nothing has been known about the biodegradation behavior of phenol and 4-cp dual-substrate system with such high substrate concentrations from 0 to 2,000 mg l−1 by C. tropicalis.
In many studies on biodegradation of phenolic compounds, the modeling of cell growth on dual substrates has been the subject of many researches, for cell growth kinetics are crucial to evaluate the biodegradation potential of organisms (Saéz and Rittmann 1993; Wang and Loh 2000, 2001; Argüelles et al. 2003). These kinetic models listed in literatures were proposed based on Monod or Haldane equation. They can describe cell growth behavior at low substrate concentrations (Paller et al. 1995; Kar et al. 1997; Paraskevi et al. 2005). However, these models can’t be used in the dual-substrate biodegradation system because of very strong inhibition from the toxicity of high-concentration substrates over 1,000 mg l−1.
Objectives of the present study are to investigate biodegradation of phenol and 4-cp as single substrate by C. tropicalis, to investigate the interaction of phenol and 4-cp in dual-substrate system, and to research the cell growth and substrate degradation kinetics of C. tropicalis in single and dual substrate biodegradation system with high substrate concentration, respectively.
Materials and methods
Microorganism and cultivation conditions
The strain and culture conditions have been described in previous studies (Jiang et al. 2005, 2006).
Phenol and 4-cp biodegradation
Experiments began with inoculating 10 ml YEPD medium with C. tropicalis (Haydée 1999). After the incubation for 18 h, 2 ml of this cell culture was added to 500 ml shaking flasks with 100 ml fresh YEPD medium. Cells (OD600 = 1.3) were harvested as inoculum. In all the experiments, 5% subculture was inoculated into 100 ml mineral salt medium with varying initial phenol and/or 4-cp concentrations. In the process of batch culture, all samples were periodically taken for biomass and substrate concentrations.
Analytical methods
Cell density was monitored spectrophotometrically by measuring the absorbance at wavelength 600 nm. Then biomass concentrations on a dry weight basis were measured by filtering cell suspension with the filler and drying the filter paper and cells to a constant weight for 24 h at 105°C. To measure concentration of residual substrate, immediately after measurements of optical density, samples of suspended culture were centrifuged at 7,500 rpm for 10 min. The cell free supernatants were used to determine the substrate concentration by high performance liquid chromatography using a LabAlliance (model SeriesШ) system, with a C18 column (250 × 4.6 mm, LabAlliance, U.S.A). Elution was performed with 400/300 (V/V) methanol/water at a flow rate of 1.0 ml min−1, and detection was realized with a UV detector (Model 500, LabAlliance, U.S.A.) at 280 nm. The retention time for phenol was 4.89 min and for 4-cp was 8.98 min.
Statistics
All the experiments were repeated three times. The data shown in the corresponding figures in Section “Results and discussions” were the mean values of the experiments.
Results and discussions
Single substrate biodegradation
Single substrate biodegradation behaviors
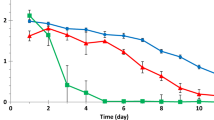
In the previous studies, the potential of C. tropicalis for phenol biodegradation reached 2,000 mg l−1. The biodegradation behavior of 4-cp was shown in Fig. 1. A total of 350 mg l−1 4-cp was degraded within 55 h. With the increase of initial 4-cp concentration, the final biomass increased slightly. However, high substrate concentration brought about the strong substrate inhibition, which was quantificationally demonstrated from the semi-log graph of cell growth and the substrate degradation curve. The slope of semi-log graph represented specific growth rate. It was clear that specific growth and degradation rates decreased with the increase of initial 4-cp concentration. Only due to the small change of 4-cp concentration, did the undistinguished changes happen to them. 360 mg l−1 4-cp was also used in the biodegradation assay. The result showed that biodegradation of C. tropicalis terminated, which was resulted form the occurrence of cell death before overcoming the lag phase.
Figure 2 compared the biodegradation behavior between 350 mg l−1 phenol and 4-cp. 350 mg l−1 phenol was completely degraded within 14 h, however, it took much more time (55 h) to consume 4-cp at the same concentration. The specific growth and degradation rates were much higher in phenol solution than in 4-cp, which indicated that 4-cp imposed the stronger substrate inhibition on the strain. In addition, when the same concentration substrate was consumed, the same biomass should be acquired. However, from the semi-log graph of cell growth, an interesting phenomenon was noticed that the final biomass yield (g/g) in 4-cp solution was smaller than that in phenol solution. The following two reasons were responsible for the matter. 4-cp inhibition on cells was stronger than phenol, which resulted in the fact that more 4-cp consumption was used to overcome substrate inhibition, but not to synthesize new cells. Besides, the content of carbon in phenol solution was 0.022 mol l−1, which was higher than that in 4-cp solution (0.016 mol l−1). Carbon was the essential element for cell synthesis. Thus for the same consumption, phenol supplied more carbon than 4-cp to synthesize new cells.
Single substrate intrinsic kinetics
Batch cultures of C. tropicalis were conducted in the mineral salt medium containing initial phenol concentrations ranging from 0 to 2,000 mg l−1 and the initial 4-cp concentrations ranging from 0 to 350 mg l−1, respectively. For each flask with a certain initial phenol or 4-cp concentration, the specific growth rate was calculated as:
where μ X was the specific growth rate (h−1), γ X was the cell growth rate, and X was cell concentration (mg l−1).
Because of the inhibition of phenol on cell growth, the Haldane’s equation was selected for assessing the dynamic behavior of C. tropicalis grown on phenol (Andrews 1968; Monteiro et al. 2000; Argüelles et al. 2003).
where S 1 was the phenol concentration (mg l−1). Based on the experimental data obtained in the tests of phenol biodegradation alone, the values of the parameters were obtained by a nonlinear least-square regression analysis using the MATLAB software (Jiang et al. 2005). The values of parameters were listed in Table 1. All these values were within the range reported in the literatures depending on cell type and culture environments (Dikshitulu et al. 1993; Kumar et al. 2005). The value of the root mean square of the residual at these parameters was small (0.035) for phenol biodegradation, indicating that the regression parameters agreed well with the experimental data.
Since 4-cp imposed a strong inhibition on cells, inhibition constant for cell growth ( \( K'{} _i \)) was appended in the equation.
The values of the parameters were obtained as μ m2 = 4.79, K S2 = 1,113 mg l−1, K i2 = 4.33 mg l−1 and \( K'{} _{i2} = 2,420 \) mg l−1. The value of the root mean square of the residual at these parameters was 0.071, which showed that revised equation adequately described the behavior of 4-cp biodegradation.
Analyzing the utilization of the substrate in cells in great detail, the consumption of substrate for growth, for maintenance and also for product formation, if possible, had to be considered (Feitkenhauer et al. 2003). The substrate consumption rates of phenol and 4-cp biodegradation were shown as following (Jiang et al. 2005):
where μ S was the specific degradation rate, A and B were all kinetic constants and they were regressed using MATLAB based on the experimental data. Thus, the following model equations were obtained for phenol h−1and 4-cp as single substrate, respectively:
Figures 3 and 4 were the comparisons between the prediction of cell growth and substrate degradation kinetics and the experimentally determined specific growth and degradation rates of C. tropicalis at different initial phenol concentrations from 0 to 2,000 mg l−1 and 4-cp concentrations from 0 to 350 mg l−1, respectively. Obviously, the simulated values of growth and degradation kinetics agreed well with the experimental data. Both the maximum specific growth and specific degradation rates occurred at low substrate concentration. With further increase of initial substrate concentration, much lower values of the specific growth and degradation rates were obtained. The phenomenon was solely due to the intense substrate inhibition at high concentration. And the higher the substrate concentration in the medium was, the stronger the substrate inhibition exhibited. Compared with the phenol inhibition, 4-cp inhibition was much higher. The higher specific growth and degradation rates in phenol solution illustrated that the strain inclined to utilize more phenol than 4-cp. A total of 2,000 mg l−1 phenol was completely degraded within 66 h, which was obviously superior to 4-cp biodegradation. It was one of the reasons why the strain C. tropicalis, isolated from acclimated activated by phenol, was easy to adjust to the environment with phenol. In addition, there were sharp drops of curves at low substrate concentration in these two figures. It was because of the lack of carbon source in the mineral salt medium (Banerjee et al. 2001).
Dual-substrate biodegradation
The effect of phenol on 4-cp biodegradation behavior
The effect of phenol at different initial concentration ranging from 100 to 800 mg l−1 on C. tropicalis to degrade 350 mg l−1 4-cp was showed in Fig. 5. It could be seen that although phenol toxicity could inhibit cell growth, the existence of low-concentration phenol still accelerated C. tropicalis biodegradation for 4-cp. When phenol concentration was from 100 to 400 mg l−1, the velocity of 4-cp biodegradation increased gradually. In the dual-substrate biodegradation system with the phenol of 400 mg l−1, all the substrates were completely degraded within 40 h, which was obviously superior to the 350 mg l−1 4-cp biodegradation alone (55 h). With the increase of phenol concentration, the acceleration of phenol existed up to 600 mg l−1, with the difference of the lower acceleration at the phenol of 400–600 mg l−1. In this phenol concentration range, phenol inhibition exposed, but phenol acceleration still played the key role, and the biodegradation was faster than 4-cp biodegradation alone. When phenol concentration was beyond 600 mg l−1, the biodegradation velocity of the strain was lower than the 4-cp biodegradation alone. However, from the figure it was clear that the biodegradation still occurred, because the phenolic acceleration couldn’t be neglected, yet phenol toxicity was dominant. In the biodegradation tests, 850 mg l−1 phenol was also used. It was found that no biodegradation occurred in the dual-substrate system. Another phenomenon was observed, that is, whatever the phenol concentration was, phenol always took precedence of 4-cp to be utilized by C. tropicalis. Rapid assimilation of 4-cp by cell occurred at the late biodegradation phase after phenol was largely degraded. For example, 400 mg l−1 phenol was completely degraded within 34 h, when the cell and 4-cp concentration was 170 and 210 mg l−1, respectively. It could be seen that phenol supplied necessary carbon source for biodegradation system to make cell accumulation and to keep cell activity high, which ensured the startup of 4-cp biodegradation. Besides, in this experiment, the higher the phenol concentration was, the longer the lag phase of cell growth was, and the higher the cell mass was. In general, phenol played two roles: one was just like glucose to accelerate biodegradation, and the other was as toxic compound to inhibit cell growth. And these two effects existed in dual-substrate biodegradation system all along. Their competition resulted in the different biodegradation velocities. In the sample of 400 mg l−1 phenol, the competitive balance was optimal between phenol toxic inhibition and the supplies of carbon and energy, leading to the optimal phenol concentration to accelerate 4-cp biodegradation.
Phenol could not only improve 4-cp biodegradation velocity but also increase 4-cp biodegradation capacity by cell (Fig. 6). It was clear that 420 mg l−1 4-cp could be completely utilized by C. tropicalis in the presence of 100–160 mg l−1 phenol. And the maximum biodegradation velocity occurred in the sample with the phenol of 140 mg l−1, where 420 mg l−1 4-cp was degraded within 62 h. Phenol still played a role to supply necessary carbon for the startup of biodegradation because of its easier utilization by cells. From the figure, within the first 27.5 h of the biodegradation, 140 mg l−1 phenol has been completely degraded by the strain, while the 4-cp biodegradation just started and the residual 4-cp concentration in the medium was 417 mg l−1. The biodegradation velocity increased gradually at the phenol concentration ranging from 100 to 140 mg l−1. However, when the phenol concentration was more than 140 mg l−1, phenol inhibition increased rapidly. It took 70 h to degrade 420 mg l−1 4-cp in the presence of 160 mg l−1 phenol. And several experiments proved that the biodegradation terminated in the sample with 170 mg l−1 phenol. Another phenomenon was noticed that 4-cp could not be utilized by cell when 80 mg l−1 phenol was in presence. However, having determined the cell and phenol concentration, we found that phenol was not detected at 21.5 h of biodegradation while the cell concentration was 23.6 mg l−1 (Fig. 7). It was because 80 mg l−1 phenol was completely degraded to synthesize new cells, but 23.6 mg l−1 cell could not overcome intense 4-cp inhibition. Before these cells overcame the lag phase of the growth, cell death occurred for 4-cp toxicity.
The effect of 4-cp on phenol biodegradation behavior
Figure 8 showed the effect of 4-cp with the concentration from 80 to 120 mg l−1 on 1,800 mg l−1 phenol biodegradation. Low-concentration 4-cp brought about inhibition on cell growth. In the sample of 80 mg l−1 4-cp, it took over 3 h to degrade 1,800 mg l−1 phenol than phenol biodegradation with the same concentration alone (Jiang et al. 2006). But the whole biodegradation ended 2 h later after phenol complete-consumption. It was obvious that although phenol concentration was much higher than 4-cp, phenol was still preferentially utilized by cell as a carbon and energy source and the rapid biodegradation of 4-cp occurred at the end of the whole biodegradation. Also, with the increase of initial 4-cp concentration, the velocity of the strain for phenol biodegradation decreased. And the biodegradation terminated in the sample beyond 120 mg l−1 4-cp.
Intrinsic kinetics of dual-substrate biodegradation
The growth behaviors of C. tropicalis in the phenol and 4-cp dual-substrate biodegradation system were described with following equations, proposed in this lab using quasi steady state approximation (Jiang et al. 2006): For phenol:
Similarly, for 4-cp:
Based on Eqs. (5) and (6), the specific growth rate for phenol and 4-cp mixed substrates could be obtained as
Where (μ m1, K S1, K i1) and (μ m2, K S2, K i2, \( K_{i2}'{} \)) could be obtained separately from the kinetics of individual cell growth on the phenol alone and 4-cp alone, respectively. S 1 and S 2 were the phenol and 4-cp concentrations in dual-substrate system. The values of parameters were obtained: f = 1.7 × 10−5, K = 6.1 × 10−9, M = 4.5 × 10−7 and Q = 6.3 × 10−5 (Table 1). The value of the root mean square of the residual at these parameters was 0.098.
The specific degradation rates of phenol and 4-cp in the dual-substrate system could be represented based on the experimental data and the proposed expressions in this lab:
According to R 2 and the root mean square of the residual, it was concluded that the regression curve was consistent with the experimental data very well. Kinetic equations adapted to describe the biodegradation behavior of C. tropicalis in dual-substrate system.
Conclusions
The presence of low-concentration phenol enhanced 4-cp biodegradation of C. tropicalis. And phenol was preferably utilized by the strain as a carbon and energy source. Furthermore, 4-cp biodegradation occurred at the end of the whole biodegradation. When 100–600 mg l−1 phenol was introduced into the mineral salt medium, 350 mg l−1 4-cp could be completely degraded within a shorter period of time than 4-cp alone. And when the phenol of 100–160 mg l−1 existed in the medium, the maximum 4-cp biodegradation could arrive at 420 mg l−1, and moreover, the maximum 4-cp biodegradation velocity was obtained at the existence of 140 mg l−1 phenol. 4-cp inhibition for C. tropicalis was much stronger than phenol. 4-cp with the concentration over 120 mg l−1 could inhibit the biodegradation of C. tropicalis for 1,800 mg l−1 phenol in the medium. In addition, the kinetic models for the specific growth and degradation rates of phenol and 4-cp as the simple and mixed substrates were obtained, and the simulated values of these models agreed well with the experimental data.
Abbreviations
- 4-cp:
-
4-chlorophenol
- HPLC:
-
high performance liquid chromatography
References
Ai Z, Yang P, Lu XH (2005) Degradation of 4-chlorophenol by a microwave assisted photocatalysis method. J Hazard Mater 124:147–152
Aleksieva Z, Ivanova D, Godjevargova T et al (2002) Degradation of some phenol derivatives by Trichosporon cutaneum R 57. Process Biochem 37:1215–1219
Alexievaa Z, Gerginova M, Zlateva P et al (2004) Comparison of growth kinetics and phenol metabolizing enzymes of Trichosporon cutaneum R57 and mutants with modified degradation abilities. Enzyme Microb Technol 34:242–247
Andrews JF (1968) A mathematical model for continuous culture of microorganisms utilizing inhibitory substrates. Biotechnol Bioeng 10:707–723
Argüelles MEA, Lora PO, Flores RE (2003) Toxicity and kinetic parameters of the aerobic biodegradation of the phenol and alkylphenols by a mixed culture. Biotechnol Lett 25:559–564
Banerjee I, Modak JM, Bandopadhyay K et al (2001) Mathematical model for evaluation of mass transfer limitations in phenol biodegradation by immobilized Pseudomonas putida. J Biotechnol 87:211–223
Bielefeldt AR, Stensel HD (1999) Biodegradation of aromatic compounds and TCE by a filamentous bacteria-dominated consortium. Biodegradation 10:1–13
Bux F, Akkinson B, Kasan K (1999) Zinc biosorption by waste activated and digested sludges. Water Sci Technol 39:127–130
Chung TP, Tseng HY, Juang RS (2003) Mass transfer effect and intermediate detection for phenol degradation in immobilized Pseudomonas putida systems. Process Biochem 38:1497–1507
Dikshitulu S, Baltzis BC, Lewandowski GA (1993) Competition between two microbial populations in a sequencing fed-batch reactor: theory, experimental verification, and implications for waste treatment applications. Biotechnol Bioeng 42:643–656
Feitkenhauer H, Schnicke S, Müller R et al (2003) Kinetic parameters of continuous cultures of Bacillus thermoleovorans sp. A2 degrading phenol at 65°C. J Biotechnol 103:129–135
Fialová A, Boschke E, Bley T (2004) Rapid monitoring of the biodegradation of phenol-like compounds by the yeast Candida maltosa using BOD measurements. Int Biodeter Biodegr 54:69–76
Haydée TG (1999) Ploidy study in Sporothrix schenkii. Fungal Genet Biol 27:49–54
Hofmann KH, Krueger A-K (1985) Induction and inactivation of phenol hydroxylase and catechol oxygenase in Candida maltosa L4 in dependence on the carbon source. J Basic Microbiol 25:373–379
Jiang Y, Wen JP, Bai J et al (2006) Phenol biodegradation by the yeast Candida tropicalis in the presence of m-cresol. Biochem Eng J 29:227–234
Jiang Y, Wen JP, Li HM et al (2005) The biodegradation of phenol at high initial concentration by the yeast Candida tropicalis. Biochem Eng J 24:243–247
Kar S, Swaminathan T, Baradarajan A (1997) Biodegradation of phenol and cresol isomer mixtures by Arthrobacter. World J Microbiol Biotechnol 13:659–663
Kavitha V, Palanivelu K (2004) The role of ferrous ion in fenton and photo-fenton processes for the degradation of phenol. Chemosphere 55:1235–1243
Kibret M, Somitsch W, Robra KH (2000) Characterization of a phenol degrading mixed population by enzyme assay. Water Res 4:1127–1134
Kim JH, Oh KK, Lee ST et al (2002) Biodegradation of phenol and chlorophenols with defined mixed culture in shake-flasks and a packed bed reactor. Process Biochem 37:1367–1373
Kumar A, Kumar S, Kumar S (2005) Biodegradation kinetics of phenol and catechol using Pseudomonas putida MTCC 1194. Biochem Eng J 22:151–159
Monteiro ÁAMG, Boaventura RAR, Rodrigues AE (2000) Phenol biodegradation by Pseudomonas putida DSM 548 in a batch reactor. Biochem Eng J 6:45–49
Müller RH, Babel W (1994) Phenol and its derivatives as heterotrophic substrates for microbial growth-An energetic comparison. Appl Microbiol Biotechnol 42:446–451
Paller G, Hommel RK, Kleber H-P (1995) Phenol degradation by Acinetobacter calcoaceticus NCIB 8250. J Basic Microbiol 35:325–335
Paraskevi N, Polymenakou C, Euripides GS (2005) Effect of temperature and additional carbon sources on phenol degradation by an indigenous soil Pseudomonad. Biodegradation 16:403–413
Santos VL, Linardi VR (2004) Biodegradation of phenol by a filamentous fungi isolated from industrial effluents-identification and degradation potential. Process Biochem 39:1001–1006
Saéz PB, Rittmann BE (1993) Biodegradation kinetics of a mixture containing a primary substrate (phenol) and an inhibitory co-metabolite (4-chlorophenol). Biodegradation 4:3–21
Vallini G, Frassinetti S, Andreac FD et al (2001) Biodegradation of 4-(1-nonyl)phenol by axenic cultures of the yeast Candida aquaetextoris: identification of microbial breakdown products and proposal of a possible metabolic pathway. Int Biodeter Biodegr 47:133–140
Wang CC, Lee CM, Lu CJ et al (2000) Biodegradation of 2,4,6-trichlorophenol in the presence of primary substrate by immobilized pure culture bacteria. Chemosphere 41:1873–1879
Wang SJ, Loh KC (2000) New cell growth pattern on mixed substrates and substrate utilization in cometabolic transformation of 4-chlorophenol. Water Res 34:3786–3794
Wang SJ, Loh KC (2001) Biotransformation kinetics of Pseudomonas putida for cometabolism of phenol and 4-chlorophenol in the presence of sodium glutamate. Biodegradation 12:189–199
Wang SJ, Loh KC (2000) Growth kinetics of Pseudomonas putida in cometabolism of phenol and 4-chlorophenol in the presence of a conventional carbon source. Biotechnol Bioeng 68:437–447
WHO (1994) Phenol, Environmental health criteria-EHC 161. World Health Organization, Geneva
Yuan SH, Lu XH (2005) Comparison treatment of various chlorophenols by electro-Fenton method: relationship between chlorine content and degradation. J Hazard Mater 118:85–92
Zumriye A, Derya A, Elif R et al (1999) Simultaneous biosorption of phenol and nickel from binary mixtures onto dried aerobic activated sludge. Process Biochem 35:301–308
Acknowledgements
The authors wish to acknowledge the financial support provided by the Key National Natural Science Foundation of China (No. 20336030), Science and Technology Innovative Talents Foundation (No. RC2006QN015018), Program for Science and Technology Development of Tianjin (No. 043185111-20), Program for New Century Excellent Talents in University, Program for Changjiang Scholars and Innovative Research Team in University, and Program of Introducing Talents of Discipline to Universities (No. B06006).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jiang, Y., Wen, J., Lan, L. et al. Biodegradation of phenol and 4-chlorophenol by the yeast Candida tropicalis . Biodegradation 18, 719–729 (2007). https://doi.org/10.1007/s10532-007-9100-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10532-007-9100-3