Abstract
In this study, the efficiency improvement of three moving bed biofilm reactors (MBBRs) was investigated by inoculation of activated sludge cells (R1), mixed culture of eight strong phenol-degrading bacteria consisted of Pseudomonas spp. and Acinetobacter spp. (R2) and the combination of both (R3). Biofilm formation ability of eight bacteria was assessed initially using different methods and media. Maximum degradation of phenol, COD, biomass growth and also changes in organic loading shock were used as parameters to measure the performance of reactors. According to the results, all eight strains were determined as enhanced biofilm forming bacteria (EBFB). Under optimum operating conditions, more than 90% of initial COD load of 2795 mg L−1 was reduced at 24 HRT in R3 while this reduction efficiency was observed in concentrations of 1290 mg L−1 and 1935 mg L−1, in R1 and R2, respectively. When encountering phenol loading shock—twice greater than optimum amount-R1, R2 and R3 managed to return to the steady-state condition within 32, 24 and 18 days, respectively. SEM microscopy and biomass growth measurements confirmed the contribution of more cells to biofilm formation in R3 followed by R2. Additionally, established biofilm in R3 was more resistant to phenol loading shock which can be attributed to the enhancer role of EBFB strains in this reactor. It has been demonstrated that the bacteria with both biofilm-forming and contaminant-degrading abilities are not only able to promote the immobilization of other favorable activated sludge cells in biofilm structure, but also cooperate in contaminant degradation which all consequently lead to improvement of treatment efficiency.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
At present, several physiochemical processes are developed for remediation of contaminated water or industrial effluents such as adsorption (Singh and Balomajumder 2016; Kilic et al. 2011), oxidation (Aneggi et al. 2017; Loloi et al. 2016; Liu et al. 2018) and membrane separation (Hemmati et al. 2015; Lin et al. 2007). However, in terms of pollution control, biological processes have superior advantages due to their ability to remove various kinds of contaminants in a cost effective and environmentally friendly manner (Moussavi et al. 2014; Rafiei et al. 2014; Pookpoosa et al. 2015; Ferro Orozco et al. 2016; Zielińska et al. 2014). In recent years, the use of specific contaminant-degrading microorganisms in wastewater treatment systems has been increased and provided an effective method to enhance the degradation of toxic organic contaminants (Zhang et al. 2012; Duque et al. 2011). For example, some strong phenol-degrading microorganisms, such as Pseudomonas sp. and Rhodococcus sp., have been successfully used to treat phenol-containing wastewater (Prieto et al. 2002). However, as a consequence of some unpleasant events such as protozoan grazing or flow washing of favorable microorganisms, high degradation rate of pollutants does not stand out for a long time. Since the performance of bioaugmented microorganism is a major prerequisite for elevating the treatment efficiency, their immobilization has been suggested as an effective strategy for maintaining them in the system. According to the literatures, different ways of immobilization have been proposed to increase the persistence of bacteria in treatment systems like immobilization in alginate beads (Guiot et al. 2000) or polyvinyl alcohol (PVA) (El-Naas et al. 2009). However, these methods may be cost prohibitive and/or complex to apply and above all, they have defined capacity, longevity and stability which all limit their application. Biofilm as a common form of bacterial life offers a natural, efficient and feasible way of immobilization which enables immobilized cells to proliferate and tolerate hostile environments. Biofilm immobilization of degrading-bacteria has been suggested as a key strategy for achieving efficient degradation in bioaugmentation system (Li et al. 2013). Biofilm treatment systems like moving bed biofilm reactors (MBBR) with elevated resistance to adverse effect of toxic pollutions and other environmental stresses are demonstrated to be useful to treat of wastewater containing xenobiotics or extremely toxic compounds (Simões et al. 2007). Biofilm treatment in this system as a well-known feature of biological wastewater treatment provides distinct advantages such as higher active biomass in a compact unit, smaller footprints, boosted resistance to the hostile environments, lower sludge production and elimination of clogging problem (Leiknes and Odegaard 2007; Bassin et al. 2016; Moussavi et al. 2009). In addition, it is more possible to control the biofilm thickness and mass transfer characteristics in MBBRs over the operation time (Moussavi et al. 2009; Nakhli et al. 2014). Nonetheless, the most critical drawback of such biofilm reactors causes by the weakness of microorganisms in terms of biofilm formation which leads to a major reduction in total biomass amount or lack of favorable microorganisms in the system (Eldyasti et al. 2013). This issue becomes even more severe when higher concentrations or sudden loading shocks of contaminants are imposed to the system which makes the process ineffectual. Immobilization of microorganisms in biofilm structure using strong biofilm producers has been proposed as an applicable solution to tackle this problem (Li et al. 2008, 2013). Recently, a variety of biofilm community compositions presented in natural or drinking water systems were studied and several strong biofilm forming strains were introduced. These bacteria generally have specific surface characteristics which enable them to aggregate with other cells in aquatic environments (Simões et al. 2008; Ramalingam et al. 2013; Metzger et al. 2009; Adav et al. 2008; Kwak et al. 2013; Malik et al. 2003; Cheng et al. 2014; Rickard et al. 2002, 2004). Among them, some strains including Acinetobacter calcoaceticus are known as bridging bacteria which function as an anchor and develop biofilm formation by aiding other microorganisms to incorporate in biofilm structure. These bacteria mainly result in formation of complex multispecies biofilms by their co-aggregation properties (Rickard et al. 2003; Simões et al. 2007, 2008). But major researchs have been done in this area have concentrated on screening of strains with co-aggregation abilities and/or explore the co-aggregation mechanism used by them (Min and Richard 2009; Stevens et al. 2015; Ren et al. 2015; Cheng et al. 2014; Simões et al. 2008; Metzger et al. 2009; Kwak et al. 2013; Malik et al. 2003; Rickard et al. 2002, 2003). Lately, few attempts have been made to take advantage of bacterial mixed-cultures with either contaminant degrading or biofilm producing abilities in biofilm treatment systems or investigate the relationships between them (Li et al. 2013, 2008; Adav et al. 2008; Di Gioia et al. 2004). For instance, Comamonas sp. PG-08 enhanced the ability of Propioniferax-like PG-02 to degrade phenol, via its aggregation but not the phenol-degrading capability (Jiang et al. 2006). Similarly, a mixed culture of Bacillus strains promoted degradation efficiency of another strain via their biofilm forming but not degradation abilities (Li et al. 2013). Nonetheless, applying a mixture of bacteria with different characteristics and origins can be cost and time prohibitive and complex to implement. It is necessary to meet the needs of all inoculated cells and provide a desired condition in which they can grow and maintain their activity. Accordingly, there is still the need to detect bacteria with dual strong biofilm forming and contaminant degrading abilities especially those with further tolerance to toxic effect of pollutants. Moreover, the activity and function of these bacteria in biofilm development and contaminant removal in wastewater treatment systems should be uncovered.
Hence, in this paper, we studied phenol degradation and biofilm formation ability of eight same-origin bacteria including Acinetobacter and Pseudomonas species, which were isolated from a contaminated soil. Then, the performance of three aerobic MBBRs inoculated with activated sludge cells, the mixed-culture of eight bacteria and their combination in stepwise increasing amount of phenol was investigated. In addition, we were interested in exploring the feedback of all reactors encountering sudden enhancement of phenol concentration.
Materials and methods
Chemicals, bacterial strains and media
All applied chemicals in this study were of analytical grade and supplied from Merck. Eight bacterial strains including four Pseudomonas spp. and four Acinetobacter spp., used in this study had been previously isolated from petroleum-contaminated soil, Tehran Refinery, Iran (Table 1) (Nafian et al. 2016) and demonstrated to degrade phenol up to 900 mg L−1 efficiently. The activated sludge used in this study was collected from a municipal sewage treatment plant (Ekbatan, Tehran, Iran; 100,000 population equivalents). Synthetic wastewater (SWW) was used in MBBR reactors including steam sterilized (mg L−1) Na2HPO4 (16.7), CaCl2·2H2O (23.5), MnSO4·7H2O (9.45), CoCl2 (0.8), ZnCl2 (10.0), CuSO4·5H2O (392.0), FeCl3·6H2O (3.3), NaHCO3 (1000.0), MgSO4·7H2O (3.1) as trace elements supplemented with following concentrations of urea (1 mg L−1 = 4.66 × 10−4 mg L−1 nitrogen (N)), K2HPO4/KH2PO4 combination (1 mg L−1 = 2.0 × 10−4 mg L−1 phosphorus (P)) and filter-sterilized phenol (the only carbon source,1 mg L−1 = COD of 2.15 mg L−1) as main components. The COD:N:P ratio in the influent SWW was retained at 100:5:1. Mineral salt medium (MSM) was prepared for biofilm formation assay containing (mg L−1) KH2PO4 (1436), K2HPO4 (2627), NH4HCO3 (325), MgSO4·7H2O (133.7), MnSO4·H2O (9.45), CaCl2·2H2O (11.91), ZnSO4·7H2O (1.44), FeCl3·6H2O (3.33) and phenol at a concentration of 900 mg L−1. Tryptic Soy Broth (TSB)—without phenol—and Plate Count Agar (PCA) were also applied for biofilm formation assay and microbial cell count, respectively.
Biofilm formation assay
Biofilm formation assay in microtiter plate
Biofilm formation was assessed using 96-well polystyrene microtiter plates. Colonies of the eight bacterial strains were individually precultured in TSB and incubated in a rotary shaking incubator at 30 ± 0.1 °C and 130 rpm overnight. The pellets of the bacterial cells were then collected by centrifugation of the cultures at 8000 rpm for 10 min, washed twice using sterilized phosphate buffer (PB) pH 6.8 to eliminate residues of the growth substrate and metabolites and next, the resulting pellets resuspended in PB. Thereupon, SWW containing 900 mg L−1 phenol was inoculated with the cell suspension of eight bacterial strains individually or equal volumes mixture of their overnight culture (diluted to absorbance 0.1 at 600 nm). Then the suspensions were added to every other row in identically three 96-well polystyrene microtiter plates (150 µL/well, three replicates per plate). The sterile culture medium was used as a control. All plates were incubated for 1, 3, 7, 10 and 20 days respectively at 80 rpm, 30 °C. The culture medium was replaced every other day using micropipette. After mentioned incubation times, the wells were drained, washed three times with 200 µL sterile PB to remove suspended cells, dried at room temperature, and the amounts of forming biofilms were quantified according to Djordjevic et al. (2002), using crystal violet method with some modification. Briefly, the wells were stained with 150 µL/well 1% (w/v) crystal violet aqueous solution for 30 min. Microtiter plates were placed in shaking incubator to enhance the penetration of dye in biofilm structure for 30 min. After removing the stain, the wells were washed three times by 200 µL PB and air dried. Finally, 200 µL ethanol-acetone (80:20 v/v) was added to each well and the absorbance measured at 590 nm wavelength. Accordingly, the strains were classified into nonbiofilm producers, Abs ≤ Abscontrol, weak biofilm producers, Abscontrol ≤ Abs ≤ 2 × Abscontrol, average biofilm producers, 2 × Abscontrol ≤ Abs ≤ 4 × Ab control and strong biofilm producers, Abs ≥ 4 × Abscontrol (Stepanovic et al. 2000). Besides, biofilm formation and planktonic growth of each eight bacterial strains and mixtures of them were examined and compared in SWW, MSM—both containing 900 mg L−1 phenol- and TSB for 24 h in the same condition as described above. Right before and after the incubation period, the planktonic growth of each experiment was measured spectrophotometrically at 600 nm.
Biofilm formation assay in flask
Flask biofilm formation assay were carried out according to the method described by Bechet and Blondeau (2003). The culture system comprised new 150-mL Erlenmeyer flasks containing 100 mL of synthetic wastewater and 20 pieces of polyvinylchloride (PVC) carriers (Pakan Ghatreh Co. Ltd., Iran). In brief, flasks were inoculated in triplicate with preculture of each eight bacterial strains or a mixture of them as described above to reach the absorbance of 1.0 OD at 600 nm. The cultures were then incubated at 30 °C with shaking at 80 rpm for 24 h. The biofilm biomass was determined through a method modified from (Zhu and Mekalanos 2003). Briefly, the SWW containing suspended cells was removed and the flasks were gently rinsed in three changes of sterile PB. Then, ten pieces of carriers were transferred to another sterile flask containing 10 mL sterile PB for further analysis. The remained carriers were stained for 30 min with 50 mL 1% (w/v) crystal violet aqueous solution to assess the number of attached biomasses. The flasks were washed thoroughly with sterile PB and dried at room temperature overnight. The residual of crystal violet dye on carriers was dissolved in 10 mL of ethanol-acetone (80:20 v/v), and related absorbance was determined at 590 nm.
Cell counts
Viable counts of the cells attached to the carriers were estimated as follows. As mentioned above, 10 of 20 carriers were transferred to a sterile flask containing 10 mL sterile PB and biofilms were detached using pulsed low-intensity ultrasonication (5 min, 30 s on/off mode, SFX250 Sonifier, Branson). The serial dilution suspension was plated onto Plate Count Agar to determine the bacterial CFU per 10 carriers.
Reactor design
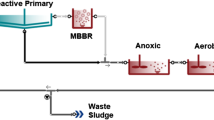
The study was performed using four cylindrical MBBR reactors (more details were illustrated in Fig. 1), made from Plexiglas with an internal diameter, height and wall thickness of 10, 70 and 0.4 cm, respectively. Three reactors named as the reactor number 1 (R1), number 2 (R2) and number 3 (R3) were inoculated with different mixed cultures, whereas the fourth was not inoculated and applied as the control reactor (RC). The total volume of each reactor was 5.5 L, of which the effective volume was 5 L and filled up to 50% with floating biofilm carrier elements. The carriers (Pakan Ghatreh Co., Iran) were composed of high-density polyethylene (HDPE), shaped as small corrugated cylinders with two crosses inside, giving an effective surface area of 389.6 m2/m3 and a density of 0.96 g/cm3, slightly lower than that of water. The aeration system supplied fine mixing bubbles to meet the oxygen demand and keep the carriers moving throughout the reactor as well. Sterile air was supplied from the bottom of the column through a 0.22 µm pore size filter. Taking samples and monitor the performance of the system, three sampling ports were designed on the reactor.
MBBR experimental setup and operation
For investigating whether the different mixed cultures would improve phenol degradation efficiency, three equivalent reactors were set up and inoculated with different inocula in a manner that each reactor was seeded up to make the final suspended solid concentration of 2400 mg L−1 just at the start point. The reactors were inoculated with activated sludge (R1), mixture an equal ratio of eight bacterial overnight cultures (R2) and a 3:1 volumetric ratio of activated sludge plus eight bacterial strains (R3), respectively. The latter was the best ratio of inoculum combination resulted from our previous experiment (data not shown here). RC was just filled up with the sterile synthetic wastewater to investigate the amount of phenol lost through either leaking or absorbing to the rubber stoppers, but according to the results, these effects were all negligible. Batch mode was run by increasing amount of phenol to study the maximum degradation efficiency. All reactors were bubbled with an air-stream and operated at the same controlled conditions, such as; room temperature (23 ± 3 °C), 4.0 ± 1 mgO2 L−1 dissolved oxygen (DO) concentration and neutral pH 7 ± 0.5. At the end of the experiment, the effect of unfavourable phenol shock load on MBBRs performance evaluated by administering twice phenol concentrations greater than the optimum amount of each reactor for just one HRT (24 h).
Analytical methods
The presented results were all obtained from the MBBR system at the steady state condition. Samples were taken and analyzed at the start point and then over 24 HRT intervals to evaluate the performance of the MBBR. The measured parameters in all taken samples were phenol, COD, pH, DO and temperature. The parameters of pH, DO and temperature of the mixed liquor were daily measured using specific electrodes (HACH-Germany) to control the optimum condition for bacterial growth in all reactors. Samples were filtered through a filter with 0.45 mm pore size before analysis to measure phenol and COD. Phenol concentrations were measured spectrophotometrically, by the colorimetric 4-aminoantipyrine method (APHA 2005), using a 2000 UV/VIS Spectrophotometer and COD was determined in accordance with Standard Methods for Water and Wastewater Examination (APHA 1998).
Biofilm biomass morphology and growth
The biofilm attached on carriers was visualized using a scanning electron microscope (SEM) (Tescan, BEGA3S). The carrier samples for SEM analysis were randomly taken out from the reactors and cut into pieces very gently to prepare a flat part using a sterile forceps and scalpel and then air dried in dark conditions for 12 h. (Moussavi et al. 2009). Afterward, they were placed in fixative (2.5% glutaraldehyde v/v in phosphate buffer saline) for an overnight. The samples were rinsed in phosphate buffer saline (2 × 10 min) and subsequently dehydrated in a series of ethanol washes (30%, 50%, 70% and 90% each for 10 min and 100% for 20 min). Finally, the samples were air dried and coated with gold/palladium (2/3 ratio).
The average values of thickness, TSS and VSS were also investigated at the end of the batch experiment and after imposing the shock. The biofilm thickness was determined according to the procedure reported by Horn et al. (2003). Total suspended solid (TSS, dried at 103–105 °C), 2540 E and volatile suspended solid (VSS, ignited at 550 °C) were measured using Standard Methods (APHA 1998). Attached growth TSS and VSS were determined by removing periodically ten carriers from the MBBRs which was followed by addition of ten clean marked ones to keep constant the filling ratio and avoid resampling (Goode and Allen 2011). Since the number of carriers and amount of surfaces provided by them were known in all MBBRs, the total attached biomass and thickness could be calculated. Over the experiment, less than 30% of the total carriers were sampled.
Results
Biofilm-forming capacity
Microtiter plate assay was applied to investigate the ability of all eight bacterial strains to form biofilm within a specific period of time (Fig. 2). For the most of cultures, the amount of biofilm formation increased by passing the time, with the exception of a common drop on day seven which may be caused by a common detachment phase of biofilm resulted in leaving single cells or macroclusters. The largest amount of biofilm was observed in the mixed culture of eight bacteria rather than their individual culture, suggesting that synergistic interspecies effects on biofilm formation were likely to occur. Generally in single strain cultures, Acinetobacter spp. formed stronger biofilms than Pseudomonas spp., for instance strains GSN3 and GSN8 with about 99% similarity to Acinetobacter calcoaceticus DSM30006, formed the strongest biofilms with a very close measured amount of biomass to that of mixed culture.
The ability of producing biofilm in different media was investigated for both single and mixed culture of eight strains by microtiter plate test. All strains were able to form biofilm and classified as enhanced biofilm forming bacteria (EBFB) in all SWW, MSM and TSB media (Fig. 3). Biofilm formation was most consistently obtained in wastewater, where eight EBFB strains formed the strongest biofilm, followed by MSM, whereas the poorest biofilm formation achieved in TSB medium. By contrast, the strongest planktonic growth was observed in TSB medium. It is noteworthy that, the amount of biofilm formation in SWW by all single or mixed culture of EBFB strains was close to those measured in MSM, indicating that all of them not only possess the ability to degrade high concentration of phenol but also can tolerate the toxic effects of it. Similar to what Nafian et al. (2016) described for planktonic forms, the biofilm forms of eight strains were able to degrade 900 mg L−1 phenol over 24 h in SWW and TSB without any significant differences observed between them (data not shown here again). These characteristics make them eligible to be used in the treatment of phenol containing wastewaters.
The biofilm biomass of each EBFB strains, along with their mixture, was measured after cultivation in Erlenmeyer flasks for 24 h at a shaking speed of 80 rpm (Fig. 4). The results indicated that the formed biofilm by the mixed culture (2.26 ± 0.15 U A590nm) was higher than those of each strain individually while the strongest biofilm was formed by GSN3 (1.66 ± 0.25 U A590nm). A visible biofilm was produced at the air–liquid interface of the flasks wall in all experiments.
The colony forming unit (CFU) of single and mixed species biofilm established on carriers were counted to estimate the number of bacteria fixed in the biofilm for 24 h (Fig. 4). It was found that the mixed culture CFU was significantly higher than those of each single species, followed by the CFU of GSN3. After 24 h, cell numbers in the mixed culture reached to 19.9 × 1020 per 10 carriers while the lowest was 31.6 × 103, observed in GSN28 biofilm culture.
Reactor startup and the effect of the inlet phenol concentration on removal efficiency
The impact of the inlet phenol concentration in an increasing step-wise fashion was assessed starting from 50 mg L−1 (equivalent to COD of 107.5 mg L−1) at hydraulic retention times of 24 h. As illustrated in Fig. 5, all reactors were conducted in batch mode to the extent that by applying higher phenol concentrations the removal efficiency decreased below 90% in a steady state operating condition; which assumed when just ± 5% removal efficiency fluctuation was detected at consecutive HRTs. The removal efficiency of both parameters, phenol and COD, was almost greater than 90% up to the inlet phenol concentrations of 600, 900 and 1300 mg L−1 corresponding to 1290, 1935 and 2795 mg L−1 COD in R1, R2, and R3, respectively (Fig. 5a–c). The reactors did not manage to reach the steady-state operation again by applying greater amounts of phenol and displayed a fluctuation in removal efficiencies. Moreover, a slight increase in the inlet phenol concentration led to a significant reduction in phenol and COD removal efficiencies which might cause inhibition on microorganism activities. For instance, adding 700 mg L−1 phenol in R1 dropped the phenol and COD removal efficiencies from 90% to about 70% and 60%, respectively. In addition, the efficiency of COD removal was always lower than that of observed in phenol removal in all reactors suggesting the accumulation of intermediates (metabolites) over the experiment and the inhibitory effect of high phenol concentrations on metabolic activities which prevented microorganisms from transforming the phenol to the end products (Moussavi et al. 2010; Ho et al. 2009).
The relatively low degradation at the startup points can be contributed to the failure of lower phenol concentrations to stimulate the metabolism activity of microorganisms which declined the biological activities such as catabolism rate, respiration rate, etc. (Hosseini and Borghei 2005; Ho et al. 2009). This outcome can be expanded to other reactors since the similar behaviour was observed in all. The inlet phenol concentration in all reactors was decreased step-wise again to confirm the ability of them to retrieve from the inhibitory effect of overloading while allowing them to attain steady-state phenol removal for each concentration. Based on the results, R3 exhibited uppermost phenol degradation capability followed by R2 and the lowermost was for R1 in compared to control. Furthermore, COD decreasing rate was relatively close to that of phenol in R3 while the substantial differences were observed between them in R1 and R2 especially in higher inlet phenol concentrations.
The optimum phenol concentration was applied as an inlet phenol concentration for the succedent phases of the experiment.
Response to organic shock loading
After applying the phenol shock load, all reactors were fed again with SWW containing optimum phenol concentrations and the concentration of outlet phenol and COD were measured until achieving the degradation above 99%. As illustrated in Fig. 6a–c an increasing tolerance to phenol loading shock was detected in R3 > R2 > R1. After applying the shock, outlet phenol degradation gradually increased, and the removal efficiency reached to around 90% after 14, 20 and 32 days in R3, R2, and R1, subsequently. According to the results, the highest tolerance was observed in R3 while it was relatively low in R2. When subjected to loading shock, R1 showed the lowest resistance.
Table 2 shows the measurements of biofilm thickness, suspended and attached growth biomass. The amount of biofilm thickness in all reactors reached to the range of active biofilm layer at the end of the batch experiment (day 0) (Tyagi and Vembu 1990); however, the thicker biofilm was detected in R3 followed by R2. The assessment of attached and suspended growth biomass was carried out considering the TSS and VSS. Attached growth was dominant over non-attached growth in all reactors; however, enhanced efficiency of MBBRs can be attributed to the cooperative effects of both which made it more steady and effective than the suspended growth. It was in well agreement with achievement of other studies which showed the greater involvement of immobilized cells in biofilm to removal efficiency than free cells (Yu et al. 2007; Li et al. 2011).
After imposing the shock, the amount of thickness, attached TSS and VSS were reduced in all reactors while it was followed by an increase in suspended growth suggesting the occurrence of biofilm detachment. In addition, results indicated that a sudden enhancement in phenol concentration led to the detachment of biofilm from carriers in R1 more than R2 and R3. The values of thickness and attached biomass growth improved gradually while the suspended growth biomass concentration declined in a stepwise manner.
Microscopic examination of biofilm
For SEM microscopy of biofilm formation, ten new labelled carriers were added to each reactor at the steady state condition of each reactor and taken out after 24 and 48 h. SEM images revealed the presence of typical biofilms with spatial heterogeneity structure formed in all MBBRs (Fig. 7). Based on all captured images and comparative analysis of attached and suspended biomass in last section, it can be proposed that the biofilm formed on carriers in R3 were more populous. Figure 8 illustrates the formation of biofilm layer on carriers.
Discussion
In the present work, the biofilm forming ability of eight same-origin bacteria was assessed in different culture media. Then, the amount of biofilm formation and phenol removal was investigated by introducing their mixed culture in an MBBR. All eight strains studied in this survey were categorized as enhanced biofilm forming bacteria (EBFB) with a greater tendency to form biofilm in wastewater. Additionally, Acinetobacter spp. especially strains GSN3 and GSN8, assigned as Acinetobacter calcoaceticus, exhibited stronger biofilm production ability in all media rather than Pseudomonas spp. In a similar study the adherence and biofilm formation of single and mixed culture of several bacteria commonly found in wastewater treatment systems were studied and showed that Acinetobacter calcoaceticus can aggregate with other cells to form biofilm in aquatic environments. This strain, known as bridging bacterium, can assist other microorganisms to incorporate into the biofilm structure mainly due to its surface-based properties (Simões et al. 2008; Andersson et al. 2008). Similar observations were reported while biofilm formation of dual or multiple-strain cultures was compared to single-strain cultures especially in the presence of Acinetobacter calcoaceticus (Simões et al. 2008; Andersson et al. 2008). Andersson et al. (2008) studied the biofilm production of several wastewater bacterial strains alone and in combination with each other. Her findings showed that a more diverse mixture of microorganisms did not necessarily lead to the formation of strong biofilm especially in such a complex environment of wastewater unless strong biofilm producers which act as a bridge, aid other cells with poor biofilm forming abilities to attach on provided surfaces. In this case, the strongest biofilm formation may be observed when a mixture of microorganisms is grown together (Li et al. 2013; Andersson et al. 2008). Accordingly, the relatively high quantity of attached biomass in the mixed culture of eight EBFB strains can be attributed to the presence of a remarkable synergy between them to form biofilm and also the bridging role of Acinetobacter spp. in their mixture.
When EBFB and the sludge cells were introduced in one reactor (R3) the noticeably greater removal of phenol and COD was observed than those of two others inoculated with either activated sludge (R1) or EBFB strains (R2). Similar deductions were previously drawn by other researchers who investigated the performance of a mixture of contaminant degrading or biofilm forming microorganisms. Kwak et al. (2013) reported that co-inoculation of Sphingomonas sp. 224 with two other biofilm-forming strains led to the more efficiently degradation of Tolclofos-methyl than Sphingomonas sp. 224 alone. In another study it was demonstrated that when two strains of Acinetobacter sp. and Sphingomonas sp. mixed at the ratio of 1:1 were able to remove 1000 mg L−1 of phenol within 60 h more efficiently (97.2%) rather than their single inoculation (78.3% and 68.1%, respectively) (Liu et al. 2009). However it is noteworthy that, such investigations mainly attempted to explore the enhancement of degradation efficiency by presenting a mixture of microorganisms with either biofilm formation or contaminant degradation properties in treatment systems.
The close amount of produced biofilm thickness and biomass growth in R2 and R3 suggests that, the activity of strong biofilm producing bacteria in these reactors had more significant role in thicker biofilm formation than sludge cells. Additionally it is more likely that the beneficial activated sludge microorganisms mostly were immobilized by EBFB strains in R3 since high phenol degradation and attached biomass growth were not observed in R1 although both reactors were inoculated with the same activated sludge inoculum. Taking all into account, it can be concluded that the presence and performance of EBFB with strong biofilm-forming ability enhanced the immobilization of other activated sludge cells in the biofilm structure which was further confirmed by SEM microscopy and growth measurements. This phenomenon led to the development of a more diverse population of microorganisms containing EBFB strains with further degrading ability and also beneficial sludge cells in R3 which ultimately boosted the efficiency of phenol removal even more than that of observed in R2. The more population immobilized in biofilm, the more cooperation occurred in degradation of phenol and its intermediates which all directed to complete degradation of phenol and transformed it up to the end products efficiently. Li et al. (2013) reported the greater efficiency improvement of a biofilm treatment system by co-inoculation of three biofilm forming Bacillus strains and a specific Acetonitrile degrading bacterium in the presence of activated sludge.
After imposing the shock, both biofilm density and thickness in all reactors were reduced, but it was accompanied by a greater decrease in removal efficiency in R1 rather than two other reactors. Observation of similar resistance in R2 and R3 and as well as higher tolerance of R2 in comparison to R1 can be elucidated by hypothesizing that the activity of EBFB strains resulted in such an enhanced stability in R3. Bridging bacteria as an enhanced biofilm forming microorganisms are able to retain the integrity of biofilm structure by attaching other microorganisms firmly to the surface of carriers even in high loading shocks due to their specific surface interactions with other cells (Li et al. 2013, 2008).
Hence, the Immobilizing of wastewater microorganisms using EBFB with dual contaminant-degrading and biofilm-forming capabilities is more efficient, easier to operate and maintain approach in comparison to artificial immobilization techniques that rely on embedding agents or applying different bacteria with diverse capabilities and requirements. It is apparent that simplicity and cost-effectiveness of a method make it technically and economically feasible for industrial application. According to the literature, this is the first study where EBFB strains assisted activated sludge cells to immobilize in biofilm structure which subsequently led to the improvement of wastewater treatment. The achievement of this study has more apparent privileges and is applicable to other biofilm treatment systems particularly those struggling with the biofilm formation challenge.
References
Adav SS, Lee DJ, Lai JY (2008) Intergeneric coaggregation of strains isolated from phenol-degrading aerobic granules. Appl Microbiol Biotechnol 79:657–661. https://doi.org/10.1007/s00253-008-1456-8
Andersson S, Rajarao GK, Land CJ, Dalhammar G (2008) Biofilm formation and interactions of bacterial strains found in wastewater treatment systems. FEMS Microbiol Lett 283:83–90. https://doi.org/10.1111/j.1574-6968.2008.01149.x
Aneggi E, Trovarelli A, Goi D (2017) Degradation of phenol in wastewaters via heterogeneous fenton-like Ag/CeO2 catalyst. J Environ Chem Eng 5(1):1159–1165. https://doi.org/10.1016/j.jece.2017.01.042
APHA, AWWA, WEF (1998) Standard methods for the examination of water and wastewater, 20th edn. American Public Health Association, Washington, DC, pp. 5–18
APHA, AWWA, WEF (2005) Standard methods for the examination of water and wastewater, 21st edn. American Public Health Association, Washington, DC
Bassin JP, Dias IN, Cao SMS, Senra E, Laranjeira Y, Dezotti M (2016) Effect of increasing organic loading rates on the performance of moving-bed biofilm reactors filled with different support media: assessing the activity of suspended and attached biomass fractions. Process Saf Environ Prot 100:131–141. https://doi.org/10.1016/j.psep.2016.01.007
Bechet M, Blondeau R (2003) Factors associated with the adherence and biofilm formation by Aeromonas caviae on glass surfaces. J Appl Microbiol 94:1072–1078. https://doi.org/10.1046/j.1365-2672.2003.01931.x
Cheng Z, Meng X, Wang H, Chen M, Li M (2014) Isolation and characterization of broad spectrum coaggregating bacteria from different water systems for potential use in bioaugmentation. PLoS ONE 9(4):e94220. https://doi.org/10.1371/journal.pone.0094220
Di Gioia D, Fambrini L, Coppini E, Fava F, Barberio C (2004) Aggregation based cooperation during bacterial aerobic degradation of polyethoxylated nonylphenols. Res Microbiol 155:761–769. https://doi.org/10.1016/j.resmic.2004.05.015
Djordjevic D, Wiedmann M, McLandsborough LA (2002) Microtiter plate assay for assessment of Listeria monocytogenes biofilm formation. Appl Environ Microbiol 68:2950–2958. https://doi.org/10.1128/AEM.68.6.2950-2958.2002
Duque AF, Bessa VS, Carvalho MF, Castro PML (2011) Bioaugmentation of a rotating biological contactor for degradation of 2-fluorophenol. Bioresour Technol 102:9300–9303. https://doi.org/10.1016/j.biortech.2011.07.003
Eldyasti A, Nakhla G, Zhu J (2013) Impact of calcium on biofilm morphology, structure, detachment and performance in denitrifying fluidized bed bioreactors (DFBBRs). Chem Eng J 232:183–195. https://doi.org/10.1016/j.cej.2013.07.084
El-Naas MH, Al-Muhtaseb SA, Souzan M (2009) Biodegradation of phenol by Pseudomonas putida immobilized in polyvinyl alcohol (PVA) gel. J Hazard Mater 164:720–725. https://doi.org/10.1016/j.jhazmat.2008.08.059
Ferro Orozco AM, Contreras EM, Zaritzky NE (2016) Biodegradation of Bisphenol A and its metabolic intermediates by activated sludge: stoichiometry and kinetics analysis. Int Biodeterior Biodegrad 106:1–9. https://doi.org/10.1016/j.ibiod.2015.10.003
Goode C, Allen DG (2011) Effect of calcium on moving-bed biofilm reactor biofilms. Water Environ Res 83(3):220–232. https://doi.org/10.2175/106143010X12780288628255
Guiot SR, Tawfiki-Hajji K, Lepine F (2000) Immobilization strategies for bioaugmentation of anaerobic reactors treating phenolic compounds. Water Sci Technol 42:245–250. https://doi.org/10.2166/wst.2000.0520
Hemmati M, Nazari N, Hemmati A, Shirazian S (2015) Phenol removal from wastewater by means of nanoporous membrane contactors. J Ind Eng Chem 21:1410–1416. https://doi.org/10.1016/j.jiec.2014.06.015
Ho KL, Lin B, Chen YY, Lee DJ (2009) Biodegradation of phenol using Corynebacterium sp. DJ1 aerobic granules. Bioresour Technol 100:5051–5055. https://doi.org/10.1016/j.biortech.2009.05.050
Horn H, Reiff H, Morgenroth E (2003) Simulation of growth and detachment in biofilm systems under defined hydrodynamic conditions. Biotechnol Bioeng 81(5):607–617
Hosseini SH, Borghei SM (2005) The treatment of phenolic wastewater using a moving bed bio-reactor. Process Biochem 40:1027–1031. https://doi.org/10.1016/S0032-9592(02)00195-4
Jiang H-L, Tay J-H, Maszenan AM, Tay ST-L (2006) Enhanced phenol biodegradation and aerobic granulation by two coaggregating bacterial strains. Environmental Science Technology 40:6137–6142. https://doi.org/10.1021/es0609295
Kilic M, Apaydin-Varol E, Pütün AE (2011) Adsorptive removal of phenol from aqueous solutions on activated carbon prepared from tobacco residues equilibrium, kinetics and thermodynamics. J Hazard Mater 189:397–403. https://doi.org/10.1016/j.jhazmat.2011.02.051
Kwak Y, Rhee IK, Shin JH (2013) Application of biofilm-forming bacteria on the enhancement of organophosphorus fungicide degradation. Bioremediat J 17:173–181. https://doi.org/10.1080/10889868.2013.807773
Leiknes TO, Odegaard H (2007) The development of a biofilm membrane bioreactor. Desalination 202:135–143. https://doi.org/10.1016/j.desal.2005.12.049
Li M, Peng L, Ji Z, Xu J, Li S (2008) Establishment and characterization of dual-species biofilms formed from a 3,5-dinitrobenzoic-degrading strain and bacteria with high biofilm-forming capabilities. FEMS Microbiol Lett 278:15–21. https://doi.org/10.1111/j.1574-6968.2007.00913.x
Li H, Han H, Du M, Wang W (2011) Removal of phenols, thiocyanate and ammonium from coal gasification wastewater using moving bed biofilm reactor. Bioresour Technol 102:4667–4673. https://doi.org/10.1016/j.biortech.2011.01.029
Li C, Li Y, Cheng X, Feng L, Xi C, Zhang Y (2013) Immobilization of Rhodococcus rhodochrous BX2 (an acetonitrile degrading bacterium) with biofilm-forming bacteria for wastewater treatment. Bioresour Technol 131:390–396. https://doi.org/10.1016/j.biortech.2012.12.140
Lin CW, Lai CY, Chen LH, Chiang WF (2007) Microbial community structure during oxygen-stimulated bioremediation in phenol-contaminated groundwater. J Hazard Mater 140:221–229. https://doi.org/10.1016/j.jhazmat.2006.06.083
Liu YJ, Kuschk P, Zhang AN, Wang XC (2009) Characterisation of phenol degradation by Acinetobacter sp. XA05 and Sphingomonas sp. FG03. Chem Ecol 25(2):107–117. https://doi.org/10.1080/02757540902849278
Liu Z, Cao J, Meng H, Zhang H, Zhou K, Lian J (2018) Highly efficient degradation of phenol wastewater by microwave induced H2O2-CuOx/GAC catalytic oxidation. Sep Purif Technol 193:49–57. https://doi.org/10.1016/j.seppur.2017.11.010
Loloi M, Rezaee A, Aliofkhazraei M (2016) Electrocatalytic oxidation of phenol from wastewater using Ti/SnO-SbO electrode: chemical reaction pathway study. Environ Sci Pollut Res 23(19):19735–19743. https://doi.org/10.1007/s11356-016-7110-6
Malik A, Sakamoto M, Ono T, Kakii K (2003) Coaggregation between Acinetobacter johnsonii S35 and microbacterium esteraromaticum strains isolated from sewage activated sludge. J Biosci Bioeng 96:10–15. https://doi.org/10.1016/j.seppur.2017.11.010
Metzger Z, Blasbalg J, Dotan M, Tsesis I, Weiss EI (2009) Characterization of coaggregation of Fusobacterium nucleatum PK1594 with six Porphyromonas gingivalis strains. J Endod 35:50–54. https://doi.org/10.1016/j.joen.2008.09.016
Min KR, Rickard AH (2009) Coaggregation by the freshwater bacterium Sphingomonas natatoria alters dual-species biofilm formation. Appl Environ Microbiol 75(12):3987–3997. https://doi.org/10.1128/AEM.02843-08
Moussavi G, Mahmoudi M, Barikbin B (2009) Biological removal of phenol from strong wastewaters using a novel MSBR. Water Res 43:1295–1302. https://doi.org/10.1016/j.watres.2008.12.026
Moussavi G, Barikbin B, Mahmoudi M (2010) The removal of high concentrations of phenol from saline wastewater using aerobic granular SBR. Chem Eng J 158:498–504. https://doi.org/10.1016/j.cej.2010.01.038
Moussavi G, Ghodrati S, Mohseni-Bandpei A (2014) The biodegradation and COD removal of 2-chlorophenol in a granular anoxic baffled reactor. J Biotechnol 184:111–117. https://doi.org/10.1016/j.jbiotec.2014.05.010
Nafian F, Gharavi S, Soudi MR (2016) Degenerate primers as biomarker for gene-targeted metagenomics of the catechol 1,2-dioxygenase-encoding gene in microbial populations of petroleum contaminated environments. Ann Microbiol 66(3):1127–1136. https://doi.org/10.1007/s13213-016-1197-3
Nakhli SAA, Ahmadizadeh K, Fereshtehnejad M et al (2014) Biological removal of phenol from saline wastewater using a moving bed biofilm reactor containing acclimated mixed consortia. SpringerPlus 3:1–10. https://doi.org/10.1186/2193-1801-3-112
Pookpoosa I, Jindal R, Morknoy D, Tantrakarnapa K (2015) Occurrence and efficacy of Bisphenol A (BPA) treatment in selected municipal wastewater treatment plants, Bangkok, Thailand. Water Sci Technol 72(3):463–471. https://doi.org/10.2166/wst.2015.232
Prieto MB, Hidalgo A, Rodríguez-Fernández C, Serra JL, Llama MJ (2002) Biodegradation of phenol in synthetic and industrial wastewater by Rhodococcus erythropolis UPV-1 immobilized in an air-stirred reactor with clarifier. Appl Microbiol Biotechnol 58:853–859. https://doi.org/10.1007/s00253-002-0963-2
Rafiei B, Naeimpoor F, Mohammadi T (2014) Bio-film and bio-entrapped hybrid membrane bioreactors in wastewater treatment: comparison of membrane fouling and removal efficiency. Desalination 337(1):16–22. https://doi.org/10.1016/j.desal.2013.12.025
Ramalingam B, Sekar R, Boxall JB, Biggs CA (2013) Aggregation and biofilm formation of bacteria isolated from domestic drinking water. Water Sci Technol Water Suppl 13:1016. https://doi.org/10.2166/ws.2013.115
Ren D, Madsen JS, Sorensen SJ et al (2015) High prevalence of bioflm synergy among bacterial soil isolates in cocultures indicates bacterial interspecifc cooperation. ISME J 9:81–89. https://doi.org/10.1038/ismej.2014.96
Rickard AH, Leach SA, Hall LS, Buswell CM, High NJ, Handley PS (2002) Phylogenetic relationships and coaggregation ability of freshwater biofilm bacteria. Appl Environ Microbiol 68(7):3644–3650. https://doi.org/10.1128/AEM.68.7.3644-3650.2002
Rickard AH, McBain AJ, Ledder RG, Handley PS, Gilbert P (2003) Coaggregation between freshwater bacteria within biofilm and planktonic communities. FEMS Microbiol Lett 220:133–140. https://doi.org/10.1016/S0378-1097(03)00094-6
Rickard AH, Gilbert P, Handley PS (2004) Influence of growth environment on coaggregation between freshwater biofilm bacteria. Appl Microbiol 96:1367–1373. https://doi.org/10.1111/j.1365-2672.2004.02297.x
Simões LC, Simões M, Vieira MJ (2007) Biofilm interactions between distinct bacterial genera isolated from drinking water. Appl Environ Microbiol 73:6192–6200. https://doi.org/10.1128/AEM.00837-07
Simões LC, Simões M, Vieira MJ (2008) Intergeneric coaggregation among drinking water bacteria: evidence of a role for Acinetobacter calcoaceticus as a bridging bacterium. Appl Environ Microbiol 74:1259–1263. https://doi.org/10.1128/AEM.00837-07
Singh N, Balomajumder C (2016) Simultaneous removal of phenol and cyanide from aqueous solution by adsorption onto surface modified activated carbon prepared from coconut shell. J Water Process Eng 9:233–245. https://doi.org/10.1016/j.jwpe.2016.01.008
Stepanovic S, Vukovic D, Dakic I, Savic B, Svabic-Vlahovic M (2000) A modified microtiter-plate test for quantification of staphylococcal biofilm formation. J Microbiol Methods 40:175–179. https://doi.org/10.1016/S0167-7012(00)00122-6
Stevens MRE, Luo TL, Vornhagen J, Jakubovics NS, Gilsdorf JR, Marrs CF, Møretrø T, Rickard AH (2015) Coaggregation occurs between microorganisms isolated from different environments. FEMS Microbiol Ecol 91(11):1–14. https://doi.org/10.1093/femsec/fiv123
Tyagi RD, Vembu K (1990) Wastewater treatment by immobilized cells. CRC Press, Boca Raton
Yu HB, Quan X, Ding YZ (2007) Medium-strength ammonium removal using a two-stage moving bed biofilm reactor system. Environ Eng Sci 24(5):595–601. https://doi.org/10.1089/ees.2006.0104
Zhang JX, Zhang YB, Quan X, Li Y, Chen S, Zhao HM, Wang D (2012) An anaerobic reactor packed with a pair of Fe-graphite plate electrodes for bioaugmentation of azo dye wastewater treatment. Biochem Eng J 63:31–37. https://doi.org/10.1016/j.bej.2012.01.008
Zhu J, Mekalanos JJ (2003) Quorum sensing-dependent biofilms enhance colonization in Vibrio cholerae. Dev Cell 5:647–656. https://doi.org/10.1016/S1534-5807(03)00295-8
Zielińska M, Cydzik-Kwiatkowska A, Bernat K, Bułkowska K, Wojnowska-Baryła I (2014) Removal of Bisphenol A (BPA) in a nitrifying system with immobilized biomass. Bioresour Technol 171:305–313. https://doi.org/10.1016/j.biortech.2014.08.087
Acknowledgements
This work partially was supported by Iran National Science Foundation [96001722].
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Irankhah, S., Abdi Ali, A., Reza Soudi, M. et al. Highly efficient phenol degradation in a batch moving bed biofilm reactor: benefiting from biofilm-enhancing bacteria. World J Microbiol Biotechnol 34, 164 (2018). https://doi.org/10.1007/s11274-018-2543-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11274-018-2543-3