Abstract
Background
Questions have been raised as to an increased risk of local recurrence with breast-conserving surgery (BCS) post NAC highlighting the uncertainty around optimal margin width in this patient population. We examined the association between margin status and local recurrence-free survival (LRFS) in patients who underwent BCS following NAC.
Methods
We performed a retrospective cohort study of adult female patients with stage I–III breast cancer who underwent NAC followed by BCS between 2012 and 2021 at two cancer centers. Margins were categorized as “close” if they were < 1 mm.
Results
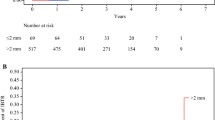
The full cohort included 544 patients with a median age of 53 years (interquartile range [IQR] 44–64). Pathologic complete response (pCR) was achieved in 41.2% of the overall cohort (n = 224). Of the 320 with residual disease, 29.4% (n = 94) had at least one close margin, and 10.9% (n = 35) had ≥2 close margins. Median follow-up was 55 months (IQR 32–83); 4.8% had an ipsilateral breast recurrence (n = 26). Patients with pCR had a higher 5-year LRFS than those with residual disease (98.0% vs. 91.6%, p = 0.02). There was no difference in 5-year LRFS between the margin categories (clear vs. 1 close margin vs. ≥2 close margins) in those with residual disease (92.2% vs. 88.9% vs. 92.9%) (p = 0.78).
Conclusions
In patients undergoing BCS post-NAC, those who achieved pCR had a significantly higher LRFS compared with those with residual disease at the time of surgery, but LRFS was not associated with margin width nor the number of close margins.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
The safety and efficacy of neoadjuvant chemotherapy (NAC) in breast cancer care has been widely validated through large prospective studies, such as the NSABP B-18 and B-27 trials.1,2,3 In large or locally advanced breast cancers, it can serve to downsize tumors in an attempt to make them eligible for breast-conserving surgery (BCS).4 Additionally, it can serve as a platform for the in vivo study of tumor response to chemotherapy and as a prognostic indicator; in fact, patients in whom pathological complete response (pCR) can be achieved with NAC have been found to have improved local recurrence free (LRFS), disease-free (DFS), and overall survival (OS).5,6,7 NAC also can be useful in downstaging a clinically positive axilla, thus allowing for de-escalation of axillary surgery.8,9,10
With regards to de-escalation of breast surgery, there are currently no guidelines regarding the optimal margin width in patients who undergo BCS following NAC. This leaves an important knowledge gap as NAC has been shown repeatedly to increase breast conservation rates.11,12,13 Some studies aim for a 2-mm negative margin;14 others have extrapolated the 2014 Society of Surgical Oncology (SSO) and American Society for Radiation Oncology (ASTRO) of “no ink on tumor” to this patient population, although patients who had received NAC were specifically excluded from these guidelines.15 A limited number of studies have tried to evaluate the optimal margin width for patients undergoing BCS post-NAC; however, these have been small retrospective cohorts with mixed margin widths and have typically included patients with frankly positive margins, who are known to have significantly lower LRFS and DFS.14,16,17 Additionally, given the small cohort sizes, event rates have been low, limiting the interpretation of the results.14 A single systematic review and meta-analysis of the literature has been performed regarding margin width in BCS, but it specifically excluded patients who had received NAC.18
Given this dearth of evidence, we evaluated the association between margin width and number of close margins, and LRFS following BCS after NAC. Secondary objectives were to try to identify clinical and histopathological features associated with recurrence.
Materials and Methods
Study Population
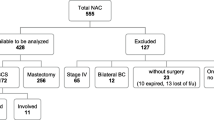
A retrospective cohort of adult female patients with stage I–III breast cancer who underwent BCS following neoadjuvant chemotherapy between 2012 and 2021 was identified at two university-affiliated academic institutions. Patients who received neoadjuvant endocrine, immuno-, or targeted therapy alone without chemotherapy were excluded. Those who underwent a completion total mastectomy after initial breast-conserving surgery for any reason, including margin positivity, and patients without surgical follow-up data also were excluded. Details regarding included and excluded patients can be found in the consort diagram (Fig. 1). Institutional ethics review board approval was obtained at both institutions before initiating the study.
Outcomes and Variables of Interest
The primary outcome of interest was local recurrence-free survival (LRFS) according to margin status (close vs. clear, and number of close margins). Electronic medical records (EMR) were interrogated to retrospectively collect demographic, clinical, pathological, and radiological data, as well as data specific to recurrences and deaths. These data included age at surgery, clinical staging information, neoadjuvant therapy regimen, type of breast and axillary surgery, pathological staging, margin and pCR details, re-excision data, and adjuvant therapy. Recurrence and mortality data were censored as of July 2023.
Staging information was standardized according to the 2018 American Joint Committee on Cancer’s Staging System (AJCC) 8th Edition.19 Clinically positive lymph nodes were defined as either palpable nodes or suspicious nodes on ultrasound with a positive biopsy. Those who had suspicious nodes on imaging without a biopsy or with a negative biopsy were considered to be clinically node-negative (cN0).
Pathological complete response (pCR) data refers to ypT0/Tis status. Margin width was defined as follows, where clear margins were defined as ≥ 1 mm, close margins were defined as < 1 mm, and positive margins were defined as “no tumour on ink.” Any invasive disease or DCIS within 1 mm of ink was considered close. Only the initial margin status was used, as those who underwent margin revision most often did not have a defined margin width. All patients with positive margins underwent margin revision and, for the aforementioned reason, were excluded from the LRFS analysis.
Recurrence data were defined as either an ipsilateral breast tumor recurrence (IBTR), a regional recurrence (ipsilateral regional nodal basin recurrence), a contralateral recurrence (nodal or in-breast), or a distant recurrence. IBTR was used to define LRFS, whereas all recurrences were grouped to define disease-free survival (DFS). Chemotherapy regimens were characterized as anthracycline-based or anthracycline-free.
Statistical Analysis
Descriptive statistics were used to summarize baseline clinical, pathological, and recurrence characteristics of the study populations. Data are represented as N (%) for categorical variables and median (interquartile range [IQR]) for continuous variables. A univariable analysis evaluating IBTR was performed using Chi-squared tests and logistic regression. The Kaplan-Meier method was used to evaluate LFRS, DFS, and OS, which were stratified based on patients’ pathological response status. Those with residual disease were then substratified based on margin width as well as number of close margins. Five-year survival estimates are presented along with 95% confidence intervals (CI). All statistical analyses were performed using the Statistical Analysis System (SAS) software version 9.4 (Cary, NC), and p-values < 0.05 were considered statistically significant.
Results
Cohort Characteristics
Clinical and pathological baseline data are summarized in Table 1. A total of 834 patients were reviewed for eligibility, of whom 544 met inclusion criteria (Fig. 1). The median age at surgery was 53 years (IQR 45–64). Nearly two-thirds of patients (65.8%) had clinical T2 (cT2) disease at diagnosis (n = 358); 95.6% of tumors (n = 525) were invasive ductal carcinoma (IDC). The vast majority of tumors were grade 2 (42.5%, n = 231) or grade 3 (53.7%, n = 294) at diagnosis. The most common biological subtype was triple-negative breast cancer (TNBC) in 34.6% (n = 190) of patients, followed by hormone receptor-positive, human epidermal growth factor receptor 2-negative (HR +/HER2 −) in 29.5% (n = 162). Just less than half of the included patients were clinically node-positive (cN +) at diagnosis (48.3%, n = 265). Preoperative magnetic resonance imaging (MRI) was performed in 49.5% (n = 269) of the cohort. Most patients received anthracycline-based chemotherapy (77.4%, n = 425). All patients with HER2+ disease received an anti-HER2 targeted agent before surgery, and 81% (n = 158) completed the full adjuvant course. Pathologic complete response was achieved in 41.2% of patients (n = 224). Nearly all patients received adjuvant radiation therapy (97.1%, n = 533), whereas 44.7% of patients (n = 243) received adjuvant endocrine therapy.
Margin Data
For the entire cohort, at the time of the initial breast conserving surgery, 82% of patients (n = 446) had clear margins (≥ 1 mm), 17.3% (n = 94) had close margins (< 1 mm), and 0.7% (n = 4) had positive margins. In those with close margins, 62.8% (n = 59) had a single close margin and 37.2% (n = 35) had two or more close margins. Of the entire cohort, 4.8% (n = 26) of patients underwent margin revision, of whom 69.2% (n = 18) had close margins, 15.4% (n = 4) had clear margins, and 15.4% (n = 4) had positive margins. Of those with close margins (n = 18) who underwent reexcision, four (22.2%) had residual disease on reexcision. Only one of those four patients with residual disease underwent an additional revision.
Outcomes of Interest
The median follow-up time was 55 (IQR 32–83 months), during which 4.8% (n = 26) had an IBTR. The crude median time to IBTR was 29 (IQR 13–49) months. Of the 26 patients who recurred, 69.2% (n = 18) had a clear initial margin, 26.9% (n = 7) had one close margin, and 3.8% (n = 1) had two or more close margins. None of the patients with initially positive margins had an IBTR event at the time of data censuring. Furthermore, 26.9% (n = 7) of patients with IBTR had HR+/HER2− disease, 3.8% (n = 1) had HR+/HER2+ disease, 30.8% (n = 8) had HR−/HER2+ disease, and 38.5% (n = 10) had HR−/HER2− disease. Of those with an IBTR, 80.8% (n = 26) had clinical T2 disease or greater, and 61.5% (n = 16) were clinically node-positive. No patients with IBTR had grade I disease, whereas 53.8% (n = 14) had grade II disease and 46.2% (n = 12) had grade III disease. Notably, 23.1% (n = 6) patients with IBTR did not receive adjuvant radiotherapy after their index surgery. The majority (76.9%) had an isolated local recurrence.
When stratifying by response to neoadjuvant chemotherapy, the 5-year LRFS was 98% (95% CI 94.9–99.3) in those with pCR compared with 91.6% (95% CI 86.9–94.7) in those with residual disease (p = 0.02; Table 2). In patients who did not achieve pCR, the group with clear margins had a 5-year LRFS of 92.2% (95% CI 87.3–95.3) compared with 88.9% (95% CI 67.8–96.4) in those with one close margin and 92.9% (95% CI 59.1–99) in those with ≥2 close margins (p = 0.78; Table 3). These LRFS estimates were not significantly different. There was also no difference in LRFS between patients with close margins who went for surgical revision versus those who did not (p = 0.41). Figures 2 and 3 demonstrate the Kaplan Meier curves for LRFS in the aforementioned subgroups.
On univariable regression analysis, the only factors associated with statistically significantly lower IBTR rates were pCR and receipt of adjuvant radiation therapy (Table 4). The unadjusted IBTR rate was 2.2% in patients with breast pCR versus 6.5% in patients who did not achieve pCR (p = 0.02). Additionally, patients who did not receive adjuvant radiation had an IBTR rate of 37.5% versus 3.8% in those who underwent radiation (p < 0.001). Conversely, neither the initial margin status (clear vs. close vs. positive) nor the number of close margins were significantly associated with IBTR (0.12). Furthermore, a sensitivity analysis was conducted by excluding the 18 patients with close margins who underwent margin revision. There remained no statistically significant difference in IBTR rate associated with margin status (p = 0.07). The unadjusted 5-year DFS and OS rates were similar in the clear and close margin groups, with p-values of 0.68 and 0.13 respectively (Table 3).
Discussion
The 2014 SSO-ASTRO guidelines confirmed “no ink on tumor” as the adequate margin width for breast-conserving surgery for invasive cancer laying to rest a longstanding clinical controversy.15 However, these guidelines specifically excluded patients having received NAC and, to date, no international consensus on the most appropriate margin distance exists for patients undergoing BCS after NAC. While early results from the landmark NSABP-B-18 trial had demonstrated higher local recurrence rates in patients who received NAC, more contemporary evidence has emerged confirming the oncologic safety of BCS in the post-NAC setting.14,16,20,21 Nonetheless, data regarding the optimal margin width in this patient population remain scarce despite the widespread use of NAC as a strategy to render patients eligible for breast conservation.12,13
In the context of this knowledge gap, we sought to examine the association between margin width and local recurrence in patients with operable breast cancer who had breast-conserving surgery after undergoing NAC in two comprehensive cancer centers in Canada. Our final cohort included 544 patients, and we defined a “close” margin as any invasive or in situ disease within < 1 mm. We also elected to evaluate whether the number of close margins (1 vs. ≥2) in addition to the margin width itself was associated with local recurrence. This was done because we felt that decisions for margin revision or completion mastectomy may be influenced by surgical pathology reports identifying more than one margin of < 1 mm. We also showed that neither the margin width, nor the number of close margins was associated with increased LRFS (p = 0.78), DFS (p = 0.68), or OS (p = 0.13). Moreover, we redemonstrated the prognostic importance of pCR as well as the strong impact of radiation therapy on reduction of local recurrence.
At a median follow-up of 55 months (IQR 32–83), our study showed a low unadjusted IBTR rate of 4.8% in the overall cohort. The recurrence rate was found to be statistically similar in patients with clear, 1 close, and ≥ 2 close margins (p = 0.12). While our series may not be the first to study the impact of margin width in patients undergoing BCS post NAC, we are the first to report on the effect of a margin width of < 1 mm in a patient cohort treated in a North American clinical context. Choi et al. published their series of 382 patients undergoing BCS after NAC from 2002 and 2014.14 At a median follow-up of 57 months, they showed an unadjusted recurrence rate of 3.9% and found no difference in LRFS, DFS, or OS rates when comparing margin widths of ≤ 2 mm and > 2 mm. However, they were unable to evaluate IBTR rates in those with margins ≤ 1 mm, as they had no events in this category. Similarly, Mrdutt et al. also evaluated the association between margin width and local recurrence post NAC in 582 patients.13 Like the previous research group, they stratified their margin width by ≤ 2 mm or > 2 mm and found no impact of margin width on their 4-year IBTR rate of 2%. Interestingly, this IBTR rate is lower than what we reported despite a comparable cohort size and treatment period. We believe the difference here is driven by a shorter follow-up period in the Mrdutt study as well as by the omission of adjuvant radiation therapy in approximately 3% of our cohort. Furthermore, although direct statistical comparisons cannot be drawn, our cohort had a numerically higher proportion of patients with a triple negative biologic subtype.
Other international groups have also reported on this clinical question with similar results.20 The largest cohort was analysed by Cheun et al., who published the results of their evaluation of 2803 South Korean patients undergoing BCS post-NAC.21 As expected, they found that the largest driver of LRFS, DFS, and OS was pCR and that there was no difference in survival outcomes when comparing the various margin statuses (positive, close [< 2 mm], or widely negative) of those with residual disease. The prognostic significance of response to neoadjuvant chemotherapy has been well-established in several prospective clinical trials.5,22 Pathologic complete response is now sought by investigators, clinicians, and patients alike as the de facto surrogate marker for improved survival outcomes. It is, therefore, unsurprising that our study results, like those of other groups, reflect this by showing a significant association between pCR and IBTR rate. On our Kaplan-Meier analysis, those with pCR had a statistically significant LRFS benefit of 6.4% compared with those with residual disease (p = 0.02; Table 3). In addition to pCR, our study demonstrated the known impact of adjuvant radiation on local control post-breast conservation.
It is noteworthy that, in our series, the margin positivity rate was very low at only 0.7% (n = 4). Margin positivity rates post-NAC have not been prospectively evaluated. As a result, evidence from retrospective series has been somewhat inconsistent. Several studies have found high rates of positive margins in those undergoing BCS following NAC.14,23,24,25 A 2018 systematic review of surgical outcomes following BCS after NAC showed a range of margin positivity rates between 2 and 40%.26 The applicability of this data to our contemporary clinical context is challenging however, as the definition of positive margins in these studies varied from “no tumor on ink” to <5 mm. Reassuringly, although this trend was not formally assessed by the authors, it is clear that as the rates of pCR increased over time with the use of modern chemotherapy/targeted therapy regimens along with improvements in diagnostic and localization techniques, the rates of positive margins declined. Our study, like other more recent ones, shows this sustained downward trend. It also is important to remember that we excluded 12 patients from our analysis who underwent completion mastectomy because of initially positive margins.
Finally, questions remain as to the standardized use of preoperative MRI in the setting of neoadjuvant chemotherapy. Routine use of MRI has not been shown to accurately predict pCR, nor has it consistently been shown to reduce rates of positive margins.27,28,29,30 Our study cohort reflects real-world practice patterns with approximately half the NAC patients undergoing preoperative MRI. We showed that preoperative MRI use in patients who received NAC was not associated with an increased incidence of IBTR (p = 0.11).
Limitations
Several important limitations must be acknowledged when contextualizing our study results to clinical practice settings. Because of the retrospective study design and the lack of synoptic reporting within and across institutions, our data lacked some important granularity. We were unable to collect certain parameters, such as the technical details of margin revisions, those of adjuvant radiation therapy protocols, or the pattern of response in patients with residual disease (i.e., concentric vs. scattered). We elected to use the “initial margin status” instead of the “final margin status” to categorize our patients as we felt that the inter- and intra-institutional heterogeneity between reexcision technique and pathology reporting made the final margin classification inaccurate. However, we conducted a sensitivity analysis excluding the 18 patients with close margins who underwent reexcision to test the effect of our decision. We found that this did not drastically change the overall conclusions (p = 0.07), suggesting that our initial findings are somewhat robust but that more data is necessary to confirm the observed trends. Moreover, we know that adding a tumor bed boost to adjuvant whole breast irradiation for patients with high-risk breast cancer can be associated with improved local control; however, we could not evaluate its use and impact in the current study.31 The association between tumor response pattern and IBTR is less clear, and it would have been interesting to study this in our cohort; however, those details were not available within our pathology reports. Finally, a longer follow-up time and larger sample size would help strengthen our results, especially given the low observed event rate. Only 26 patients in our study had an IBTR and, as a result, we were unable to perform a statistically sound multivariable analysis. Our results should therefore be interpreted with caution.
Conclusions
In our real-world cohort of patients undergoing BCS post NAC, neither a margin width of <1 mm, nor the number of close margins was associated with an increased IBTR rate. Residual disease post-NAC and omission of adjuvant radiation were drivers of increased local recurrence. Our study supports the recommendation of “no ink on tumor” in this patient population, although a larger sample size and longer follow-up would help to reinforce these conclusions.
References
Wolmark N, Wang J, Mamounas E, Bryant J, Fisher B. Preoperative chemotherapy in patients with operable breast cancer: nine-year results from National Surgical Adjuvant Breast and Bowel Project B-18. J Natl Cancer Inst Monogr. 2001;30:96–102. https://doi.org/10.1093/oxfordjournals.jncimonographs.a003469.
Mamounas EP. NSABP Protocol B-27. Preoperative doxorubicin plus cyclophosphamide followed by preoperative or postoperative docetaxel. Oncology (Williston Park). 1997;11(6 Suppl 6):37–40.
Abdel-Razeq H, Marei L, Saadeh SS, et al. From clinical trials to clinical practice: outcome of NSABP-B27 neoadjuvant chemotherapy regimen for high-risk early-stage breast cancer. Breast Cancer Res Treat. 2017;165(3):771–7. https://doi.org/10.1007/s10549-017-4359-5.
Fisher B, Brown A, Mamounas E, et al. Effect of preoperative chemotherapy on local-regional disease in women with operable breast cancer: findings from National Surgical Adjuvant Breast and Bowel Project B-18. J Clin Oncol. 1997;15(7):2483–93. https://doi.org/10.1200/jco.1997.15.7.2483.
Spring LM, Fell G, Arfe A, et al. Pathologic complete response after neoadjuvant chemotherapy and impact on breast cancer recurrence and survival: a comprehensive meta-analysis. Clin Cancer Res. 2020;26(12):2838–48. https://doi.org/10.1158/1078-0432.Ccr-19-3492.
von Minckwitz G, Untch M, Blohmer J-U, et al. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J Clin Oncol. 2012;30(15):1796–804. https://doi.org/10.1200/jco.2011.38.8595.
Bonnefoi H, Litière S, Piccart M, et al. Pathological complete response after neoadjuvant chemotherapy is an independent predictive factor irrespective of simplified breast cancer intrinsic subtypes: a landmark and two-step approach analyses from the EORTC 10994/BIG 1–00 phase III trial. Ann Oncol. 2014;25(6):1128–36. https://doi.org/10.1093/annonc/mdu118.
Caudle AS, Yang WT, Krishnamurthy S, et al. Improved axillary evaluation following neoadjuvant therapy for patients with node-positive breast cancer using selective evaluation of clipped nodes: implementation of targeted axillary dissection. J Clin Oncol. 2016;34(10):1072–8. https://doi.org/10.1200/jco.2015.64.0094.
Boughey JC, Ballman KV, Le-Petross HT, et al. Identification and resection of clipped node decreases the false-negative rate of sentinel lymph node surgery in patients presenting with node-positive breast cancer (T0–T4, N1–N2) who receive neoadjuvant chemotherapy: results from ACOSOG Z1071 (Alliance). Ann Surg. 2016;263(4):802–7. https://doi.org/10.1097/sla.0000000000001375.
Boileau JF, Poirier B, Basik M, et al. Sentinel node biopsy after neoadjuvant chemotherapy in biopsy-proven node-positive breast cancer: the SN FNAC study. J Clin Oncol. 2015;33(3):258–64. https://doi.org/10.1200/jco.2014.55.7827.
Cortazar P, Zhang L, Untch M, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet. 2014;384(9938):164–72. https://doi.org/10.1016/s0140-6736(13)62422-8.
Golshan M, Cirrincione CT, Sikov WM, et al. Impact of neoadjuvant chemotherapy in stage II-III triple negative breast cancer on eligibility for breast-conserving surgery and breast conservation rates: surgical results from CALGB 40603 (Alliance). Ann Surg. 2015;262(3):434–9. https://doi.org/10.1097/sla.0000000000001417. (discussion 438-9).
Golshan M, Cirrincione CT, Sikov WM, et al. Impact of neoadjuvant therapy on eligibility for and frequency of breast conservation in stage II-III HER2-positive breast cancer: surgical results of CALGB 40601 (Alliance). Breast Cancer Res Treat. 2016;160(2):297–304. https://doi.org/10.1007/s10549-016-4006-6.
Choi J, Laws A, Hu J, Barry W, Golshan M, King T. Margins in breast-conserving surgery after neoadjuvant therapy. Ann Surg Oncol. 2018;25(12):3541–7. https://doi.org/10.1245/s10434-018-6702-4.
Moran MS, Schnitt SJ, Giuliano AE, et al. Society of Surgical Oncology-American Society for Radiation Oncology consensus guideline on margins for breast-conserving surgery with whole-breast irradiation in stages I and II invasive breast cancer. J Clin Oncol. 2014;32(14):1507–15. https://doi.org/10.1200/jco.2013.53.3935.
Mrdutt M, Heerdt A, Sevilimedu V, Mamtani A, Barrio A, Morrow M. Margin width and local recurrence in patients undergoing breast conservation after neoadjuvant chemotherapy. Ann Surg Oncol. 2022;29(1):484–92. https://doi.org/10.1245/s10434-021-10533-w.
Bodilsen A, Bjerre K, Offersen BV, et al. Importance of margin width in breast-conserving treatment of early breast cancer. J Surg Oncol. 2016;113(6):609–15. https://doi.org/10.1002/jso.24224.
Bundred JR, Michael S, Stuart B, et al. Margin status and survival outcomes after breast cancer conservation surgery: prospectively registered systematic review and meta-analysis. BMJ. 2022;378:e070346. https://doi.org/10.1136/bmj-2022-070346.
Zhu H, Doğan BE. American Joint Committee on Cancer’s staging system for breast cancer, eighth edition: summary for clinicians. Eur J Breast Health. 2021;17(3):234–8. https://doi.org/10.4274/ejbh.galenos.2021.2021-4-3.
Lin J, Lin K-J, Wang Y-F, Huang L-H, Chen SL-S, Chen D-R. Association of surgical margins with local recurrence in patients undergoing breast-conserving surgery after neoadjuvant chemotherapy. BMC Cancer. 2020;20(1):451. https://doi.org/10.1186/s12885-020-06955-6.
Cheun JH, Lee YJ, Lee JH, et al. Surgical margin status and survival outcomes of breast cancer patients treated with breast-conserving surgery and whole-breast irradiation after neoadjuvant chemotherapy. Breast Cancer Res Treat. 2022;194(3):683–92. https://doi.org/10.1007/s10549-021-06500-4.
van Mackelenbergh MT, Loibl S, Untch M, et al. Pathologic complete response and individual patient prognosis after neoadjuvant chemotherapy plus anti-human epidermal growth factor receptor 2 therapy of human epidermal growth factor receptor 2-positive early breast cancer. J Clin Oncol. 2023;41(16):2998–3008. https://doi.org/10.1200/jco.22.02241.
Spronk PER, Volders JH, van den Tol P, Smorenburg CH, Vrancken Peeters M. Breast conserving therapy after neoadjuvant chemotherapy; data from the Dutch Breast Cancer Audit. Eur J Surg Oncol. 2019;45(2):110–7. https://doi.org/10.1016/j.ejso.2018.09.027.
Devane LA, Baban CK, O’Doherty A, Quinn C, McDermott EW, Prichard RS. The impact of neoadjuvant chemotherapy on margin re-excision in breast-conserving surgery. World J Surg. 2020;44(5):1547–51. https://doi.org/10.1007/s00268-020-05383-8.
Cen C, Chun J, Kaplowitz E, et al. Margin assessment and re-excision rates for patients who have neoadjuvant chemotherapy and breast-conserving surgery. Ann Surg Oncol. 2021;28(9):5142–8. https://doi.org/10.1245/s10434-020-09524-0.
Volders JH, Negenborn VL, Spronk PE, et al. Breast-conserving surgery following neoadjuvant therapy-a systematic review on surgical outcomes. Breast Cancer Res Treat. 2018;168(1):1–12. https://doi.org/10.1007/s10549-017-4598-5.
Turnbull L, Brown S, Harvey I, et al. Comparative effectiveness of MRI in breast cancer (COMICE) trial: a randomised controlled trial. Lancet. 2010;375(9714):563–71. https://doi.org/10.1016/s0140-6736(09)62070-5.
Peters NH, van Esser S, van den Bosch MA, et al. Preoperative MRI and surgical management in patients with nonpalpable breast cancer: the MONET—randomised controlled trial. Eur J Cancer. 2011;47(6):879–86. https://doi.org/10.1016/j.ejca.2010.11.035.
Houssami N, Turner R, Morrow M. Preoperative magnetic resonance imaging in breast cancer: meta-analysis of surgical outcomes. Ann Surg. 2013;257(2):249–55. https://doi.org/10.1097/SLA.0b013e31827a8d17.
Marinovich ML, Houssami N, Macaskill P, et al. Meta-analysis of magnetic resonance imaging in detecting residual breast cancer after neoadjuvant therapy. J Natl Cancer Inst. 2013;105(5):321–33. https://doi.org/10.1093/jnci/djs528.
Bartelink H, Maingon P, Poortmans P, et al. Whole-breast irradiation with or without a boost for patients treated with breast-conserving surgery for early breast cancer: 20-year follow-up of a randomised phase 3 trial. Lancet Oncol. 2015;16(1):47–56. https://doi.org/10.1016/s1470-2045(14)71156-8.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure
None of the authors has any relevant conflicts of interest to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Di Lena, É., Iny, E., Wong, S.M. et al. Impact of Margin Status on Local Recurrence in Patients with Breast Cancer Undergoing Breast-Conserving Surgery After Neoadjuvant Chemotherapy: A Retrospective Multi-Institutional Cohort Study. Ann Surg Oncol 31, 6786–6794 (2024). https://doi.org/10.1245/s10434-024-15716-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-024-15716-9