Abstract
Background
Optimal margin width for breast-conserving therapy (BCT) after neoadjuvant chemotherapy (NAC) is unknown. We sought to determine the impact of margin width on local recurrence and survival after NAC and BCT.
Methods
Patients treated with NAC and BCT for stage I–III breast cancer from 2002 to 2014 were identified. Multivariate Cox regression was performed to determine the relationship between margin width and local recurrence free-survival (LRFS), disease-free survival (DFS), and overall survival (OS).
Results
A total of 382 patients were included. Median age was 51 years [range 22–79], median tumor size 3.0 cm [range 0.6–11.0], and receptor subtypes included 144 (37.7%) HR−/HER2−, 47 (12.3%) HR−/HER2+, 118 (30.9%) HR+/HER2−, and 70 (18.3%) HR+/HER2+. Breast pathologic complete response (pCR) was achieved in 105 (27.5%) patients. Final margin status was positive in 8 (2.1%) patients, ≤ 1 mm in 65 (17.0%), 1.1–2 mm in 30 (7.9%), and > 2 mm in 174 (45.5%). The 5-year LRFS was 96.3% (95% CI 94.0–98.6), DFS was 85.5% (95% CI 81.8–90.7), and OS was 90.8% (95% CI 87.4–94.2). There was no difference in LRFS, DFS, or OS for margins ≤ 2 versus > 2 mm, and no difference in DFS or OS for margins ≤ 1 versus > 1 mm. HR+ subtype (p = 0.04) and pCR (p = 0.03) were correlated with favorable DFS and node negativity (p < 0.001) with favorable DFS and OS.
Conclusions
In this cohort treated with NAC and BCT, there was no association between margin width and LRFS, DFS, or OS. Although further studies are needed, the excellent long-term outcomes demonstrated in patients with close (≤ 2 mm) margins following NAC suggest that a margin of “no-ink-on-tumor” may be acceptable in appropriately selected patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Originally reserved for management of locally advanced breast cancer, neoadjuvant chemotherapy (NAC) is now increasingly used among patients with earlier-stage disease. The NSABP B-18 and EORTC 10902 trials showed no survival advantage or disadvantage with neoadjuvant versus adjuvant chemotherapy, yet an important secondary endpoint was increased: rates of breast-conserving surgery (BCS) following NAC.1,2 Although early results from NSABP-B-18 suggested that rates of local recurrence may be higher among patients downstaged for BCS, generating controversy over the safety of this approach, excellent local control (95% 5-year local recurrence-free survival) has now been reported in several single-institution series, and a meta-analysis of 5500 patients randomized to NAC versus adjuvant chemotherapy demonstrated no difference in locoregional recurrence by type of surgical procedure (BCS vs. mastectomy, χ2 = 0.01, 1 d.f., p = 0.92).3,4,5
An important criterion for minimizing local recurrence following BCS is achieving a negative margin.6 The 2014 SSO/ASCO/ASTRO consensus guideline recommends no-tumor-on-ink for early-stage breast cancer patients treated with BCS and whole breast irradiation (WBI).7 However, patients treated with NAC were excluded from the analysis. A limited number of studies have investigated the impact of margin width on local recurrence in the NAC population with differing results.4,8,9 Notably, only one study includes patients treated after 2000. In recent years, the use of NAC has been further accelerated by the recognition of high rates of pathologic complete response (pCR) among the high-risk molecular subtypes.10 As such, determining the optimal margin width for patients pursuing BCS after NAC is critical.
In this study, we sought to determine the impact of margin width on local recurrence and survival outcomes in breast cancer patients treated in the modern era with NAC, BCS, and WBI.
Methods
Institutional databases were reviewed to identify stage I–III breast cancer patients older than 18 years of age treated with NAC, BCS, and WBI from 2002 to 2014. All patients received a complete course of NAC, surgical axillary staging with sentinel lymph node biopsy (SLNB) and/or axillary lymph node dissection (ALND), and adjuvant WBI. Patients treated with neoadjuvant endocrine therapy were excluded. Monitoring of clinical response to NAC, eligibility for BCS, and use of adjuvant endocrine therapy, extended adjuvant chemotherapy, and targeted therapy was at the discretion of the treating surgeon and oncologist. Chart review was performed to collect demographics, presenting clinical oncologic features (tumor size, nodal stage, focality), tumor characteristics (histology, grade, lymphovascular invasion, receptor subtype), surgical and adjuvant treatment details, and final pathology (ypT, ypN, margin width).
Clinical tumor and nodal stages were collected based on physical examination and preoperative imaging at presentation and described according to the 7th edition of the American Joint Committee guidelines. Overall pathologic complete response (pCR) was defined as no residual invasive carcinoma or in situ carcinoma in the breast or axillary lymph nodes. Patients who had axillary surgery before NAC were classified as pathologic Nx if node-positive at presentation or N0 if node-negative at presentation. The presence of axillary isolated tumor cells (ITC) or micrometastases on final pathology was defined as node-positive.
Margin widths were collected as reported on final pathology, then grouped as positive (ink-on-tumor), close (≤ 2 mm), or negative (> 2 mm) for primary analysis. The closest margin width was used for analysis, irrespective of type of disease (invasive or in situ carcinoma) near the margin. A second analysis was performed using margin widths grouped as positive (ink-on-tumor), close (≤ 1 mm), or negative (> 1 mm). All immediate and delayed reexcisions for inadequate margins were abstracted and accounted for when determining final margin status. If additional “directed” margins were taken at initial BCS or if the shaved cavity margin technique was used, the margin was considered negative (> 2 mm) if no tumor was present in the reexcised margin or shaved margin specimen.
The primary end points were local recurrence-free survival (LRFS), disease-free survival (DFS), and overall survival (OS). Local recurrence was defined as ipsilateral breast tumor recurrence. Descriptive statistics were used to characterize the cohort’s baseline features. Survival analysis was performed using Kaplan–Meier methods, with time zero defined as date of diagnosis. Patients who later chose to pursue prophylactic mastectomy were censored at the time of this operation. Univariate and multivariate Cox proportional hazards regression analyses were performed to determine the relationship between margin width and the primary outcomes, and a sensitivity analysis was performed excluding patients with a breast pCR. Patients with a positive margin were included in the close margin group for analysis due to small sample size. The impact of pCR on LRFS, DFS, and OS among four receptor subtypes was further studied by univariate Cox proportional hazards model. All statistical analysis was performed using R version 3.3.1.11 This study was approved by the institutional review board at Dana-Farber/Harvard Cancer Center.
Results
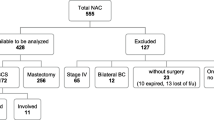
We identified 382 patients who received NAC followed by BCS and WBI during the study period, with a median follow-up of 57 months (range 10–148). Median patient age was 51 years (range 22–79), median tumor size at presentation was 3.0 cm (range 0.6–11.0), and 179 (46.9%) patients presented with clinically node-positive disease. On initial core biopsy, 359 (94.0%) patients had invasive ductal carcinoma, and 284 (74.3%) had grade III tumors. Breast cancer subtypes included 144 (37.7%) patients with HR−/HER2− disease, 118 (30.9%) with HR+/HER2− disease, 70 (18.3%) with HR+/HER2+ disease, and 47 (12.3%) with HR−/HER2+ disease. Among 117 HER2+ patients, 110 (94.0%) received neoadjuvant trastuzumab, and among 188 HR+ patients, 170 (90.4%) received adjuvant endocrine therapy. Axillary lymph node dissection was performed in 156 (40.8%) patients. The cohort’s clinicopathologic characteristics are presented in Table 1.
Breast pCR was achieved in 105 (27.5%) patients. Among 277 patients with residual disease after NAC, 75 (27.1%) had residual invasive cancer with 13 (17.3%) requiring reexcision, 175 (63.2%) had residual invasive cancer and DCIS with 75 (42.9%) requiring reexcision, and 27 (9.7%) had residual DCIS alone with 13 (48.2%) requiring reexcision. The overall rate of return to the operating room for reexcision due to inadequate margins therefore was 26.4% (101 patients). Of these 101 patients, reexcision was performed for positive margins at initial BCS in 32 (31.6%) patients and close margins at initial BCS in 69 (68.3%) patients. Stratified by margin status at initial BCS, the rate of residual disease (invasive carcinoma or DCIS) in reexcisions performed for positive margins was 40.6 versus 24.6% in reexcisions performed for close margins. Final margin status after all reexcisions was positive (ink-on-tumor) in 8 (2.1%) patients, ≤ 1 mm in 65 (17.0%), 1.1–2 mm in 30 (7.9%), and > 2 mm in 174 (45.5%). All final positive margins were in the anterior or posterior position and were documented as not amenable to reexcision by the operating surgeon.
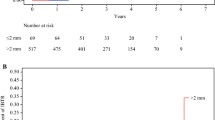
The unadjusted local recurrence (LR) rate was 3.9% (15 patients, 95% CI 2.2–6.4) and 5-year LRFS was 96.3% (95% CI 94.0–98.6). Local recurrence occurred in 3 of 103 (2.9%) patients with 1.1 to 2 mm margins, 11 of 174 (6.3%) patients with > 2 mm margins, and 1 of 105 (1.0%) patients with a breast pCR. Cox regression analysis was performed for 369 patients; 10 were excluded due to unknown pathologic nodal status (Nx) and 3 due to unknown HR/HER2 status. There was no association between margin width and LRFS in the primary multivariate analysis for the comparison between margins ≤ 2 versus > 2 mm (HR 0.33, 95% CI 0.07–1.52, p = 0.37). Multivariate analysis for LRFS could not be performed for margins ≤ 1 versus > 1 mm, because there were no local recurrence events in the ≤ 1 mm group. No other clinical or pathologic features, including age, presenting clinical T stage, receptor subtype, final pathologic nodal status, or overall pCR, were found to be significantly associated with LRFS. The 5-year DFS was 85.5% (95% CI 81.8–90.7) and 5-year OS was 90.8% (95% CI 87.4–94.2). On multivariate analysis, margin width was not associated with DFS or OS when close margins were defined as ≤ 2 mm (DFS: HR 0.99, 95% CI 0.53–1.86, p = 0.93; OS: HR 0.78, 95% CI 0.34–1.79, p = 0.83) or ≤ 1 mm (DFS: HR 1.13, 95% CI 0.56–2.25, p = 0.89; OS: HR 1.11, 95% CI 0.46–2.64, p = 0.96). When patients with breast pCR were excluded, the results did not change; margin width was not associated with LRFS, DFS, or OS. Furthermore, when the 13 patients excluded due to Nx and unknown HR/HER2 status were included in the model, the results again did not change.
On multivariate analysis, hormone receptor-negative subtypes (p = 0.04, HR−/HER2−: HR 2.47, 95% CI 1.22–5.00; HR−/HER2+: HR 1.72, 95% CI 0.61–4.85), residual breast or axillary disease (p = 0.03, HR 7.8, 95% CI 1.16–53.87), and positive post-NAC pathologic nodal status (p < 0.001, HR 2.95, 95% CI 1.56–5.57) were associated with inferior DFS. Only positive post-NAC pathologic nodal status (p < 0.001, HR 4.24, 95% CI 1.86–9.64) was associated with inferior OS on multivariate analysis. Local recurrence and survival outcomes are presented in Fig. 1 and Supplemental Table 1, and results of the primary multivariate Cox regression analysis are presented in Table 2.
The overall pCR rate in the breast and axillary nodes was 24.6% (94 patients) and varied by receptor subtype. Overall, pCR was 40.4% (19/47) in HR−/HER2+ patients, 33.8% (48/144) in HR−/HER2− patients, 18.6% (13/70) in HR+/HER2+ patients, and 11.9% (14/118) in HR+/HER2− patients. Furthermore, the effect of overall pCR on DFS and OS was significant specifically in the HR−/HER2− subtype (5-year DFS 97.9%, 95% CI 93.6–100.0 vs. 77.9%, 95% CI 69.2–87.8, p = 0.003; 5-year OS 98.0%, 95% CI 93.4–100.0 vs. 82.5%, 95% CI 93.4–100.0, p = 0.03). The effect of overall pCR on survival by receptor subtype is presented in Table 3.
Discussion
In this retrospective cohort of breast cancer patients treated with NAC, BCS, and WBI, wider final margins ≥ 2 mm did not confer improved local recurrence-free survival, and margins ≥ 1 or ≥ 2 mm did not confer improved disease-free survival or overall survival. Despite high rates of locoregionally advanced disease at presentation and aggressive receptor subtypes, the observed local recurrence rate was low, and LRFS did not differ by margin width. By excluding patients with residual in situ carcinoma from the pCR group, our analysis captures the effect of in situ margins as well as invasive disease. We found that hormone receptor-negative subtypes and lack of overall pCR was correlated with poorer DFS, and positive post-NAC pathologic nodal status was correlated with poorer DFS and OS.
While margin widths greater than no-tumor-on-ink are unnecessary in early breast cancer patients treated with primary BCS and WBI, adequate margin widths in the neoadjuvant setting are unknown.7 Achieving negative margins can be particularly challenging after NAC, because breast stroma becomes less edematous with treatment, and residual carcinoma often exists as small foci scattered throughout the tumor bed.12,13 These effects result in a more indistinct appearance on breast imaging and less obvious borders on palpation. The high rate of wire-localization in our cohort (96%) does suggest the majority of tumors were nonpalpable post-NAC, making decisions on volume of excision to ensure complete resection more difficult. A population-based study from the Netherlands found higher rates of tumor-involved margins in patients treated with NAC and BCS versus primary BCS (23 vs. 10%), with close margins (≤ 1 mm) identified in an additional 17.7% after NAC.14
Consistent with this literature, we found a high reexcision rate (26%) in our population, particularly in those with residual DCIS present. The rate of “ink-on-tumor” margins requiring reexcision was actually quite low (8%). Most reexcisions were instead for close (≤ 2 mm) margin widths; yet in this group of patients only one in four had residual disease in the reexcision specimen. Clearly, uncertainty regarding safe oncologic margins in this population contributes to high reexcision rates. Our findings therefore lend further support for the need to determine safe margins in this population to minimize unnecessary reexcisions and avoid the risk of conversion to mastectomy. The advent of newer localization strategies for nonpalpable tumors, such as radioactive seeds, also may impact reexcision rates and warrants future investigation in BCS after NAC.
Few other studies have examined the effect of margin width on prognosis in breast cancer patients treated with NAC, BCS, and WBI, and results have been mixed. Rouzier et al. reported on 257 patients treated from 1985 to 1994 and found that margins ≤ 2 mm were associated with increased ipsilateral breast tumor recurrence (IBTR) (HR 2.48, p = 0.04), along with age < 40, S-phase fraction > 4% on final pathology and clinical tumor size > 2 cm. Furthermore, LR was a strong predictor for distant metastases.8 Conversely, in a cohort of 347 patients treated between 1987 and 2000 at MD Anderson Cancer Center, there was no difference in 5-year LRFS in patients with a negative (> 2 mm) versus close/positive (≤ 2 mm) margin (92 vs. 89%, p = 0.2). Clinical N2/N3 disease, residual tumor > 2 cm on final pathology, a multifocal pattern of residual disease, and lymphovascular invasion were correlated with IBTR and LR.4 Jwa et al.9 also did not find an association between margin status and IBTR or LR (p = 0.59, p = 0.21) in a population of 335 patients treated from 2002 to 2009 but did find that triple-negative subtype as well as clinical T stage 3/4 were correlated with increased IBTR and LR. All studies have been retrospective and limited by small proportions of patients having close or positive margins.
Besides margin status, other important determinants of overall prognosis, notably tumor biology, are increasingly recognized. Receptor subtype is known to impact treatment selection and tumor response to systemic therapy.15,16 We too found that rates of overall pCR were highest in patients with HR−/HER2+ and HR−/HER2− subtypes. When the effect of pCR on DFS and OS was examined by receptor subtype, only the HR−/HER2− profile demonstrated a significant improvement in these outcomes when pCR was achieved. While our analysis was limited by small sample sizes and low event rates in each subgroup, von Minckwitz et al. found that the effect of pCR on DFS did vary by breast cancer subtype. In their pooled analysis of 6377 patients from seven randomized control trials treated with NAC, pCR was correlated with improved DFS in those with luminal B/HER2− (p = 0.005), nonluminal/HER2+ (p < 0.001), and triple-negative subtypes (p < 0.001) but not with luminal A (p = 0.39) or luminal B/HER2+ (p = 0.45).17
Our study is limited by its retrospective design, as well as lack of uniform pathology reporting during earlier years of study. As a result, we did not include the effect of extensive intraductal component, pattern of response to NAC and residual lymphovascular invasion, which are known to be associated with margin status.18,19 Residual tumor burden (RCB) has been advocated as an important consideration in the neoadjuvant setting but was only reported in 253 patients (66.2%) in our cohort and therefore could not be incorporated into the analysis.12 We also were unable to account for specific radiation protocols, including which patients received a boost to the tumor bed, which may have an effect on local recurrence. Finally, given the low incidence of local recurrence and small sample size, we lacked power to adequately detect statistically significant differences in the LRFS analysis, or make meaningful conclusions with respect to LRFS between patients with ≤ 1- versus ≤ 2-mm margins. This may explain why factors previously found to be associated with local recurrence, such as breast cancer subtype, did not demonstrate an association in our LRFS analysis.20,21 Our cohort also had only a small number of patients with “ink-on-tumor” margins. As a result, this subset had to be combined with the close margin group for analysis. Further studies with larger cohorts, likely in the setting of a meta-analysis, are needed to characterize definitively the safe oncologic margin width in BCS and WBI after NAC.
Conclusions
In this cohort of stage I–III breast cancer patients treated with NAC, BCS, and WBI, local recurrence rates were low. On multivariate analysis, there was no association between margin width and local recurrence-free survival, disease-free survival, or overall survival. Hormone receptor-negative subtype, lack of pCR, and positive pathologic nodal status were associated with inferior outcomes. Although further studies are needed to determine how the pattern of response and margin width are related to local recurrence in this population, the excellent long-term outcomes demonstrated in patients with close (≤ 2 mm) margins following NAC suggest that a margin of “no-ink-on-tumor” may be acceptable in appropriately selected patients.
Change history
25 August 2018
In the original article, there is an error in Jungeun Choi’s affiliation. It is corrected as reflected here.
25 August 2018
In the original article, there is an error in Jungeun Choi?s affiliation. It is corrected as reflected here.
References
Rastogi P, Anderson SJ, Bear HD, et al. Preoperative chemotherapy: updates of National Surgical Adjuvant Breast and Bowel Project Protocols B-18 and B-27. J Clin Oncol. 2008;26:778–85.
van der Hage JA, van de Velde CJ, Julien JP, Tubiana-Hulin M, Vandervelden C, Duchateau L. Preoperative chemotherapy in primary operable breast cancer: results from the European Organization for Research and Treatment of Cancer trial 10902. J Clin Oncol. 2001;19:4224–37.
Swisher SK, Vila J, Tucker SL, et al. Locoregional control according to breast cancer subtype and response to neoadjuvant chemotherapy in breast cancer patients undergoing breast-conserving therapy. Ann Surg Oncol. 2016;3:749–56.
Chen AM, Meric-Bernstam F, Hunt KK, et al. Breast conservation after neoadjuvant chemotherapy: the MD Anderson Cancer Center Experience. J Clin Oncol. 2004;22:2303–12.
Mieog JS, van der Hage JA, van de Velde CJ. Neoadjuvant chemotherapy for operable breast cancer. Br J Surg. 2007;94:1189–200.
Houssami N, Macaskill P, Marinovich ML, Dixon JM, Irwig L, Brennan ME, Solin SJ. Meta-analysis of the impact of surgical margins on local recurrence in women with early-stage invasive breast cancer treated with breast-conserving therapy. Eur J Cancer. 2010;46:3219–32.
Moran MS, Schnitt SJ, Giuliano AE, et al. Society of Surgical Oncology-American Society for Radiation Oncology consensus guideline on margins for breast-conserving surgery with whole-breast irradiation in stages I and II invasive breast cancer. Ann Surg Oncol. 2014;21:704–16.
Rouzier R, Extra JM, Carton M, et al. Primary chemotherapy for operable breast cancer: incidence and prognostic significance of ipsilateral breast tumor recurrence after breast-conserving surgery. J Clin Oncol. 2001;19:3828–35.
Jwa E, Shin KH, Kim JY, et al. Locoregional recurrence by tumor biology in breast cancer patients after preoperative chemotherapy and breast conservation treatment. Cancer Res Treat. 2016;48:1363–72.
King TA, Morrow M. Surgical issues in patients with breast cancer receiving neoadjuvant chemotherapy. Nat Rev Clin Oncol. 2015;12:335–43.
R Development Core Team. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria (2008). http://www.R-project.org. ISBN 3-900051-07-0.
Bossuyt V, Provenzano E, Symmans WF, et al. Recommendations for standardized pathological characterization of residual disease for neoadjuvant clinical trials of breast cancer by the BIG-NABCG collaboration. Ann Oncol. 2015;26:1280–91.
Mukhtar RA, Yau C, Rosen M, Tandon VJ, I-SPY 1 TRIAL and ACRIN 6657 Investigators, Hylton N, Esserman LJ. Clinically meaningful tumor reduction rates vary by prechemotherapy MRI phenotype and tumor subtype in the I-SPY 1 TRIAL (CALGB 150007/150012; ACRIN 6657). Ann Surg Oncol. 2013;20:3823–30.
Volders JH, Haloua MH, Krekel NM, et al. Neoadjuvant chemotherapy in breast-conserving surgery—consequences on margin status and excision volumes: a nationwide pathology study. Eur J Surg Oncol. 2016;42:986–93.
Teshome M, Kuerer HM. Breast conserving surgery and locoregional control after neoadjuvant chemotherapy. Eur J Surg Oncol. 2017;43:865–74.
Boughey JC, McCall LM, Ballman KV et al. Tumor biology correlates with rates of breast-conserving surgery and pathologic complete response after neoadjuvant chemotherapy for breast cancer: findings from the ACOSOG Z1071 (Alliance) Prospective Multicenter Clinical Trial. Ann Surg 2014;260:608–14 (discussion 614–6).
Von Minckwitz G, Untch M, Blohmer JU, et al. Definition and impact of pathological complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J Clin Oncol. 2012;30:1796–9.
Lovrics PJ, Cornacchi SD, Farrokhyar F, Garnett A, Chen V, Franic S, Simunovic M. Technical factors, surgeon case volume and positive margin rates after breast conservation surgery for early-stage breast cancer. Can J Surg. 2010;53:305–12.
Hanna J, Lannin D, Killelea B, Horowitz N, Chagpar AB. Factors associated with persistently positive margin status after breast-conserving surgery in women with breast cancer: an analysis of the national cancer database. Am Surg. 2016;82:748–52.
Lowery AJ, Kell MR, Glynn RW, Kerin MJ, Sweeney KJ. Locoregional recurrence after breast cancer surgery: a systematic review by receptor phenotype. Breast Cancer Res Treat. 2012;133:831–41.
Caudle AS, Yu TK, Tucker SL, et al. Local-regional control according to surrogate markers of breast cancer subtypes and response to neoadjuvant chemotherapy in breast cancer patients undergoing breast conserving therapy. Breast Cancer Res. 2012;14:R83.
Disclosures
The authors have no disclosures to declare.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Choi, J., Laws, A., Hu, J. et al. Margins in Breast-Conserving Surgery After Neoadjuvant Therapy. Ann Surg Oncol 25, 3541–3547 (2018). https://doi.org/10.1245/s10434-018-6702-4
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-018-6702-4