Abstract
Background
Laparoscopic gastrectomy (LG) is a standard approach for patients with clinical stage I gastric cancer in East Asia; however, following surgery, these patients may be pathologically diagnosed with stage II or III cancer. The prognosis of patients with gastric cancer migration from clinical stage I to pathological stage II or III after LG has not been completely clarified.
Methods
To compare the prognosis following LG and open gastrectomy (OG) in patients with pathological stage II or III gastric cancer who were preoperatively diagnosed with stage I cancer, we conducted a retrospective analysis using a multicenter dataset comprising details of 3480 patients who underwent gastrectomy between 2010 and 2014 at nine participating institutions. We used propensity score matching to reduce selection bias.
Results
After propensity score matching, 146 patients were finally selected. There were no significant differences in the number of dissected lymph nodes. Morbidity rates, length of postoperative hospital stay, and time between surgery and initiation of adjuvant chemotherapy were comparable between the two groups. Moreover, there were no significant differences in the overall, disease-specific, and relapse-free survival rates between the LG and OG groups. The LG group tended to have more patients with hematogenous recurrence, whereas the OG group tended to have more patients with peritoneal recurrence.
Conclusions
Our multicenter dataset analysis indicated that the prognosis of patients with gastric cancer migration from clinical stage I to pathological stage II or III was independent of the surgical approach.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Gastric cancer is the third most common cause of cancer-related deaths in Japan.1,2 The 4th edition of the Japanese Gastric Cancer Treatment Guidelines state that laparoscopic gastrectomy (LG) is recommended for clinical stage I gastric cancer;3 however, the accuracy of preoperative staging is limited.4 It is not uncommon for patients who were preoperatively diagnosed with clinical stage I gastric cancer to be postoperatively diagnosed with pathological stage II or III gastric cancer.5 Little information is available regarding the prognosis of such patients with gastric cancer migration from clinical stage I to pathological stage II or III after LG.
Several retrospective studies from centralized centers and a meta-analysis have indicated that LG for advanced gastric cancer provides long-term outcomes comparable with those of open gastrectomy (OG).6,7,8 However, those studies selected patients on the basis of preoperative clinical stage and faced issues such as limited information on postoperative adjuvant treatment, recurrence patterns, and inclusion of patients with cancer who had inversely migrated from clinical stage II or III to pathological stage I. Thus, those previous studies and some ongoing clinical trials comparing long-term outcomes between patients who underwent LG and those who underwent OG can hardly be expected to resolve the clinical question regarding the prognosis of patients with gastric cancer migration from clinical stage I to pathological stage II or III after LG.9
We constructed a multicenter database comprising details of 3480 patients who underwent gastrectomy between 2010 and 2014 at nine participating institutions, and retrospectively analyzed the prognostic factors for gastric cancer.10,11,12 Using this database, we evaluated the postoperative outcomes of patients with pathological stage II or III gastric cancer (according to surgical approaches [LG or OG]) who were preoperatively diagnosed with stage I; the evaluation was performed by propensity score matching.
Materials and Methods
Patients
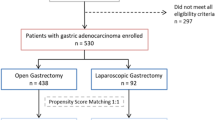
Figure 1 illustrates the flowchart of patient enrollment. We retrospectively reviewed clinical data procured from the medical records of 3480 patients who underwent gastrectomy for gastric cancer at nine institutions between January 2010 and December 2014. Of these, we selected 221 patients with pathological stage II to III gastric cancer according to the TNM Classification of Malignant Tumors, 7th Edition, who were preoperatively diagnosed with clinical stage I gastric cancer.13 The exclusion criteria were preoperative treatment (n = 188), gastric stump cancer (n = 88), extended surgery (e.g. pancreaticoduodenectomy or esophagectomy; n = 6), the presence of other primary malignant cancer (n = 456), and missing data for estimation of the propensity score (n = 2). This study conformed with the ethical guidelines of the World Medical Association Declaration of Helsinki–Ethical Principles for Medical Research Involving Human Subjects. Written informed consent for surgery and use of clinical data was obtained from patients, as required by the Institutional Review Board of each participating institutions. We employed opt-out recruitment according to the policy of the Japanese government because this clinical research was conducted using only retrospective clinical data without intervention. The purpose, design, and objectives of the study were posted on the homepage of the Nagoya University Graduate School of Medicine (https://www.med.nagoya-u.ac.jp/medical_J/ethics/rinsyoukansatsu.html(https://www.med.nagoya-u.ac.jp/medical_J/ethics/rinsyoukansatsu.html) to provide an opportunity for patients to decline to contribute to our study.
Patient Management
Upper gastrointestinal endoscopy and contrast-enhanced chest and abdominal computed tomography (CT) were performed for all patients for preoperative staging, and magnetic resonance imaging was considered as necessary. Patients underwent gastrectomy with D1 + or D2 lymphadenectomy according to clinical stage, and the reconstruction method was selected at the surgeon’s discretion. Postoperative follow-up included physical examinations and laboratory tests every month, as well as enhanced computed tomography scans (chest and abdominal cavity) once every 6 months for 5 years or until death.3 S-1 (an oral fluoropyrimidine derivative) monotherapy or doublet chemotherapy was administered to all patients as postoperative adjuvant treatment unless contraindicated by the patient’s condition or unless the patient refused.14,15 Treatment after recurrence was determined according to the information available at the time and the patient’s condition, with the patient’s consent.
Propensity Score Matching
We used propensity score matching to reduce potential bias. Propensity scores were estimated using a logistic regression model according to the following 11 factors: age, body mass index, sex, performance status, tumor size, type of gastrectomy, extent of lymph node dissection, pathological stage, vascular invasion, lymphatic involvement, and adjuvant chemotherapy. After propensity score matching, 146 patients were subjected to the analysis. Propensity scores were matched using one-to-one nearest-neighbor matching with no replacement and no caliper width. Postoperative complications were defined according to the Clavien–Dindo classification16.
Statistical Analysis
Qualitative variables were compared between the two patient groups using Fisher’s exact test, and quantitative variables were compared using the t test. Survival rates were estimated using the Kaplan–Meier method. The hazard ratio (HR) and 95% confidence interval (CI) were estimated using the univariate Cox proportional hazards model. Statistical analysis was performed using EZR statistical software,17 and a p value< 0.05 indicated a statistically significant difference.
Results
Demographics and Clinical Characteristics
Patient demographics and clinical characteristics before and after propensity score matching are presented in Table 1. After propensity score matching, the LG and OG groups showed similar distributions with respect to demographics, type of gastrectomy, extent of lymphadenectomy, pathological stage, and administration of adjuvant chemotherapy. Before propensity score matching, the number of patients with clinical T1N0, T1N1, and T2N0 was 39, 1, and 33 in the LG group, and 38, 8, and 100 in the OG group, respectively. After propensity score matching, the number of patients with clinical T1N0, T1N1, and T2N0 was 24, 6, and 43 in the OG group. The median observation period was 51.4 months in the LG group and 57.3 months in the OG group.
Clinical characteristics after propensity score matching between the LG and OG groups are shown in electronic supplementary Table 1. The rates of patients who had a history of laparotomy in the LG and OG groups were 12.3% and 5.5%, respectively. The margin-positive rates were 1.4% for both groups. There were no statistically significant differences in comorbidities that might affect the overall survival, such as diabetes, hypertension, respiratory disease, heart disease, and cerebrovascular disease. The number of patients who received S-1 monotherapy, capecitabine plus oxaliplatin, S-1 plus docetaxel, and cisplatin-based doublet chemotherapy was 38, 1, 0, and 2 in the LG group, and 40, 0, 2, and 0 in the OG group, respectively (electronic supplementary Table 1).
Short-Term Outcomes
Information regarding operative findings and postoperative complications is summarized in Table 2. The mean operative time was significantly longer in the LG group than in the OG group (285 vs. 237 min; p < 0.001), and the mean intraoperative blood loss volume was significantly smaller in the LG group than in the OG group (87 vs. 265 ml; p < 0.001). There were no significant differences in the number of dissected lymph nodes (mean 33.2 vs. 34.6), incidence of postoperative complications, length of postoperative hospitalization, rate of readmission, or rate of reoperation between the LG and OG groups. The median number of retrieved lymph nodes in patients who underwent non-D2 lymph node dissection was 28.5 in the LG group and 26.5 in the OG group. The LG group had a larger number of retrieved lymph nodes compared with the OG group (mean 31.7 vs. 25.4; p = 0.044). The proportions of patients who had < 15 retrieved lymph nodes in the LG and OG groups were 4.1% and 9.6%, respectively. There was no significant difference in the time from surgery to initiation of adjuvant chemotherapy between the LG and OG groups (44.4 vs. 45.2 day; p = 0.883).
Long-Term Outcomes
The 1-, 2-, 3-, and 5-year relapse-free survival rates in the LG group were 97%, 92%, 90%, and 90%, respectively, and 99%, 92%, 91%, and 87%, respectively, in the OG group. The LG group had similar relapse-free survival compared with the OG group (HR 0.811, 95% CI 0.294–2.236, p = 0.685) (Fig. 2a). The 5-year disease-specific survival rate was 91% and 93% in the LG and OG groups, respectively; There was no statistically significant difference between the two groups (HR 1.377, 95% CI 0.388–4.879, p = 0.621) (Fig. 2b). With respect to overall survival, no statistically significant differences were observed between the LG and OG groups (HR 0.589, 95% CI 0.259–1.363, p = 0.216). The 5-year overall survival rates for the LG and OG groups were 89% and 80%, respectively.
Of 1578 patients who were preoperatively diagnosed with clinical stage I, the numbers of patients with pathological stage I and stage II/III gastric cancer were 1343 and 221, respectively. The 5-year overall survival, disease-specific survival, and relapse-free survival rates of patients pathologically diagnosed as stage I were 94.0%, 99.0%, and 98.5%, respectively, and 81.8%, 90.7%, and 83.9%, respectively, for patients pathologically diagnosed as stage II/III.
Disease recurrence was observed in seven patients (9.6%) in the LG group and eight patients (11.0%) in the OG group. Although there were no statistically significant differences in the prevalence of recurrence pattern between the LG and OG groups, the OG group tended to have more patients with peritoneal recurrence, whereas the LG group tended to have more patients with hematogenous recurrence (Fig. 3).
Discussion
According to the current Japanese gastric cancer treatment guideline, LG is only recommended for clinical stage I gastric cancer. On the other hand, in clinical practice, it is not uncommon for patients who were preoperatively diagnosed with clinical stage I gastric cancer to be postoperatively diagnosed as pathological stage II or III gastric cancer. In this study, we aimed to clarify the prognosis of patients with pathological stage II or III gastric cancer following LG who were preoperatively diagnosed with clinical stage I. For this purpose, we compared the outcomes between the LG and OG patients using propensity score matching and found that prognosis was comparable between the two surgical approaches.
LG for early gastric cancer has become common, and the safety of this procedure has proven to be equivalent to that of OG in large-scale prospective clinical trials, such as the JCOG0912 and KLASS-01 trials.18,19 In those clinical trials, conducted by high-volume centers, patients with stage I gastric cancer were strictly selected before enrollment to minimize the risk of stage migration. Nevertheless, accurate diagnosis of the clinical stage of gastric cancer is difficult, and the clinical stage is sometimes unexpectedly different from the pathological stage.4,5 In fact, in the KLASS-01 trial, 196 of 1359 patients (14.4%) were finally diagnosed with pathological stage II or III gastric cancer, although they were preoperatively diagnosed with clinical stage I gastric cancer.19 Similarly, in the JCOG0912 trial, 88 of 912 (9.6%) patients who were finally diagnosed with pathological stage II or III gastric cancer were preoperatively diagnosed with clinical stage I gastric cancer preoperatively.18 In this study, 14.0% of patients showed gastric cancer migration from clinical stage I to pathological stage II or III after LG. Knowledge regarding the prognosis of such patients, particularly after LG, remains limited. Single-center studies generally suffer from insufficient statistical power owing to the limited number of patients and difficulty in propensity score matching, thereby causing substantial selection bias. To answer this clinical question, we herein analyzed a multi-institute dataset focusing on patients with gastric cancer migration from clinical stage I to pathological stage II or III after LG. As we aimed to primarily evaluate long-term outcomes, we included several important intraoperative and postoperative factors that are closely related with prognosis to allow for accurate propensity score matching.
In this study, patients who underwent LG had similar morbidity rates, smaller intraoperative blood loss, and longer operative time compared with those who underwent OG, consistent with the results of the KLASS-01 and JCOG0912 trials.18,19 Regarding long-term outcomes, survival of the LG group was comparable with that of the OG group. Our results indicate that the surgical approach has limited prognostic impact in patients with pathological stage II or III gastric cancer who were preoperatively diagnosed with stage I cancer. However, surgeons could have hesitated to perform LG and selected OG for patients with advanced age, history of abdominal surgery, comorbidities, or poor performance status, although patient background was adjusted by propensity score matching.20 We speculate that this is a potential factor resulting in the slight differences observed in the overall survival curves between the LG and OG groups.
In this study, we investigated the long-term outcomes of all patients with pathological stage II or III gastric cancer, with a 5-year relapse-free survival rate of 64.8%. The long-term prognosis of patients in the LG group with pathological stage II or III gastric cancer who were preoperatively diagnosed with clinical stage I was more favorable compared with that of all patients diagnosed pathologically at the same stages. A possible reason was a large proportion of patients with clinical stage I were finally diagnosed as pathological stage II in the present study. Moreover, the mean number of retrieved lymph nodes in patients who received non-D2 lymphadenectomy during the LG was 31.7, comparable with that of patients who received D2 lymphadenectomy. This might be another reason for excellent relapse-free survival in the LG group.
There was no statistically significant difference in the site of initial disease recurrence; however, the LG group tended to have more patients with hematogenous recurrence, whereas the OG group tended to have more patients with peritoneal recurrence. It is difficult to draw a conclusion from the limited number of events, although these findings imply that the laparoscopic approach and subsequent pneumoperitoneum did not increase the risk of peritoneal recurrence. Another important finding is the low nodal recurrence rate in both groups (1.3% each), although 63% of patients in the LG group underwent non-D2 lymphadenectomy.
This study has several potential limitations. Although propensity score matching was performed to minimize selection bias, this was a retrospective study. Because the surgical indication of OG for clinical stage I gastric cancer depends on the surgeons’ discretion, patient selection might have been quite biased. We used performance status for propensity score matching, although the American Society of Anesthesiologists (ASA) physical status classification, which reflects patients’ underlying condition in more detail, might be a better tool. Unfortunately, our multi-institutional dataset did not include sufficient information to determine the ASA physical status classification, such as history of smoking and the severity of diabetes.
Conclusions
We evaluated the prognosis of patients with gastric cancer migration from clinical stage I to pathological stage II or III following LG using a multicenter dataset and found that it was comparable with that of patients who underwent OG.
References
Katai H, Ishikawa T, Akazawa K, Isobe Y, Miyashiro I, Oda I, et al., Five-year survival analysis of surgically resected gastric cancer cases in Japan: a retrospective analysis of more than 100,000 patients from the nationwide registry of the Japanese Gastric Cancer Association (2001–2007). Gastric Cancer. 2018;21:144–54.
Kanda M, Fujiwara M, Tanaka C, Kobayashi D, Iwata N, Mizuno A, et al. Predictive value of drain amylase content for peripancreatic inflammatory fluid collections after laparoscopic (assisted) distal gastrectomy. Surg Endosc. 2016;30:4353–62.
Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2014 (ver. 4). Gastric Cancer. 2017;20:1–19.
Fukagawa T, Katai H, Mizusawa J, Nakamura K, Sano T, Terashima M, et al. A prospective multi-institutional validity study to evaluate the accuracy of clinical diagnosis of pathological stage III gastric cancer (JCOG1302A). Gastric Cancer. 2018;21:68–73.
Wu CW, Hsiung CA, Lo SS, Hsieh MC, Chen JH, Li AF, et al., Stage migration influences on stage-specific survival comparison between D1 and D3 gastric cancer surgeries. Eur J Surg Oncol. 2005;31:153–7.
Kinoshita T, Uyama I, Terashima M, Noshiro H, Nagai E, Obama K, et al., Long-term outcomes of laparoscopic versus open surgery for clinical Stage II/III gastric cancer: a multicenter cohort study in Japan (LOC-a study). Ann Surg. 2019;269(5):887–94.
Martinez-Ramos D, Miralles-Tena JM, Cuesta MA, Escrig-Sos J, Van der Peet D, Hoashi JS, et al., Laparoscopy versus open surgery for advanced and resectable gastric cancer: a meta-analysis. Rev Esp Enferm Dig. 2011;103:133–41.
Chen K, Xu XW, Mou YP, Pan Y, Zhou YC, Zhang RC, et al., Systematic review and meta-analysis of laparoscopic and open gastrectomy for advanced gastric cancer. World J Surg Oncol. 2013;11:182.
Inaki N, Etoh T, Ohyama T, Uchiyama K, Katada N, Koeda K, et al., A multi-institutional, prospective, Phase II feasibility study of laparoscopy-assisted distal gastrectomy with D2 Lymph node dissection for locally advanced gastric cancer (JLSSG0901). World J Surg. 2015;39:2734-41.
Hayashi S, Kanda M, Ito S, Mochizuki Y, Teramoto H, Ishigure K, et al., Number of retrieved lymph nodes is an independent prognostic factor after total gastrectomy for patients with stage III gastric cancer: propensity score matching analysis of a multi-institution dataset. Gastric Cancer. 2019;22(4):853–63.
Ryo S, Kanda M, Ito S, Mochizuki Y, Teramoto H, Ishigure K, et al. The controlling nutritional status score serves as a predictor of short- and long-term outcomes for patients with Stage 2 or 3 gastric cancer: analysis of a multi-institutional data set. Ann Surg Oncol. 2019;26(2):456–64.
Ito Y, Kanda M, Ito S, Mochizuki Y, Teramoto H, Ishigure K, et al., Intraoperative Blood Loss is Associated with Shortened Postoperative Survival of Patients with Stage II/III Gastric Cancer: Analysis of a Multi-institutional Dataset. World J Surg. 2019;43:870–7.
Liu JY, Peng CW, Yang XJ, Huang CQ, Li Y. The prognosis role of AJCC/UICC 8(th) edition staging system in gastric cancer, a retrospective analysis. Am J Transl Res. 2018;10:292–303.
Kanda M, Kodera Y, Sakamoto J. Updated evidence on adjuvant treatments for gastric cancer. Expert Rev Gastroenterol Hepatol. 2015;9:1549–60.
Kanda M, Murotani K, Kobayashi D, Tanaka C, Yamada S, Fujii T, et al. Postoperative adjuvant chemotherapy with S-1 alters recurrence patterns and prognostic factors among patients with stage II/III gastric cancer: a propensity score matching analysis. Surgery. 2015;158:1573–80.
Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. 2009;250:187-96.
Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transpl. 2013;48:452–8.
Katai H, Mizusawa J, Katayama H, Takagi M, Yoshikawa T, Fukagawa T, et al. Short-term surgical outcomes from a phase III study of laparoscopy-assisted versus open distal gastrectomy with nodal dissection for clinical stage IA/IB gastric cancer: japan clinical oncology group study JCOG0912. Gastric Cancer. 2017;20:699–708.
Kim HH, Han SU, Kim MC, Kim W, Lee HJ, Ryu SW, et al. Effect of laparoscopic distal gastrectomy vs open distal gastrectomy on long-term survival among patients with Stage I gastric cancer: the KLASS-01 randomized clinical trial. JAMA Oncol. 2019;5(4):506–13.
Hikage M, Tokunaga M, Makuuchi R, Irino T, Tanizawa Y, Bando E, et al. Surgical outcomes after gastrectomy in very elderly patients with gastric cancer. Surg Today. 2018;48(8):773–82.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
DISCLOSURE
Yuki Ito, Mitsuro Kanda, Seiji Ito, Yoshinari Mochizuki, Hitoshi Teramoto, Kiyoshi Ishigure, Toshifumi Murai, Takahiro Asada, Akiharu Ishiyama, Hidenobu Matsushita, Chie Tanaka, Daisuke Kobayashi, Michitaka Fujiwara, Kenta Murotani, and Yasuhiro Kodera declare they have no conflicts of interest and no sources of financial support were used in the preparation of this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ito, Y., Kanda, M., Ito, S. et al. Prognosis After Laparoscopic Gastrectomy in Patients with Pathological Stage II or III Gastric Cancer Who Were Preoperatively Diagnosed with Clinical Stage I: Propensity Score Matching Analysis of a Multicenter Dataset. Ann Surg Oncol 27, 268–275 (2020). https://doi.org/10.1245/s10434-019-07781-2
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-019-07781-2