Abstract
Background
It remains unclear whether laparoscopic conversation to open gastrectomy causes higher morbidity and has an adverse effect on the long-term survival outcomes of patients with gastric cancer. This study was designed to evaluate the impact of the conversion on short and long-term outcomes of patients with locally advanced gastric cancer (AGC).
Methods
We retrospectively investigated 871 patients who initially underwent laparoscopic gastrectomy (LG) for pathologically confirmed diagnosis of AGC between February 2009 and April 2018. The patients were grouped as the conversion (CONV) group and completed laparoscopic (LAP) group. The 1:2 propensity score matching was performed to reduce the effect of bias due to the imbalanced baseline features between the two groups. Multivariate analyses were performed to identify risk factors for conversion and poor survival.
Results
After propensity-score matching, 168 patients (56 in the CONV group and 112 in the LAP group) were studied. The CONV group was associated with significantly longer operation time (252.4 vs. 216.7 min, P < 0.001) and greater estimated blood loss (234.8 vs. 171.2 ml, P < 0.001) as compared with the LAP group. The time to first flatus (3.8 vs. 3.3 days, P = 0.043), time to start a liquid diet (4.1 vs. 3.5 days, P = 0.021), and postoperative hospital stay (8.7 vs. 7.6 days, P = 0.020) were significantly longer in the CONV group than that in the LAP group. The overall complication rate did not differ significantly between the CONV group and the LAP group (16.1% vs. 12.5%, P = 0.692). Both 5-year overall survival (OS) and 5-year disease-free survival (DFS) did not differ significantly between the CONV group and the LAP group (P = 0.805, P = 0.945, respectively). Multivariate analysis showed that lymphovascular invasion and stage III were independent prognostic factors for poor OS and DFS, whereas conversion was not.
Conclusions
The conversion from laparoscopic to open gastrectomy had no negative impact on morbidity and long-term survival outcomes for patients with locally AGC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Laparoscopic gastrectomy(LG) for gastric cancer has increasingly gained popularity since it was first reported by Kitano et al. in 19941. Large randomized controlled trials (RCTs) have demonstrated that LG is a safe and feasible procedure with better short-term outcomes and equivalent long-term prognosis for early gastric cancer (EGC) as compared with open gastrectomy (OG)2,3,4. Based on the experience accumulation of EGC, increasing numbers of experienced laparoscopic surgeons have applied the LG for patients in locally advanced gastric cancer5,6,7,8. Conversion of LG to OG is always unavoidable because of various reasons such as technical difficulty, large tumor size, abdominal adhesion, obesity, and uncontrollable intraoperative complications. The conversion rate has been reported up to 17.4% in a recent study9. A multicenter randomized controlled trial revealed that the conversion rate is 6.4% even by experienced laparoscopic surgeons in high-volume centers 10. To date, extensive studies have demonstrated that postoperative complications are associated with adverse survival outcomes after radical resection of gastric cancer. It remains unclear whether the conversion causes higher morbidity and has an adverse effect on the long-term survival outcomes of patients in gastric cancer. Additionally, about 80% patients are diagnosed at advanced stages in China, and gastrectomy with D2 lymph node dissection has been recommended as a standard procedure for these cases. These cases may be at high risk of conversion due to tumor-related reasons.
We, therefore, designed this study to evaluate the impact of the conversion on short and long-term outcomes of patients with locally advanced gastric cancer (AGC).
Patients and Methods
Patients
We initially screened our prospectively maintained gastric cancer database including patients with pathological diagnoses of locally AGC who initially underwent laparoscopic D2 gastrectomy between February 2009 and April 2018. The exclusion criteria included EGC, older than 70 years, stage IV disease, D1 or D1+ lymph node dissection, distant metastasis or invasion to adjacent organs, combined with other malignancy, combined with adjacent organ resection, emergency surgery, and patients underwent neoadjuvant chemotherapy. Patients chose the surgical procedure by their individual decision after they were informed of the advantages and possible complications of surgery. Written informed consent was obtained from all patients before the surgery. The operative procedures have been described in detail previously 11,12,13. The tumor staging was recorded according to the 8th Union for International Cancer Control (UICC)/American Joint Committee on Cancer (AJCC) staging system of gastric cancer14. This study was approved by the Institutional Review Board of our institution.
Postoperative Evaluation and Follow-up
In this study, postoperative complications were determined according to the Clavien–Dindo (C-D) classification system 15, 16 Patients were followed up every 3 months during the first 2 years and then every 6 months from 2 to 5 years, and then annually. We routinely administered postoperative adjuvant chemotherapy with 5-fluorouracil (5-FU)-based regimens (5-FU with cisplatin, capecitabine with oxaliplatin, and S-1 alone) for the patients with stage II or more advanced cancer.
Statistical Analysis
We performed 1:2 propensity score matching using R Statistics version 3.4.0. with the following variables: age, sex, body mass index (BMI), extent of resection, tumor size, histology type, pathologic T stage, pathologic N stage, adjuvant chemotherapy, comorbidities, and year of surgery. Statistical analyses were performed using SPSS, ver.22.0 (SPSS Inc., Chicago IL, USA). The chi-square test or Fisher’s exact test was used to compare categorical variables between the two groups. To evaluate the risk factors for conversion, a multivariate analysis was conducted with the binary logistic regression model. OS and DFS were compared using the Kaplan–Meier method and log-rank test. Multivariate analyses were performed by Cox proportional hazard model to identify the independent risk factors for OS and DFS. P < 0.05 was considered statistically significant.
Patient Characteristics
Table 1 summarizes the baseline characteristics of patients in the entire and propensity score-matched cohort. Finally, 871 patients who underwent initially LG were included in our analysis. Among these patients, 62 cases were converted to open surgery and 809 cases were successfully performed by the laparoscopic procedure. The patients were grouped as the conversion (CONV) group and completed the laparoscopic (LAP) group. Overall, no significant differences were noted in sex, age, the extent of resection, histological type, pT stage, adjuvant chemotherapy, and comorbidities. However, BMI, tumor size, pN stage, pTNM stage, and year of surgery were found to be significantly different between the two groups. After propensity-score matching, the baseline characteristics were well balanced between the two groups. Finally, 168 patients (56 in the CONV group, and 112 in the LAP group) were studied.
Surgical Outcomes and Complications
Table 2 summarizes the surgical outcomes of patients in the propensity score-matched cohort. The CONV group was associated with significantly longer operation time (252.4 vs. 216.7 min, P < 0.001), greater estimated blood loss (234.8 vs. 171.2 ml, P < 0.001). The number of retrieved lymph nodes was similar between the LAG and OTG groups (28.2 vs. 29.2, P = 0.404). The time to first flatus (3.8 vs. 3.3 days, P = 0.043), time to start liquid diet (4.1 vs. 3.5 days, P = 0.021), and postoperative hospital stay (8.7 vs. 7.6 days, P = 0.020) were significantly longer in the CONV group than that in the LAP group.
The postoperative complications of propensity score-matched cohort are shown in Table 3. The overall complication rate did not differ significantly between the CONV group and the LAP group (16.1% vs. 12.5%, P = 0.692). Moreover, no significant differences were noted in the minor (C–D grade II) and severe complication (C–D grade >II) rates between the two groups (7.1% vs. 5.4%, P = 0.908; 8.9% vs. 7.1%, P=0.919; respectively). Regarding the major individual complications, the incidence of intra-abdominal abscess, anastomotic leakage, duodenal stump leakage, and wound infection were also comparable between the two groups (all P > 0.05).
Analysis of Risk Factors for Conversion
Among the 56 patients in the CONV group, the primary reasons for the conversion were large tumor size or advanced gastric cancer (29 cases, 51.8 %), followed by adhesions (15 cases, 26.8 %), obesity (7 cases, 12.5 %), and others (5 cases, 8.9 %). Univariate analysis showed that BMI (P < 0.001), tumor size (P < 0.001), abdominal adhesion (P < 0.001), tumor stage (P = 0.003), and surgeon’s experience (P < 0.001) were significantly related to conversion (Table 4). Multivariate analysis revealed that BMI ≥28 (OR, 2.970; 95%CI, 1.580–5.583, P = 0.001), tumor size >5cm (OR, 2.317; 95%CI, 1.334–4.026, P = 0.003), the presence of abdominal adhesion (OR, 3.202; 95%CI, 1.482–6.918, P = 0.003), and surgeon’s experience < 50 cases (OR, 2.259; 95%CI, 1.259–4.054, P = 0.006) were independent risk factors for conversion (Table 4).
Long-Term Survival Outcomes
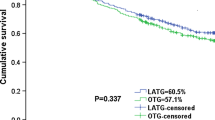
After a median follow-up period of 69 months, the 5-year overall survival (OS) rate was 37.5% in the CONV group and 41.1% in the LAP group (Fig. 1), and the 5-year disease-free survival (DFS) rate was 33.9% in the CONV group and 38.4% in the LAP group (Fig. 2). Both 5-year OS and 5-year DFS did not differ significantly between the CONV group and LAP group (P = 0.805, P = 0.945, respectively). The stage-specific analysis for patients with stage III showed that the 5-year OS rate was 29.5% in the CONV group and 34.6% in the LAP group (Fig. 3), and the 5-year DFS rate was 27.3% in the CONV group and 32.1% in the LAP group (Fig. 4). No significant differences were observed between the CONV and LAP groups in terms of 5-year OS and DFS for patients with stage III (P = 0.875, P = 0.987, respectively). In multivariate analysis, the presence of lymphovascular invasion (OR, 2.184; 95%CI, 1.445–3.302, P < 0.001) and high pTNM stage (OR, 2.549; 95%CI, 1.469–4.422, P = 0.001) were independent prognostic factors for OS (Table 5), and the presence of lymphovascular invasion (OR, 1.952; 95%CI, 1.308–2.912, P = 0.001) and high pTNM stage (OR, 2.463; 95%CI, 1.458–4.160, P = 0.001) were also independent prognostic factors for DFS (Table 6).
Discussion
Many studies have demonstrated that LG with D2 lymph node dissection is a safe and feasible procedure for locally AGC17, 18. However, it is a technically demanding procedure even for experienced surgeons, and the conversion rate has been reported up to 17.4%9. It remains unclear whether laparoscopic converted to open colectomy causes higher morbidity and harms the long-term survival outcomes of patients with gastric cancer. In this study, we evaluate the impact of the conversion on short and long-term outcomes and identify risk factors for conversion and long-term survival in patients with locally AGC.
In the present study, the baseline characteristics of the two groups were not comparable in the entire cohort and, therefore, it could be argued that the direct comparison of survival rate is not appropriate for all patients because some factors could be independently responsible for survival outcome, regardless of the conversion. We used the propensity score matching method to reduce the effect of selection bias and potential confounding due to the limits of the respective study.
Our results indicated that patients in the CONV group are associated with increased operation time and greater blood loss as compared with the LAP group. Additionally, patients in the CONV group have a delayed recovery after surgery when we take time to first flatus, time to start a liquid diet, time to ambulation, and postoperative hospital stays into consideration. Postoperative morbidity is always regarded as one of the major concerns in clinical practice. Studies have demonstrated that the occurrence of postoperative complications has an adverse impact on short and long-term outcomes of the patient after radical resection of gastric cancer 19,20,21. In the present study, the overall postoperative complication rate of the CONV and LAP group was 16.1% and 12.5%, respectively. Although there is a tendency favoring the LAP group in terms of overall complication rate, we did not observe a significant difference between the two groups. Our recent research reported that the occurrence of severe complication was an independent risk factor of poor survival for patients with AGC underwent radical gastrectomy22. In the present study, further analysis showed that the minor and severe complication rates did not significantly differ between the two groups. Regarding major individual complications, no significant difference was found in terms of anastomosis leakage, intra-abdominal abscess, and wound infection.
Studies have reported that the learning curve of LG for surgeons with rich experience is about 50 cases, and the conversion from laparoscopic to open gastrectomy is always unavoidable for surgeons in the initial learning phase23, 24. Between 2009 and 2010, two surgeons were at the initial phase of the learning curve. So, we consider the surgeon’s experience. The results of our multivariate analysis confirmed that the surgeon’s experience in less than 50 cases was an independent risk factor for conversion, along with obesity, large tumor size, and the presence of abdominal adhesion. Hence, we suggest that LG should be performed with care for patients in advanced stages, large tumor size, and combined with abdominal adhesion, especially for surgeons at the learning phase.
Long-term survival outcomes are a key indicator for assessing oncological safety, and few studies have assessed the impact of conversion from laparoscopic to open surgery on long-term outcomes for gastric cancer. A previous meta-analysis reported that conversion from laparoscopic to open colorectal cancer surgery may be associated with adverse long-term oncological outcomes25. However, we found that the baseline information was unmatched in the study which may lower the statistical power of the conclusion. Up to now, only two small retrospective studies investigate the impact of the conversion on long-term outcomes for patients with gastric cancer9, 26. Studies on this topic are always limited by inadequate follow-up, unmatched groups, and small sample size, indicating the evidence is still lacking. Yue et al.9 reported that the 5-year OS of patients with gastric cancer in the CONV group and LAP group was 51% and 57% with a median follow-up of 37 months, respectively; the difference was not statistically significant. Ye et al.26 reported the 5-year OS of patients with gastric cancer for the LAG group was similar to that in the LAP group with a median follow-up of 38 months. Comparing with previous studies, our study is in a relatively large sample size with sufficient follow-up. In this study, the propensity score-matched cohort analysis showed no statistically significant differences for 5-year OS or DFS with a median follow-up of 57 months. In addition, the multivariate analysis showed that the presence of lymphovascular invasion and high pTNM stage were independent risk factors for adverse long-term survival, whereas the conversion was not.
In this study, we could not evaluate the influence of timing of conversion due to the limited number of patients who underwent an early conversion. Further studies with a large sample size are needed to identify the impact of timing of conversion on survival outcomes.
It has been generally accepted that gastrectomy with a sufficient number of lymph nodes dissection could improve the long-term prognosis of gastric cancer patients27,28,29. Our results revealed that there is no significant difference between the CONV and LAP groups in terms of the number of retrieved lymph nodes. Extensive studies have confirmed that the positive resection margin is associated with poor oncological outcomes for gastric cancer30,31,32. Large tumor size is always identified as an independent risk factor for positive margin33. In the current study, patients with tumor size larger than 5cm account for more than 50%, and R0 resection could also be performed for these high-risk cases. These results suggest that patients in the CONV group could also obtain radical gastrectomy, indicating the conversion did not reduce the oncological safety.
Patients in previous studies are always in a relatively early stage, indicating a favorable prognosis for these cases. In China, most patients with gastric cancer are diagnosed at an advanced stage. In this study, patients in stage III account for 74.4% of the propensity score-matched cohort. Patients with Stage III GC still have a high incidence of recurrence and a poor prognosis. The stage-specific analysis showed that 5-year OS and DFS rates did not significantly differ between CONV and LAP patients for patients with stage III. This suggested that patients who underwent conversion could also have comparable long-term survival outcomes as compared with those who underwent successful laparoscopic operations for cases with more advanced stage.
Some limitations of our present study need to be noted. First, this is a single-center non-randomized study and some inherent confounding factors could not be offset by the propensity score matching method. Also, the CONV group is in a small sample size which may lead to an unpowered conclusion. Finally, there is currently no consensus on the specific definition of conversion, and this may differ between surgeons. Despite the limitations of this study, our results still provide valuable evidence in clinical practice.
In conclusion, the conversion from laparoscopic to open gastrectomy did not adversely influence morbidity and long-term survival outcomes for patients with locally AGC. Large tumor size, abdominal adhesion, obesity, and insufficient surgeon’s experience are independent risk factors for conversion.
References
Kitano S IY, Moriyama M, Sugimachi K. Laparoscopy-assisted Billroth I gastrectomy. Surg Laparosc Endosc 1994;4:146–8.
Zeng YK, Yang ZL, Peng JS, Lin HS, Cai L. Laparoscopy-assisted versus open distal gastrectomy for early gastric cancer: evidence from randomized and nonrandomized clinical trials. Annals of surgery. 2012;256(1):39-52. https://doi.org/10.1097/SLA.0b013e3182583e2e.
Kim YW, Yoon HM, Yun YH, Nam BH, Eom BW, Baik YH et al. Long-term outcomes of laparoscopy-assisted distal gastrectomy for early gastric cancer: result of a randomized controlled trial (COACT 0301). Surgical endoscopy. 2013;27(11):4267-76. https://doi.org/10.1007/s00464-013-3037-x.
Deng Y, Zhang Y, Guo TK. Laparoscopy-assisted versus open distal gastrectomy for early gastric cancer: a meta-analysis based on seven randomized controlled trials. Surgical oncology. 2015;24(2):71-7. https://doi.org/10.1016/j.suronc.2015.02.003.
Goh PM KA, So JB, Lomanto D, Cheah WK, Muthiah R, Gandhi A. Early experience with laparoscopic radical gastrectomy for advanced gastric cancer. Surg Laparosc Endosc Percutan Tech. 2001;11(2):83-7.
Zhao Y, Yu P, Hao Y, Qian F, Tang B, Shi Y et al. Comparison of outcomes for laparoscopically assisted and open radical distal gastrectomy with lymphadenectomy for advanced gastric cancer. Surgical endoscopy. 2011;25(9):2960-6. https://doi.org/10.1007/s00464-011-1652-y.
Shinohara T, Satoh S, Kanaya S, Ishida Y, Taniguchi K, Isogaki J et al. Laparoscopic versus open D2 gastrectomy for advanced gastric cancer: a retrospective cohort study. Surgical endoscopy. 2013;27(1):286-94. https://doi.org/10.1007/s00464-012-2442-x.
Li Z, Ji G, Bai B, Yu D, Liu Y, Lian B et al. Laparoscopy-assisted distal gastrectomy versus laparoscopy-assisted total gastrectomy with D2 lymph node dissection for middle-third advanced gastric cancer. Surgical endoscopy. 2018;32(5):2255-62. https://doi.org/10.1007/s00464-017-5919-9.
Yue F GX. Impact of conversion during laparoscopic gastrectomy on outcomes of patients with gastric cancer. J BUON. 2017;22(4):926-31.
Hu Y, Huang C, Sun Y, Su X, Cao H, Hu J et al. Morbidity and mortality of laparoscopic versus open D2 distal gastrectomy for advanced gastric cancer: a randomized controlled trial. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2016;34(12):1350-7. https://doi.org/10.1200/JCO.2015.63.7215.
Shuang J, Qi S, Zheng J, Zhao Q, Li J, Kang Z et al. A case-control study of laparoscopy-assisted and open distal gastrectomy for advanced gastric cancer. J Gastrointest Surg. 2011;15(1):57-62. https://doi.org/10.1007/s11605-010-1361-1.
Du J ZJ, Li Y, Li J, Ji G, Dong G, Yang Z, Wang W, Gao Z. Laparoscopy-assisted total gastrectomy with extended lymph node resection for advanced gastric cancer--reports of 82 cases. Hepatogastroenterology 2010;57.(104):1589-94.
Fang C, Hua J, Li J, Zhen J, Wang F, Zhao Q et al. Comparison of long-term results between laparoscopy-assisted gastrectomy and open gastrectomy with D2 lymphadenectomy for advanced gastric cancer. American journal of surgery. 2014;208(3):391-6. https://doi.org/10.1016/j.amjsurg.2013.09.028.
Sano T, Coit DG, Kim HH, Roviello F, Kassab P, Wittekind C et al. Proposal of a new stage grouping of gastric cancer for TNM classification: International Gastric Cancer Association staging project. Gastric cancer: official journal of the International Gastric Cancer Association and the Japanese Gastric Cancer Association. 2017;20(2):217-25. https://doi.org/10.1007/s10120-016-0601-9.
Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD et al. The Clavien-Dindo classification of surgical complications: five-year experience. Annals of surgery. 2009;250(2):187-96. https://doi.org/10.1097/SLA.0b013e3181b13ca2.
Dindo D, Demartines N, Clavien P-A. Classification of surgical complications. Annals of surgery. 2004;240(2):205-13. https://doi.org/10.1097/01.sla.0000133083.54934.ae.
Park YK, Yoon HM, Kim YW, Park JY, Ryu KW, Lee YJ et al. Laparoscopy-assisted versus open D2 distal gastrectomy for advanced gastric cancer: results from a randomized phase II multicenter clinical trial (COACT 1001). Annals of surgery. 2017;42(9):S98-S9. https://doi.org/10.1097/SLA.0000000000002168.
Shi Y, Xu X, Zhao Y, Qian F, Tang B, Hao Y et al. Short-term surgical outcomes of a randomized controlled trial comparing laparoscopic versus open gastrectomy with D2 lymph node dissection for advanced gastric cancer. Surgical endoscopy. 2017. https://doi.org/10.1007/s00464-017-5942-x.
Fujiya K, Tokunaga M, Mori K, Makuuchi R, Tanizawa Y, Bando E et al. Long-term survival in patients with postoperative intra-abdominal infectious complications after curative gastrectomy for gastric cancer: a propensity score matching analysis. Annals of surgical oncology. 2016;23(Suppl 5):809-16. https://doi.org/10.1245/s10434-016-5577-5.
Tsujimoto H, Ichikura T, Ono S, Sugasawa H, Hiraki S, Sakamoto N et al. Impact of postoperative infection on long-term survival after potentially curative resection for gastric cancer. Annals of surgical oncology. 2009;16(2):311-8. https://doi.org/10.1245/s10434-008-0249-8.
Li QG, Li P, Tang D, Chen J, Wang DR. Impact of postoperative complications on long-term survival after radical resection for gastric cancer. World journal of gastroenterology. 2013;19(25):4060-5. https://doi.org/10.3748/wjg.v19.i25.4060.
Li Z, Bai B, Zhao Y, Yu D, Lian B, Liu Y et al. Severity of complications and long-term survival after laparoscopic total gastrectomy with D2 lymph node dissection for advanced gastric cancer: a propensity score-matched, case-control study. International journal of surgery. 2018;54(54):62-9. https://doi.org/10.1016/j.ijsu.2018.04.034.
Yoo CH, Kim HO, Hwang SI, Son BH, Shin JH, Kim H. Short-term outcomes of laparoscopic-assisted distal gastrectomy for gastric cancer during a surgeon’s learning curve period. Surgical endoscopy. 2009;23(10):2250-7. https://doi.org/10.1007/s00464-008-0315-0.
Jung DH, Son SY, Park YS, Shin DJ, Ahn HS, Ahn SH et al. The learning curve associated with laparoscopic total gastrectomy. Gastric cancer: official journal of the International Gastric Cancer Association and the Japanese Gastric Cancer Association. 2016;19(1):264-72. https://doi.org/10.1007/s10120-014-0447-y.
Clancy C, O'Leary DP, Burke JP, Redmond HP, Coffey JC, Kerin MJ et al. A meta-analysis to determine the oncological implications of conversion in laparoscopic colorectal cancer surgery. Colorectal Disease. 2015;17(6):482-90. https://doi.org/10.1111/codi.12875.
Ye M JK, Xu G, Lin F, Zhou Q, Tao K, Tao F. Short- and long-term outcomes after conversion of laparoscopic total gastrectomy for gastric cancer: a single-center study. J BUON. 2017;22(1):126-33.
Zhao B, Zhang J, Chen X, Sun T, Wang Z, Xu H et al. The retrieval of at least 25 lymph nodes should be essential for advanced gastric cancer patients with lymph node metastasis: a retrospective analysis of single-institution database study design: cohort study. International journal of surgery. 2017;48:291-9. https://doi.org/10.1016/j.ijsu.2017.11.036.
Zheng G, Feng F, Guo M, Xu G, Liu S, Liu Z et al. Harvest of at least 23 lymph nodes is indispensable for stage N3 gastric cancer patients. Annals of surgical oncology. 2017;24(4):998-1002. https://doi.org/10.1245/s10434-016-5667-4.
Shen Z, Ye Y, Xie Q, Liang B, Jiang K, Wang S. Effect of the number of lymph nodes harvested on the long-term survival of gastric cancer patients according to tumor stage and location: a 12-year study of 1,637 cases. American journal of surgery. 2015;210(3):431-40 e3. https://doi.org/10.1016/j.amjsurg.2015.01.029.
Cho BC, Jeung HC, Choi HJ, Rha SY, Hyung WJ, Cheong JH et al. Prognostic impact of resection margin involvement after extended (D2/D3) gastrectomy for advanced gastric cancer: a 15-year experience at a single institute. Journal of surgical oncology. 2007;95(6):461-8. https://doi.org/10.1002/jso.20731.
Kim SY, Hwang YS, Sohn TS, Oh SJ, Choi MG, Noh JH et al. The predictors and clinical impact of positive resection margins on frozen section in gastric cancer surgery. Journal of Gastric Cancer. 2012;12(2):113. https://doi.org/10.5230/jgc.2012.12.2.113.
Cunningham SC, Kamangar F, Kim MP, Hammoud S, Haque R, Maitra A et al. Survival after gastric adenocarcinoma resection: eighteen-year experience at a single institution. J Gastrointest Surg. 2005;9(5):718-25. https://doi.org/10.1016/j.gassur.2004.12.002.
Wang SY, Yeh CN, Lee HL, Liu YY, Chao TC, Hwang TL et al. Clinical impact of positive surgical margin status on gastric cancer patients undergoing gastrectomy. Annals of surgical oncology. 2009;16(10):2738-43. https://doi.org/10.1245/s10434-009-0616-0.
Acknowledgements
The authors are thankful to the medical staff of Xijing Hospital of Digestive Diseases for their management of the database.
Author information
Authors and Affiliations
Contributions
Fen-gni Xie, Jie Chen, and Zheng-yan Li initiated the study design and wrote the manuscript. Zheng-yan Li and Bing Bai helped with implementation. Dan Song, Shuai Xu, and Xiao-tian Song contributed to the acquisition, analysis, or interpretation of data. Jie Chen and Gang Ji revised and supervised the study. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Disclosures
Fengni Xie, Jie Chen, Zhengyan Li, Bing Bai, Dan Song, Shuai Xu, Xiaotian Song, and Gang Ji have no conflicts of interest or financial ties to disclose.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Xie, Fn., Chen, J., Li, Zy. et al. Impact of Laparoscopic Converted to Open Gastrectomy on Short- and Long-Term Outcomes of Patients with Locally Advanced Gastric Cancer: A Propensity Score-Matched Analysis. J Gastrointest Surg 25, 2484–2494 (2021). https://doi.org/10.1007/s11605-021-04975-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11605-021-04975-6