Abstract
Background
The frequency of intraperitoneal free tumor cells (IPTC) is considered to reflect the severity of peritoneal metastasis (PM). We quantified the relative number of IPTC against leukocytes in peritoneal fluid and evaluated its clinical relevance in gastric cancer (GC) patients, particularly those with PM.
Methods
Cells recovered from ascites or peritoneal lavage fluid were immunostained with monoclonal antibodies (mAb) to CD45 and CD326 (EpCAM). Using flow cytometry (FACS), CD326(+) and CD45(+) cells were classified as either tumor cells (T) or leukocytes (L) and the T/L ratio (TLR) was calculated in a total of 506 samples obtained from 300 patients with GC and 33 patients with liver cirrhosis (LC).
Results
Median (M) of the TLR of the initial samples obtained from 199 patients with PM(+) GC was 1.32 % (0–1,868.44 %), which was significantly higher than that in patients with PM(−) GC (M = 0 %, 0–0.35 %; n = 101) or LC (M = 0 %, 0–0.031 %; n = 33). In 104 PM(+) patients who received combination chemotherapy including intraperitoneal paclitaxel, the TLR was repeatedly measured in peritoneal fluid obtained from the port. In these patients, the TLR showed a strong correlation with clinical features as well as cytological findings and carcinoembryonic antigen messenger RNA status. Finally, the median survival time of the 11 patients with initial TLR > 10 % was significantly shorter than that of the 52 patients with TLR < 10 % (271 vs. 627 days; p = 0.0002).
Conclusion
The TLR excellently reflected tumor burden in the peritoneal cavity, and could be a reliable biomarker to determine the outcome, as well as the effectiveness, of chemotherapy in patients with PM(+) GC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Peritoneal metastasis (PM) is the most common form of recurrence in gastric cancer (GC), particularly in cases with serosal exposure and undifferentiated/signet-ring cell-type histological features.1 – 3 Peritoneal recurrence is considered to be caused by intraperitoneal free tumor cells (IPTC) that have been exfoliated from the serosal surface of GC.4,5 At present, IPTC are diagnosed by conventional cytological methods using hematoxylin eosin or Papanicolaou staining (CY) as the gold standard. Many reports have shown that CY is the most important determinant in the prediction of the development of peritoneal recurrence in patients with various types of abdominal malignancies, including GC.6 – 12 However, CY evaluated by the conventional method is totally qualitative with low sensitivity, and largely dependent on the institution as well as the pathologist, which sometimes results in confusion in the clinical evaluation of the outcome of CY(+) patients.13 – 15
Recently, an reverse transcription polymerase chain reaction (RT-PCR) method of detecting tumor cell-specific messenger RNA (mRNA), such as carcinoembryonic antigen (CEA) or cytokeratin (CK) 19 or 20, has been introduced, which has improved the sensitivity in detecting IPTC, with a better correlation with peritoneal recurrence in GC.13,16 – 19 However, the amplified mRNA may be derived from dead cells or phagocytes that have engulfed tumor cells, and can be released from hematopoietic cells in an inflammatory context.20 Therefore, the clinical issue of false-positive cases remains to be addressed.
We have recently developed a new method to quantify the relative number of IPTC in the abdominal cavity by calculating the tumor cell/leukocyte ratio (TLR) using flow cytometry (FACS).21 In this study, we evaluated the clinical value of the TLR in a large number of samples derived from patients with GC, particularly those who received intraperitoneal chemotherapy against PM.
Materials and Methods
Patients and Samples
A total of 506 samples of ascites or peritoneal lavage fluid were recovered from 333 patients and then analyzed in this study. Among these, 392 samples were obtained from 219 patients who underwent abdominal surgery for GC at The University of Tokyo between August 2009 and March 2014. In patients who underwent open abdominal surgery, peritoneal washing was performed using 200 ml of normal saline, and a 100 ml sample was recovered by lavage before operative manipulation. In cases with ascites, 20 ml of ascitic fluid was obtained soon after laparotomy. Among these cases, 104 patients with PM received combination chemotherapy using intravenous and intraperitoneal paclitaxel together with oral S-1.22,23 In short, paclitaxel was administered intravenously at 50 mg/m2 and intraperitoneally at 20 mg/m2 on days 1 and 8, respectively, while S-1 was administered at 80 mg/m2/day for 14 consecutive days, followed by 7 days of rest. In these patients, peritoneal fluids were repeatedly obtained from a subcutaneously implanted peritoneal access port and used to measure the TLR as well as conventional cytological examination and/or CEA mRNA analysis during the course of chemotherapy. Pathologists performed peritoneal cytological evaluation of all samples, and CEA mRNA was examined in 194 samples using the RT-PCR method, as described previously.17 In addition, ascitic samples obtained from 114 patients with peritoneal carcinomatosis due to GC (n = 81) or liver cirrhosis (LC) (n = 33) who underwent paracentesis at Kanamecho Hospital were also included in the analysis. Written informed consent was obtained from all patients. This study was carried out in accordance with the Declaration of Helsinki and was approved by the Institutional Review Board of The University of Tokyo.
Cell Processing and Calculation of the Tumor Cell/Leukocyte Ratio (TLR)
After centrifugation of ascites or peritoneal lavage fluid at 1,500 rpm for 10 min, the pellets were resuspended in PBS + 0.02 % EDTA and overlayed on Ficoll-Hypaque solution (Pharmacia Biotech, Piscataway, NJ, USA). After centrifugation at 3,000 rpm for 10 min, the intermediate layer was taken and washed twice with PBS + 0.02 % EDTA. The cells were suspended in 100 μl PBS + 0.02 % EDTA, incubated with 10 μl Fc-blocker for 20 min, and then immunostained with fluorescein isothiocyanate (FITC)-conjugated monoclonal antibody (mAb) to CD45 (Becton-Dickinson, San-Jose, CA, USA), phycoerythrin (PE)-conjugated mAb to CD326 (Miltenyi Biotec, Auburn, CA, USA) and 7-amino-actinomycin D (7AAD) for 30 min at 4 °C according to the manufacturers’ recommendations. After washing, at least 104 cells were acquired in the 7AAD-negative with FACS-Caliber. All the CD326(+) cells showed the morphological characteristics of malignant cells (electronic supplementary Fig. 1).
TLR in 300 patients with GC with or without PM, and 33 patients with LC. The data of the first samples in 333 patients were plotted. The y-axis was plotted as a logarithmic scale. The TLR of the samples derived from PM(−) GC or LC was very low, with a medial TLR of 0 %. The TLR of the samples derived from PM(+) GC patients was highly variable, and was significantly higher than that of PM(−) GC or LC. TLR tumor cell/leukocyte ratio, GC gastric cancer, PM peritoneal metastasis, LC liver cirrhosis, M median
Calculation of the TLR was performed by a modification of the previously described method.21 In brief, the FL-1 (FITC) and FL-2 (PE) intensities were plotted against side scatter (SSC) in the 7AAD-negative region (R1), and negative areas were determined by the staining intensities with negative control mouse IgG (mIgG) mAbs, respectively (electronic supplementary Fig. 2). The cut-off line for fluorescein intensity usually increased as SSC increased, and thus the positive areas for CD45 (R2) and CD326 (R3) showed a ‘sox-like’ shape. The number of CD45(+) leukocytes and CD326(+) tumor cells were then calculated as the number of dots located in the gated areas R1 + R2 and R1 + R3, respectively, and the TLR was calculated using the following formulae.
Change of TLR by combination chemotherapy in patients with PM. The TLR was calculated before and after one cycle of combination chemotherapy in 39 patients. In 33 cases that showed a negative change in CY, TLR was greatly reduced (a), but not in the remaining six patients in whom CY remained positive (b). TLR tumor cell/leukocyte ratio, PM peritoneal metastasis
Statistics
For comparison of the TLR, p values were calculated using Wilcoxon’s non-parametric analysis with JUMP software. The Kaplan–Meier method was used to estimate the survival curve, and the log-rank test was used to compare the outcome. A p value <0.05 was considered significant for all statistical analyses.
Results
The TLR of Peritoneal Fluid in Patients with Gastric Cancer or Liver Cirrhosis
Figure 1 shows the initial TLR of peritoneal fluid obtained from 300 patients with GC with or without PM, and 33 patients with LC. In general, samples derived from PM(−) patients contained few CD326-reactive cells, and the median (M) of the TLR was 0 % (0–0.35 %; n = 101). Indeed, the TLR of 70 of the 101 patients was 0 %, and was less than 0.01 % in the remaining seven patients. Ascitic fluid of LC patients also showed a very low TLR (M = 0 %, 0–0.031 %; n = 33). In comparison, the TLR of samples recovered from PM(+) patients showed a significantly higher TLR (M = 1.32 %, 0–1,868.44 %; n = 199) (p < 0.001). However, the TLR was highly variable among the samples from PM(+) patients. Ascitic fluid of 22 (11 %) patients contained extremely high levels of CD326(+) cells with a TLR above 100 %, while the TLR was less than 0.1 % in 50 (25 %) patients.
Change of TLR in Patients Who Received Intraperitoneal Chemotherapy
We treated PM(+) GC patients with combination chemotherapy including intraperitoneal paclitaxel. Figure 2 shows the change of TLR in 39 patients after one course of chemotherapy. As shown in Fig. 2a, the TLR showed a rapid decrease in 33 patients who showed a negative change in cytological findings (M = 0.58 %, 0.01–87.9 % vs. M = 0 %, 0–3.3 %; p < 0.01). In contrast, the TLR did not show such a clear reduction in six patients whose cytological findings remained positive (M = 8.03 %, 0.04–339.01 % vs. M = 1.77 %, 0.08–35.4 %) (Fig. 2b).
Figure 3 shows an example of the whole time course of the TLR in one patient. After three courses of chemotherapy, the TLR was greatly reduced from 42.8 to 0 %, with a negative change in cytological findings after three courses of chemotherapy. Eventually, the patient had a recurrence in the meninges and died at 20 months from the start of chemotherapy, but the TLR was maintained below 1.0 % with negative cytological findings, whereas CEA mRNA remained positive during the whole period of intraperitoneal chemotherapy.
Example of TLR changes during the course of chemotherapy. Bars show the TLR, and lines show serum CA19-9 level. In this patient, TLR showed a marked decrease with the first cycle of combination chemotherapy and remained low during the whole period, suggesting that PM was well controlled by this chemotherapeutic regimen. TLR tumor cell/leukocyte ratio, PM peritoneal metastasis, CA19-9 carbohydrate antigen 19-9, CEA carcinoembryonic antigen, mRNA messenger RNA
We then summarized the TLR of peritoneal fluid obtained from the 104 PM(+) patients who received combination chemotherapy. As shown in Fig. 4a, the median TLR of CY(+) samples was 0.59 % (0–533.77 %; n = 120). In comparison, the TLR of CY(−) samples was much lower (M = 0 %, 0–1.13 %; n = 141) (p < 0.001), which was mostly the same as the TLR in samples from the PM(−) patients at laparotomy. Similarly, the TLR in CEA mRNA(+) samples was significantly higher than that in CEA mRNA(−) samples (M = 0.11 %, 0–533.77 %, n = 139 vs. M = 0 %, 0–0.94 %, n = 55) (p < 0.001; Fig. 4b).
Relationship between TLR and CY or CEA mRNA in samples derived from patients who received combination chemotherapy. a TLR of CY(+) peritoneal fluid was significantly higher than that of CY(−) samples. b Similarly, TLR of CEA mRNA(+) peritoneal fluid was significantly higher than that of CEA mRNA(−) samples. TLR tumor cell/leukocyte ratio, CEA carcinoembryonic antigen, mRNA messenger RNA, M median
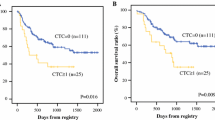
Initial TLR and Outcome of Patients Who Received Intraperitoneal Chemotherapy
Finally, we examined the impact of the initial TLR before treatment in 63 patients. The median TLR was 0.68 % (0–533.77 %) and median survival time (MST) was 394 days. However, as shown in Fig. 5, the MST of the 52 patients whose initial TLR was <10 % was 627 days, which was markedly longer than that of patients with a TLR >10 % (MST = 271 days; p = 0.0002).
Overall survival curves of 63 PM(+) patients who received combination chemotherapy. MST was 627 days in the 52 patients whose initial TLR before chemotherapy was <10 %, and 271 days in the remaining 11 patients with TLR >10 % (p = 0.0002). PM peritoneal metastasis, MST median survival time, TLR tumor cell/leukocyte ratio
Discussion
Although cytological detection of IPTC in peritoneal fluid is the most important prognostic factor for peritoneal recurrence,11,12 the results evaluated by the conventional method varied greatly among institutions and pathologists, with low sensitivity.13 Recently, immunostaining methods using specific mAbs to tumor cell-associated antigens have been clinically introduced to increase sensitivity.24,25 We used an immunostaining method using pan-leukocyte markers CD45 and CD326, which are widely overexpressed in a variety of human cancers,26 – 28 and investigated the relative number of CD326(+) tumor cells against CD45(+) leukocytes as TLR using flow cytometry.21 Although staining is performed in cell suspensions and within approximately 1 h, the value of the TLR is highly reproducible when more than 104 living cells can be counted in 7AAD(−) area in the FACS profile. Recently, it has been reported that the relative number of CD326(+) tumor cells can be examined with a computerized image in patients with malignant ascites.29,30 Although our method has basically the same concept, it can be practiced more widely without specialized equipment.
One possible concern is that tumor cells are often observed to form clusters in classical cytological examination, which might not be accurately evaluated in FACS analysis. In fact, the presence of clustered tumor cells in CY was reported not to have a significant association with patient outcome,31 or to have rather favorable prognosis for GC patients.32 However, we found that few clusters were observed in all samples after repeated washing with EDTA-containing medium. Clusters were often observed in the sediment after Ficoll-Hypaque centrifugation; however, most were positive for Propidium iodide as well as 7-AAD (electronic supplementary Fig. 3), suggesting that those clustered IPTC may consist mainly of dead or dying cells. In some cases, doublets or small clusters were included in the intermediate layer even after thorough washing with EDTA. Therefore, we also examined the TLR after additional enzymatic digestion with 0.25 % trypsin; however, the treatment did not produce marked changes in the TLR values (electronic supplementary Fig. 4). From these findings, we consider that the TLR well reflects the density of living IPTC with significant biological activity and thus our calculation method could be acceptable enough as semi-quantitative analysis.
In our series, the value of the TLR varied markedly in samples from patients with PM(+) GC, ranging from less than 0.1 % to more than 100 %. This indicates that the amount of IPTC was highly variable among PM(+) patients, and thus accurate quantification of IPTC is clinically important to classify the severity of the patients with PM. Whereas the TLR was calculated as 0 % in the majority of samples derived from patients with PM(−) GC or LC, which means CD326(+) cells rarely exist in the peritoneal cavity in physiological conditions. Thirty-one of 101 (30 %) samples from PM(−) GC also showed a TLR of 0.01–0.35 %. Among these, five cases that showed TLRs of more than 0.1 % were pathologically diagnosed as positive exposure to the serosal surface. Thus, it may be more likely that their lavage fluid actually contained a small number of IPTC, although they were diagnosed as CY(−) with the conventional method. Follow-up of the outcome of these patients may give us a cut-off value of the TLR that distinguishes the real presence of IPTC and contamination.
We have recently treated PM(+) patients with combination chemotherapy—intraperitoneal and intravenous paclitaxel with oral S-1, which showed a marked response of peritoneal lesions, with excellent survival.22,23,33 In these patients, peritoneal fluid can be easily obtained from the peritoneal access port, and thus the TLR could be non-invasively monitored during treatment. In fact, the TLR showed a marked reduction with a negative change of CY in the majority of cases after one cycle of chemotherapy. Moreover, the TLR showed an excellent correlation with CY and CEA mRNA data. Compared with qualitative results on CY or CEA mRNA, the quantitative value of the TLR is useful to determine the degree of response to chemotherapy. Furthermore, the initial TLR showed a significant association with the outcome of PM(+) patients.
Conclusions
The presence of IPTC is certainly associated with high risk of peritoneal recurrence and a worse outcome in GC, irrespective of the methodology. Although some error range exists, this method to quantify the relative volume of IPTC is simple, takes a short time, and is highly objective. Recent data have shown that some of the circulating tumor cells lack expression of CD326, which may also be observed in IPTC, and thus the FACS methodology should be improved by the introduction of multicolor analysis in the future. However, our results suggest that the TLR calculated with this method is a reliable biomarker to predict outcome, as well as to evaluate the efficacy of treatment, particularly in patients with PM(+) GC who have received chemotherapy. How TLR or quantitative RT-PCR data correlate with their clinical outcome should be comparatively investigated in future studies.
References
Nakamura K, Ueyama T, Yao T, et al. Pathology and prognosis of gastric carcinoma: findings in 10,000 patients who underwent primary gastrectomy. Cancer. 1992;70:1030–1037.
Isobe Y, Nashimoto A, Akazawa K, et al. Gastric cancer treatment in Japan: 2008 annual report of the JGCA nationwide registry. Gastric Cancer. 2011;14:301–316.
Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90.
Koga S, Kaibara N, Iitsuka Y, et al. Prognostic significance of intraperitoneal free cancer cells in gastric cancer patients. J Cancer Res Clin Oncol. 1984;108:236–238.
Sodek KL, Murphy KJ, Brown TJ, Ringuette MJ. Cell-cell and cell-matrix dynamics in intraperitoneal cancer metastasis. Cancer Metastasis Rev. 2012;31:397–414.
Warshaw AL. Implications of peritoneal cytology for staging of early pancreatic cancer. Am J Surg. 1991;161:26–29; discussion 29–30.
Ziselman EM, Harkavy SE, Hogan M, et al. Peritoneal washing cytology. Uses and diagnostic criteria in gynecologic neoplasms. Acta Cytol. 1984;28:105–110.
Hayes N, Wayman J, Wadehra V, et al. Peritoneal cytology in the surgical evaluation of gastric carcinoma. Br J Cancer. 1999;79:520–524.
Vogel P, Ruschoff J, Kummel S, et al. Prognostic value of microscopic peritoneal dissemination: comparison between colon and gastric cancer. Dis Colon Rectum. 2000;43:92–100.
Horattas MC, Evasovich MR, Topham N. Colorectal carcinoma and the relationship of peritoneal cytology. Am J Surg. 1997;174:334–337; discussion 337–338.
Bonenkamp JJ, Songun I, Hermans J, van de Velde CJ. Prognostic value of positive cytology findings from abdominal washings in patients with gastric cancer. Br J Surg. 1996;83:672–674.
Bando E, Yonemura Y, Takeshita Y, et al. Intraoperative lavage for cytological examination in 1,297 patients with gastric carcinoma. Am J Surg. 1999;178:256–262.
Leake PA, Cardoso R, Seevaratnam R, et al. A systematic review of the accuracy and utility of peritoneal cytology in patients with gastric cancer. Gastric Cancer. 2012;15 Suppl 1:S27–S37.
Konishi M, Kinoshita T, Nakagohri T, et al. Prognostic value of cytologic examination of peritoneal washings in pancreatic cancer. Arch Surg. 2002;137:475–480.
Lorenzen S, Panzram B, Rosenberg R, et al. Prognostic significance of free peritoneal tumor cells in the peritoneal cavity before and after neoadjuvant chemotherapy in patients with gastric carcinoma undergoing potentially curative resection. Ann Surg Oncol. 2010;17:2733–2739.
Katsuragi K, Yashiro M, Sawada T, et al. Prognostic impact of PCR-based identification of isolated tumour cells in the peritoneal lavage fluid of gastric cancer patients who underwent a curative R0 resection. Br J Cancer. 2007;97:550–556.
Kodera Y, Nakanishi H, Ito S, et al. Quantitative detection of disseminated free cancer cells in peritoneal washes with real-time reverse transcriptase-polymerase chain reaction: a sensitive predictor of outcome for patients with gastric carcinoma. Ann Surg. 2002;235:499–506.
Rossi Del Monte S, Ranieri D, Mazzetta F, et al. Free peritoneal tumor cells detection in gastric and colorectal cancer patients. J Surg Oncol. 2012;106:17–23.
Dalal KM, Woo Y, Kelly K, et al. Detection of micrometastases in peritoneal washings of gastric cancer patients by the reverse transcriptase polymerase chain reaction. Gastric Cancer. 2008;11:206–213.
Kowalewska M, Chechlinska M, Nowak R. Carcinoembryonic antigen and cytokeratin 20 in peritoneal cells of cancer patients: are we aware of what we are detecting by mRNA examination? Br J Cancer. 2008;98:512–513; author reply 514.
Kitayama J, Emoto S, Yamaguchi H, et al. Flow cytometric quantification of intraperitoneal free tumor cells in patients with peritoneal metastasis. Cytometry B Clin Cytom. 2014;86:56–62.
Ishigami H, Kitayama J, Kaisaki S, et al. Phase II study of weekly intravenous and intraperitoneal paclitaxel combined with S-1 for advanced gastric cancer with peritoneal metastasis. Ann Oncol. 2010;21:67–70.
Yamaguchi H, Kitayama J, Ishigami H, et al. A phase 2 trial of intravenous and intraperitoneal paclitaxel combined with S-1 for treatment of gastric cancer with macroscopic peritoneal metastasis. Cancer. 2013;119:3354–3358.
Benevolo M, Mottolese M, Cosimelli M, et al. Diagnostic and prognostic value of peritoneal immunocytology in gastric cancer. J Clin Oncol. 1998;16:3406–3411.
Vogel P, Ruschoff J, Kummel S, et al. Immunocytology improves prognostic impact of peritoneal tumour cell detection compared to conventional cytology in gastric cancer. Eur J Surg Oncol. 1999;25:515–519.
Went PT, Lugli A, Meier S, et al. Frequent EpCam protein expression in human carcinomas. Hum Pathol. 2004;35:122–128.
Baeuerle PA, Gires O. EpCAM (CD326) finding its role in cancer. Br J Cancer. 2007;96:417–423.
Patriarca C, Macchi RM, Marschner AK, Mellstedt H. Epithelial cell adhesion molecule expression (CD326) in cancer: a short review. Cancer Treat Rev. 2012;38:68–75.
Burges A, Wimberger P, Kumper C, et al. Effective relief of malignant ascites in patients with advanced ovarian cancer by a trifunctional anti-EpCAM x anti-CD3 antibody: a phase I/II study. Clin Cancer Res. 2007;13:3899–3905.
Heiss MM, Murawa P, Koralewski P, et al. The trifunctional antibody catumaxomab for the treatment of malignant ascites due to epithelial cancer: results of a prospective randomized phase II/III trial. Int J Cancer. 2010;127:2209–2221.
Miyashiro I, Takachi K, Doki Y, et al. When is curative gastrectomy justified for gastric cancer with positive peritoneal lavage cytology but negative macroscopic peritoneal implant? World J Surg. 2005;29:1131–1134.
Iitsuka Y, Shiota S, Matsui T, et al. Relationship between the cytologic characteristics of intraperitoneal free cancer cells and the prognosis in patients with gastric cancer. Acta Cytol. 1990;34:437–442.
Kitayama J, Ishigami H, Yamaguchi H, et al. Salvage gastrectomy after intravenous and intraperitoneal paclitaxel (PTX) administration with oral S-1 for peritoneal dissemination of advanced gastric cancer with malignant ascites. Ann Surg Oncol. 2014;21(2):539–546.
Acknowledgment
We thank Ms. Chieko Uchikawa, Ikuko Nieda, and Yu Saito for their excellent technical and clerical support. This study was financially supported by the Ministry of Health, Labor and Welfare of Japan. This work was funded by the Ministry of Education, Culture, Sports, Science and Technology of Japan, and the Ministry of Health, Labor and Welfare of Japan, and by a Grant-in-aid of the Public Trust Surgery Research Fund, Tokyo, Japan.
Disclosure
Joji Kitayama, Shigenobu Emoto, Hironori Yamaguchi, Hironori Ishigami, Haruna Onoyama, Hiroharu Yamashita, Yasuyuki Seto, Keisuke Matsuzaki, and Toshiaki Watanabe have no financial disclosures.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kitayama, J., Emoto, S., Yamaguchi, H. et al. Flow Cytometric Quantification of Intraperitoneal Free Tumor Cells is a Useful Biomarker in Gastric Cancer Patients with Peritoneal Metastasis . Ann Surg Oncol 22, 2336–2342 (2015). https://doi.org/10.1245/s10434-014-4238-9
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-014-4238-9