Abstract
Background
Recent studies have shown an association in non-metastatic colorectal cancer between patient survival and immunoprofiling (expression of CD3, CD4, CD8, CD45, and FOXP3 T cells at the invasive margin (IM) and the tumor center (TC)) regardless of stage. Patients with peritoneal carcinomatosis have a dismal prognosis, but survival can be significantly improved in selected patients who undergo cytoreductive surgery and hyperthermic intraperitoneal chemotherapy (CRS/HIPEC). However, current patient selection for CRS/HIPEC is suboptimal. The purpose of this study is to evaluate immune profiles of patients with peritoneal carcinomatosis and their correlation with overall survival (OS).
Methods
The study cohort included patients from a prospectively maintained database of adults with colorectal peritoneal carcinomatosis who underwent CRS/HIPEC. Immunohistochemistry (IHC) using antibodies to CD3, CD4, CD8, CD45RO, and FOXP3 T cells was performed. IHC image density was calculated using ImageJ software, and an immunoscore was determined.
Results
Eighty tumors were evaluated from 66 patients. These included 14 primary sites and 66 metastatic sites. R0/R1 resection was achieved in 44 (66.7%) patients. Known prognostic factors including resection status (HR 1.99, p = 0.004) and lymph node status (HR 3.49, p = 0.002) were associated with overall survival. On multivariate analysis, increased CD3/CD4 IM (HR 0.54, p = 0.03) ratio positively was associated with improved OS.
Discussion
This is the first study to assess the utility of subtypes of T cells as prognostic markers in patients with colorectal peritoneal carcinomatosis, which may play a role in patients with low-volume disease. Further studies into immune mechanisms may improve patient selection for cytoreductive surgery and HIPEC as well as provide novel pathways for effective immunotherapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Immune profiling has recently been shown to be an important prognostic factor in colorectal cancer (CRC). 1,2,3,4,5 The presence of tumor-infiltrating lymphocytes (TILs) is a marker of the patients’ immune response to the tumor and its presence is associated with a favorable immune microenvironment that can translate to greater survival. Recently, our group demonstrated that TILs, including CD3 (total helper and cytotoxic T cells), CD4 (helper T cells), CD8 (cytotoxic T cells), and FOXP3 (regulatory T cells), correlated with higher disease-free survival in a multicenter prospective clinical trial in stage 1–3 colon cancer.2 An “immunoscore” in CRC has been developed using TILs, specifically CD3+ and CD8+, to both prognosticate and direct treatment.4,6,7 Little is known about its prognostic value in patients with metastatic disease particularly those patients with peritoneal carcinomatosis. We have found that appendiceal cancers with peritoneal dissemination have better outcomes if they have an immune enhanced signature, rather than one which is oncogene dominant.8 Typically patients with peritoneal involvement have a poor survival and quality of life.
Cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC) are complex procedures; the goals of which are to remove all visible cancer and perfuse heated chemotherapy to eliminate micrometastases. In 2003, Verwaal et al. showed that CRS and HIPEC improve survival by about a year in patients with peritoneal metastases of colorectal origin.9 This often requires resection of multiple organs and is associated with considerable morbidity in over a third of patients.10 In the appropriate patients, however, a significant improvement in the 5 year survival was improved by 50%, but it is 50% has been reported.11,12
The selection of patients for this procedure who are most likely to benefit continues to be challenging. A better understanding of the dynamic role between the immune environment and outcomes after surgery will help surgeons with patient selection as well as quality of life discussions related to long-term prognosis. The purpose of this study is to identify immune profile pattern changes with peritoneal disease progression of colon cancer and evaluate if those patterns are predictive of recurrence and overall survival.
Methods
All tissue and clinical history were obtained for research from a prospectively maintained database with institutional review board (IRB) approval at Wake Forest University Medical Center, Winston-Salem, NC. Data sharing between Wake Forest Baptist Medical Center and John Wayne Cancer Institute was approved by the IRBs of both institutions (IRB 2019000171). All tumor specimens were identified grossly by a pathologist and processed as formalin-fixed paraffin-embedded (FFPE) tumor blocks and maintained in a tissue repository.
Patient selection used a prospectively managed database of patients undergoing CRS/HIPEC for biopsy-proven peritoneal metastases from colorectal cancer at Wake Forest University Medical Center between 2010 and 2014. Tissue was included only from a patient’s first CRS/HIPEC, and not subsequent resections. Excluded patients were those < 18 years of age, those with appendiceal cancers, and those who were not perfused with chemotherapy. Techniques for CRS/HIPEC have been described elsewhere.13 The Peritoneal Cancer Index (PCI) was tabulated at the time of surgery. This was calculated by dividing the abdomen into 9 sections and the bowel into 4 sections. Scores in each area are given 0–3 (0 for no gross disease, 1 for 0–0.5 cm of disease, 2 for 0.5–5.0 cm, and 3 for > 5.0 cm).14 Patients were stratified as low or high PCI using a cutoff PCI score of 11 based on a recent French PRODIGE 7 randomized trial, which compared cytoreduction alone with cytoreduction and HIPEC15. This cutoff was chosen as the trial suggested that cytoreduction alone may be adequate in this low PCI group and the addition of HIPEC benefited those with a PCI score of 11–1515. Additionally, 12 was the median PCI, making the groups equally distributed. Neoadjuvant chemotherapy was defined as any therapy within 3 months prior to the CRS/HIPEC.
Immunohistochemistry (IHC) was performed on 4-μm formalin-fixed paraffin-embedded slides by NeoGenomics Laboratories, Inc (Aliso Viejo, CA). Primary polyclonal antibodies included anti-CD3 clone 2GV6, anti-CD4 clone SP35, anti-CD45 clone UCHL1, anti-CD8 clone C8/144B, and anti-FOXP3 clone 236A/E7. Tissue was evaluated for adenocarcinoma implants and IHC-stained slides were reviewed to evaluate the stain density at the tumor center (TC) and invasive margin (IM) as previously described by Lavotshkin et al.1 The most representative image was captured for data analysis by a pathologist blinded to clinical data. Image analysis and density calculation were done using the ImageJ Java-based processing program (National Institutes of Health, Bethesda, MD) by a single author who was blinded to the clinical data at the time of analysis (Fig. 1). Prior reports identified good correlation between blinded pathologists in image selection. 16 Low versus high density scores were calculated by comparing the lowest and highest quartiles.1,2 Unless otherwise stated, all comparisons were made using metastatic tissue as this was consistently available for all patients.
All statistical analyses were performed using SAS version 9.4. Scores were compared using the non-parametric Kruskal-Wallis test. Survival curves were produced by the Kaplan-Meier method. The Wilcoxon signed-rank test was used to compare paired observations. Univariable and multivariable survival analyses were performed by the Cox proportional hazards method.
Results
Patient Demographics and Tumor Status
Sixty-six unique patients were included in this study, with 14 of them having both primary and metastatic tumor tissue available for review. Thirty-six (54.5%) were female and most had an R0/R1 resection (66.7%), and the median PCI score was 12. The median survival was 21.2 months, 26.3 months for R0/R1 (Table 1). Most (93%) received chemotherapy within 3 months prior to cytoreduction and HIPEC, typically FOLFOX or FOLFIRI ± bevacizumab.
Immunohistochemistry Analysis of Tumor Tissue
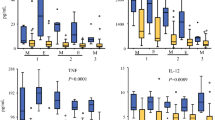
Digital images of tumor tissue from a single patient with peritoneal carcinomatosis of colon origin, stained for CD3+, CD4+, CD8+, CD45RO+, and FOXP3+ T cells, are shown in Fig. 1. Densities of each marker in the primary and metastatic tissue for all patients are shown in Table 2 and a comparison of cell densities at the invasive margin and tumor center is seen in Fig. 2. Cell densities were consistently higher at the invasive margin than the tumor center.
When comparing the 14 primary samples to the metastatic tissue, both CD3+ and FOXP3+ cells in the IM were more prevalent in the primary tumor than in the metastatic tissue (p = 0.01 and p = 0.02, respectively). The Wilcoxon signed-rank test was used to compare the paired primary tissue to the metastatic tissue for the 14 patients with both available. CD3+ cells in the IM (p = 0.01), CD3+ cells in the tumor center (TC) (p = 0.04), CD8+ cells in the TC (p = 0.05), and FOXP3+ cells in the IM (p < 0.01) were all higher in the primary tumor compared to the metastatic tissue. None of the metastatic tissues had higher cell densities than the primary tumor tissues.
Obese patients (BMI ≥ 30) were found to have lower CD4+ cell IM levels (p = 0.03) than non-obese patients. Neoadjuvant chemotherapy had no effect on TILs (all p > 0.05). There was no difference in TIL densities between lymph node–positive and lymph node–negative patients. Both FOXP3 IM and CD4/CD8 TC had higher densities in those that were able to achieve R0/R1 resection status.
Associations with OS and DFS
Univariate analysis was performed on TILs, BMI, resection status, grade, lymph node status, neoadjuvant therapy, and PCI. On multivariate analysis, the higher CD3+/CD4+ IHC density ratio in the invasive margin (IM) of the tumor was significantly associated with overall survival (OS) (p = 0.04) (Table 3). Other independent factors associated with OS were lymph node status (p < 0.001) and an R0/R1 resection (p = 0.01). CD3+ tumor cell density in the IM was significant in univariate, but not in multivariate, analysis (Table 3). No TILs, including the Immunoscore, other than the CD3+/CD4+ ratio was associated with OS.
Although lymph node positivity was associated with OS, the density of TILs was not significantly different in those patients with lymph node positivity versus those without positive lymph nodes. Only 7% of patients did not receive neoadjuvant chemotherapy, but there appeared to be no difference in the number of TILs according to therapy.
The IM CD3+/CD4+ cell ratio, R status, tumor grade, and lymph node status were associated with disease-free survival (DFS) in univariate analysis, but only tumor grade (p < 0.01) remained significant on multivariate analysis (Table 4). When stratifying patients by PCI (< 11 or 11+), higher CD3+/CD4+ IM was associated with better OS (p = 0.007) and DFS (p = 0.009) (Fig. 3). No other TILs, including the Immunoscore, were associated with improvement in OS or DFS.
Overall survival and disease-free survival for PCI < 11 based on the CD3+/CD4+ ratio at the IM. Top row is PCI < 11. Bottom row is PCI 11+. Kaplan-Meier analysis of overall survival (OS) and disease-free survival (DFS) in months for patients with a high versus low CD3+/CD4+ IM ratio with a follow-up of 60 months. The statistical significance (p value) of the difference between the survival curves of patients with high or low PCI is shown on each figure
Samples of metastatic tissue from patients with low (< 11) versus high (11+) PCI were compared with low PCI having a higher density of CD3 IM than high PCI (p = 0.04). OS and DFS in these groups were then assessed. The low PCI group, but not the high PCI patients, showed better survival with the CD3+/CD4+ IM ratio in both OS (HR 0.32, p = 0.01) and DFS (HR 0.36, p = 0.01). In the high PCI group, increased CD8+ IM was associated with decreased DFS (HR 2.75, p = 0.04).
Discussion
Immune profiling has been proposed as a useful adjunct to the standard American Joint Committee on Cancer (AJCC) TNM staging in colon cancer.2 The immune environment of tumors plays a crucial role in the prognosis and treatment of both earlier stage colon cancers and metastatic colon cancers.2,17 Our study demonstrated an improved response to CRS/HIPEC in patients with peritoneal carcinomatosis who had higher IM CD3+/CD4+ cell ratios, particularly with lower PCI scores.
TILs have been studied across many cancer types. Their role in cancer immunogenesis is complex. CD4+ cells are known to interact with major histocompatibility complex (MHC) II molecules to recruit CD8+ cells for tumor suppression. High levels of both CD4+ and CD8+ cells have been associated with slower tumor growth, but these cells can also become inactive over time. This is seen in cancer recurrence and rapid disease growth. Reactivation of these cells is one of the targets of immunotherapy. To further complicate this dynamic relationship, CD4+ cells can differentiate into regulatory T cells such as CD25+ or FOXP3+ cells, thereby suppressing the immune response to the tumor and allowing for growth.18 The data regarding the role of FOXP3+ cells is inconsistent. Previous research from our institution by Flaherty et al. showed that an increased FOXP3+/CD8+ cell ratio in stage II and III was associated with poorer DFS.2 Another study, however, showed that in stage II and III colorectal cancers, high FOXP3+ alone was associated with improved OS.19 Similarly, in this study, the FOXP3+ IM densities were higher in the primary than the metastases and associated with the ability to achieve R0/R1 cytoreduction. FOXP3+ in head and neck cancers has been similarly shown to be associated with locoregional control.20 How these regulatory cells are interacting with each other as tumor burden increases needs further study.
Low levels of CD45RO+ memory T cells are associated with poor prognosis.21 Non-metastatic CRC patients with low numbers of CD3+ and CD45RO+ cells have a prognosis similar to those with metastatic disease. These memory T cells have a long life and are hypothesized to decrease relapse.22 The data in this study does not suggest a survival advantage or longer disease-free interval with expression of this memory T cell once the disease is already metastatic.
Galon and colleagues created an Immunoscore, based on CD3+ and CD8+ cell positivity after concluding that these cells provided the most accurate prognostic information.5 Flaherty et al. demonstrated the prognostic value of the tumor immune environment for patients with stages I–III colon cancer, showing that total TC+IM CD8+ cells and total TC+IM CD8+/FOXP3+ cells were significantly associated with DFS.2 In univariate analysis, increased CD3+ IM cells and decreased CD4+/CD8+ ratio in TC+IM were associated with improved DFS too. Additionally, studies looking at brain, lung, and liver cancers have suggested that immune profiling can still predict prognosis in the metastatic setting.17,23,24In this study, however, the Immunoscore calculation was not prognostic of DFS and OS and a higher CD4+/CD8+ TC ratio was associated with better cytoreduction, suggesting that peritoneal carcinomatosis affects the immune system differently than solid organ metastases and early-stage diseases.
Immunoscore has been evaluated in relation to microsatellite instability (MSI). The high Immunoscore group had the largest percent of MSI patients, while 5% of the MSI patients were in the lowest Immunoscore group.7 Another study had only 14% of MSI in the lowest group and 56% of MSI in the highest Immunoscore group.25 In metastatic melanoma and non-small cell lung cancer, higher numbers of tumor-infiltrating lymphocytes predicted better response to immunotherapy.26 The evaluation of the role of Immunoscore in immunotherapy is an important next step. Additionally, evaluation of the role of neoadjuvant and adjuvant therapies, both in non-metastatic and metastatic disease, should be further examined. In stage III colon cancer, however, the Immunoscore was recently shown to determine efficacy of adjuvant chemotherapy. Pages et al. compared 3-month duration and 6-month duration of mFOLFOX treatment and found that patients with an intermediate or high Immunoscore were the only ones who benefited from longer treatment.7 Although Immunoscore was not significant for carcinomatosis, the fact that the immune environment affects response to chemotherapy is important for future study. Since most patients receive neoadjuvant chemotherapy for peritoneal carcinomatosis, it is possible that resection prior to chemotherapy may be beneficial in some patients in an effort to stimulate the immune system.
In this study of colorectal carcinomatosis, in contrast to previous studies of non-metastatic colon and gastric cancers, only a higher CD3+/CD4+ cell ratio appears to be associated with decreased death and recurrence. This does not apply to patients with more advanced carcinomatosis (PCI 11+), suggesting that the immune system may become overwhelmed. In primary tumors, increased CD4+ expression is thought to represent a more active adaptive immune system. However, in metastatic disease, a larger fraction of these cells may have differentiated into regulatory T cells, thus contributing to the ability of tumors to metastasize. The higher number of TILs in primary tumors compared to matched metastases as well as higher CD3+ IM in low PCI patients supports this hypothesis of eventual escape from the immune system. Similarly, Lynch syndrome patients are less likely to develop metastases and this has been attributed to the increased role of TILs.27 Treatment to reinvigorate the immune system within the peritoneum is therefore an important area for exploration.
Limitations of this study include that it is a retrospective, single-institution analysis of a modest number of patients. Additionally, to standardize the surgery, those who were not perfused were omitted. These patients generally were those that were deemed unresectable, often with a high PCI. They might, however, represent a different immune environment. This study also focuses on a subset of TILs, and other subsets may also be involved in control of carcinomatosis. This study, however, concurs with others that show that increased TILs and immune profiling are valuable prognostic tools, even in metastatic disease.
Conclusions
This is the first analysis of TILs in patients with peritoneal metastases of colorectal origin. Analysis of the CD3+/CD4+ IM ratio using IHC is simple and inexpensive and has prognostic value in patients with a low PCI score. Furthermore, lack of correlation in advanced disease suggests that the immune environment is less significant in patients with high PCI. Although at this time we would still offer CRS/HIPEC to low PCI patients with a low CD3+/CD4+ IM ratio, this too warrants further study. The immune environment has previously been associated with response to chemotherapy and this relationship should be further studied in metastatic disease.7 Exploring individualized treatment utilizing immune environment, genetic profiles, and disease burden to create algorithms of debulking surgery, systemic chemotherapy, intraperitoneal therapy, and targeted therapy is a crucial next step.
Abbreviations
- AJCC:
-
American Joint Committee on Cancer
- BMI:
-
body mass index
- CRC:
-
colorectal cancer
- CRS:
-
cytoreductive surgery
- DFS:
-
disease-free survival
- FFPE:
-
formalin-fixed paraffin-embedded
- HIPEC:
-
heated intraperitoneal chemotherapy
- IM:
-
invasive margin
- IRB:
-
institutional review board
- mFOLFOX:
-
modified folinic acid, fluorouracil, and oxaliplatin treatment
- MSI:
-
microsatellite instability
- MSS:
-
microsatellite stability
- OS:
-
overall survival
- PCI:
-
Peritoneal Cancer Index
- R:
-
residual tumor status
- R0:
-
cure/complete remission
- R1:
-
microscopic residual tumor
- R2:
-
macroscopic residual tumor
- TC:
-
tumor center
- TILs:
-
tumor-infiltrating lymphocytes
References
Lavotshkin S, Jalas JR, Torisu-Itakura H, et al. Immunoprofiling for Prognostic Assessment of Colon Cancer: a Novel Complement to Ultrastaging. J Gastrointest Surg. 2015;19(6):999-1006. https://doi.org/10.1007/s11605-015-2759-6
Flaherty DC, Lavotshkin S, Jalas JR, et al. Prognostic Utility of Immunoprofiling in Colon Cancer: Results from a Prospective, Multicenter Nodal Ultrastaging Trial. J Am Coll Surg. 2016;223(1):134-140. https://doi.org/10.1016/j.jamcollsurg.2016.03.003
Mlecnik B, Bindea G, Kirilovsky A, et al. The tumor microenvironment and Immunoscore are critical determinants of dissemination to distant metastasis. Sci Transl Med. 2016;8(327):327ra26. https://doi.org/10.1126/scitranslmed.aad6352
Galon J, Angell HK, Bedognetti D, Marincola FM. The Continuum of Cancer Immunosurveillance: Prognostic, Predictive, and Mechanistic Signatures. Immunity. 2013;39(1):11-26. https://doi.org/10.1016/J.IMMUNI.2013.07.008
Galon J, Costes A, Sanchez-Cabo F, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313(5795):1960-1964. https://doi.org/10.1126/science.1129139
Pagès F, Mlecnik B, Marliot F, et al. International validation of the consensus Immunoscore for the classification of colon cancer: a prognostic and accuracy study. Lancet. 2018;391(10135):2128-2139. https://doi.org/10.1016/S0140-6736(18)30789-X
Pages F, Andre T, Taieb J, et al. Progostic and predictive value of the Immunoscore in stage III colon cancer patients treated with mFOLFOX6 (3 vs 6 months) in the prospective IDEA France cohort study. Gastrointest Cancers Symp. 2020;Jan 23-25(San Francisco, CA):Abstract.
Levine EA, Votanopoulos KI, Qasem SA, et al. Prognostic Molecular Subtypes of Low-Grade Cancer of the Appendix. J Am Coll Surg. 2016;222(4):493-503. https://doi.org/10.1016/j.jamcollsurg.2015.12.012
Verwaal VJ, van Ruth S, de Bree E, et al. Randomized trial of cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy and palliative surgery in patients with peritoneal carcinomatosis of colorectal cancer. J Clin Oncol. 2003;21(20):3737-3743. https://doi.org/10.1200/JCO.2003.04.187
Jafari MD, Halabi WJ, Stamos MJ, et al. Surgical outcomes of hyperthermic intraperitoneal chemotherapy: analysis of the american college of surgeons national surgical quality improvement program. JAMA Surg. 2014;149(2):170-175. https://doi.org/10.1001/jamasurg.2013.3640
Ahmed S, Stewart JH, Shen P, Votanopoulos KI, Levine EA. Outcomes with cytoreductive surgery and HIPEC for peritoneal metastasis. J Surg Oncol. 2014;110(5):575-584. https://doi.org/10.1002/jso.23749
Elias D, Lefevre JH, Chevalier J, et al. Complete cytoreductive surgery plus intraperitoneal chemohyperthermia with oxaliplatin for peritoneal carcinomatosis of colorectal origin. J Clin Oncol. 2009;27(5):681-685. https://doi.org/10.1200/JCO.2008.19.7160
Levine EA, Stewart JH, Shen P, Russell GB, Loggie BL, Votanopoulos KI. Intraperitoneal Chemotherapy for Peritoneal Surface Malignancy: Experience with 1,000 Patients. J Am Coll Surg. 2014;218(4):573-585. https://doi.org/10.1016/j.jamcollsurg.2013.12.013
Berthet B, Sugarbaker TA, Chang D, Sugarbaker PH. Quantitative methodologies for selection of patients with recurrent abdominopelvic sarcoma for treatment. Eur J Cancer. 1999;35(3):413-419. https://doi.org/10.1016/s0959-8049(98)00375-x
Quenet F, Elias D, Roca L, et al. A UNICANCER phase III trial of hyperthermic intra-peritoneal chemotherapy (HIPEC) for colorectal peritoneal carcinomatosis (PC): PRODIGE 7. J Clin Oncol. 2018;36(18_suppl):LBA3503. https://doi.org/10.1200/JCO.2018.36.18_suppl.LBA3503
Uppal A, Stern S, Thompson JF, et al. Regional Node Basin Recurrence in Melanoma Patients: More Common After Node Dissection for Macroscopic Rather than Clinically Occult Nodal Disease. Ann Surg Oncol. 2019:1-8. https://doi.org/10.1245/s10434-019-08086-0
Mlecnik B, Van den Eynde M, Bindea G, et al. Comprehensive Intrametastatic Immune Quantification and Major Impact of Immunoscore on Survival. JNCI J Natl Cancer Inst. 2018;110(1):97-108. https://doi.org/10.1093/jnci/djx123
Ostroumov D, Fekete-Drimusz N, Saborowski M, Kühnel F, Woller N. CD4 and CD8 T lymphocyte interplay in controlling tumor growth. Cell Mol Life Sci. 2018;75(4):689-713. https://doi.org/10.1007/s00018-017-2686-7
Salama P, Phillips M, Grieu F, et al. Tumor-infiltrating FOXP3+ T regulatory cells show strong prognostic significance in colorectal cancer. J Clin Oncol. 2009;27(2):186-192. https://doi.org/10.1200/JCO.2008.18.7229
Badoual C, Hans S, Rodriguez J, et al. Prognostic value of tumor-infiltrating CD4+ T-cell subpopulations in head and neck cancers. Clin Cancer Res. 2006;12(2):465-472. https://doi.org/10.1158/1078-0432.CCR-05-1886
Pagès F, Kirilovsky A, Mlecnik B, et al. In Situ Cytotoxic and Memory T Cells Predict Outcome in Patients With Early-Stage Colorectal Cancer. J Clin Oncol. 2009;27(35):5944-5951. https://doi.org/10.1200/JCO.2008.19.6147
Pagès F, Berger A, Camus M, et al. Effector Memory T Cells, Early Metastasis, and Survival in Colorectal Cancer. N Engl J Med. 2005;353(25):2654-2666. https://doi.org/10.1056/NEJMoa051424
Berghoff AS, Fuchs E, Ricken G, et al. Density of tumor-infiltrating lymphocytes correlates with extent of brain edema and overall survival time in patients with brain metastases. Oncoimmunology. 2016;5(1):e1057388. https://doi.org/10.1080/2162402X.2015.1057388
Wang C, Jette N, Moussienko D, Bebb DG, Lees-Miller SP. ATM-Deficient Colorectal Cancer Cells Are Sensitive to the PARP Inhibitor Olaparib. Transl Oncol. 2017;10(2):190-196. https://doi.org/10.1016/J.TRANON.2017.01.007
Wirta E-V, Seppälä T, Friman M, et al. Immunoscore in mismatch repair-proficient and -deficient colon cancer. J Pathol Clin Res. 2017;3(3):203-213. https://doi.org/10.1002/cjp2.71
Uryvaev A, Passhak M, Hershkovits D, Sabo E, Bar-Sela G. The role of tumor-infiltrating lymphocytes (TILs) as a predictive biomarker of response to anti-PD1 therapy in patients with metastatic non-small cell lung cancer or metastatic melanoma. Med Oncol. 2018;35(3):25. https://doi.org/10.1007/s12032-018-1080-0
Drescher KM, Sharma P, Lynch HT. Current Hypotheses on How Microsatellite Instability Leads to Enhanced Survival of Lynch Syndrome Patients. Clin Dev Immunol. 2010;2010:1-13. https://doi.org/10.1155/2010/170432
Funding
This study was supported, in part, by the Smith Family Fund and the Comprehensive Cancer Center of Wake Forest University, and the tumor tissue pathology shared resource supported by NCI CCSG P30CA012197.
Author information
Authors and Affiliations
Contributions
Garland-Kledzik: project development, data analysis, data collection, manuscript writing/editing; Uppal: project development, manuscript writing/editing; Naeini: data analysis; Stern: data analysis; Erali: project development; Yi: data collection; Cummins-Perry: data collection; Scholer: data analysis; Khader: data analysis; Santamaria-Barria: data analysis; Votanopoulos: data collection; Shen: data collection; Levine: project design, data collection; Bilchik: project development, manuscript writing/editing.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Garland-Kledzik, M., Uppal, A., Naeini, Y.B. et al. Prognostic Impact and Utility of Immunoprofiling in the Selection of Patients with Colorectal Peritoneal Carcinomatosis for Cytoreductive Surgery (CRS) and Heated Intraperitoneal Chemotherapy (HIPEC). J Gastrointest Surg 25, 233–240 (2021). https://doi.org/10.1007/s11605-020-04886-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11605-020-04886-y