Abstract
Background
The presence of circulating tumor cells (CTCs) is negatively associated with survival after resection of colorectal liver metastases (CLM). The current study aimed to determine the prognostic value of CTCs and disseminated tumor cells (DTCs) at the time of surgery and the prognostic value of CTCs at follow-up assessment, for patients scheduled to undergo two-stage hepatectomy with portal vein embolization (PVE) for CLM.
Methods
Samples were collected at surgery (blood and bone marrow) and at follow-up assessment (blood) for the period 2008 through 2011. In this study, CTCs were detected with the CellSearch system, and DTCs were detected using standard immunocytochemical analysis.
Results
Of 24 patients, 18 completed both stages, and no patients were lost to follow-up. The median overall survival (OS) was 37 months, and the median recurrence-free survival (RFS) was 7 months. At surgery, CTCs were found in nine patients (38 %), and their presence was associated with reduced OS (p < 0.001) and RFS (p = 0.006). Follow-up CTC status was available for 11 patients. All eight patients with positive CTC status experienced recurrence. Two of three patients with negative CTC status remained recurrence free. In seven patients (32 %), DTCs were detected but were not associated with OS or RFS.
Conclusions
The presence of CTCs at surgery is associated with worse OS and RFS for patients undergoing two-stage hepatectomy with PVE for CLM. Analysis of CTCs should be explored further for their potential to assist in treatment decisions and monitoring for CLM patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Colorectal cancer (CRC), the third most common cancer worldwide, accounts for more than 600,000 deaths annually.1 Most deaths can be attributed to metastatic disease, which is confined to the liver in about 50 % of cases, and resection still is considered the only curative treatment option for colorectal liver metastases (CLM).2,3 Coordinated management by oncologists, radiologists, and hepatobiliary surgeons combined with improved imaging, modern chemotherapy, and surgical advances has increased the proportion of patients with CLM eligible for surgery.4 The optimal therapeutic approach is not always obvious, and identifying patients with the greatest potential for survival benefit is a challenge.5
For patients with bilateral CLM, the size of the future liver remnant (FLR) and the risk of postoperative liver failure may limit the possibility of completing the surgical resection. Two-stage surgery with induction of liver hypertrophy by portal vein embolization (PVE) may convert some of these patients to a resectable status.6,7 Associating liver partition and portal vein ligation for staged hepatectomy (ALPPS) is another recently proposed method of extended liver resection that may provide increased hypertrophy compared with PVE, but the safety aspects of the method remain controversial.8,9 Nevertheless, tools that could improve prognostication and assist in the difficult patient selection for extensive surgery are highly relevant for these borderline resectable patients.
Recent reports indicate that circulating tumor cells (CTCs) in blood, disseminated tumor cells (DTCs), or both in bone marrow may serve as prognostic markers in primary and metastatic CRC10–15. However, few studies have evaluated the impact of CTCs and DTCs in various clinical settings, and even fewer have included follow-up CTC assessments after surgery.16,17 The current study aimed to examine the prognostic value of CTC and DTC assessments both at surgery and at follow-up visits for patients with bilateral CLM scheduled for two-stage liver surgery with PVE.
Methods
The Regional Ethical Committee (Oslo, Norway) and the Hospital’s Patient-Surveillance Service approved the study before patient inclusion. Permission for bio-banking was obtained from the National Health Department. The current study population constituted a subgroup of patients from a prospective cohort of 194 consecutive patients referred for resection of CLM between May 2008 and December 2011.17
Patient Management
Patients were evaluated by a multidisciplinary team to determine resectability and treatment strategy. After written informed consent, the study enrolled 24 patients scheduled to undergo two-stage hepatectomy with PVE.
Synchronous liver metastases were defined as metastases detected within 6 months after diagnosis of the primary tumor.2 Of the 24 patients, 20 presented with synchronous CLM to be treated using the following resection strategies: primary tumor first approach (n = 17), liver first approach (n = 2), and combined first-stage liver and primary tumor approach (n = 1).3
Computed tomography (CT)-based volumetry of the liver was performed for all patients before PVE and 4 weeks afterward. The second stage of the two-stage hepatectomy was discontinued for two patients because the FLR before the second resection was considered insufficient (20 and 23 %, respectively). One patient did not undergo the second stage due to presentation of bone metastasis in the interval between the stages. Three patients were found to be unresectable during the second stage because of intrahepatic tumor progression.
For this study, R0 (negative resection margin) was defined as a resection margin more than 1 mm from cancer tissue. Radiofrequency ablation (RFA) was used in combination with first-stage hepatectomy for two patients.
All the patients received four cycles of oxaliplatin or irinotecan and 5-fluorouracil-based preoperative chemotherapy: FLOX (n = 14), FLIRI (n = 2), and FLIRI with bevacizumab (n = 8).18 The choice of chemotherapy was based on the history of previously administered chemotherapy, tolerance, and comorbidity. The response to preoperative chemotherapy was assessed according to current grading systems.19–21Chemotherapy was not used in the interval between the two surgical stages for any patient. Postoperative (adjuvant) FLOX was used for 14 of the 18 patients completing both stages.
Follow-up consultations were performed every 4 months with clinical and radiologic assessments. At the first and/or second follow-up visit, CTCs were assessed, and the highest number of cells detected was used for analyses. The main end points of the study were overall survival (OS) and recurrence-free survival (RFS). For the purpose of analyzing the survival of the whole group (n = 24), progression was assessed as recurrence in the six patients who did not undergo complete resection at the second stage.15 Patient characteristics including demographic data, primary tumor details, clinical history and chemotherapy, preoperative radiologic workup, surgical procedures, and clinical outcome were registered prospectively. Patients were followed from the date of bone marrow aspiration/blood sampling at the first liver resection until the date of relapse, progression, death, or end of the follow-up period. Clinicians were blinded from the CTC and DTC results, and the laboratory staff had no access to the clinical data.
Detection of CTCs
The Food and Drug Administration (FDA)-approved automatic CellSearch system (Janssen Diagnostics LLC, Beerse, Belgium) was used for CTC detection. The method, described in detail elsewhere, is clinically validated and considered the gold standard of CTC detection.22 Samples were collected into CellSave preservation tubes (Janssen Diagnostics LLC, Beerse, Belgium), maintained at room temperature, and processed within 96 h at the Micrometastasis Laboratory, Oslo University Hospital. From peripheral blood, CTCs were captured by an anti-epithelial cell adhesion molecule (EpCAM) antibody-bearing ferrofluid. Subsequently, captured cells were immunostained for pancytokeratin and leucocyte common antigen (CD45) and with 4,6-diamidino-2-phenylindole (DAPI) nuclear stain and presented on the CellSearch screen. An experienced technician reviewed the captured elements, which were subsequently reevaluated by a pathologist (E.B.). Only complete cells satisfying the standard CellSearch CTC definition criteria were scored as CTCs.23 The cutoff for positivity was determined to be one or more 7.5-ml CTC/samples of blood.13
Bone Marrow Preparation and Detection of DTCs
The bone marrow aspirations were performed from the anterior iliac crest bilaterally, 5 ml from each site, and processed as described previously.17,24 After separation by density centrifugation, mononuclear cells (MNC) were collected, and cytospins were prepared with a 5 × 105 MNC/slide. The immunocytologic staining was performed by incubating four slides (2 × 106 bone marrow MNC) with the anticytokeratin monoclonal antibodies (mAbs) AE1 and AE3 (Merck Millipore, Billerica, MA, USA), and the same number of slides were incubated with an irrelevant control mAb of the same immunoglobulin (Ig) subtype (IgG1; MOPC21, Sigma-Aldrich, St. Louis, MO, USA). The cytospins were screened by the Ariol SL 50 (Leica Biosystems, Nusslock, Germany) automated screening system.25 All immune-positive candidate cells were evaluated by a pathologist (E.B.). Only cells with immune morphology satisfying the standardized criteria for DTCs were scored as positive.26,27
Statistical Methods
Demographic and clinical parameters are shown as median values with range or proportions. Fisher’s exact test and the χ 2 test were used to compare ratios. Survival analyses were performed using the Kaplan–Meier method and the log-rank test. Overall survival was defined as the time from the second stage to death or last follow-up visit. Recurrence-free survival was defined as time from the second stage to recurrence or last follow-up visit without recurrence. No patient death was recorded without a recorded recurrence. Data were recorded in FileMaker Pro 12 (Filemaker Inc., Santa Clara, CA, USA) and analyzed using SPSS 20 (IBM SPSS, Chicago, IL, USA).
Results
Demographics and Surgical and Oncologic Outcomes
The median age of the 24 study patients (eight women and 16 men) was 63 years (range 39–75 years). Both resection stages were completed for 18 patients (Fig. 1). The median follow-up period was 29 months (range 7–73 months) after the first liver resection. No patients were lost to follow-up evaluation. A R0 resection was achieved for 20 of the 24 patients (including two patients who received RFA) at the first stage and 14 of 18 patients at the second stage (Table 1). The postoperative morbidity constituted bile leakage (n = 3), subphrenical abscess (n = 2), and pneumonia (n = 1). No postoperative liver failures or perioperative mortalities occurred.
Patient selection and management flow chart. The 24 patients included in the current report represent a subgroup from a larger study17 investigating the impact of circulating tumor cells (CTCs) and disseminated tumor cells (DTCs) in 194 patients evaluated for resection of colorectal liver metastases (CLMs)
Recurrence occurred for 22 patients. The median time to recurrence was 7 months (range 1–24 months). Recurrence presented at the following sites: liver (n = 15), lung (n = 8), extrahepatic intraabdominal site (n = 7), bone (n = 2), and brain (n = 1). Nine patients presented with multifocal recurrence. During the follow-up period, 11 patients died, and all the deaths were cancer related. The median survival period for all 24 patients was 37 months (range 7–73 months). The OS rate was 88 % at 1 year, 56 % at 3 years, and 48 % at 5 years.
Circulating Tumor Cells and Survival
Assessment of CTC status was performed for 21 of the 24 patients at the first stage of the two-stage hepatectomy and for 16 of 18 patients at the second stage. The findings showed CTCs in six patients (1–5 cells) at the first stage and in five patients (1–9 cells) at the second stage (Table 1). Of the 24 patients, 9 (38 %) had CTCs present (CTC-positive) at one or both surgical stages.
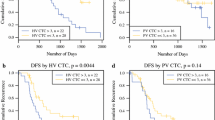
The presence of CTCs at surgery (first and/or second stage) was significantly associated with impaired OS (median, 24 vs 37 months; p < 0.001) and impaired RFS (median, 6 vs 10 months; p = 0.006) in all the patients and for the 18 patients completing both stages (median OS, 24 vs 37 months; p = 0.004 and median RFS, 7 vs 11 months; p = 0.031; Fig. 2). Among the 21 patients with CTCs available at the first stage, impaired OS and RFS were observed for the patients with CTC presence at this stage (median OS, 24 months vs not reached; p = 0.001 and median RFS, 6 vs 9 months; p = 0.019). Furthermore, three of six patients with positive CTC status at the first stage were found to be unresectable at the second stage, whereas only three of 15 patients with negative CTC status were unresectable at the second stage (p = 0.075).
At follow-up assessment, CTC status was assessed in 11 patients (seven patients at the first follow-up visit, two patients at the second follow-up visit, and two patients at both follow-up visits). Of the 11 patients, three had CTCs present at surgery, and all remained positive at follow-up assessment. Of the 11 patients, eight were CTC-negative at surgery, three remained negative, and five had CTCs present during follow-up assessment. All eight patients with positive CTC status at follow-up assessment experienced recurrence compared with only one of three patients who had negative follow-up CTC status (p = 0.048; Fig. 3).
Circulating tumor cell (CTC) status (negative or positive) and number of positive cells (y-axis) at the time of surgery (first and second stage combined) (x-axis) and at follow-up assessment (first or second follow-up visit combined) for 11 patients who underwent two-stage hepatectomy. Black circles results at surgery. Open circles patient recorded with recurrence at follow-up assessment. Open square patient without recurrence at follow-up assessment
Disseminated Tumor Cells and Survival
Bone marrow aspiration was performed for 21 of the 24 patients during the first stage and 15 of 21 patients during the second stage of the liver resection (Table 1). The findings showed DTCs in three patients during first stage, in five patients during second stage, and in one patient during both stages. For the two stages combined, DTCs were present in seven of the 24 patients (29.2 %) at either the first or second stage. The presence of DTCs was not associated with survival (Fig. 4). Considering the first and second stages combined, only two patients were positive for both CTCs and DTCs.
Discussion
In the current study, the presence of CTCs at the time of surgery (first and/or second stage) was associated with poor RFS and OS after two-stage hepatectomy combined with PVE for patients with CLM. This result is in agreement with recent reports, but more importantly, it confirms that the prognostic value of CTC presence is preserved for patients with extensive metastatic liver disease requiring staged resection and PVE to clear all disease. Furthermore, recurrence was detected in all patients with positive CTC status at follow-up assessment but only in one of three patients with negative CTC status, suggesting a potential postoperative role for CTC assessment. The latter may promote an earlier change of treatment strategy such as selection to chemotherapy or targeted therapy.
To date, most reports on CTCs and CRCs have been based on patients with unresectable metastatic disease. Cohen et al.11,28 reported that the numbers of CTCs before and during treatment were independent predictors of progression-free and overall survival in a large multinational cohort of patients with metastatic CRCs. In contrast, few studies have explored the value of CTC assessments in a surgical setting.16
Our group recently published a prospective study of 194 patients and could confirm that the findings of Cohen et al. also apply to patients undergoing resection of CLM.17 The current report presents data from a subgroup of high-risk patients with extensive metastatic disease undergoing two-stage hepatectomy and PVE, for whom modeling of prognosis preoperatively may be even more important.
Clinicopathologic parameters such as size and number of liver metastases and tumor-node-metastasis (TNM) stage have been losing their prognostic value for patients undergoing resection of CLM, and this could be attributed the use of perioperative modern chemotherapy.29,30 In this study, all the patients had extensive disease and received perioperative chemotherapy. As a consequence, traditional prognostic scoring models would have reduced yield for identifying patients with a favorable outcome. On the basis of this, the current report is especially important because the presence of CTCs provided identification of patients with favorable outcome regardless of the extensive disease. A new generation of prognostic factors has emerged for modeling prognosis after resection of CLM. Rat sarcoma viral oncogene (RAS) mutations, pathologic response to chemotherapy, and tumor immune cell infiltration are among the most promising to date.20,31,32 However, whereas these require tumor tissue for analysis, CTCs can be determined easily and affordably in patient blood samples preoperatively and repeatedly during the course of the disease.
We recently reported that CTCs were detected in 19.6 % of 194 patients being evaluated for resection of CLM, which was significantly lower than the 38 % positive rate in the current cohort.17 This discrepancy may be explained by several factors. First, patients selected for two-stage hepatectomy have more extensive disease according to previous reports suggesting higher CTC rates for patients with unresectable disease.11,33 Second, CTC status was assessed at both surgical stages, which may have increased the number of CTCs detected. Third, manipulation of liver and tumor during the first stage and the subsequent PVE may have caused release of CTCs being detected at the second stage.34 However, the standardized and FDA-approved CellSearch system for detection of CTCs in peripheral blood allows comparison of results between patients and centers.27,35
The number of CTCs and DTCs detected at the second stage was higher than at the first stage in the current study, although tumor load was reduced. Using proliferation markers and liver volumetry, Kokudo et al.36 reported increased proliferation and tumor volume after PVE than in PVE-naïve tumors. Others have made similar observations, and the increased tumor growth may be explained by upregulation of cytokines and growth factors in response to reduced portal flow and increased arterial perfusion after PVE34,37–39. In the current patient series, PVE was performed shortly after the first stage, but it remains uncertain whether this could facilitate release of cells becoming detectable as CTCs at the second stage.
In 32 % of the patients, DTCs were detected, and detection was not associated with OS or RFS. This is in agreement with our previous results and a recent meta-analysis by Rahbari et al.13 establishing CTCs but not DTCs as a prognostic factor in CRC.17 Interestingly, only two patients were dual-positive for CTCs and DTCs during surgery, indicating the possibility of an underlying biologic difference between tumors seeding CTCs and DTCs.
The main limitation of the current study was the small sample size with the subsequent risk of underpowered analyses. This is a challenge in single-center studies because patients eligible for two-stage hepatectomy and PVE represent a small subset of patients with resectable CLM. Nevertheless, the presence of CTCs had a significant impact on prognosis, and this is consistent with results from larger series of patients undergoing conventional liver resection of CLM without PVE.17 The association between CTC status at follow-up assessment and postoperative recurrence should be interpreted with caution because follow-up samples were available for only 11 patients. This, however, is an interesting association, and findings in metastatic breast cancer have shown that repeated CTC assessment predicts treatment response and prognosis.40
Another limitation of the study was that the different sensitivities of the methods used to detect CTCs and DTCs could have influenced the significance of their survival impact. We cannot exclude the possibility that optimized detection methods in the future could show an association between DTCs and survival. Finally, detection of one or more tumor cells was defined as CTC positivity in the current study, whereas others have used different definitions, and the ideal cutoff for CTC positivity in CLM patients remains to be determined.11,17,41
In conclusion, the prognostic value of CTCs is preserved for patients with multiple bilateral CLM undergoing PVE and two-stage hepatectomy. This finding is important because these patients represent a subgroup with extensive disease for which selection of surgical candidates is especially challenging. The presented results support further investigations of CTC to determine the role for identification of patients who may not benefit from two-stage hepatectomy with PVE, as well as for disease monitoring after completion of surgery.
References
Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–917.
Manfredi S, Lepage C, Hatem C, Coatmeur O, Faivre J, Bouvier AM. Epidemiology and management of liver metastases from colorectal cancer. Ann Surg. 2006;244:254–9.
Adam R, De Gramont A, Figueras J, et al. The oncosurgery approach to managing liver metastases from colorectal cancer: a multidisciplinary international consensus. Oncologist. 2012;17:1225–39.
Alberts SR, Poston GJ. Treatment advances in liver-limited metastatic colorectal cancer. Clin Colorect Cancer. 2011;10:258–65.
Gallagher DJ, Kemeny N. Improving response and outcomes for patients with liver-limited metastatic colorectal cancer. Clin Colorect Cancer. 2010;9(Suppl 1):S36–43.
Adam R, Laurent A, Azoulay D, Castaing D, Bismuth H. Two-stage hepatectomy: a planned strategy to treat irresectable liver tumors. Ann Surg. 2000;232:777–85.
Narita M, Oussoultzoglou E, Jaeck D, et al. Two-stage hepatectomy for multiple bilobar colorectal liver metastases. Br J Surg. 2011;98:1463–75.
Aloia TA, Vauthey JN. Associating liver partition and portal vein ligation for staged hepatectomy (ALPPS): what is gained and what is lost? Ann Surg. 2012;256:e9; author reply e16–9.
Schadde E, Ardiles V, Slankamenac K, et al. ALPPS offers a better chance of complete resection in patients with primarily unresectable liver tumors compared with conventional-staged hepatectomies: results of a multicenter analysis. World J Surg. 2014;38:1510–9.
Pantel K, Alix-Panabieres C, Riethdorf S. Cancer micrometastases. Nat Rev Clin Oncol. 2009;6:339–51.
Cohen SJ, Punt CJ, Iannotti N, et al. Relationship of circulating tumor cells to tumor response, progression-free survival, and overall survival in patients with metastatic colorectal cancer. J Clin Oncol. 2008;26:3213–21.
Allen JE, El-Deiry WS. Circulating tumor cells and colorectal cancer. Curr Colorect Cancer Rep. 2010;6:212–20.
Rahbari NN, Aigner M, Thorlund K, et al. Meta-analysis shows that detection of circulating tumor cells indicates poor prognosis in patients with colorectal cancer. Gastroenterology. 2010;138:1714–26.
Tol J, Koopman M, Miller MC, et al. Circulating tumour cells early predict progression-free and overall survival in advanced colorectal cancer patients treated with chemotherapy and targeted agents. Ann Oncol. 2010;21:1006–12.
Koerkamp BG, Rahbari NN, Buchler MW, Koch M, Weitz J. Circulating tumor cells and prognosis of patients with resectable colorectal liver metastases or widespread metastatic colorectal cancer: a meta-analysis. Ann Surg Oncol. 2013;20:2156–65.
Negin BP, Cohen SJ. Circulating tumor cells in colorectal cancer: past, present, and future challenges. Curr Treat Options oncol. 2010;11:1–13.
Seeberg LT, Waage A, Brunborg C, et al. Circulating tumor cells in patients with colorectal liver metastasis predict impaired survival. Ann Surg. 2015;261:164–71.
Sharif S, O’Connell MJ, Yothers G, Lopa S, Wolmark N. FOLFOX and FLOX regimens for the adjuvant treatment of resected stage II and III colon cancer. Cancer Invest. 2008;26:956–63.
Blazer DG III, Kishi Y, Maru DM, et al. Pathologic response to preoperative chemotherapy: a new outcome end point after resection of hepatic colorectal metastases. J Clin Oncol. 2008;6:5344–51.
Rubbia-Brandt L, Giostra E, Brezault C, et al. Importance of histological tumor response assessment in predicting the outcome in patients with colorectal liver metastases treated with neo-adjuvant chemotherapy followed by liver surgery. Ann Oncol. 2007;18:299–304.
Shindoh J, Loyer EM, Kopetz S, et al. Optimal morphologic response to preoperative chemotherapy: an alternate outcome end point before resection of hepatic colorectal metastases. J Clin Oncol. 2012;30:4566–72.
Riethdorf S, Fritsche H, Muller V, et al. Detection of circulating tumor cells in peripheral blood of patients with metastatic breast cancer: a validation study of the CellSearch system. Clin Cancer Res. 2007;13:920–8.
Allard WJ, Matera J, Miller MC, et al. Tumor cells circulate in the peripheral blood of all major carcinomas but not in healthy subjects or patients with nonmalignant diseases. Clin Cancer Res. 2004;10:6897–904.
Wiedswang G, Borgen E, Karesen R, et al. Detection of isolated tumor cells in bone marrow is an independent prognostic factor in breast cancer. J Clin Oncol. 2003;21:3469–78.
Borgen E, Naume B, Nesland JM, et al. Use of automated microscopy for the detection of disseminated tumor cells in bone marrow samples. Cytometry. 2001;46:215–21.
Borgen E, Naume B, Nesland JM, et al. Standardization of the immunocytochemical detection of cancer cells in BM and blood: I. establishment of objective criteria for the evaluation of immunostained cells. Cytotherapy. 1999;1:377–88.
Fehm T, Braun S, Muller V, et al. A concept for the standardized detection of disseminated tumor cells in bone marrow from patients with primary breast cancer and its clinical implementation. Cancer. 2006;107:885–92.
Cohen SJ, Punt CJ, Iannotti N, et al. Prognostic significance of circulating tumor cells in patients with metastatic colorectal cancer. Ann Oncol. 2009;20:1223–9.
Zakaria S, Donohue JH, Que FG, et al. Hepatic resection for colorectal metastases: value for risk scoring systems? Ann Surg. 2007;246:183–91.
Spolverato G, Ejaz A, Azad N, Pawlik TM. Surgery for colorectal liver metastases: the evolution of determining prognosis. World J Gastrointest Oncol. 2013;5:207–21.
Katz SC, Pillarisetty V, Bamboat ZM, et al. T cell infiltrate predicts long-term survival following resection of colorectal cancer liver metastases. Ann Surg Oncol. 2009;16:2524–30.
Vauthey JN, Zimmitti G, Kopetz SE, et al. RAS mutation status predicts survival and patterns of recurrence in patients undergoing hepatectomy for colorectal liver metastases. Ann Surg. 2013;258:619–26; discussion 26–7.
Kaifi JT, Kunkel M, Zhu J, et al. Circulating tumor cells are associated with diffuse spread in stage IV colorectal cancer patients. Cancer Biol Ther. 2013;14:1174–81.
Pamecha V, Levene A, Grillo F, Woodward N, Dhillon A, Davidson BR. Effect of portal vein embolisation on the growth rate of colorectal liver metastases. Br J Cancer. 2009;100:617–22.
Alix-Panabieres C, Pantel K. Challenges in circulating tumour cell research. Nat Rev Cancer. 2014;0(14):623–31.
Kokudo N, Tada K, Seki M, et al. Proliferative activity of intrahepatic colorectal metastases after preoperative hemihepatic portal vein embolization. Hepatology. 2001;34:267–72.
Hoekstra LT, van Lienden KP, Doets A, Busch OR, Gouma DJ, van Gulik TM. Tumor progression after preoperative portal vein embolization. Ann Surg. 2012;256:812–7; discussion 7–8.
Abdalla EK. Portal vein embolization (prior to major hepatectomy) effects on regeneration, resectability, and outcome. J Surg Oncol. 2010;102:960–7.
van Gulik TM, van den Esschert JW, de Graaf W, et al. Controversies in the use of portal vein embolization. Dig Surg. 2008;25:436–44.
Wallwiener M, Riethdorf S, Hartkopf AD, et al. Serial enumeration of circulating tumor cells predicts treatment response and prognosis in metastatic breast cancer: a prospective study in 393 patients. BMC Cancer. 2014;14:512.
Gazzaniga P, Raimondi C, Gradilone A, et al. Circulating tumor cells in metastatic colorectal cancer: do we need an alternative cutoff? J Cancer Res Clin Oncol. 2013;139:1411–6.
Acknowledgment
Kristoffer Watten Brudvik was awarded the Unger-Vetlesen Medical Fund for 2014, and Lars Thomas Seeberg received founding from The Eckbo Foundation, The Finn Wilhelmsen Foundation, and The Blix Family Foundation.
Conflict of interest
There are no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Kristoffer Watten Brudvik and Lars Thomas Seeberg have contributed equally.
Rights and permissions
About this article
Cite this article
Brudvik, K.W., Seeberg, L.T., Hugenschmidt, H. et al. Detection of Circulating Tumor Cells at Surgery and at Follow-Up Assessment to Predict Survival After Two-Stage Liver Resection of Colorectal Liver Metastases. Ann Surg Oncol 22, 4029–4037 (2015). https://doi.org/10.1245/s10434-015-4482-7
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-015-4482-7