Abstract
Dissolution testing and solubility determinations in different biorelevant media have gained considerable interest in the pharmaceutical industry from early-stage development of new products to forecasting bioequivalence. Among all biorelevant fluids, the preparation of fed-state simulated gastric fluid (FeSSGF) and handling of samples from dissolution/solubility testing in FeSSGF is considered to be relatively challenging. Challenges include maintaining the stability of FeSSGF medium upon sampling, filtration, and mitigating analytical interference of excipients and milk components. To overcome these challenges, standard and uniform working practices are required that are not only helpful in preparation of stable FeSSGF but also serve as a harmonizing guide for the collection of dissolution/solubility samples and their subsequent processing (i.e., handling and assay). The optimization of sample preparation methodology is crucial to reduce method-related variance by ensuring specificity, robustness, and reproducibility with acceptable recovery of the analytes. The sample preparation methodology includes a combination of techniques including filtration, solvent treatment, and centrifugation to remove the interfering media-related components and excipients from the analyte. The analytes of interest were chromatographically separated from the interfering analytes to quantify the drug concentration using the new high-performance liquid chromatography methods with ultraviolet detection. The methods developed allow rapid sample preparation, acceptable specificity, reproducible recoveries (greater than 95% of label claim), and quantification of study drugs (ibuprofen and ketoconazole). The sample preparation technique and method considerations provided here for ibuprofen and ketoconazole can serve as a starting point for solubility and dissolution testing of other small molecules in FeSSGF.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
One of the challenges in drug product development is the availability and selection of appropriate dissolution methods for predicting in vivo behavior of a drug product. Study test model drugs ibuprofen (a weak acid) and ketoconazole (a weak base) have been in the market since the early 1980s. There are many publications on their solubility, in vitro dissolution and absorption characteristics, approaches for improving their bioavailability, and on methodology exploring in vitro and in vivo correlations. Ibuprofen and ketoconazole were selected as good examples of drugs in Biopharmaceutics Classification System Class II (BCS Class II), exhibiting low solubility and high permeability. BCS Class II drugs represent approximately 40% of the drug products undergoing development (1,2,3). Improving their solubility creates a path for improving their bioavailability. These drugs are further categorized as weakly acidic and weakly basic drugs. Given their pH-dependent solubility characteristics, it is equally important to properly select and design solubility and dissolution testing methods as the obtained results and data can be considered just as informative as in vivo studies during formulation development. Very often dissolution testing of poorly soluble drug products is studied in biorelevant media (BRM) mimicking fasted and fed-state gastric and intestinal environment for guiding formulation development (4,5). Compendial dissolution tests typically do not provide the level of information that can be obtained by solubility and dissolution testing in BRM mimicking a physiologic environment under fed and fasted conditions (6,7,8,9,10). The BRM components often contain bile acids, lipid micelles, and similar components which are reported to enhance the dissolution rate and solubility of drugs with poor aqueous solubility (11). To assess these effects, a weakly acidic drug, ibuprofen and a weakly basic drug, ketoconazole, were selected as model drugs to understand the possible behavior of weakly acidic or basic drugs.
Dissolution results obtained using BRM have proven helpful in predicting in vivo performance of drug products (12). For example, as published extensively, FeSSGF has been informative in studying in vitro and in vivo performance of drug products, particularly, poorly soluble drugs and in identifying formulations of poorly soluble drugs which are affected by the presence of food (13,14,15,16).
Other reasons for studying in vitro performance of drug products in FeSSGF, in addition to studying formulation optimization under fed and fasted conditions, include richness of the body of knowledge with FeSSGF. FeSSGF serves as a well-studied benchmark for comparisons, and it is versatile to address specific questions. The established recipe for FeSSGF contains pH 5 acetate buffer and full-fat milk (3.5% fat) (4,17). Full-fat milk is preferred because its ratio of carbohydrate/protein/fat is similar to that observed in the stomach after meals (18). For studying different clinical settings and patient populations, modifications of FeSSGF can be prepared with homogenized meals, liquid meals, emulsions, and baby formula replacing milk in the FeSSGF recipe (4,19).
The main challenge in quantifying a drug in in vitro dissolution studies and solubility determinations using FeSSGF was to obtain a stable analytical sample (dissolution medium containing the API). Unlike most dissolution studies, simple filtration techniques alone do not work on samples obtained from the dissolution medium containing milk or any other comparable fluids (20,21,22,23,24). Therefore, the samples withdrawn from the dissolution medium needed extra steps for processing before quantitation of ibuprofen and ketoconazole.
The objective of this study was to develop and demonstrate applications of a standard working practice for processing dissolution samples collected during dissolution testing in FeSSGF. Having access to such a robust method will enable in vitro product performance assessments under conditions simulating in vivo-fed conditions. A step-wise approach was followed. The first aim was to study solubility of the two model drug substances (ibuprofen and ketoconazole) and in vitro dissolution of their immediate release (IR) tablets in the standard FeSSGF, USP compendial medium (0.1 N HCl and pH 7.2 phosphate buffer), and in pH 5 acetate buffer. The second aim of the study was to develop the method and approaches that will ensure high recovery (95% or better) of ibuprofen and ketoconazole during solubility and dissolution testing in FeSSGF. The third aim was to develop and validate a robust and efficient HPLC method for quantifying the study model drugs.
MATERIALS AND METHODS
To test the robustness, repeatability, and effectiveness of the sample preparation technique, high-performance liquid chromatography method with ultraviolet-visible detectors (HPLC-UV) methods were developed and dissolution studies in fed-state gastric conditions were performed by two analysts.
Standards and Drug Substances
All active pharmaceutical ingredient (API) standards used for method development and validation were of analytical grade. Ibuprofen (batch No. LRAA8931) and ketoconazole (batch No. LRAA9173) were procured from Sigma-Aldrich Corporation (St. Louis, MO, USA). All API standards were secondary standards traceable to USP, PhEUR, and BP and came with a certificate of analysis confirming that they met product specification and had a purity of > 99%. The drug substances, ibuprofen and ketoconazole, were purchased from Fagron Chemicals (St. Paul, MN, USA). Table I lists the physicochemical properties of both APIs that might affect chromatographic performance (25,26,27,28). Chemical structures of ibuprofen and ketoconazole are given in Fig. 1 (25), respectively.
Drug Products
Commercially available ibuprofen tablets (200 mg) and ketoconazole tablets (200 mg) were purchased from Wellcure Pharmacy (Audobon, NJ, USA). The products studied are ibuprofen tablets (Major Pharmaceuticals, Livonia, MI, USA; lot number: P97327, expiration date: June 2018) and ketoconazole tablets (Teva Pharmaceuticals, Sellersville, PA, USA; lot number: 30227503A, expiration date: May 2017).
Reagents
Glacial acetic acid was purchased from Merck KGaA (Darmstadt, Hesse, Germany), sodium acetate and sodium chloride were purchased from ThermoFisher Scientific (Waltham, MA, USA), and hydrochloric acid was purchased from AppliChem GmbH (Darmstadt, Hesse, Germany). HPLC-grade acetonitrile and orthophosphoric acid for HPLC analysis were purchased from VWR Chemicals (Fontenay-sous-Bois, France). Water for mobile phases and sample preparation was obtained from a Milli-Q Reference Ultrapure water purification system from Merck KGaA ((Darmstadt, Hesse, Germany) and filtered through a EMD Millipore Durapore 0.22-μm pore-size polyvinylidene fluoride (PVDF) membranes (hydrophilic) filter obtained from MilliporeSigma (Burlington, MA, USA). Whole milk ultra heat treated (UHT) was purchased from Parmalat USA Corp (Wallington, NJ, USA). All other compounds were of analytical grade and sourced from commercial suppliers.
FeSSGF Dissolution Sample Treatment
Different techniques including filtration, centrifugation, pH adjustment, use of organic solvents, and/or some combination of these techniques were studied to successfully extract the drug from the complex FeSSGF dissolution medium. Samples (1.5 mL) withdrawn from the dissolution medium were filtered using 10 μm cellulose filter (Distek Inc., New Brunswick, NJ, USA), and the filtered samples were centrifuged at 4000 rpm for 15 min using tabletop centrifuge (Model TJ-6, Beckman Instruments Inc., Palo Alto, CA, USA). An aliquot (750 μL) of the filtered sample obtained from FeSSGF and pH 5 acetate buffer dissolution medium was then mixed with 750 μL of acetonitrile either as is for ketoconazole or after it is made alkaline for ibuprofen. Samples were centrifuged at 4000 rpm for 15 min. After centrifugation, the supernatant liquid was analyzed using HPLC.
Analytical Method Development and Validation
High Performance Liquid Chromatography Method with Ultraviolet-Visible Detectors Instrumentation
Method development and validation were performed, separately for ibuprofen and ketoconazole, using Waters HPLC-UV system (Waters Corporation, Milford, MA, USA), consisting of a 2690 separation module providing quaternary solvent, high-performance solvent delivery, integral helium sparge for solvent conditioning, integral plunger seal-wash system, 120-vial capacity sample management system with sample heater/cooler, an automatic injector equipped with a 100-μL loop, a thermostated column compartment, and a 2487 UV/Visible detector/2998 photodiode Array (PDA) detector. System control, data acquisition, and integration were accomplished with the Empower 3 software (Waters Corporation).
Chromatographic Separation and Conditions for Ibuprofen Sample Analysis
The analytical method for quantitation of ibuprofen was developed and validated. Chromatographic separation of the analytes in the dissolution sample of ibuprofen tablet was achieved on a 150 × 4.6 mm and 5 μm particle size, Zorbax SB RP-18 column (Agilent Technologies Deutschland GmbH, Waldbronn, Germany). HPLC-UV analyses were performed under isocratic conditions using optimized solvent ratio and chromatographic conditions as described in Tables II and III. The chromatographic run time to provide acceptable resolution was determined to be 10 min.
Chromatographic Separation and Conditions for Ketoconazole Sample Analysis
The analytical method for quantitation of ketoconazole was developed and validated. Chromatographic separation of the analytes in the dissolution sample of ketoconazole tablet was achieved on a 150 × 4.6 mm and 5 μm particle size Zorbax XDB RP-18 column (Agilent, Germany). Isocratic HPLC analyses were carried out using optimized solvent ratio and chromatographic conditions as described in Tables II and III. pH 3.3 phosphate buffer was prepared by adding 0.075% (v/v) phosphoric acid and 0.015% triethylamine in water and the pH adjusted to 3.3 using 0.01 N sodium hydroxide. The chromatographic run time to provide acceptable resolution was determined to be 8 min.
Prior to sample analysis, the HPLC-UV system was equilibrated with the mobile phase for 30 min. All mobile phase components were filtered through a 0.22-μm polyethersulfon filter (Millipore Express Plus, Merck KGaA, Darmstadt, Germany) before use.
Analytical Method Validation
The developed and validated HPLC-UV method successfully separates the BRM components from the analyte peak and was performed based on the International Conference on Harmonization (ICH-Q2(R1)) (29) and United States Pharmacopeia (USP) validation guidelines, USP General Chapters — <1225> and <1092> (30,31). The validated parameters include linearity and range, accuracy, precision, limit of detection (LOD), limit of quantification (LOQ), specificity, system suitability, and robustness. The acceptance criteria for validation parameters are included in Table IV. The HPLC-UV method was developed for quantifying the ibuprofen and ketoconazole dissolved from the IR tablets in FeSSGF at predetermined sampling times. The validated concentration range for ibuprofen and ketoconazole was from 25.0 to 120.0% of the maximum expected analyte concentration upon the complete dissolution of a tablet. The developed analytical method was validated over 100–1000.00 μg/mL for both analytes.
System suitability was performed by determining the tailing factor (T), retention factor (k), number of theoretical plates (N), height equivalent to the theoretical plate (HETP), resolution of the respective analytes, and the reproducibility of peak areas and retention times. The % relative standard deviation (RSD) for system suitability of peaks areas and retention times should be < 2.0% (31). The k value should be between 1 and 10 (32).
Linearity was established by plotting the absorbance (as determined from the chromatographic peak areas) obtained from different concentrations of the analyte versus the corresponding concentrations followed and further by least squares linear regression and calculation of the slope, intercept, and coefficient of determination. Measurements were performed in triplicates. The R-squared value must be ≥ 0.9990 (31).
Accuracy, expressed as mean absolute recovery and percent relative standard deviation (% RSD), for all analytes was assessed in triplicate for 80.0, 100.0, and 120.0% of the maximum expected analyte concentration. The % RSD for all the levels must be < 2.0% (31).
The precision of the assay method was expressed as repeatability. Precision was validated by injecting six individual samples at 100.0% concentration for each drug. The % RSD must be < 2.0% (31).
Specificity, which is an essential part of method validation, was assessed as follows. First, a set of standard solutions (API reference material dissolved in a simple solvent or dissolution medium) were prepared using each API. Different standard solutions were prepared using simple solvents (e.g., acetonitrile/water) and the compendial media and FeSSGF BRM listed in Table III. Following sample analysis, the chromatograms obtained were examined for peak area and interference from excipients or any other sources at the API retention times.
Signal-to-noise (S/N) ratio was used to calculate LOD and LOQ for each drug using Empower 3 software (Waters Corporation). In the current work, the ratio was found by comparing the peak height values of analyte and comparing it the measured signals of blank samples and establishing the minimum concentration at which the analyte can be reliably detected and quantified. A signal-to-noise ratio greater than 3:1 or 2:1 is usually considered acceptable for determining the LOD and a signal-to-noise ratio of 10:1 or greater for LOQ (29).
As described in “FeSSGF Dissolution Sample Treatment” method section, the dissolution samples were filtered and diluted with ACN in 1:1 ratio. Therefore, it was important to determine the stability of drug in a solution mixture of ACN and FeSSGF at 1:1. The solution stability in the current research work refers to the stability of drug in the mentioned solution mixture for up to 24 h at ambient temperature (25°C). Solution stability was determined by preparing a 100.0% of the maximum expected analyte concentration in the mobile phase and the FeSSGF medium and sampled and analyzed using HPLC-UV method at 0, 6, 12, and 24 h timepoint. The % recovery of drug must be > 95.0%, and the % RSD of peak area at different timepoints and initial timepoint should be < 2.0%.
While setting up the HPLC-UV sample injection sequence, the check standards were injected after every ten injections and five standards with different concentrations were randomly distributed into the routine dissolution sample sequence for analysis (determination of repeatability of the measurements, including showing, i.e., lack of chromatographic interference from the previously measured samples) to track the performance of the system and ensure the accuracy of the unknown samples being analyzed.
Dissolution Media Preparation and Surface Tension Measurements
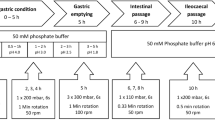
FeSSGF was prepared according to directions provided in Jantratid et al. (4,17,33). pH 5 acetate buffer was prepared by adding 0.57 g of acetic acid, 1.36 g of sodium acetate, and 8.87 g of sodium chloride into 500 mL of the purified water. Four hundred eighty milliliters of the prepared acetate buffer was mixed with 500 mL of milk (“heat-treated” and homogenized milk containing 3.5% fat) to form a mixture. The pH of the mixture was adjusted to 5.0 using 0.1 N HCl with continuous stirring. The volume was made up to 1000 mL using pH 5 acetate buffer. Phosphate buffer (pH 7.2) was prepared by adding 5.78 g of potassium phosphate monobasic to 1000 mL purified water and pH was adjusted to 7.2 using 0.2 M sodium hydroxide. 0.1 N HCl was prepared by adding 10 mL of 10 N hydrochloric acid to 1000 mL purified water (24). FeSSGF could be stored for not more than 2 days at 2–8°C.
A force Tensiometer (K6, KRUSS, Germany) based on the du nouy ring method was used to determine the surface tension of the dissolution medium at room temperature each time FeSSGF was prepared. The instrument has a sensitive micro-balance and precise mechanics to vertically move the sample liquid in a glass beaker. The platinum ring was used for the measurements. The du nouy ring was first immersed into the dissolution media in a vessel, and then the vessel height was adjusted manually until the ring is just below the surface of dissolution media. For measurement purposes, the vessel is lowered, which forms a meniscus which pulls the hang-down wire. The point, at which ring loses contact with the dissolution media, was registered as a deflection on a scale calibrated for the direct measurement of surface tension in dyne/cm, which was visually observed and recorded. The accuracy of the du nouy ring used in surface tensiometer was verified by measuring the surface tension of water (72 millinewton per meter (mN/m)) (34).
Solubility and In Vitro Dissolution Studies
Equilibrium Solubility
An accurately weighed drug substance (700 mg) was transferred into a 100-mL glass bottles containing 50 mL of FeSSGF. The solubility studies were performed at room temperature and 37°C using magnetic stirrer by placing the cap on the glass bottle. The agitation was continued for 24 h at 400 rpm. Solubility studies were performed in triplicates at each condition. The concentrations of the drug substance from the solubility studies were analyzed with necessary dilutions such that the unknown concentrations fall within the established linearity range and were determined by the validated HPLC-UV method. The final pH at the end of equilibrium solubility was also measured.
In Vitro Dissolution Test
The USP apparatus II (paddle method) containing 900 or 500 mL of dissolution medium (compendial or biorelevant), maintained at 37 ± 0.5°C and stirred at 75 rpm, was used to dissolve 12 tablets per experiment by two analysts on two occasions (each occasion, n = 6 units). The aliquots were withdrawn using an autosampler (EVOLUTION 4300, Distek inc) at 10, 20, 30, 45, 60, and 75 min (75 min was the infinity time point) for the 12 units tested. The paddle speed was increased to 150 rpm after 60 min. Samples were filtered through a syringe membrane filter (10 μm cellulose filter) and further processed as described in dissolution sample treatment method for further analysis. A 1.5-mL of sample was withdrawn at each time point without replacement from the dissolution medium consisting of FeSSGF or USP compendial medium (pH 7.2 phosphate buffer for ibuprofen and 0.1 N HCl for ketoconazole) or pH 5 acetate buffer.
The dissolution data were also studied for inter-occasion (Day 1 vs. Day 2) repeatability by comparing the mean dissolution profiles obtained from six experiments (n = 6 units) carried out by each analyst per occasion. Comparison of mean dissolution profiles based on the data generated by two analysts from 12 experiments (i.e., n = 12 units in 12 vessels), explores repeatability of results when the experiments are carried out by different analysts. It is important to note that inherent differences in these experiments are acknowledged; the two analysts performed the 12 of experiments on two different occasions (i.e., n = 6 per occasion).
RESULTS AND DISCUSSION
The top three considerations in the current work were ensuring stability of the analytes during dissolution testing and assay, preventing interactions and interferences, and optimizing recovery. This was accomplished firstly, by developing a technique to process samples obtained during dissolution testing, secondly, developing and validating quantitative HPLC-UV methods for the collected FeSSGF medium samples containing ibuprofen and ketoconazole, and lastly by performing the dissolution studies and solubility determinations in FeSSGF and pH 5 acetate buffer.
Method Development and Optimization of the FeSSGF Sample Preparation Technique of the Dissolution Samples
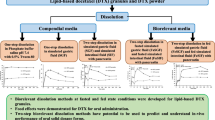
For each drug, a separate sample preparation technique was required, although there is always a dependence of the HPLC-UV method on the extraction conditions (and vice versa). In addition to this, the sample preparation is very sensitive to the FeSSGF medium due to presence of milk components along with the excipients present in the tablets. The literature indicated the availability of sample preparation techniques that required use of different methods that include diafiltration, high heat treatment, calcium chloride addition, liquid-liquid extraction, using rotary evaporator, and low-temperature ultra-centrifugation (21,23,35). However, these methods are very lengthy, complex, time consuming, and involved multiple steps. The main purpose of this work was to develop a new sample preparation technique, which is more precise, robust, reproducible, accurate, simple, and time efficient, to quantitatively extract the drug from the dissolution medium containing milk proteins and drug product excipients with optimal recovery. In addition, for the new method, achieving drug recoveries greater than 95.0% of label claim was targeted (29,36). To quantify the extracted drug, the HPLC-UV method discussed in the previous section was employed.
System suitability for the peak areas (% RSD) was less than 2.0%, and linearity (R2 values) was greater than 0.9990 for the standards injected within 90 min of the standard preparation in FeSSGF using only 10 μm filter. However, when the same standards were injected after 6 h, the % RSD of peak area was 8.03% and R2 values for linearity of the same standards were 0.96. The possible reason for the observed changes may be attributed to the instability and/or formation of complexes of milk components (e.g., proteins and fats). It should be noted that proteins and fats also have binding tendencies that may either make the drug unavailable for analysis or it may result in degradation of the analytes of interest.
Attempts were then made to pre-process standards in FeSSGF to improve the system suitability and R2 of the HPLC-UV analysis method. Filters starting with the smallest pore size (0.10, 0.45, and 1.20 μm) were tested sequentially to get rid of what appeared as complexation of dissolved and undissolved drug substance, excipients with the solid milk components present in FeSSGF. The initial filtering attempts were unsuccessful because the dissolution samples were viscous compared with FeSSGF as the dissolution medium, likely due to complex/agglomerate formation from protein denaturation and solidifying milk fat.
Therefore, the samples were filtered using 10 μm filter followed by centrifugation at 4000 rpm for 15 min, so as to sediment any undissolved drug particles. The supernatant liquid was then treated with acetonitrile to precipitate out the milk protein and the solidified fat. The mixture was then centrifuged again for 15 min at 4000 rpm to separate the drug from the denatured milk protein and unwanted solid contents. The supernatant liquid was subsequently transferred into 2 mL HPLC glass vials for analysis/quantitation. The supernatant liquid was clear in appearance; however, it did contain some dissolved lipids, which were successfully separated from drug during HPLC method development (Figs. 2 and 3). This method was successful for ketoconazole, and the linearity, accuracy, precision, system suitability, and solution stability were all within the acceptance criterion. However, the linearity and system suitability criteria were not met for ibuprofen. Reviewing the physico-chemical properties of the ibuprofen, it was found that the pKa of ibuprofen is 4.8, while the pH of FeSSGF was 5.0. To rule out the possibility of the partial unionization of ibuprofen during sample extraction, the pH of the aqueous acetonitrile mixture supernatant liquid was raised to 9.0 using 0.1 N NaOH and then mixed with the FeSSGF dissolution samples (pH 5.0). Final pH of the solution was determined to be 7.2 ensuring the 100.0% ionization of the drug. Using this sample preparation method for the ibuprofen dissolution samples, linearity, accuracy, precision, system suitability, and solution stability were all within the acceptance criteria. Following is the step-by-step procedure for processing samples obtained from FeSSGF dissolution studies:
-
a.
A 1.5-mL sample withdrawn at each time point from 500 mL FeSSGF dissolution medium using autosampler was filtered using 10 μm filter and centrifuged at 4000 rpm for 15 min. Centrifugation allows sedimentation of undissolved solids in the withdrawn sample.
-
b.
Of the filtered and centrifuged sample, 750 μL was taken in 2 mL HPLC vial.
-
c.
For ibuprofen dissolution samples, 750 μL of aqueous ACN (adjusted to pH 9.0 with 0.1 N NaOH) was added to 750 μL filtered sample in HPLC vial. For ketoconazole dissolution samples, 750 μL of ACN was added to 750 μL filtered sample in HPLC vial.
-
d.
The vial was shaken well to ensure uniform dispersion of solution. (At this point, most of the milk proteins will precipitate out and some remain suspended in the solution).
-
e.
The HPLC vial was subjected to centrifugation for 15 min at 4000 rpm
-
f.
The supernatant that was a clear liquid in the HPLC vials was used for analysis.
HPLC chromatogram of ibuprofen a dissolution sample in FeSSGF at 75 min (after sample treatment), b standard (400 μg/mL) in FeSSGF (after sample treatment), c ibuprofen standard (400 μg/mL) in FeSSGF (before treatment), d ibuprofen standard (400 μg/mL) in pH 5.0 buffer, e ibuprofen standard (400 μg/mL) in pH 7.2 USP buffer, and f FeSSGF (blank—after sample treatment)
HPLC chromatogram of ketoconazole a dissolution sample in FeSSGF at 75 min (after sample treatment), b standard (60 μg/mL) in FeSSGF (after sample treatment), c standard (60 μg/mL) in FeSSGF (before treatment), d standard (60 μg/mL) in pH 5.0 buffer, e standard (60 μg/mL) in 0.1 N HCl USP buffer, and f FeSSGF (blank—after sample treatment)
Optimizing the HPLC-UV Method Development and Validation for Drug Content Analysis
HPLC-UV Method Development
USP monographs for ibuprofen and ketoconazole recommends using traditional UV-visible spectroscopic methods for quantification of drug content in dissolution samples; however, the presence of BRM components and excipients made UV methods unusable as published. Despite the use of the USP-recommended HPLC-UV method for assay/drug content analysis, complete separation of BRM components from the main API peak could not be achieved for either product studied (i.e., ibuprofen and ketoconazole). There are a large number of HPLC-UV methods available for the detection and quantification of ketoconazole and ibuprofen (37,38,39,40); however, the drug products were always tested in the USP compendial medium (14). As discussed in the “INTRODUCTION,” interference from the milk components present in FeSSGF made it more challenging to quantify the compounds even with minor modifications to the published methods (31,35,38). Therefore, new HPLC-UV methods were developed for each compound to resolve the interfering dissolved milk components and the drugs. The purpose of the current study was to develop a cost- and time-efficient chromatographic method with the acceptable performance and suitable analyte recoveries by taking into consideration the following parameters: maintaining physical and chemical integrity of its stationary phase upon exposure to a wide range of pH ranges (pH 1.6 to 6.5 covering the pH range observed with fasted and fed gastric and intestinal biorelevant media), components of biorelevant media (such as bile salts, lecithin, etc.), milk proteins, and analyte peak resolution.
Reverse-phase chromatography using C18 column was employed and drug and BRM component peak resolution was achieved by optimizing the ratio of aqueous to organic solvent in the mobile phase. For method development optimization and selection of the method, criterion for USP Symmetry factor (USP tailing) at less than 2.0%, number of theoretical plates at more than 4000, and % RSD for peak areas at less than 2.0% were targeted. Based on the physicochemical properties of the APIs, the isocratic separation needs to begin with aqueous mobile phase progressing to more of the organic solvents. For the analysis of ibuprofen, 0.1% (v/v) phosphoric acid was chosen as aqueous solvent (solvent A), while acetonitrile was selected as organic solvent (solvent B). For the analysis of ketoconazole, pH 3.30 phosphate buffer was used as aqueous phase (solvent A) and acetonitrile was selected as organic solvent (solvent B). Different ratios of mobile phase (aqueous to organic) were studied for both drugs. The ratio, at which desired analyte peak resolution from interfering milk components was achieved, was selected for further analysis. Table II shows the optimized mobile phase composition for each drug.
In addition to the mobile phase ratio optimization, injection volume, flow rate, column temperature, and detection wavelength were optimized, to demonstrate system suitability in terms of symmetry factor, retention factor, number of plates, and peak area (% RSD). Finalized injection volume, column temperature, and detection wavelength are detailed in Table III. The developed HPLC-UV methods for ibuprofen and ketoconazole were further validated.
HPLC-UV Method Validation Results
System Suitability
Primary parameters to evaluate system suitability including the symmetry factor, retention factor, and number of theoretical plates were determined for a 100.0% of target concentration for each analyte (Table IV). Both ibuprofen and ketoconazole showed excellent peak symmetry. Moreover, the analyte peaks showed consistent low variability in peak area and retention time.
Linearity
The linear relationship of drug concentrations and peak areas is expressed by the coefficient of determination (R2). Linearity for both compounds over the concentration ranges stated earlier was acceptable (intraday and interday) with all R2 values greater than 0.9990 (see Table IV; Fig. 4).
Accuracy, Precision
Results for accuracy and precision are presented in Table IV. For each drug analyte, a consistent and high absolute recovery and low % RSD within the acceptance limit of ± 2.0% of 100.0% drug recovery of label claim was demonstrated at three concentration levels (high, medium, and low). The % RSD results for repeatability were within the acceptance limit of ± 2.0% of 100.0% drug recovery and thus regarded as acceptable.
Specificity
The specificity of the method was evaluated by analyzing blank solvents/media and then samples containing the drug standard in the FeSSGF media. The method showed good chromatographic separation of the drug and milk components. No peak interference with the drug from the blank media, dissolved BRM components, or excipients of the marketed products was observed. Figure 2 shows the chromatogram of FeSSGF using the ibuprofen HPLC-UV method, standard solution of ibuprofen in FeSSGF, and dissolution sample of ibuprofen in FeSSGF. Figure 3 shows a chromatogram of FeSSGF using the ketoconazole HPLC-UV method, a standard solution of ketoconazole in FeSSGF, and a dissolution sample of ketoconazole in FeSSGF. The retention times were 3.86 and 1.89 min for ibuprofen and ketoconazole, respectively. The dissolved BRM components were observed between retention times of 0.70 and 1.30 min as multiple peaks. These peaks were well resolved and did not interfere with the API peaks (Figs. 2 and 3).
LOD and LOQ
The LOD and LOQ for each drug are expressed as lowest detectable and low quantifiable concentrations for each drug. LOD and LOQ for both compounds were acceptable with all signal-to-noise ratio (S/N) greater than 3:1 and 10:1, respectively. The S/N and concentrations at LOD and LOQ are reported in Table IV, and HPLC chromatograms are shown in Fig. 5.
Solution Stability in ACN and FeSSGF Mixture
Stability in solution (ACN:FeSSGF in 1:1 ratio) was evaluated by the standard solution of ibuprofen and ketoconazole, respectively. The responses were measured in terms of percent recovery at the end of 24 h and evaluated by comparison with freshly prepared solutions. For ibuprofen, the % recovery at the end of 24 h was quantified to be 99.33 ± 0.51%, and for ketoconazole, % recovery was quantified to be 99.18 ± 0.61. Both compounds were determined to be stable in FeSSGF media for up to 24 h at ambient temperature (25°C), therefore, the dissolution studies were planned in such a manner that the dissolution samples could be analyzed within 24 h.
The HPLC-UV method developed using a reverse phase column with the optimized isocratic separation method demonstrated excellent chromatographic separation of ibuprofen and ketoconazole in the presence of complex matrices (including the excipients and ingredients from BRM) within a relatively short (< 10 min) overall run time. The method was validated in accordance with ICH guidelines (ICH-Q2(R1)) and USP General Chapters <1225> and <1092> (29,30,31), producing a robust, efficient, and reliable method that can be extended to the content uniformity, assay, and drug-release studies of drug products in all BRM and compendial media, including low-volume (as low as 40 mL) dissolution studies.
Surface Tension Measurements
Measurements of surface tension each time after preparation of dissolution media was used as a quality control test to ensure that a similar medium is used through all the in vitro dissolution studies. It was reported that the surface tension of the BRM provides information about the physical stability of the medium (41). The surface tension of freshly prepared FeSSGF was between 43 and 45 mN/m for all samples tested at RT. This is in the range of the surface tension of human gastric fluid, which has been reported as ranging from 35 to 50 mN/m (17,41,42).
Solubility and In Vitro Dissolution Studies
Equilibrium Solubility
Solubility is one of the major parameters in drug discovery and product development. The model test drugs used in the current research are BCS Class II drugs; therefore, it was important to determine the maximum concentration each drug can reach in FeSSGF. The equilibrium solubility of ibuprofen and ketoconazole was determined in FeSSGF. Solubility of ibuprofen in FeSSGF was found to be 1895.46 μg/mL (± 0.52) at RT and 1995.26 μg/mL (± 0.63) at 37°C. Solubility of ketoconazole in FeSSGF was found to be 195.03 μg/mL (± 3.40) at RT and 395.96 μg/mL (± 1.65) at 37°C. The pH measured for ibuprofen-saturated solution at 24 h was 4.73 and that of ketoconazole was pH 5.32.
In Vitro Dissolution Studies
With the development of the sample preparation technique and the validated HPLC-UV methods for ibuprofen and ketoconazole complete, the in vitro dissolution studies were initiated. To study the effect of BRM components on the release profiles, the drug products were tested in FeSSGF, in pH 5 acetate buffer, and in USP dissolution medium (0.1 N HCl for ketoconazole tablets and pH 7.2 phosphate buffer for ibuprofen tablets).
Dissolution profiles generated by different research analysts were also compared to test the reproducibility and repeatability of dissolution test results. Additional data analysis was further carried out to understand the contribution of multiple factors on dissolution test results. As observed in Figs. 6 and 7, dissolution profiles obtained by the two research analysts were superimposable. The theoretical target concentrations for 200 mg dose were 400.00 μg/mL in 500 mL.
Dissolution in FeSSGF Versus pH 5 Acetate Buffer Versus USP Buffer
In vitro dissolution experiments on the marketed ibuprofen and ketoconazole products were performed in: (a) 0.1 N HCl or in pH 7.2 phosphate buffer as specified in the drug product monographs, (b) pH 5 acetate buffer, and (c) in FeSSGF. The main objective of including dissolution testing results in compendial media (0.1 N HCl for ketoconazole and pH 7.2 phosphate buffer for ibuprofen) in this study was to assess differences in dissolution profiles in the compendial media versus the BRM and whether dissolution testing in one medium may be more predominantly influenced by solubility and likely to be less influenced by the drug product attributes. Approximately 80% or more of the label claim of ibuprofen and ketoconazole was dissolved by 30 min when dissolution testing was performed in the compendial media (i.e., solubility of ibuprofen is approximately 3400 μg/mL in pH 7.2 buffer and approximately 20,000 μg/mL for ketoconazole in 0.1 N HCl (35,43)). Ibuprofen and ketoconazole dissolution profiles in the compendial media show that the primary effect influencing the dissolution results is their solubility in the compendial medium. Because they display pH-dependent solubility, contribution of other factors, if they were to be detected, has to be of comparable magnitude with their solubilization in 0.1 N HCl for ketoconazole and pH 7.2 phosphate buffer for ibuprofen. Their dissolution in BRM is more gradual and may better reflect in vivo dissolution of the drug products.
Furthermore, pH 5 acetate buffer was used to study the differences in the drug release profile of the drug product in the presence and absence of FeSSGF components under similar pH conditions. The percent drug released from ibuprofen tablets after 45, 60, and 75 min was comparable, and it was the highest in the pH 7.2 USP buffer compared with the other medium (Fig. 6).
The mean (n = 12) percentage of ibuprofen dissolved at 60 min in 900 mL of pH 7.2 phosphate buffer (compendial medium) was 107.02 and 95.24% in 500 mL of pH 5 acetate buffer and 89.10% in 500 mL FeSSGF (Fig. 6). The similar trend that is the highest percentage of ketoconazole dissolved was in 0.1 N HCl (compendial medium) compared with the other medium (Fig. 7). The mean (n = 12) percentage of ketoconazole dissolved at 60 min in 900 mL USP compendial medium (0.1 N HCl) was 95.83 and 25.38% in 500 mL of pH 5.0 acetate buffer and 23.82% in 500 mL of FeSSGF (Fig. 7).
Inter-occasion and Repeatability
The inter-occasion differences including repeatability of dissolution testing by the two analysts were evaluated to assess the robustness of the methods for both ibuprofen and ketoconazole tablets in FeSSGF. The ibuprofen and ketoconazole dissolution profiles in FeSSGF obtained on two different occasions by two analysts were lower than the dissolution profiles obtained in pH 5 acetate buffer. The two ibuprofen and ketoconazole profiles (mean, n = 12/analyst) in FeSSGF are almost superimposable for the respective products, supporting reproducibility of the results even when solubility and dissolution characteristics of each API is significantly different in FeSSGF. The methods are validated for each drug and include sample preparation, extraction, handling and the HPLC-UV quantitation techniques for the drugs used in the current research work.
CONCLUSION
There has been significant progress in the past several years in predicting the in vivo performance of the solid oral dosage forms based on dissolution testing. Dissolution testing in BRM serves as a viable tool to study both water soluble and poorly water-soluble drugs. FeSSGF components pose some unique challenges for both preparation of samples for assay and quantification of the dissolved API during dissolution testing, but these challenges may be overcome using the sample preparation techniques and methods as described in this report. The sample preparation techniques demonstrated that the selection of pH is crucial to enable consistent drug ionization and also that the use of organic solvents for precipitating the milk components (e.g. carbohydrate (lactose), fat, and protein) from the FeSSGF can produce samples which are analyzable by HPLC-UV. These methods allowed rapid sample preparation, reproducible high recoveries and quantification of ibuprofen and ketoconazole by HPLC-UV without interference from either the excipients or the ingredients of the FeSSGF. These techniques may serve as a workflow outline for BRM dissolution testing and should require only minor modifications based on the physico-chemical properties of the drug, excipients, and variations in the BRM components.
References
Williams HD, Trevaskis NL, Charman SA, Shanker RM, Charman WN, Pouton CW, et al. Strategies to address low drug solubility in discovery and development. Pharmacol Rev. 2013;65(1):315–499.
Di L, Fish PV, Mano T. Bridging solubility between drug discovery and development. Drug Discov Today. 2012;17(9–10):486–95.
Di L, Kerns EH, Carter GT. Drug-like property concepts in pharmaceutical design. Curr Pharm Des. 2009;15(19):2184–94.
Klein S. The use of biorelevant dissolution media to forecast the in vivo performance of a drug. AAPS J. 2010;12(3):397–406.
Dressman JB, Reppas C. In vitro–in vivo correlations for lipophilic, poorly water-soluble drugs. Eur J Pharm Sci. 2000;11:S73–80.
Bou-Chacra N, Melo KJC, Morales IAC, Stippler ES, Kesisoglou F, Yazdanian M, et al. Evolution of choice of solubility and dissolution media after two decades of biopharmaceutical classification system. AAPS J. 2017;19(4):989–1001.
Bhagat NB. A review on development of biorelevant dissolution medium. Journal of Drug Delivery and Therapeutics. 2014;4(2):140–8.
Nicolaides E, Symillides M, Dressman JB, Reppas C. Biorelevant dissolution testing to predict the plasma profile of lipophilic drugs after oral administration. Pharm Res. 2001;18(3):380–8.
Vertzoni M, Dressman J, Butler J, Hempenstall J, Reppas C. Simulation of fasting gastric conditions and its importance for the in vivo dissolution of lipophilic compounds. Eur J Pharm Biopharm. 2005;60(3):413–7.
Shono Y, Jantratid E, Janssen N, Kesisoglou F, Mao Y, Vertzoni M, et al. Prediction of food effects on the absorption of celecoxib based on biorelevant dissolution testing coupled with physiologically based pharmacokinetic modeling. Eur J Pharm Biopharm. 2009;73(1):107–14.
Takács-Novák K, Szőke V, Völgyi G, Horváth P, Ambrus R, Szabó-Révész P. Biorelevant solubility of poorly soluble drugs: rivaroxaban, furosemide, papaverine and niflumic acid. J Pharm Biomed Anal. 2013;83:279–85.
Kalantzi L, Goumas K, Kalioras V, Abrahamsson B, Dressman JB, Reppas C. Characterization of the human upper gastrointestinal contents under conditions simulating bioavailability/bioequivalence studies. Pharm Res. 2006;23(1):165–76.
Fang JB, Robertson VK, Rawat A, Flick T, Tang ZJ, Cauchon NS, et al. Development and application of a biorelevant dissolution method using USP apparatus 4 in early phase formulation development. Mol Pharm. 2010;7(5):1466–77.
Galia E, Nicolaides E, Hörter D, Löbenberg R, Reppas C, Dressman J. Evaluation of various dissolution media for predicting in vivo performance of class I and II drugs. Pharm Res. 1998;15(5):698–705.
Jantratid E, De Maio V, Ronda E, Mattavelli V, Vertzoni M, Dressman JB. Application of biorelevant dissolution tests to the prediction of in vivo performance of diclofenac sodium from an oral modified-release pellet dosage form. Eur J Pharm Sci. 2009;37(3–4):434–41.
Sunesen VH, Pedersen BL, Kristensen HG, Müllertz A. In vivo in vitro correlations for a poorly soluble drug, danazol, using the flow-through dissolution method with biorelevant dissolution media. Eur J Pharm Sci. 2005;24(4):305–13.
Jantratid E, Dressman J. Biorelevant dissolution media simulating the proximal human gastrointestinal tract: an update. Dissolut Technol. 2009;16(3):21–5.
Early R. Dairy products and milk-based food ingredients. Natural food additives, ingredients and flavourings: Elsevier; 2012. p. 417–45.
Klein S, Dressman JB, Butler J, Hempenstall JM, Reppas C. Media to simulate the postprandial stomach. I. Matching the physicochemical characteristics of standard breakfasts. J Pharm Pharmacol. 2004;56(5):605–10.
Cluskey F, Thomas E, Coulter S. Precipitation of milk proteins by sodium carboxymethylcellulose1. J Dairy Sci. 1969;52(8):1181–5.
Maubois J. Separation, extraction and fractionation of milk protein components. Lait. 1984;64(645–646):485–95.
Fríguls B, Joya X, García-Algar O, Pallás C, Vall O, Pichini S. A comprehensive review of assay methods to determine drugs in breast milk and the safety of breastfeeding when taking drugs. Anal Bioanal Chem. 2010;397(3):1157–79.
Diakidou A, Vertzoni M, Dressman J, Reppas C. Estimation of intragastric drug solubility in the fed state: comparison of various media with data in aspirates. Biopharm Drug Dispos. 2009;30(6):318–25.
Shah H, Shah V, Parikh D, Butani S, Mehta T. Dissolution improvement of nebivolol hydrochloride using solid dispersion adsorbate technique. Asian Journal of Pharmaceutics (AJP): Free full text articles from Asian J Pharm. 2015;9(1):49–55.
Kim S, Thiessen PA, Bolton EE, Chen J, Fu G, Gindulyte A, et al. PubChem substance and compound databases. Nucleic Acids Res. 2015;44(D1):D1202–D13.
Davanzo R, Bua J, Paloni G, Facchina G. Breastfeeding and migraine drugs. Eur J Clin Pharmacol. 2014;70(11):1313–24.
Gillespie W, DiSanto A, Monovich R, Albert K. Relative bioavailability of commercially available ibuprofen oral dosage forms in humans. J Pharm Sci. 1982;71(9):1034–8.
Huang Y, Colaizzi J, Bierman R, Woestenborghs R, Heykants J. Pharmacokinetics and dose proportionality of ketoconazole in normal volunteers. Antimicrob Agents Chemother. 1986;30(2):206–10.
ICH Q2 (R1): validation of analytical procedures: text and methodology. International Conference on Harmonization, Geneva; 2005.
United States Pharmacopeia, USP 39-NF34. General chapter on the dissolution procedure: development and validation <1092>. United States Pharmacopeial Convention Rockville, MD; 2016.
United States Pharmacopeia, USP 39–NF34. General chapter on validation of compendial procedures < 1225>. United States Pharmacopeial Convention Rockville, MD; 2016.
Crawford Scientific. The theory of HPLC chromatographic parameters. Available from https://www.chromacademy.com/lms/sco2/Theory_Of_HPLC_Chromatographic_Parameters.pdf.
Dressman JB, Amidon GL, Reppas C, Shah VP. Dissolution testing as a prognostic tool for oral drug absorption: immediate release dosage forms. Pharm Res. 1998;15(1):11–22.
Vargaftik N, Volkov B, Voljak L. International tables of the surface tension of water. J Phys Chem Ref Data. 1983;12(3):817–20.
Ghazal HS, Dyas AM, Ford JL, Hutcheon GA. The impact of food components on the intrinsic dissolution rate of ketoconazole. Drug Dev Ind Pharm. 2015;41(10):1647–54.
United States Food and Drug Administration. Guidance for industry - bioanalytical method validation. Available from https://wwwfdagov/downloads/drugs/guidances/ucm070107pdf. 2018. Accessed 30 Nov 2019.
Savu SN, Silvestro L, Mircioiu C, Anuta V. Development of in vitro in vivo correlation models for clopidogrel tablets to describe administration under fasting and fed conditions. Therapy. 2016;11(16):18.
Farrar H, Letzig L, Gill M. Validation of a liquid chromatographic method for the determination of ibuprofen in human plasma. J Chromatogr B. 2002;780(2):341–8.
Velikinac I, Čudina O, Janković I, Agbaba D, Vladimirov S. Comparison of capillary zone electrophoresis and high performance liquid chromatography methods for quantitative determination of ketoconazole in drug formulations. Il Farmaco. 2004;59(5):419–24.
Valko K, Nunhuck S, Bevan C, Abraham MH, Reynolds DP. Fast gradient HPLC method to determine compounds binding to human serum albumin. Relationships with octanol/water and immobilized artificial membrane lipophilicity. J Pharm Sci. 2003;92(11):2236–48.
Jantratid E, Janssen N, Reppas C, Dressman JB. Dissolution media simulating conditions in the proximal human gastrointestinal tract: an update. Pharm Res. 2008;25(7):1663–76.
Jantratid E, Janssen N, Chokshi H, Tang K, Dressman JB. Designing biorelevant dissolution tests for lipid formulations: case example–lipid suspension of RZ-50. Eur J Pharm Biopharm. 2008;69(2):776–85.
Levis KA, Lane ME, Corrigan OI. Effect of buffer media composition on the solubility and effective permeability coefficient of ibuprofen. Int J Pharm. 2003;253(1–2):49–59.
Disclaimer
The approaches and conclusions reported in this publication have not been formally disseminated by the US Food and Drug Administration and should not be construed to represent any Agency determination or policy.
Funding
We gratefully acknowledge the US Food and Drug Administration (USFDA) and the National Institute for Pharmaceutical technology and Education (NIPTE) for financial support. This study was funded by the FDA Grant to NIPTE titled “The Critical Path Manufacturing Sector Research Initiative (U01)”; Grant No. 5U01FD004275.
Author information
Authors and Affiliations
Corresponding author
Additional information
Guest Editors: Ajaz S. Hussain, Kenneth Morris, and Vadim J. Gurvich
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shah, H.S., Sardhara, R., Nahar, K. et al. Development and Validation of Sample Preparation and an HPLC Analytical Method for Dissolution Testing in Fed-State Simulated Gastric Fluid—Illustrating Its Application for Ibuprofen and Ketoconazole Immediate Release Tablets. AAPS PharmSciTech 21, 172 (2020). https://doi.org/10.1208/s12249-020-01702-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-020-01702-3