Abstract
Background
The causality of overactive bladder syndrome (OAB) is still not fully understood. Several studies indicate a significant increase of prostaglandin E2 (PGE2) in patients with OAB. However, in order to clarify whether these compounds can help to objectify the clinical diagnosis, further studies are needed. This prospective study aims to analyze PGE2 blood levels (sPGE2) in patients with OAB before and after botulinum toxin type A (BoNT-A) therapy.
Methods
Blood samples were obtained from 56 patients (52y, 18–87) with idiopathic OAB. sPGE2 levels were measured before and 4 weeks after BoNT-A treatment by enzyme linked immunosorbent assay (ELISA). 31 healthy persons with normal bladder function served as control group (59 y, 21–72). sPGE2 was set in relation to clinical data and the severity of OAB (wet/dry). The statistical data analysis was performed by using the non-parametric Mann–Whitney U test and paired t-test.
Results
Significant higher sPGE2 levels were detected in patients with OAB compared to members of the control group (2750 pg/ml vs. 1674 pg/ml, p < 0.005). Furthermore sPGE2 levels were increased in patients with OAB wet compared to OAB dry (p <0.01). In 30 patients sPGE2 levels decreased significantly after BoNT-A treatment compared to baseline (2995 pg/ml vs. 1486 pg/ml, p <0.005). Patients reported an average drug effect of 9 month (0–19); incontinence pads were needed significantly less frequent (p < 0.05). 3 patients reported no postoperative effect. sPGE2 increased in two patients compared to initial levels, a single patient showed a remotely decreased sPGE2. Six patients were treated repeatedly with BoNT-A after showing an sPGE2 re-rise.
Conclusions
sPGE2-level is increased in patients with OAB. We could prove a significant decrease of sPGE2 after BoNT-A treatment. In this small cohort we could demonstrate a correlation between OAB and sPGE2, especially in the non-responder group. The use of sPGE2 as a biomarker in diagnostics and follow-up after therapy seems promising. To what extent sPGE2 can be useful as such needs to be examined prospectively in a larger population.
Similar content being viewed by others
Background
The prevalence of idiopathic overactive bladder syndrome (OAB) in Europe is estimated at 16% in men and women [1]. Sixty-eight percent of the women and 60% of the men are bothered by OAB. It is a serious problem that negatively affects the quality of life [1, 2]. According to the International Continence Society (ICS), OAB syndrome is defined as urinary urgency usually accompanied by frequency and nocturia, with or without urge incontinence, in the absence of causative infection or pathological conditions [3]. The reasons for these symptoms are still unknown, although already many studies could uncover compounds and pathways inducing detrusor contractions and/or detrusor over activity (DO) [4–6].
One of these compounds originates from arachidonic acid. Prostaglandin E2 (PGE2) is a cytoprotective eicosanoid, which is synthesized de novo from the detrusor muscle or urothelium [7]. It has been shown that PGE2 is increased in carcinogenesis, urinary tract infections and overactive bladder syndrome [8, 9]. The urinary PGE2 level is significantly increased in OAB and correlates negatively with the maximum cystometric capacity [10]. In rats, intravesical instillation of PGE2 triggers detrusor contractions, but topical application to the urethra leads to urethral relaxation in rats [11]. Bladder storage dysfunction and elevated urinary PGE2 levels have been proven to be enhanced in rats after causing severe damage to the urothelial barrier [12].
Once a patient is diagnosed with OAB syndrome, lifestyle modifications and bladder retraining is suggested. Pharmacological treatments are needed to improve quality of life if behavioural training fails. Due to many side effects the majority of patients discontinue anticholinergic medication [13]. Botulinum toxin (BTX) is a potent neurotoxin, used to treat different pathologies such as blepharospasm or spastic torticollis. It attacks one of the fusion proteins (SNAP-25, syntaxin or synaptobrevin) at the neuromuscular junction, preventing vesicles from anchoring to the membrane to release acetylcholine. By inhibiting acetylcholine release, the toxin interferes with nerve impulses and causes flaccid paralysis of muscles [14].
BTX type A (BoNT-A) and Onabotulinum toxin are used to treat neurogenic and/or overactive bladder when oral anticholinergic medication is not successful or not tolerated.
Injected into the detrusor muscle, it reduces urgency and frequency and improves quality of life. Common but rare side effects are urinary retention and urinary tract infections [15–17].
Cause and development of OAB are still unknown, thus further studies are needed to enhance diagnosis of OAB as well as to identify markers useful for daily clinical routine in verifying the presence of OAB and establishing treatment monitoring [18–20].
The aim of this prospectively designed study was to test PGE2 in OAB and its changes after BoNT-A therapy.
Methods
Patients
56 patients (48 female, 8 male, mean age of 63 years, range 18–87) with the typical symptoms of OAB were enrolled in this study (Group 1).
Overactive bladder specifically is defined as urgency, with or without urge incontinence, usually with frequency and nocturia [3]. So, all patients in the group of cases showed each of the symptoms with or without urge incontinence and have been in treatment accordingly. All patients were non-responder to medical treatment or discontinued anticholinergic therapy due to severe side effects. None of them underwent BoNT-A therapy before. The patients underwent a washout either by getting no more anticholinergic medication before treatment with BoNT-A, or terminated taking the medication after application of BoNT-A immediately. So until the full insertion of the BoNT-A effect after 14 days a sufficient wash out has taken place in any case.
In addition, none of the patients took medications with anticholinergic side effects in the investigated group.
31 healthy persons (19 female, 12 male, mean age of 59 years, range 21–72) with normal bladder function served as control group (Group 2). None of the control group showed lower urinary tract symptoms in the 14 days before as well on the day of blood collection.
Patient data are summarized in Table 1.
The study was approved by the ethical review committee of the Philipps University Marburg (AZ 12/11). Informed written consent was obtained from all participants before collecting blood samples for measurement.
Inclusion criteria
Inclusion criteria were urodynamic proved non-neurogenic overactive bladder with detrusor hyperactivity or hypersensitive low capacity bladder without detrusor hyperactivity. Detrusorhyperactivity was diagnosed if a pattern of bladder muscle contraction was observed while urodynamics correlated to the symptoms of overactive bladder. On the other side hypersensitive low capacity bladder without detrusor hyperactivity was defined as the occurrence of early urgency before filling to 150 ml and a maximum bladder capacitiy less than 350 ml without pattern of bladder muscle contraction during urodynamics.
All patients with proven neurogenic bladder dysfunction, chronic pelvic pain, prostate hyperplasia (weight >30 gramm) or bladder outlet obstructions were not included in this study.
Evaluation included patient history, urine analysis, a voiding diary and urodynamic studies. Patients were only included into the control group if patient history and urine analysis were without pathological findings and “International Consultation on Incontinence Questionnaire – Short Form” (ICIQ-SF) and “Kings Health Questionnaire” (KHQ) scores showed no impaired quality of life.
All patients were investigated thoroughly and excluded if they did not meet the criteria.
BoNT-A application
BoNT-A (500 MU Dysport®, Ipsen Pharma, Ettlingen, Germany) was injected into the detrusor muscle including trigone at 20 different locations in general or local anaesthesia under visual control.
Therapeutic success
Before and 4 weeks after treatment therapeutic success was assessed on the basis of standardized questionnaires ICIQ-SF and KHQ. Additionally, all patients were interviewed face-to-face about their individual quality of life, changes in number of used pads and leakage before and after BoNT-A treatment.
Sample handling and PGE2 measurement
Before and 4 weeks after BoNT-A treatment, blood samples were collected in serum-gel-tubes and processed within 1 h. After centrifugation (3500 rpm for 10 min) serum samples were aliquoted and stored at -20°C. After defrosting, all samples were diluted (150 μL of sample +300 μL of Calibrator Diluent). Serum PGE2 (sPGE2) blood levels were measured at room temperature (18 – 23°C) by a commercialized enzyme linked immunosorbent assay (PGE2 Immunoassay, R&D Systems, Minneapolis, USA). Standard (25,000 pg/mL) was used to produce a dilution series (2500 pg/mL standard served as high standard, Calibrator Diluent as zero standard). The Microplate was prepared with Calibrator Diluent, zero standard and sample according to manufacturer’s instructions. After 1 h incubation at room temperature on a microplate shaker PGE2 Conjugate (50 μl) was added to each well, a 2 h incubation followed. Each well was washed with Wash Buffer (400 μl). Remaining Wash Buffer was aspirated and the plate blotted. Substrate Solution was added to each well (200 μl) and incubated for 30 minutes at room temperature. Stop Solution (100 μL) was added to each well. The optical density of each well was determined within 30 minutes, using a microplate reader set to 450 nm. Wavelength correction was set to 540 or 570 nm.
Statistical analysis
The statistical data analysis was performed using the non-parametric Mann–Whitney U test and paired t-test to compare the different groups and the individual changes (IBM SPSS® Statistics for Windows Version 22, Ehningen, Germany).
Results
General
Baseline data and sPGE2 levels were available for all 56 patients before BoNT-A therapy, hereof 44 with wet OAB (Group 1A), and 12 with dry OAB (Group 1B). Median Follow-up was 28 month (range 15–32). For 30 patients control visits 4 weeks after BoNT-A therapy were realized in order to assess the sPGE2 course in postoperative samples. In this group median follow-up was 30 month (range 15–32).
Therapeutic success
OAB symptoms improved. This is reflected in significantly decreased urinary frequency (p < 0.01) and significant reduction of incontinence episodes (p < 0.05). Pads were needed significantly less frequent (4 (range 1–18) vs. 1(range 0–8), p < 0,05).
KHQ indicated a significant improvement of quality of life in all individual life situations (p < 0.05).
Mean duration of BoNT-A effect was 9 month (range 0–19). 3 patients (2 female, 1 male) reported no adequate effects after BoNT-A treatment and were ranked as non-responders.
sPGE2 levels before BoNT-A
The average concentration of sPGE2 in Group 1 was significantly higher (2749.5 pg/ml, SD 2375.7, range 332–9422) compared to Group 2 (1674.3 pg/ml SD 873, range 611–4038, p < 0.005, see Figure 1).
Subgroup analysis showed significantly increased sPGE2 levels in Group 1A compared to Group 1B (3241 pg/ml versus 1734 pg/ml, p < 0.01).
sPGE2 levels after BoNT-A
Postoperative sPGE2 levels could be determined in 30 patients of Group 1. Compared to baseline, mean sPGE2 levels significantly decreased 4 weeks after BoNT-A therapy (2995 pg/ml, SD 2022, range 389–8861 versus 1486 pg/ml, SD 2414, range 201–11286; p <0.005, see Figure 2).
sPGE2 levels of the different groups are summarized in Table 2.
Decrease of sPGE2 levels was correlated with the mean duration of drug effect (9 month). The subgroup with a shorter drug effect <9 month presented a significantly lower sPGE2 decrease (22.2%) compared to patients with a drug effect >9 month (57.4%, p < 0.05).
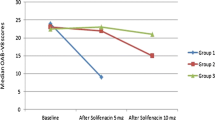
Additionally, in 2 of 3 non-responders (66.6%) sPGE2 levels increased after treatment up to 51% compared to baseline (Pat 1: 1815 pg/ml to 2749 pg/ml (+51.5%)/Pat 2: 1797 pg/ml to 2333 pg/ml (+29.8%)).Percental decrease/increase of each patient is illustrated in Figure 3.
In the study period 6 patients (5 female, 1 male, mean age of 63.7 years, range 40–76) re-visited our department due to progressively increasing urgency and frequency as the BoNT–A effects wore off after a median time of 8.5 month (range 6–12). Measurements confirmed a re-rise of sPGE2 in all patients after initial sPGE2 decrease. After reapplication of BoNT-A a re-decrease of sPGE 2 could be detected.
Discussion
OAB represents a rising health problem massively affecting quality of life. Intravesical BoNT–A administration has been shown to be a safe and effective therapy for refractory idiopathic OAB. It is a reasonable treatment of patients impossible to take anticholinergic medication [15–17, 21, 22]. To date several studies tried to elucidate cause and mechanism of OAB, but it is still poorly understood [4, 18, 19, 23]. Up to now a marker for OAB is lacking. In general a biomarker should be able to indicate and define both the presence and severity of a disease as well as progression/relapse and response on therapy [20]. Prostanoids are inflammatory mediators induced by mitogens or proinflammatory agents. PGE2 associated physiological responses are found in the whole human system. Typical prostanoid-induced actions are smooth muscle relaxation and contraction and neuromodulation (inhibition or release of neurotransmitters, regulation of inflammatory mediation, thermoregulation or sleep induction). In the urinary bladder, PGE2 is released from the urothelium, smooth muscle [24, 25], glial cells and neurons as required [26, 27]. Our results clearly demonstrate the relationship between PGE2 and OAB reflected in significantly elevated serum levels of PGE2 in patients suffering from OAB. It is particularly noticeable that there is a wide range of sPGE2 levels with pronounced inter-individual differences. These findings are in line with studies trying to find a urinary marker to differentiate OAB, DO or interstitial cystitis (IC)/bladder pain syndrome (BPS). Recent studies showed significantly increased urinary PGE2 in male and female OAB patients, underlining the important but still unclear role of PGE2 [5, 8, 28]. Moreover, our data detect a correlation of sPGE2 levels with the severity of OAB reflected by significant higher levels in wet compared to dry OAB. This possible potential to differentiate between vairous OAB stages reveals PGE2 as promising for future use as a marker and additional helpful tool for therapeutic decision in OAB.
For the first time we present data that sPGE2 development is influenced by intravesical BoNT-A therapy in OAB. We found a significant decrease of sPGE2 levels 4 weeks after BoNT-A application. Furthermore, we detected a correlation between the success of therapy as well as the degree and duration of decreased sPGE2 levels. A shorter drug effect (<9 month) was significantly associated with less sPGE decrease. Non-responders even displayed a sPGE2 increase or only a slight decrease. A recent study examined PGE2 urine levels in female OAB patients treated with anticholinergics and no significant changes of PGE2 urine levels could be detected after 4 weeks of treatment [28]. These differences in PGE2 detection in urine and serum may be due to the different methods and body fluids used and due to the diverging administered therapeutic substances. But how far are local urinary levels of the inflammatory mediators relevant to the inflammatory process at all? In particular, anticholinergics and BoNT-A are acting by different receptor mediated signal transduction pathways affecting Ca++ influx with impact on PGE2 release [29, 30]. The regulation and action of prostaglandins in the urinary bladder are still obscure. In addition, we verified a re-rise of sPGE2 when BoNT-A effects wore off and OAB relapsed. A limitation of this prospective single-center examination was the relatively small patient cohort of 58 OAB-patients. These first promising results, that show the potential of sPGE2 as a biomarker in OAB, have to be confirmed in further investigations with a larger population.
Conclusions
These promising first data are indicate the helpfulness of sPGE2 in OAB. sPGE2 seems to have the potential as a biomarker for OAB defining the presence, severity and response to therapy and relapse. Future investigations have to evaluate the relevance of sPGE2 to diagnose and monitor patients with OAB. BoNT-A treated patients could be monitored using sPGE2 as an early marker to anticipate decreasing effects of BoNT–A in combination with a voiding diary. Regarding the non-responder group, an efficient monitoring could filter these patients and lead to an alternative treatment. To what extent sPGE2 can be useful as such needs to be examined in further investigations.
Consent
Written informed consent was obtained from the patient for the publication of this report and any accompanying images.
References
Milsom I, Abrams P, Cardozo L, Roberts RG, Thuroff J, Wein AJ: How widespread are the symptoms of an overactive bladder and how are they managed? A population-based prevalence study. BJU Int. 2001, 87: 760-766.
Milsom I, Kaplan SA, Coyne KS, Sexton CC, Kopp ZS: Effect of bothersome overactive bladder symptoms on health-related quality of life, anxiety, depression, and treatment seeking in the United States: results from EpiLUTS. Urology. 2012, 80: 90-96. 10.1016/j.urology.2012.04.004.
Abrams P, Cardozo L, Fall M, Griffiths D, Rosier P, Ulmsten U, Van Kerrebroeck P, Victor A, Wein A, Standardisation Sub-Committee of the International Continence Society: The standardisation of terminology in lower urinary tract function: report from the standardisation sub-committee of the International Continence Society. Urology. 2003, 61: 37-49. 10.1016/S0090-4295(02)02243-4.
Knippschild S, Frohme C, Olbert P, Hofmann R, Hegele A: Value of nerve growth factor levels in overactive bladder syndrome: alterations after botulinum toxin therapy. Urologe A. 2012, 51: 379-383. 10.1007/s00120-011-2726-0.
Liu HT, Tyagi P, Chancellor MB, Kuo HC: Urinary nerve growth factor but not prostaglandin E2 increases in patients with interstitial cystitis/bladder pain syndrome and detrusor overactivity. BJU Int. 2010, 106: 1681-1685. 10.1111/j.1464-410X.2009.08851.x.
Cartwright R, Afshan I, Derpapas A, Vijaya G, Khullar V: Novel biomarkers for overactive bladder. Nat Rev Urol. 2011, 8: 139-145. 10.1038/nrurol.2011.7.
Rastogi P, Rickard A, Dorokhov N, Klumpp DJ, McHowat J: Loss of prostaglandin E2 release from immortalized urothelial cells obtained from interstitial cystitis patient bladders. Am J Physiol Renal Physiol. 2008, 294: F1129-F1135. 10.1152/ajprenal.00572.2007.
Kim JC, Park EY, Hong SH, Seo SI, Park YH, Hwang TK: Changes of urinary nerve growth factor and prostaglandins in male patients with overactive bladder symptom. Int J Urol. 2005, 12: 875-880. 10.1111/j.1442-2042.2005.01140.x.
Kim JC, Park EY, Seo SI, Park YH, Hwang TK: Nerve growth factor and prostaglandins in the urine of female patients with overactive bladder. J Urol. 2006, 175: 1773-1776. 10.1016/S0022-5347(05)00992-4.
Takagi-Matsumoto H, Ng B, Tsukimi Y, Tajimi M: Effects of NSAIDs on bladder function in normal and cystitis rats: a comparison study of aspirin, indomethacin, and ketoprofen. J Pharmacol Sci. 2004, 95: 458-465. 10.1254/jphs.FP0040098.
Yokoyama O, Miwa Y, Oyama N, Aoki Y, Ito H, Akino H: Antimuscarinic drug inhibits detrusor overactivity induced by topical application of prostaglandin E2 to the urethra with a decrease in urethral pressure. J Urol. 2007, 178: 2208-2212. 10.1016/j.juro.2007.06.044.
Shioyama R, Aoki Y, Ito H, Matsuta Y, Nagase K, Oyama N, Miwa Y, Akino H, Imamura Y, Yokoyama O: Long-lasting breaches in the bladder epithelium lead to storage dysfunction with increase in bladder PGE2 levels in the rat. Am J Physiol Regul Integr Comp Physiol. 2008, 295: R714-R718. 10.1152/ajpregu.00788.2007.
Brostrom S, Hallas J: Persistence of antimuscarinic drug use. Eur J Clin Pharmacol. 2009, 65: 309-314. 10.1007/s00228-008-0600-9.
Andersson KE: New developments in the management of overactive bladder: focus on mirabegron and onabotulinumtoxinA. Ther Clin Risk Manag. 2013, 9: 161-170.
Frohme C, Varga Z, Olbert P, Schrader AJ, Hofmann R, Hegele A: Effects of botulinum toxin type A in the single and repeated treatment of overactive bladder. A prospective analysis. Urologe A. 2010, 49: 639-644. 10.1007/s00120-009-2208-9.
Nitti VW, Dmochowski R, Herschorn S, Sand P, Thompson C, Nardo C, Yan X, Haag-Molkenteller C, EMBARK Study Group: OnabotulinumtoxinA for the treatment of patients with overactive bladder and urinary incontinence: results of a phase 3, randomized, placebo controlled trial. J Urol. 2013, 189: 2186-2193. 10.1016/j.juro.2012.12.022.
Mohee A, Khan A, Harris N, Eardley I: Long-term outcome of the use of intravesical botulinum toxin for the treatment of overactive bladder (OAB). BJU Int. 2013, 111: 106-113. 10.1111/j.1464-410X.2012.11282.x.
Dickson MJ, Anders NR, Cox S: Overactive bladder symptoms have a variety of causes. BMJ. 2012, 344: e3191-10.1136/bmj.e3191.
Dmochowski RR: The puzzle of overactive bladder: controversies, inconsistencies, and insights. Int Urogynecol J Pelvic Floor Dysfunct. 2006, 17: 650-658. 10.1007/s00192-005-0032-3.
Bhide AA, Cartwright R, Khullar V, Digesu GA: Biomarkers in overactive bladder. Int Urogynecol J. 2013, 24: 1065-1072. 10.1007/s00192-012-2027-1.
Coyne KS, Sexton CC, Irwin DE, Kopp ZS, Kelleher CJ, Milsom I: The impact of overactive bladder, incontinence and other lower urinary tract symptoms on quality of life, work productivity, sexuality and emotional well-being in men and women: results from the EPIC study. BJU Int. 2008, 101: 1388-1395. 10.1111/j.1464-410X.2008.07601.x.
Tincello DG, Kenyon S, Abrams KR, Mayne C, Toozs-Hobson P, Taylor D, Slack M: Botulinum toxin a versus placebo for refractory detrusor overactivity in women: a randomised blinded placebo-controlled trial of 240 women (the RELAX study). Eur Urol. 2012, 62: 507-514. 10.1016/j.eururo.2011.12.056.
Wagg AS, Cardozo L, Chapple C, De Ridder D, Kelleher C, Kirby M, Milsom I, Vierhout M: Overactive bladder syndrome in older people. BJU Int. 2007, 99: 502-509. 10.1111/j.1464-410X.2006.06677.x.
Masunaga K, Yoshida M, Inadome A, Iwashita H, Miyamae K, Ueda S: Prostaglandin E2 release from isolated bladder strips in rats with spinal cord injury. Int J Urol. 2006, 13: 271-276. 10.1111/j.1442-2042.2006.01274.x.
Pinna C, Zanardo R, Puglisi L: Prostaglandin-release impairment in the bladder epithelium of streptozotocin-induced diabetic rats. Eur J Pharmacol. 2000, 388: 267-273. 10.1016/S0014-2999(99)00833-X.
Maggi CA, Giuliani S, Conte B, Furio M, Santicioli P, Meli P, Gragnani L, Meli A: Prostanoids modulate reflex micturition by acting through capsaicin-sensitive afferents. Eur J Pharmacol. 1988, 145: 105-112. 10.1016/0014-2999(88)90221-X.
Narumiya S, Sugimoto Y, Ushikubi F: Prostanoid receptors: structures, properties, and functions. Physiol Rev. 1999, 79: 1193-1226.
Cho KJ, Kim HS, Koh JS, Kim JC: Changes in urinary nerve growth factor and prostaglandin E(2) in women with overactive bladder after anticholinergics. Int Urogynecol J. 2013, 24: 325-330. 10.1007/s00192-012-1854-4.
Morita T, Ando M, Kihara K, Kitahara S, Ishizaka K, Matsumura T, Oshima H: Effects of prostaglandins E1, E2 and F2 alpha on contractility and cAMP and cGMP contents in lower urinary tract smooth muscle. Urol Int. 1994, 52: 200-203. 10.1159/000282608.
Su X, Leon LA, Wu CW, Morrow DM, Jaworski JP, Hieble JP, Lashinger ES, Jin J, Edwards RM, Laping NJ: Modulation of bladder function by prostaglandin EP3 receptors in the central nervous system. Am J Physiol Renal Physiol. 2008, 295: F984-F994. 10.1152/ajprenal.90373.2008.
Pre-publication history
The pre-publication history for this paper can be accessed here:http://www.biomedcentral.com/1471-2490/14/85/prepub
Acknowledgements
E. Oplesch is to be acknowledged for her excellent technical assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
AH: Project development, Data collection, Data analysis, Manuscript writing. SK: Project development, Data collection, Data analysis, Manuscript writing. CF: Data collection, Manuscript editing. JH: Manuscript editing. PO: Manuscript editing. RH: Manuscript editing. All authors read and approved the final manuscript.
Axel Hegele, Sonja Knippschild contributed equally to this work.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Hegele, A., Knippschild, S., Frohme, C. et al. Changes in prostaglandin E2 in patients with idiopathic overactive bladder syndrome after botulinum toxin type A treatment: is there a clinical benefit?. BMC Urol 14, 85 (2014). https://doi.org/10.1186/1471-2490-14-85
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1471-2490-14-85






